Abstract
Polyethylene terephthalate (PET) is one of the most used polymeric substances in production of packaging materials, fibers, textiles, coatings, and engineering materials. This paper elucidates the transport parameters of hydrogen gas through a PET membrane, which was selected to be a sufficiently permeable substrate for setting up an empirical strategy that aims at developing hydrogen barrier coatings. An examination of the structural degradation of PET by prolonged hydrogen exposure was performed. Hydrogen permeation tests were performed on a PET membrane with a thickness of 50 μm. To investigate the behavior of the material by prolonged hydrogen treatment, hydrogen-exposure experiments were carried out at a certain hydrogen pressure and time. Comparisons of the mechanical properties of the material were documented both before and after hydrogen exposure. A strong impact of comparatively transient hydrogen exposure on the mechanical and hydrogen transport properties of PET was observed. After 72 h of hydrogen exposure at 103 hPa and 300 K, the tensile strength decreased by 19%, the diffusion coefficients more than doubled, and material fracture behavior changed from ductile to distinctly brittle. This underlines the importance of developing effective hydrogen barrier coatings in case PET tubing is intended for use in hydrogen transport or storage.
1. Introduction
Today, in various industries, polymeric materials, known for their cost-effectiveness and versatile properties, have become ubiquitous. The hydrogen industry also identified interesting properties and already incorporated polymer-based coatings and pipelines crafted from these material class [1,2,3,4,5]. The hydrogen embrittlement in metallic materials is well recognized for its potential to cause issues, including corrosion, fractures, and safety-related concerns [6,7,8]. From this perspective the use of polymeric materials and barrier coatings can significantly reduce the risks associated with the structure degradation of materials, reduce costs and weight, and avoid the loss of hydrogen as fuel [9,10,11]. In contrast to metallic materials, this distinction arises from the physisorption of hydrogen in polymers and the absence of chemical interactions between hydrogen and the polymer matrix. However, during the prolongated permeation of hydrogen in polymeric materials (spec. at high pressures in hydrogen pipelines, tanks, and storage), there is a risk for the structural degradation of material caused by fatigue fracture [12]. Exemplarily, when designing tanks for storing media, e.g., vertical non-pressurized tanks made of thermoplastics for hydrogen storage, the effect of the stored medium on the polymer needs to be considered and reduction factors for characteristic mechanical properties are to be assessed [13]. All such phenomena increasingly emphasize the importance of barrier coatings in preventing the structural degradation of materials exposed to hydrogen.
Recently, different approaches for developing hydrogen barrier coatings have been presented [14,15]. Some approaches involve using carbides and silicates as protective coatings to reduce permeation, while others rely on oxide and metal coatings [16,17,18,19,20]. The choice of approach depends on the substrate and its stability with respect to the coating process. In general, metal and oxide barrier coatings exhibit lower transport properties compared to polymer materials. This is expected from the dissociation process of hydrogen molecules on metal surfaces and the diffusion in ionic or atomic form through the denser metal crystal lattice. Polymer materials exhibit more internal cavities, and a larger free volume is required for particle diffusion within the material. However, the development of hydrogen permeation barrier coatings using polymer-based materials may have several advantages such as a relatively easy coating process and the avoidance of high temperatures, compared with metal and oxide coatings. Such gas permeation barrier coatings based solely on polymeric materials for permeation resistance have already been successfully developed, e.g., Yang and al. reported the development of polymeric gas barrier films utilizing polyethylenimine (PEI) and polyacrylic acid (PAA) crosslinking [21]. This approach significantly reduces the material internal volume available for gas molecule permeation, effectively slowing oxygen permeation by several orders of magnitude. The development of gas barrier coatings based on PEI and modified graphene oxide (GO), as reported by Li, is also noteworthy [22]. We infer that durable barrier coatings may boost the use of polymeric materials in hydrogen technology, and we suggest that understanding hydrogen transport through polymers will support a targeted development of barrier coatings. The permeation through solid material sheets involves three stages: adsorption of the permeant on the contact surface, diffusion through the bulk material, and final desorption of the permeant on the opposite surface. When permeating through polymers, the gas saturates the material, penetrating the cavities and free spaces between the polymer chains, causing local structure changes. Repeated treatments result in an escalating degree of cracks and structural damages, leading to the formation of voids (“blisters”) in the polymer matrix, exhibiting various geometric shapes [23,24,25,26]. The degradation of the polymer structure itself under the influence of various factors is an undesirable process and typical for rubber seals and O-rings in diverse gas storage equipment [23].
A more detailed assessment of the gas diffusion process within the polymer matrix reveals that diffusion is not reliant on the gas pressure at the contact surface of the membrane but is influenced by the size and nature of the diffusing particles, the materials’ porosity, and the geometry of these pores (tortuosity). Sorption is an intrinsic material property also influenced by the presence of free volume between molecular chains. The semi-crystalline polymers exhibit superior barrier properties compared to amorphous polymers due to their non-absorbing crystalline phase [24,25,26]. However, the structural organization, the orientation of molecular chains within the material and at the adsorbing surface, plays a significant role in sorption properties as well. Nevertheless, the significant influencing factor is not merely the absence of diffusant sorption by polymer crystallites but rather the semi-crystalline morphology. The presence of crystalline sites within the material increases the tortuosity, thereby impending diffusion, influencing the sorption, and resulting in a heterogeneous distribution of the diffusant throughout the material [27,28]. Hydrogen usually has a higher diffusion and permeation coefficient compared to other gases, due to its small size, minor polarizability, and the absence of a dipole moment, which implies a lower accumulation potential in the material. However, the degradation of the polymer structure after hydrogen treatment is observed [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Models for predicting and characterizing these kinds of structural degradations are based mainly on theoretical concepts, molecular dynamics simulations, and several assumptions [33,34,35]. In essence, they elucidate the degradation of the polymer structure subjected to mechanical stress and strain elongation, use a mathematical idealization of the crack propagation geometry, as well as an empirical relationship between deformation and internal structural stress in the material.
This study is dedicated to examining the practical degradation of a PET structure induced by hydrogen exposure, with a primary emphasis on alterations in transport parameters resulting from structural modifications. A sample of semi-crystalline PET, known for its enhanced barrier properties, was utilized to examine the material’s behavior during hydrogen exposure and permeation. A key consideration in characterizing materials for the hydrogen industry includes transport parameters and structural stability. The work presented here is part of the authors’ strategy that aims to develop hydrogen barrier layers for versatile (substrate) materials. Thin PET membranes were selected as reference substrates that were expected to be permeable for hydrogen and allow an assessment of the hydrogen permeability with measurements over a few hours of time and facilitate revealing and differentiating the effects of distinct optimal barrier coatings in an empirical screening approach. In industrial applications, the thickness of polymer materials, such as in pipe walls, liners, or coatings, is notably greater than the thickness of specimen discussed in this paper. However, the extended service life at relatively high pressures is a characteristic of materials intended for industrial use. Thus, comprehending and assessing the degradation processes of polymeric material structure during prolonged hydrogen permeation holds paramount significance for achieving the defined objectives.
