Changes in the Characteristics of Pine Logging Residue during Storage in Forest Stands
Abstract
1. Introduction
2. Materials and Methods
2.1. Storage of Residues and Collecting the Samples
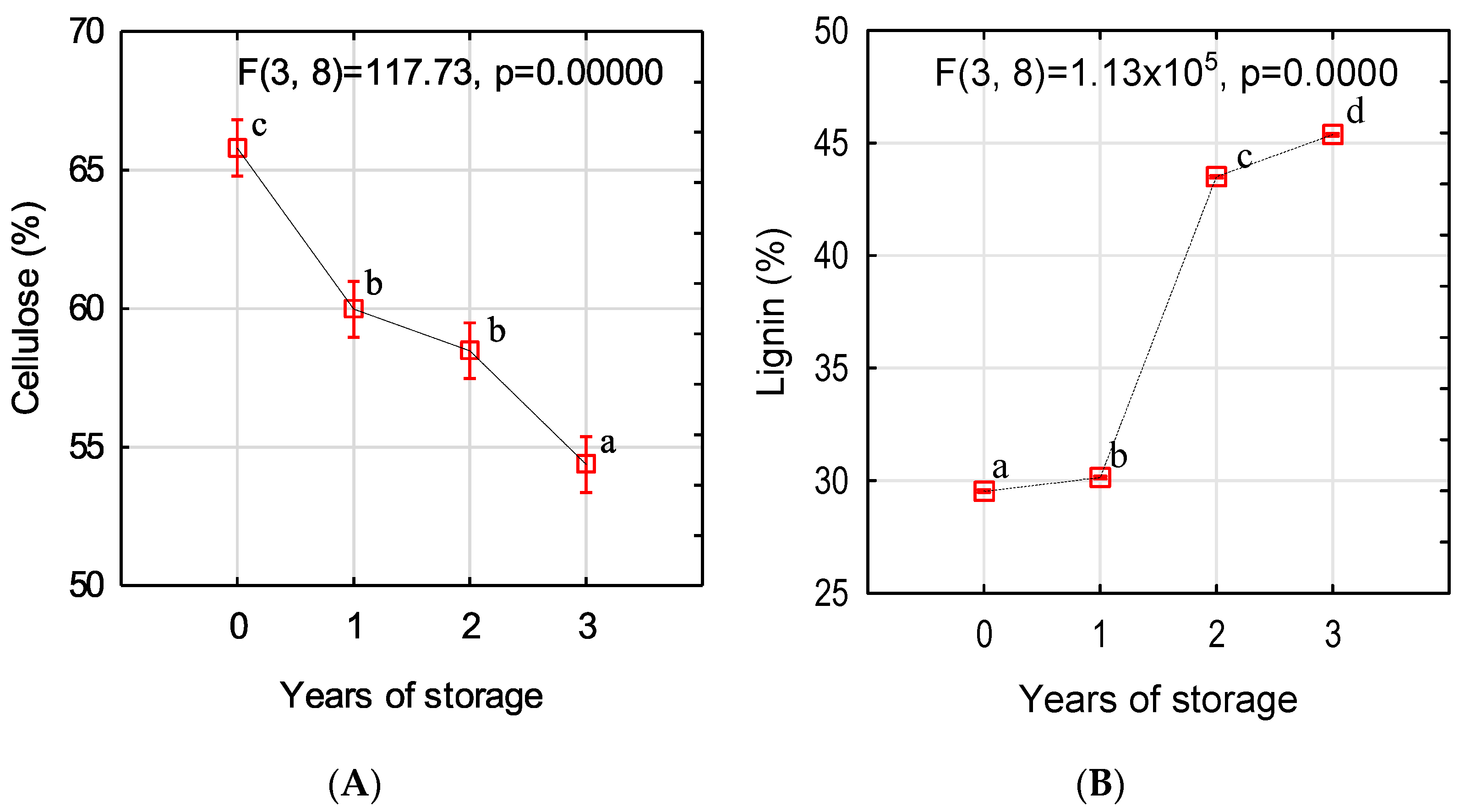
2.2. Determination of Cellulose and Lignin Content
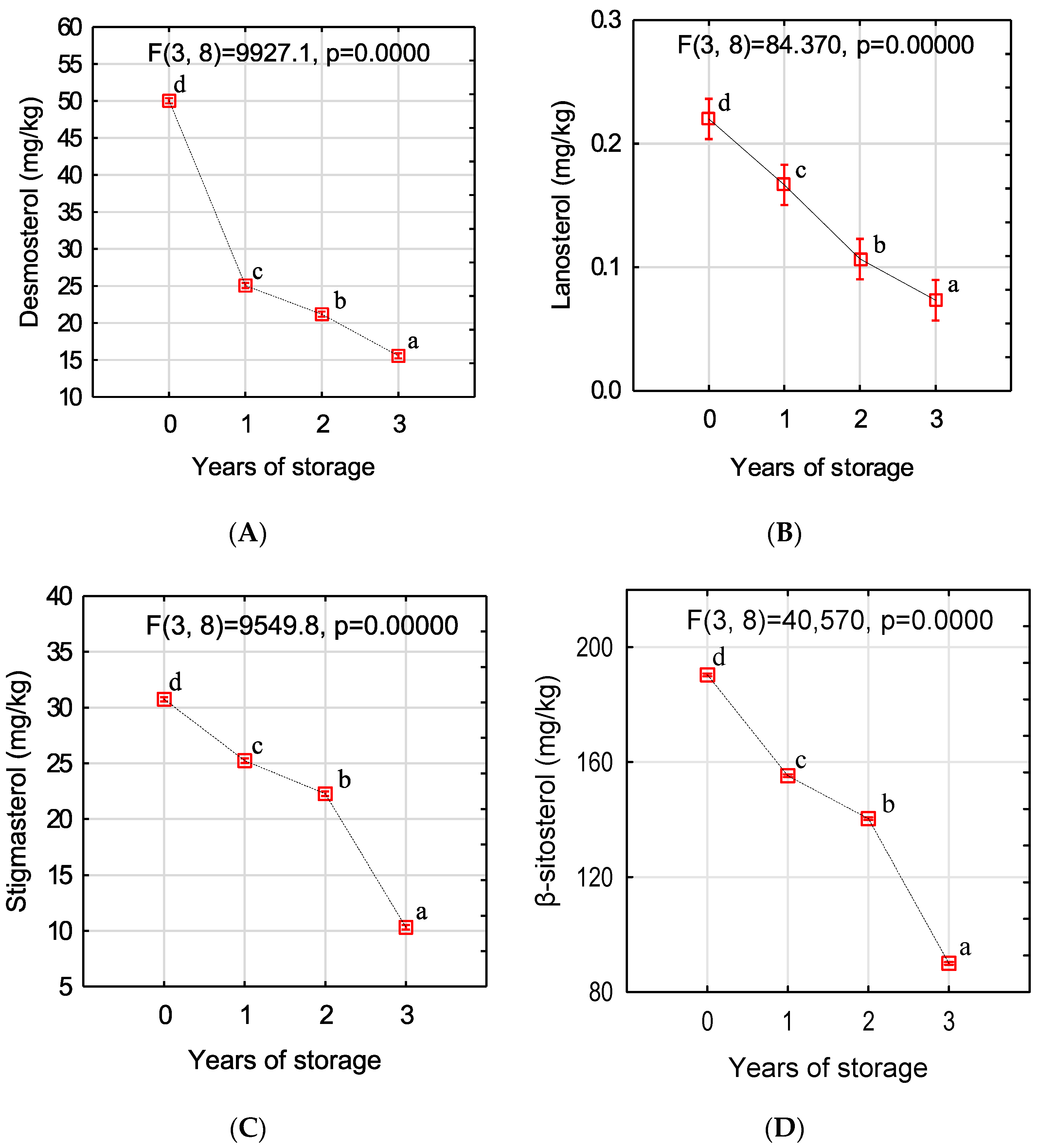
2.3. Determination of Sterol Content
2.4. Determination of Low Molecular Weight Organic Acid (LMOWA) Content
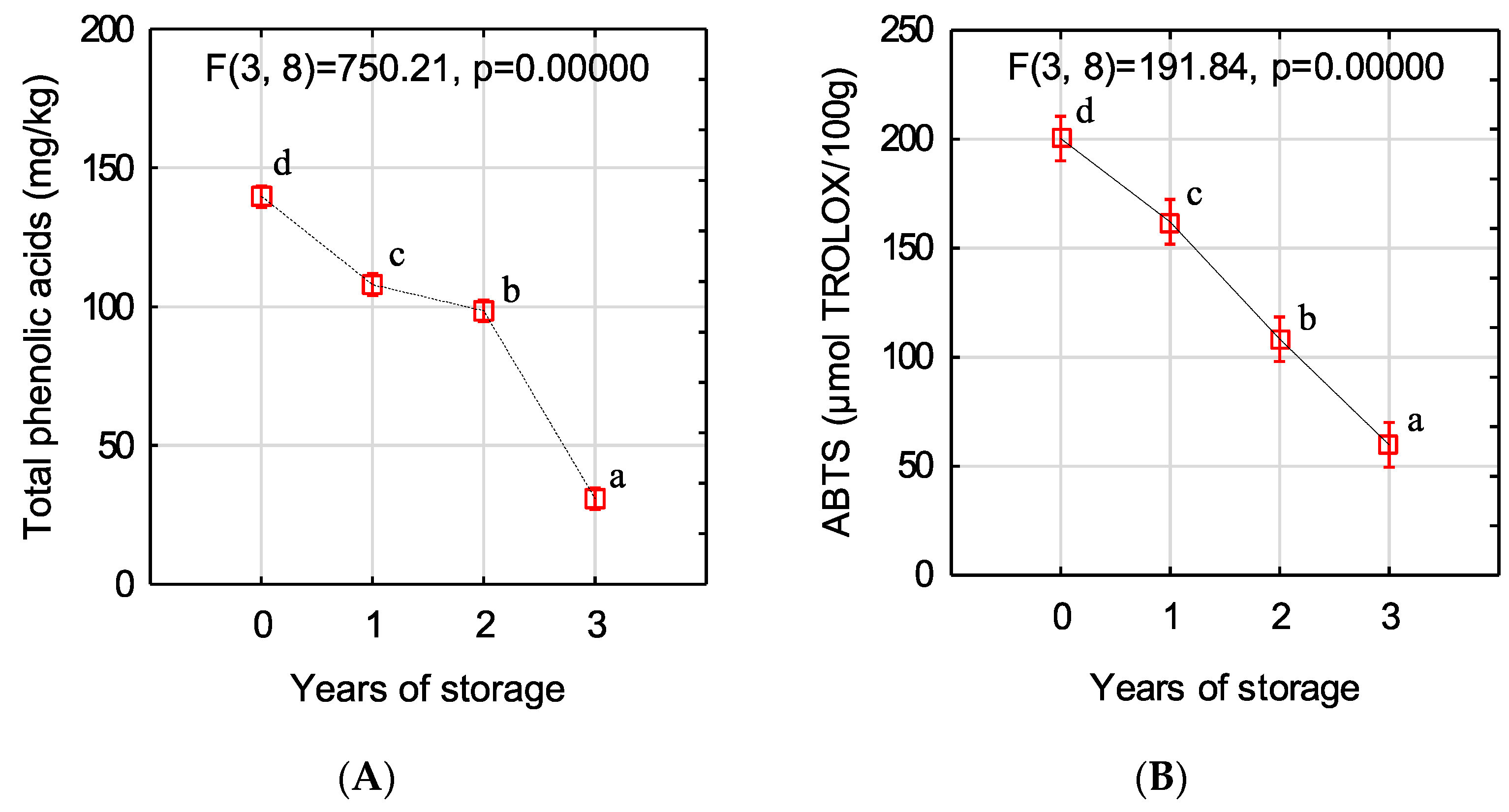
2.5. Determination of Total Phenolic Acid (TPA) Content
2.6. Antioxidant Activity (ABTS+ Method)
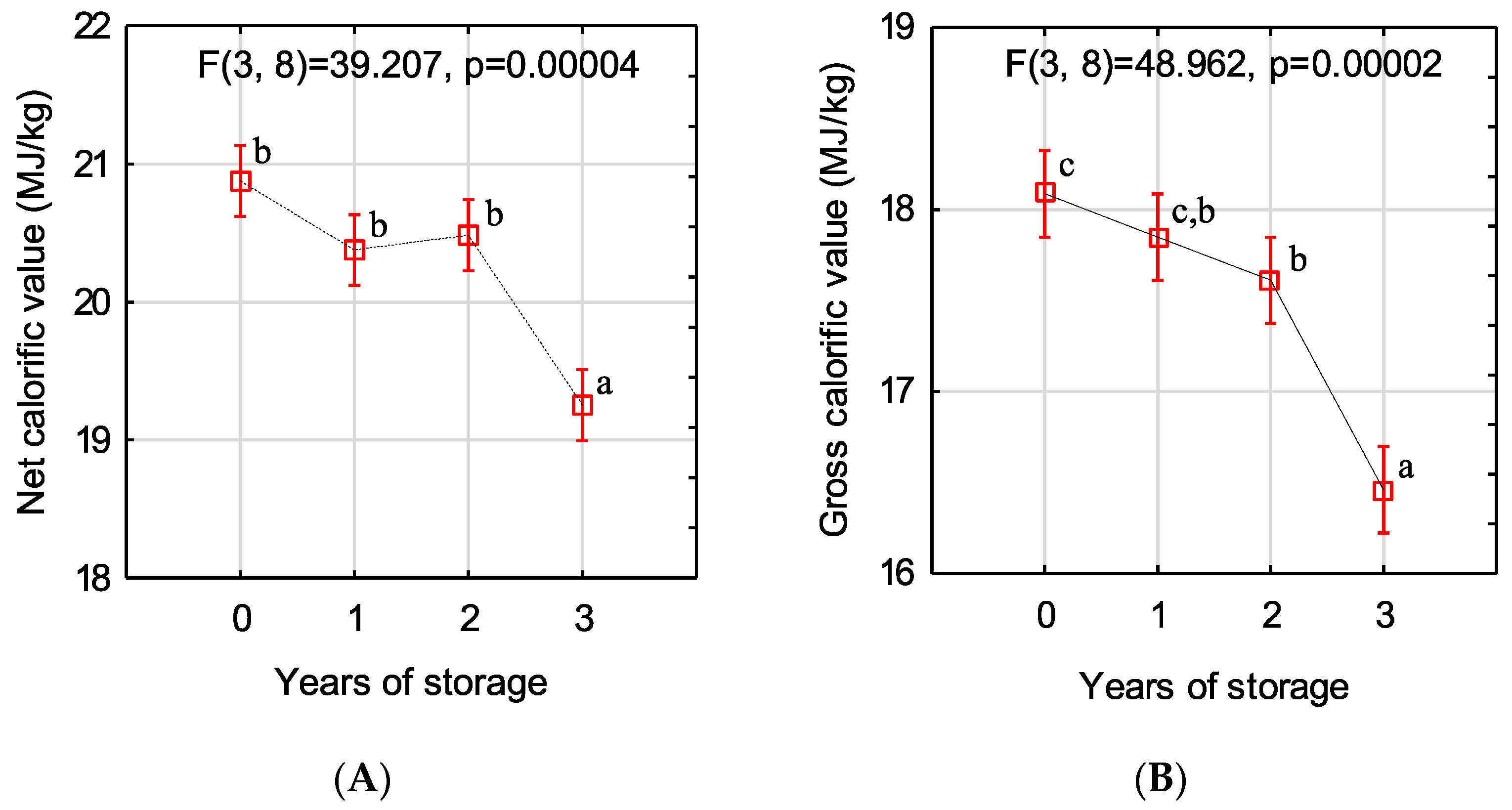
2.7. Determination of Ash Content and Net and Gross Calorific Values
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shmulsky, R.; Jones, P.D. Forest Products and Wood Science: An Introduction; John Wiley & Sons: New York, NY, USA, 2019; ISBN 9780813820743. [Google Scholar]
- Wieruszewski, M.; Turbański, W.; Mydlarz, K.; Sydor, M. Economic Efficiency of Pine Wood Processing in Furniture Production. Forests 2023, 14, 688. [Google Scholar] [CrossRef]
- Ranius, T.; Hämäläinen, A.; Egnell, G.; Olsson, B.; Eklöf, K.; Stendahl, J.; Rudolphi, J.; Sténs, A.; Felton, A. The Effects of Logging Residue Extraction for Energy on Ecosystem Services and Biodiversity: A Synthesis. J. Environ. Manag. 2018, 209, 409–425. [Google Scholar] [CrossRef]
- Amiandamhen, S.O.; Kumar, A.; Adamopoulos, S.; Jones, D.; Nilsson, B. Bioenergy Production and Utilization in Different Sectors in Sweden: A State of the Art Review. BioResources 2020, 15, 9834. [Google Scholar] [CrossRef]
- Bessaad, A.; Bilger, I.; Korboulewsky, N. Assessing Biomass Removal and Woody Debris in Whole-Tree Harvesting System: Are the Recommended Levels of Residues Ensured? Forests 2021, 12, 807. [Google Scholar] [CrossRef]
