Novel Batch and Repeated-Batch Butanol Fermentation from Sweet Sorghum Stem Juice by Co-Culture of Arthrobacter and Immobilized Clostridium in Scaled-Up Bioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Inoculum Preparation
2.2. Raw Materials
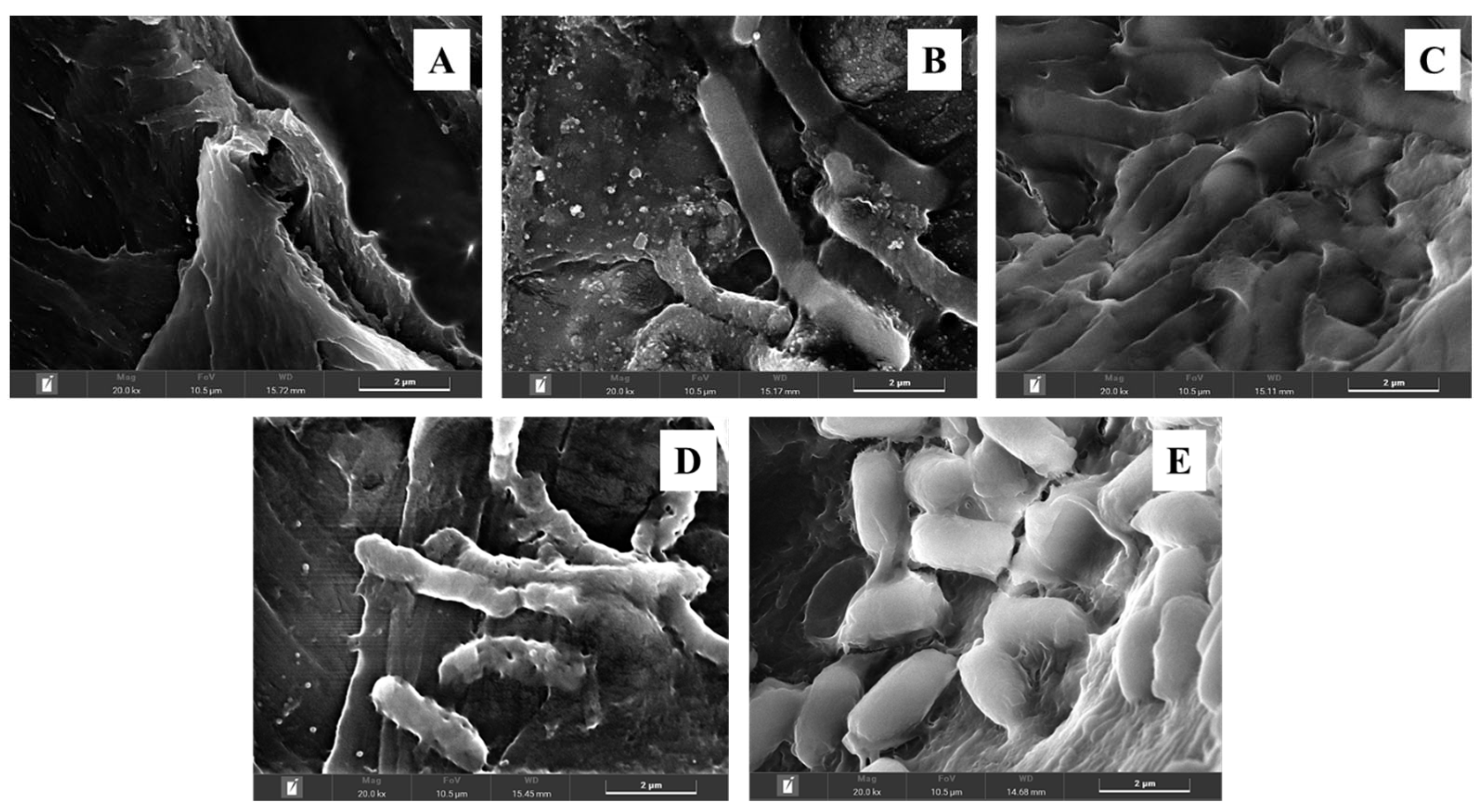
2.3. Carriers
2.4. Fermentation Procedures
2.4.1. Batch Butanol Fermentation by Co-Culture of Arthrobacter sp. and Immobilized C. beijerinckii Cells in Screw-Capped Bottles
2.4.2. Batch Butanol Fermentation by Co-Culture of Arthrobacter sp. and Immobilized C. beijerinckii Cells in Scaled-Up Bioreactors
2.4.3. Repeated-Batch Butanol Fermentation by Co-Culture of Arthrobacter sp. and Immobilized C. beijerinckii Cells
2.5. Analytical Methods
2.6. Statistical Analyses
3. Results and Discussion
3.1. Batch Butanol Fermentation by Co-Culture of Arthrobacter sp. and Immobilized C. beijerinckii Cells in Screw-Capped Bottles
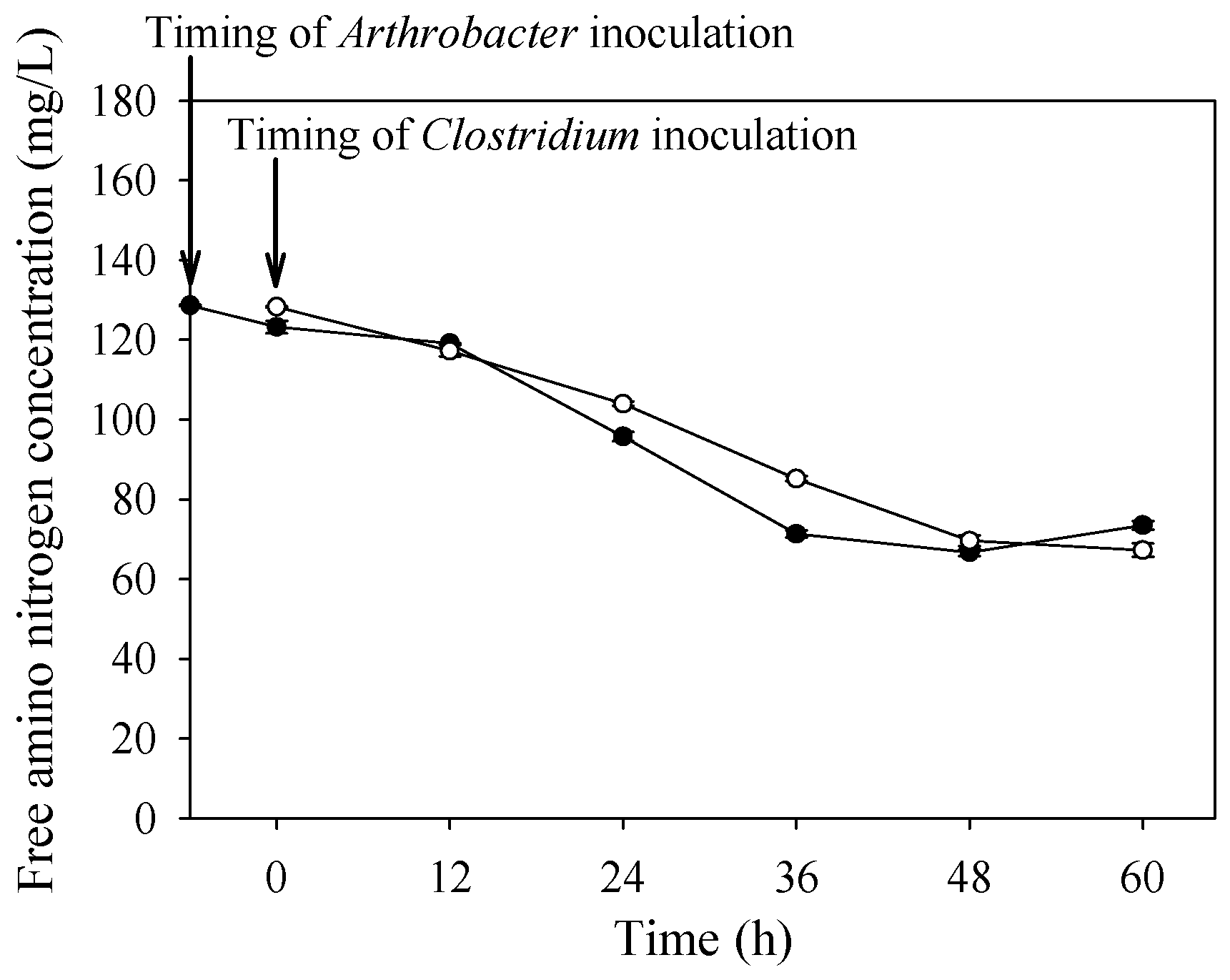
3.2. Batch Butanol Fermentation by Co-Culture in Scaled-Up Bioreactors
3.3. Repeated-Batch Butanol Fermentation in Co-Culture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.W.; Yu, W.S.; Zheng, Z.X.; Cheng, Y.S.; Li, S.Y. Waste valorization through acetone-butanol-ethanol (ABE) fermentation. J. Taiwan Inst. Chem. Eng. 2023, 105280. [Google Scholar] [CrossRef]
- Lee, H.Y.; You, T.S.; Chen, C.L. Energy efficient design of bio-butanol purification process from acetone butanol ethanol fermentation. J. Taiwan Inst. Chem. Eng. 2022, 130, 104015. [Google Scholar] [CrossRef]
- Xue, C.; Cheng, C. Chapter Two—Butanol Production by Clostridium; Li, Y., Ge, X., Eds.; Advances in Bioenergy: Oxford, UK, 2019; pp. 35–37. ISSN 2468-0125. [Google Scholar]
- Li, H.-G.; Zhang, Q.-H.; Yu, X.-B.; Wei, L.; Wang, Q. Enhancement of butanol production in Clostridium acetobutylicum SE25 through accelerating phase shift by different phases pH regulation from cassava flour. Bioresour. Technol. 