Enhancing the Storage Performance and Thermal Stability of Ni-Rich Layered Cathodes by Introducing Li2MnO3
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurements
2.4. Thermal Stability Test
3. Results and Discussion
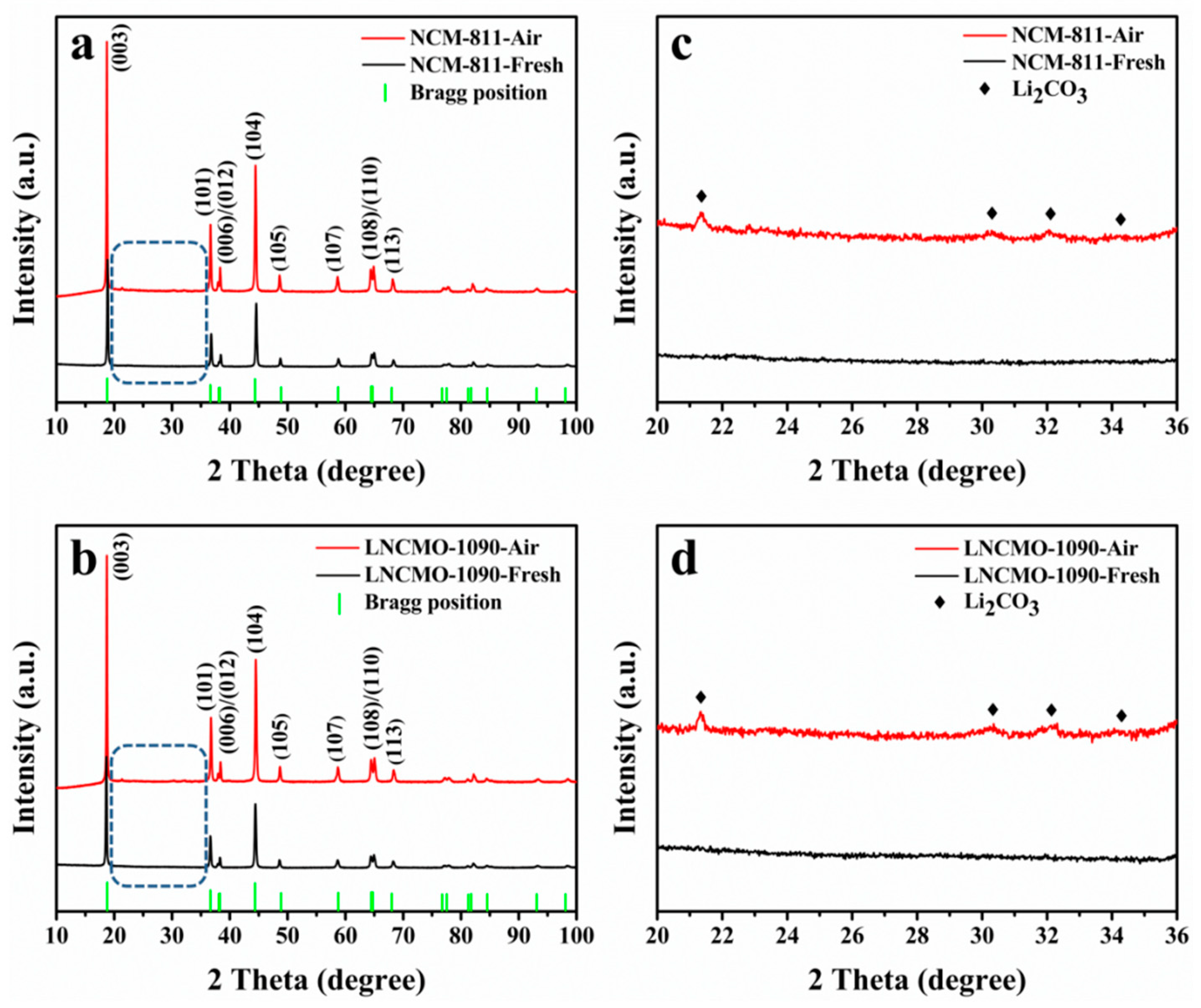
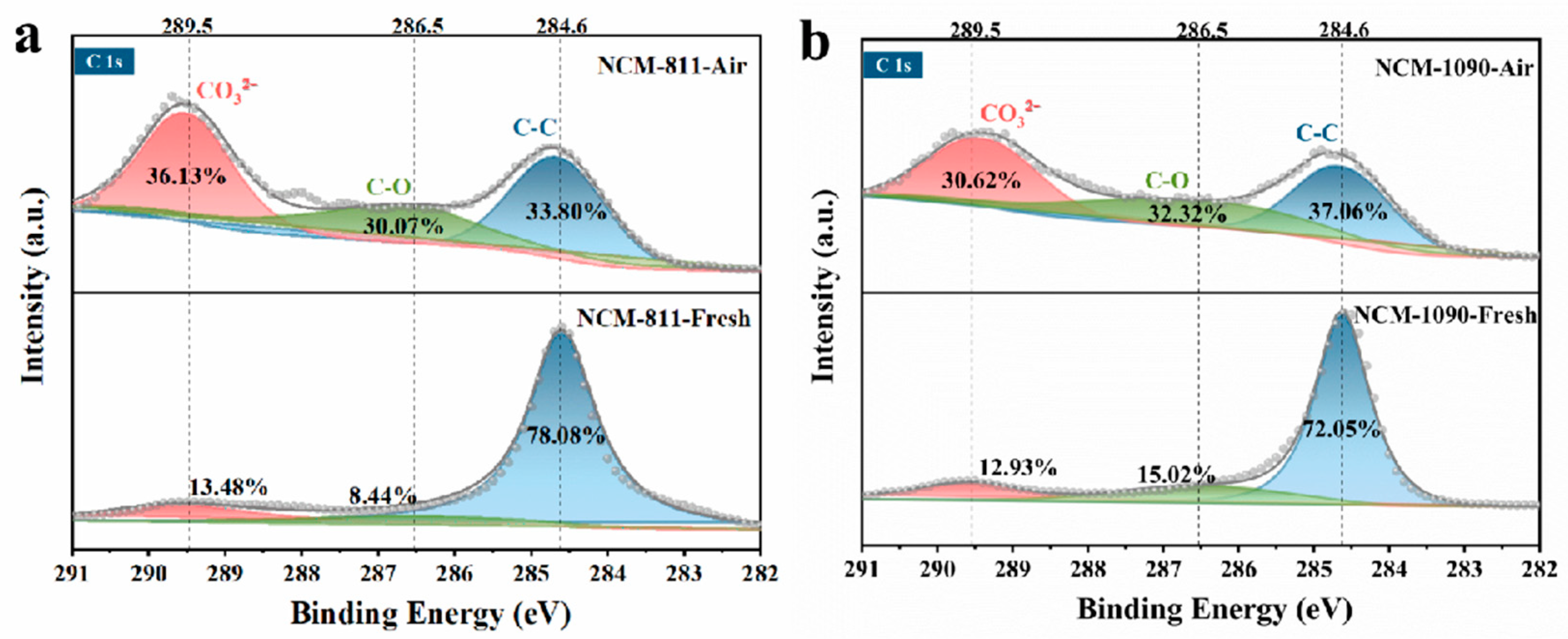
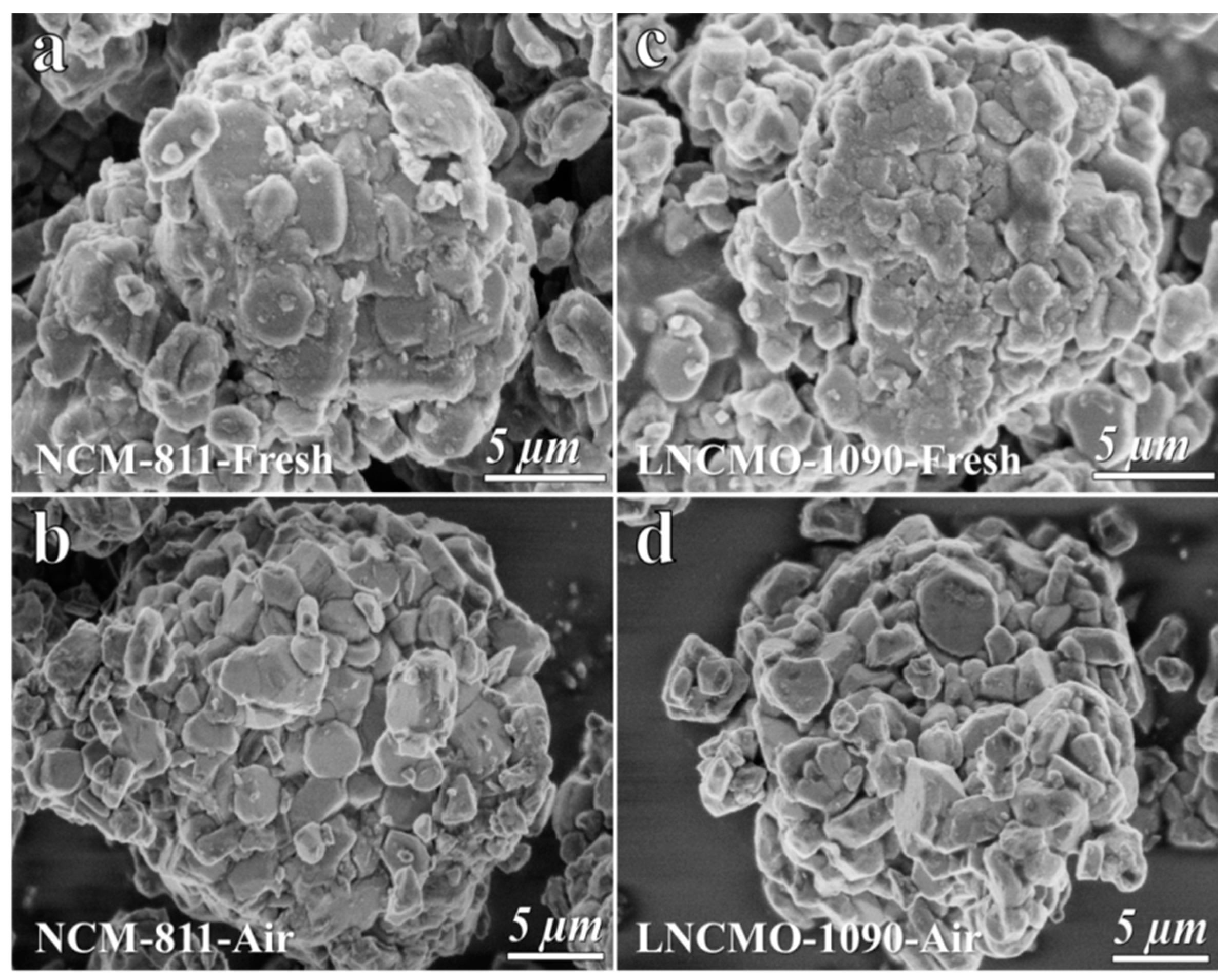
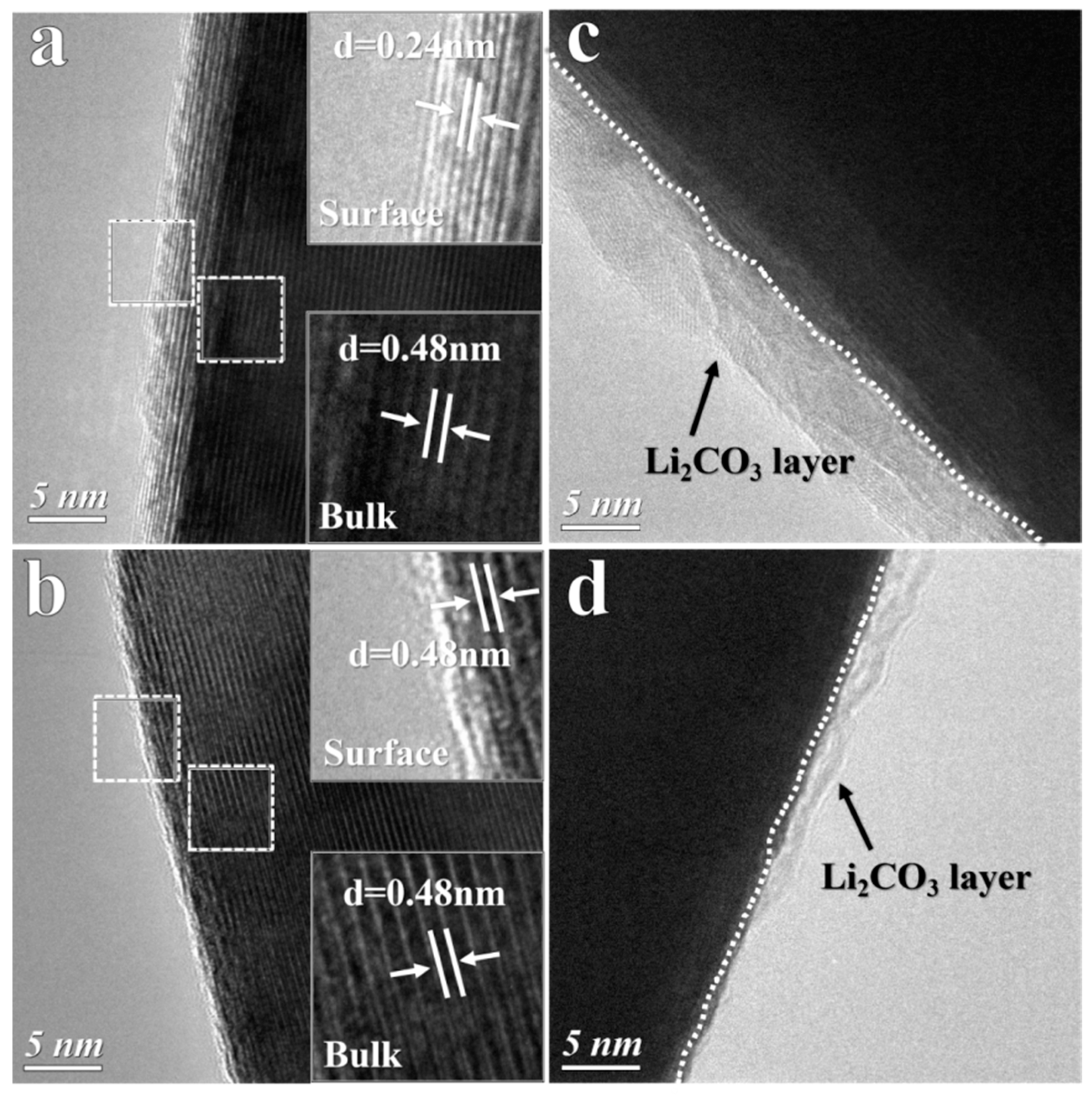
3.1. Structure Change of the Ni-Rich Cathode during Storage in Air
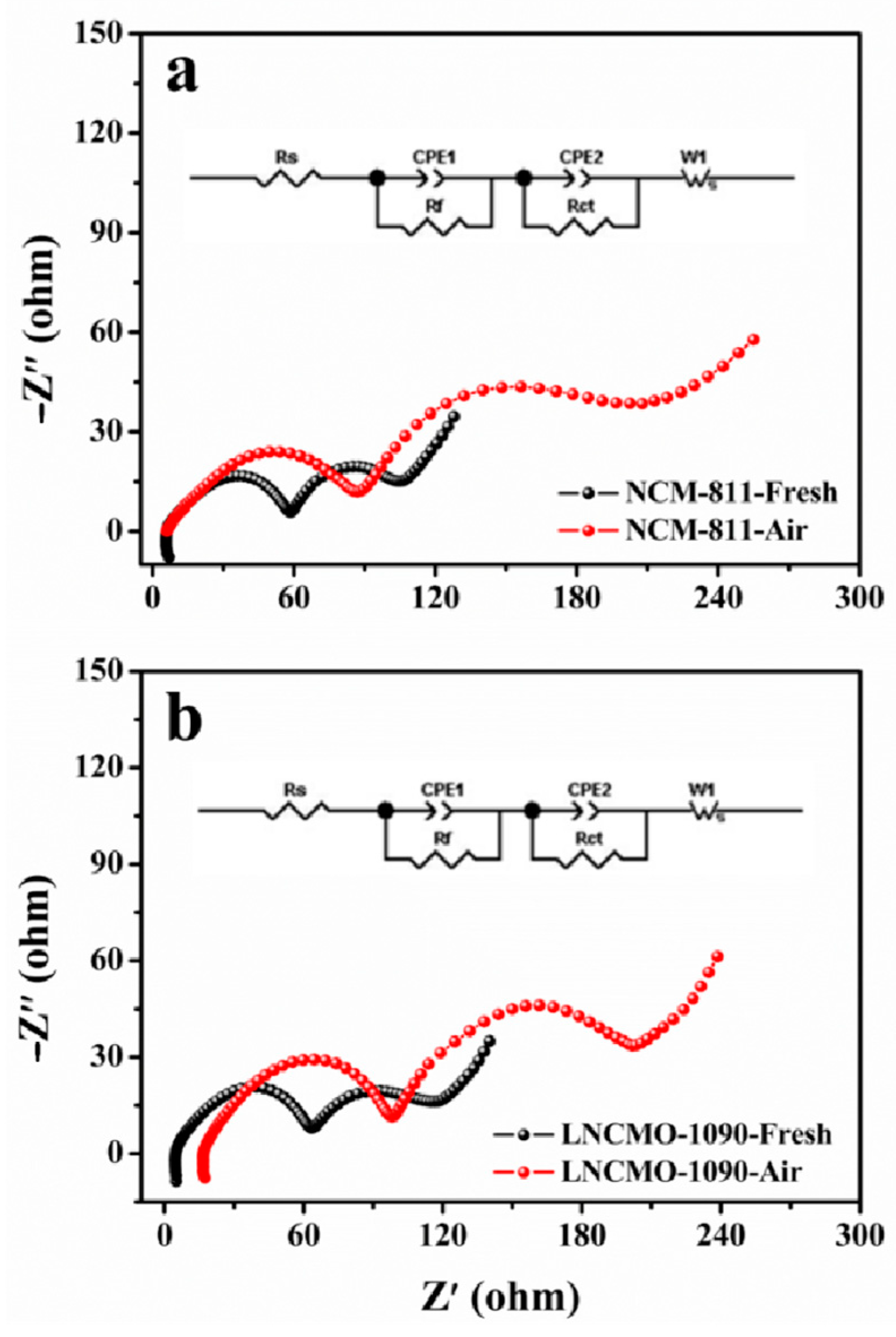
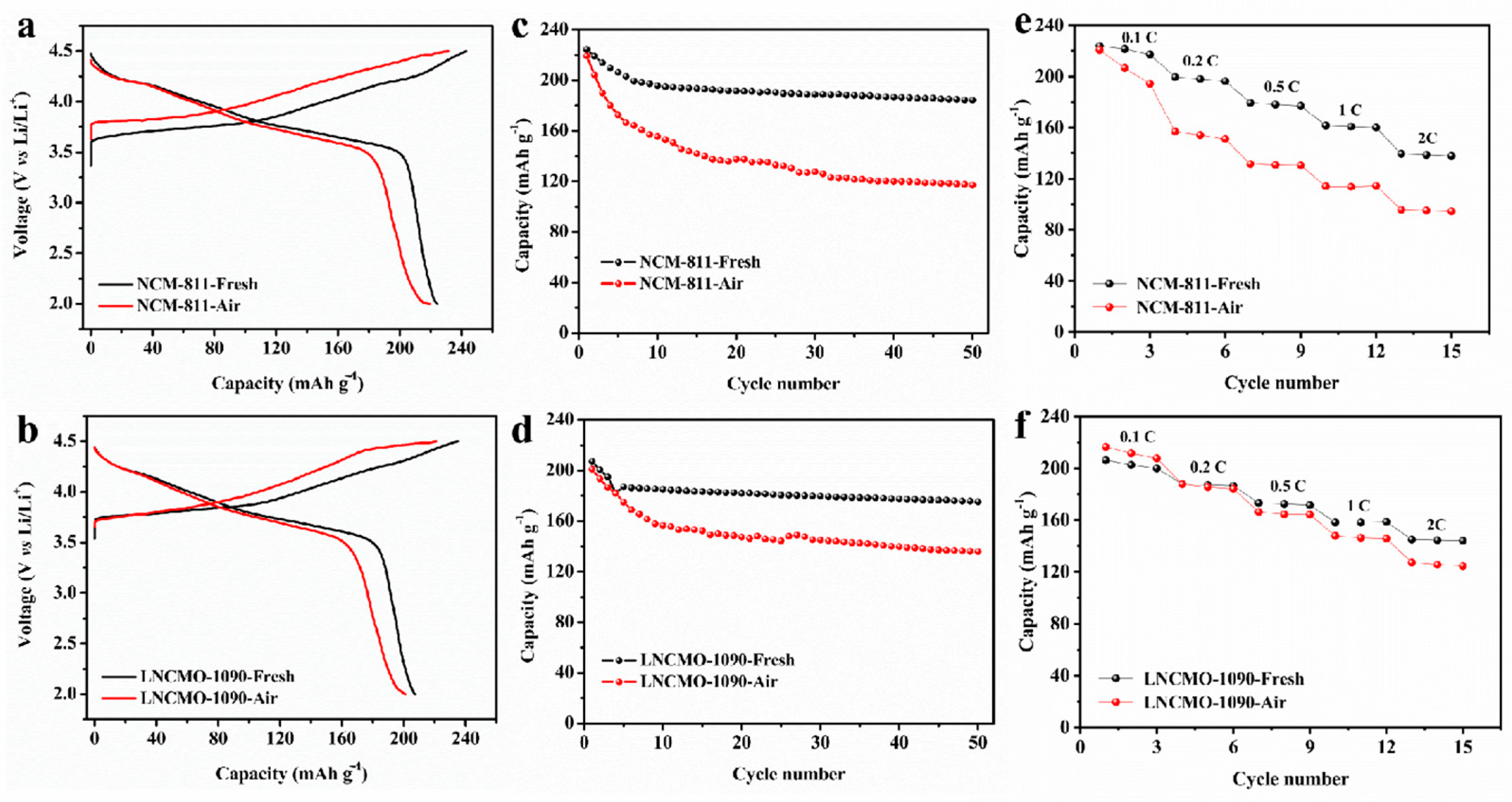
3.2. Changes in Electrochemical Properties of Ni-Rich Cathodes during Storage
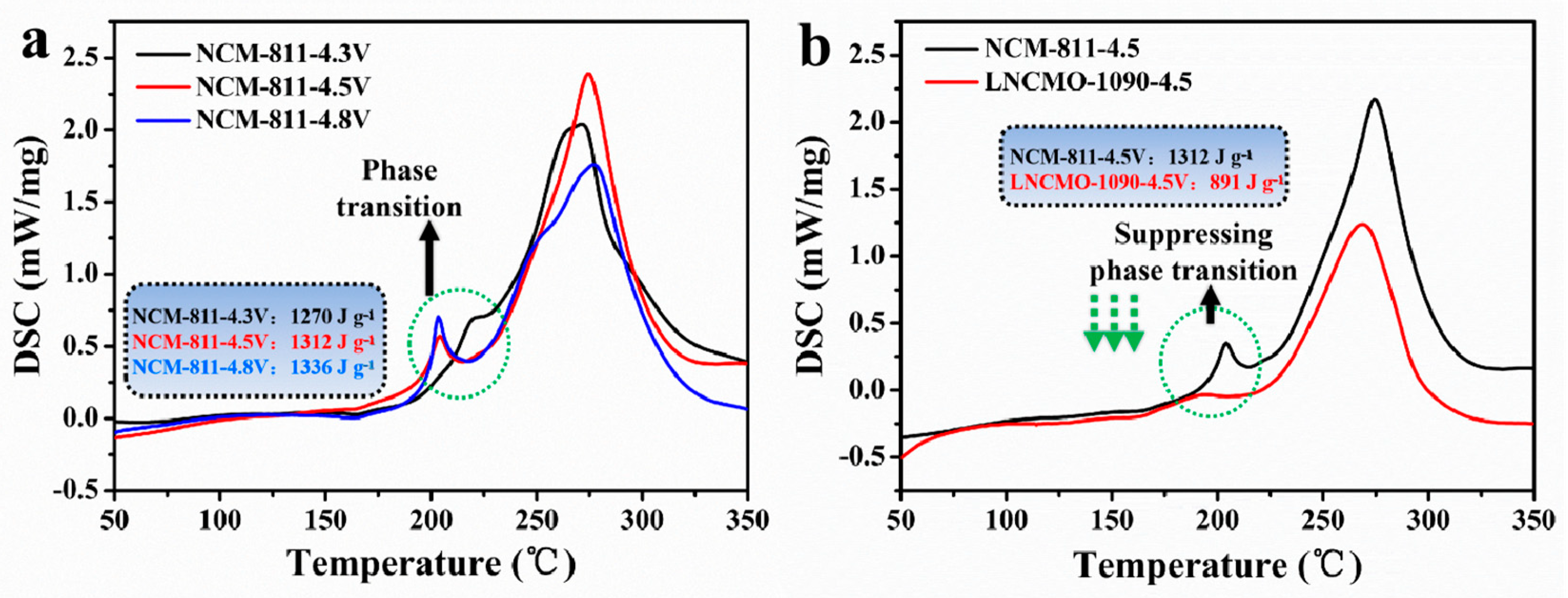
3.3. Thermal Stability of Ni-Rich Layered Cathodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, C.-G.; Peng, X.; Dai, P.; Xiao, P.; Zheng, W.-C.; Li, H.-Y.; Li, H.; Indris, S.; Mangold, S.; Hong, Y.-H.; et al. Investigation and Suppression of Oxygen Release by LiNi0.8Co0.1Mn0.1O2 Cathode under Overcharge Conditions. Adv. Energy Mater. 2022, 12, 2200569. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zeng, T.Y.; Li, G.T.; Wan, T.; Li, M.Y.; Zhang, X.Y.; Li, M.Q.; Su, M.R.; Dou, A.C.; Zeng, W.S.; et al. The surface double-coupling on single-crystal LiNi0.8Co0.1Mn0.1O2 for inhibiting the formation of intragranular cracks and oxygen vacancies. Energy Storage Mater. 2022, 52, 534–546. [Google Scholar] [CrossRef]
- Freiberg, A.T.S.; Sicklinger, J.; Solchenbach, S.; Gasteiger, H.A. Li2CO3 decomposition in Li-ion batteries induced by the electrochemical oxidation of the electrolyte and of electrolyte impurities. Electrochim. Acta 2020, 346, 136271. [Google Scholar] [CrossRef]
