Temperature Reduction as Operando Performance Recovery Procedure for Polymer Electrolyte Membrane Fuel Cells
Abstract
1. Introduction
2. Experimental
2.1. Test Hardware
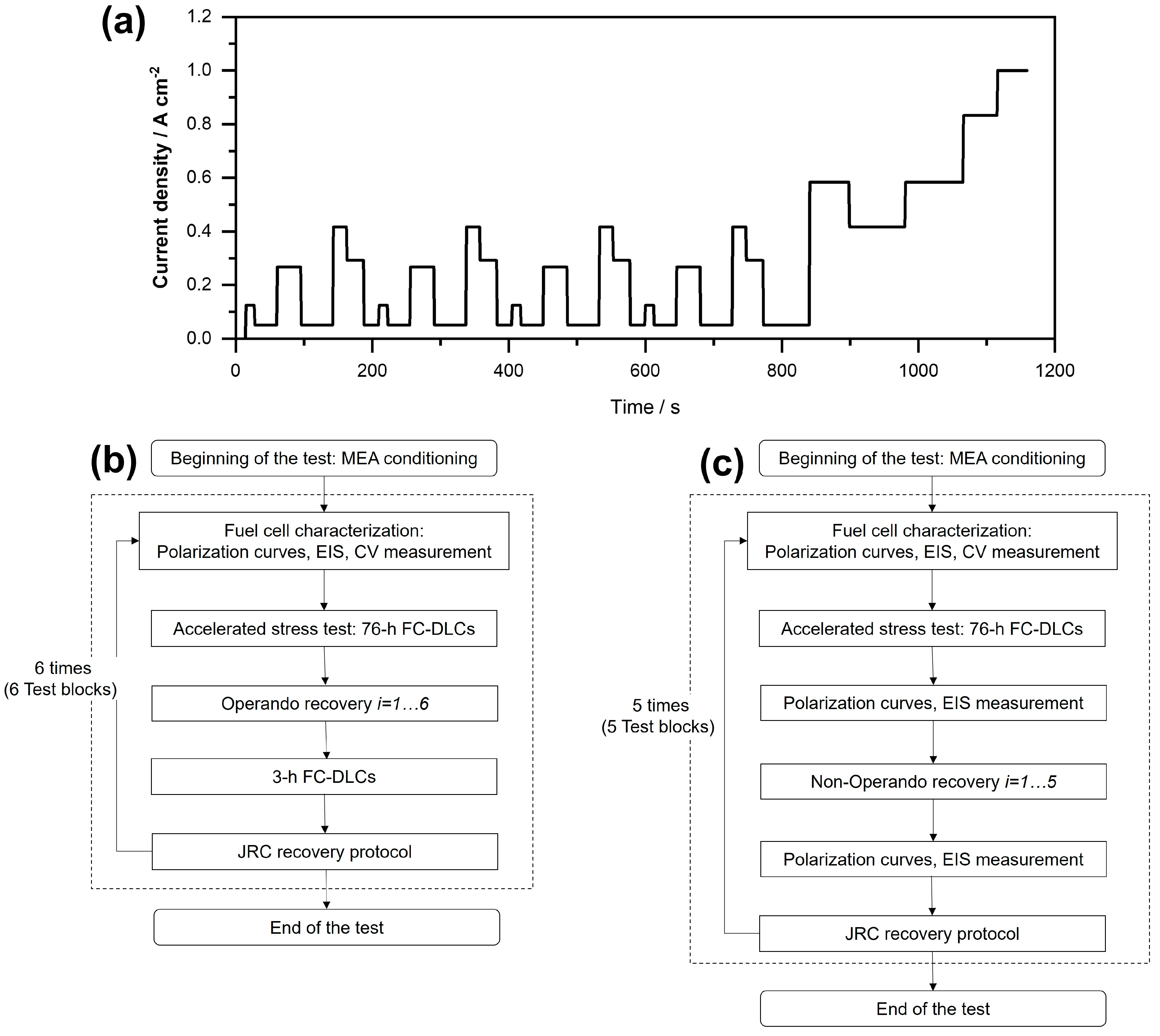
2.2. Operation Conditions and Test Protocols
- The first durability test comprises 6 test blocks, as indicated in Figure 1b. At the beginning of each test block, a performance characterization was conducted, followed by a 76 h period of load cycling. Subsequently, an operando recovery procedure was implemented; specifically, the cell temperature was reduced to a specific set point and then increased to the nominal operating temperature. After reaching the nominal operating temperature, FC-DLC operation was continued for an additional 3 h before applying the JRC recovery procedure [35] as a benchmark (see Supplementary Materials for more details). The JRC protocol, proposed by the Joint Research Center (JRC) of the European Commission [35], was chosen for this work based on our previous study [34], assuming 100% recovery. After each test block, it is imperative to implement the JRC protocol to prevent the accumulation of non-recovered reversible losses throughout the experiment [34]. This is crucial to avoid misinterpretation and misevaluation of the effectiveness of the operando recovery procedure.
- The second durability test (Figure 1c) consists of 5 test blocks. After each 76 h load cycling period, the cycling was paused, and performance characterization measurements were conducted before and after each non-operando temperature reduction procedure. Specifically, the cell temperature was reduced once the electrical load was switched off (leading to OCV), while the other operating parameters (gas flow and pressure) were kept constant. To compare the efficiency of the recovery due to temperature reduction with the efficiency of the JRC recovery protocol, at the end of each test block the JRC recovery protocol was performed, followed by an additional performance characterization.
