Preparation of Samples for the Study of Rheological Parameters of Digested Pulps in a Bioreactor of an Agricultural Biogas Plant
Abstract
1. Introduction
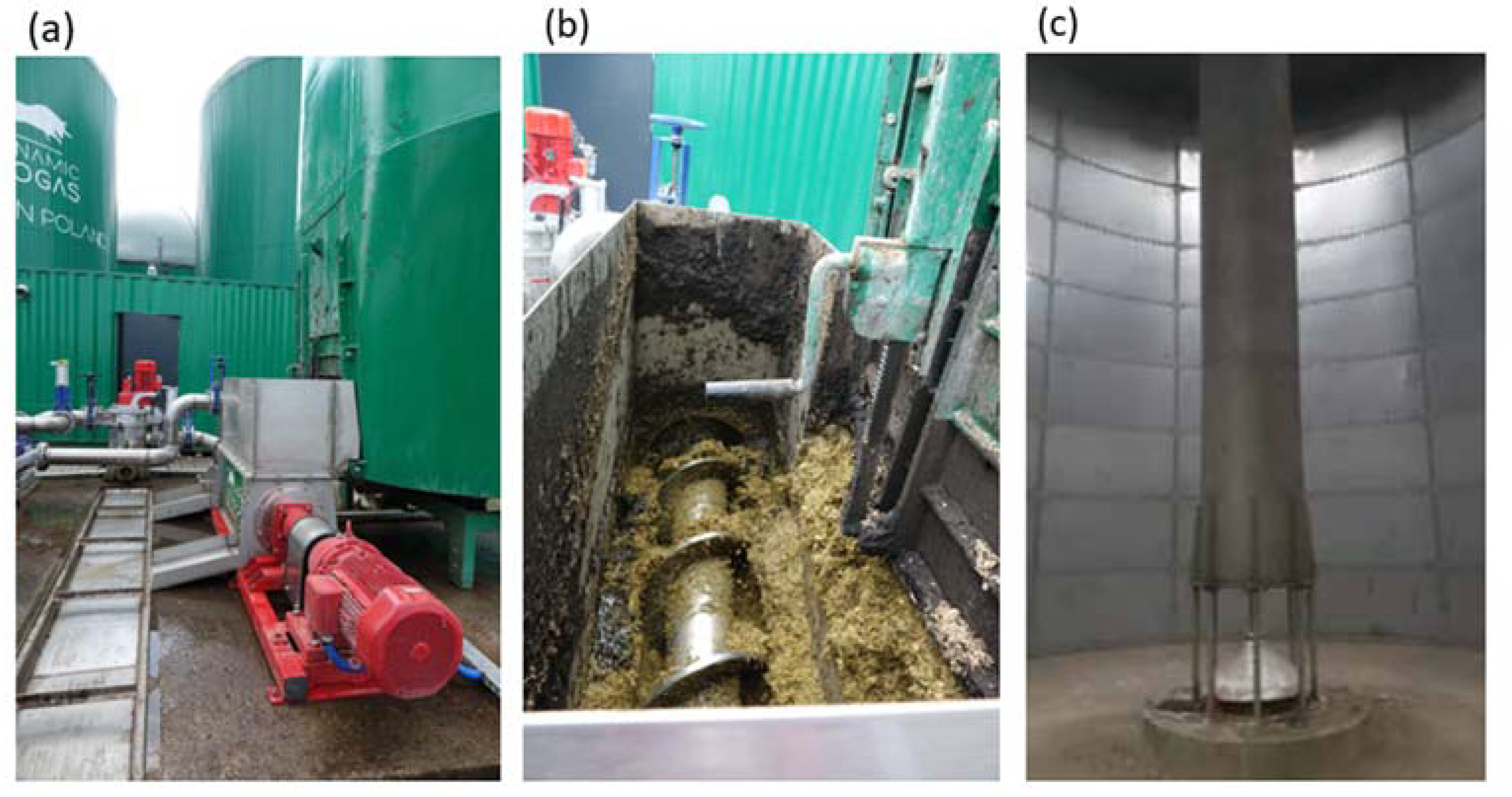
2. Test Object—Agricultural Biogas Plant
3. Inspiration for Research
4. Proposed Methodology
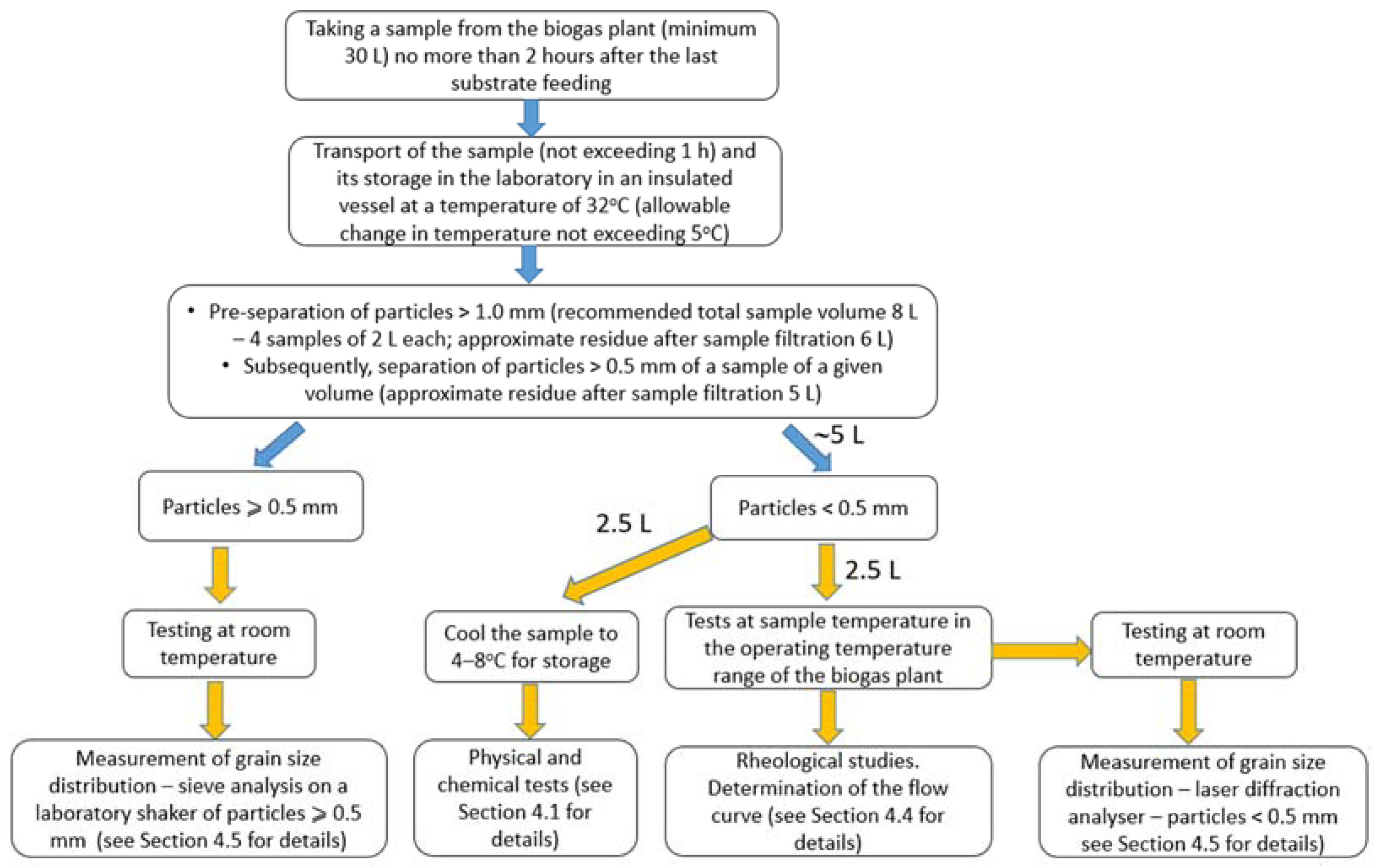
4.1. Collection and Transport
- Take a sample from the sampling pipe after draining a volume of digested pulp equivalent to twice the volume of the sampling pipe.
- Collect the sample into a sealed, thermally insulated container with a working volume of 30 dm3.
- Take the sample to an isolation chamber for transport.
- Transport the sample to the analysis site under constant temperature conditions.
- Carry the sample for no more than 1 h after collection (alternatively, through a check valve, release the accumulated gases into a container to measure the volume of produced (released) gases).
- The sample should not change its temperature by more than 5 °C.
- After delivery to the test site, take intermediate samples to determine the parametric characteristics of the digested pulp, i.e., the following:
- pH value;
- temperature;
- dry matter content;
- organic dry matter content;
- C/N ratio;
- ammonium nitrogen content;
- granulometric characteristics (during rheological studies);
- qualitative characteristics (optionally as supplementary data, e.g., total protein, crude fat, crude fibre, fatty acid profile, and mineral composition).
