Structural Characterization and Molecular Model Construction of Lignite: A Case of Xianfeng Coal
Abstract
1. Introduction
2. Coal Sample and Experimental Method
2.1. Characteristics of Coal Sample
2.2. Experiments
2.3. Lignite Structure Model Construction Method
3. Analysis and Results
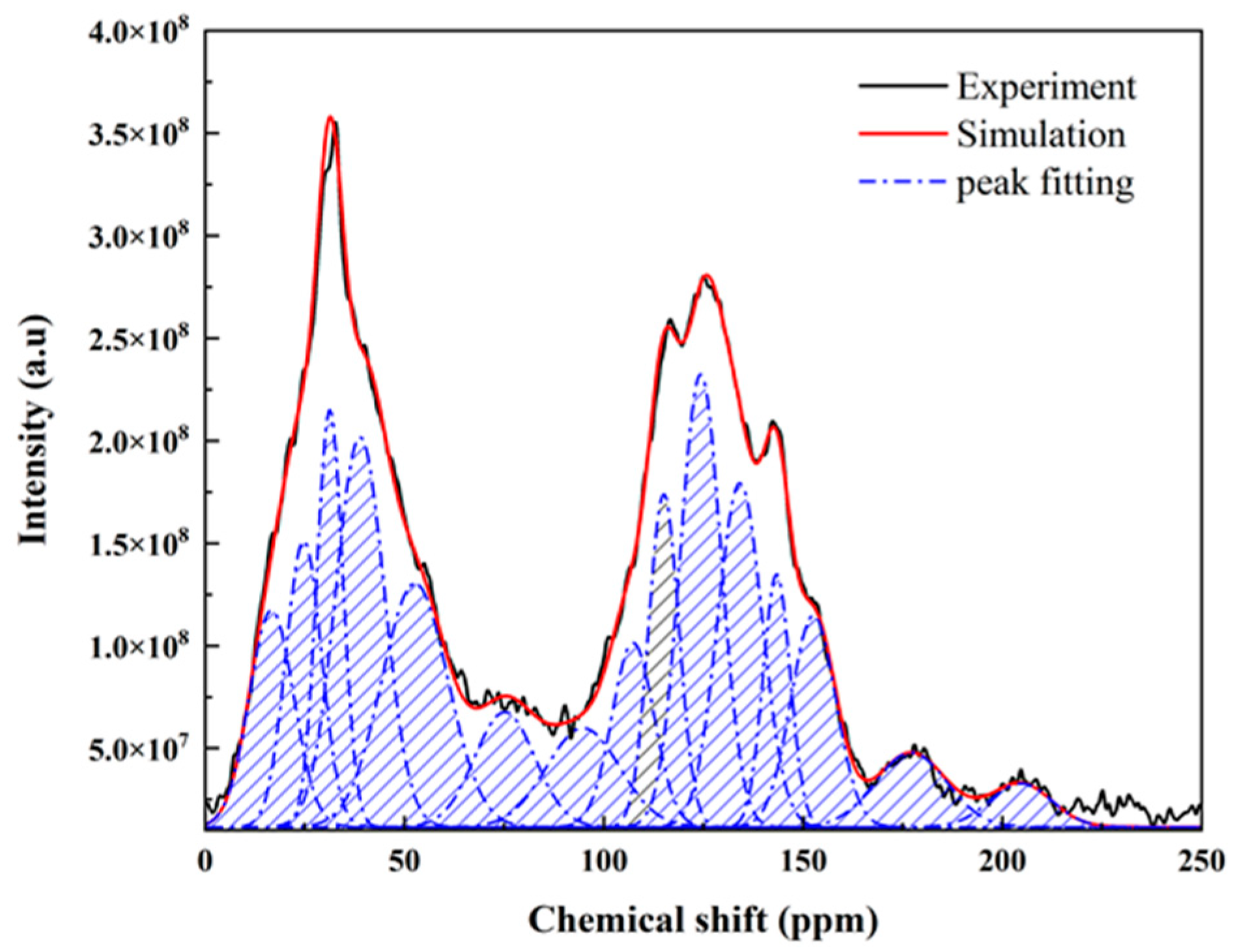
3.1. 13C NMR
3.2. FTIR
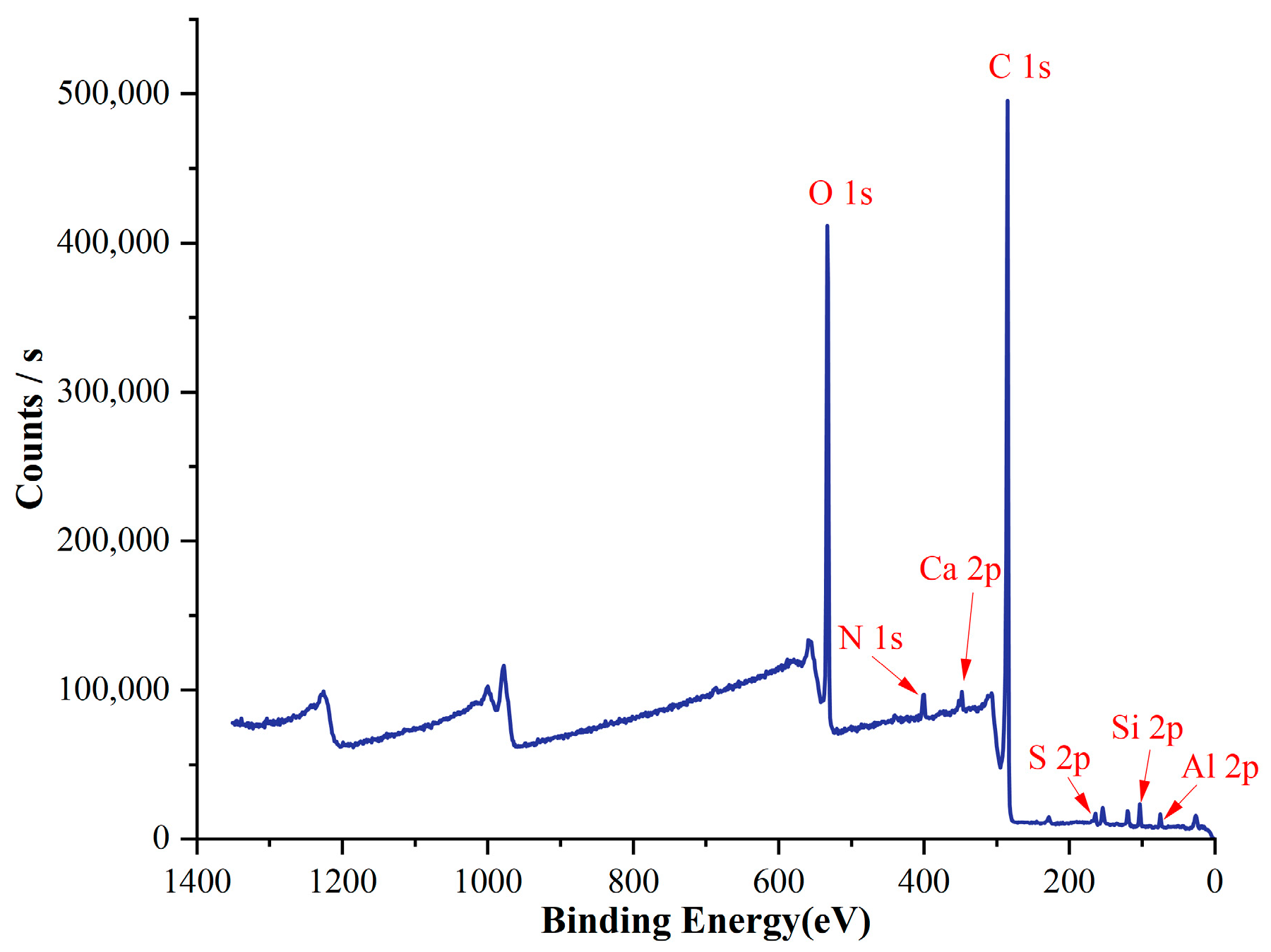
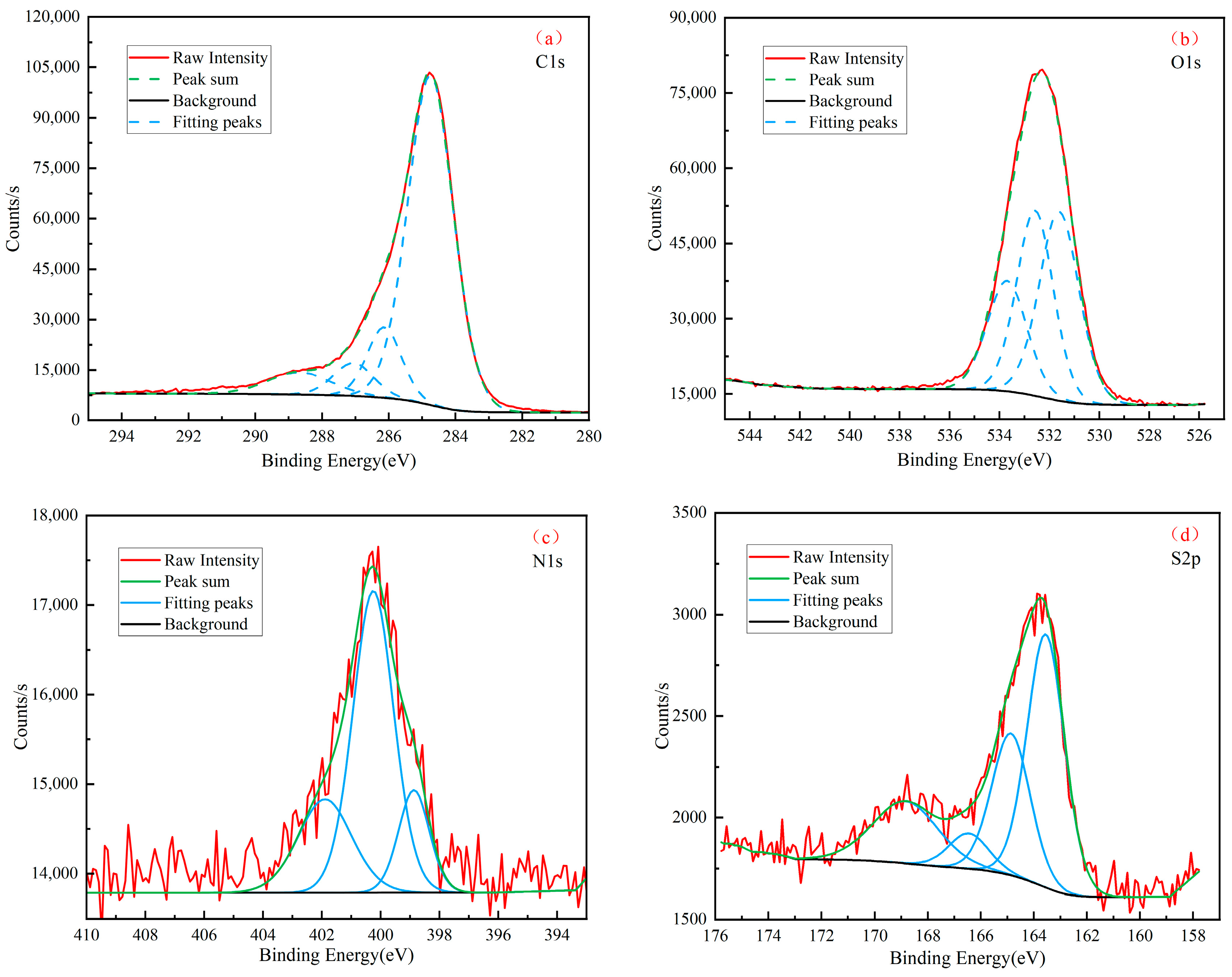
3.3. XPS
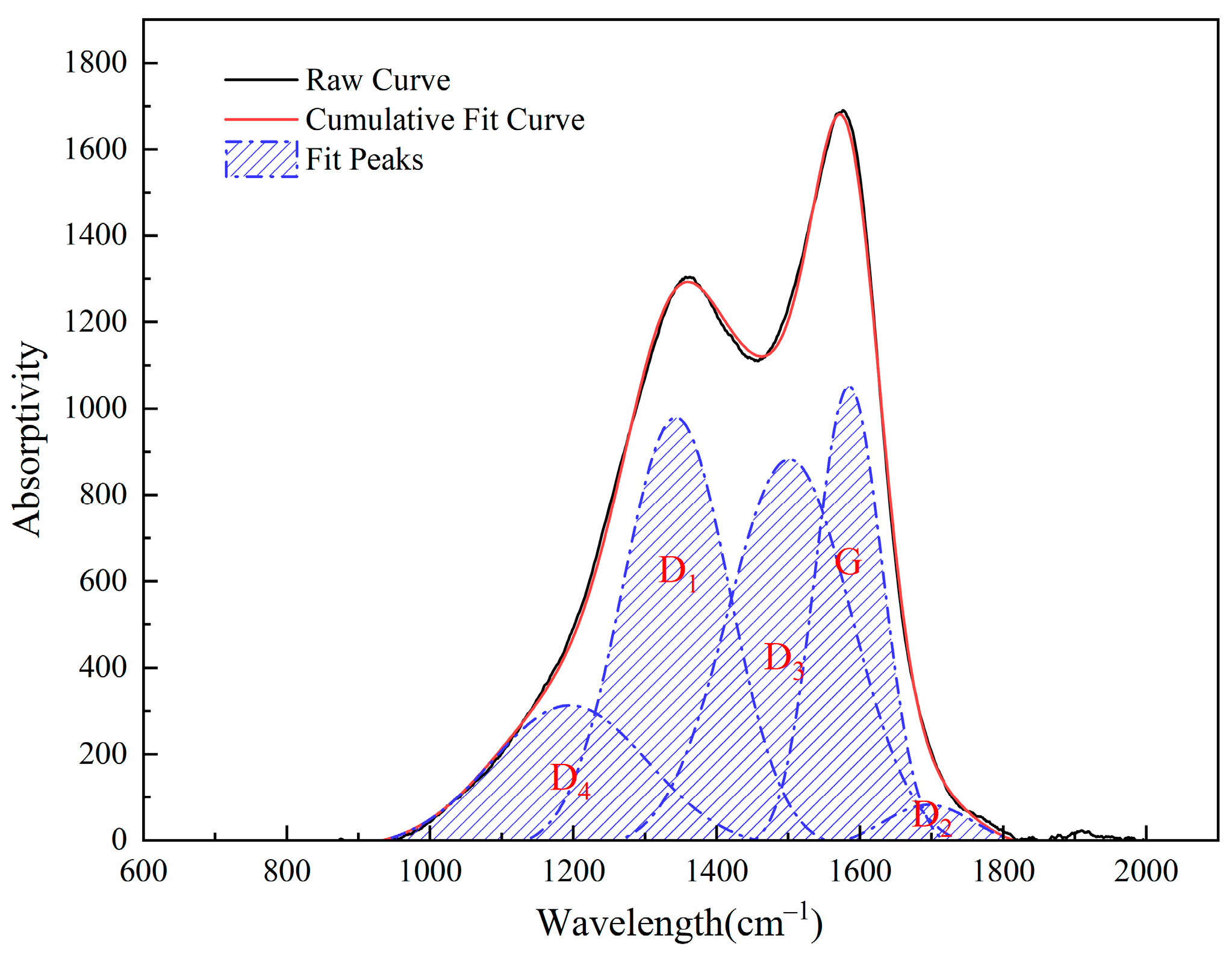
3.4. Raman
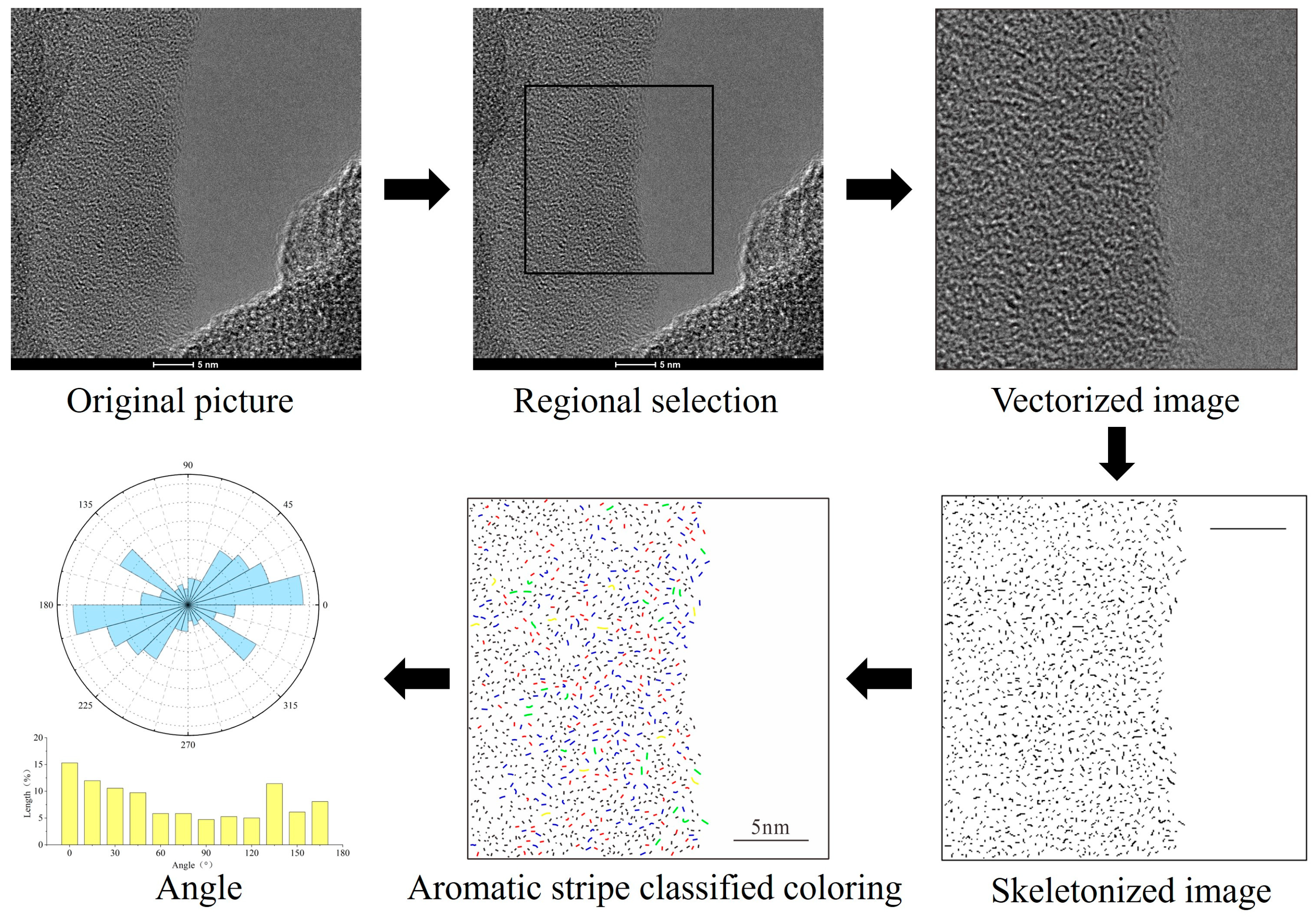
3.5. HRTEM
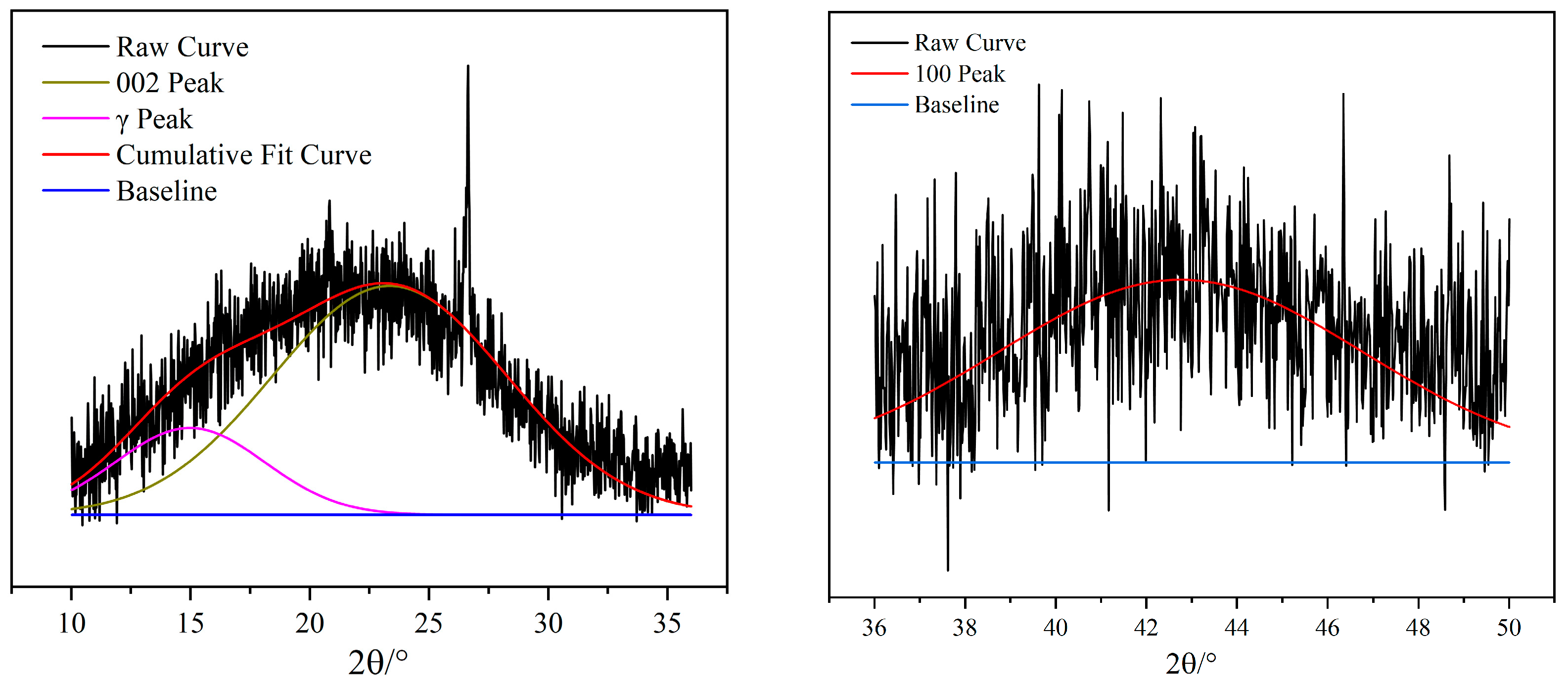
3.6. XRD
4. Construction and Model Validation of Lignite Molecular Structure Model
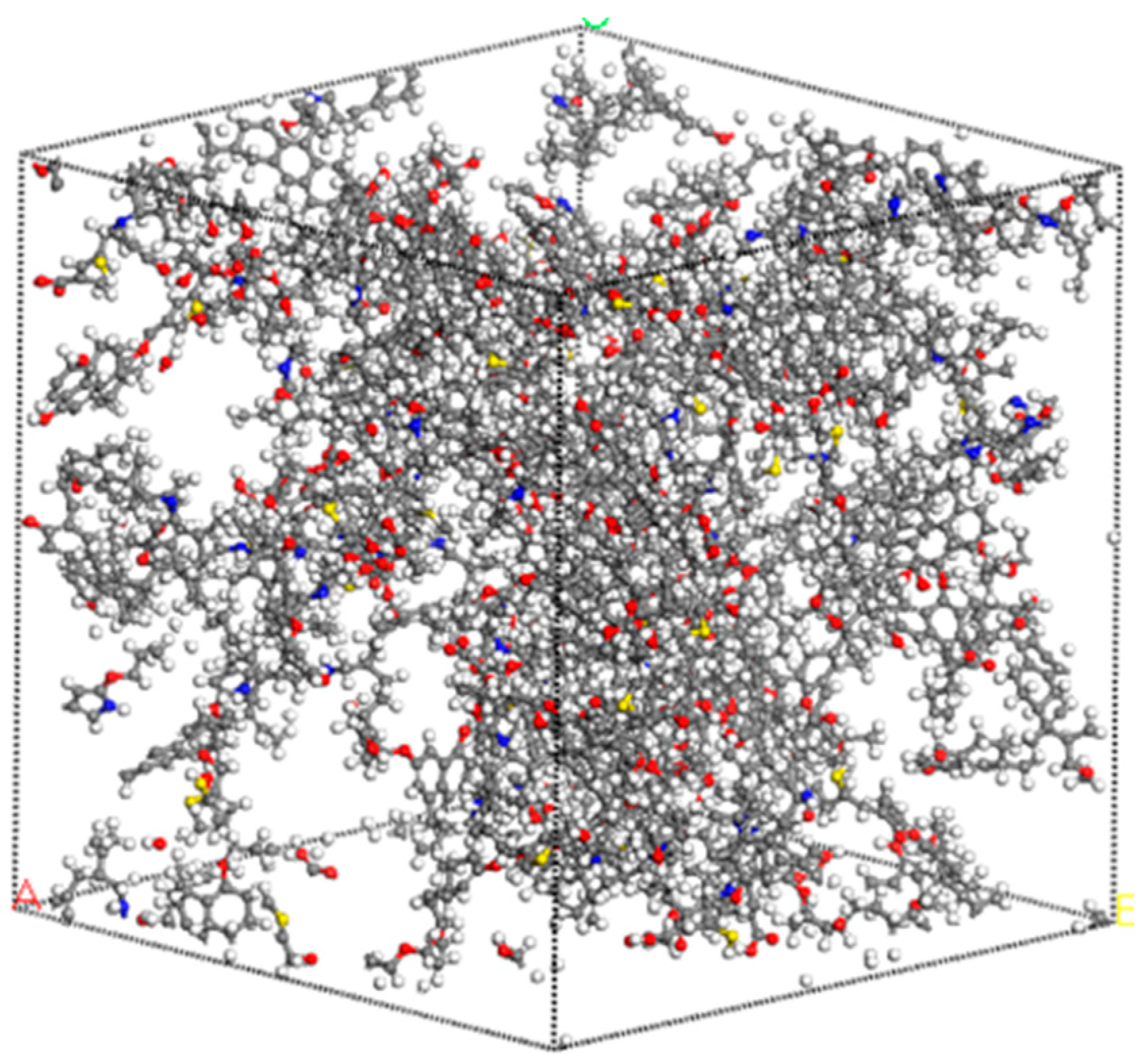
4.1. Construction of a Molecular Structure Model of Lignite
4.2. Optimization and Validation of Coal Molecular Structure Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Guan, Y.; Li, C.; Shi, L.; Yang, S.; Rajasekhar Reddy, B.; Ye, G.; Zhang, Q.; Liew, R.K.; Zhou, J.; et al. Free-radical behaviors of co-pyrolysis of low-rank coal and different solid hydrogen-rich donors: A critical review. Chem. Eng. J. 2023, 474, 145900. [Google Scholar] [CrossRef]
- Meng, X.; Gao, M.; Chu, R.; Miao, Z.; Wu, G.; Bai, L.; Liu, P.; Yan, Y.; Zhang, P. Construction of a macromolecular structural model of Chinese lignite and analysis of its low-temperature oxidation behavior. Chin. J. Chem. Eng. 2017, 25, 1314–1321. [Google Scholar] [CrossRef]
- Bian, J.; Li, X.; Zeng, F.; Wang, X. Construction and Evaluation of a Medium-Rank Coal Molecular Model Using a Hybrid Experimental–Simulation–Theoretical Method. Energy Fuels 2019, 33, 12905–12915. [Google Scholar] [CrossRef]
- Landais, P.; Gerard, L.; Chyi, L.L.; Medlin, J.H.; Coleman, S.L. Coalification stages from confined pyrolysis of an immature humic coal. Int. J. Coal Geol. 1996, 30, 285–301. [Google Scholar] [CrossRef]