- Kang, H.S.; Santhoshkumar, P.; Park, J.W.; Sim, G.S.; Nanthagopal, M.; Lee, C.W. Glass ceramic coating on LiNi0.8Co0.1Mn0.1O2 cathode for Li-ion batteries. Korean J. Chem. Eng. 2020, 37, 1331–1339. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.L.; Ruther, R.E.; Du, Z.J.; Self, E.C.; Meyer, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L. Chemical stability and long-term cell performance of low-cobalt, Ni-rich cathodes prepared by aqueous processing for high-energy Li-ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Zheng, W.-C.; Shi, C.-G.; Dai, P.; Huang, Z.; Lin, J.-X.; Chen, H.; Sun, M.-L.; Shen, C.-H.; Luo, C.-X.; Wang, Q.; et al. A functional electrolyte additive enabling robust interphases in high-voltage Li||LiNi0.8Co0.1Mn0.1O2 batteries at elevated temperatures. J. Mater. Chem. A 2022, 10, 21912–21922. [Google Scholar] [CrossRef]
- Liu, J.X.; Wang, J.Q.; Ni, Y.X.; Zhang, K.; Cheng, F.Y.; Chen, J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater. Today 2021, 43, 132–165. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.N.; Ren, D.S.; Wu, Y.; Xu, G.L.; Lu, L.G.; Hou, J.X.; Zhang, W.F.; et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Co0.1Mn0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Xue, W.J.; Huang, M.J.; Li, Y.T.; Zhu, Y.G.; Gao, R.; Xiao, X.H.; Zhang, W.X.; Li, S.P.; Xu, G.Y.; Yu, Y.; et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 2021, 6, 495–505. [Google Scholar] [CrossRef]
- Wang, C.H.; Shao, L.; Guo, X.; Xi, X.M.; Yang, L.S.; Huang, C.H.; Zhou, C.X.; Zhao, H.H.; Yin, D.L.; Wang, Z.C. Air-Induced Degradation and Electrochemical Regeneration for the Performance of Layered Ni-Rich Cathodes. ACS Appl. Mater. Inter. 2019, 11, 44036–44045. [Google Scholar] [CrossRef]
- Sun, P.P.; Du, F.H.; Zhou, Q.; Hu, D.; Xu, T.; Mei, C.X.; Hao, Q.; Fan, Z.X.; Zheng, J.W. Efficient preservation of surface state of LiNi0.82Co0.15Al0.03O2 through assembly of hydride terminated polydimethylsiloxane. J. Power Sources 2021, 495, 229761. [Google Scholar] [CrossRef]
