Catalyst-Free Depolymerization of Methanol-Fractionated Kraft Lignin to Aromatic Monomers in Supercritical Methanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Extraction
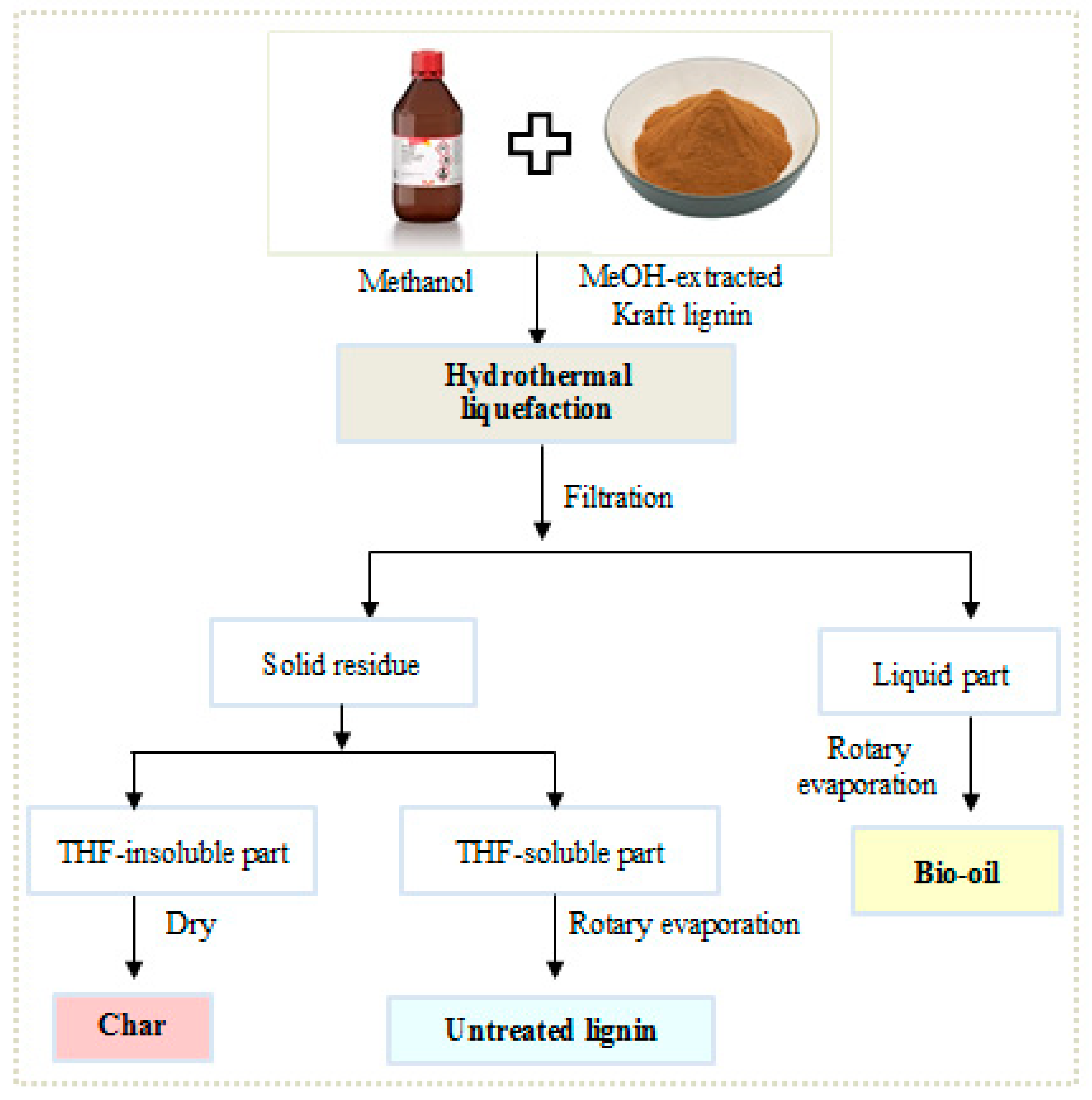
2.3. Lignin Depolymerization
2.4. Product Analyses
3. Results and Discussion
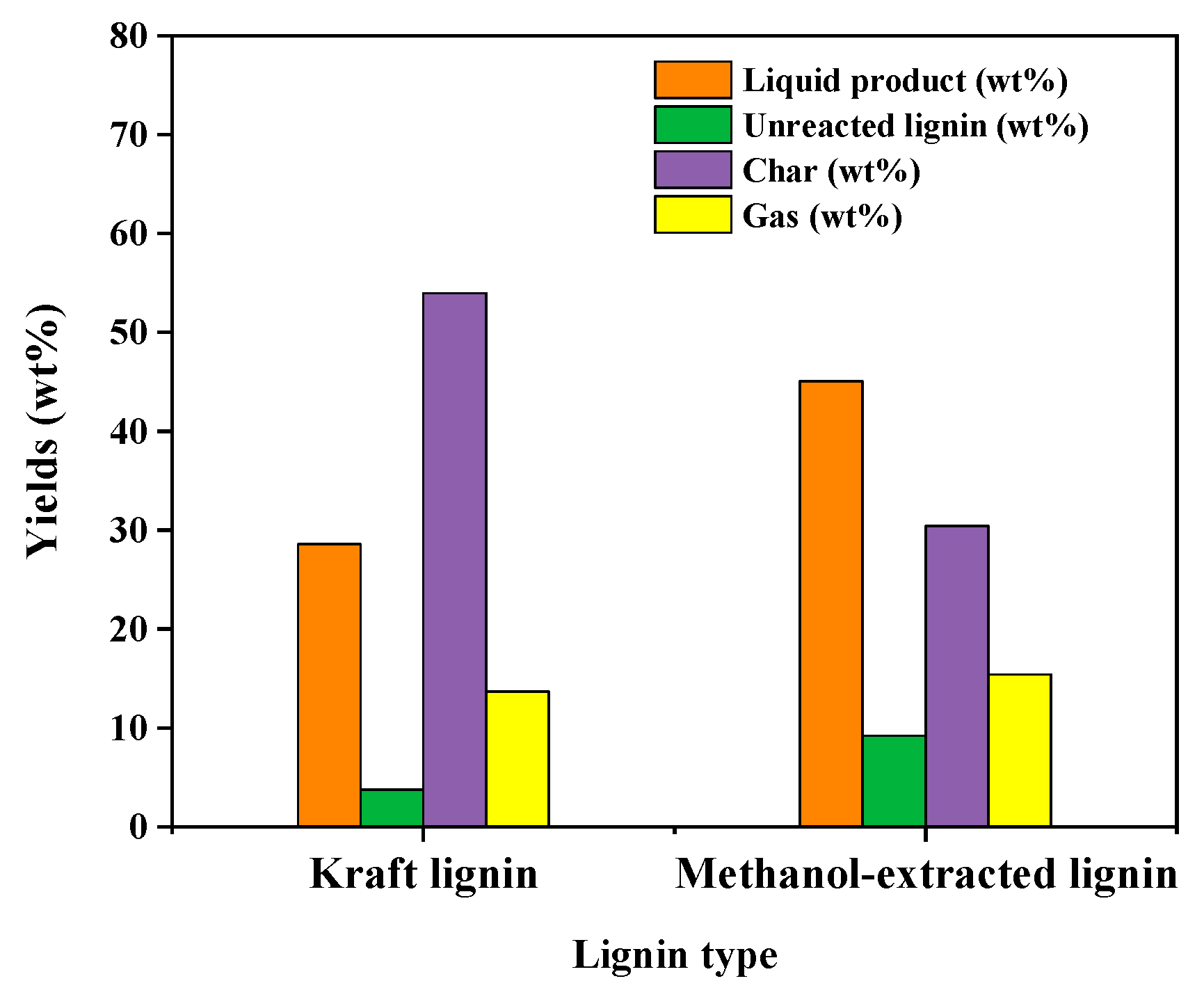
3.1. Characterization of MeOH-Soluble Lignin and Raw Kraft Lignin
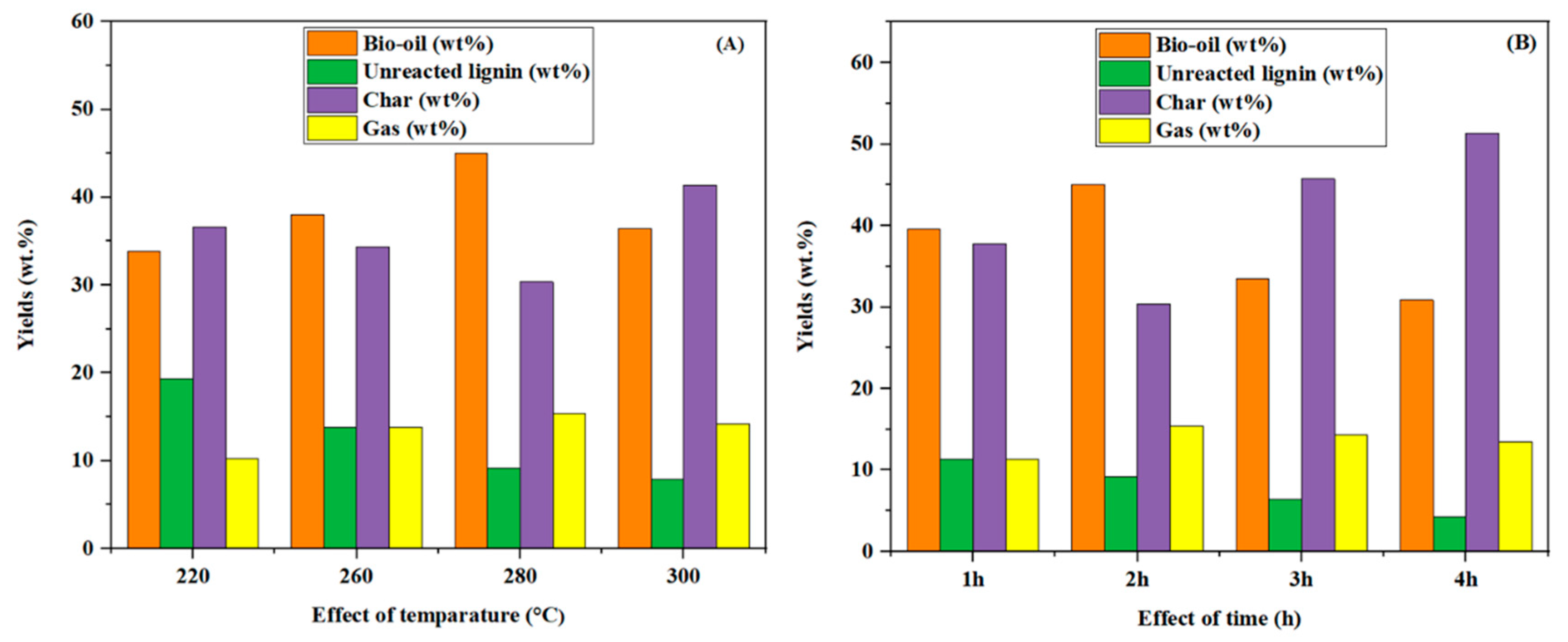
3.2. Effects of the Reaction Temperature and Time on the Product Yields
3.3. Bio-Oil Characterization
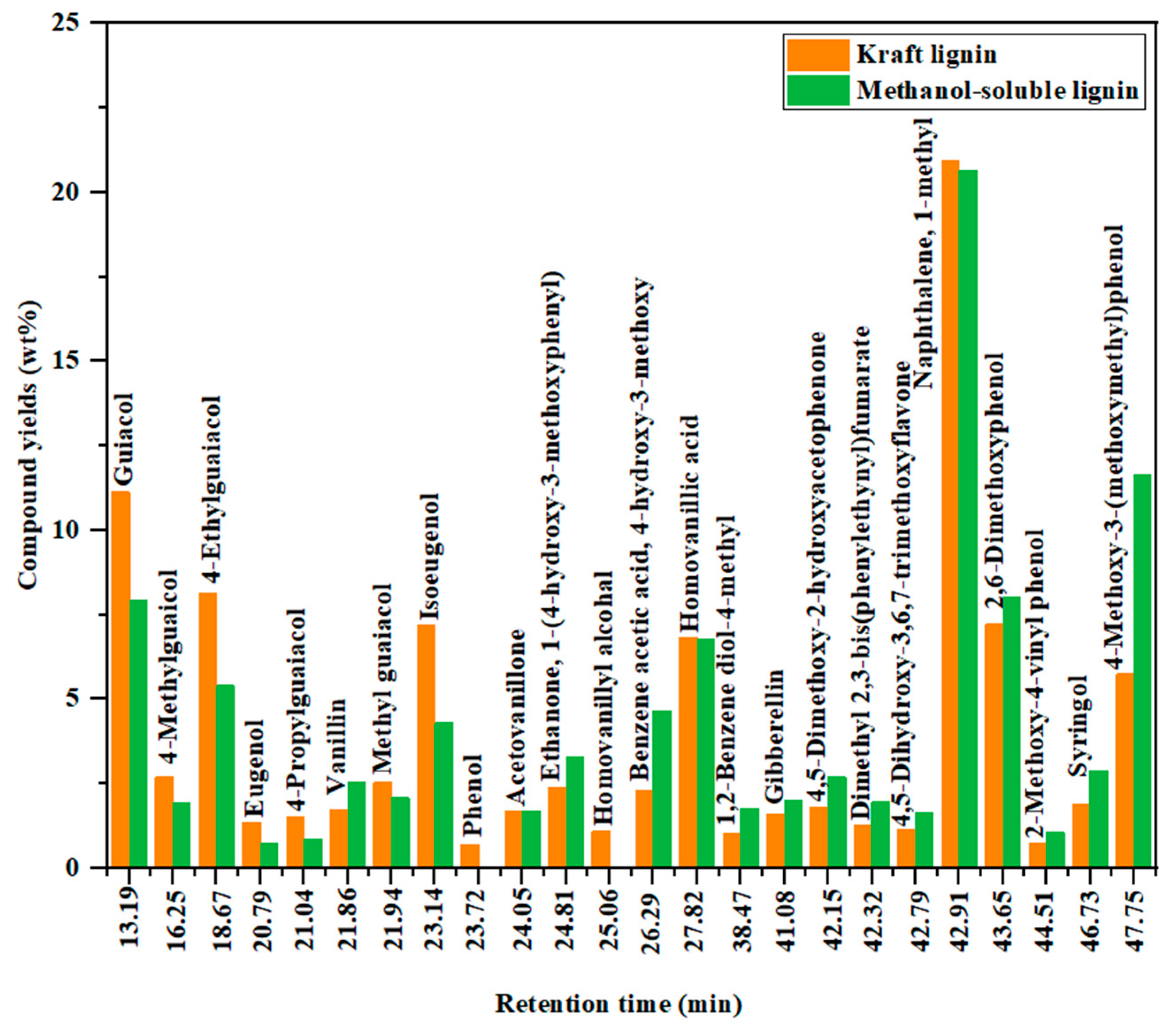
3.3.1. GC-MS Analysis of Bio-Oils
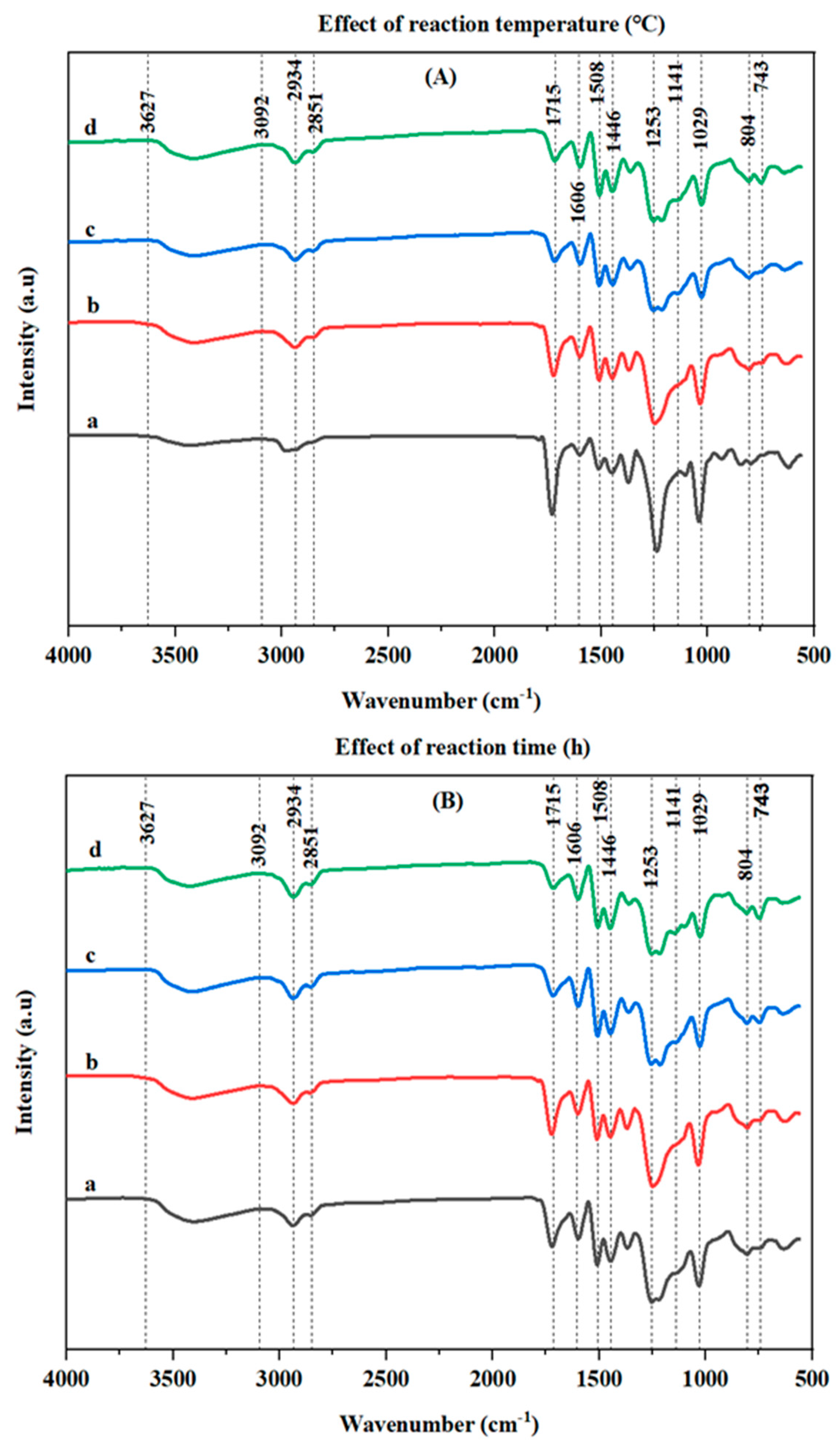
3.3.2. FT-IR Analysis of the Bio-Oils
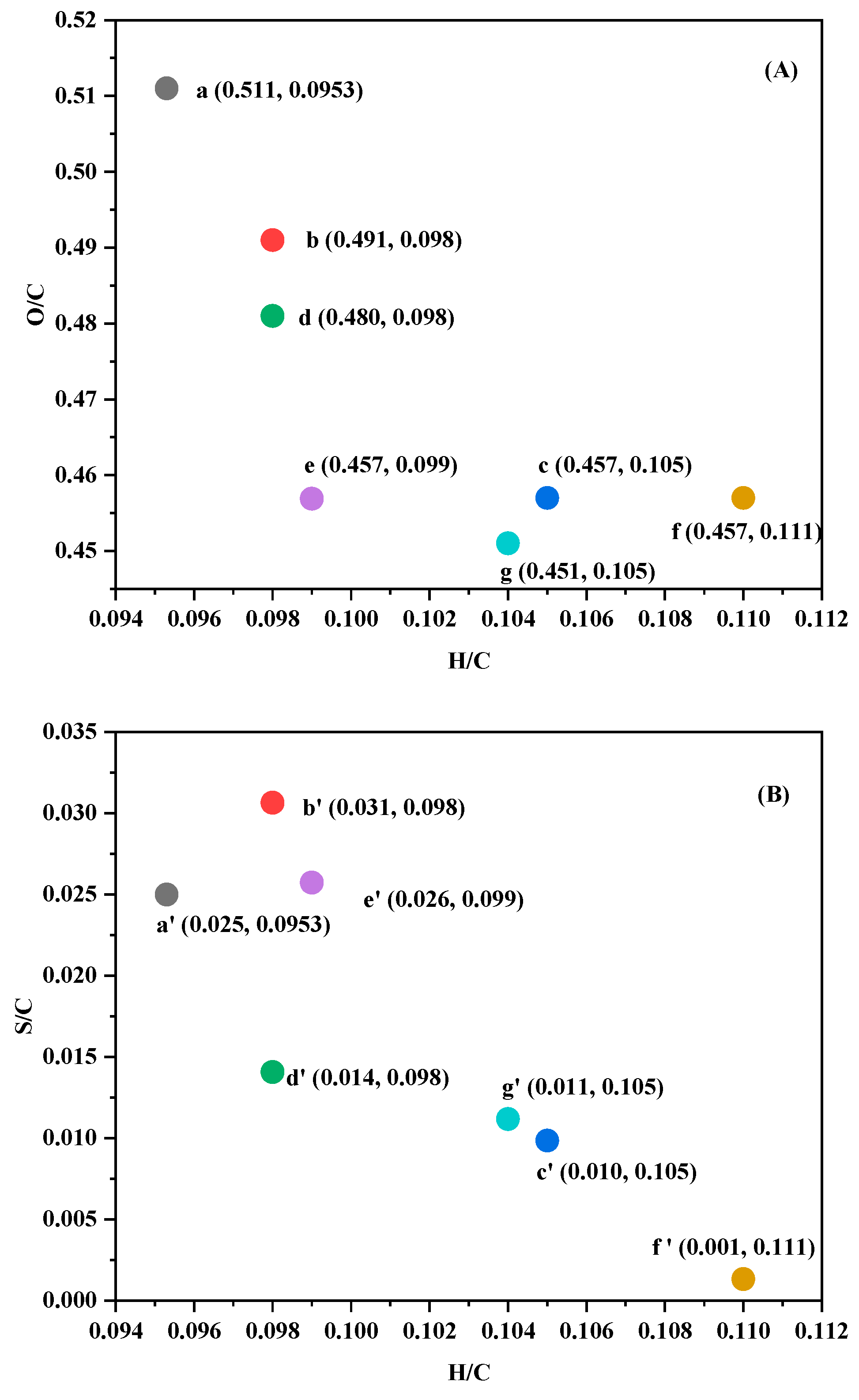
3.3.3. Elemental Analysis
3.4. Reaction Route and Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream Processing of Lignin Derived Feedstock into End Products. Chem. Soc. Rev. 2020, 49, 5510–5560. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.J.A.; Teunissen, W.; van Dam, J.E.G.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P.M. Lignin Depolymerisation in Supercritical Carbon Dioxide/Acetone/Water Fluid for the Production of Aromatic Chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Nshizirungu, T.; Park, J.H. Effect of Simultaneous Use of Microwave and Ultrasound Irradiation on the Sulfuric Acid Hydrolysis Lignin (SAHL) Depolymerization. Sustain. Energy Fuels 2022, 6, 861–878. [Google Scholar] [CrossRef]
- Rana, M.; Ghosh, S.; Nshizirungu, T.; Park, J.H. Catalytic Depolymerization of Kraft Lignin to High Yield Alkylated-Phenols over CoMo/SBA-15 Catalyst in Supercritical Ethanol. RSC Adv. 2023, 13, 30022–30039. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rana, M.; Park, J.H. Advancement in Technologies for the Depolymerization of Lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Jokinen, N.; Eronen, E.; Salami, A.; Hyttinen, M.; Jänis, J.; Vepsäläinen, J.; Lappalainen, R.; Tomppo, L. Valorization Potential of the Aqueous Products from Hydrothermal Liquefaction and Stepwise Slow Pyrolysis of Wood Bark and Hemp Hurds with Yields and Product Comparison. Bioresour. Technol. Rep. 2023, 21, 101385. [Google Scholar] [CrossRef]
- Marx, S.; Laubscher, A.N.E.; Bunt, J.R.; Venter, R.J.; Uwaoma, R.C.; Strydom, C.A. Evaluation of Sugar Cane Bagasse Hydrothermal Liquefaction Products for Co-Gasification with Coal as Green Coal Pellet Production. Bioresour. Technol. Rep. 2023, 22, 101503. [Google Scholar] [CrossRef]