- Ghaffariyan, M.R. Remaining Slash in Different Harvesting Operation Sites in Australian Plantations. Silva Balc. 2013, 14, 83–93. [Google Scholar]
- Ghaffariyan, M.R.; Acuna, M.; Wiedemann, J.; Mitchell, R. Productivity of the Bruks Chipper When Harvesting Forest Biomass in Pine Plantations; CRC for Forestry Bulletin 16; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Conway, S. Logging Practices: Principles of Timber Harvesting Systems, Revised ed.; Miller Freeman Inc.: San Francisco, CA, USA, 1982. [Google Scholar]
- Watson, W.F.; Stokes, B.J.; Savelle, I.W. Comparisons of Two Methods of Harvesting Biomass for Energy. For. Prod. J. 1986, 36, 63–68. [Google Scholar]
- Spinelli, R.; Visser, R.; Björheden, R.; Röser, D. Recovering Energy Biomass in Conventional Forest Operations: A Review of Integrated Harvesting Systems. Curr. For. Rep. 2019, 5, 90–100. [Google Scholar] [CrossRef]
- Cuchet, E.; Roux, P.; Spinelli, R. Performance of a Logging Residue Bundler in the Temperate Forests of France. Biomass Bioenergy 2004, 27, 31–39. [Google Scholar] [CrossRef]
- Spinelli, R.; Lombardini, C.; Magagnotti, N. The Effect of Mechanization Level and Harvesting System on the Thinning Cost of Mediterranean Softwood Plantations. Silva Fenn. 2014, 48, 1003. [Google Scholar] [CrossRef]
- Hytönen, J.; Moilanen, M. Effect of Harvesting Method on the Amount of Logging Residues in the Thinning of Scots Pine Stands. Biomass Bioenergy 2014, 67, 347–353. [Google Scholar] [CrossRef]
- Ghaffariyan, M.R.; Apolit, R. Harvest Residues Assessment in Pine Plantations Harvested by Whole Tree and Cut-to-Length Harvesting Methods (a Case Study in Queensland, Australia). Silva Balc. 2015, 16, 113–122. [Google Scholar]
- Koelling, C.; Göttlein, A.; Rothe, A. Energieholz Nachhaltig Nutzen–Biomassenutzung Und Nährstoffentzug. LWF Aktuell 2007, 32–36. [Google Scholar]