2. Materials and Methods
2.1. Material
A PET membrane (Bleher Folientechnik GmbH, (UST Umweltsensortechnik GmbH, Geratal, Germany) with a thickness of 50 μm was used for the experiments.
2.2. Hydrogen Permeation Measurement
The first methods for the practical determination of transport parameters by transmembrane gas transport were reported by Daynes and Barrer [36,37]. Over time, several methods based on adsorption and further diffusion of gas through the polymeric materials have been proposed and described [38,39,40,41,42,43,44,45]. Typically, in such experiments, a permeation cell is used, which provides a gradient of chemical potential on different surfaces of the specimen. Permeation proceeds by a solution–diffusion mechanism, and diffusion occurs in the direction opposite to the concentration gradient. Transport parameters—permeation coefficient, diffusion coefficient, and solubility constant—can be calculated based on the behavior of permeation. Experiments on hydrogen permeation through the studied materials were carried out using a permeation cell (Figure 1) equipped with a high-resolution hydrogen sensor (UST Umweltsensorik GmbH). The permeation cell was split into two chambers by the test specimen, where the permeant hydrogen gas (CANgas hydrogen 99.999%, Messer SE & Co. KGaA, Bad Soden am Taunus, Germany) flowed from the first chamber, a reservoir, with a constant partial pressure into the second chamber, the acceptor, from where it was removed by constant degassing through purging lines. This provided a gradient of chemical potential on different surfaces of specimens and a certain concentration profile of permeated hydrogen in the acceptor chamber, due to the controlled hydrogen permeation flux and acceptor degassing. The concentration profile in the acceptor allowed us to directly determine the permeation rate. Since the thickness of the specimen (50 μm) was significantly small compared to the permeation area (6.28 cm2), the transverse permeation of hydrogen was neglected. When the test specimen was fully saturated with a permeant, a steady-state diffusion profile was established, and the flux of gas passing through the specimen was equalized. To model hydrogen permeation, the resulting differential curve of the flux was converted into an integral curve, showing the change in the total amount of gas permeated through the specimen in a time unit. The initial conditions determined the zero concentration of hydrogen (C0) at the interface between the specimen surfaces at the start of the experiment, the change in the concentration of hydrogen due to the experiment, and the establishment of an equilibrium concentration (C∞) during prolonged permeation. At a certain pressure, the dependence of the equilibrium gas concentration in the test specimen was assumed to follow Henry’s law [46]:
where S represents the ambient pressure solubility, and p is the partial pressure of permeants in contact with one of the specimen surfaces. Considering the one-dimensional gas diffusion through the polymer membrane, the change in the concentration of the diffusant in the polymer matrix could be expressed in terms of Fick’s second law [46]:
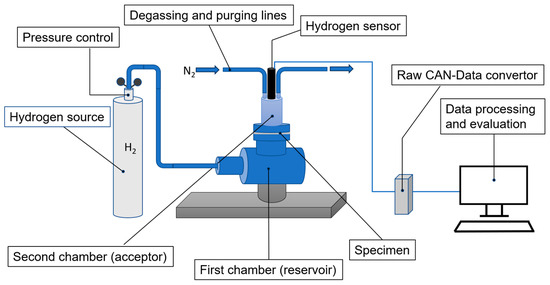
Figure 1.
Schematic representation of the differential method of hydrogen permeability measurement.
The solution of this partial differential equation of diffusion, proposed by Crank for the concentration profile evaluation can be written in the following form [47]:
where D and l represent the diffusion coefficient and specimen thickness, respectively, and n is an integer from one to infinity. The evaluation of the concentration profile allowed the formulation of the gas flux as well, using Fick’s first law [42]:
where A is a permeation area. By rearranging the variables, a general relation for the flux evaluation from the specimen over time could be written in the following form [42,43]:
where u = Dt/l2. The flux curve by non-steady-state diffusion has a typically asymmetrical sigmoidal shape, which is caused by the time-oriented achievement of saturation (or equilibrium concentration of diffusant) in the specimen. In the case of non-steady-state diffusion, the estimation of transport parameters can be achieved through several methodologies, including the finite-element method, conversion to a functional scale, and further analysis of breakthrough curves, and the application of the Einstein–Smoluchowski equation for the integral curve of permeation [42,47,48]:
where tlag represents the “lag-time”. When the steady-state diffusion is established, a constant flux of permeant J∞ is reached, from which the permeation coefficient can be calculated [42,49]:
where Δm expresses the total amount of gas permeated through the membrane in a unit of time Δt (Δt = t − tlag). The resulting parameters of the equilibrium concentration and solubility constant can be calculated from Equation (7).
2.3. Hydrogen Exposure Repetitions
To perform the hydrogen exposure repetitions on the test specimen, five cycles (12 h, 24 h, 36 h, 48 h, 72 h) of exposure in 99.999% hydrogen (CAN Messer® AG Schweiz, Lenzburg, Switzerland) were carried out at a partial pressure of 0.1 MPa and a temperature 300 K. Each cycle lasted twelve hours. After each cycle, we waited 24 h at atmospheric pressure for the complete degasification of the sample and equipment. After complete degassing, hydrogen permeation experiments were performed to re-determine the transport parameters.
2.4. Scanning Electron Microscopy
Scanning electron microscopy was carried out on a Helios Nanolab 600 machine (Thermo Fisher Scientific, Eindhoven, The Netherlands) with a resolution of about 1 nm. Prior to imaging, about 5 nm of Iridium was sputtered onto the sample surfaces to prevent charging. In addition, all images were acquired at 10 kV using a concentric back-scattered (CBS) detector to rule out any charging artefacts in the images.
2.5. Mechanical Analysis
The mechanical characterization of test specimens was performed via a Modular Force Stage (Linkam Scientific Instruments, Salfords, UK) at room temperature and a strain rate of 2 mm/min. The experiments utilized a flat sample with a rectangular geometry, featuring a length of 40 mm and a width of 10 mm.
2.6. ATR-FTIR Analysis
The experiments of infrared spectroscopy were carried out on Bruker Alpha II Platinum ATR Spectrometer, a diamond crystal with a reflection of 1, 32 scans, and a 2 cm−1 spectral resolution.
2.7. DSC Analysis
DSC measurements were performed using DSC Discovery (TA Instruments, New Castle, DE, USA). According to DIN EN ISO 11357-3 [50], two heating experiments were carried out with initial and exposed PET specimens. In increments of 10 K/min, the samples were heated from 323 to 573 K, isothermally maintained for 5 min at 573 K. After reverse cooling (10 K/min) and 5 min of exposure at 323 K, they were heated again at a rate of 10 K/min up to 573 K. In line with ISO 11357-3, a secondary heating procedure was employed to assess and quantify the heat capacity and crystallinity calculation.