2016, 201, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rochón, E.; Ferrari, M.D.; Lareo, C. Integrated ABE fermentation-gas striping process for enhanced butanol production from sugarcane-sweet sorghum juices. Biomass Bioenergy 2017, 98, 153–160. [Google Scholar] [CrossRef]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. 14-Biofuels: Their characteristics and analysis. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 277–325. ISBN 978-0-08-1024263. [Google Scholar]
- Oliva-Rodríguez, A.G.; Quintero, J.; Medina-Morales, M.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; Aroca, G.; Rios González, L.J. Clostridium strain selection for co-culture with Bacillus subtilis for butanol production from agave hydrolysates. Bioresour. Technol. 2019, 275, 410–415. [Google Scholar] [CrossRef]
- Tri, C.L.; Kamei, I. Butanol production from cellulosic material by anaerobic co-culture of white-rot fungus Phlebia and bacterium Clostridium in consolidated bioprocessing. Bioresour. Technol. 2020, 305, 123065. [Google Scholar] [CrossRef]
- Silva, C.R.; Souza, J.C.; Araújo, L.S.; Kagohara, E.; Garcia, T.P.; Pelizzari, V.H.; Andrade, L.H. Exploiting the enzymatic machinery of Arthrobacter atrocyaneus for oxidative kinetic resolution of secondary alcohols. J. Mol. Catal. B Enzym. 2012, 83, 23–28. [Google Scholar] [CrossRef]
- Daengbussadee, C.; Laopaiboon, L.; Kaewmaneewat, A.; Sirisantimethakom, L.; Laopaiboon, L. Novel methods using an Arthrobactor sp. to create anaerobic conditions for biobutanol production from sweet sorghum juice by Clostridium beijerinckii. Processes 2021, 9, 178. [Google Scholar] [CrossRef]
- López-Sandin, I.; Zavala-Gracía, F.; Gutiérrez-Soto, G.; Leza, H.R. Conversion of sweet sorghum juice to bioethanol. In Bioethanol: Biochemistry and Biotechnological Advances; Almanza, A.Y.H., Balagurusamy, N., Leza, H.R., Aguilar, C.N., Eds.; CSC Press: Boca Raton, FL, USA; Apple Academic Press Inc.: Palm Bay, FL, USA, 2023; pp. 315–337. ISBN 9781003277132. [Google Scholar]