- Ahmad, W.; Nisar, J.; Anwar, F.; Muhammad, F. Future Prospects of Biomass Waste as Renewable Source of Energy in Pakistan: A Mini Review. Bioresour. Technol. Rep. 2023, 24, 101658. [Google Scholar] [CrossRef]
- Rana, M.; Nshizirungu, T.; Park, J.H. Synergistic Effect of Water-Ethanol-Formic Acid for the Depolymerization of Industrial Waste (Black Liquor) Lignin to Phenolic Monomers. Biomass Bioenergy 2021, 153, 106204. [Google Scholar] [CrossRef]
- Rana, M.; Islam, M.N.; Agarwal, A.; Taki, G.; Park, S.J.; Dong, S.; Jo, Y.T.; Park, J.H. Production of Phenol-Rich Monomers from Kraft Lignin Hydrothermolysates in Basic-Subcritical Water over MoO3/SBA-15 Catalyst. Energy Fuels 2018, 32, 11564–11575. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Islam, M.N.; Taki, G.; Rana, M.; Park, J.H. Yield of Phenolic Monomers from Lignin Hydrothermolysis in Subcritical Water System. Ind. Eng. Chem. Res. 2018, 57, 4779–4784. [Google Scholar] [CrossRef]
- Dunn, K.G.; Hobson, P.A. Hydrothermal Liquefaction of Lignin. In Sugarcane-Based Biofuels and Bioproducts; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 165–206. [Google Scholar] [CrossRef]
- Mazaheri, H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Sub/Supercritical Liquefaction of Oil Palm Fruit Press Fiber for the Production of Bio-Oil: Effect of Solvents. Bioresour. Technol. 2010, 101, 7641–7647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, S.; Fu, H.; Chen, J. Liquefaction of Macroalgae Enteromorpha Prolifera in Sub-/Supercritical Alcohols: Direct Production of Ester Compounds. Energy Fuels 2012, 26, 2342–2351. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Dutta, S. Review and Outlook of Hydrogen Production through Catalytic Processes. Energy Fuels 2024, 38, 2601–2629. [Google Scholar] [CrossRef]
- Mankar, A.R.; Ahmad, E.; Pant, K.K. Insights into Reductive Depolymerization of Kraft Lignin to Produce Aromatics in the Presence of Pt/HZSM-23 Catalyst. Mater. Sci. Energy Technol. 2021, 4, 341–348. [Google Scholar] [CrossRef]
- Wu, Y.; Dang, Q.; Wu, T.; Lei, T.; Wang, K.; Luo, Z. Efficient Lignin Depolymerization Process for Phenolic Products with Lignin-Based Catalysts and Mixed Solvents. Energy Fuels 2023, 37, 5206–5219. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Riaz, A.; Park, J.; Insyani, R.; Verma, D.; Kim, J. Depolymerization of Concentrated Sulfuric Acid Hydrolysis Lignin to High-Yield Aromatic Monomers in Basic Sub- and Supercritical Fluids. Chem. Eng. J. 2017, 317, 9–19. [Google Scholar] [CrossRef]