3. Results and Discussion
To process the results of the permeation experiment, the results were normalized, and the original function u was determined using the scientific computation library (SciPy) in python by a 1D interpolation of the initial F(u) function. After the determination of the values of the u function, the curve was fitted using the linear least-squares method for functional scale (Figure 2a). The evaluated new values of u (fitting results) were used in the reconstruction of the normalized flux function (Figure 2b) as a functional scale for the Fourier form (Equation (5)). By performing a reverse denormalization of the reconstructed permeation curve, optimized differential curves for initial and exposed PET specimens were compiled (Figure 2d). Further, to determine the diffusion coefficient and solubility constant, the permeation curve was integrated, and subsequently, the time-lag method was applied on the integral curve (Figure 2c). The graph differential curve of hydrogen permeation exhibited an asymmetric sigmoid shape, suggesting a gradual saturation of the test specimen with hydrogen and the stabilization of the gas flow at the membrane outlet. The graph for the untreated PET specimen indicated that permeation reached a steady state around 3 × 103 s. The integral curve graph depicts the overall diffusant quantity that traversed the specimen in the experiment, along with the asymptotic line indicating a steady-state diffusion phase, used for determining the diffusion coefficient. The transport parameters calculated according to the expressions given in the experimental section are listed in Table 1. The lower graph in Figure 2c illustrates a notable alteration in permeation behavior upon varying the exposure of the test sample to hydrogen. It is worth mentioning that the saturation time of the specimen with hydrogen and the stabilization of the diffusant flux at the outlet membrane surface underwent changes. Longer sample exposure to hydrogen resulted in earlier saturation and transition to a steady-state diffusion. Also, the transition to the steady-state diffusion phase with prolonged exposure revealed a relatively smooth hydrogen flux curve. Moreover, the mathematical expressions derived from Fick’s laws in the experimental section employ an approach based on the phenomenological thermodynamics of irreversible processes. This method treats diffusion and transport as external phenomena, allows the determination of the diffusion coefficient in the space direction and time but disregards atomic processes and particle diffusion geometry [51,52]. Yet, as previously stated, diffusion is contingent upon the size of the diffusing particle, the material’s porosity, and the geometric characteristics of the pores and cavities facilitating the diffusion process. In line with this, a decrease in the size of pores and cavities within the material corresponds to increased storage potential (solubility) and decreased diffusion rate. Conversely, larger cavities within the material result in higher particle diffusion rates and reduced storage potential. To provide a more detailed explanation of this dependency, one can refer to Knudsen’s description of diffusion as an alternative [53,54]:
where Dm represents the diffusion coefficient in the medium, Dp the diffusion coefficient in pores, ϴ the porosity, τ the tortuosity, r the pore radius, and M the molar mass of the diffusant. The challenge in this methodology stems from the intricacy of accurately determining the complex geometry of pores or cavities within the material, which have irregular structures and cannot be described by simplified models. However, the empirical description suggests a direct relationship between the diffusion coefficient and escalating porosity of the material, as well as the adoption of a simplified pore geometry. The calculated transport parameters of hydrogen for PET membrane before and after exposure in Table 1 show an increase in the diffusion coefficient and a decrease in the solubility constant with prolonged hydrogen exposure. This outcome is interpreted to stem from an augmentation in material cavities following exposure, leading to a diminished accumulation potential. Consequently, the diffusant exhibits accelerated movement within the polymer matrix, against the concentration gradient. In our case, the permeation process could be conceptually classified into three stages: diffusion from the reservoir into the sample, internal diffusion within the sample from the contact surface to the opposite outlet surface, and diffusion from the outlet surface into the acceptor. In the assessment of transport parameters using Fick’s and Henry laws, the determination of the solubility constant relies on establishing the diffusant’s equilibrium concentration within the material, achieved after saturation at a specified partial pressure (initiating stationary diffusion through the material). Here, the equilibrium concentration (C∞) denotes the equilibrium between inflowing and outflowing diffusant within the test sample. While the material’s diffusion coefficient increases, less diffusant is retained, consequently lowering the equilibrium concentration in the sample. This, in turn, leads to a decrease in the effective solubility constant, as determined by the diffusant’s equilibrium concentration during the diffusion process.
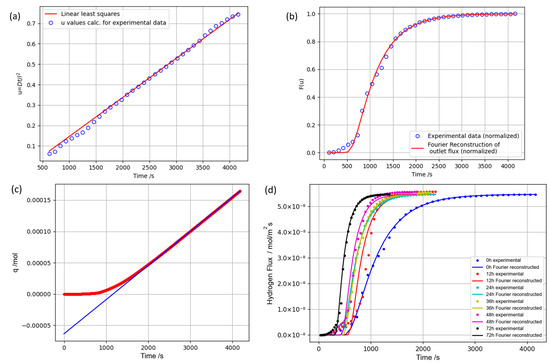
Figure 2.
The permeation graphs of hydrogen through a PET membrane: (a) functional curve of interpolated u values (u = Dt/l2, as detailed in the text), calculated for experimental data of the initial PET (not exposed) and fitting results for u by the linear least-squares method; (b) normalized experimental data of hydrogen permeation of the initial PET sample (differential curve) and Fourier reconstruction of the permeation curve using the fitting results of the functional curve u; (c) integral curve of permeated hydrogen amount through the initial PET sample, (d) experimental and reconstructed permeation curves of hydrogen through the initial PET sample and after hydrogen exposure.

Table 1.
Hydrogen transport parameters of the PET membrane before and after hydrogen exposure.
Significant changes were also observed in the behavior of the material during tensile stretching experiments (Figure 3). For the reproductive analysis of changes in mechanical properties, four analyses were performed (two initial samples and two samples after hydrogen exposure). After hydrogen exposure, the material showed a relative decrease in mechanical properties—Young’s modulus and ultimate tensile strength (Table 2). A decrease in the Young’s modulus and ultimate tensile strength indicated the structural degradation of the material after hydrogen exposure. While the formation of visible voids and “blisters” following hydrogen exposure did not occur, notable differences were observed via electron scanning microscopy of the material fracture regions by tensile strength. The fracture edges of both the initial PET sample and after 72 h of hydrogen exposure were primarily oriented either parallel or perpendicular to the tensile direction at the ultimate tensile strength. However, the stronger offset of neighboring perpendicular edges in the tensile direction (exemplified by blue arrows in Figure 4a,d) and the more frayed local arrangement of the fracture area in the thickness direction of the foil (exemplified by green circles in Figure 4a,d) differentiated the fracture image of the initial PET specimen from the exposed one. As is known, when mechanical stress is applied to a polymeric material, the ductile fracture can be caused by the ultimate tensile stress from the outer surface or an internal defect of the material. In that case, the ridges are usually directed at an angle to the fault surfaces, and with a rapid crack propagation, bulges or stretched films are formed. In the case of a brittle fracture of the polymer, due to the heterogeneity of the material, the rupture occurs in multiple areas, which are reunited later along the failure. As a result of the relatively low-tension stress and the brittle nature of the failure in areas with a structure irregularity, so called ladder-shaped patterns are formed [55]. Figure 4 presents scanning electron microscope images of the PET specimens’ fracture regions after the tensile strength experiments, both before and after hydrogen exposure. The images of the initial PET sample (Figure 4a–c) clearly show the ductile nature of the fracture, in which the material shows typical ridges and stretched films, whereas the sample after hydrogen exposure shows a more pronounced brittle fracture and typical ladder-shaped patterns (Figure 4d–f). There is also a significant difference in the structure of the sample surfaces before and after hydrogen exposure in the regions of rupture. The surface of the PET sample without hydrogen exposure in the regions of rupture is smooth, whereas the sample exposed to hydrogen shows a predominant roughness and fractures on its surface. Notably, such surface differences are evident only in the regions of rupture of the specimen following the tensile strength. The main factor influencing the nature of the rupture is the temperature effect, since plastic materials express brittle properties at low temperatures, but in the absence of a temperature difference in tensile strength experiment, the ductile–brittle transition in the rupture of the polymer can occur due to the structural alterations in material [56,57,58,59].
