Charge Storage and Magnetic Properties Nitrogen-Containing Nanoporous Bio-Carbon
Abstract
1. Introduction
2. Conceptual Provisions and Experimental Methodology
2.1. Sample Preparation
2.2. Research Methods
3. Results and Discussion
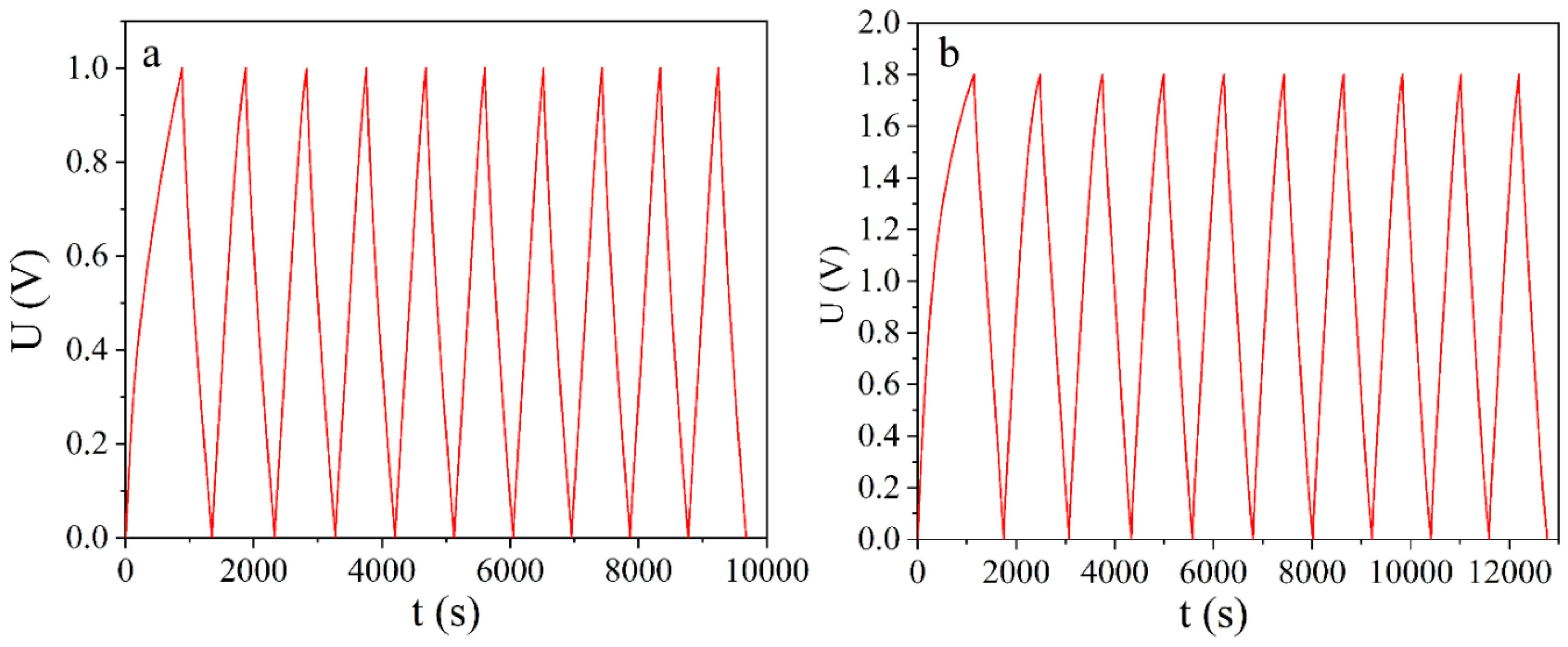
3.1. Galvanostatic Studies
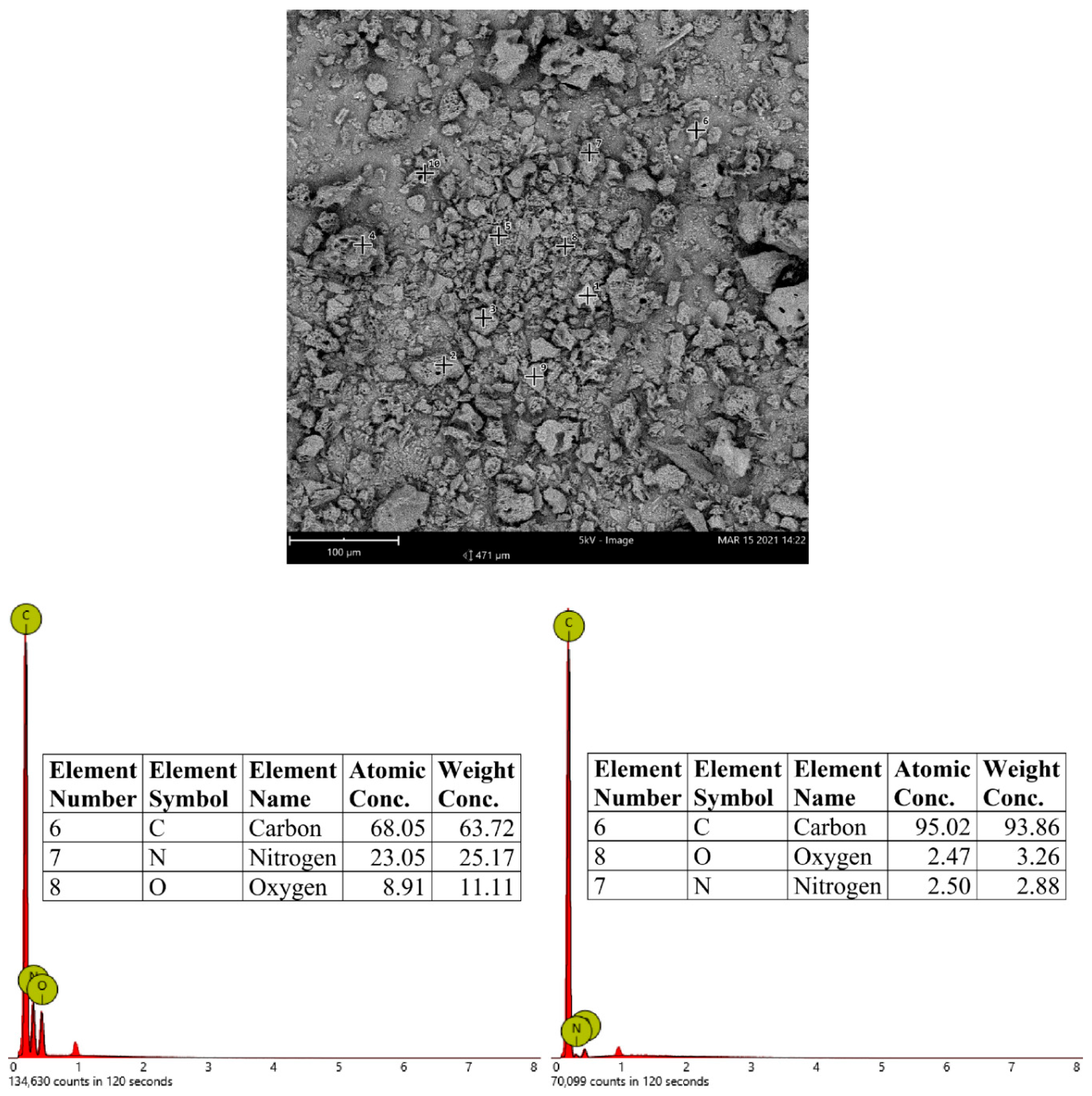