2.3. Characterization Methods
2.4. Recovery Procedures
3. Results
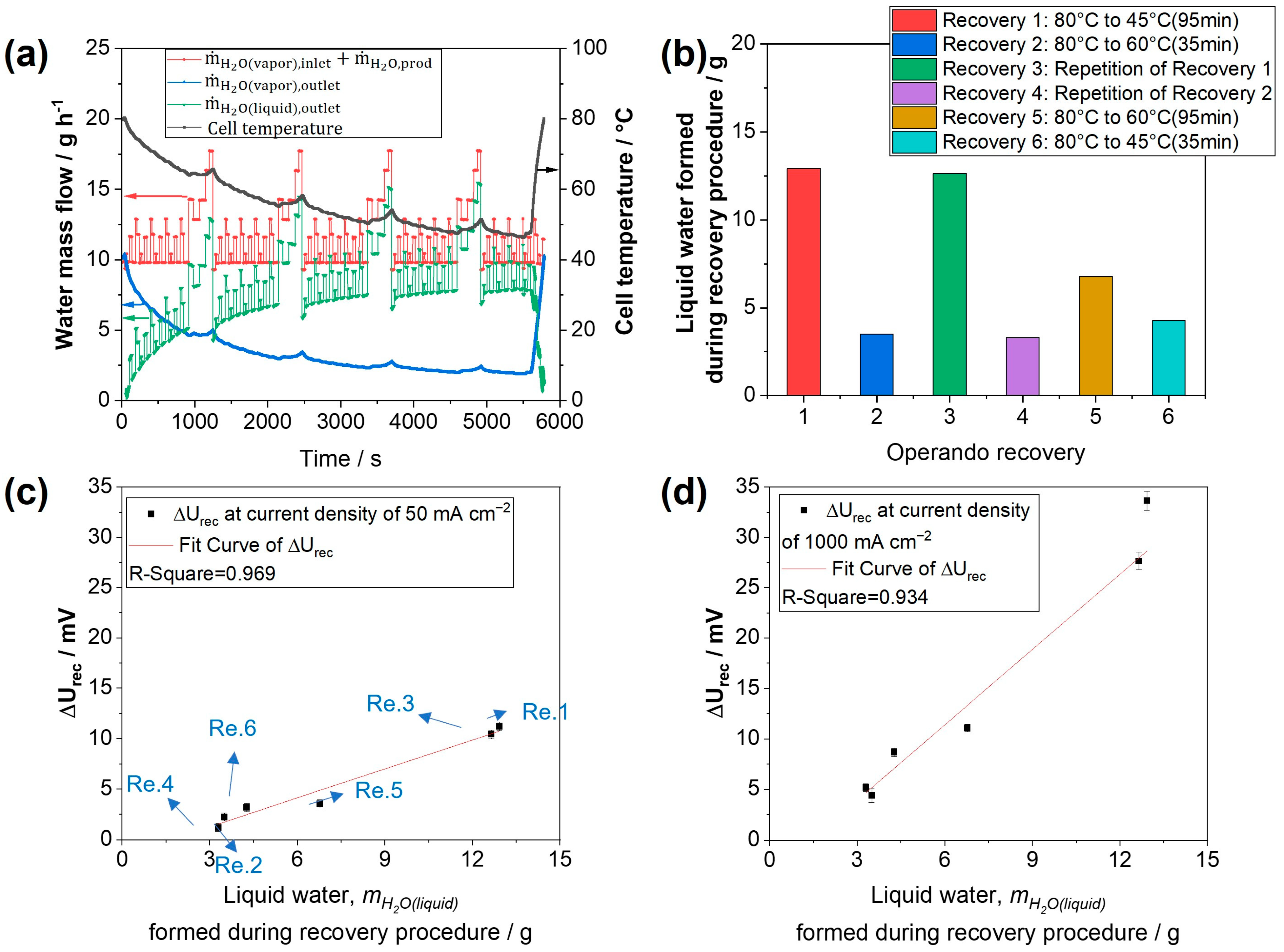
3.1. Operando Performance Recovery Procedures Based on Temperature Reduction
- , the total water mass flow into the cell (red data points). It consists of the vaporous water mass flow in the inlet air ( at 100% RH at 80 °C and a constant flow of 0.83 L min−1), plus product water () from the electrochemical reaction. It is calculated according to:with being the air flow rate of 0.83 L min−1, and indicating the air pressure and vapor pressure in the cathode inlet, respectively. The constants include , the molar volume of an ideal gas of 22.414 L mol−1, the Faraday constant, F = 96,485 C⋅mol−1, and , the molar mass of water of 18.015 g mol−1. The current is denoted by I;
- , the maximum output of vaporous water mass flow (blue data points) at the cathode outlet (depending on gas outlet pressure and cell temperature), is calculated using:with and being the air pressure and vapor pressure in the cathode outlet, respectively.
