A Review of Renewable Energy Technologies in Municipal Wastewater Treatment Plants (WWTPs)
Abstract
1. Introduction
2. WWTP Overview
Sewage Sludge
3. Energy Resources in WWTP
3.1. Site-Specific Sources
3.1.1. Anaerobic Digestion
3.1.2. Thermochemical Processes
- (a)
- Combustion
- (b)
- Gasification
- (c)
- Pyrolysis
- (d)
- Hydrothermal treatment
- (e)
- Supercritical water
| Ref. | Sewage Sludge | Feedstock Characteristics | Operation Parameters | Generated Products |
|---|---|---|---|---|
| [88] | DSS | HHV 13.5 MJ/kg dry, 8.6% MC, 51.8% VM, 43.1% ash. UA: 51% , 7.3% , 7.9% , 2.05% , and 31.8% . | 100 FBR, DSS feeding rate of 9–16 kg/h, temperature 790–815 °C, air flow 11–15 m3/h, | Syngas LHV 3.6–4.5 MJ/m3 dry, gas composition includes 4.6–6.6% , 8.4–10.2% , 7.3–9.9% , 15.2–16.5% and other gases. |
| [89] | DSS + sp (mix ST) | HHV 13.2 MJ/kg dry, 29.9% MC, 67.7% VM, 9.8% ash. UA: 45.2% , 5.8% , 1.2% , 0.4% , and 37.4% . | 100 LTCFB reactor, 45 kg of dry SS gasified for 28 h, temperature 500–750 °C, Air eq. ratio of 0.2–0.3 | Syngas LHV and HHV as 1.76 and 1.88 MJ/m3 dry. Gas composition includes 2.5% , 1.6% , 8% , 18% and other gases. |
| Dry SS + sp (BJ) | HHV 14.8 MJ/kg dry, 12.5% MC, 66.5% VM, 13.3% ash. UA: 43.5% , 4.2% , 1.2% , 0.4% , and 35.7% . | 6 LTCFB reactor, 7.5 ton of dry SS gasified for 30 h under 500–750 °C, Air eq. ratio of 0.3 | Syngas LHV and HHV as 3.84 and 4.2 MJ/m3 dry. Gas composition includes 4.2% , 9% , 11.8% , 19.4% and other gases. | |
| Dry SS (SLU BJ) | HHV 11.4 MJ/kg dry, 12.5% MC, 42.7% VM, 43.1% ash. UA: 27.6% , 4.3% , 3.9% , 0.8% , and 19.8% . | 100 LTCFB reactor, 240 kg of dry SS gasified for 14 h, temperature 500–750 °C, Air eq. ratio of 0.2–0.3 | Syngas LHV and HHV as 1.88 and 2.05 MJ/m3 dry. Gas composition includes 2.6% , 5% of , 4.5% , 18.5% and other gases. | |
| Dry SS (SLU RA) | HHV 12.2 MJ/kg dry, 4.6% MC, 43.1% VM, 43.8% ash. UA: 28.7% , 4.2% , 3.9% , 1.3% , and 18.1% . | 100 LTCFB reactor, 800 kg of dry SS gasified for 40 h under 600–750 °C, Air eq. ratio of 0.2–0.3 | Syngas LHV and HHV as 1.96 and 2.16 MJ/m3 dry. Gas composition includes 2.4% , 6.6% , 4.2% , 17% and other gases. | |
| [90] | SS | SS: HHV 2.84 MJ/kg dry, 80.1% MC, 9.78% VM, 8.3% ash. UA: 6.27% , 1.1% , 0.77% , 0.3% , 3.2% . | Fluidised-bed gasifier, temperature of 784–822 °C with 0.3 air:fuel ratio. SS feeding rate of 25 kg/min, | Syngas LHV 2.2–2.6 MJ/m3. Gas composition: 2.9–3.4% , 3.3–3.5% , 5.6–6.9% , 23.2–27.8% . Gas yield: 28.5 m3/min, 46.6–53% CGE. |