- Umakanth, A.V.; Kumar, A.A.; Vermerris, W.; Tonapi, V.A. Chapter 16—Sweet Sorghum for Biofuel Industry; Breeding Sorghum for Diverse End Uses; Aruna, C., Visarada, K.B.R.S., Venkatesh Bhat, B., Tonapi, V.A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 255–270. ISBN 978-0-08-101879-8. [Google Scholar]
- Khalifa, K.K.; Al-Tabib, A.I.; Kalil, M.S. High yield of butanol production in repeated batch culture fermentation by Clostridium acetobutylicum YM1. J. Teknol. 2018, 1, 7–14. [Google Scholar] [CrossRef]
- Vieira, C.F.D.S.; Filho, F.M.; Filho, R.M. Isopropanol-butanol-ethanol (IBE) production in repeated-batch cultivation of Clostridium beijerinckii DSM 6423 immobilized on sugarcane bagasse. Fuel 2020, 263, 116708. [Google Scholar] [CrossRef]
- Chang, Z.; Cai, D.; Wang, Y.; Chen, C.J.; Fu, C.H.; Wang, G.Q.; Qin, P.Y.; Wang, Z.; Tan, T.W. Effective multiple stages continuous acetone-butanol-ethanol fermentation by immobilized bioreactors: Making full use of fresh corn stalk. Bioresour. Technol. 2016, 205, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Cai, D.; Wang, C.; Li, L.; Han, J.; Qin, P.; Wang, Z. Sweet sorghum bagasse as an immobilized carrier for ABE fermentation by using Clostridium acetobutylicum ABE 1201. RSC Adv. 2014, 4, 21819–21825. [Google Scholar] [CrossRef]
- Vichuviwat, R.; Boonsombuti, A.; Luengnaruemitchai, A.; Wongkasemjit, S. Enhanced butanol production by immobilized Clostridium beijerinckii TISTR 1461 using zeolite 13X as a carrier. Bioresour. Technol. 2014, 172, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, P.; Chen, C.; Wang, Y.; Hu, S.; Cui, C.; Qin, P.; Tan, T. Effect of chemical pretreatments on corn stalk bagasse as immobilizing carrier of Clostridium acetobutylicum in the performance of a fermentation-pervaporation coupled system. Bioresour. Technol. 2016, 220, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.; Zhou, W.; Fan, S.; Qiu, B.; Wang, Y.; Xiao, Z.; Tang, X.; Wang, W.; Jian, S.; Qin, Y. Coproduction of hydrogen and butanol by Clostridium acetobutylicum with the biofilm immobilized on porous particulate carriers. Int. J. Hydrogen Energy 2019, 44, 11617–11624. [Google Scholar] [CrossRef]