4.2. Storage of Samples
- The sample for testing should be taken from the transport container in the required portion for further analysis.
- The sample should be vigorously stirred for about 20 s using, for example, a twin-bladed construction stirrer with a diameter of 60 to 80 mm. The stirrer’s rotational speed should be maintained within the range of 1400 rpm. There must be clearly visible movement during stirring. The entire volume of the sample should be stirred. If possible, several “subsamples” (components of an aggregate sample) should be taken at several locations in the tank and at several depths before stirring is completed. These samples should then be added together to form the aggregate sample.
- The sample for indirect testing should be cooled to 4–8 °C.
- Samples for the actual rheological tests should be maintained at a constant temperature, the same as how they were delivered to the research unit.
- The sample is suitable for testing for a maximum of 8 h due to the processes involved.
4.3. Preparation of Samples for Rheological Measurements
- A tank with a volume of about 30 dm3, containing the sample taken from the reactor, should be placed in an incubator at a temperature of 32 °C. The sample should be vigorously stirred for about 20 s using a twin-bladed construction stirrer with a diameter of 60 to 80 mm. The stirrer’s rotational speed should be maintained within the range of 1400 rpm. There must be clearly visible movement during stirring and the stirring must involve the entire volume of the sample.
- Samples of approximately 2 dm3 in volume should be collected each time. Sample collection should be repeated four times.
- About 6 dm3 of digested pulp should be separated using a 1.0 mm sieve. When separating particles larger than the mesh, the sample should be moved across the sieve in a circular motion, pressing it gently against the mesh. The process of separating the carrier fluid from particles larger than 1.0 mm should continue until the liquid ceases to flow through the sieve and no liquid appears on the surface of the grouped solid particles.
- After passing the sample through the 1.0 mm sieve, the sample is then separated using a sieve with a # 0.5 mm mesh. The sample should be gently moved across the sieve to separate particles larger than the mesh until visible liquid ceases to appear on the surface of the separated particles. The volume of the prepared sample should be about 5 dm3.
- The sample should be placed in a covered container that is protected from excessive evaporation, and should be stored in an incubator at a temperature of 32 °C.
- The durability and suitability for rheological measurements of the sample are estimated to be about 8 h.
- The sample for rheological measurements is about 1 dm3, while 2.5 dm3 is needed to determine the mass concentration. The sample of about 1.5 dm3 serves as an averaging volume and reserve volume.
- After completion of the measurements, i.e., up to 8 h after separation of the fractions above 0.5 mm, three samples should be prepared for concentration measurements.
4.4. Procedure for Flow Curve Measurements
Measurement Procedure for the Prepared Sample’s Flow Curve
- Set the desired temperature in the thermostat bath system and wait for the temperature to reach the set point (due to thermal inertia, it may take a considerable amount of time to reach the desired temperature; ensure that the heated bath temperature is achieved before removing the sample from the incubator to avoid unnecessary sample aging).
- Remove the container with approximately 5 dm3 of the sample from the incubator after passing the sample through the 0.5 mm sieve. Shake the entire volume of the sample for about 20 s to stir any potential sediments that may have settled at the bottom of the container.
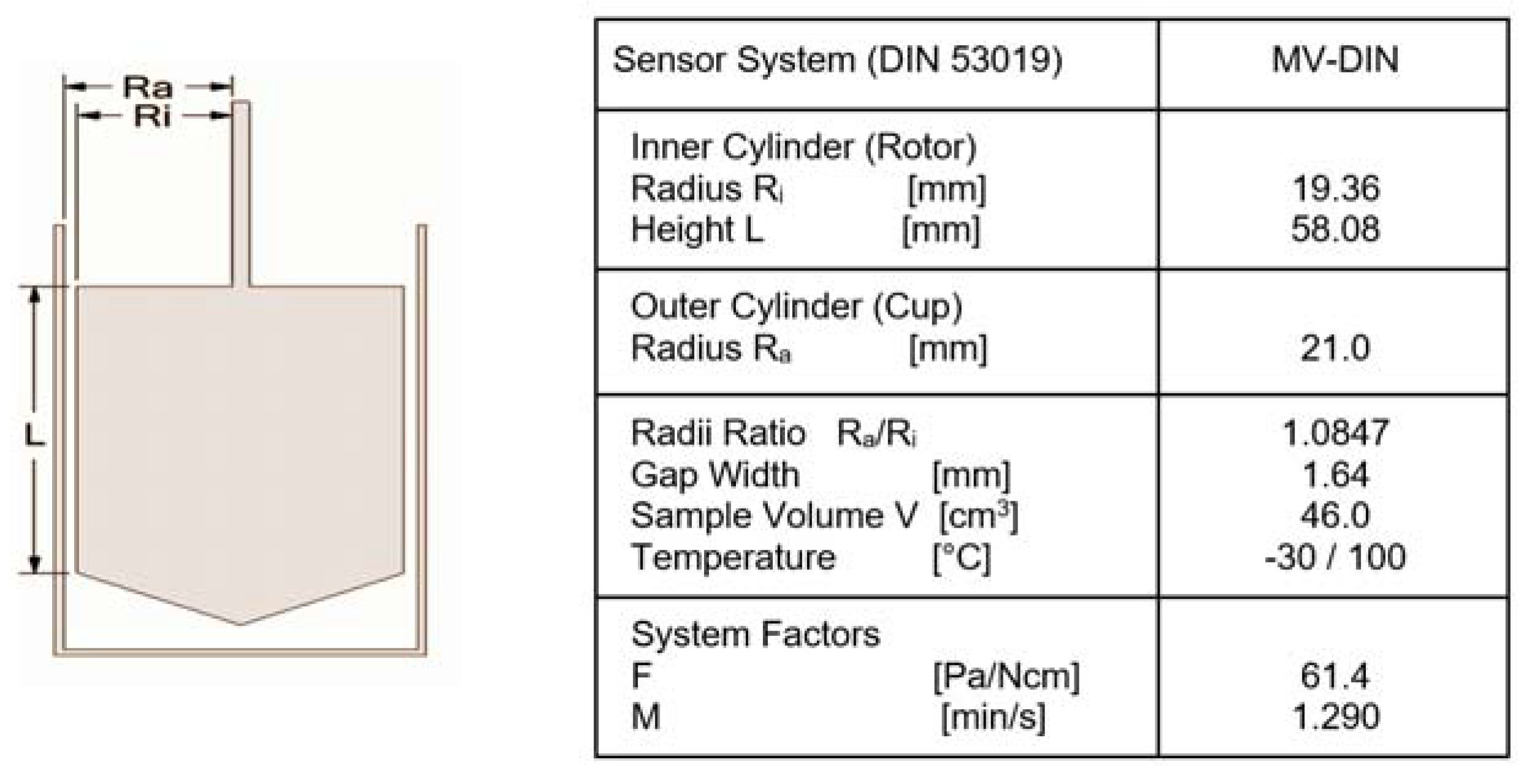
- Extract a sample and fill the rotational rheometer’s cylinder with a sample volume of 0.05–0.10 dm3.
- Place the sample-filled cylinder in the rheometer’s temperature control system using the thermostat bath system.
- Perform a direct and continuous temperature measurement of the sample in the cylinder until the desired temperature is reached. During heating or cooling of the sample, cover the measurement cylinder with a damp material to reduce evaporation and concentration changes.