| SS and PMS | PMS characteristics: HHV 4.25 MJ/kg dry, 51.4% MC, 29.9% VM, 18.33% ash. UA: 13.9% , 2.03% , 0.42% , 0.15% , and 13.8% . | Fluidized-bed gasifier, 1:1/1:2 SS:PMS mix ratios at 816–858 °C and 0.3 air:fuel ratio. SS feeding rate of 8.3–12.5 kg/min, and 16.7–12.5 kg/min for PMS. | Syngas LHV 1.7–2.42 MJ/m3 dry. Gas composition: 2.6–3.65% of , 2.6–4.13% of , 3.95–4.7% of , 17.4–25.42% of and other gases. Gas yield of 29.4 m3/min and CGE around 45.6–61.6%. | |
| [91] | Dry SS | 6.5% MC, 48.1% VM, 47.6% ash, 4.3% fc. UA: 51% , 6.9% , 7.5% , 2.4% , and 32% . | 20 kW bubbling fluidised bed reactor, temperature of 850 °C, air ratio of 0.25, fuel mass flow of 7 kg/h. | Syngas yield of 1.3 m3/kg. Gas composition in fraction mole: 6% of , 40% of , 20% of , 32% of and other gases. |
| [92] | Dry SS | 6.8% MC, 38% VM, 50% ash, 12% fc. UA: 29.7% , 4.3% , 4.6% , 1.4% , 59.9% . 11.73 MJ/kg HHV. | 20 kW reactor operating for 12 h/day at 20 kg/h feeding rate (max of 240 kg/day) at 1000–1150 °C. | Gas composition in fraction mole: 1.2% , 23.3% , 18.5% , 13.4% and 43.6% . 1.2 kg SS could produce up to 1 kWh |
| [93] | Dry SS | LHV 9.2 MJ/kg dry, 7.8% MC, 47.8% VM, 47.5% ash. UA: 48.8% , 7.4% , 7.1% , 2.3% , and 34.2% . | 20 kW fluidised bed reactor at 800 °C. Parameters: 2.7 kg/h steam, 3.6–5.4 kg/h fuel, 1.5 mol/mol steam:carbon. | Gas yield: 0.85 m3/kg (800 °C), 0.4 m3/kg (710 °C). CGE: 60% (800 °C), 45% (710 °C). Gas composition in m3/m3: 0.1 , 0.46 , 0.12 , 0.3 . |
| [94] | Dry SS | Raw SS: HHV 10.98 MJ/kg dry, 3.2% fc, 55% VM, 41.8% ash. UA: 21.86% , 3.4% , 3.8% , 0.64% , 28.5% . | Reactor at 700–900 °C and 1–60 min reaction. SS:SD mix ratios of 1:0, 1:3, 1:1, 3:1, and 0:1. SS upgraded via HTC. | At 900 °C, syngas yield was 0.93 m3/kg, LHV 5.62 MJ/kg dry and 67% efficiency. Gas composition: 2% , 5% , 35% and 58% . |
| Dry SS and SD | SD-SS: HHV 12–17 MJ/kg, 13–21% fc, 46–65% VM, 13–41% ash. A total of 33–49% , 3–5% , 2.5% , 0.5% , 19–30% | 130 W and 20 kHz (ultrasound) and 180 W (ozonation) applied to a 0.2 L of sewage sludge. | At 900 °C and 3:1 SS:SD ratio, syngas yield was 1.04 m3/kg, LHV 8.15 MJ/kg dry and 77.7% efficiency. Gas content: 7% , 8% , 45% , 40% . | |
| [95] | SS | 64.2% VM. UA: 41.2% , 5.2% , 3.2% , 20.7% , and calorific value of 14.1 MJ/kg. | Temperature around 750–850 °C, SS mass rate around 170–260 g/h. | Gas yield was 0.45–1.13 m3/kg (20–23% and 4–13% ), LHV was 6.5–9.7 MJ/m3. Biochar HHV up to 15 MJ/kg |
| [96] | Dry SS | LHV 13.2 MJ/kg, 7.1% MC, 51.53% VM, 4% fc, 37.4% ash. UA: 31.3% , 4.56% , 4.72% , 1.3% , 20.7% . | Fluidized bed reactor at 813–817 °C, 60–260 min reaction time, and usage of additives (AC, Ni, Fe) | Syngas LHV 5.04–6.52 MJ/m3 dry, 67.5–88.87% CGE. Gas content: 4–6.5% , 13.4–28.13% , 10–13.4% , 9.4–14.5% , 44–51.7% and others. |