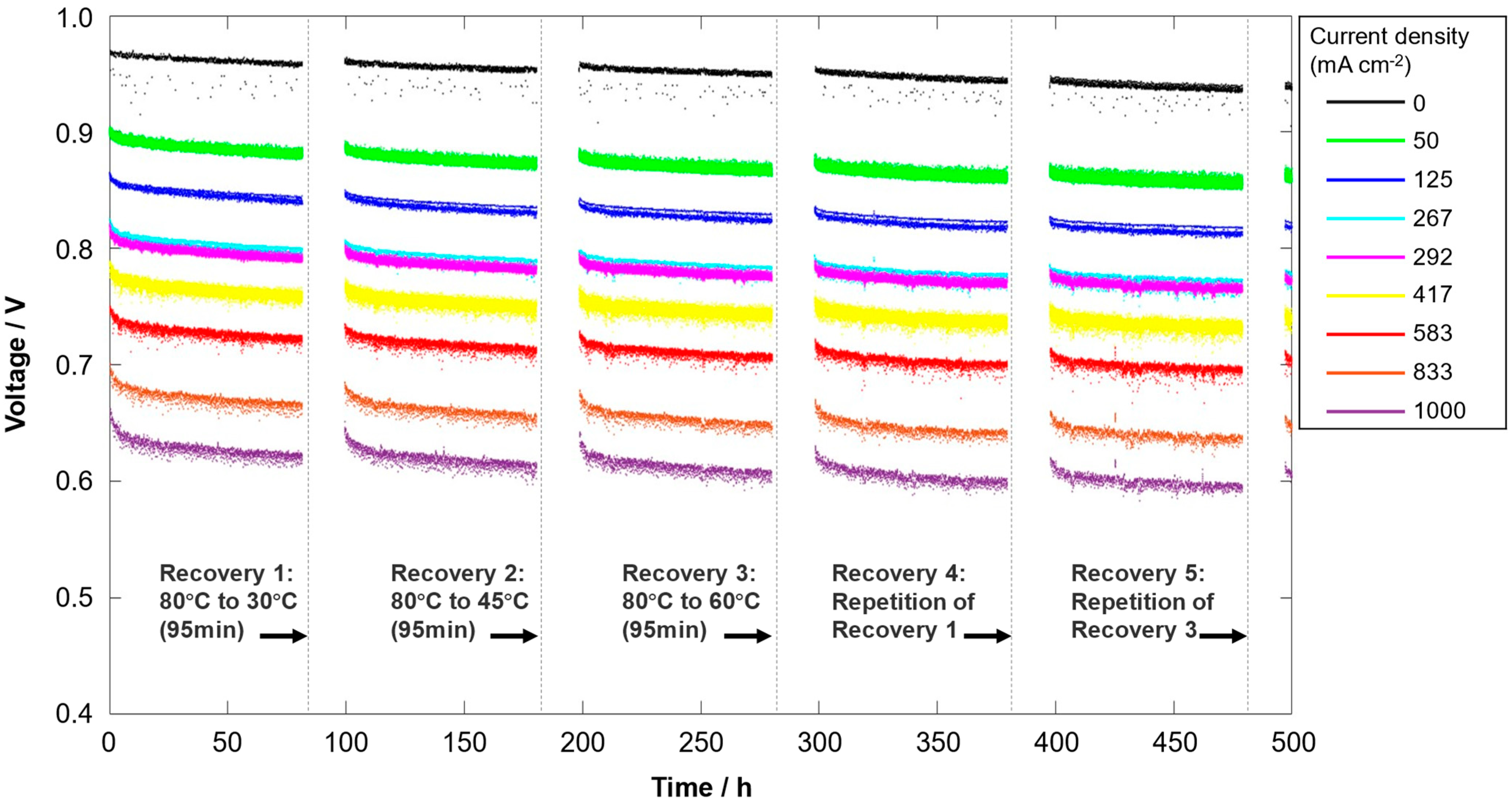
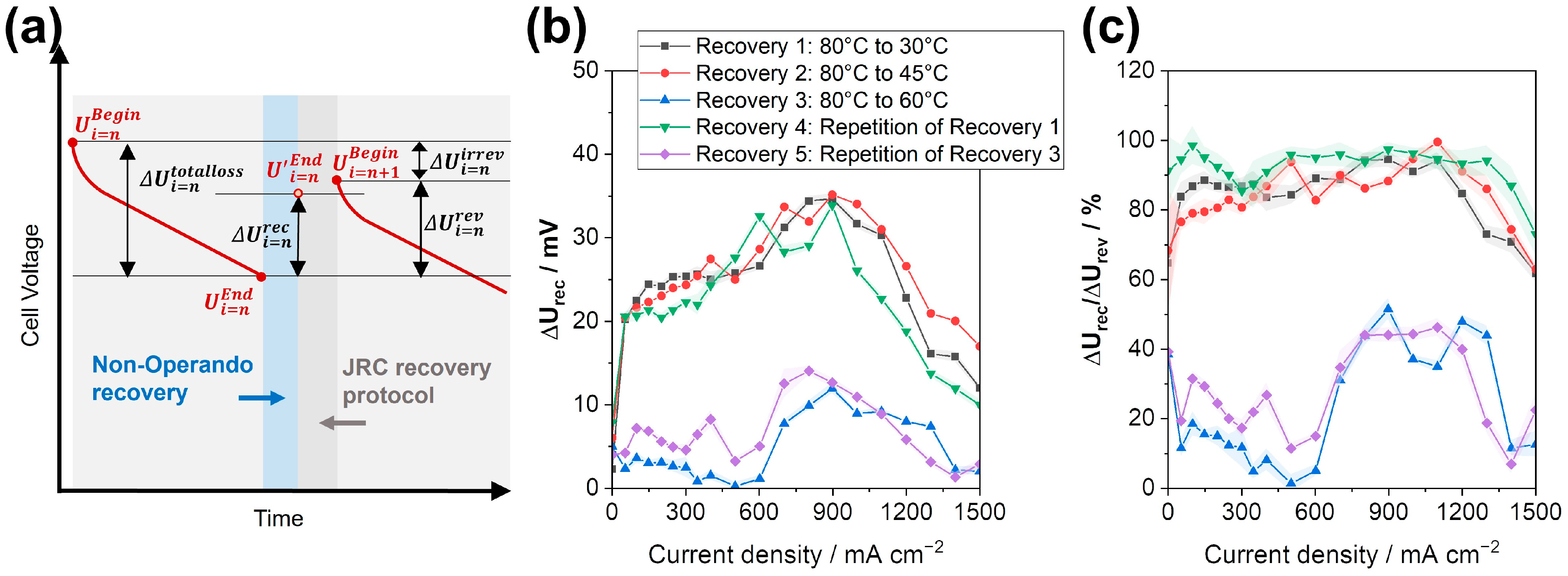
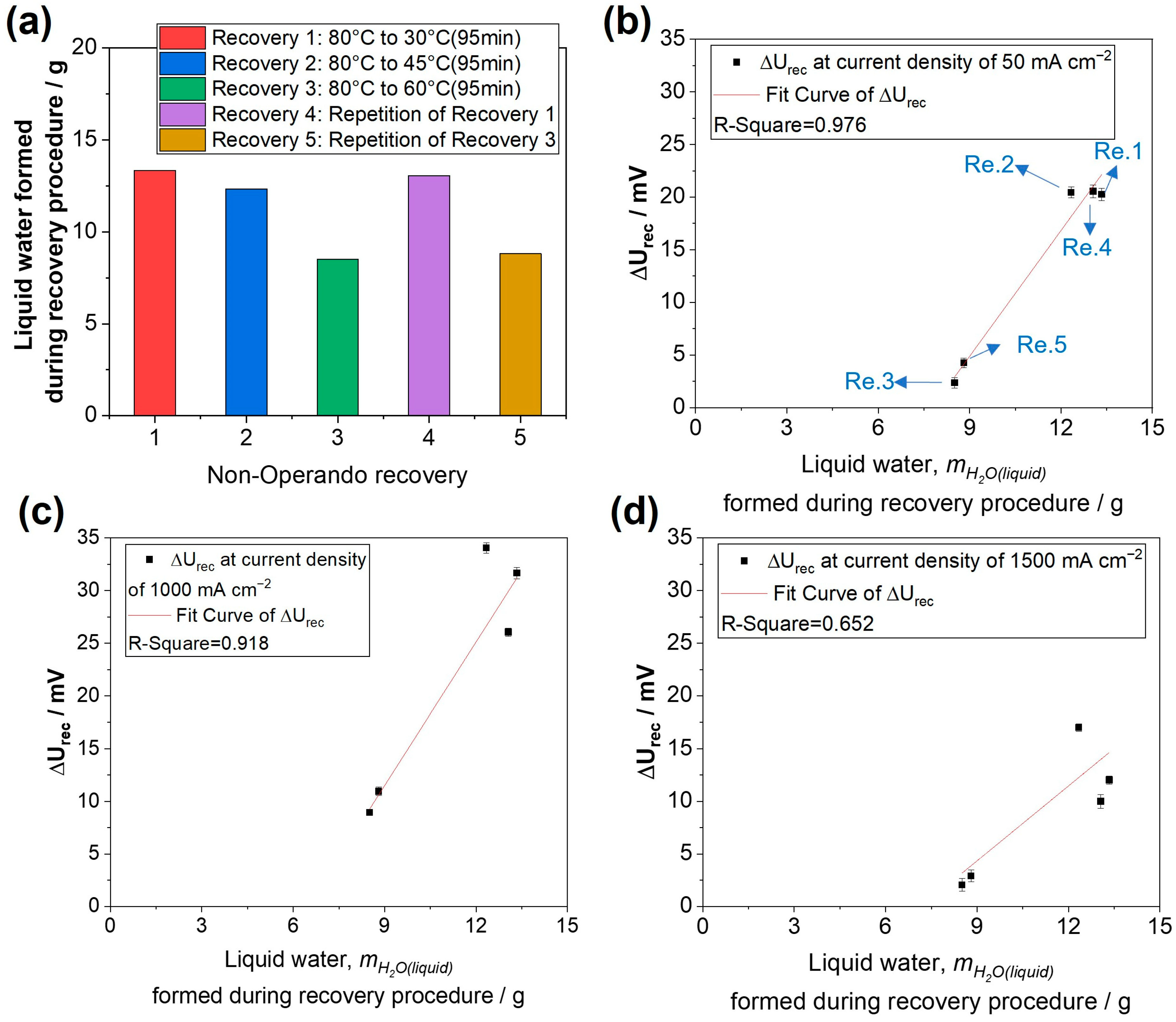
3.2. Recovery Effect of the Non-Operando Performance Recovery Procedures with Temperature Reduction
4. Conclusions
- Temperature reduction (associated with water condensation) is often attributed to the recovery of voltage losses resulting from the regeneration/redistribution of the ionomer structure, representing a primary effect. Therefore, its efficacy is anticipated to be influenced by specific operation conditions, particularly the level of relative humidity (RH). To avoid overinterpretation of our study, it is crucial to emphasize that the conclusions drawn from our study are applicable to MEAs operated at 100% RH; variations in RH levels are expected to result in quantitative differences in the observed outcomes;
- The amount of liquid water generated in the cathode during the operando recovery procedure exhibits a positive linear correlation with the absolute recovered voltage, particularly within a current density range below 1 A cm−2. This finding confirms the pivotal role of liquid water in the recovery of performance losses, likely associated with ionomer hydration and the resultant structure changes. Consequently, the observed results are anticipated to be applicable irrespective of the catalyst type, whether Pt/C (as in this study) or an alloyed catalyst (e.g., Pt-Co/C) is utilized;
- Operando temperature reduction during load cycling leads to 60–70% of the recovery of the JRC protocol at a much shorter duration (1.5 h versus 10.5 h). If the cell is kept at OCV during temperature reduction, the relative recovery is higher than 80%, likely due to avoiding flooding since no product water can be generated;
- The reduction in temperature from 80 °C to 45 °C is sufficient, and a further reduction to 30 °C does not lead to further recovery (at a similar duration). Longer durations of the cooling-down process have a slightly positive effect on the recovery of performance losses;
- Based on EIS data, the main contribution to recovery is the reduction in charge transfer resistance, followed by the reduction in mass transport resistance;