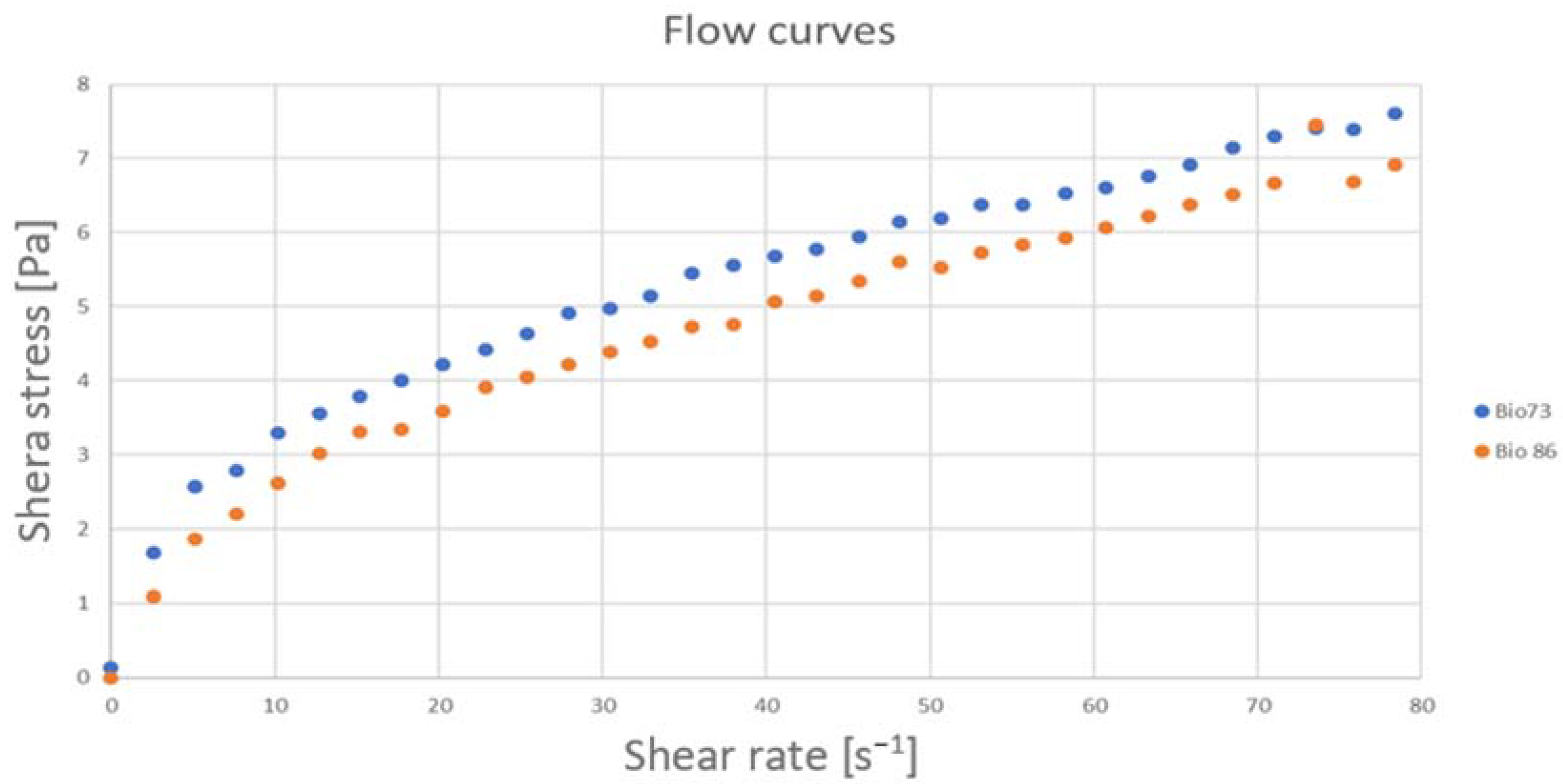
- Measure the flow curve in the deformation gradient range of 0–200 1/s with steps of 2–3 1/s increment.
- Perform a direct temperature measurement after obtaining the flow curve. If the sample temperature has changed by more than 2 °C, the measurement should be repeated.
- Empty, clean, and dry the measurement cylinder. Repeat the measurement three times.
- -
- The temperature of the sample before and after the test should be measured directly by placing the thermometer head in the sample.
- -
- During temperature changes (reaching the heated bath temperature), the samples should be protected from drying out.
- -
- The results should be interpreted immediately after the measurement to identify any gross errors and repeat the measurement if needed.
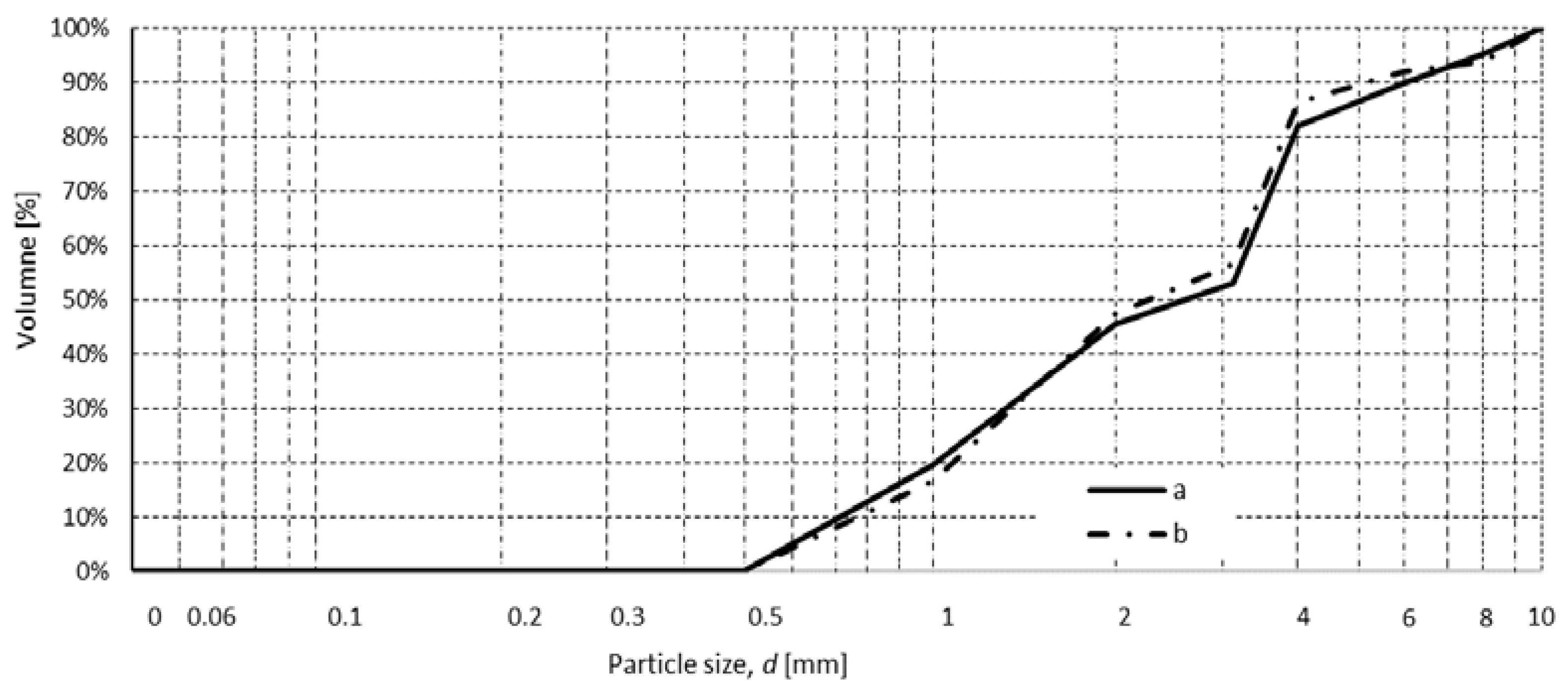
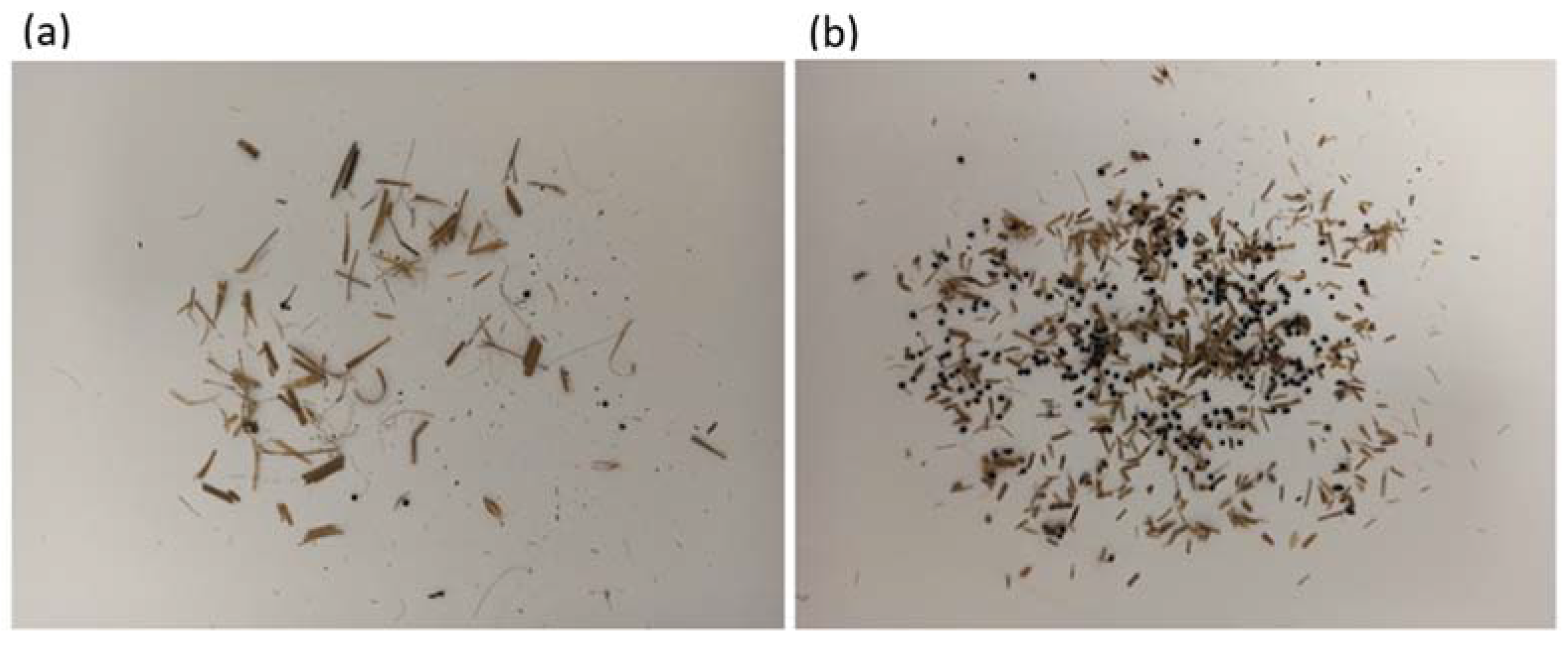
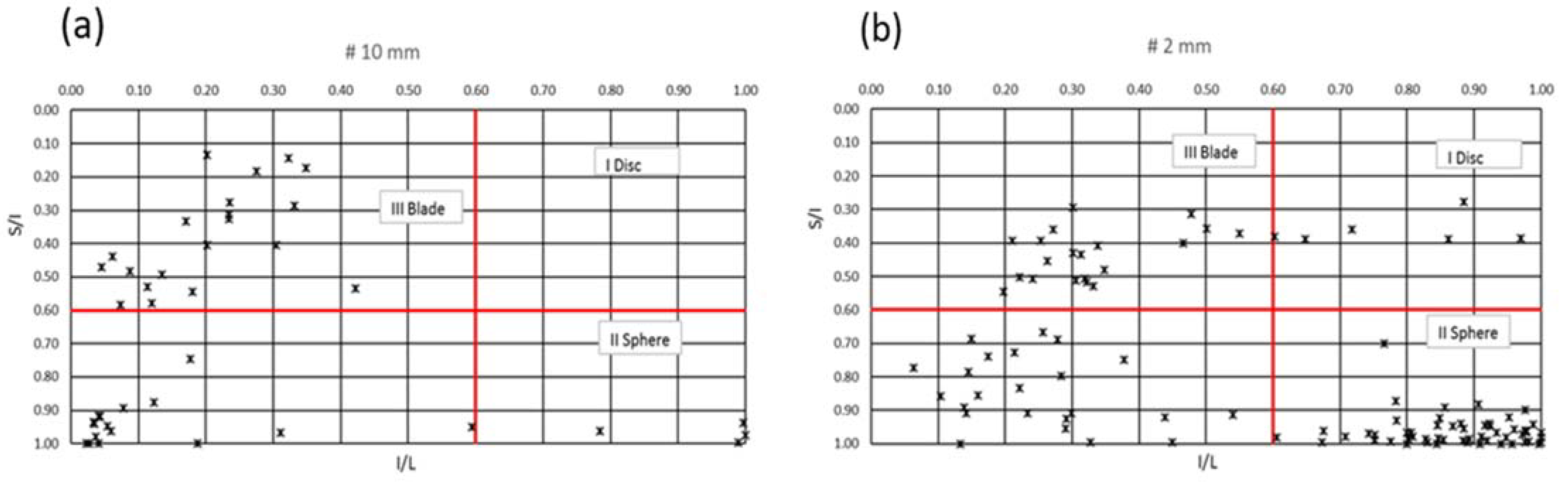
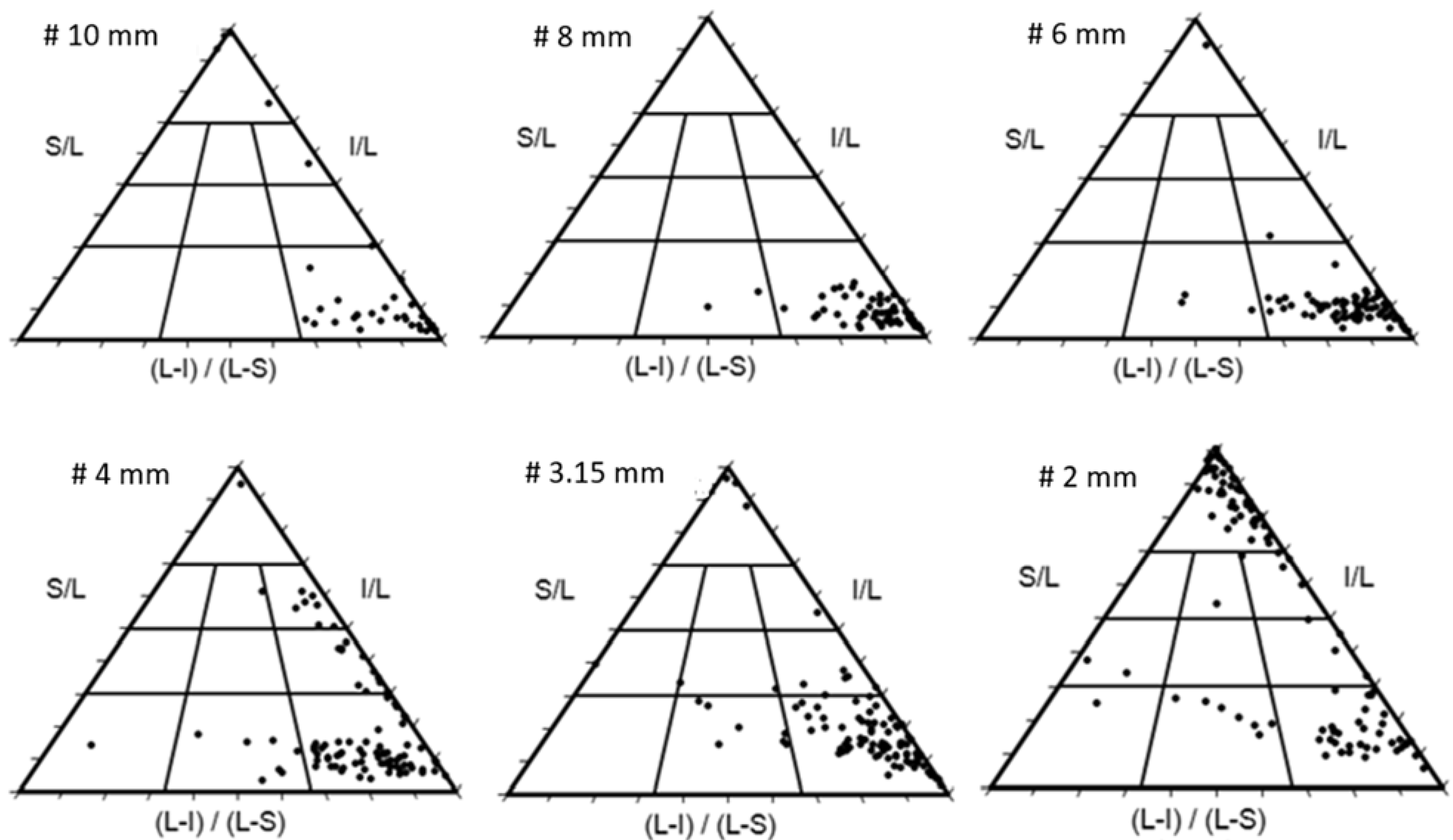
4.5. Particle Characteristics of the Feed
4.5.1. Particle Size Distribution Curve
4.5.2. Distribution of Particle Shapes
5. Discussion of Results
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMS | Ball measuring system |
| CFD | Computational fluid dynamics |
| CR | Controlled shear rate |
| CS | Controlled shear stress |
| DEM | Discrete element method |
| MAE | Mean absolute error |
| MAPE | Mean absolute percentage error |
| MPS | Moving particle semi-implicit |
| RES | Renewable energy sources |
| PE | Percentage error |
References
- Alao, M.A.; Popoola, O.M.; Ayodele, T.R. Waste-to-energy nexus: An overview of technologies and implementation for sustainable development. Clean Energy Syst. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Kałuża, T.; Hämmerling, M.; Zawadzki, P.; Czekała, W.; Kasperek, R.; Sojka, M.; Mokwa, M.; Ptak, M.; Szkudlarek, A.; Czechlowski, M.; et al. The hydropower sector in Poland: Historical development and current status. Renew. Sustain. Energy Rev. 2022, 158, 112150. [Google Scholar] [CrossRef]
- Kałuża, T.; Hämmerling, M.; Zawadzki, P.; Czekała, W.; Kasperek, R.; Sojka, M.; Mokwa, M.; Ptak, M.; Szkudlarek, A.; Czechlowski, M.; et al. The hydropower sector in poland: Barriers and the outlook for the future. Renew. Sustain. Energy Rev. 2022, 163, 112500. [Google Scholar] [CrossRef]
- Mazurkiewicz, J. Energy and Economic Balance between Manure Stored and Used as a Substrate for Biogas Production. Energies 2022, 15, 413. [Google Scholar] [CrossRef]
- Pochwatka, P.; Kowalczyk-Juśko, A.; Sołowiej, P.; Wawrzyniak, A.; Dach, J. Biogas plant exploitation in a middle-sized dairy farm in Poland: Energetic and economic aspects. Energies 2020, 13, 6058. [Google Scholar] [CrossRef]
- Czekała, W. Agricultural Biogas Plants as a Chance for the Development of the Agri-Food Sector. J. Ecol. Eng. 2018, 19, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, K.; Mazurkiewicz, J.; Chełkowski, D.; Jeżowska, A.; Cieślik, M.; Brzoski, M.; Smurzyńska, A.; Dongmin, Y.; Wei, Q. The effect of mixing during laboratory fermentation of maize straw with thermophilic technology. J. Ecol. Eng. 2018, 19, 93–98. [Google Scholar] [CrossRef]
- Annas, S.; Jantzen, H.-A.; Scholz, J.; Janoske, U. A scale-up strategy for the fluid flow in biogas plants. Chem. Eng. Technol. 2018, 41, 739–746. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digested pulp as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Pochwatka, P.; Zaborowicz, M.; Czekała, W.; Mazurkiewicz, J.; Mazur, A.; Janczak, D.; Marczuk, A.; Dach, J. Energy value estimation of silages for substrate in biogas plants using an artificial neural network. Energy 2020, 202, 117729. [Google Scholar] [CrossRef]