- Zeng, L.C.; Shi, K.X.; Qiu, B.; Liang, H.Y.; Li, J.H.; Zhao, W.; Li, S.L.; Zhang, W.G.; Liu, Z.P.; Liu, Q.B. Hydrophobic surface coating against chemical environmental instability for Ni-rich layered oxide cathode materials. Chem. Eng. J. 2022, 437, 135276. [Google Scholar] [CrossRef]
- Xie, Q.; Li, W.D.; Manthiram, A. A Mg-Doped High-Ni Layered Oxide Cathode Enabling Safer, High-Energy-Density Li-Ion Batteries. Chem. Mater. 2019, 31, 938–946. [Google Scholar] [CrossRef]
- Han, G.-M.; Kim, Y.-S.; Ryu, H.-H.; Sun, Y.-K.; Yoon, C.S. Structural Stability of Single-Crystalline Ni-Rich Layered Cathode upon Delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Li, L.J.; Fu, L.Z.; Li, M.; Wang, C.; Zhao, Z.X.; Xie, S.C.; Lin, H.C.; Wu, X.W.; Liu, H.D.; Zhang, L.; et al. B-doped and La4NiLiO8-coated Ni-rich cathode with enhanced structural and interfacial stability for lithium-ion batteries. J. Energy Chem. 2022, 71, 588–594. [Google Scholar] [CrossRef]
- Fan, X.M.; Ou, X.; Zhao, W.G.; Liu, Y.; Zhang, B.; Zhang, J.F.; Zou, L.F.; Seidl, L.; Li, Y.Z.; Hu, G.R.; et al. In situ inorganic conductive network formation in high-voltage single-crystal Ni-rich cathodes. Nat. Commun. 2021, 12, 5320. [Google Scholar] [CrossRef]
- Yin, S.Y.; Deng, W.T.; Chen, J.; Gao, X.; Zou, G.Q.; Hou, H.S.; Ji, X.B. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 2021, 83, 105854. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Enhancement on the Cycling Stability of the Layered Ni-Rich Oxide Cathode by In-Situ Fabricating Nano-Thickness Cation-Mixing Layers. J. Electrochem. Soc. 2016, 163, A2665–A2672. [Google Scholar] [CrossRef]
- Zybert, M.; Ronduda, H.; Dabrowska, K.; Ostrowski, A.; Sobczak, K.; Moszynski, D.; Hamankiewicz, B.; Rogulski, Z.; Rarog-Pilecka, W.; Wieczorek, W. Suppressing Ni/Li disordering in LiNi0.6Mn0.2Co0.2O2 cathode material for Li-ion batteries by rare earth element doping. Energy Rep. 2022, 8, 3995–4005. [Google Scholar] [CrossRef]