3.2. SEM Microphotography and Surface Elemental Analysis
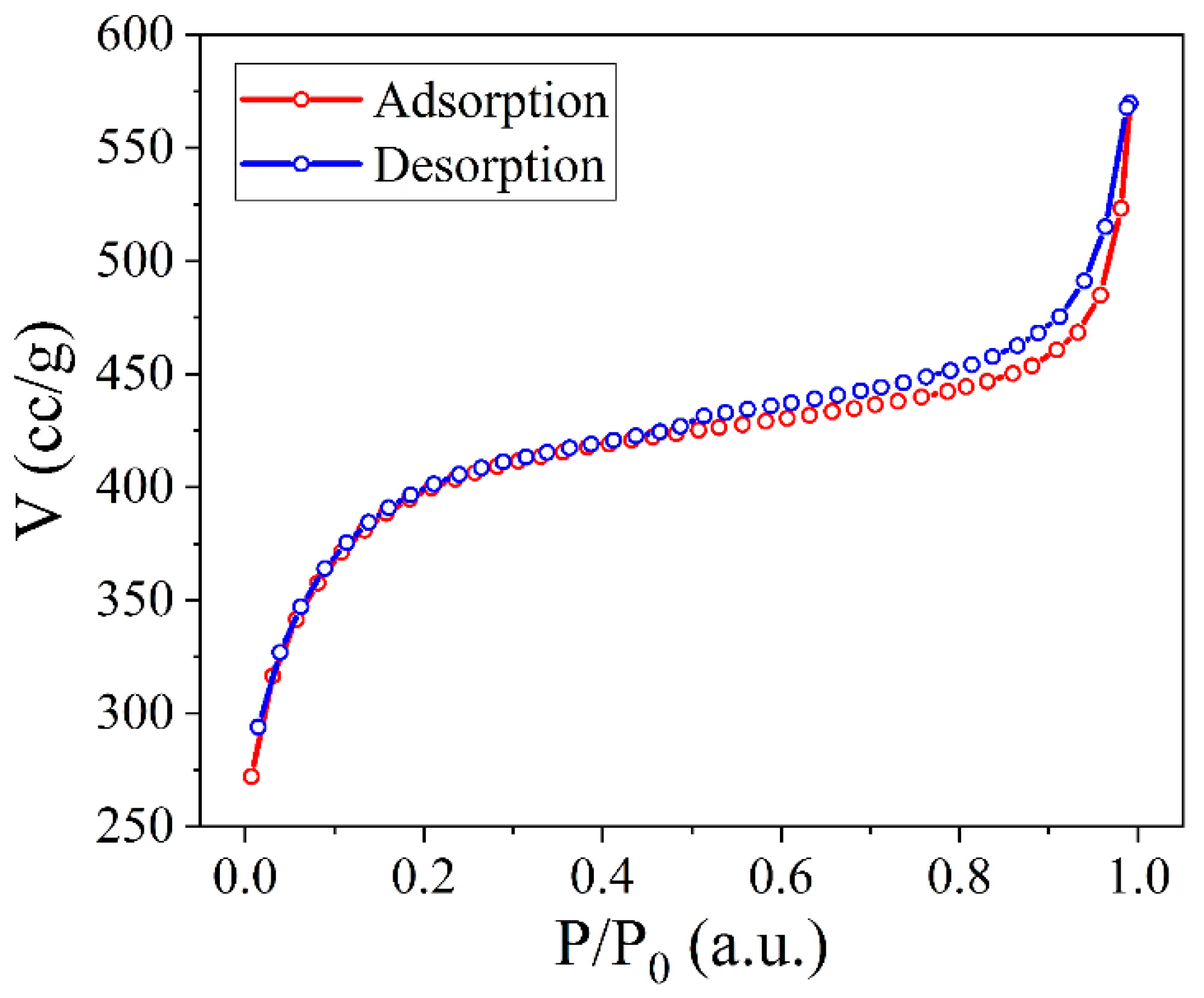
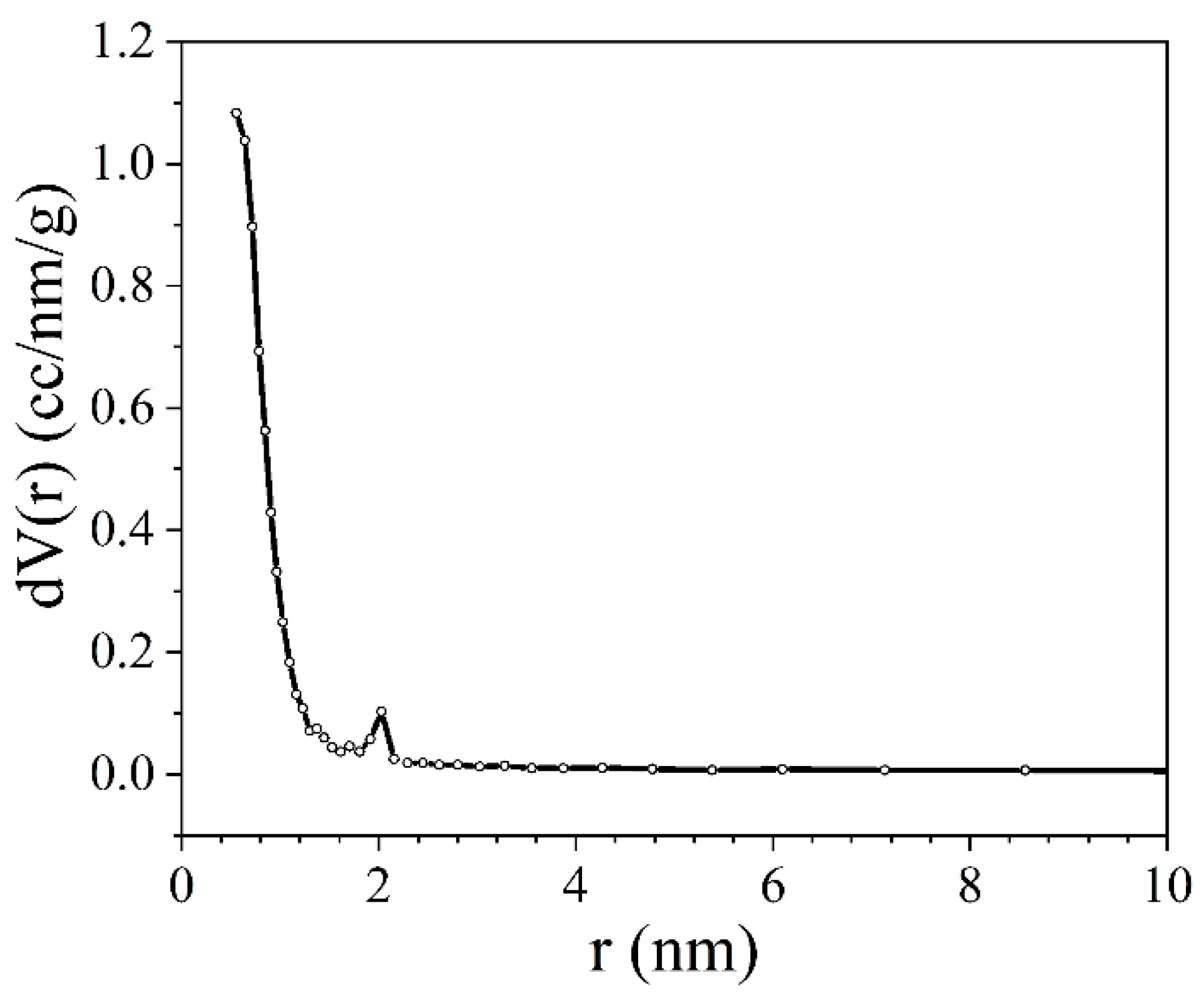
3.3. Nitrogen Adsorption/Desorption Porometry
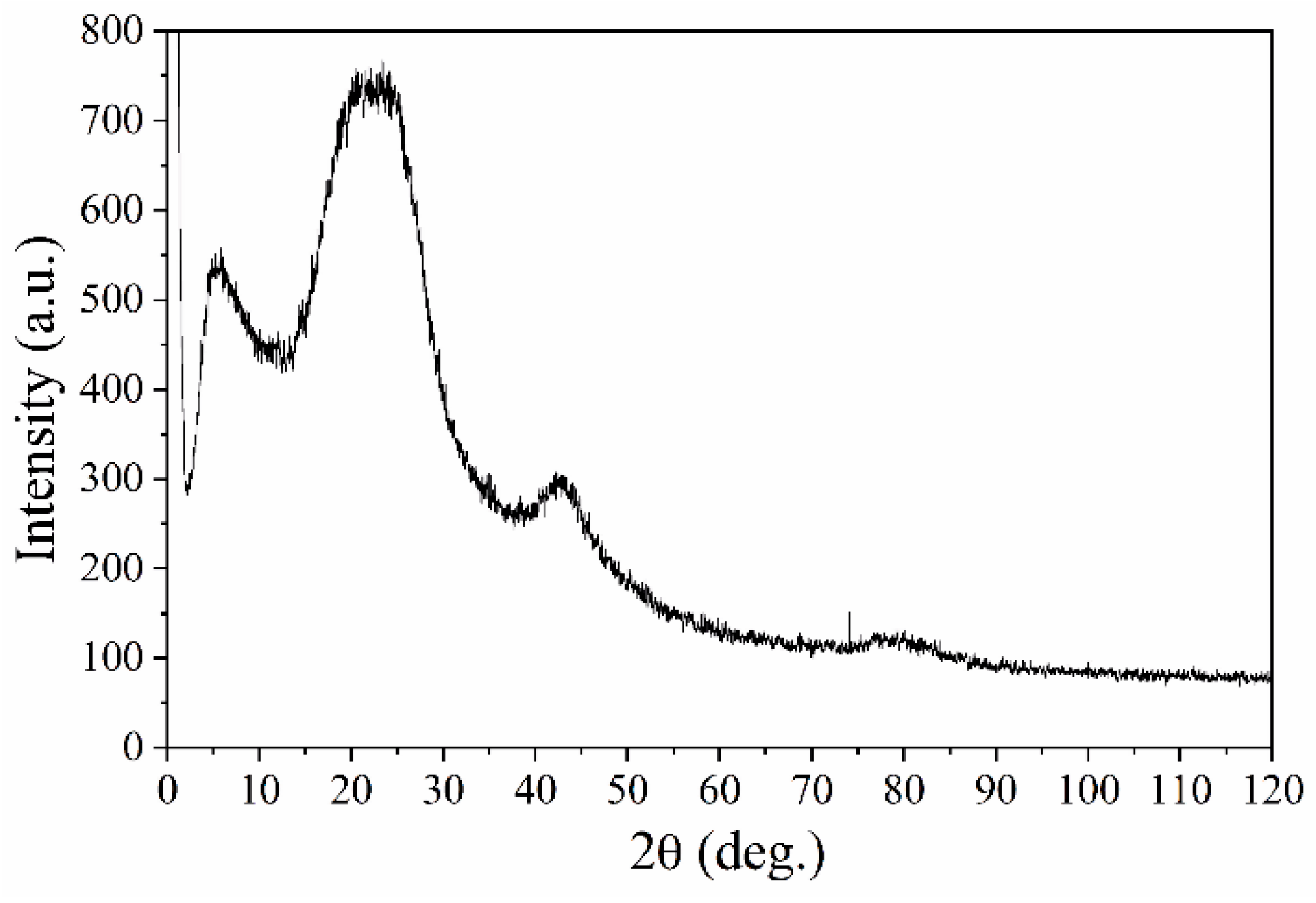
3.4. X-ray Diffraction Analysis
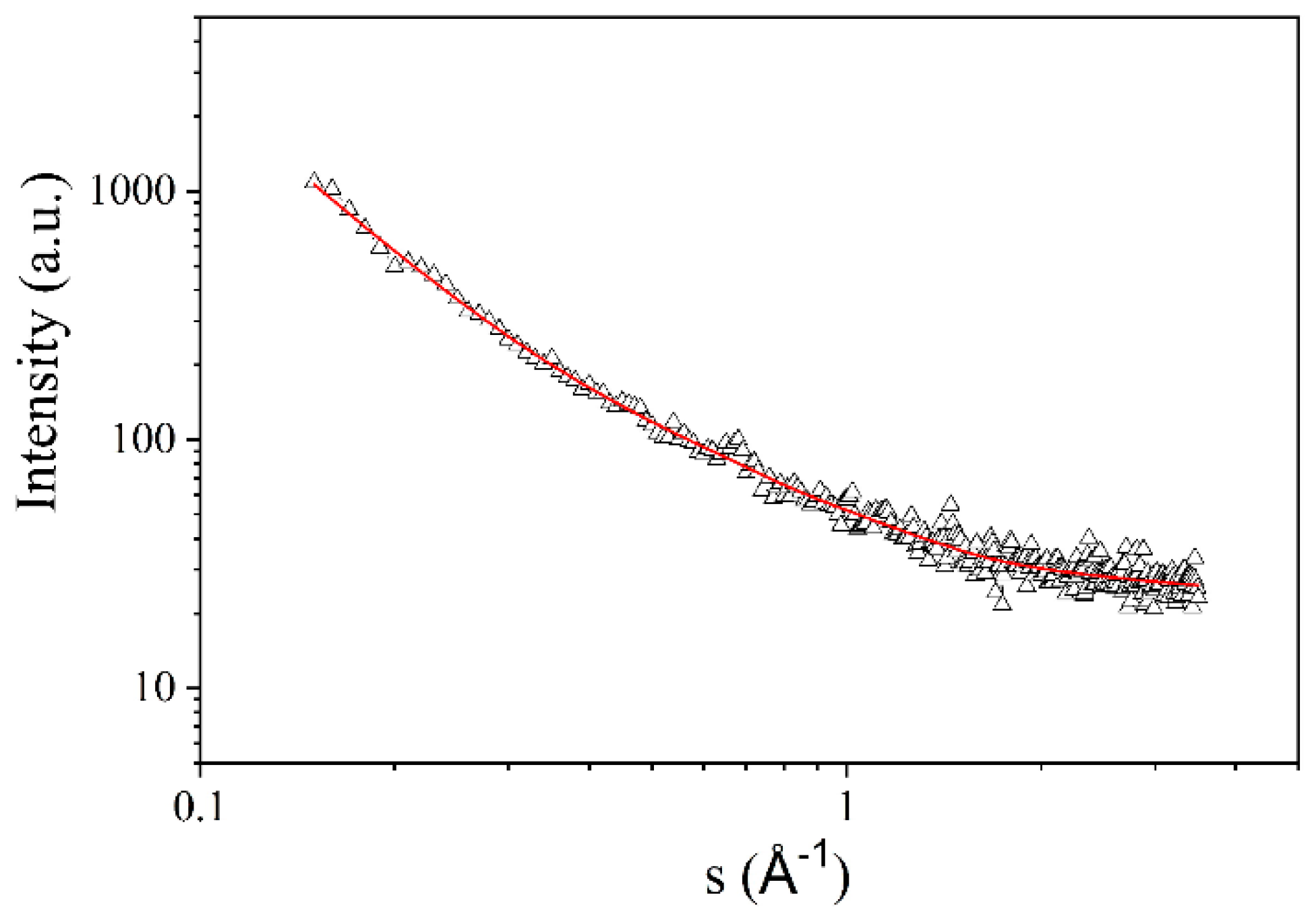
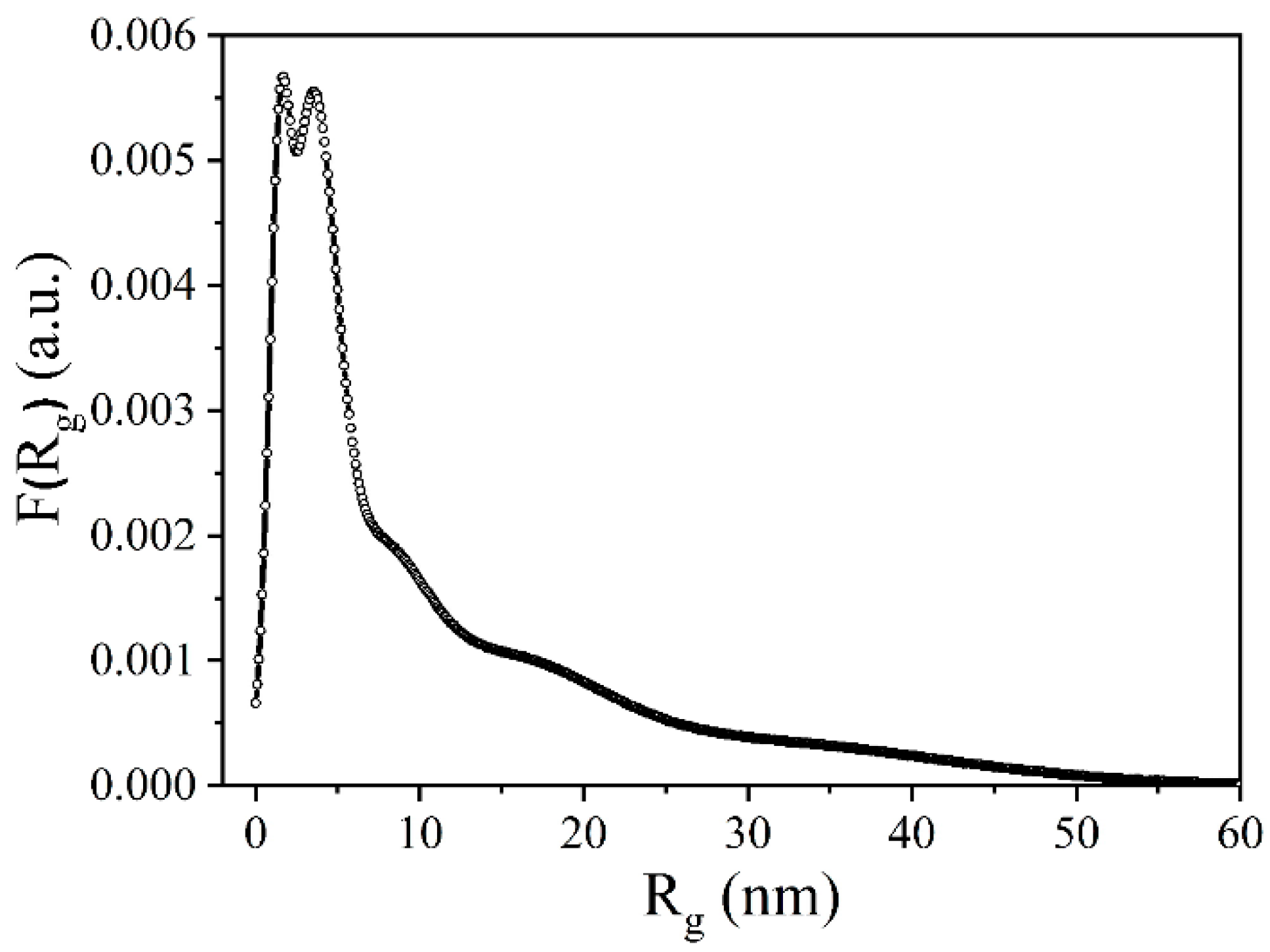
3.5. Small-Angle X-ray Scattering
- 1.
- A tangent is drawn to the scattering curve constructed in Guinier coordinates at the point of intersection with the ordinate axis;
- 2.
- The slope of the tangent determines the radius of inertia of the smallest particles Rg1, and the point of intersection of the tangent with the ordinate axis gives the value I1(0);
- 3.
- The contribution to the scattering intensity of the smallest particles is computed using the Guinier formula:
- 4.
- The difference scattering curve is calculated, and by performing similar calculations for this curve I2(s), the scattering invariants for the particles of the second fraction I2(0), Rg2 are determined;
- 5.
- Applying the above method iteratively, the scattering invariants for particles of all fractions are determined until the scattering intensity curve calculated using formula (3) coincides with the experimental curve.
3.6. Magnetometry
- Chains of interacting radicals;
- Carbon structures containing trivalent elements, such as P, N, and B;
- Structures that are a combination of sp2- and sp3-coordinated carbon atoms;
- Graphite and nanographite;
- Fullerenes.