- It could be argued that the recovery due to water management, which was investigated using a specific flow field design, cannot be generalized because it strongly depends on the flow field design. Water management and the corresponding performances of PEMFCs have been shown to vary based on factors such as the flow field design (e.g., straight parallel channels, single or multiple serpentine channels, interdigitated channel or pin-type structures), channel geometry, and surface properties [57,58]. However, many studies have comparatively investigated, both experimentally and theoretically, the differences between flow field geometries, allowing variances for other configurations to be assessed [59,60].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Wang, C.; Zhang, C.; Bai, H.; Xu, L. Adaptive estimation of road slope and vehicle mass of fuel cell vehicle. eTransportation 2019, 2, 100023. [Google Scholar] [CrossRef]
- Greene, D.L.; Ogden, J.M.; Lin, Z. Challenges in the designing, planning and deployment of hydrogen refueling infrastructure for fuel cell electric vehicles. eTransportation 2020, 6, 100086. [Google Scholar] [CrossRef]
- Tanaka, S.; Nagumo, K.; Yamamoto, M.; Chiba, H.; Yoshida, K.; Okano, R. Fuel cell system for Honda CLARITY fuel cell. eTransportation 2020, 3, 100046. [Google Scholar] [CrossRef]
- Strategic Research and Innovation Agenda Clean Hydrogen Joint Undertaking 2021–2027. Available online: https://www.clean-hydrogen.europa.eu/about-us/key-documents/strategic-research-and-innovation-agenda_en (accessed on 1 October 2023).
- Marcinkoski, J. Hydrogen Class 8 Long Haul Truck Targets 2019. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/19006_hydrogen_class8_long_haul_truck_targets.pdf?Status=Master (accessed on 1 October 2023).
- Zhao, J.; Li, X. Fuel cell durability under automotive driving cycles—Fundamentals and experiments. In Fuel Cells for Transportation; Elsevier: Amsterdam, The Netherlands, 2023; pp. 419–462. [Google Scholar] [CrossRef]
- Lin, R.; Li, B.; Hou, Y.; Ma, J. Investigation of dynamic driving cycle effect on performance degradation and micro-structure change of PEM fuel cell. Int. J. Hydrogen Energy 2009, 34, 2369–2376. [Google Scholar] [CrossRef]
- Garzon, F.H.; Lopes, T.; Rockward, T.; Sansinena, J.M.; Kienitz, B.; Mukundan, R.; Springer, T. The Impact of Impurities On Long Term PEMFC Performance. ECS Trans. 2009, 25, 1575–1583. [Google Scholar] [CrossRef]
- Dhanushkodi, S.R.; Kundu, S.; Fowler, M.W.; Pritzker, M.D. Study of the effect of temperature on Pt dissolution in polymer electrolyte membrane fuel cells via accelerated stress tests. J. Power Sources 2014, 245, 1035–1045. [Google Scholar] [CrossRef]