| [97] | Dry SS | LHV 13.1 MJ/kg dry, 8.7% MC, 51.3% OM, 41.7% ash. UA: 29.5% , 4.9% , 4.1% , 1.6% , and 15.0% . | Fluidised bed gasified with 0.3 air: fuel ratio and 800 °C operating temperature. A total of 1.2–3.7 g/min SS feeding rate, and use Do as catalyst (10% mix with sludge) | Without De: Syngas LHV 2.9–3.6 MJ/m3, 35.7–43.2% CGE of, gas yield 2.7–3 m3/kg. Gas content: 2.3–3.3% , 8.5–10.3% , 6.1–9% , 13.2–13.9% . With De: Syngas LHV 2.9–3.9 MJ/m3, 35–49% CGE, gas yield 2.8–3.2 m3/kg. Gas content: 2.2–3.2% , 9.6–14% , 5.3–9.7% , 12.6–15% . |
| [98] | Dry SS | LHV 17 MJ/kg dry, 5.3% MC, 61.56% VM, 26.14% ash, 7% fc. UA: 39.5% , 5.8% , 5.3% , 0.9% , 24.4% . | 2-stage gasifier in series. A total of 2 kg/h feeding rate of, and use additives (AC, ). A 0.25 eq. ratio and 785–820 °C. | 52.5–66% syngas, 19.2–23% char, 14.3–20.3% condensate liquid and tar. Syngas LHV was 9.2–11.7 MJ/m3 dry and 51.7–80.1% CGE. Gas content: 7.2–8.5% , 28–52.2% , 15.9–19.3% , 20–32.4% and 3.8–8.9% . |
| [99] | DSS | SS Characteristics: LHV 14.26 MJ/kg dry, 18.75% MC, 33.4% VM, 11.6% ash, 36.3% fc. UA: 45.55% , 6.6% , 1.1% , 1.2% , and 33.9% . | 2-stage gasifier (fluidized bed and tar-cracking reactors) connected in series. Operating temperature of 800 °C, air flow rate 13–17 L/min, operating time 60 min. | 65.5–75.3% syngas, 15–22.4% char, 9% condensate liquid and tar. Syngas LHV of 5.35–6.1 MJ/m3 dry and 67–92.4% CGE. Gas composition: 4.5–5.4% , 14.6–22.6% , 9.7–12.4% , 10.64–10.9 and 51.6–54.5% . |
| DSS and coal | Coal Characteristics: 1% MC, 22.5% VM, 17.8% ash, 64.95% fc. UA: 78.5% , 0.6% , 0.43% , 0.4% , and 2.32% . Coal:DSS mix ratio of 70:30, 50:50, and 30:70. | 74% syngas, 12.5–13.7% char, 11.8–13.3% condensate liquid and tar. Syngas LHV of 5.1–5.4 MJ/m3 dry, 80.6–84.3% CGE. Gas composition: 3.8–4% , 24–26.6% , 9.46–10.4% , 11.12–12.5% and 46.2–50.8% . | ||
| [100] | Dry SS | LHV 15.1 MJ/kg dry, 8.2% MC, 56.9% VM, 30.3% ash, 4.6% fc. UA: 39.8% , 6.4% , 5.6% , 1.2% , and 24.7% . | 2-stage gasifier (fluidized bed and tar-cracking reactors) connected in series, 760–815 °C temperature, 90–100 min reaction, and use of AC as additive. | 68.6–76.9% syngas, 12–19.5% char, 6.2–20% condensate liquid and tar. Syngas LHV of 5.4–7.5 MJ/m3 dry. Gas composition: 2.7–7% , 9.6–34.1% , 9.2–17.2% , 6.5–14.6 , 38.4–64.4% and other elements. |
| [101] | Dry SS | LHV 14.1 MJ/kg dry, 4.7% MC, 51.3% VM, 24.1% ash. UA: 39.46% , 5.8% , 5.35% , 0.9% , and 24.4% . | 2-stage gasifier, 13 L/min ratio, and 785–810 °C. A 10.4–16.6 g/min feeding rate for 75–220 min, and used additives. | Syngas LHV was 5.65–7.1 MJ/m3 dry. Gas content: 3.2–5.8% , 11.8–31.3% , 9.1–18.4% , 7.6–14.7% of , 39.5–54.4% of and other elements. |