- Beardsell, M.G. Integrated Harvesting Systems to Incorporate the Recovery of Logging Residues with the Harvesting of Conventional Forest Products; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 1983. [Google Scholar]
- Nurmi, J.; Hillebrand, K. Storage Alternatives Affect Fuelwood Properties of Norway Spruce Logging Residues. N. Z. J. For. Sci. 2001, 31, 289–297. [Google Scholar]
- Zyryanov, M.; Medvedev, S.; Mokhirev, A. Study of the Possibility of Using Logging Residue for the Production of Wood Processing Enterprises. J. Appl. Eng. Sci. 2020, 18, 15–18. [Google Scholar] [CrossRef]
- Schnepf, C.; Graham, R.T.; Kegley, S.; Jain, T.B. Managing Organic Debris for Forest Health; Pacific Northwest Extension Publication PNW, PNW609; Washington State University: Pullman, WA, USA, 2009. [Google Scholar]
- Smolander, A.; Saarsalmi, A.; Tamminen, P. Response of Soil Nutrient Content, Organic Matter Characteristics and Growth of Pine and Spruce Seedlings to Logging Residues. For. Ecol. Manag. 2015, 357, 117–125. [Google Scholar] [CrossRef]
- Richardson, J.; Björheden, R.; Hakkila, P.; Lowe, A.T.; Smith, C.T. Bioenergy from Sustainable Forestry: Guiding Principles and Practice; Springer: Berlin, Germany, 2002. [Google Scholar]
- Mälkönen, E. Effect of Whole-Tree Harvesting on Soil Fertility. Silva Fenn 1976, 10, 157–164. [Google Scholar] [CrossRef]
- Evans, A.M. Ecology of Dead Wood in the Southeast; Forest Guild: Santa Fe, NM, USA, 2011; p. 37. [Google Scholar]
- Hakkila, P. Operations with Reduced Environmental Impact; Klewer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 244–261. [Google Scholar]
- Wall, A.; Hytönen, J. The Long-Term Effects of Logging Residue Removal on Forest Floor Nutrient Capital, Foliar Chemistry and Growth of a Norway Spruce Stand. Biomass Bioenergy 2011, 35, 3328–3334. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying Consequences of Removing Harvesting Residues on Forest Soils and Tree Growth–A Meta-Analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Kuiper, L.; Oldenburger, J. The Harvest of Forest Residues in Europe. In Quick-Scans on Upstream Biomass: Yearbook; Wageningen University & Research: Wageningen, The Netherlands, 2006; pp. 13–18. [Google Scholar]
- Thiffault, E.; Béchard, A.; Paré, D.; Allen, D. Recovery Rate of Harvest Residues for Bioenergy in Boreal and Temperate Forests: A Review. In Advances in Bioenergy: The Sustainability Challenge; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 293–316. [Google Scholar]
- Titus, B.D.; Brown, K.; Helmisaari, H.-S.; Vanguelova, E.; Stupak, I.; Evans, A.; Clarke, N.; Guidi, C.; Bruckman, V.J.; Varnagiryte-Kabasinskiene, I. Sustainable Forest Biomass: A Review of Current Residue Harvesting Guidelines. Energy Sustain. Soc. 2021, 11, 1–32. [Google Scholar] [CrossRef]
- Malinowski, Z.; Kawalerczyk, J.; Walkiewicz, J.; Dziurka, D.; Mirski, R. The Effect of the Tree Dieback Process on the Mechanical Properties of Pine (Pinus sylvestris L.) Wood. Forests 2023, 14, 274. [Google Scholar] [CrossRef]
- Bekhta, P.; Kozak, R.; Gryc, V.; Sebera, V.; Tippner, J. Effects of Wood Particles from Deadwood on the Properties and Formaldehyde Emission of Particleboards. Polymers 2022, 14, 3535. [Google Scholar] [CrossRef]
- Lebreton, A.; Zeng, Q.; Miyauchi, S.; Kohler, A.; Dai, Y.-C.; Martin, F.M. Evolution of the Mode of Nutrition in Symbiotic and Saprotrophic Fungi in Forest Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 385–404. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Tian, P.; Yao, X.; Sun, H.; Wang, Q.; Delgado-Baquerizo, M. Decoupled Diversity Patterns in Bacteria and Fungi across Continental Forest Ecosystems. Soil Biol. Biochem. 2020, 144, 107763. [Google Scholar] [CrossRef]
- Available online: www.lasy.gov.pl (accessed on 30 June 2023).