- Westerholm, S.B.M.; Qiao, W.; Mahdy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe2+ and Ni2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Šlepetys, J.; Cesevičienė, J. Towards a Full Circular Economy in Biogas Plants: Sustainable Management of Digested pulp for Growing Biomass Feedstocks and Use as Biofertilizer. Energies 2021, 14, 4272. [Google Scholar] [CrossRef]
- Satjaritanun, P.; Khunatorn, Y.; Vorayos, N.; Shimpalee, S.; Bringley, E. Numerical analysis of the mixing characteristic for napier grass in the continuous stirring tank reactor for biogas production. Biomass Bioenergy 2016, 86, 53–64. [Google Scholar] [CrossRef]
- Ghanimeh, S.A.; Al-Sanioura, D.N.; Saikaly, P.E.; El-Fadel, M. Correlation between system performance and bacterial composition under varied mixing intensity in thermophilic anaerobic digestion of food waste. J. Environ. Manag. 2018, 206, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, J.; Thorin, E.; Fdhila, R.B.; Dahlquista, E. Effects of mixing on the result of anaerobic digestion: Review. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Huang, Y.K.; Dehkordy, F.M.; Li, Y.; Emadi, S.; Bagtzoglou, A.; Li, B.K. Enhancing Anaerobic Fermentation Performance Through Eccentrically Stirred Mixing: Experimental and Modeling Methodology. Chem. Eng. J. 2018, 334, 1383–1391. [Google Scholar] [CrossRef]
- Annas, S.; Elfering, M.; Jantzen, H.-A.; Scholz, J.; Janoske, U. Experimental analysis of mixing-processes in biogas plants. Chem. Eng. Science. 2022, 258, 11776. [Google Scholar] [CrossRef]
- Chang, C.-C.; Kuo-Dahab, C.; Chapman, T.; Mei, Y. Anaerobic digestion, mixing, environmental fate, and transport. Water Environ. Res. 2019, 91, 1210–1222. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. Impact of mixing intensity and duration on biogas production in an anaerobic digester: A review. Crit. Rev. Biotechnol. 2020, 40, 508–521. [Google Scholar] [CrossRef]
- Naegele, H.J.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Electric energy consumption of the full-scale research biogas plant” unterer lindenhof”: Results of longterm and full detail measurements. Energies 2012, 5, 5198–5214. [Google Scholar] [CrossRef]
- Low, S.C.; Eshtiaghi, N.; Slatter, P.; Baudez, J.-C.; Parthasarathy, R. Mixing characteristics of sludge simulant in a model anaerobic digester. Bioproc. Biosyst. Eng. 2016, 39, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jobst, K.; Lomtscher, A.; Deutschmann, A.; Fogel, S.; Rostalski, K.; Stempin, S.; Brehmer, M.; Kraume, M. Optimierter Betrieb von Rührsystemen in Biogasanlagen. In Biogas in der Landwirtschaft: Stand und Perspektiven; KTBL: Darmstadt, Germany, 2015; pp. 140–150. Available online: https://www.ktbl.de/fileadmin/user_upload/Artikel/Energie/Biogastagung/11508.pdf (accessed on 2 January 2024).
- Lemmer, A.; Naegele, H.-J.; Sondermann, J. How Efficient are Agitators in Biogas Digesters? Determination of the Efficiency of Submersible Motor Mixers and Incline Agitators by Measuring Nutrient Distribution in Full-Scale Agricultural Biogas Digesters. Energies 2013, 6, 6255–6273. [Google Scholar] [CrossRef]
- Hernandez-Shek, A.; Mottelet, S.; Peultier, P.; Pauss, A.; Ribeiro, T. Development of devices for the determination of the rheological properties of coarse biomass treated by dry anaerobic digestion. Bioresour. Technol. Reports. 2021, 15, 100686. [Google Scholar] [CrossRef]
- Šafarič, L. Anaerobic Digester Fluid Rheology and Process Efficiency. Interactions of Substrate Composition, Trace Element Availability, and Microbial Activity, Linköping Studies in Arts and Science No. 768 Faculty of Arts and Sciences, Linköping. 2019. Available online: https://www.diva-portal.org/smash/get/diva2:1301565/FULLTEXT01.pdf (accessed on 2 January 2024).