- Roberts, V.M.; Stein, V.; Reiner, T.; Lemonidou, A.; Li, X.; Lercher, J.A. Towards Quantitative Catalytic Lignin Depolymerization. Chem. A Eur. J. 2011, 17, 5939–5948. [Google Scholar] [CrossRef]
- Kristianto, I.; Limarta, S.O.; Lee, H.; Ha, J.M.; Suh, D.J.; Jae, J. Effective Depolymerization of Concentrated Acid Hydrolysis Lignin Using a Carbon-Supported Ruthenium Catalyst in Ethanol/Formic Acid Media. Bioresour. Technol. 2017, 234, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Limarta, S.O.; Kim, H.; Ha, J.M.; Park, Y.K.; Jae, J. High-Quality and Phenolic Monomer-Rich Bio-Oil Production from Lignin in Supercritical Ethanol over Synergistic Ru and Mg-Zr-Oxide Catalysts. Chem. Eng. J. 2020, 396, 125175. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Lu, X.; Xu, J.; Zhou, Q.; Zhang, G.; Xin, J.; Yan, D.; Sayed, I.E.I.T.E.I. The Ionic Liquids upon Perchlorate to Promote the C-C/C-O Bonds Cleavage in Alkali Lignin under Photothermal Synergism. Int. J. Biol. Macromol. 2024, 255, 128125. [Google Scholar] [CrossRef]
- Zhan, X.; Cai, C.; Pang, Y.; Qin, F.; Lou, H.; Huang, J.; Qiu, X. Effect of the Isoelectric Point of PH-Responsive Lignin-Based Amphoteric Surfactant on the Enzymatic Hydrolysis of Lignocellulose. Bioresour. Technol. 2019, 283, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Seidi, F.; Wu, W.; Pan, Y.; Xiao, H. Dual-Functional Lignin-Based Hydrogels for Sustained Release of Agrochemicals and Heavy Metal Ion Complexation. Int. J. Biol. Macromol. 2023, 235, 123701. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. Organic Quinones towards Advanced Electrochemical Energy Storage: Recent Advances and Challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Wang, J.; Seidi, F.; Huang, Y.; Xiao, H. Smart Lignin-Based Polyurethane Conjugated with Corrosion Inhibitor as Bio-Based Anticorrosive Sublayer Coating. Ind. Crops Prod. 2022, 188, 115719. [Google Scholar] [CrossRef]