- Ghaffariyan, M.R.; Spinelli, R.; Magagnotti, N.; Brown, M. Integrated Harvesting for Conventional Log and Energy Wood Assortments: A Case Study in a Pine Plantation in Western Australia. South. For. J. For. Sci. 2015, 77, 249–254. [Google Scholar] [CrossRef]
- Smethurst, P.J.; Nambiar, E.K.S. Distribution of Carbon and Nutrients and Fluxes of Mineral Nitrogen after Clear-Felling a Pinus Radiata Plantation. Can. J. For. Res. 1990, 20, 1490–1497. [Google Scholar] [CrossRef]
- Palacka, M.; Vician, P.; Holubčík, M.; Jandačka, J. The Energy Characteristics of Different Parts of the Tree. Procedia Eng. 2017, 192, 654–658. [Google Scholar] [CrossRef]
- Singh, T.; Kostecky, M.M. Calorific Value Variations in Components of 10 Canadian Tree Species. Can. J. For. Res. 1986, 16, 1378–1381. [Google Scholar] [CrossRef]
- Miao, Z.; Grift, T.E.; Hansen, A.C.; Ting, K.C. Energy Requirement for Comminution of Biomass in Relation to Particle Physical Properties. Ind. Crops Prod. 2011, 33, 504–513. [Google Scholar] [CrossRef]
- Jewiarz, M.; Wróbel, M.; Mudryk, K.; Szufa, S. Impact of the Drying Temperature and Grinding Technique on Biomass Grindability. Energies 2020, 13, 3392. [Google Scholar] [CrossRef]
- T264-cm-07; Preparation of Wood for Chemical Analysis. Technical Association of the Pulp and Paper Industry: New York, NY, USA, 2001.
- Browning, B.L. The Chemistry of Wood; Interscience: Geneva, Switzerland, 1963. [Google Scholar]
- T 222 cm-06; Acid Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp and Paper Industry: New York, NY, USA, 2006; p. 5.
- Szwajkowska-Michałek, L.; Rogoziński, T.; Mirski, R.; Stuper-Szablewska, K. Wood Processing Waste–Contamination with Microscopic Fungi and Contents of Selected Bioactive Compounds. BioResources 2020, 15, 1763–1772. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Rogoziński, T.; Perkowski, J. Contamination of Pine and Birch Wood Dust with Microscopic Fungi and Determination of Its Sterol Contents. Arh. Hig. Rada Toksikol. 2017, 68, 127. [Google Scholar] [CrossRef] [PubMed]
- Stuper-Szablewska, K.; Szablewski, T.; Przybylska-Balcerek, A.; Szwajkowska-Michałek, L.; Krzyżaniak, M.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z. Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine. Molecules 2023, 28, 244. [Google Scholar] [CrossRef] [PubMed]
- Mirski, R.; Kawalerczyk, J.; Dziurka, D.; Stuper-Szablewska, K.; Wieruszewski, M. Mold Fungi Development during the Short-Term Wood-Chips Storage Depending on the Storage Method. Wood Mater. Sci. Eng. 2023, 18, 1243–1251. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- STN ISO 1928; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method, and Calculation of Net Calorific Value. European Committee for Standardization (CEN-CENELEC): Brussels, Belgium, 2003.
- EN ISO 18122; Solid Biofuels: Determination of Ash Content. European Committee for Standardization (CEN-CENELEC): Brussels, Belgium, 2016.