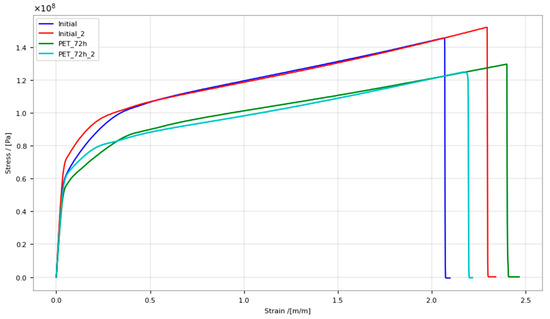
Figure 3.
Stress–strain curve of initial PET specimen and after 72 h hydrogen exposure.

Table 2.
Mechanical properties of the PET membrane before and after hydrogen exposure.
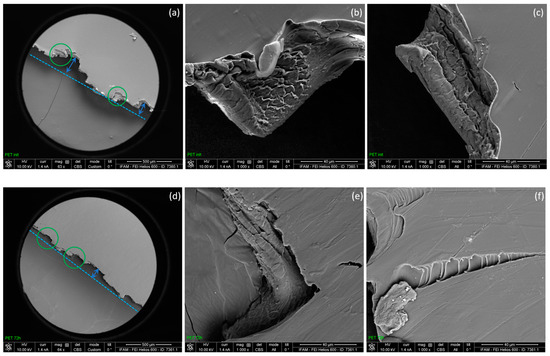
Figure 4.
Specimen fracture surface SEM micrographs (fracture edge and 1000× magnification) of PET after tension tests: initial (a–c) and after hydrogen exposure (d–f).
For a comprehensive understanding of structure degradation mechanisms, it is essential to highlight the nature of the PET structure. PET exhibits a linear, unbranched polar molecular configuration, facilitating robust intermolecular interactions among its constituent groups, thereby fostering the formation of a semi-crystalline macrostructure. For technical applications, PET materials with diverse crystallization levels are viable, with even highly amorphous PET (as in our case) potentially containing trace amounts of crystalline regions. The macrostructure of PET is primarily governed by dipole–dipole interactions among ester groups and π–π interactions among benzene moieties. While the molecular chains of the polymer can traverse both crystalline and amorphous regions, the terminals of these chains predominantly accumulate within the mobile amorphous domains. The PET structure incorporates ethylene glycol segments existing in two conformations: trans and gauche. Simultaneously, the gauche conformation induces an increased entanglement and disorder among molecular chains, attributed to additional interactions. S. J. M. Van Den Heuvel et al. reported a conformational transition from gauche to trans in PET yarns observed during mechanical stretching [60]. There is also a tendency toward the crystallization of amorphous PET induced by mechanical stretching between glass transition and melting temperatures [61,62]. These interactions and resulting structural characteristics play a crucial role in determining the mechanical properties of the material. Two methods, IR spectroscopy and DSC analysis, were chosen to study this kind of structural features caused by hydrogen exposure in PET. For a comprehensive analysis, three FTIR analyses were carried out for each sample, both before and after exposure to hydrogen (Figure 5a). To determine the content of certain conformations, the intensities defining these conformations were used [63,64,65]. As can be seen from Figure 5a, it is evident that there was no discernible pattern of intensities change within the specified conformations, nor was there any significant increase in the content of one conformation accompanied by a decrease in another following exposure to hydrogen compared to the initial sample. Here, it is necessary to highlight that the ATR-FTIR experiments were performed within a randomly chosen region of the sample surfaces, both before and after hydrogen exposure. While certain alterations in the peaks assigned to the C-H wagging vibrations of trans and gauche conformations were observable (1370 cm−1 and 1386 cm−1), deriving a definitive conclusion regarding conformational changes throughout the sample volume based on these ATR-FTIR experiments is unattainable. For a more detailed study of changes in crystallinity, DSC analyses of the initial sample and the sample after hydrogen exposure were performed. The following expression was used to determine the degree of crystallinity [63]:
where Xc is the weight fraction extent of crystallinity, ΔHm is the specific melting enthalpy, and ΔHm0 is the empirically calculated specific enthalpy of fusion of the theoretically 100% crystalline polymer. The calculated crystallinity degrees of the initial PET membrane and after hydrogen exposure are given in Table 3. To determine the specific enthalpy of melting according to the standard mentioned in the experimental section, the integral peak area of the heat flow rate at the melting temperature with a given basis line was used. The DSC study did not show any significant changes in the crystalline part of the sample before and after hydrogen exposure. Typically, a higher crystalline part content in a material leads to an increased ultimate tensile strength, due to the presence of additional interactions between molecular segments and oriented structure, which requires more energy to rupture [66,67]. In this case, as with the FTIR experiments, randomly selected regions of the material were used for the calorimetric analysis, before and after hydrogen exposure. Since the amorphous content prevailed in material, the heterogeneity of the material structure across different regions could not be excluded.
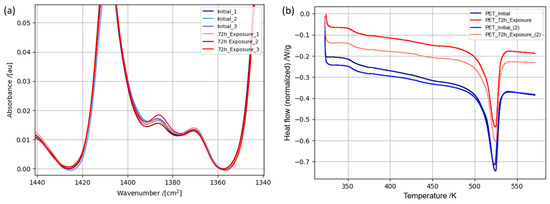
Figure 5.
ATR-FTIR spectra of initial PET specimen and after 72 h hydrogen exposure (a); DSC curves of initial PET specimen and after 72 h hydrogen exposure (b).

Table 3.
Calculated crystallinity degrees of PET specimen before and after 72 h hydrogen exposure.