- Asada, C.; Basnet, S.; Otsuka, M.; Sasaki, C.; Nakamura, Y. Epoxy Resin Synthesis Using Low Molecular Weight Lignin Separated from Various Lignocellulosic Materials. Int. J. Biol. Macromol. 2015, 74, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, M.; Huo, W.; Cai, D.; Qin, P.; Cao, H.; Tan, T. Fractionation and Oxypropylation of Corn-Stover Lignin for the Production of Biobased Rigid Polyurethane Foam. Ind. Crops Prod. 2020, 143, 111887. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Yang, W.; Jiao, L.; Zhang, S.; Dai, H. Efficient Production of Low Molecular Weight Lignin from Eucalyptus Wood through Methanol-Alkali System. Ind. Crops Prod. 2024, 207, 117728. [Google Scholar] [CrossRef]
- Goldmann, W.M.; Ahola, J.; Mikola, M.; Tanskanen, J. Solubility and Fractionation of Indulin AT Kraft Lignin in Ethanol-Water Media. Sep. Purif. Technol. 2019, 209, 826–832. [Google Scholar] [CrossRef]
- Dastpak, A.; Lourençon, T.V.; Balakshin, M.; Farhan Hashmi, S.; Lundström, M.; Wilson, B.P. Solubility Study of Lignin in Industrial Organic Solvents and Investigation of Electrochemical Properties of Spray-Coated Solutions. Ind. Crops Prod. 2020, 148, 112310. [Google Scholar] [CrossRef]
- Drame, K.; Likozar, B.; Tofani, G. Evaluation of Differences in Solubility in Organic Solvents of Softwood/Hardwood-Based Industrial Kraft Lignins Using Hansen Parameters and FTIR. Separations 2024, 11, 250. [Google Scholar] [CrossRef]
- Available online: https://d1wqtxts1xzle7.cloudfront.net/94752235/BioRes_15_4_8577_Ribeiro_LM_Solubility_Parameter_Eucalyptus_Kraft_Lignin_17761-libre.pdf?1669252252=&response-content-disposition=inline%3B+filename%3DSolubility_parameters_analysis_of_Eucaly.pdf&Expires=1734961531&Signature=Wn9Re1XY9c8iuZgJ03MNVoVR3ySoeGycekOD4oNoWUX2qd~oMRfvlrJYECxWgblhwhfUJju9yXtrDgUrCPi-bEgJXcOl7kIw3BbmvdRAOjLMqf7B7b~G7QNyuVMs8rIQAGatH3SmCHl5eiOScwQAQnxKf-r-2ns2oS0L3iFBppGA3WadfcXF6qs0H0t1ElbeDK~RwdMSVWdz6IuvlxqYj4WuhOJKFSgX8yQiKR9JVuZVtSQBRe-tIlEaUQHr3BuSk8eI-9LkSrO~9wlww8jES-9Xjm48hnDQEj1HmOY2XQicCooawUVj4-GoIKjZ7WwsClLch72RrYB-Vvxbi9Yr4Q__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 18 December 2024).
- Araújo, L.C.P.; Yamaji, F.M.; Lima, V.H.; Botaro, V.R. Kraft Lignin Fractionation by Organic Solvents: Correlation between Molar Mass and Higher Heating Value. Bioresour. Technol. 2020, 314, 123757. [Google Scholar] [CrossRef]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent Screening for the Fractionation of Industrial Kraft Lignin. Holzforschung 2016, 70, 11–20. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Vautard, F.; Meyer, H.M.; Messman, J.M.; Tolnai, B.; Naskar, A.K. Methanol Fractionation of Softwood Kraft Lignin: Impact on the Lignin Properties. ChemSusChem 2014, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bai, X.; Lusi, A.; Wan, C. High-Solid Lignocellulose Processing Enabled by Natural Deep Eutectic Solvent for Lignin Extraction and Industrially Relevant Production of Renewable Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 12205–12216. [Google Scholar] [CrossRef]