| Ref. | Sludge Collection | Feedstock Characteristics | Operation Parameters | Generated Products |
|---|---|---|---|---|
| [102] | WWTP in Medellin, Colombia | 6.56 MJ/kg dry HHV, 6.1% MC, 27.24% VM, 3.3% fc, 63.4% ash. UA: 12.8% , 1.74% , 1.2% , 0.55% , 16.22% . | Fluidised bed reactor operated at an atm pressure. Operating temperature between 300 °C and 800 °C. | At 500 °C and 600 °C, the biochar, bio-oil and gas yields were 14–27%, 28–39%, and 45–47%, respectively. At 800 °C, gas content included 50% , 20% , 20% , and 10% . At 600 °C, it was 43% , 37% , 12% , and 8% . |
| [103] | Municipal WWTP in Dalian, China | 13.6 MJ/kg dry HHV, 76.6% MC, 27.24% VM, 3.3% fc, 63.4% ash. UA: 12.8% , 1.74% , 1.2% , 0.55% , and 16.22% . | Quartz cylindrical reactor. Sludge pyrolised for 4 h with heating rate of 3 °C/min at temperatures ranging from 500 °C to 800 °C. | The char, bio-oil and gas yields were around 17.5–25%, 38–43.5%, and 37–42%, respectively. Highest yields for char, bio-oil and gas were at 800 °C, 600 °C, and 500 °C. The lowest value was at 600 °C, 500 °C/800 °C, and 800 °C, respectively. |
| [104] | Carter’s Creek WWTP in Texas, USA | 18.5 MJ/kg dry HHV, 1.9% MC, 68.1% VM, 14.1% fc, 17.8% ash. UA: 39.4% , 5.6% , 7.8% , 0.8% , and 28.6% . | Fast pyrolysis using a bench-scale bubbling fluidized bed reactor. Temperature between 425 °C and 550 °C. | Highest bio-oil yield was 35.7%, biochar was 28.7% and biogas 23.5%, and 11.8% losses. The bio-oil HHV ranged between 24.3 MJ/kg (at 425 °C) and 37.6 MJ/kg (at 550 °C). The generated biochar HHV was 7.4 MJ/kg. |
| [105] | Urban WWTP in Barcelona, Spain. | 11.1 MJ/kg dry HHV, 5.6% MC, 54.2% VM, 8.6% fc, 37.2% ash. UA: 25.5% , 4.5% , 4.9% , 2.1% , and 25.8% . | Flash pyrolysis using a conical spouted bed reactor (CSBR) at between 450 °C and 600 °C. | Char and bio-oil yields at 450 °C, 500 °C, 550 °C, 600 °C were 51%, 46%, 44%, 43%, and 45%, 48.5%, 48.5%, 46%, respectively. Gas yield of 4–11%. |
| [106] | Fast co-pyrolysis with SS and lignocellulosic biomass in a conical spouted bed reactor. | At 500 °C, the gas, bio-oil and biochar yields were 12%, 55%, and 33%, respectively, | ||
| [107] | WWTP in Minnesota, USA. | 4.5% MC, 68.6% VM, 0.3% fc, 16.4% ash. UA: 53.24% , 7.4% , 6.12% , and 33.25% . A total of 24.42 MJ/kg HHV. | A cfMAP with 1 kW power and 2450 MHz was used. Temperature of 450–600 °C. Used ZSM-5 as catalyst (activated at 550 °C for 4 h) | The energy value for bio-oil, biochar and gas varied between 2.2 and 7.7 MJ/kg, 2.14 and 5.4 MJ/kg, and 5.6 and 9.4 MJ/kg, respectively. The char, bio-oil and gas yields were between 33 and 62.5%, 16 and 40%, and 21.5 and 40%, respectively. |