- Zybert, M.; Ronduda, H.; Ostrowski, A.; Sobczak, K.; Moszynski, D.; Rarog-Pilecka, W.; Hamankiewicz, B.; Wieczorek, W. Structural analysis and electrochemical investigation of dual-doped NMC622 cathode material: Effect of sodium and neodymium on the performance in Li-ion batteries. Energy Rep. 2023, 10, 1238–1248. [Google Scholar] [CrossRef]
- Zybert, M.; Ronduda, H.; Rarog-Pilecka, W.; Wieczorek, W. Application of rare earth elements as modifiers for Ni-rich cathode materials for Li-ion batteries: A mini review. Front. Energy Res. 2023, 11, 1248641. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Yang, J.; Hou, M.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. Improving the Cycling Performance of the Layered Ni-Rich Oxide Cathode by Introducing Low-Content Li2MnO3. Electrochim. Acta 2016, 189, 101–110. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Suppressing the Phase Transition of the Layered Ni-Rich Oxide Cathode during High-Voltage Cycling by Introducing Low-Content Li2MnO3. ACS Appl. Mater. Inter. 2016, 8, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.-J.; Lee, E.; Yoon, W.-S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sour. 2020, 474, 228592. [Google Scholar] [CrossRef]
- Becker, D.; Boerner, M.; Noelle, R.; Diehl, M.; Klein, S.; Rodehorst, U.; Schmuch, R.; Winter, M.; Placke, T. Surface Modification of Ni-Rich LiNi0.8Co0.1Mn0.1O2 Cathode Material by Tungsten Oxide Coating for Improved Electrochemical Performance in Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2019, 11, 18404–18414. [Google Scholar] [CrossRef]
- Andersen, H.L.; Cheung, E.A.; Avdeev, M.; Maynard-Casely, H.E.; Abraham, D.P.; Sharma, N. Consequences of long-term water exposure for bulk crystal structure and surface composition/chemistry of Ni-rich layered oxide materials for Li-ion batteries. J. Power Sources 2020, 470, 228370. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, P.; Gu, M.; Xiao, J.; Browning, N.D.; Yan, P.; Wang, C.; Zhang, J.-G. Structural and Chemical Evolution of Li- and Mn-Rich Layered Cathode Material. Chem. Mater. 2015, 27, 1381–1390. [Google Scholar] [CrossRef]