Alterations in the material’s behavior under mechanical stress following the hydrogen exposure signified changes in its structural features. When examining the stress–strain curve of the test specimen, observable alterations became apparent at yield strength points and in plastic deformation regions. The emergence of localized voids in the material upon gas decompression impacted on its resilience and elasticity. Apparently, material saturation with a diffusant during hydrogen exposure triggered the uncoiling of molecular chains, facilitating the merging of resultant cavities, and inducing disruptions in the material’s structural integrity and local density. For a comprehensive understanding of the ongoing changes occurring in the structure, one can refer to the three-phase model of the PET structure (Figure 6a). According to the three-phase model, the physical structure of PET consists of a crystalline (CF), a rigid amorphous (RAF), and a mobile amorphous fraction (MAF) [69,70]. The content of mobile and rigid amorphous fractions in PET may vary depending on the material. Thus, Menczel determined a 45%/18% ratio of mobile and rigid amorphous fractions, and Arnoult 75%/17% in PET—defined as amorphous [71,72]. In this work, no studies were performed to determine the ratio between the mobile and rigid fractions of amorphous phase, since this was not the purpose of this work. As noted in the introduction part, the presence of crystallites within the polymer matrix enhances sorption capabilities due to the semi-crystalline morphology of the polymer and changes in the tortuosity compared to a confined amorphous material. The mobile fraction of the amorphous phase implies a highly chaotic arrangement of the terminals of polymer chains, the absence of any ordered structure, and, accordingly, a higher mobility of segments in the region. Additionally, it is worth considering the structural orientation of PET molecular chains in the amorphous regions, which is established during the material’s manufacturing process at a specific temperature. In gas permeation through a membrane, the driving force for diffusion is the chemical potential gradient, signifying a concentration gradient. A higher elevated concentration gradient corresponds to an increased diffusion rate. In initial experiments without hydrogen exposure, the equilibrium concentration of the diffusant in the sample was relatively high (Figure 6b), resulting in a comparatively lower difference in hydrogen concentration between the saturated sample and the reservoir (with a constant hydrogen partial pressure). This lower concentration difference correlated with a reduced diffusion coefficient, especially from the reservoir to the sample. In that case, the polymer’s molecular chains acted as a barrier, impending hydrogen diffusion and maintaining a high equilibrium concentration. Upon hydrogen exposure, we noticed a deterioration in the mechanical properties, an increase in the diffusion coefficient, a decrease in the equilibrium concentration of the diffusant in the polymer matrix, which indicated a faster transport of hydrogen through the material. It is likely that changes in the diffusion coefficient and equilibriums’ concentration (solubility constant) without changes in the crystalline phase of the material may indicate the process of a morphological perturbation, occurring specifically in the amorphous regions as a result of hydrogen sorption by exposure and permeation processes (Figure 6c). Hydrogen sorption may allow the overcoming of energy barriers in the physical polymer network of oriented PET and promote the mobility of chain segments, contributing to relaxation and an increase in conformational entropy, e.g., following a Rouse-like relaxation of chain segments as suggested by Oultache et al. for thermally induced mobility changes in chain segments in extended PET specimens [73]. In this context, molecular simulation studies are essential for a deeper understanding of sorption processes and morphological perturbation [74,75,76]. We suppose that this type of relaxation may microscopically lead to conformational changes, influence physical interactions between neighboring macromolecule segments, which macroscopically result in alterations in the transport and mechanical properties of the polymer. The permeation coefficient exhibited a rise corresponding to the product of the diffusion coefficient and solubility. When comparing the diffusion coefficient experimentally calculated for the initial sample and the sample after 72 h of hydrogen exposure, a notable increase in the diffusion coefficient by more than twofold was observed (Figure 6d). By combining Knudsen’s diffusion definition with the effective hydrogen diffusion coefficient experimentally defined for PET after 72 h of hydrogen exposure, it was assumed that an increase in the porosity of the material was equivalent to an increase in the diffusion coefficient. For achieving an in-depth understanding of the effects of hydrogen exposure on the structural/macromolecular arrangement, physical and conformational changes in the PET material, hydrogen exposure for significantly more than 72 h appears to be recommendable in order to potentially even further increase the magnitude of the observed changes in the diffusion coefficient and the solubility constant that were still ongoing between 48 and 72 h of exposure (Table 1). However, aiming at developing a material platform for the empirical design of barrier coatings, we expected to gather pathbreaking insights already within a few hours of hydrogen exposure, exemplarily when measuring permeation graphs (Figure 2) and observing the changed inset times of hydrogen flux as compared to uncoated specimens of PET membranes. In addition, as previously mentioned, composite and polymer materials are currently used in the construction of third- and fourth-generation hydrogen tanks [31]. In this context, PET can be evaluated as a cost-effective barrier coating substrate, capable of incorporating various pigments, nano-flakes, and other structures to enhance hydrogen permeation resistance.
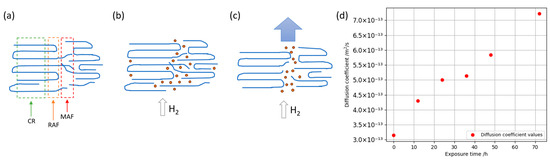
Figure 6.
Physical structure or PET (a) and illustration of the effect of polymer structure degradation on the diffusion of hydrogen through the specimen. A structure with a preliminary orientation of chains, segments, and diffusant flux passing through the amorphous phase of the polymer (b); the modified structure of the amorphous phase, which, as result of changes, provides a faster local diffusant flux and lower equilibrium concentration (c); graphical representation illustrating the change in the diffusion coefficient with respect to hydrogen exposure time (d).
Summarizing the results of our analyses before and after hydrogen exposure, the following changes in the PET specimen can be highlighted: an increase in the diffusion coefficient and a decrease in the equilibrium concentration of hydrogen during the permeation. There was also a change in the mechanical properties and rupture behavior of the material during the tensile strength experiments. The material exhibited altered mechanical properties characterized by a reduction in ultimate tensile strength following exposure to hydrogen. The SEM analysis clearly revealed significant changes in fracture behavior from the ductile to the brittle fracture after hydrogen exposure. The results of these studies indicated the degradation of the physical structure of PET after hydrogen exposure in the amorphous phase of the material. This in turn led to a decrease in mechanical properties and a change in the parameters of hydrogen transport through PET.
4. Conclusions
To investigate the degradation of the PET structure after hydrogen exposure and gas decompression, experiments involving hydrogen permeation, ATR-FTIR, DSC, and mechanical stretching were performed. The results of hydrogen permeation experiments revealed significant alterations in both the diffusion coefficient and solubility constant, suggesting an increase in cavities and pores within the material. Experiments on the mechanical stretching of the PET specimen showed a decrease in the modulus of elasticity and ultimate tensile strength after hydrogen exposure, in contrast to the original material. Scanning electron microscopy revealed noticeable differences in the nature of the material’s rupture in samples before and after hydrogen exposure as well. Thus, the initial sample (without hydrogen exposure) showed a ductile nature of the material fracture, whereas the sample after hydrogen exposure shows the brittle nature of the fracture at the ultimate tensile strength.