| [108] | WWTP in Shaanxi, China | 4.57% MC, 63.13% VM, 5.47% fc, 26.83% ash. UA: 29.12% , 5.98%, 3.98% , 1.64% , 21% . A total of 16.7 MJ/kg HHV. | Fast pyrolysis using a fixed bed quartz tube reactor at operating temperature between 500 °C and 900 °C. | Char, bio-oil and gas yields ranged between 14 and 35%, 37.5 and 71%, and 15 and 27.5%, respectively. The generated gas included 8–36% and below 7% . content was 35%, at 900 °C, and below 10% for the other temperatures |
| [109] | urban WWTP in Madrid, Spain. | 7% MC, 50% VM, 3% fc, 40% ash. UA: 27.9% , 4.7% , 4.5% , 1.4% , and 34.6% . A total of 12.5 MJ/kg HHV. | Bench-scale stirred batch reactor at 525 °C under atmosphere. | At 525 °C, the biochar, bio-oil and gas yields were approx. 50%, 41%, and 9%, respectively. LHV of the pyrolised SS was 10 MJ/kg. |
| [110] | WWTP in Beijing, China. | SS: 2.25% MC, 61.52% VM, 6.7% fc, 29.5% ash. UA: 53.2% , 7.5% , 6.4% , 2% , and 30.9% . Cb: 4.6% MC, 78.1% VM, 15.64% fc, 1.7% ash. UA: 49.2% , 6.3% , 0.5% , 0.3% , and 43.7% . | Tube furnace reactor under 400–800 °C. Pyrolysis rate of 20 °C/min under protection (99.99%, flow rate = 25 mL/min). Mix ratio of SS:Corncob of 1:1 | The yields of char, bio-oil and gas varied from 30.4% (800 °C) to 75.1% (400 °C), 10.2% (400 °C) to 51.8% (800 °C), and 14.7% (400 °C) to 20% (700 °C), respectively. Gas content: and below 10%, ranged from 22% to 59% and from 35% to 59%. |
| [111] | Nanjing urban WWTP in Nanjing, Jiangsu province, China. | SS: 79% MC, 31.52% VM, 5.25% fc, 63.23% ash. UA: 20.9% , 8.7% , 3.5% , 0.9% , 2.8% . HHV 12.5 MJ/kg. SD: 6.3% MC, 73.6% VM, 14.4% fc, 5.7% ash. UA: 49.5% , 7% , 0.3% , 0.4% , 42.7% . | Co-pyrolysis conducted in a screw moving bed reactor. Operating parameters included 900 °C at 20 °C/min rate and kept for 30 min based on the 16 g/min feeding rate. SD content mixed with SS were 0, 20%, 40%, 60% and 80%. | Generated gas had an HHV of 14 MJ/kg, 13.4 MJ/kg, 13.45 MJ/kg, 13.41 MJ/kg and 13 MJ/kg for a mix of SD about 0, 20%, 40%, 60% and 80%, respectively. Dry gas yield for a mix of SD of 0, 20%, 40%, 60% and 80% was 0.24 m3/kg, 0.36 m3/kg, 0.55 m3/kg, 0.66 m3/kg, 0.74 m3/kg, respectively. The , , and were 29.13–42.35%, 10.5–18.9%, 14.6–24.1%, and 26.8–31.4%, respectively. |