- Schneider, N.; Gerber, M. Rheological properties of digested pulp from agricultural biogas plants: An overview of measurement techniques and influencing factors. Renew Sustain. Energy. Rev. 2020, 121, 109709. [Google Scholar] [CrossRef]
- Pohn, S.; Kamarad, L.; Kirchmayr, R.; Harasek, M. Design, calibration and numerical investigation of a macro viscosimeter. In Proceedings of the 19th International Congress of Chemical and Process Engineering CHISA, Prague, Czech, 28 August–1 September 2010. [Google Scholar]
- Monch-Tegeder, M.; Lemmer, A.; Hinrichs, J.; Oechsner, H. Development of an in-line process viscometer for the full-scale biogas process. Bioresour. Technol. 2015, 178, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley-VCH: New York, NY, USA, 1994. [Google Scholar]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 3rd Revised ed.; European Coatings Tech Files; Vincentz Network: Hanover, Germany, 2020. [Google Scholar]
- Ewoldt, R.H.; Johnston, M.T.; Caretta, L.M. Experimental challenges of shear rheology: How to avoid bad data. In Complex Fluids in Biological Systems; Spagnolie, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Ismail, N.I.; Kuang, S.; Zheng, E.; Yu, A. Modeling and analysis of fluid rheology effect on sand screen performance. Powder Technol. 2022, 411, 117961. [Google Scholar] [CrossRef]
- Seyssiecq, I.; Karrabi, M.; Roche, N. In situ rheological characterisation of wastewater sludge: Comparison of stirred bioreactor and pipe flow configurations. Chem. Eng. J. 2015, 259, 205–212. [Google Scholar] [CrossRef]
- Gienau, T.; Kraume, M.; Rosenberger, S. Rheological characterization of anaerobic sludge from agricultural and bio-waste biogas plants. Chem. Ing. Tec. 2018, 90, 988–997. [Google Scholar] [CrossRef]
- Jobst, K.; Lincke, M. Modification of measuring systems for the application to flow behaviour determination of fibrous suspensions. In Collection of Methods for Biogas: Methods to Determine Parameters for Analysis Purposes and Parameters That Describe Processes in the Biogas Sector; Leipzig, J., Liebetrau, D., Pfeiffer, D.T., Eds.; DBFZ: Leipzig, Germany, 2016; pp. 114–124. [Google Scholar]
- Brehme, M.K. Measurement methods for the rheologic characterisation of fermentation substrates. In Collection of Methods for Biogas: Methods to Determine Parameters for Analysis Purposes and Parameters That Describe Processes in the Biogas Sector; Leipzig, J., Liebetrau, D., Pfeiffer, D.T., Eds.; DBFZ: Leipzig, Germany, 2016; pp. 105–124. [Google Scholar]
- Schatzmann, M.; Bezzola, G.R.; Minor, H.E.; Windhab, E.J.; Fischer, P. Rheometry for large-particulated fluids: Analysis of the ball measuring system and comparison to debris flow rheometry. Rheol Acta 2009, 48, 715–733. [Google Scholar] [CrossRef]
- Fakhari, A.; Galindo-Rosales, F.J. Numerical assessment of the penetroviscometer approach for large, rapid and transient shear deformations. arXiv 2020. [Google Scholar] [CrossRef]
- Cruz, N.; Forster, J.; Bobicki, E.R. Slurry rheology in mineral processing unit operations, A critical review. Can. J. Chem. Eng. 2019, 97, 2102–2120. [Google Scholar] [CrossRef]
- Lefebvre, L.P.; Whiting, J.; Nijikovsky, B.; Brika, S.E.; Fayazfar, H.; Lyckfeldt, O. Assessing the robustness of powder rheology and permeability measurements. Addit. Manuf. 2020, 35, 101203. [Google Scholar] [CrossRef]
- de Goede, T.C.; de Bruin, K.G.; Bonn, D. High-velocity impact of solid objects on Non-Newtonian Fluids. Sci. Rep. 2019, 9, 1250. [Google Scholar] [CrossRef]
- Wolski, P. Analysis of Rheological Models of Modified Sewage Sludge. Annu. Set Environ. Prot. 2017, 19, 230–239. Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-18656c22-ab15-41d2-b74f-992b8a9f010f/c/13_wolski_analysis_ROS_2017.pdf (accessed on 2 January 2024).