- Chacón, S.J.; Matias, G.; Ezeji, T.C.; Filho, R.M.; Mariano, A.P. Three-stage repeated-batch immobilized cell fermentation to produce butanol from non-detoxified sugarcane bagasse hemicellulose hydrolysates. Bioresour. Technol. 2021, 321, 124504. [Google Scholar] [CrossRef]
- Cai, D.; Chang, Z.; Gao, L.; Chen, C.; Niu, Y.; Qin, P.; Wang, Z.; Tan, T. Acetone– butanol–ethanol (ABE) fermentation integrated with simplified gas stripping using sweet sorghum bagasse as immobilized carrier. Chem. Eng. J. 2015, 277, 176–185. [Google Scholar] [CrossRef]
- Bangkokbiznews. Effects of Bamboo Chopsticks Waste. 2019. Available online: https://www.bangkokbiznews.com/pr-news/biz2u/268129 (accessed on 15 March 2020).
- Thanapornsin, T.; Laopaiboon, P.; Laopaiboon, L. An alternative approach to improve the butanol production efficiency from sweet sorghum stem juice using immobilized cell combined with an in situ gas stripping system. Fermentation 2022, 8, 464. [Google Scholar] [CrossRef]
- Thanapornsin, T.; Laopaiboon, L.; Laopaiboon, P. Capability of immobilized Clostridium beijerinckii for batch and repeated-batch butanol fermentation from sweet sorghum stem juice in various bioreactors. Bioresour. Technol. Rep. 2023, 23, 101590. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Abernathy, D.G.; Spudding, G.; Starcher, B. Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J. Inst. Brew. 2009, 115, 122–127. [Google Scholar] [CrossRef]
- Junker, B.H. Scale-up methodologies for Escherichia coli and yeast fermentation processes. J. Biosci. Bioeng. 2004, 97, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Tinôco, D.; Freire, D.M.G. Scale-up of 2,3-butanediol production by Paenibacillus peoriae NRRL BD-62 using constant oxygen transfer rate-based strategy. Fuel 2023, 340, 127603. [Google Scholar] [CrossRef]
- SRM University. The Design of Fermentation. 2019. Available online: https://webstor.srmist.edu.in/web_assets/srm_mainsite/files/files/UNIT-II-FERMENTOR.pdf (accessed on 30 January 2024).