- Okolo, G.N.; Neomagus, H.W.J.P.; Everson, R.C.; Roberts, M.J.; Bunt, J.R.; Sakurovs, R.; Mathews, J.P. Chemical-structural properties of South African bituminous coals: Insights from wide angle XRD-carbon fraction analysis, ATR-FTIR, solid state 13C NMR, and HRTEM techniques. Fuel 2015, 158, 779–792. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, J.; Zhu, J.; Zhou, Q.; Wang, Z.; Zhou, J.; Cen, K. Effect of microwave irradiation on the propensity for spontaneous combustion of Inner Mongolia lignite. J. Loss Prevent. Proc. 2016, 44, 390–396. [Google Scholar] [CrossRef]
- Lin, H.; Li, K.; Zhang, X.; Wang, H. Structure Characterization and Model Construction of Indonesian Brown Coal. Energy Fuels 2016, 30, 3809–3814. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Li, H.; Yu, J.; Xie, W.; Wei, H. The molecular structure of Inner Mongolia lignite utilizing XRD, solid state 13C NMR, HRTEM and XPS techniques. Fuel 2017, 203, 764–773. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Wu, J. Pyrolysis characteristics and kinetics of low rank coals by distributed activation energy model. Energy Convers. Manag. 2016, 126, 1037–1046. [Google Scholar] [CrossRef]
- Jia, J.; Yang, Q.; Liu, B.; Wang, D. Structural characterization and macromolecular structure construction of non-caking coal in Chicheng Mine. Sci. Rep. 2023, 13, 16931. [Google Scholar] [CrossRef]
- Yin, N.; Song, Y.; Wang, C.; Zhang, X.; Wu, L.; Jing, X.; Zhou, J.; Lan, X. Molecular structure model construction and pyrolysis characterization of four typical coals: A case study. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 6837–6855. [Google Scholar] [CrossRef]
- GB/T6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. China Standard Press: Beijing, China, 2008.