- Casas, A.; Alonso, M.V.; Oliet, M.; Rojo, E.; Rodríguez, F. FTIR Analysis of Lignin Regenerated from Pinus Radiata and Eucalyptus Globulus Woods Dissolved in Imidazolium-Based Ionic Liquids. J. Chem. Technol. Biotechnol. 2012, 87, 472–480. [Google Scholar] [CrossRef]
- Tabasso, S.; Grillo, G.; Carnaroglio, D.; Gaudino, E.C.; Cravotto, G. Microwave-Assisted γ-Valerolactone Production for Biomass Lignin Extraction: A Cascade Protocol. Molecules 2016, 21, 413. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Li, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. Effects of Organosolv and Ammonia Pretreatments on Lignin Properties and Its Inhibition for Enzymatic Hydrolysis. Green Chem. 2017, 19, 2006–2016. [Google Scholar] [CrossRef]
- Jiang, W.; Lyu, G.; Liu, Y.; Wang, C.; Chen, J.; Lucia, L.A. Quantitative Analyses of Lignin Hydrothermolysates from Subcritical Water and Water-Ethanol Systems. Ind. Eng. Chem. Res. 2014, 53, 10328–10334. [Google Scholar] [CrossRef]
- Riaz, A.; Verma, D.; Zeb, H.; Lee, J.H.; Kim, J.C.; Kwak, S.K.; Kim, J. Solvothermal Liquefaction of Alkali Lignin to Obtain a High Yield of Aromatic Monomers While Suppressing Solvent Consumption. Green Chem. 2018, 20, 4957–4974. [Google Scholar] [CrossRef]
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Beckham, G.T.; Román-Leshkov, Y. Flowthrough Reductive Catalytic Fractionation of Biomass. Joule 2017, 1, 613–622. [Google Scholar] [CrossRef]
- Cheah, Y.W.; Intakul, R.; Salam, M.A.; Sebastian, J.; Ho, P.H.; Arora, P.; Öhrman, O.; Creaser, D.; Olsson, L. Slurry Co-Hydroprocessing of Kraft Lignin and Pyrolysis Oil over Unsupported NiMoS Catalyst: A Strategy for Char Suppression. Chem. Eng. J. 2023, 475, 146056. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, P.; Wolcott, M.P.; Zhang, X.; Zhang, J. Partial Depolymerization of Enzymolysis Lignin via Mild Hydrogenolysis over Raney Nickel. Bioresour. Technol. 2014, 155, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Taki, G.; Islam, M.N.; Agarwal, A.; Jo, Y.-T.; Park, J.-H. Effects of Temperature and Salt Catalysts on Depolymerization of Kraft Lignin to Aromatic Phenolic Compounds. Energy Fuels 2019, 33, 6390–6404. [Google Scholar] [CrossRef]