- Xu, C.L.; Xiang, W.; Wu, Z.G.; Qiu, L.; Ming, Y.; Yang, W.; Yue, L.C.; Zhang, J.; Zhong, B.H.; Guo, X.D.; et al. Dual-site lattice modification regulated cationic ordering for Ni-rich cathode towards boosted structural integrity and cycle stability. Chem. Eng. J. 2021, 403, 126314. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B.H.; Manthiram, A. An in-depth understanding of the effect of aluminum doping in high-Ni cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Tang, M.; Yang, J.; Chen, N.; Zhu, S.; Wang, X.; Wang, T.; Zhang, C.; Xia, Y. Overall structural modification of a layered Ni-rich cathode for enhanced cycling stability and rate capability at high voltage. J. Mater. Chem. A 2019, 7, 6080–6089. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, S.F.; Li, S.; Yu, Z.J.; Liu, Q.S.; Dai, A.; Cao, C.T.; Toney, M.F.; Ge, M.Y.; Xiao, X.H.; et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 2020, 11, 3050. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, H.; Wang, B.; Tuo, K.; Wang, P.; Wang, S.; Liang, W.; Lu, H.; Li, S. Structural evolution of Ni-rich layered cathode material LiNi0.8Co0.1Mn0.1O2 at different current rates. Ionics 2021, 27, 517–526. [Google Scholar] [CrossRef]
- Yin, E.; Grimaud, A.; Rousse, G.; Abakumov, A.M.; Senyshyn, A.; Zhang, L.; Trabesinger, S.; Iadecola, A.; Foix, D.; Giaume, D.; et al. Structural evolution at the oxidative and reductive limits in the first electrochemical cycle of Li1.2Ni0.13Mn0.54Co0.13O2. Nat. Commun. 2020, 11, 1252. [Google Scholar] [CrossRef]
- Xiong, C.; Fu, H.K.; Wu, L.J.; Yuan, G.Q. Enhanced Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Material for lithium ion batteries by WO3 surface coating. Int. J. Electrochem. Sc. 2020, 15, 8990–9002. [Google Scholar] [CrossRef]