- Zago, M.; Baricci, A.; Bisello, A.; Jahnke, T.; Yu, H.; Maric, R.; Zelenay, P.; Casalegno, A. Experimental analysis of recoverable performance loss induced by platinum oxide formation at the polymer electrolyte membrane fuel cell cathode. J. Power Sources 2020, 455, 227990. [Google Scholar] [CrossRef]
- Qi, Z.G.; Tang, H.; Guo, Q.H.; Du, B. Investigation on “saw-tooth” behavior of PEM fuel cell performance during shutdown and restart cycles. J. Power Sources 2006, 161, 864–871. [Google Scholar] [CrossRef]
- Stumper, E.; Lohr, M.; Hamada, S. Diagnostic tools for liquid water in PEM fuel cells. J. Power Sources 2005, 143, 150–157. [Google Scholar] [CrossRef]
- Mitzel, J.; Zhang, Q.; Gazdzicki, P.; Friedrich, K.A. Review on mechanisms and recovery procedures for reversible performance losses in polymer electrolyte membrane fuel cells. J. Power Sources 2021, 488, 229375. [Google Scholar] [CrossRef]
- Shi, W.Y.; Yi, B.L.; Hou, M.; Jing, F.N.; Ming, P.W. Hydrogen sulfide poisoning and recovery of PEMFC Pt-anodes. J. Power Sources 2007, 165, 814–818. [Google Scholar] [CrossRef]
- Du, F.M.; Dao, T.A.; Peitl, P.V.J.; Bauer, A.; Preuss, K.; Bonastre, A.M.; Sharman, J.; Spikes, G.; Perchthaler, M.; Schmidt, T.J.; et al. Effects of PEMFC Operational History under Dry/Wet Conditions on Additional Voltage Losses due to Ionomer Migration. J. Electrochem. Soc. 2020, 167, 144513–144527. [Google Scholar] [CrossRef]
- Inaba, M.; Yamada, H.; Tokunaga, J.; Tasaka, A. Effect of agglomeration of Pt/C catalyst on hydrogen peroxide formation. Electrochem. Solid. St. 2004, 7, A474. [Google Scholar] [CrossRef]
- Paul, D.K.; Karan, K. Conductivity and Wettability Changes of Ultrathin Nafion Films Subjected to Thermal Annealing and Liquid Water Exposure. J. Phys. Chem. C 2014, 118, 1828–1835. [Google Scholar] [CrossRef]
- Ott, S.; Bauer, A.; Du, F.M.; Dao, T.A.; Klingenhof, M.; Orfanidi, A.; Strasser, P. Impact of Carbon Support Meso-Porosity on Mass Transport and Performance of PEMFC Cathode Catalyst Layers. Chemcatchem 2021, 13, 4759–4769. [Google Scholar] [CrossRef]
- Langlois, D.A.; Lee, A.S.; Macauley, N.; Maurya, S.; Hawley, M.E.; Yim, S.D.; Kim, Y.S. A rejuvenation process to enhance the durability of low Pt loaded polymer electrolyte membrane fuel cells. J. Power Sources 2018, 396, 345–354. [Google Scholar] [CrossRef]