- Jakob, M.; Mahendran, A.R.; Gindl-Altmutter, W.; Bliem, P.; Konnerth, J.; Mueller, U.; Veigel, S. The Strength and Stiffness of Oriented Wood and Cellulose-Fibre Materials: A Review. Prog. Mater. Sci. 2022, 125, 100916. [Google Scholar] [CrossRef]
- Rodriguez-Fabia, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobisation of Lignocellulosic Materials Part I: Physical Modification. Cellulose 2022, 29, 5375–5393. [Google Scholar] [CrossRef]
- Tripathi, S.; Mishra, O.P.; Gangwar, A.; Chakrabarti, S.K.; Varadhan, R. Impact of Wood Storage on Pulp and Paper Making Properties. IPPTA J. 2011, 23, 161–164. [Google Scholar]
- Chang, S.-T. Photodegradation and Photoprotection of Wood Surfaces; US Forest Products Laboratory: Madison, WI, USA, 1982. [Google Scholar]
- Alakoski, E.; Jämsén, M.; Agar, D.; Tampio, E.; Wihersaari, M. From Wood Pellets to Wood Chips, Risks of Degradation and Emissions from the Storage of Woody Biomass—A Short Review. Renew. Sustain. Energy Rev. 2016, 54, 376–383. [Google Scholar] [CrossRef]
- Przybysz Buzała, K.; Kalinowska, H.; Małachowska, E.; Boruszewski, P.; Krajewski, K.; Przybysz, P. The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose. Energies 2019, 12, 2952. [Google Scholar] [CrossRef]
- Täubel, M.; Jalanka, J.; Kirjavainen, P.V.; Tuoresmäki, P.; Hyvärinen, A.; Skevaki, C.; Piippo-Savolainen, E.; Pekkanen, J.; Karvonen, A.M. Fungi in Early-Life House Dust Samples and the Development of Asthma: A Birth Cohort Study. Ann. Am. Thorac. Soc. 2023, 20, 1456–1464. [Google Scholar] [CrossRef]
- Mirski, R.; Kawalerczyk, J.; Dziurka, D.; Stuper-Szablewska, K.; Walkiewicz, J. Selected Chemical and Physical Properties of Pine Wood Chips Inoculated with Aspergillus and Penicillium Mold Fungi. Drv. Ind. 2023, 74, 317–326. [Google Scholar] [CrossRef]
- Gutarowska, B.; Cichocka, A. Zastosowanie Metody Oznaczania Ergosterolu Do Szybkiej Oceny Zanieczyszczenia Grzybami Na Różnych Etapach Produkcji Papieru. Przegląd Papierniczy 2010, 66, 45–47. [Google Scholar]
- Leppänen, H.K.; Nevalainen, A.; Vepsäläinen, A.; Roponen, M.; Täubel, M.; Laine, O.; Rantakokko, P.; Von Mutius, E.; Pekkanen, J.; Hyvärinen, A. Determinants, Reproducibility, and Seasonal Variation of Ergosterol Levels in House Dust. Indoor Air 2014, 24, 248–259. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Yli-Pietilä, K.; Pasanen, P.; Kalliokoski, P.; Tarhanen, J. Ergosterol Content in Various Fungal Species and Biocontaminated Building Materials. Appl. Environ. Microbiol. 1999, 65, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Rogoziński, T.; Szwajkowska-Michałek, L.; Matysiak, A.; Stuper-Szablewska, K. The content of bioactive compounds in chips from the wood processing line. Chip Chipless Woodwork. Process. 2018, 11, 145–150. [Google Scholar]
- Rogowska, A.; Szakiel, A. The Role of Sterols in Plant Response to Abiotic Stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Del Rio, J.C.; Martínez-Íñigo, M.J.; Martínez, M.J.; Martínez, Á.T. Production of New Unsaturated Lipids during Wood Decay by Ligninolytic Basidiomycetes. Appl. Environ. Microbiol. 2002, 68, 1344–1350. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Dou, J.; Xu, H. The Effect of Low-Molecular-Weight Organic-Acids (LMWOAs) on Treatment of Chromium-Contaminated Soils by Compost-Phytoremediation: Kinetics of the Chromium Release and Fractionation. J. Environ. Sci. 2018, 70, 45–53. [Google Scholar] [CrossRef]
- Plassard, C.; Fransson, P. Regulation of Low-Molecular Weight Organic Acid Production in Fungi. Fungal Biol. Rev. 2009, 23, 30–39. [Google Scholar] [CrossRef]