No special changes were observed in the FTIR and DSC analyses of the samples before and after hydrogen exposure. This can be explained by the localized specificity of these methods, which characterize only specific small regions of the material, where in turn, structural heterogeneities cannot be entirely excluded. These methods indicate changes in the crystallinity of the material, which represents only a small portion of the crystalline phase. Additionally, the crystalline phase itself does not absorb hydrogen; instead, hydrogen sorption and permeation occur through the material’s amorphous phase.
The experimental findings demonstrated significant alterations in the material’s properties attributed to structural modifications, with a focus on structural degradation induced by extended exposure to hydrogen. Despite performing the experiments using a relatively thin specimen, which may expedite earlier structural changes, the hydrogen exposure was also carried out at a relatively low pressure. We found a significant and readily measurable hydrogen permeability for PET membranes. Surprisingly, we found a strong impact of rather mild and short-term hydrogen contact on the mechanical and hydrogen permeation properties of PET. We discussed our findings in the context of available literature. As we expect a suitable barrier layer to decrease hydrogen flow by several orders of magnitude, we consider the selected PET membranes a reasonable reference substrate for our further developments.
Author Contributions
Conceptualization, E.A. and B.M.; Methodology, E.A. and P.-L.M.N.; Software, E.A.; Validation, T.F.; Formal analysis, B.M.; Investigation, E.A. and P.-L.M.N.; Resources, T.F.; Data curation, E.A. and P.-L.M.N.; Writing—original draft, E.A. and P.-L.M.N.; Writing—review & editing, T.F. and B.M.; Visualization, E.A.; Supervision, T.F.; Project admin-istration, E.A.; Funding acquisition, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated or analysed during this study are included in this manuscript. More detailed information on the datasets generated or analysed during the current study is available from the authors on reasonable request.
Acknowledgments
The authors would like to thank Karsten Thiel (Fraunhofer Institute for Manufacturing and Applied Materials Research IFAM, Bremen, Germany) for performing the scanning electron microscope (SEM) investigations. We confirm that all the individuals included in this section agree to the acknowledgement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Szczurek, A.; Tran, T.N.L.; Kubacki, J.; Gąsiorek, A.; Startek, K.; Mazur-Nowacka, A.; Dell’Anna, R.; Armellini, C.; Varas, S.; Carlotto, A.; et al. Mazur-Nowacka, Polyethylene terephtalate (PET) optical properties deterioration induced by temperature and protective effect of organically modified SiO2-TiO2 coating. Mat. Chem. Phys. 2023, 306, 128016. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, L.; Scholes, C.A.; Kentish, S.E. Crosslinked PVA based polymer coatings with shear-thinning behaviour and ultralow hydrogen permeability to prevent hydrogen embrittlement. Int. J. Hydrogen Energy 2024, 54, 947–954. [Google Scholar] [CrossRef]
- Barth, R.R.; Simmons, K.L.; San Marchi, C. Polymers for Hydrogen Infrastructure and Vehicle Fuel Systems: Applications, Properties, and Gap Analysis; Sandia Report; Pacific Northwest National Laboratory: Richland, WA, USA, 2013. [Google Scholar]
- Karki, S.; Hazarika, G.; Yadav, D.; Ingole, P.G. Polymeric membranes for industrial applications: Recent progress, challenges and perspectives. Desalination 2024, 573, 117200. [Google Scholar] [CrossRef]
- Condé-Wolter, J.; Ruf, M.G.; Liebsch, A.; Lebelt, T.; Koch, I.; Drechsler, K.; Gude, M. Hydrogen permeability of thermoplastic composites and liner systems for future mobility applications. Comp. Part A Appl. Sci. Manuf. 2023, 167, 107446. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Low Carbon Structural Steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Li, J.; Yu, B.; Wang, J.; Lyu, R.; Xi, Q. Research progress on corrosion and hydrogen embrittlement in hydrogen-natural gas pipeline transportation. Nat. Gas Ind. B 2023, 10, 570–582. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, Y.; Yang, C.; Zhang, Y.; Cong, C.; Yuan, Y. A novel dual-functional epoxy-based composite coating with exceptional anti-corrosion and enhanced hydrogen gas barrier properties. Chem. Eng. J. 2022, 449, 137876. [Google Scholar] [CrossRef]
- Wan, H.; Cheng, Z.; Song, D.; Chen, C. Preparation and performance study of waterbone epoxy resin/non-covalent modified graphene oxide hydrogen barrier coatings. Int. J. Hydrogen Energy 2024, 53, 218–228. [Google Scholar] [CrossRef]
- Laadel, N.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation barriers for hydrogen embrittlement prevention in metals- A review on mechanisms, materials suitability and efficiency. Int. J. Hydrogen Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Rueda, F.; Torres, J.P.; Machado, M.; Frontini, P.M.; Otegui, J.L. External pressure induced buckling collapse of high density polyethylene (HDPE) liners: FEM modelling and predictions. Thin-Walled Struct. 2015, 96, 56–63. [Google Scholar] [CrossRef]
- Beber, V.C.; Abels, G.; Hesebeck, O. Material Selection of Tanks for Storage and Transport of Liquid Organic Hydrogen Carriers: A Lightweight and Lifecycle Assessment Comparative Study of Metal, Polymer, and Composite Alternatives. Energy Technol. 2024, 2401297. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Oelrich, R.L.; Gates, R.O. Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review. Molecules 2022, 27, 6528. [Google Scholar] [CrossRef]
- Xiao, S.; Meng, X.; Shi, K.; Liu, L.; Wu, H.; Lian, W. Hydrogen permeation barriers and preparation techniques: A review. J. Vac. Sci. Technol. 2022, A40, 060803. [Google Scholar] [CrossRef]
- Shi, K.; Meng, X.; Xiao, S.; Chen, G.; Wu, H.; Zhou, C.; Jiang, S.; Chu, P.K. MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials 2021, 11, 2737. [Google Scholar] [CrossRef]
- Ke, N.; Huang, H.; Wang, F.; Dong, B.; Huang, A.; Hao, L.; Xu, X. Study on the hydrogen barrier performance of the SiOC coating. Int. J. Hydrogen Energy 2023, 48, 8286–8295. [Google Scholar] [CrossRef]