- GB/T212-2008; Proximate Analysis of Coal. China Standard Press: Beijing, China, 2008.
- GB/T476-2008; Determination of Carbon and Hydrogen in Coal. China Standard Press: Beijing, China, 2008.
- GB/T19227-2008; Determination of Nitrogen in Coal. China Standard Press: Beijing, China, 2008.
- GB/T214-2007; Determination of Total Sulfur in Coal. China Standard Press: Beijing, China, 2008.
- Cui, X.; Yan, H.; Zhao, P.; Yang, Y.; Xie, Y. Modeling of molecular and properties of anthracite base on structural accuracy identification methods. J. Mol. Struct. 2019, 1183, 313–323. [Google Scholar] [CrossRef]
- Niekerk, D.V.; Mathews, J.P. Molecular representations of Permian-aged vitrinite-rich and inertinite-rich South African coals. Fuel 2010, 89, 73–82. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhang, R.; Zhang, Y. The molecular model of Marcellus shale kerogen: Experimental characterization and structure reconstruction. Int. J. Coal Geol. 2021, 246, 103833. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Zhu, D.; Chappell, M.A.; Miller, L.F.; Mao, J. Characterization of coals and their laboratory-prepared black carbon using advanced solid-state 13C nuclear magnetic resonance spectroscopy. Fuel Process. Technol. 2012, 96, 56–64. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, S.; Longhurst, P.; Yang, W.; Zheng, S. Molecular structure characterization of bituminous coal in Northern China via XRD, Raman and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119724. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Yang, J.; Liang, C. Investigation of changes in surface properties of bituminous coal during natural weathering processes by XPS and SEM. Appl. Surf. Sci. 2014, 293, 293–298. [Google Scholar] [CrossRef]
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef]