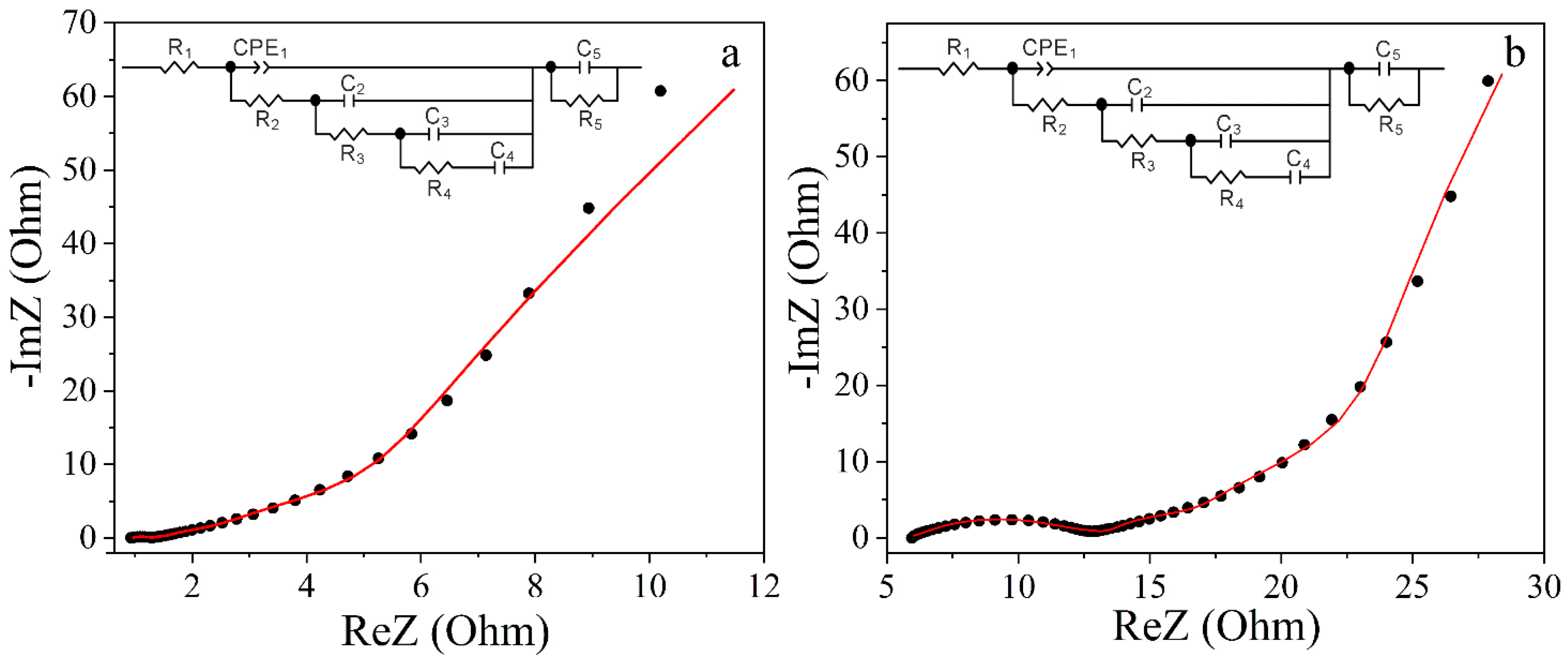
3.7. Cyclic Voltammetry and Impedance Studies
4. Conclusions
- A technology for synthesising the nitrogen-containing bio-carbon NCNM, characterised by a high degree of chemical composition homogeneity and a well-developed porous structure, has been introduced. This structure contributes to the superior energy performance of supercapacitors.
- The correlation of the results obtained using the nitrogen adsorption/desorption method and small-angle X-ray analysis indicates the accessibility of pores for the electrolyte and their full contribution to the specific capacity for NCNM.
- The NCNM exhibited specific saturation magnetisation values of σs = 1.4 A∙m2∙kg−1 and coercive force Hc = 10 kA/m when measured in a magnetic field of 800 kA/m. These magnetic properties arose from a graphite-like material containing numerous unpaired spins, enabling exchange interactions. The material formed a three-dimensional structure comprising both sp2- and sp3-carbon atoms.
- In a symmetrical electrochemical supercapacitor (SC), the specific capacitance reached 180 F/g, surpassing existing market analogues by more than 50%. The differential capacitance of the synthesised nanoporous carbon in a 30% aqueous solution of KOH was ~14.4 μF/cm2, and in a 1 M aqueous solution of Na2SO4,9.6 μF/cm2. According to the results of impedance spectroscopy, it was found that, in the case of the 1 M Na2SO4 electrolyte, diffusion processes had a significantly greater influence on charge accumulation than in the KOH-based electrolyte.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maksymych, V.; Całus, D.; Ivashchyshyn, F.; Pidluzhna, A.; Chabecki, P.; Shvets, R. Quantum energy accumulation in semiconductor < ionic liquid > layered clathrates. Appl. Nanosci. 2022, 12, 1147–1153. [Google Scholar] [CrossRef]
- Chabecki, P. Quantum Energy Storage in Dielectric Porous Clathrates. Energies 2022, 15, 6069. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Puziy, A.M.; Poddubnaya, O.I.; Suárez-García, F.; Tascón, J.M.; Lu, G.Q. Highly stable performance of supercapacitors from phosphorus-enriched carbons. J. Am. Chem. Soc. 2009, 131, 5026–5027. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogendoped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2015, 4, 1144–1173. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Generation of nitrogen functionalities on activated carbons by amidation reactions and Hofmann rearrangement: Chemical and electrochemical characterization. Carbon 2015, 91, 252–262. [Google Scholar] [CrossRef]
- Xiang, S.; Yang, X.; Lin, X.; Chang, C.; Que, H.; Li, M. Nitrogen and sulfur co-doped polyurethane-based porous carbon materials as supercapacitors exhibit excellent electrochemical performance. J. Solid State Electrochem. 2017, 21, 1457–1465. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhang, T.; Ouyang, L.; Yuan, S. Single-Step Preparation of Ultrasmall Iron Oxide-Embedded Carbon Nanotubes on Carbon Cloth with Excellent Superhydrophilicity and Enhanced Supercapacitor Performance. ACS Appl. Mater. Interfaces 2021, 13, 45670–45678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Kwon, T.; Nishihara, H.; Itoi, H.; Yang, Q.-H.; Kyotani, T. Enhancement mechanism of electrochemical capacitance in nitrogen- /boron-doped carbons with uniform straight nanochannels. Langmuir 2009, 25, 11961–11968. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, D.; Chen, X.; Fu, R. Nitrogen-enriched nanocarbons with a 3-D continuous mesopore structure from polyacrylonitrile for supercapacitor application. J. Phys. Chem. C 2010, 114, 8581–8586. [Google Scholar] [CrossRef]
- Lei, Z.; Christov, N.; Zhang, L.L.; Zhao, X.S. Mesoporous carbon nanospheres with an excellent electrocapacitive performance. J. Mater. Chem. 2011, 21, 2274–2281. [Google Scholar] [CrossRef]
- Ornelas, O.; Sieben, J.M.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D.; Geng, J.; Soin, N.; Siores, E.; Johnson, B.F.G. On the origin of the high capacitance of nitrogen-containing carbon nanotubes in acidic and alkaline electrolytes. Chem. Commun. 2014, 50, 11343–11346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel di-functional material for enhancing CO2 capture and super-capacitance. J. Mater. Sci. Technol. 2022, 99, 73–81. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, X.; Wei, J.; Gao, S. Functional groups and pore size distribution do matter to hierarchically porous carbons as high-rate-performance supercapacitors. Chem. Mater. 2016, 28, 445–458. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Garcia, B.B.; Liu, D.; Cao, G. Nitrogen modification of highly porous carbon for improved supercapacitor performance. J. Mater. Chem. 2012, 22, 9884–9889. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Shiraishi, S.; Morallón, E.; Cazorla-Amorós, D. Improvement of carbon materials performance by nitrogen functional groups in electrochemical capacitors in organic electrolyte at severe conditions. Carbon 2015, 82, 205–213. [Google Scholar] [CrossRef]