- Cetinbas, F.C.; Ahluwalia, R.K.; Kariuki, N.N.; De Andrade, V.; Myers, D.J. Effects of Porous Carbon Morphology, Agglomerate Structure and Relative Humidity on Local Oxygen Transport Resistance. J. Electrochem. Soc. 2019, 167, 013508. [Google Scholar] [CrossRef]
- Van Dine, L.L.; Steinbugler, M.M.; Reiser, C.A.; Scheffler, G.W. Procedure for Shutting Down A Fuel Cell System Having An Anode Exhaust Recycle Loop. U.S. Patent 6514635B2, 4 April 2004. Available online: https://patents.google.com/patent/US6514635B2/en (accessed on 1 October 2023).
- Colombo, E.; Baricci, A.; Bisello, A.; Guetaz, L.; Casalegno, A. PEMFC performance decay during real-world automotive operation: Evincing degradation mechanisms and heterogeneity of ageing. J. Power Sources 2023, 553, 232246. [Google Scholar] [CrossRef]
- Guétaz, L.; Escribano, S.; Sicardy, O. Study by electron microscopy of proton exchange membrane fuel cell membrane-electrode assembly degradation mechanisms: Influence of local conditions. J. Power Sources 2012, 212, 169–178. [Google Scholar] [CrossRef]
- Steinbach, A.J.; Hamilton, C.V.; Debe, M.K. Impact of micromolar concentrations of externally-provided chloride and sulfide contaminants on PEMFC reversible stability. ECS Trans. 2007, 11, 889. [Google Scholar] [CrossRef]
- Prass, S.; Friedrich, K.A.; Zamel, N. Tolerance and Recovery of Ultralow-Loaded Platinum Anode Electrodes upon Carbon Monoxide and Hydrogen Sulfide Exposure. Molecules 2019, 24, 3514. [Google Scholar] [CrossRef]
- Pivac, I.; Barbir, F. Impact of Shutdown Procedures on Recovery Phenomena of Proton Exchange Membrane Fuel Cells. Fuel Cells 2020, 20, 185–195. [Google Scholar] [CrossRef]
- Kabir, S.; Myers, D.J.; Kariuki, N.; Park, J.; Wang, G.X.; Baker, A.; Macauley, N.; Mukundan, R.; More, K.L.; Neyerlin, K.C. Elucidating the Dynamic Nature of Fuel Cell Electrodes as a Function of Conditioning: An ex Situ Material Characterization and in Situ Electrochemical Diagnostic Study. ACS Appl. Mater. Interfaces 2019, 11, 45016–45030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Litteer, B.A.; Coms, F.D.; Makharia, R. Recoverable Performance Loss Due to Membrane Chemical Degradation in PEM Fuel Cells. J. Electrochem. Soc. 2012, 159, F287. [Google Scholar] [CrossRef]
- Robb, G.M.; Folmsbee, D.T. Procedure for Stack Voltage Recovery. U.S. Patent 9343760B2, 17 May 2014. Available online: https://patents.google.com/patent/US9343760B2/en (accessed on 1 October 2023).