- Jia, J.; Wang, D.; Li, B. Study on molecular structure characteristic and optimization of molecular model construction of coal with different metamorphic grade. J. Mol. Struct. 2024, 1295, 136655. [Google Scholar] [CrossRef]
- Yan, J.; Lei, Z.; Li, Z.; Wang, Z.; Ren, S.; Kang, S.; Wang, X.; Shui, H. Molecular structure characterization of low-medium rank coals via XRD, solid state 13C NMR and FTIR spectroscopy. Fuel 2020, 268, 117038. [Google Scholar] [CrossRef]
- Ding, D.; Liu, G.; Fu, B. Influence of carbon type on carbon isotopic composition of coal from the perspective of solid-state 13C NMR. Fuel 2019, 245, 174–180. [Google Scholar] [CrossRef]
- Zhu, B.; Dong, X.; Fan, Y.; Ma, X.; Yao, S.; Fu, Y.; Chen, R.; Chang, M. Structural Characterization and Molecular Model Construction of High-Ash Coal from Northern China. Molecules 2023, 28, 5593. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Xie, R.; Liu, F.; Li, P.; Zong, Z. Structural Characterization of Typical Organic Species in Jincheng No. 15 Anthracite. Energy Fuels 2015, 29, 595–601. [Google Scholar] [CrossRef]
- Dun, W.; Guijian, L.; Ruoyu, S.; Xiang, F. Investigation of Structural Characteristics of Thermally Metamorphosed Coal by FTIR Spectroscopy and X-ray Diffraction. Energy Fuels 2013, 27, 5823–5830. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, J.; Yu, C.; Wang, X.; Zhou, Y.; Zheng, Y.; Ji, B.; Zhang, Q. Experimental and theoretical study on molecular structure construction of Hongliulin coal. Fuel 2023, 349, 128708. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, W. Characterization of surface properties of Inner Mongolia coal using FTIR and XPS. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1190–1194. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Wang, C.; Xing, Y.; Shi, K.; Wang, S.; Xia, Y.; Li, J.; Gui, X. Chemical Structure Characteristics and Model Construction of Coal with Three Kinds of Coalification Degrees. ACS Omega 2024, 9, 1881–1893. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, M.; Liu, X.; Xue, S. Model construction and optimization of coal molecular structure. J. Mol. Struct. 2023, 1290, 135960. [Google Scholar] [CrossRef]
- Marshall, C.P.; Edwards, H.G.M.; Jehlicka, J. Understanding the application of Raman spectroscopy to the detection of traces of life. Astrobiology 2010, 10, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Wu, C.; Song, Y.; Zhou, D.; Niu, Q.; Zhou, H.; Jiang, X. Structural characterization of high fidelity for bituminous and semi-anthracite: Insights from spectral analysis and modeling. Fuel 2022, 315, 123183. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Mathews, J.P.; Sharma, A. The structural alignment of coal and the analogous case of Argonne Upper Freeport coal. Fuel 2012, 95, 19–24. [Google Scholar] [CrossRef]
- Pan, J.; Lv, M.; Bai, H.; Hou, Q.; Li, M.; Wang, Z. Effects of Metamorphism and Deformation on the Coal Macromolecular Structure by Laser Raman Spectroscopy. Energy Fuels 2017, 31, 1136–1146. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; He, R. Mechanistic analysis of chemical structure evolution for coals under igneous intrusion. Fuel 2024, 357, 130055. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, F. Molecular Structure Characterization of CS2–NMP Extract and Residue for Malan Bituminous Coal via Solid-State 13C NMR, FTIR, XPS, XRD, and CAMD Techniques. Energy Fuels 2020, 34, 12142–12157. [Google Scholar] [CrossRef]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P.K. FT-IR and XRD analysis of coal from Makum coalfield of Assam. J. Earth Syst. Sci. 2007, 116, 575–579. [Google Scholar] [CrossRef]