- Kleinert, M.; Gasson, J.R.; Barth, T. Optimizing Solvolysis Conditions for Integrated Depolymerisation and Hydrodeoxygenation of Lignin to Produce Liquid Biofuel. J. Anal. Appl. Pyrolysis 2009, 85, 108–117. [Google Scholar] [CrossRef]
- Xu, W.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Depolymerization and Hydrodeoxygenation of Switchgrass Lignin with Formic Acid. ChemSusChem 2012, 5, 667–675. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Production of Polyols via Direct Hydrolysis of Kraft Lignin: Effect of Process Parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Kim, C.S.; Kim, Y.; Kim, J. High-Yield and High-Calorific Bio-Oil Production from Concentrated Sulfuric Acid Hydrolysis Lignin in Supercritical Ethanol. Fuel 2016, 172, 238–247. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Jensen, A.; Madsen, L.R.; Larsen, F.H.; Felby, C.; Jensen, A.D. Noncatalytic Direct Liquefaction of Biorefinery Lignin by Ethanol. Energy Fuels 2017, 31, 7223–7233. [Google Scholar] [CrossRef]
- Daniel, D.; Khachatryan, L.; Astete, C.; Asatryan, R.; Marculescu, C.; Boldor, D. Sulfur Contaminations Inhibit Depolymerization of Kraft Lignin. Bioresour. Technol. Rep. 2019, 8, 100341. [Google Scholar] [CrossRef]
- Rohde, V.; Böringer, S.; Tübke, B.; Adam, C.; Dahmen, N.; Schmiedl, D. Fractionation of Three Different Lignins by Thermal Separation Techniques—A Comparative Study. GCB Bioenergy 2019, 11, 206–217. [Google Scholar] [CrossRef]
- Singh, S.K.; Nandeshwar, K.; Ekhe, J.D. Thermochemical Lignin Depolymerization and Conversion to Aromatics in Subcritical Methanol: Effects of Catalytic Conditions. N. J. Chem. 2016, 40, 3677–3685. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of Organosolv Lignin to Aromatic Compounds over Cu-Doped Porous Metal Oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Li, H.; Song, G. Ru-Catalyzed Hydrogenolysis of Lignin: Base-Dependent Tunability of Monomeric Phenols and Mechanistic Study. ACS Catal. 2019, 9, 4054–4064. [Google Scholar] [CrossRef]



| RT (min) | Compound | Wt% of Dry Lignin | |||
|---|---|---|---|---|---|
| 260 °C | 280 °C | 300 °C | 320 °C | ||
| 13.19 | Guaiacol | 14.91 | 7.95 | 14.79 | 15.71 |
| 16.25 | 4-methylguaicol | 1.02 | 1.93 | 4.33 | 6.83 |
| 17.57 | 2-methoxy-4-vinylphenol | - | - | 0.76 | 2.28 |