- Seredych, M.; Hulicova-Jurcakova, D.; Lu, G.Q.; Bandosz, T.J. Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance. Carbon 2008, 46, 1475–1488. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.-G.; Neff, D.; Fulvio, P.F.; Mayes, R.T.; Zhamu, A.; Fang, Q.; Chen, G.; Meyer, H.M.; Jang, B.Z.; et al. Nitrogen-enriched ordered mesoporous carbons through direct pyrolysis in ammonia with enhanced capacitive performance. J. Mater. Chem. A 2013, 1, 7920. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A.; Find, J.; Wild, U.; Schlögl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R. Synthesis of ordered mesoporous carbon and nitrogen-doped carbon materials with graphitic pore walls via a simple chemical vapor deposition method. Adv. Mater. 2004, 16, 1553–1558. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Synthesis of mesoporous carbons using ordered and disordered mesoporous silica templates and polyacrylonitrile as carbon precursor. J. Phys. Chem. B 2005, 109, 9216–9225. [Google Scholar] [CrossRef]
- Bleda-Martínez, M.J.; Peng, C.; Zhang, S.; Chen, G.Z.; Morallón, E.; CazorlaAmorós, D. Electrochemical methods to enhance the capacitance in activated carbon/polyaniline composites. J. Electrochem. Soc. 2008, 155, A672–A678. [Google Scholar] [CrossRef][Green Version]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Nishihara, H.; Kyotani, T. Templated nanocarbons for energy storage. Adv. Mater. 2012, 24, 4473–4498. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Nitrogen doped superporous carbon prepared by a mild method. Enhancement of supercapacitor performance. Int. J. Hydrogen Energy 2016, 41, 19691–19701. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; González-Gaitán, C.; Morallón, E.; Cazorla-Amorós, D. Effect of carbonization conditions of polyaniline on its catalytic activity towards ORR. Some insights about the nature of the active sites. Carbon 2017, 119, 62–71. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; et al. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef] [PubMed]
- Ostafiychuk, B.K.; Budzulyak, I.M.; Kuzyshyn, M.M.; Rachiy, B.I.; Zatorskiy, R.A.; Lisovskiy, R.P.; Mandzyuk, V.I. Nitrogen-Containing Nanoporous Coal for Electrodes of Supercapacitors. J. Nano Electron. Phys. 2013, 5, 03049. [Google Scholar]
- Tian, W.; Zhang, H.; Sun, H.; Tadé, M.O.; Wang, S. Template-free synthesis of N-doped carbon with pillared-layered pores as bifunctional materials for supercapacitor and environmental applications. Carbon 2017, 118, 98–105. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Puthusseri, D.; Aravindan, V.; Madhavi, S.; Ogale, S. 3D micro-porous conducting carbon beehive by single step polymer carbonization for high performance supercapacitors: The magic of in situ porogen formation. Energy Environ. Sci. 2014, 7, 728–735. [Google Scholar] [CrossRef]
- Bichat, M.P.; Raymundo-Piñero, E.; Béguin, F. High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte. Carbon 2010, 48, 4351–4361. [Google Scholar] [CrossRef]
- Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. A symmetric carbon/carbon supercapacitor operating at 1.6 V by using a neutral aqueous solution. Electrochem. Commun. 2010, 12, 1275–1278. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Li, M.; Yang, C.; Zhang, L.; Wang, C.; Lu, H. Strong interface coupling and few-crystalline MnO2/Reduced graphene oxide composites for supercapacitors with high cycle stability. Electrochim. Acta 2018, 292, 115–124. [Google Scholar] [CrossRef]
- Gao, Q.; Demarconnay, L.; Pineroa, E.R.; Beguin, F. Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte. Energy Environ. Sci. 2012, 5, 9611–9617. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Zheng, X.; Tian, X.; Yuan, S. Enhancing Energy Storage via Confining Sulfite Anions onto Iron Oxide/Poly(3,4-Ethylenedioxythiophene) Heterointerface. ACS Appl. Mater. Interfaces 2023, 15, 59413–59421. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M. Structure and Texture of Carbon Materials. Carbons Electrochem. Energy Storage Convers. Syst. 2009, 37–76. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Zavalij, P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bordun, I.; Pidluzhna, A.; Ivashchyshyn, F.; Borysiuk, A.; Całus, D.; Chwastek, K. Structural and magnetic properties of Ni/C composites synthesized from beet pulp and corn stems. Magnetochemistry 2021, 7, 31. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1117. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Lozano-Castelló, D.; Suárez-Garsía, F.; Cazorla-Amoróz, D.; Linares-Solano, Á. Porous texture of carbons. In Carbon for Electrochemical Energy Storage Systems; Beguin, F., Frackowiak, E., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA; New York, NY, USA, 2002. [Google Scholar]