- Beugre, E.Y.-M.; Gnagne, T. Vane geometry for measurement of influent rheological behavior in dry anaerobic digestion. Renew. Sust. Energ. Rev. 2022, 155, 111928. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Cui, W.; Wang, Y.; Yang, P. Rheological Properties of Municipal Sewage Sludge: Dependency on Solid Concentration and Temperature. Proc. Environ. Sci. 2016, 31, 113–121. [Google Scholar] [CrossRef]
- Sozański, M.; Kempa, E.; Grocholski, K.; Bień, J. The rheological experiment in sludge properties research. Water Sci. Technol. 1997, 36, 69–78. [Google Scholar] [CrossRef]
- Baroutian, S.; Eshtiaghi, N.; Gapes, D.J. Rheology of a primary and secondary sewage sludge mixture: Dependency on temperature and solid concentration. Bioresour. Technol. 2013, 140, 227–233. [Google Scholar] [CrossRef]
- Chmiel, H.; Takors, R.; Weuster-Botz, D. Bioprozesstechnik; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Redlinger-Pohn, J.D.; Jagiello, L.A.; Bauer, W.; Radl, S. Mechanistic understanding of size-based fiber separation in coiled tubes. Int. J. Multiph. Flow. 2016, 83, 239–253. [Google Scholar] [CrossRef][Green Version]
- Schmid, C.F.; Switzer, L.H.; Klingenberg, D.J. Simulations of fiber flocculation: Effects of fiber properties and interfiber friction. J. Rheol. 2000, 44, 781–809. [Google Scholar] [CrossRef]
- VDI 4630; Fermentation of Organic Materials—Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Verlag des Vereins Deutscher Ingenieure: Düsseldorf, Germany, 2016. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1932350 (accessed on 2 January 2024).
- Vasilyev, A.L.; Lavrenko, S.L.; Gruszczynski, M. Experimental studies of rheological properties of stowing pulps. J. Phys. Conf. Ser. 2018, 1118, 012045. [Google Scholar] [CrossRef]
- Dhodapkar, S.; Jacob, K.; Hu, S. Fluid-solid flow in duct. In Multiphase Flow Handbook; Crowe, C.T., Ed.; CRC Press Taylor & Francis Group: Abingdon, UK, 2006; Chapter 4. [Google Scholar]
- Mazur, R.; Kałuża, T.; Chmist, J.; Walczak, N.; Laks, I.; Strzeliński, P. Influence of deposition of fine plant debris in river floodplain shrubs on flood flow conditions—The Warta River case study. Phys. Chem. Earth 2016, 94, 106–113. [Google Scholar] [CrossRef]
- Jewell, R.J.; Fourie, A.B. Paste and Thickened Tailings: A Guide, 2nd ed.; Australian Centre for Geomechanics, The University of Western Australia: Nedlands, Australia, 2006. [Google Scholar]
- Wilson, K.C.; Addie, G.R.; Sellgren, A.; Clift, R. Slurry Transport Using Centrifugal Pumps; Springer: New York, NY, USA, 1997. [Google Scholar]
- Shook, C.A.; Roco, M.C. Slurry Flow: Principles and Practice; Butterworth–Heinemann, a division of Reed Publishing: Oxford, UK, 1991. [Google Scholar] [CrossRef]
- Dziubiński, M.; Kiljańsk, T.; Sęk, J. Theoretical Basis and Measurement Methods of Rheology; Lodz University of Technology Publishers: Łódź, Poland, 2014. (In Polish) [Google Scholar]
- May, C.J.; Henderson, K.O. Rheological Measurement Methods and Equipment. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013; pp. 2777–2787. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry, 2nd ed.; Thermo Electron (Karlsruhe) GmbH, Federal Republic of Germany: Karlsruhe, Germany, 2004; Available online: https://www.ifi.es/wp-content/uploads/2021/06/Rheology-Book.pdf (accessed on 6 July 2023).
- Barnes, H.A. Measuring the viscosity of large-particle (and flocculated) suspensions—A note on the necessary gap size of rotational viscometer. J. Non-Newton. Fluid Mech. 2020, 94, 213–217. [Google Scholar] [CrossRef]
- DIN 53019-1; Viscometry-Measurement of viscosities and flow curves by means of rotational viscometers-Part 1: Principles and geometry of measuring system. Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 2008.
- Montgomery, L.F.R.; Schoepp, T.; Fuchs, W.; Bochmann, G. Design, Calibration and Validation of a Large Lab-Scale System for Measuring Viscosity in Fermenting Substrate from Agricultural Anaerobic Digesters. Biochem. Eng. J. 2016, 115, 72–79. [Google Scholar] [CrossRef]
- Koll, C. Aufnahme, Auswertung und Beurteilung rheologischer Parameter zur Auslegung und Simulation von Fördereinheiten sowie Rühraggregaten in Biogasanlagen. Master’s Thesis, Hochschule Hannover, Hannover, Germany, 2012. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Zhou, J.; Chen, Q.; Yang, C. Feasibility of Recycling Ultrafine Leaching Residue by Backfill: Experimental and CFD Approaches. Minerals 2017, 7, 54. [Google Scholar] [CrossRef]
- Bridge, J.S.; Bennett, S.J. A model for entrainment and transport of sediment grains of mixed sizes, shapes and densities. Water Resour. Res. 1992, 28, 337–363. [Google Scholar] [CrossRef]