| 18.67 | 4-ethylguaiacol | 3.15 | 5.41 | 10.46 | 10.46 |
| 20.79 | Eugenol | - | 0.75 | 0.73 | 0.93 |
| 21.04 | 4-proylguaiacol | - | 0.88 | 1.86 | 5.76 |
| 21.86 | Vanillin | 5.76 | 2.53 | 0.52 | 0.80 |
| 21.94 | Methyl guaiacol | 5.97 | 2.08 | - | - |
| 23.14 | Isoeugenol | 4.22 | 4.31 | 2.60 | 0.50 |
| 24.05 | Acetovanillone | 1.90 | 1.68 | 1.21 | - |
| 24.81 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl) | 3.50 | 3.28 | 1.56 | - |
| 26.29 | Benzene acetic acid, 4-hydroxy-3-methoxy | 1.63 | 2.98 | 2.35 | - |
| 26.58 | Ethyl vanillate | - | - | 0.57 | 0.90 |
| 27.82 | Homovanillic acid | 5.13 | 6.78 | 5.26 | 4.01 |
| 38.47 | 1,2-benzene diol-4-methyl | 2.06 | 1.76 | 1.08 | 0.98 |
| 41.08 | Gibberellin | 1.45 | 2.02 | 1.99 | 1.68 |
| 42.15 | 4,5-Dimethoxy-2-hydroxyacetophenone | 2.32 | 2.70 | 2.00 | 1.17 |
| 42.32 | Dimethyl 2,3-bis(phenylethynyl)fumarate | 1.34 | 1.95 | 1.66 | 0.87 |
| 42.79 | 4,5-Dihydroxy-3,6,7-trimethoxyflavone | 1.40 | 1.64 | 1.60 | 1.73 |
| 42.91 | Naphthalene, 1-methyl | 20.64 | 20.65 | 25.45 | 27.76 |
| 43.65 | 2,6-Dimethoxyphenol | 8.70 | 8.00 | 7.13 | 4.27 |
| 44.51 | 4-Propylguaiacol | - | 1.05 | 0.77 | - |
| 46.73 | Syringol | 0.63 | 2.90 | 1.27 | 0.45 |
| 47.75 | 4-Methoxy-3-(methoxymethyl)phenol | 8.93 | 11.62 | 3.64 | - |
| RT (min) | Compound | Wt% of Dry Lignin | |||
|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | ||
| 13.19 | Guaiacol | 6.98 | 7.95 | 5.67 | 15.86 |
| 16.25 | 4-Methylguaicol | 1.37 | 1.93 | 1.42 | 3.52 |
| 18.67 | 4-Ethylguaiacol | 3.75 | 5.41 | 4.65 | 9.64 |
| 20.79 | Eugenol | 0.76 | 0.75 | - | - |
| 21.04 | 4-Propylguaiacol | 0.58 | 0.88 | - | 1.72 |
| 21.86 | Vanillin | 3.56 | 2.53 | - | - |
| 21.94 | Methyl guaiacol | 3.23 | 2.08 | - | - |
| 23.14 | Isoeugenol | 5.05 | 4.31 | 2.42 | 2.55 |
| 24.05 | Acetovanillone | 2.03 | 1.68 | 1.88 | 1.62 |
| 24.81 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl) | 2.89 | 3.28 | 2.84 | 1.64 |
| 26.29 | Benzene acetic acid, 4-hydroxy-3-methoxy | 3.39 | 2.98 | 2.47 | 2.03 |
| 27.82 | Homovanillic acid | 6.72 | 6.78 | 7.78 | 6.49 |
| 38.47 | 1,2-Benzene diol-4-methyl | 1.69 | 1.76 | 1.73 | 1.51 |
| 41.08 | Gibberellin | 1.60 | 2.02 | 2.92 | 2.41 |
| 42.15 | 4,5-Dimethoxy-2-hydroxyacetophenone | 2.42 | 2.71 | 3.35 | 2.80 |
| 42.32 | Dimethyl 2,3-bis(phenylethynyl)fumarate | 1.38 | 1.95 | 2.18 | 1.92 |
| 42.79 | 4,5-Dihydroxy-3,6,7-trimethoxyflavone | 1.48 | 1.64 | 2.22 | 1.83 |