- Liu, X.S.; Zheng, B.Z.; Zhao, J.; Zhao, W.M.; Liang, Z.T.; Su, Y.; Xie, C.P.; Zhou, K.; Xiang, Y.X.; Zhu, J.P.; et al. Electrochemo-Mechanical Effects on Structural Integrity of Ni-Rich Cathodes with Different Microstructures in All Solid-State Batteries. Adv. Energy Mater. 2021, 11, 2003583. [Google Scholar] [CrossRef]
- Li, X.L.; Jin, L.B.; Song, D.W.; Zhang, H.Z.; Shi, X.X.; Wang, Z.Y.; Zhang, L.Q.; Zhu, L.Y. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, G.J.; Sim, S.J.; Jin, B.S.; Kim, H.S. Improved electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode via SiO2 coating. J. Alloy. Compd. 2019, 791, 193–199. [Google Scholar] [CrossRef]
- Li, Y.J.; Deng, S.Y.; Chen, Y.X.; Gao, J.; Zhu, J.; Xue, L.L.; Lei, T.X.; Cao, G.L.; Guo, J.; Wang, S.L. Dual functions of residue Li-reactive coating with C4H6CoO4 on high-performance LiNiO2 cathode material. Electrochim. Acta 2019, 300, 26–35. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Katayama, Y.; Tatara, R.; Giordano, L.; Yu, Y.; Fraggedakis, D.; Sun, J.G.W.; Maglia, F.; Jung, R.; Bazant, M.Z.; et al. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy. Energ. Environ. Sci. 2020, 13, 183–199. [Google Scholar] [CrossRef]
- Cheng, W.D.; Hao, S.; Ji, Y.Y.; Li, L.; Liu, L.; Xiao, Y.; Wu, Y.X.; Huo, J.S.; Tang, F.; Liu, X.Q. Optimizing surface residual alkali and enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode by LiH2PO4. Nanotechnology 2022, 33, 045404. [Google Scholar] [CrossRef]
- Zuo, T.-T.; Ruess, R.; Pan, R.; Walther, F.; Rohnke, M.; Hori, S.; Kanno, R.; Schroeder, D.; Janek, J. A mechanistic investigation of the Li10GeP2S12|LiNi1-x-yCoxMnyO2 interface stability in all-solid-state lithium batteries. Nat. Commun. 2021, 12, 6669. [Google Scholar] [CrossRef]