- Qian, L.; Tao, C.; Ma, C.; Xue, J.; Guo, F.; Jia, X.; Yan, W. Construction of a Macromolecular Structure Model for Zhundong Subbituminous Coal. J. Mol. Struct. 2022, 1248, 131496. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, B.; Song, Y.; Hou, C. The tectonic stress-driving alteration and evolution of chemical structure for low- to medium-rank coals–by molecular simulation method. Arab. J. Geosci. 2019, 12, 726. [Google Scholar] [CrossRef]
- Feng, W.; Li, Z.; Gao, H.; Wang, Q.; Bai, H.; Li, P. Understanding the molecular structure of HSW coal at atomic level: A comprehensive characterization from combined experimental and computational study. Green Energy Environ. 2021, 6, 150–159. [Google Scholar] [CrossRef]






| Proximate Analysis/% | Ultimate Analysis/% | Atomic Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | Cdaf | Hdaf | Odaf | Ndaf | St,d | H/C | O/C | N/C | S/C |
| 4.22 | 5.66 | 49.47 | 47.67 | 70.31 | 5.47 | 19.73 | 1.90 | 2.44 | 0.933 | 0.210 | 0.023 | 0.013 |
| Number | Chemical Shift/δ | FWHM | Relative Area | Symbol | Carbon Type |
|---|---|---|---|---|---|
| 1 | 16.64 | 13.12 | 0.0617 | fal3 | Aliphatic methyl |
| 2 | 24.72 | 10.49 | 0.0653 | fala | Aromatic methyl |
| 3 | 31.28 | 7.69 | 0.0696 | fal2 | Methylene |
| 4 | 38.85 | 14.35 | 0.1217 | fal1 | Methine |
| 5 | 52.63 | 19.57 | 0.1036 | falO | Oxy-aliphatic carbons |
| 6 | 75.16 | 17.85 | 0.0445 | falO | Oxy-aliphatic carbons |
| 7 | 94.83 | 21.62 | 0.0464 | falO | Oxy-aliphatic carbons |
| 8 | 107.37 | 12.41 | 0.0497 | faH | Protonated aromatic carbons |
| 9 | 115.07 | 8.90 | 0.0644 | faH | Protonated aromatic carbons |
| 10 | 124.32 | 12.04 | 0.1185 | faH | Protonated aromatic carbons |
| 11 | 134.19 | 13.15 | 0.0983 | faB | Bridged carbons |
| 12 | 143.40 | 8.03 | 0.0441 | faS | Alkylated aromatic carbons |
| 14 | 177.14 | 21.07 | 0.0341 | faCC1 | Carboxyl |