| 42.91 | Naphthalene, 1-methyl | 19.85 | 20.65 | 36.99 | 27.3 |
| 43.65 | 2,6-Dimethoxyphenol | 7.35 | 8.00 | 11.30 | 8.42 |
| 44.51 | 2-Methoxy-4-vinyl pheno | 0.923 | 1.05 | 1.303 | 0.89 |
| 46.73 | Syringol | 2.612 | 2.891 | - | - |
| 47.75 | 4-Methoxy-3-(methoxymethyl)phenol | 11.06 | 11.62 | 6.542 | 3.63 |
| Wavenumber (cm−1) | Characteristics |
|---|---|
| 3092 to 3627 | O–H groups |
| 2934 | C–H (aliphatic and aromatic groups) |
| 2851 | C–H (methoxy groups) |
| 1715 | C=O in unconjugated ketone, carbonyl, and ester groups) |
| 1606 | Aromatic ring (C=C) vibrations |
| 1508 | Aromatic ring (C=C) vibrations |
| 1253, 1141, and 1110 | G ring and C–O vibrations |
| 1029 | C–O vibrations in ether, acid, or ester groups |
| 804 and 743 | Para-substituted aromatic rings |
| Entry | Sample | Elemental Composition (%) | HHV (MJ/kg) * | ||||
|---|---|---|---|---|---|---|---|
| C (%) | H (%) | O (%) | N (%) | S (%) | |||
| 1 | Kraft lignin | 60.83 | 5.80 | 31.09 | 0.77 | 1.52 | 25.19 |
| 2 | Methanol-soluble lignin | 63.31 | 6.20 | 27.52 | 1.04 | 1.94 | 26.98 |
| 3 | Kraft lignin bio-oil 1 | 68.03 | 7.16 | 23.81 | 0.32 | 0.67 | 29.85 |
| 4 | Methanol-soluble lignin bio-oil 2 | 64.69 | 6.34 | 27.55 | 0.51 | 0.91 | 27.38 |
| 5 | Methanol-soluble lignin bio-oil 3 | 68.04 | 6.76 | 23.03 | 0.43 | 1.75 | 29.64 |
| 6 | Methanol-soluble lignin bio-oil 4 | 67.98 | 7.52 | 24.12 | 0.29 | 0.09 | 30.13 |
| 7 | Methanol-soluble lignin bio-oil 5 | 68.88 | 7.20 | 22.84 | 0.31 | 0.77 | 30.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Rana, M.; Park, J.-H. Catalyst-Free Depolymerization of Methanol-Fractionated Kraft Lignin to Aromatic Monomers in Supercritical Methanol. Energies 2024, 17, 6482. https://doi.org/10.3390/en17246482
Ghosh S, Rana M, Park J-H. Catalyst-Free Depolymerization of Methanol-Fractionated Kraft Lignin to Aromatic Monomers in Supercritical Methanol. Energies. 2024; 17(24):6482. https://doi.org/10.3390/en17246482
Chicago/Turabian StyleGhosh, Shubho, Masud Rana, and Jeong-Hun Park. 2024. "Catalyst-Free Depolymerization of Methanol-Fractionated Kraft Lignin to Aromatic Monomers in Supercritical Methanol" Energies 17, no. 24: 6482. https://doi.org/10.3390/en17246482
APA StyleGhosh, S., Rana, M., & Park, J.-H. (2024). Catalyst-Free Depolymerization of Methanol-Fractionated Kraft Lignin to Aromatic Monomers in Supercritical Methanol. Energies, 17(24), 6482. https://doi.org/10.3390/en17246482