- Liu, S.F.; Ji, X.; Yue, J.; Hou, S.; Wang, P.F.; Cui, C.Y.; Chen, J.; Shao, B.W.; Li, J.R.; Han, F.D.; et al. High Interfacial-Energy Interphase Promoting Safe Lithium Metal Batteries. J. Am. Chem. Soc. 2020, 142, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, X.H.; Ryu, H.H.; Yoon, C.S.; Sun, Y.K. Ni-Rich Layered Cathodes for Lithium-Ion Batteries: From Challenges to the Future. Energy Storage Mater. 2023, 63, 102969. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Inter. 2014, 6, 22594–22601. [Google Scholar]
- Wu, L.; Kyung-Wan, N.; Wang, X.; Zhou, Y.; Zheng, J.-C.; Yang, X.-Q.; Zhu, Y. Structural Origin of Overcharge-Induced Thermal Instability of Ni-Containing Layered-Cathodes for High-Energy-Density Lithium Batteries. Chem. Mater. 2011, 23, 3953–3960. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Lee, D.-J.; Lee, Y.J.; Chen, Z.; Myung, S.-T. Cobalt-Free Ni Rich Layered Oxide Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2013, 5, 11434–11440. [Google Scholar] [CrossRef]
- Yang, J.; Lim, J.-M.; Park, M.; Lee, G.-H.; Lee, S.; Cho, M.; Kang, Y.-M. Thermally Activated P2-O3 Mixed Layered Cathodes toward Synergistic Electrochemical Enhancement for Na Ion Batteries. Adv. Energy Mater. 2021, 11, 2102444. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, U.H.; Kim, J.H.; Jun, S.G.; Yoon, C.S.; Jung, Y.S.; Sun, Y.K. Ni-Rich Layered Cathode Materials with Electrochemo-Mechanically Compliant Microstructures for All-Solid-State Li Batteries. Adv. Energy Mater. 2020, 10, 1903360. [Google Scholar] [CrossRef]
- Xu, G.L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.H.; Amine, K. Challenges and Strategies to Advance High-Energy Ni-Rich Layered Lithium Transition Metal Oxide Cathodes for Harsh Operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Wang, Q.D.; Yao, Z.P.; Zhao, C.L.; Verhallen, T.; Tabor, D.P.; Liu, M.; Ooms, F.; Kang, F.Y.; Aspuru-Guzik, A.; Hu, Y.S.; et al. Interface chemistry of an amide electrolyte for highly reversible lithium metal batteries. Nat. Commun. 