| 15 | 204.65 | 18.28 | 0.0173 | faCC2 | Carboxyl |
| fal | fal3 | fala | fal2 | fal1 | falO |
|---|---|---|---|---|---|
| 0.5129 | 0.0617 | 0.0653 | 0.0696 | 0.1217 | 0.1945 |
| fa | faH | faB | faS | faO | faC |
| 0.4357 | 0.2325 | 0.0983 | 0.0441 | 0.0608 | 0.0513 |
| Wavenumber/cm−1 | Group |
|---|---|
| 3300 | hydrogen bond |
| 3030 | hydrogen bond–C–H– |
| 2950 | –CH3 |
| 1735, 1690–1720, 1650–1630, 1600, 1560–1590 | C=O |
| 2920, 2850 | Fatty bond–CH, CH2, –CH3 |
| 1600 | Aromatic ring stretching vibration |
| 1490 | Aromatic ring stretching vibration |
| 1450 | –CH2 and –CH3 bending vibration |
| 1375 | –CH3 |
| 1300–1110 | C–O stretching vibration, O–H bending vibration |
| 1100–1000 | Aliphatic ethers |
| 700–900 | Aromatic C–H bending vibration |
| Name | Peak BE | FWHM eV | Area (P) CPS.eV | Atomic % |
|---|---|---|---|---|
| C1s | 284.29 | 2.82 | 1,381,579.04 | 68.90 |
| O1s | 531.85 | 3.27 | 1,124,898.65 | 23.22 |
| N1s | 399.83 | 3.65 | 73,904.70 | 2.38 |
| Si2p | 102.09 | 2.72 | 42,064.26 | 2.09 |
| Al2p | 74.05 | 2.68 | 27,376.63 | 2.15 |
| S2p | 163.36 | 3.15 | 27,832.19 | 0.69 |
| Ca2p | 347.21 | 2.99 | 65,965.51 | 0.57 |
| Elemental | Functionality | Binding Energy/eV | Molar Content% |
|---|---|---|---|
| C1s | C–C, C–H | 284.78 | 82.13 |
| C–O | 286.48 | 7.51 | |
| O–C–O | 287.38 | 4.32 | |
| COO– | 288.58 | 6.03 | |
| O1s | C=O | 531.61 | 40.21 |
| –OH | 532.58 | 36.28 | |
| COO– | 533.69 | 23.51 | |
| N1s | Pyridinic nitrogen | 398.87 | 15.42 |
| Pyrrolic nitrogen | 400.25 | 60.16 | |
| Quaternary nitrogen | 401.89 | 24.42 | |
| S2p | Thiophenes | 163.55 | 46.47 |
| Sulphoxides | 164.87 | 26.90 | |
| Sulphones | 166.46 | 6.88 | |
| Sulphates | 168.84 | 19.75 |
| Aromatic Sheet | Grouping | MinL (Å) | MaxL (Å) | Mean (Å) | Freq (%) |
|---|---|---|---|---|---|
| Noise | <0.25 Å | / | / | / | / |