- Shokhen, V.; Strandberg, L.; Skoglundh, M.; Wickman, B. Impact of Accelerated Stress Tests on the Cathodic Catalytic Layer in a Proton Exchange Membrane (PEM) Fuel Cell Studied by Identical Location Scanning Electron Microscopy. ACS Appl. Energy Mater. 2022, 5, 11200–11212. [Google Scholar] [CrossRef]
- Gazdzick, P.; Mitzel, J.; Sanchez, D.G.; Schulze, M.; Friedrich, K.A. Evaluation of reversible and irreversible degradation rates of polymer electrolyte membrane fuel cells tested in automotive conditions. J. Power Sources 2016, 327, 86–95. [Google Scholar] [CrossRef]
- Talukdar, K.; Gazdzicki, P.; Friedrich, K.A. Comparative investigation into the performance and durability of long and short side chain ionomers in Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2019, 439, 227078. [Google Scholar] [CrossRef]
- Sanchez, D.G.; Ortiz, A.; Friedrich, K.A. Oscillation of PEFC under low cathode humidification: Effect of gravitation and bipolar plate design. J. Electrochem. Soc. 2013, 160, F636. [Google Scholar] [CrossRef]
- Zhang, Q.; Schulze, M.; Gazdzicki, P.; Friedrich, K.A. Comparison of different performance recovery procedures for polymer electrolyte membrane fuel cells. Appl. Energy 2021, 302, 117490. [Google Scholar] [CrossRef]
- Tsotridis, G.; Pilenga, A.; De Marco, G.; Malkow, T. EU Harmonised Test Protocols for PEMFC MEA Testing in Single Cell Configuration for Automotive Applications; JRC Science for Policy report 2015; European Commission: Brussels, Belgium, 2015; Volume 27632. [CrossRef]
- Schulze, M.; Wagner, N.; Kaz, T.; Friedrich, K.A. Combined electrochemical and surface analysis investigation of degradation processes in polymer electrolyte membrane fuel cells. Electrochim. Acta 2007, 52, 2328–2336. [Google Scholar] [CrossRef]
- Tang, Z.P.; Huang, Q.A.; Wang, Y.J.; Zhang, F.Z.; Li, W.H.; Li, A.J.; Zhang, L.; Zhang, J.J. Recent progress in the use of electrochemical impedance spectroscopy for the measurement, monitoring, diagnosis and optimization of proton exchange membrane fuel cell performance. J. Power Sources 2020, 468, 228361. [Google Scholar] [CrossRef]
- Schneider, I.A.; Kramer, D.; Wokaun, A.; Scherer, G.G. Oscillations in gas channels—II. Unraveling the characteristics of the low frequency loop in air-fed PEFC impedance spectra. J. Electrochem. Soc. 2007, 154, B770. [Google Scholar] [CrossRef]
- Yin, Y.; Li, R.T.; Bai, F.Q.; Zhu, W.K.; Qin, Y.Z.; Chang, Y.F.; Zhang, J.F.; Guiver, M.D. Ionomer migration within PEMFC catalyst layers induced by humidity changes. Electrochem. Commun. 2019, 109, 106590. [Google Scholar] [CrossRef]
- Morawietz, T.; Handl, M.; Oldani, C.; Friedrich, K.A.; Hiesgen, R. Quantitative in Situ Analysis of Ionomer Structure in Fuel Cell Catalytic Layers. ACS Appl. Mater. Interfaces 2016, 8, 27044–27054. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Ye, X.; LeVan, M.D. Water transport properties of Nafion membranes: Part I. Single-tube membrane module for air drying. J. Membr. Sci. 2003, 221, 147–161. [Google Scholar] [CrossRef]
- Monroe, C.W.; Romero, T.; Mérida, W.; Eikerling, M. A vaporization-exchange model for water sorption and flux in Nafion. J. Membr. Sci. 2008, 324, 1–6. [Google Scholar] [CrossRef]
- Aleksandrova, E.; Hiesgen, R.; Eberhard, D.; Friedrich, K.A.; Kaz, T.; Roduner, E. Proton Conductivity Study of a Fuel Cell Membrane with Nanoscale Resolution. ChemPhysChem 2007, 8, 519–522. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kusoglu, A.; Lucas, I.T.; Clark, K.; Weber, A.Z.; Kostecki, R. Correlating humidity-dependent ionically conductive surface area with transport phenomena in proton-exchange membranes. J. Phys. Chem. B 2011, 115, 11650–11657. [Google Scholar] [CrossRef]
- Adachi, M.; Navessin, T.; Xie, Z.; Frisken, B.; Holdcroft, S. Correlation of in situ and ex situ measurements of water permeation through Nafion NRE211 proton exchange membranes. J. Electrochem. Soc. 2009, 156, B782. [Google Scholar] [CrossRef]