- Książek, L.; Mitka, B.; Mrokowska, M.; Nones, M.; Phan, C.N.; Przyborowski, Ł.; Strużyński, A.; Wojak, S.; Wyrębek, M. Application of digital close-range photogrammetry to determine changes in gravel bed surface due to transient flow conditions. Publ. Inst. Geophys. Pol. Acad. Sci. 2021, 434, 95–96. [Google Scholar]
- Mumot, J.; Tymiński, T. Hydraulic research of sediment transport in the vertical slot fish passes. J. Ecol. Eng. 2016, 17, 143–148. [Google Scholar] [CrossRef][Green Version]
- Szałkiewicz, E.; Dysarz, T.; Kałuża, T.; Malinger, A.; Radecki-Pawlik, A. Analysis of in-stream restoration structures impact on hydraulic condition and sedimentation in the Flinta River, Poland, Carpathian. J. Earth Environ. Sci. 2019, 14, 275–286. [Google Scholar] [CrossRef]
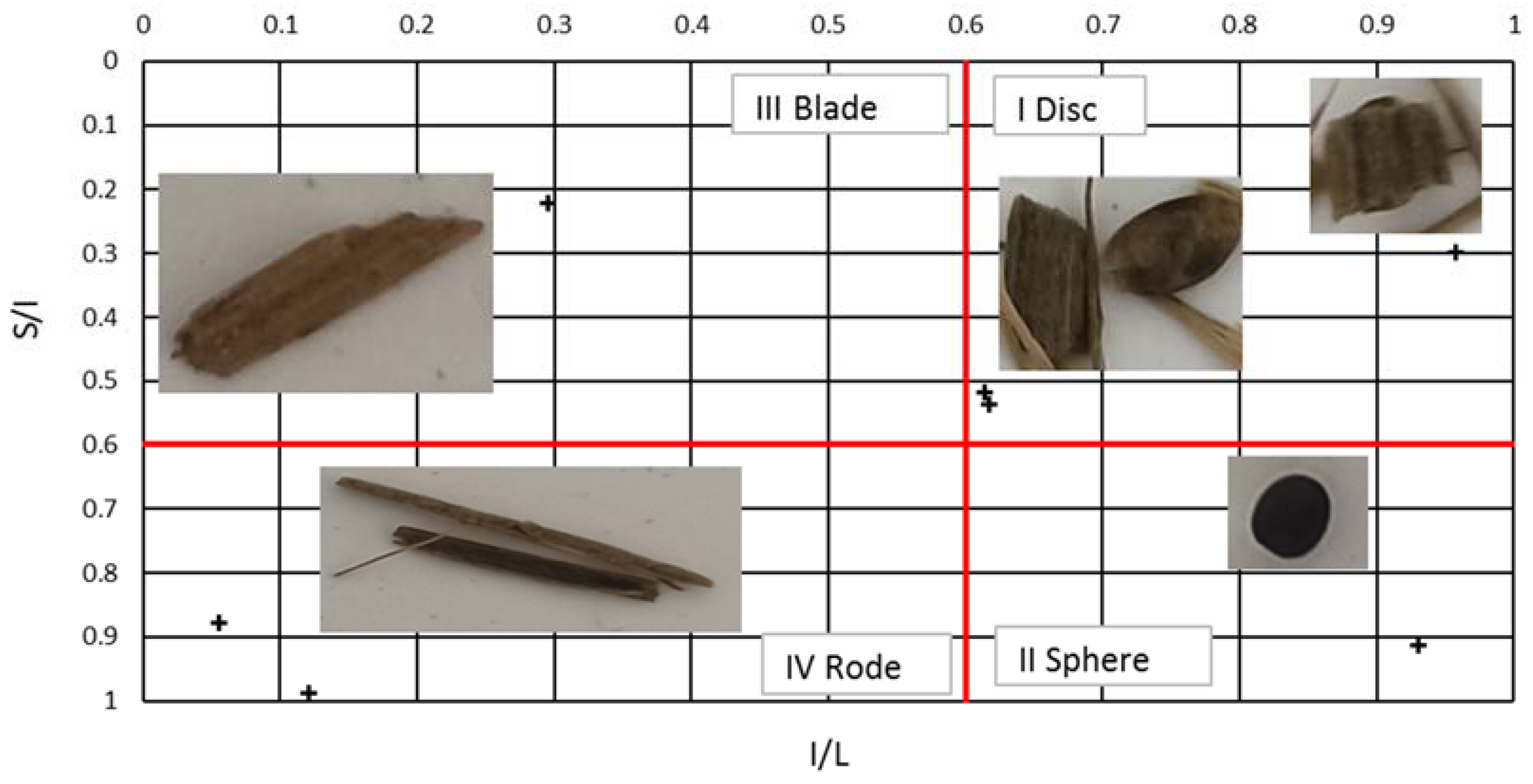
- Blott, S.J.; Pye, K. Particle shape: A review and new methods of characterization and classification. Sedimentology 2008, 55, 31–63. [Google Scholar] [CrossRef]
- Szabó, T.; Domokos, G. A new classification system for pebble and crystal shapes based on static equilibrium points. Cent. Eur. Geol. 2010, 53, 1–19. [Google Scholar] [CrossRef]
- Zingg, T. Beitrag zur Schotteranalyse.—Schweizeriscke Mineralogische und Petrologische Mitteilungen. Doctoral Thesis, ETH Zurich Research Collection, Zurich, Switzerland, 1935. [Google Scholar] [CrossRef]
- Sneed, E.; Folk, R.L. Pebbles in the lower Colorado River, Texas, a study in particle morphogenesis. J. Geol. 1958, 66, 114–150. Available online: https://www.journals.uchicago.edu/doi/abs/10.1086/626490 (accessed on 2 January 2024). [CrossRef]
- Javris, P.; Jefferson, B.; Parsons, S.A. Measuring floc structural characteristics. Rev. Environ. Sci. Bio/Technol. 2005, 4, 1–18. [Google Scholar] [CrossRef]
- Graham, D.J. TRI-PLOT. Ternary Diagram Plotting Software. 2000. Available online: https://triplot.software.informer.com/download/ (accessed on 2 January 2024).
- Graham, D.J.; Midgley, N.G. Graphical representation of particle shape using triangular diagrams: An excel spreadsheet method. Earth Surf. Process. Landf. 2000, 25, 1473–1477. [Google Scholar] [CrossRef]
- Fraunhofer IKTS/TU Berlin/KSB AG, Entwicklung eines Steuerungs- und Regelkonzeptes für Mischprozesse in Biogasfermentern auf der Basis zu validierender Prozessmodelle. Final Report, Dresden. 2017. Available online: https://www.fnr.de/index.php?id=11150&fkz=22023012 (accessed on 8 August 2023).
- Kamarád, L.; Pohn, S.; Bochmann, G.; Harasek, M. Determination of mixing quality in biogas plant digesters using tracer tests and computational fluid dynamics, Acta Universit. Agricult. Et Silvic. Mendel. Brun. 2013, 61, 1269–1278. [Google Scholar] [CrossRef]
- Kube, J.; Köhnlechner, M.; Thurner, F. Einfache Methode zur online-Bestimmung der Viskosität von Gärsubstraten in Biogasanlagen. 2011, pp. 83–91. Available online: https://serwiss.bib.hs-hannover.de/frontdoor/deliver/index/docId/386/file/MA-Koll-2012.pdf (accessed on 2 January 2024).
- Tian, L.; Shen, F.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Reducing Agitation Energy-Consumption by Improving Rheological Properties of Corn Stover Substrate in Anaerobic Digestion. Bioresour. Technol. 2014, 168, 86–91. [Google Scholar] [CrossRef]
- Basedau, D.; Lüdersen, U.; Glasmacher, B. Rheologische Charakterisierung von Fermentersuspensionen. Chemie Ingenieur Technik. 2015, 87, 543–548. [Google Scholar] [CrossRef]
- Hreiz, R.; Adouani, N.; Fünfschilling, D.; Marchal, P.; Pons, M.-N. Rheological Characterization of Raw and Anaerobically Digested Cow Slurry. Chem. Eng. Res. Design. 2017, 119, 47–57. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K. Kraft lignin grafted with polyvinylpyrrolidone as a novel microbial carrier in biogas production. Energies 2018, 11, 3246. [Google Scholar] [CrossRef]
- Bansil, R.; Celli, J.P.; Hardcastle, J.M.; Turner, B.S. The influence of mucus microstructure and rheology in Helicobacter pylori infection. Front. Immunol. 2013, 4, 310. [Google Scholar] [CrossRef] [PubMed]
- Persat, A.; Nadell, C.D.; Kim, M.K.; Ingremeau, F.; Siryaporn, A.; Drescher, K.; Wingreen, N.S.; Bassler, B.L.; Gitai, Z.; Stone, H.A. The mechanical world of bacteria. Cell 2015, 161, 988–997. [Google Scholar] [CrossRef]
- Jankowska, H.; Dzido, A.; Krawczyk, P. Determination of Rheological Parameters of Non-Newtonian Fluids on an Example of Biogas Plant Substrates. Energies 2023, 16, 1128. [Google Scholar] [CrossRef]