| [112] | WWTP in Australia. | Biosolids: 20–80% MC. UA: 35.7% , 5.2% , 3.5% , 25.4% , 0.7% on wt%. | 450–850 °C, 1 atm and feed rate of 265 kg/h. Costs included AUD 1000/kW (reactor and turbine), AUD 600/m3 () plant, AUD 3/m3 for water, and AUD 0.03/kW for electricity. | The biochar, bio-oil and gas composition was 43.2–53%, 37.7–40.4% and 9.3–17.2%, respectively. From 650 °C to 850 °C, the gas increases (HHV decreased from 23.2 to 20 MJ/kg) and oil decreases. For a 30-year plant operation, the NPV was AUD 2.3–2.6. |
| Ref. | Type | Sludge Collection | Feedstock Characteristics | Parameters | Generated Products |
|---|---|---|---|---|---|
| [113] | HTC | Jiangxinzhou WWTP in China. | 82.5% MC. UA: 39.88% , 6.20% , 6.04% , 20.5% , 5.62% . A total of 17.97 MJ/kg HHV. | 1 L batch reactor with maximum pressure and temperature of 35 MPa and 500 °C. | Gas production of 1.59 L at 200 °C under 2 MPa and 2.86 L at 360 °C under 19.4 MPa. At 380 °C, and yields were 0.14 and 0.24 mol/kg, respectively. |
| [114] | WWTP in Nanjing, China. | 89.2% MC, 56.9% VM. UA: 25.6% , 4.4% , 4.6% , 22% , 0.2% . 11 MJ/kg HHV. | 5 reactors operation under 200–250 °C and 2–26 MPa. | yield reached 0.7 mol/kg at 450 °C, accounting for 11.2 v/v% of the syngas. At 400 °C, yield was below 0.15 mol/kg. | |
| [115] | Il-San municipal WWTP, in Korea | 66.9% VM. UA: 38.55% , 6.46% , 8.05% , 46.50% , 0.44% . HHV of 16.5 MJ/kg. | 1 L autoclave reactor under 180–280 °C and 30 min reaction time. | The HHV of the solid fuel was around 17.3–22.4 MJ/kg. Energy recovery efficiency decreased (from 92.2% to 89.6%) with the increase in temperature. | |
| [116] | WWTP in Japan. | 85.94% MC. UA: 51.20% , 6.64% , 8.85% , 31.94% , 1.37% on db. 18.81 MJ/kg HHV | Operating temperature around 180–240 °C and 15–45 min reaction time. | Lowest HHV was 18.30 MJ/kg at 180 °C and 15 min. Highest value was 20.17 MJ/kg at 240 °C and 45 min. Recovery efficiency of 40% (>200 °C). | |
| [117] | WWTP in Changsha, China. | 89.3% MC, 47.5% VM. UA: 25% , 4.2% , 4.8% , 15.3% , 0.74% , 11 MJ/kg HHV | 0.5 L 316 stainless steel reactor (180–300 °C temperature and 30–480 min reaction time. | Maximum HVV was 12.06 MJ/kg at 260 °C and 1 h reaction. Lowest value (9.8 MJ/kg) was for 3 h reaction and 260 °C (high reaction time does not increase HHV). | |
| [118] | WWTP in Ratmal-na, Sri Lanka | 81% MC. UA: 34.41% , 5.21% , 4.75% , 23.3% , 1.73% on db. 15.2 MJ/kg HHV | Temperature between 100 and 200 °C and reaction up to 1 h. | Maximum (89%) and minimum (73.7%) char yields. Highest HHV (16.17 MJ/kg) at 150 °C and lowest HHV (13.57 MJ/kg) at 100 °C. | |
| [119] | WWTP in Kar-miel city, Israel. | 81% MC. UA: 40.3% , 5.8% , 4.7% , 19.4% on db. A total of 18.0 MJ/kg HHV | 0.5 L stainless steel stirred reactor, 200–300 °C and 0.5, 1, and 2 h retention times. | Hydrochar HHV ranged from 18.2 MJ/kg (200 °C, 0.5 h) to 21.5 MJ/kg (300 °C, 0.5 h). Highest and lowest BMP yield were 236.0 and 25.7 mL /gCOD. | |