- Fite, S.; Zukerman, I.; Ben Shabat, A.; Barzilai, S. Hydrogen protection using CrN coatings: Experimental and theoretical study. Sur. Int. 2023, 37, 102629. [Google Scholar] [CrossRef]
- Lotkov, A.; Latushkina, S.; Kopylov, V.; Grishkov, V.; Baturin, A.; Girsova, N.; Zhapova, D.; Timkin, V. Nanostructured Coatings (Ti,Zr)N as a Barrier to Hydrogen Diffusion into Ti0.16Pd (wt.%) Alloy. Metals 2021, 11, 1332. [Google Scholar] [CrossRef]
- Zirbel, A.; Müller, M.; Ulrich, S. Plasma Deposition of Thin Hydrogen Barrier Coatings. 2024. Available online: https://ssrn.com/abstract=4959171 (accessed on 15 September 2024). [CrossRef]
- Yang, Y.H.; Haile, M.; Park, Y.T.; Malek, F.A.; Grunlan, J.C. Super gas barrier of all-polymer multilayer thin films. Macromolecules 2011, 44, 1450–1459. [Google Scholar] [CrossRef]
- Li, P.; Chen, K.; Zhao, L.; Zhang, H.; Sun, H.; Yang, X.; Kim, N.H.; Lee, J.H.; Niu, Q.J. Preparation of modified graphene oxide/polyethyleneimine film with enhanced hydrogen barrier properties by reactive layer-by-layer self-assembly. Composites 2019, 166, 663–672. [Google Scholar] [CrossRef]
- Hiroaki, O.; Hirotada, F.; Kiyoaki, O.; Shin, N. Influence of repetitions of the high-pressure hydrogen gas exposure on the internal damage quantity of high-density polyethylene evaluated by transmitted light digital image. Int. J. Hydrogen Energy 2019, 44, 23303–23319. [Google Scholar] [CrossRef]
- Lin, J.; Shenogin, S.; Nazarenko, S. Oxygen solubility and specific volume of rigid amorphous fraction in semicrystalline poly(ethylene terephtalate). Polymer 2002, 43, 4733–4743. [Google Scholar] [CrossRef]
- Michaels, A.S.; Vieth, W.R.; Barrie, J.J. Diffusion of Gases in Polyethylene Terephtalate. J. Appl. Phys. 1963, 34, 13–20. [Google Scholar] [CrossRef]
- Sekelik, D.J.; Stepanov, E.V.; Nazarenko, S.; Schiraldi, D.; Hiltner, A.; Baer, E. Oxygen barrier properties of crystallized and talc-filled poly(ethylene terephatalate). J. Polym. Sci. 1999, 37, 847. [Google Scholar] [CrossRef]
- Minelli, M.; Baschetti, M.G.; Doghieri, F. A comprehensive model for mass transport properties in nanocomposites. J. Membr. Sci. 2011, 381, 10–20. [Google Scholar] [CrossRef]
- Chen, X.; Papathanosiou, T.D. Barrier Properties of Flake-Filled Membranes: Review and Numerical Evaluation. J. Plast. Film Sheeting 2007, 23, 319–345. [Google Scholar] [CrossRef]
- Federico, R.; José Luis, O.; Patricia, F. Numerical tool to model collapse of polymeric liners in pipelines. Eng. Fail. Anal. 2012, 20, 25–34. [Google Scholar] [CrossRef]
- Bo, K.; Feng, H.; Jiang, Y.; Deng, G.; Wang, D.; Zhang, Y. Study of blister phenomena on polymer liner of type IV hydrogen storage cylinders. Int. J. Hydrogen Energy 2024, 54, 922–936. [Google Scholar] [CrossRef]
- Kis, D.I.; Kókai, E. A review on the factors of liner collapse in type IV hydrogen storage vessels. Int. J. Hydrogen Energy 2024, 50, 236–253. [Google Scholar] [CrossRef]
- Koga, A.; Yamabe, T.; Sato, H.; Uchida, K.; Nakayama, J.; Yamabe, J.; Nishimura, S. A Visualizing Study of Blister Initiation Behavior by Gas Decompression. Tribol Online 2013, 8, 68–75. [Google Scholar] [CrossRef]
- Persson, N. Fracture of poylmers. J. Chem. Phys. 1999, 110, 19. [Google Scholar] [CrossRef]
- Langer, J.S. Models of crack propagation. Phys. Rev. A 1992, 46, 3123. [Google Scholar] [CrossRef] [PubMed]
- Sixou, B. Molecular dynamics simulation of the first stages of the cavitation process in amorphous polymers. Mol. Simul. 2007, 33, 965–973. [Google Scholar] [CrossRef][Green Version]
- Daynes, H.A. The process of diffusion through a rubber membrane. Proc. R. Soc. London 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Barrer, R.M.; Rideal, R.M. Permeation, diffusion and solution of gases in organic polymers. Trans. Faraday Soc. 1939, 35, 628–643. [Google Scholar] [CrossRef]
- Brubaker, W.; Kammermeyer, K. Flow of Gases through Plastic Membranes. Ind. Eng. Chem. 1953, 45, 1148–1152. Available online: https://pubs.acs.org/doi/abs/10.1021/ie50521a069 (accessed on 15 September 2024). [CrossRef]
- Fraga, S.C.; Monteleone, M.; Lanc, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnacek, K.; Friess, K.; Carta, M.; McKeown, N.B.; et al. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Kim, K.T. Evaluation of hydrogen permeation characteristics in rubbery polymers. J. App. Phys. 2021, 21, 43–49. [Google Scholar] [CrossRef]
- Humpenoder, J. Gas Permeation of Fibre Reinforced Plastics. Cryogenics 1998, 38, 1. Available online: https://api.semanticscholar.org/CorpusID:119536134 (accessed on 15 September 2024). [CrossRef]
- Beckman, I.N.; Syrtsova, D.A.; Shalygin, M.G.; Kandasamy, P.; Teplyakov, V.V. Transmembrane gas transfer: Mathematics of diffusion and experimental practice. J. Membr. Sci. 2020, 601, 117737. [Google Scholar] [CrossRef]
- Zafra, A.; Harris, Z.; Korec, E.; Martínez-Pañeda, E. On the relative efficacy of electropermeation and isothermal desorption approaches for measuring hydrogen diffusivity. Int. J. Hydrogen Energy 2023, 48, 1218–1233. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C. Research on hydrogen permeability of polyamide 6 as liner material for type IV hydrogen storage tank. Int. J. Hydrogen Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-pressure gaseous hydrogen permeation test method -property of polymeric materials for high-pressure hydrogen devices (1)-. Int. J. Hydrogen Energy 2020, 45, 29082–29094. [Google Scholar] [CrossRef]
- Baehr, H.D.; Stephan, K. Wärme- und Stoffübertragung, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–273. ISBN 978-3-642-36557-7. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Claredon Press: Oxford, UK, 1975; pp. 47–61. ISBN 0-19-853344-6. [Google Scholar]
- Mehrer, H. Diffusion in Solids; Springer: Berlin/Heidelberg, Germany, 2007; pp. 59–60. ISBN 978-3-540-71486-6. [Google Scholar]
- Extrand, W.; Monson, L. Gas permeation resistance of a perfluoroalkoxy-tetrafluoroethylene copolymer. J. Appl. Polym. Sci. 2006, 100, 2122–2125. [Google Scholar] [CrossRef]
- DIN EN ISO 11357-3-2018-07; Determination of Temperature and Enthalpy of Melting and Crystallization (ISO 11357-3:2018). German Version EN ISO 11357-3:2018. DIN Media: Berlin, Germany, 2018. Available online: https://www.dinmedia.de/de/norm/din-en-iso-11357-3/282639092 (accessed on 2 January 2024).