- Shimada, M.; Akamtsu, Y.; Tokimatsu, T.; Mii, K.; Hattori, T. Possible Biochemical Roles of Oxalic Acid as a Low Molecular Weight Compound Involved in Brown-Rot and White-Rot Wood Decays. J. Biotechnol. 1997, 53, 103–113. [Google Scholar] [CrossRef]
- Johansson, E.M.; Fransson, P.M.; Finlay, R.D.; van Hees, P.A. Quantitative Analysis of Root and Ectomycorrhizal Exudates as a Response to Pb, Cd and As Stress. Plant Soil 2008, 313, 39–54. [Google Scholar] [CrossRef]
- Takao, S. Organic Acid Production by Basidiomycetes: I. Screening of Acid-Producing Strains. Appl. Microbiol. 1965, 13, 732–737. [Google Scholar] [CrossRef]
- Mäkelä, M.; Galkin, S.; Hatakka, A.; Lundell, T. Production of Organic Acids and Oxalate Decarboxylase in Lignin-Degrading White Rot Fungi. Enzym. Microb. Technol. 2002, 30, 542–549. [Google Scholar] [CrossRef]
- Hofrichter, M.; Vares, T.; Kalsi, M.; Galkin, S.; Scheibner, K.; Fritsche, W.; Hatakka, A. Production of Manganese Peroxidase and Organic Acids and Mineralization of 14C-Labelled Lignin (14C-DHP) during Solid-State Fermentation of Wheat Straw with the White Rot Fungus Nematoloma Frowardii. Appl. Environ. Microbiol. 1999, 65, 1864–1870. [Google Scholar] [CrossRef]
- Galkin, S.; Vares, T.; Kalsi, M.; Hatakka, A. Production of Organic Acids by Different White-Rot Fungi as Detected Using Capillary Zone Electrophoresis. Biotechnol. Tech. 1998, 12, 267–271. [Google Scholar] [CrossRef]
- Mansour, M.M.; Hamed, S.A.E.-K.M.; Salem, M.Z.; Ali, H.M. Illustration of the Effects of Five Fungi on Acacia Saligna Wood Organic Acids and Ultrastructure Alterations in Wood Cell Walls by HPLC and TEM Examinations. Appl. Sci. 2020, 10, 2886. [Google Scholar] [CrossRef]
- Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Rogoziński, T.; Stuper-Szablewska, K. Phenolic Compounds in Trees and Shrubs of Central Europe. Appl. Sci. 2020, 10, 6907. [Google Scholar] [CrossRef]
- Bending, G.D.; Read, D.J. Lignin and Soluble Phenolic Degradation by Ectomycorrhizal and Ericoid Mycorrhizal Fungi. Mycol. Res. 1997, 101, 1348–1354. [Google Scholar] [CrossRef]
- Di Lella, S.; La Porta, N.; Tognetti, R.; Lombardi, F.; Nardin, T.; Larcher, R. White Rot Fungal Impact on the Evolution of Simple Phenols during Decay of Silver Fir Wood by UHPLC-HQOMS. Phytochem. Anal. 2022, 33, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Jellison, J.; Liu, J.; Daniel, G.; Paszczynski, A.; Fekete, F.; Krishnamurthy, S.; Jun, L.; Xu, G. Low Molecular Weight Chelators and Phenolic Compounds Isolated from Wood Decay Fungi and Their Role in the Fungal Biodegradation of Wood. J. Biotechnol. 1997, 53, 133–162. [Google Scholar] [CrossRef]
- Diouf, P.-N.; Merlin, A.; Perrin, D. Antioxidant Properties of Wood Extracts and Colour Stability of Woods. Ann. For. Sci. 2006, 63, 525–534. [Google Scholar] [CrossRef]
- Antoniewicz, J.; Kochman, J.; Jakubczyk, K.; Janda-Milczarek, K. The Influence of Time and Storage Conditions on the Antioxidant Potential and Total Phenolic Content in Homemade Grape Vinegars. Molecules 2021, 26, 7616. [Google Scholar] [CrossRef] [PubMed]
- Orémusová, E.; Tereňová, L.; Réh, R. Evaluation of the Gross and Net Calorific Value of the Selected Wood Species. In Proceedings of the Advanced Materials Research; Trans Tech Publishers: Zurich, Switzerland, 2014; Volume 1001, pp. 292–299. [Google Scholar]
- Gendek, A.; Piętka, J.; Aniszewska, M.; Malaťák, J.; Velebil, J.; Tamelová, B.; Krilek, J.; Moskalik, T. Energy Value of Silver Fir (Abies Alba) and Norway Spruce (Picea Abies) Wood Depending on the Degree of Its Decomposition by Selected Fungal Species. Renew. Energy 2023, 215, 118948. [Google Scholar] [CrossRef]