| [120] | WWTP in Shimodate, Japan | 79% VM. UA: 48.94% , 7.09% , 2.51% , 33.4% , 0.65% on db. 21.0 MJ/kg HHV | 0.2 L reactor for 0.5 h and 180 °C, at 6:1, 4:1, 3:1 and 2:1 mixing ratios | SS hydrochar HHV was 21.59 MJ/kg (180 °C and 30 min) and HTC input energy was 115.96–117.7 MJ. Hydrochar HHV was about 23.46–25.34 MJ/kg. | |
| [121] | WWTP in Gwangju, Korea. | 72.33% VM. UA: 52.29% , 7.89% , 6.39% , 32.62% , 0.81% on db. A total of 20.6 MJ/kg | 1 L reactor operating under 180–270 °C and 30 reaction time. | Hydrochar HHV was around 18.66–23.44 MJ/kg. Maximum (93.13%) and minimum (40.78%) char yields were achieved at 180 °C and 270 °C. | |
| [122] | HTL | Adelaide plant, Ontario, Canada. | 62.2% VM, 96.1% MC. UA: 38.0% , 5.23% , 7.2% , 25.2% , 0.75% . 16.0 MJ/kg HHV | 0.1 L stirred reactor under 2 MPa, 200–350 °C, 10–60 min reaction. | Composition of oil, solid and WSP were 11.3–33.6 wt%, 9.9–61.2 wt%, and 27.3–62.3 wt%. From WSP, biogas was recovered (up to 0.8 L in 31 days). |
| [123] | WWTP at Viborg, Denmark. | Dry matter of 4 wt%. UA: 46.5% , 6.1% , 3.3% , 31.2% , 0.4% . A total of 19.8 MJ/kg HHV | Reactor with 1.66 L/min feed capacity (20 L vol.), 350 °C temperature and 5 h reaction. | Bio-crude average yield of 24.5 wt%, chemical energy recovery of about 33.6%, and an average of HHV of around 26.9 MJ/kg. | |
| [124] | Marselisborg WWTP, Denmark | Not informed | HTC batch reactor (20 mL) at 340 °C and 20 min reaction time. | With catalyst, the HHV and chemical energy recovery increased from 36.1 to 38 MJ/kg and 56 to 67%, respectively. | |
| [125] | Daugavgriva plant in Riga, Latvia. | 80.5% MC, 56.8% VM. UA: 52.0% , 7.6% , 7.5% , 30.4% , 2.6% . A total of 15.3 MJ/kg HHV | Batch stainless steel autoclave reactor at 200–300 °C and 10–100 min reaction time. | Highest bio-oil yield (47.8%) and 70% recovery achieved under 5.0 MPa, 300 °C, and 40 min time. Lowest bio-oil yield (34.5%) with 36.2 MJ/kg HHV at 200 °C. | |
| [126] | WWTP in Aalborg Forsyning, Denmark. | 73.4% MC, 50.5% VM. UA: 50.9% , 7.4% , 6.9% , 34.8% , 0.8% . A total of 22.15 MJ/kg HHV | 0.1 L stainless steel reactor at 350–400 °C, 10 MPa, and 15 min reaction time. | At 350 °C and no catalyst, bio-crude HHV was 35.3 MJ/kg and 64% energy recovery. With catalyst, HHV was 36.6 MJ/kg and 74.6% energy recovery. | |