- Onsager, L. Reciprocal Relations in Irreversible Processes. Phys. Rev. 1931, 37, 405. [Google Scholar] [CrossRef]
- Agren, J. The Onsager Reciprocity Relations Revisited. J. Phase Equil. Diff. 2022, 43, 640–647. [Google Scholar] [CrossRef]
- Mason, E.A.; Malinauskas, A.P. Gas Transport in Porous Media: The Dusty-Gas Model; Elsevier: Amsterdam, The Netherlands, 1983; p. 13. ISBN 10-0444421904. [Google Scholar]
- Veldsink, J.W.; Van Damme, R.M.J.; Versteeg, G.F.; Van Swaaij, W.P.M. The use of the dusty-gas model for the description of mass transport with chemical reaction in porous media. Chem. Eng. J. 1997, 57, 115–125. [Google Scholar] [CrossRef]
- Sangyoon, P.; Sarinthip, T.; Hojun, S.; Youngsoo, L.; Guman, T.; Jongchul, S. PET/Bio-Based Terpolyester Blends with High Dimensional Thermal Stability. Polymers 2021, 13, 728. [Google Scholar] [CrossRef]
- Schmidt, P.F. Praxis der Rasterelektronenmikroskopie und Mikrobereichsanalyse; Renningen-Malmsheim Expert-Verlag: Tübingen, Germany, 1994; pp. 585–588. ISBN 3-8169-1038-6. [Google Scholar]
- Matsushige, K.; Radcliffe, S.V.; Baer, E. The pressure and temperature effects on brittle-to-ductile transition in PS and PMMA. J. Appl. Polym. Sci. 1976, 20, 1853. [Google Scholar] [CrossRef]
- Jang, B.Z.; Uhlman, D.R.; Vander Sande, J.B. Ductile-brittle transition in polymers. J. Appl. Polym. Sci. 1984, 29, 3409–3420. [Google Scholar] [CrossRef]
- Hocker, S.J.A.; Kim, W.T.; Schniepp, H.C.; Krnbuehl, D.E. Polymer crystallinity and the ductile to brittle transition. Polymer 2018, 158, 72–76. [Google Scholar] [CrossRef]
- Van Den Heuvel, C.J.M.; Heuvel, H.M.; Faassen, W.A.; Veurink, J.; Lucas, L.J. Molecular changes of PET yarns during stretching measured with rheo-optical infrared spectroscopy and other techniques. J. App. Pol. Sci. 1993, 49, 925–934. [Google Scholar] [CrossRef]
- Elsner, P.; Eyerer, P.; Hirth, P.; Hrsg, T. Kunststoffe—Eigenschaften und Anwendungen, 7th ed.; Springer: Berlin, Germany, 2007; pp. 1057–1067. ISBN 978-3-540-72400-1. [Google Scholar]
- Haji, R.S. Rahbar, Structure evolution and mechanical behavior of poly(ethylene terephtalate) fibers drawn at different number of drawing stages. Chem. Ind. Chem. Eng. 2012, 18, 233–243. [Google Scholar] [CrossRef]
- Dieval, F.; Khoffi, F.; Mir, R.; Chaouch, W.; Nouen, D.L.; Chakfe, N.; Durand, B. Long-Term Biostability of PET Vascular Prostheses. Int. J. Pol. Sci. 2012, 2012, 646578. [Google Scholar] [CrossRef]
- Perret, E.; Sharma, K.; Braun, O.; Tritsch, S.; Muff, R.; Hufenus, R. 2D Raman, ATR-FTIR, WAXD, SAXS and DSC data of PET mono- and PET/PA6 bicomponent filaments. Data Brief 2021, 38, 107416. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.H. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Kaisersberger, E.; Knappe, S.; Möhler, H. TA for Polymer Engineering DSC-TG-DMA. In NETZSCH Annual for Science and Industry; TGA-DSC-DMA: Würzburg, Germany, 1993; Volume 2, pp. 13–14. [Google Scholar]
- Kale, R.D.; Banerjee, A.; Katre, G. Dyeing of polyester and polyamide at low temperature using solvent crazing technique. Fib. Pol. 2015, 16, 54–56. [Google Scholar] [CrossRef]
- Teli, M.D.; Kale, R.D. Polyester nanocomposite fibers with improved flame retardancy and thermal stability. Pol. Eng. Sci. 2012, 52, 1148–1154. [Google Scholar] [CrossRef]
- Menczel, J.; Wunderlich, B. Heat capacity hysteresis of semicrystalline macromolecular glasses. J. Pol. Sci. Pol. Lett. Ed. 1981, 19, 261–264. [Google Scholar] [CrossRef]
- Heidrich, D.; Gehde, M. The 3-Phase Structure of Polyesters (PBT, PET) after Isothermal and Non-Isothermal Crystallization. Polymers 2022, 14, 793. [Google Scholar] [CrossRef] [PubMed]
- Menczel, J.D. The rigid amorphous fraction in semicrystalline macromolecules. J. Therm. Anal. Calorim. 2011, 106, 7–24. [Google Scholar] [CrossRef]
- Arnoult, M.; Dargent, E.; Mano, J.F. Mobile amorphous phase fragility in semi-crystalline polymers: Comparison of PET and PLLA. Polymer 2007, 48, 1012–1019. [Google Scholar] [CrossRef]
- Oultache, A.K.; Kong, X.; Pellerin, C.; Brisson, J.; Pezolet, M.; Prud’homme, R.E. Orientation and relaxation of orientation of amorphous poly(ethylene terephthalate). Polymer 2001, 42, 9051–9058. [Google Scholar] [CrossRef]
- Atiq, O.; Ricci, E.; Bashcetti, M.G.; Grazia De Angelis, M. Multi-scale modeling of gas solubility in semi-crystalline polymers: Bridging Molecular Dynamics with Lattice Fluid. Fluid Phase Eq. 2023, 570, 113798. [Google Scholar] [CrossRef]
- Atiq, O.; Ricci, E.; Baschetti, M.G.; Grazia De Angelis, M. Molecular Simulations of Hydrogen Sorption in Semicrystalline High-Density Polyethylene: The Impact of the Surface Fraction of Tie-Chains. J. Phys. Chem. 2024, 128, 2799–2810. [Google Scholar] [CrossRef]
- Stalker, M.R.; Grant, J.; Yong, C.W.; Ohene-Yeboah, L.A.; Mays, T.J.; Parker, S.C. Molecular simulation of hydrogen storage and tansport in cellulose. Mol. Sim. 2021, 43, 170–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).