- Guinier, A.; Fournet, G. Small-Angle Scattering of X-rays; Wiley: New York, NY, USA, 1955. [Google Scholar]
- Porod, G. General theory. In Small-Angle X-ray Scattering; Glatter, O., Kratky, O., Eds.; Academic Press: London, UK, 1982. [Google Scholar]
- Soloviy, C.; Malovanyy, M.; Bordun, I.; Ivashchyshyn, F.; Borysiuk, A.; Kulyk, Y. Structural, magnetic and adsorption characteristics of magnetically susceptible carbon sorbents based on natural raw materials. J. Water Land Dev. 2020, 47, 160–168. [Google Scholar] [CrossRef]
- Makarova, T.L. Magnetic properties of carbon structures. Semiconductors 2004, 38, 615–638. [Google Scholar] [CrossRef]
- Murata, K.; Ushijima, H.; Ueda, H.; Kawaguchi, K. A stable carbon-based organic magnet. J. Chem. Soc. Chem. Commun. 1992, 7, 567–569. [Google Scholar] [CrossRef]
- Ushijima, H.; Murata, K.; Ueda, H.; Kawaguchi, K. Preparation of amorphouslike carbons by pyrolysis of organic compounds and their magnetic properties. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1993, 233, 351–360. [Google Scholar] [CrossRef]
- Setnescu, R.; Jipa, S.; Setnescu, T.; Kappel, W.; Kobayashi, S.; Osawa, Z. IR and X-ray characterization of the ferromagnetic phase of pyrolysed polyacrylonitrile. Carbon 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Helm, L. Defect-induced magnetism in graphene. Phys. Rev. B—Condens. Matter Mater. Phys. 2007, 75, 125408. [Google Scholar] [CrossRef]
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Half-metallic graphene nanoribbons. Nature 2006, 444, 347–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Talapatra, S.; Kar, S.; Vajtai, R.; Nayak, S.K.; Ajayan, P.M. First-principles study of defect-induced magnetism in carbon. Phys. Rev. Lett. 2007, 99, 10720. [Google Scholar] [CrossRef] [PubMed]
- Bordun, I.; Chabecki, P.; Malovanyy, M.; Pieshkov, T.; Chwastek, K. Changes in the Electrical Charge Accumulation Ability of Nanoporous Activated Carbon under Ultrasonic Radiation Exposure. Isr. J. Chem. 2020, 60, 615–623. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Cheng, H.M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor. J. Power Sources 2008, 185, 1563–1568. [Google Scholar] [CrossRef]
- Taberna, P.; Simon, P.; Fauvarque, J. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 2003, 150, 292–300. [Google Scholar] [CrossRef]
- Frackowiak, E.; Jurewicz, K.; Delpeux, S.; Béguin, F. Nanotubular materials for supercapacitors. J. Power Sources 2001, 97–98, 822–825. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Mandzyuk, V.I.; Myronyuk, I.F.; Sachko, V.M.; Rachiy, B.I.; Kulyk, Y.O.; Mykytyn, I.M. Structure and Electrochemical Properties of Saccharide-derived Porous Carbon Materials. J. Nano-Electron. Phys. 2018, 10, 02018. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Mesostructured carbon-based nanocages: An advanced platform for energy chemistry. Sci. China Chem. 2020, 63, 665–681. [Google Scholar] [CrossRef]




| Electrolyte | Synthesis Temperature NCNM, °С | |||
|---|---|---|---|---|
| 600 | 700 | 800 | ||
| C, F/g | 30% KOH | 20 | 109 | 180 |
| 1 M Na2SO4 | 9 | 68 | 124 | |
| Sample | S, m2/g | V, cm3/g | , nm | Correlation Coefficient according to the BET Model |
|---|---|---|---|---|
| NCNM | 1247 | 0.88 | 1.42 | 0.996 |
| Sample | Kp·104, nm−4 | Qp·103, nm−3 | Rgs, nm | Sp, m2/cm3 |
|---|---|---|---|---|
| NCNM | 0.883 | 49.5 | 2.95 | 592 |
| Electrolyte | R1, Оhm | CPE1 | R2, Оhm | C2, F | R3, Ohm | C3, F | R4, Оhm | C4, F | R5, Ohm | C5, mF/g | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | ||||||||||
| 30% KOH | 0.958 | 0.052 | 0.785 | 0.84 | 0.037 | 3.839 | 0.066 | 23.7 | 0.063 | 0.315 | 0.131 |
| 1 M Na2SO4 | 5.639 | 0.007 | 0.429 | 5.608 | 0.032 | 6.376 | 0.099 | 32 | 0.098 | 3.114 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczykiewicz, E.; Bordun, I.; Maksymych, V.; Klapchuk, M.; Kohut, Z.; Borysiuk, A.; Kulyk, Y.; Ivashchyshyn, F. Charge Storage and Magnetic Properties Nitrogen-Containing Nanoporous Bio-Carbon. Energies 2024, 17, 903. https://doi.org/10.3390/en17040903
Szymczykiewicz E, Bordun I, Maksymych V, Klapchuk M, Kohut Z, Borysiuk A, Kulyk Y, Ivashchyshyn F. Charge Storage and Magnetic Properties Nitrogen-Containing Nanoporous Bio-Carbon. Energies. 2024; 17(4):903. https://doi.org/10.3390/en17040903
Chicago/Turabian StyleSzymczykiewicz, Ewelina, Ihor Bordun, Vitalii Maksymych, Myroslava Klapchuk, Zenoviy Kohut, Anatoliy Borysiuk, Yuriy Kulyk, and Fedir Ivashchyshyn. 2024. "Charge Storage and Magnetic Properties Nitrogen-Containing Nanoporous Bio-Carbon" Energies 17, no. 4: 903. https://doi.org/10.3390/en17040903
APA StyleSzymczykiewicz, E., Bordun, I., Maksymych, V., Klapchuk, M., Kohut, Z., Borysiuk, A., Kulyk, Y., & Ivashchyshyn, F. (2024). Charge Storage and Magnetic Properties Nitrogen-Containing Nanoporous Bio-Carbon. Energies, 17(4), 903. https://doi.org/10.3390/en17040903