- Domingos, I.; Ayata, U.; Ferreira, J.; Cruz-Lopes, L.; Sen, A.; Sahin, S.; Esteves, B. Calorific Power Improvement of Wood by Heat Treatment and Its Relation to Chemical Composition. Energies 2020, 13, 5322. [Google Scholar] [CrossRef]
- Lieskovský, M.; Jankovský, M.; Trenčiansky, M.; Merganič, J. Ash Content vs. the Economics of Using Wood Chips for Energy: Model Based on Data from Central Europe. BioResources 2017, 12, 1579–1592. [Google Scholar] [CrossRef]
- Mancini, M.; Rinnan, Å.; Pizzi, A.; Toscano, G. Prediction of Gross Calorific Value and Ash Content of Woodchip Samples by Means of FT-NIR Spectroscopy. Fuel Process. Technol. 2018, 169, 77–83. [Google Scholar] [CrossRef]

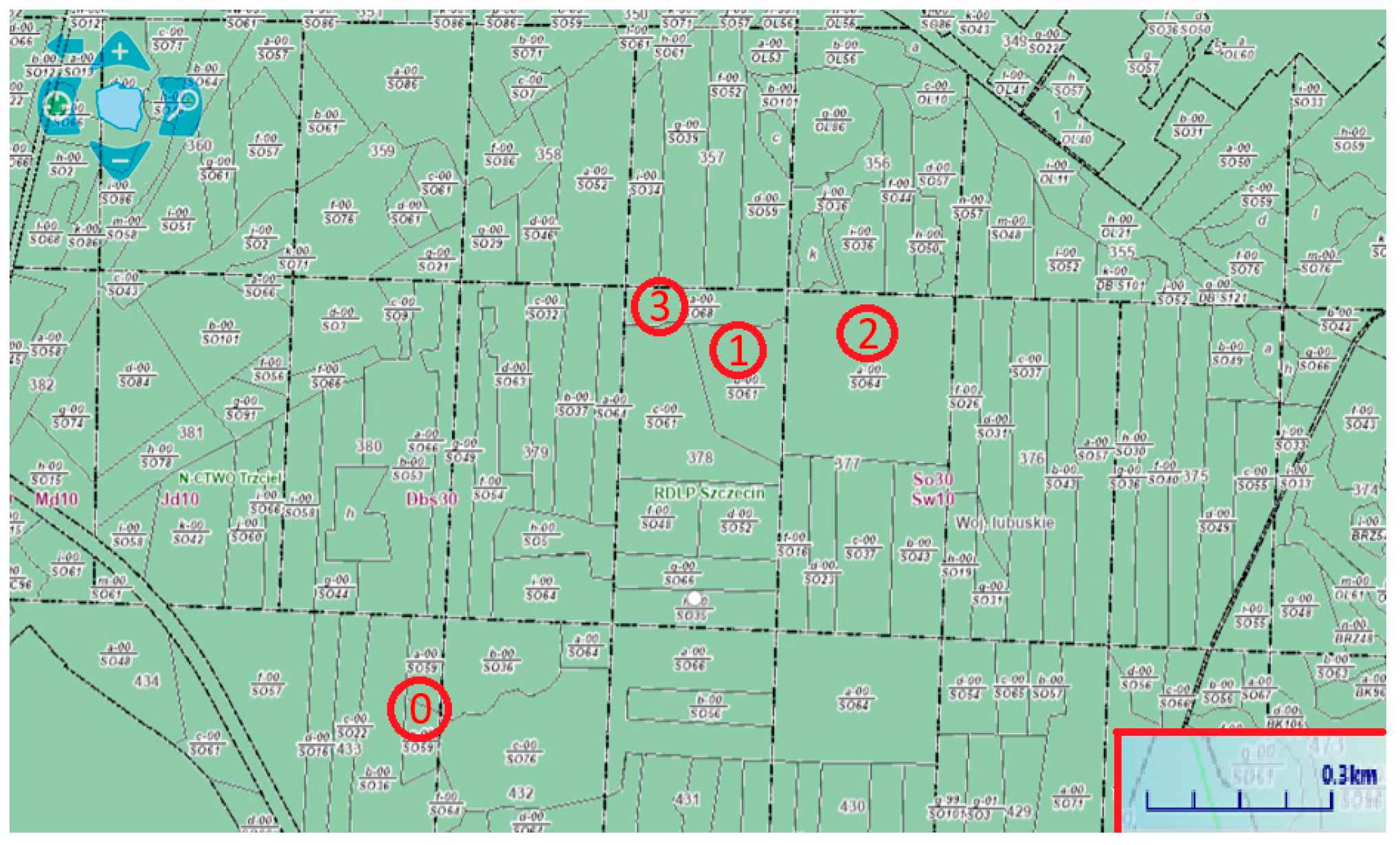


| Characteristics | Fresh Residues | Residues Stored for 1 Year | Residues Stored for 2 Years | Residues Stored for 3 Years |
|---|---|---|---|---|
| Coordinates | 52°37′93″ N 15°72′24″ E | 52°38′61″ N 15°73′33″ E | 52°38′64″ N 15°73′65″ E | 52°38′76″ N 15°73′16″ E |
| Forest address | 10-29-1-04-433-g-00 | 10-29-1-04-378-b-00 | 10-29-1-04-377-a-00 | 10-29-1-04-378-a-00 |
| Area (ha) | 1.20 | 4.87 | 13.58 | 2.68 |
| Forest district | Trzciel, PL | Trzciel, PL | Trzciel, PL | Trzciel, PL |
| Forest habitat | Fresh mixed forest | Fresh mixed forest | Fresh mixed forest | Fresh mixed forest |
| Dominant species | Scots pine | Scots pine | Scots pine | Scots pine |
| Age of dominant species | 60 | 61 | 64 | 68 |
| Type of Acid | Acid Concentration (µg/g) | |||
|---|---|---|---|---|
| 0 Years | 1 Year | 2 Years | 3 Years | |
| Lactic | 0.11 a ± 0.02 | 0.22 b ± 0.01 | 0.82 c ± 0.04 | 1.53 d ± 0.08 |
| Acetic | 0.29 a ± 0.06 | 0.79 b ± 0.04 | 2.23 c ± 0.03 | 8.16 d ± 0.03 |
| Malonic | 9.36 a ± 0.03 | 15.39 b ± 0.09 | 16.55 c ± 0.02 | 32.51 d ± 0.06 |
| Maleic | 20.33 a ± 0.08 | 23.22 b ± 0.07 | 38.24 c ± 0.05 | 70.24 d ± 0.08 |
| Formic | 5.25 a ± 0.05 | 6.11 b ± 0.04 | 6.37 c ± 0.07 | 7.05 d ± 0.03 |
| Oxalic | 5.11 a ± 0.09 | 40.36 b ± 0.11 | 50.74 c ± 0.14 | 89.35 d ± 0.17 |
| Malic | 10.25 a ± 0.04 | 50.33 b ± 0.12 | 78.52 c ± 0.16 | 198.33 d ± 0.09 |
| Citric | 152.31 a ± 0.09 | 155.70 b ± 0.04 | 159.71 c ± 0.06 | 161.44 d ± 0.04 |
| Succinic | 1.32 a ± 0.05 | 10.22 b ± 0.03 | 11.36 c ± 0.04 | 21.35 d ± 0.08 |
| Fumaric | 10.06 a ± 0.07 | 30.06 b ± 0.06 | 48.22 c ± 0.05 | 106.52 d ± 0.07 |
| Sum | 214.39 | 332.40 | 412.76 | 696.48 |
| Ash Content (%) | |||
|---|---|---|---|
| 0 Years | 1 Year | 2 Years | 3 Years |
| 0.65 a ± 0.05 | 0.86 b ± 0.08 | 2.34 c ± 0.53 | 4.17 d ± 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieruszewski, M.; Kawalerczyk, J.; Stuper-Szablewska, K.; Walkiewicz, J.; Lieskovský, M.; Jarzębski, M.; Mirski, R. Changes in the Characteristics of Pine Logging Residue during Storage in Forest Stands. Energies 2024, 17, 843. https://doi.org/10.3390/en17040843
Wieruszewski M, Kawalerczyk J, Stuper-Szablewska K, Walkiewicz J, Lieskovský M, Jarzębski M, Mirski R. Changes in the Characteristics of Pine Logging Residue during Storage in Forest Stands. Energies. 2024; 17(4):843. https://doi.org/10.3390/en17040843
Chicago/Turabian StyleWieruszewski, Marek, Jakub Kawalerczyk, Kinga Stuper-Szablewska, Joanna Walkiewicz, Martin Lieskovský, Maciej Jarzębski, and Radosław Mirski. 2024. "Changes in the Characteristics of Pine Logging Residue during Storage in Forest Stands" Energies 17, no. 4: 843. https://doi.org/10.3390/en17040843
APA StyleWieruszewski, M., Kawalerczyk, J., Stuper-Szablewska, K., Walkiewicz, J., Lieskovský, M., Jarzębski, M., & Mirski, R. (2024). Changes in the Characteristics of Pine Logging Residue during Storage in Forest Stands. Energies, 17(4), 843. https://doi.org/10.3390/en17040843