- Smuts, E.M.; Deglon, D.A.; Meyer, C.J. A coupled CFD-DEM model for simulating the rheology of particulate suspensions. Prog. Comput. Fluid Dyn. 2017, 17, 290–301. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J. Dam-Break of Mixtures Consisting of Non-Newtonian Liquids and Granular Particles. Powder Technol. 2018, 338, 493–505. [Google Scholar] [CrossRef]
- Zhong, W.; Yu, A.; Liu, X.; Tong, Z.; Zhang, H. DEM/CFD-DEM Modelling of Non-spherical Particulate Systems: Theoretical Developments and Applications. Powder Technol. 2016, 302, 108–152. [Google Scholar] [CrossRef]
- Li, J.-J.; Qiu, L.-C.; Tian, L.; Yang, Y.-S.; Han, Y. Modeling 3D non-Newtonian solid–liquid flows with a free-surface using DEM-MPS. Eng. Anal. Bound. Elem. 2019, 105, 70–77. [Google Scholar] [CrossRef]






| Rheological Models | Ostwald–de Waele Relationship, Model | Bingham Model | Herschel–Bulkley Model |
|---|---|---|---|
| formula | |||
| symbols | γ [1/s]: shear rate τ [Pa]: shear stress KO [Pa·sn]: flow consistency index nO [-]: flow behaviour index | τo [Pa]: Bingham yield stress ηB [kg/m·s]: Bingham viscosity | τH [Pa]: Herschel–Bulkley yield stress KH [Pa·sn]: flow consistency index, flow coefficient nH [-]: flow behaviour index, Herschel–Bulkley index |
| The Bingham Model | The Ostwald–de Waele Model | The Herschel–Bulkley Model | ||||||||||||||
| Fermenter No. 2 | Parameters | Fit | Parameters | Fit | Parameters | Fit | ||||||||||
| Date | τo | ηB | R | MAE | MAPE | KO | nO | R | MAE | MAPE | τo | KH | nH | R | MAE | MAPE |
| [-] | [Pa] | [Pa·s] | [-] | [Pa] | [%] | [N·s^(n/m2)] | [-] | [-] | [Pa] | [%] | [Pa] | [N·s^(n/m2)] | [-] | [-] | [Pa] | [%] |
| 12 June 2021 | 2.977 | 0.243 | 0.960 | 3.084 | 14.4 | 0.378 | 0.928 | 0.957 | 3.414 | 16.7 | 8.452 | 0.018 | 1.485 | 0.966 | 2.875 | 20.8 |
| 13 June 2021 | 8.471 | 0.151 | 0.935 | 2.546 | 13.0 | 1.922 | 0.556 | 0.943 | 2.199 | 8.8 | 2.185 | 1.271 | 0.624 | 0.943 | 2.184 | 8.5 |
| PE | 64.9% | 60.3% | 80.3% | 66.8% | 286.8% | 98.6% | 137.8% | |||||||||
| The Bingham Model | The Ostwald–de Waele model | The Herschel–Bulkley model | ||||||||||||||
| Lagoon | Parameters | Fit | Parameters | Fit | Parameters | Fit | ||||||||||
| Date | τo | ηB | R | MAE | MAPE | KO | nO | R | MAE | MAPE | τo | KH | nH | R | MAE | MAPE |
| [-] | [Pa] | [Pa·s] | [-] | [Pa] | [%] | [N·s^(n/m2)] | [-] | [-] | [Pa] | [%] | [Pa] | [N·s^(n/m2)] | [-] | [-] | [Pa] | [%] |
| 12 June 2021 | 3.711 | 0.044 | 0.968 | 0.501 | 8.3 | 1.068 | 0.453 | 0.988 | 0.250 | 2.9 | 0.459 | 0.869 | 0.485 | 0.988 | 0.243 | 2.7 |
| 13 June 2021 | 3.035 | 0.045 | 0.982 | 0.372 | 8.6 | 0.767 | 0.510 | 0.998 | 0.128 | 2.0 | 0.183 | 0.699 | 0.525 | 0.998 | 0.123 | 1.9 |
| PE | 22.3% | 3.0% | 39.2% | 11.1% | 151.3% | 24.4% | 7.5% | |||||||||
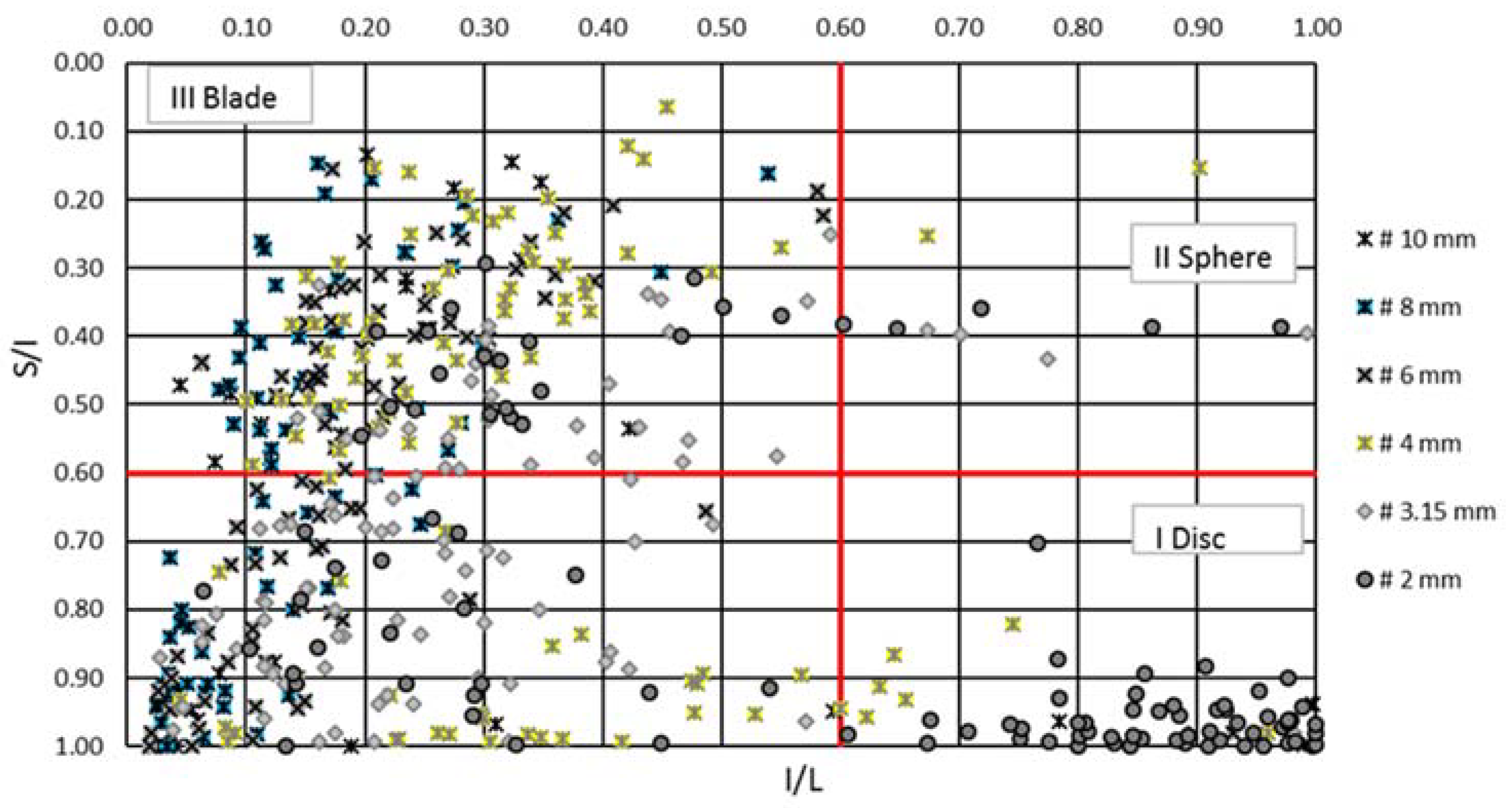
| Class | # 10 mm | # 8 mm | # 6 mm | # 4 mm | # 3.15 mm | # 2 mm | Total |
|---|---|---|---|---|---|---|---|
| C | 10.00 | 0.00 | 1.19 | 1.05 | 3.16 | 47.62 | 12.07 |
| CP | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CB | 0.00 | 0.00 | 0.00 | 1.50 | 0.00 | 0.95 | 0.41 |
| CE | 2.50 | 0.00 | 0.00 | 7.37 | 1.05 | 4.76 | 2.86 |
| P | 0.00 | 0.00 | 0.00 | 0.00 | 1.05 | 1.9 | 0.61 |
| B | 0.00 | 0.00 | 0.00 | 0.00 | 2.11 | 0.00 | 0.41 |
| E | 2.50 | 0.00 | 1.19 | 11.58 | 5.26 | 3.81 | 4.50 |
| VP | 0.00 | 0.00 | 0.00 | 1.05 | 0.00 | 0.95 | 0.40 |
| VB | 0.00 | 2.86 | 3.57 | 7.37 | 7.37 | 6.67 | 5.32 |
| VE | 85.00 | 97.14 | 94.05 | 70.53 | 80.00 | 33.34 | 73.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszczyński, M.; Kałuża, T.; Mazurkiewicz, J.; Zawadzki, P.; Pawlak, M.; Matz, R.; Dach, J.; Czekała, W. Preparation of Samples for the Study of Rheological Parameters of Digested Pulps in a Bioreactor of an Agricultural Biogas Plant. Energies 2024, 17, 965. https://doi.org/10.3390/en17040965
Gruszczyński M, Kałuża T, Mazurkiewicz J, Zawadzki P, Pawlak M, Matz R, Dach J, Czekała W. Preparation of Samples for the Study of Rheological Parameters of Digested Pulps in a Bioreactor of an Agricultural Biogas Plant. Energies. 2024; 17(4):965. https://doi.org/10.3390/en17040965
Chicago/Turabian StyleGruszczyński, Maciej, Tomasz Kałuża, Jakub Mazurkiewicz, Paweł Zawadzki, Maciej Pawlak, Radosław Matz, Jacek Dach, and Wojciech Czekała. 2024. "Preparation of Samples for the Study of Rheological Parameters of Digested Pulps in a Bioreactor of an Agricultural Biogas Plant" Energies 17, no. 4: 965. https://doi.org/10.3390/en17040965
APA StyleGruszczyński, M., Kałuża, T., Mazurkiewicz, J., Zawadzki, P., Pawlak, M., Matz, R., Dach, J., & Czekała, W. (2024). Preparation of Samples for the Study of Rheological Parameters of Digested Pulps in a Bioreactor of an Agricultural Biogas Plant. Energies, 17(4), 965. https://doi.org/10.3390/en17040965