- Daengbussadee, C.; Laopaiboon, L.; Laopaiboon, P. Butanol production by a novel efficient method using mixed cultures of Clostridium beijerinckii and Arthrobacter sp. in stirred-tank and gas-lift bioreactors. Fermentation 2022, 8, 160. [Google Scholar] [CrossRef]
- Rochón, E.; Cebreiros, F.; Ferrari, M.D.; Lareo, C. Isopropanol-butanol production from sugarcane and sugarcane-sweet sorghum juices by Clostridium beijerinckii DSM 6423. Biomass Bioenergy 2019, 128, 105331. [Google Scholar] [CrossRef]


| Bioreactor | Culture | Products (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids | |||||
| 1-L screw-capped bottle | Single culture (control) | 4.31 ± 0.13 c | 10.62 ± 0.20 b | 0.60 ± 0.02 b | 15.23 ± 0.33 b | 4.03 ± 0.23 b,c | 71.30 ± 1.29 a | 0.26 ± 0.01 a | 0.22 ± 0.01 b |
| Co-culture | 5.34 ± 0.21 a | 12.09 ± 0.27 a | 0.71 ± 0.01 a | 18.14 ± 0.37 a | 4.19 ± 0.15 b | 74.73 ± 2.16 a | 0.28 ± 0.01 a | 0.25 ± 0.01 a | |
| 5-L stirred-tank bioreactor | Co-culture | 5.18 ± 0.05 a,b | 12.22 ± 0.15 a | 0.74 ± 0.03 a | 18.21 ± 0.32 a | 4.71 ± 0.34 a | 74.02 ± 1.76 a | 0.28 ± 0.01 a | 0.26 ± 0.01 a |
| 30-L stirred-tank bioreactor | Co-culture | 4.93 ± 0.29 b | 11.92 ± 0.19 a | 0.74 ± 0.02 a | 17.80 ± 0.34 a | 3.64 ± 0.24 c | 73.80 ± 2.08 a | 0.28 ± 0.01 a | 0.25 ± 0.01 a |
| Cycle | Product (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) | PB* (g) | t* (h) | QB* (g/h) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids | |||||||
| 1 | 3.96 ± 0.37 b | 11.24 ± 0.22 b | 0.69 ± 0.04 b | 15.77 ± 0.11 b | 3.54 ± 0.34 b | 71.40 ± 2.11 a | 0.27 ± 0.01 a | 0.23 ± 0.01 b | 8.43 | 70 | 0.12 |
| 2 | 4.68 ± 0.59 a | 12.12 ± 0.33 a | 0.75 ± 0.02 a | 17.45 ± 0.53 a | 3.26 ± 0.48 b | 74.83 ± 2.23 a | 0.28 ± 0.01 a | 0.25 ± 0.01 a | 17.52 | 118 | 0.15 |
| 3 | 1.14 ± 0.10 c | 6.36 ± 0.29 c | 0.66 ± 0.01 b,c | 8.17 ± 0.40 c | 6.84 ± 0.58 a | 43.47 ± 2.35 b | 0.27 ± 0.01 a | 0.13 ± 0.01 c | 22.29 | 166 | 0.13 |
| 4 | 1.19 ± 0.04 c | 3.66 ± 0.26 d | 0.64 ± 0.01 c | 5.50 ± 0.33 d | 6.22 ± 0.46 a | 24.49 ± 2.99 c | 0.27 ± 0.01 a | 0.08 ± 0.01 d | 25.04 | 214 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanapornsin, T.; Laopaiboon, L.; Laopaiboon, P. Novel Batch and Repeated-Batch Butanol Fermentation from Sweet Sorghum Stem Juice by Co-Culture of Arthrobacter and Immobilized Clostridium in Scaled-Up Bioreactors. Energies 2024, 17, 1009. https://doi.org/10.3390/en17051009
Thanapornsin T, Laopaiboon L, Laopaiboon P. Novel Batch and Repeated-Batch Butanol Fermentation from Sweet Sorghum Stem Juice by Co-Culture of Arthrobacter and Immobilized Clostridium in Scaled-Up Bioreactors. Energies. 2024; 17(5):1009. https://doi.org/10.3390/en17051009
Chicago/Turabian StyleThanapornsin, Thanawat, Lakkana Laopaiboon, and Pattana Laopaiboon. 2024. "Novel Batch and Repeated-Batch Butanol Fermentation from Sweet Sorghum Stem Juice by Co-Culture of Arthrobacter and Immobilized Clostridium in Scaled-Up Bioreactors" Energies 17, no. 5: 1009. https://doi.org/10.3390/en17051009
APA StyleThanapornsin, T., Laopaiboon, L., & Laopaiboon, P. (2024). Novel Batch and Repeated-Batch Butanol Fermentation from Sweet Sorghum Stem Juice by Co-Culture of Arthrobacter and Immobilized Clostridium in Scaled-Up Bioreactors. Energies, 17(5), 1009. https://doi.org/10.3390/en17051009