2020, 11, 4188. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Guo, W.B.; Wu, H.L.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.S.; Liu, P.F.; Zheng, H.F.; Wang, L.S.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2005937. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.S.; Feng, X.N.; Liu, L.S.; Hsu, H.J.; Lu, L.G.; Wang, L.; He, X.M.; Ouyang, M.G. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Csernica, P.M.; Kalirai, S.S.; Gent, W.E.; Lim, K.; Yu, Y.S.; Liu, Y.Z.; Ahn, S.J.; Kaeli, E.; Xu, X.; Stone, K.H.; et al. Persistent and partially mobile oxygen vacancies in Li-rich layered oxides. Nat. Energy 2021, 6, 642–652. [Google Scholar] [CrossRef]
| Sample | First Discharge Capacity (mAh g−1) | 50th Discharge Capacity (mAh g−1) | Capacity Retention (%) | |
|---|---|---|---|---|
| NCM-811 | Before storage | 224 | 184 | 82.2 |
| After storage | 219 | 117 | 53.4 | |
| LNCMO-1090 | Before storage | 207 | 175 | 84.6 |
| After storage | 201 | 126 | 62.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yang, P.; Wang, H. Enhancing the Storage Performance and Thermal Stability of Ni-Rich Layered Cathodes by Introducing Li2MnO3. Energies 2024, 17, 810. https://doi.org/10.3390/en17040810
Yang J, Yang P, Wang H. Enhancing the Storage Performance and Thermal Stability of Ni-Rich Layered Cathodes by Introducing Li2MnO3. Energies. 2024; 17(4):810. https://doi.org/10.3390/en17040810
Chicago/Turabian StyleYang, Jun, Pingping Yang, and Hongyu Wang. 2024. "Enhancing the Storage Performance and Thermal Stability of Ni-Rich Layered Cathodes by Introducing Li2MnO3" Energies 17, no. 4: 810. https://doi.org/10.3390/en17040810
APA StyleYang, J., Yang, P., & Wang, H. (2024). Enhancing the Storage Performance and Thermal Stability of Ni-Rich Layered Cathodes by Introducing Li2MnO3. Energies, 17(4), 810. https://doi.org/10.3390/en17040810