- Adachi, M.; Navessin, T.; Xie, Z.; Li, F.H.; Tanaka, S.; Holdcroft, S. Thickness dependence of water permeation through proton exchange membranes. J. Membr. Sci. 2010, 364, 183–193. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R. Evolution of permanent deformations (or memory) in Nafion 117 membranes with changes in temperature, relative humidity and time, and its importance in the development of medium temperature PEMFCs. Fuel Cells 2009, 9, 410–420. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R. On the decay of Nafion proton conductivity at high temperature and relative humidity. J. Power Sources 2006, 162, 141–145. [Google Scholar] [CrossRef]
- Fumagalli, M.; Lyonnard, S.; Prajapati, G.; Berrod, Q.; Porcar, L.; Guillermo, A.; Gebel, G. Fast water diffusion and long-term polymer reorganization during Nafion membrane hydration evidenced by time-resolved small-angle neutron scattering. J. Phys. Chem. B 2015, 119, 7068–7076. [Google Scholar] [CrossRef] [PubMed]
- Gebel, G.; Lyonnard, S.; Mendil-Jakani, H.; Morin, A. The kinetics of water sorption in Nafion membranes: A small-angle neutron scattering study. J. Phys. Condens. Matter 2011, 23, 234107. [Google Scholar] [CrossRef] [PubMed]
- Gierke, T.D.; Munn, G.; Wilson, F. The morphology in nafion perfluorinated membrane products, as determined by wide-and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Safiollah, M.; Melchy, P.-E.A.; Berg, P.; Eikerling, M. Model of water sorption and swelling in polymer electrolyte membranes: Diagnostic applications. J. Phys. Chem. B 2015, 119, 8165–8175. [Google Scholar] [CrossRef]
- Mittelsteadt, C.K.; Staser, J. Simultaneous water uptake, diffusivity and permeability measurement of perfluorinated sulfonic acid polymer electrolyte membranes. ECS Trans. 2011, 41, 101. [Google Scholar] [CrossRef]
- Takamatsu, T.; Hashiyama, M.; Eisenberg, A. Sorption phenomena in Nafion membranes. J. Appl. Polym. Sci. 1979, 24, 2199–2220. [Google Scholar] [CrossRef]
- Sauermoser, M.; Kizilova, N.; Pollet, B.G.; Kjelstrup, S. Flow Field Patterns for Proton Exchange Membrane Fuel Cells. Front. Energy Res. 2020, 8, 13. [Google Scholar] [CrossRef]
- Lim, B.H.; Majlan, E.H.; Daud, W.R.W.; Husaini, T.; Rosli, M.I. Effects of flow field design on water management and reactant distribution in PEMFC: A review. Ionics 2016, 22, 301–316. [Google Scholar] [CrossRef]
- Pan, W.T.; Wang, P.H.; Chen, X.L.; Wang, F.C.; Dai, G.C. Combined effects of flow channel configuration and operating conditions on PEM fuel cell performance. Energy Convers. Manag. 2020, 220, 113046. [Google Scholar] [CrossRef]
- Arif, M.; Cheung, S.C.; Andrews, J. Numerical investigation of effects of different flow channel configurations on the 100 cm2 PEM fuel cell performance under different operating conditions. Catal. Today 2022, 397, 449–462. [Google Scholar] [CrossRef]
- Bonnet, C.; Didierjean, S.; Guillet, N.; Besse, S.; Colinart, T.; Carré, P. Design of an 80 kWe PEM fuel cell system: Scale up effect investigation. J. Power Sources 2008, 182, 441–448. [Google Scholar] [CrossRef]



| # | Operando | Non-Operando |
|---|---|---|
| Recovery 1 | 80 °C to 45 °C (95 min) | 80 °C to 30 °C (95 min) |
| Recovery 2 | 80 °C to 60 °C (35 min) | 80 °C to 45 °C (95 min) |
| Recovery 3 | Repetition of Recovery 1 | 80 °C to 60 °C (95 min) |
| Recovery 4 | Repetition of Recovery 2 | Repetition of Recovery 1 |
| Recovery 5 | 80 °C to 60 °C (95 min) | Repetition of Recovery 3 |
| Recovery 6 | 80 °C to 45 °C (35 min) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Schulze, M.; Gazdzicki, P.; Friedrich, K.A. Temperature Reduction as Operando Performance Recovery Procedure for Polymer Electrolyte Membrane Fuel Cells. Energies 2024, 17, 774. https://doi.org/10.3390/en17040774
Zhang Q, Schulze M, Gazdzicki P, Friedrich KA. Temperature Reduction as Operando Performance Recovery Procedure for Polymer Electrolyte Membrane Fuel Cells. Energies. 2024; 17(4):774. https://doi.org/10.3390/en17040774
Chicago/Turabian StyleZhang, Qian, Mathias Schulze, Pawel Gazdzicki, and Kaspar Andreas Friedrich. 2024. "Temperature Reduction as Operando Performance Recovery Procedure for Polymer Electrolyte Membrane Fuel Cells" Energies 17, no. 4: 774. https://doi.org/10.3390/en17040774
APA StyleZhang, Q., Schulze, M., Gazdzicki, P., & Friedrich, K. A. (2024). Temperature Reduction as Operando Performance Recovery Procedure for Polymer Electrolyte Membrane Fuel Cells. Energies, 17(4), 774. https://doi.org/10.3390/en17040774