| Benzene | 0.25 Å–3.0 Å | 2.51 | 2.99 | 2.73 | 41.50 |
| Naphthalene | 3.0 Å–4.4 Å | 3.00 | 4.37 | 3.56 | 49.03 |
| Phenanthrene/Anthracene | 4.4 Å–5.9 Å | 4.42 | 5.87 | 4.93 | 6.69 |
| 2 × 2 | 5.9 Å–7.4 Å | 5.98 | 7.36 | 6.48 | 2.79 |
| Peak | 2θ/° | FWHM | Peak Shape |
|---|---|---|---|
| γ | 14.98 | 7.39 | Gaussian |
| 002 | 23.35 | 11.58 | Gaussian |
| 100 | 42.75 | 9.45 | Gaussian |
| d002 | Lc | La | Nave |
|---|---|---|---|
| 4.42 | 0.15 | 0.37 | 0.03 |
| Type | Aromatic Unit Structure | Number | Type |
|---|---|---|---|
| Naphthalene | 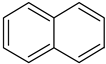 | 3 | Naphthalene |
| Anthracene | 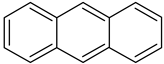 | 2 | Anthracene |
| Phenanthrene | 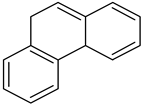 | 1 | Phenanthrene |
| Pyrrole | 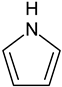 | 1 | Pyrrole |
| Thiophene |  | 1 | Thiophene |
| Type | Aromatic unit structure | Number | Type |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhu, Y.; Chen, S.; Wang, Y.; Song, Y. Structural Characterization and Molecular Model Construction of Lignite: A Case of Xianfeng Coal. Energies 2024, 17, 1049. https://doi.org/10.3390/en17051049
Shi Y, Zhu Y, Chen S, Wang Y, Song Y. Structural Characterization and Molecular Model Construction of Lignite: A Case of Xianfeng Coal. Energies. 2024; 17(5):1049. https://doi.org/10.3390/en17051049
Chicago/Turabian StyleShi, Ying, Yanming Zhu, Shangbin Chen, Yang Wang, and Yu Song. 2024. "Structural Characterization and Molecular Model Construction of Lignite: A Case of Xianfeng Coal" Energies 17, no. 5: 1049. https://doi.org/10.3390/en17051049
APA StyleShi, Y., Zhu, Y., Chen, S., Wang, Y., & Song, Y. (2024). Structural Characterization and Molecular Model Construction of Lignite: A Case of Xianfeng Coal. Energies, 17(5), 1049. https://doi.org/10.3390/en17051049