| [127] | Beishiqiao plant in Xi’an, China | 90% MC. UA: 33.9% , 5.1% , 5.8% , 16.5% , 3.2% . A total of 16.1 MJ/kg HHV | 4.4 mL mini-batch reactors at 18 MPa with temperature ranging 260–350 °C. | Highest biocrude yield (23 wt.%), 35.4 MJ/kg HHV and energy recovery (50.2%) at 340 °C. Lowest energy recovery (32.6%) with 34.84 MJ/kg HHV at 260 °C. | |
| [128] | WWTP in State College, PA, USA. | 97.8% MC. UA: 46.5% , 7.0% , 2.1% , 33.3% , 0.8% . A total of 19.9 MJ/kg HHV | 4.1 mL reactor at isothermal HTL (673 K, 1 h) and non-isothermal HTL (773 K, 1 min). | With 10 wt% and 50 wt% additives, biocrude yield ranged around 18.9–21.7% and 10.2–18.6% (isothermal), and 24.8–29.1% and 10.9–27.5% (non-isothermal). | |
| [129] | Qinghe WWTP in Beijing, China | 84.5% MC. UA: 46.7% , 6.8% , 8.1% , 37.6% , 0.8% | 0.6 L batch stainless 316 reactor, at 210–330 °C, 30 MPa and 0.5 h. | At 210 °C, biocrude yield was 39.9% and 86.3% HTL conversion. At 270 °C, it increased to 47.5% and 97.7%, and at 330 °C, it decreased to 41% and 90.1%. | |
| [130] | Municipal WWTP in Changsha, China. | UA: 28.9% , 4.5% , 4.2% , 13.9% , 0.6% . | 0.5 L autoclave reactor (316 stainless steel) at 350–400 °C, 35 MPa (max), and 0.5 h. | Bio-crude HHV about 37.8–39 MJ/kg at 350 °C and 0.5 h reaction time. For SS with no pyrolysis, bio-crude HHV was 37.35 MJ/kg at 350 °C and 30 min reaction. | |
| [131] | WWTP in Doha, Qatar. | 83.6% MC. UA: 30.5% , 6.2% , 5.5% . A total of 16.9 MJ/kg HHV | 0.1 L reactor under 275–400 °C and 30–120 min reaction time. | For a 0.5h reaction time and 350 °C, maximum biocrude yield was 44.8%, whereas at 275 °C, the lowest biocrude yield was reached (less than 20%). | |
| [132] | H.C. Morgan Water Facility, in Alabama, USA. | 82.4% MC, 52.9% VM. UA: 33.1% , 5.5% , 5.0% , 25.9% , 0.7% . A total of 14.1 MJ/kg HHV | 1.8 L reactor at 350 °C, and 1 h. Used red mud (RM) catalyst: calcined RM, reduced RM at 500 °C, and reduced RM at 700 °C | At 25 wt% of CRM ethylene, RM at 500 °C ethylene and RM at 700 °C ethylene, the biocrude yield reached 27.1%, 31.3% and 38.3%, respectively, and HHV was 30.43 MJ/kg, 28.29 MJ/kg and 28.44 MJ/kg, respectively. | |
| [133] | WWTP in Shenyang, China | 84.9% MC. UA: 40.6% , 4.7% , 3.7% , 49.6% , 1.2% . A total of 14.3 MJ/kg HHV | Batch-type 0.5 L reactor at 340 °C and 40 min reaction time. | Bio-oil HHV was 32.2 MJ/kg. After using treatment methods, it increased to 33.5–35.3 MJ/kg, and maximum HHV was reached (37.2 MJ/kg). | |
| [134] | Qinghe WWTP, in Beijing, China. | 53.5% OM. UA: 44.7% , 7.6% , 7.2% , 39.6% , 1.0% . A total of 21.3 MJ/kg HHV | 1 L stainless 316 steel reactor with 400 °C and 20 MPa. | Bio-oil HHV was 29.05 MJ/k (control). With treatment, Bio-oil HHV was 25.3–41.5 MJ/kg. Bio-oil HHV treated with reached up to 45.2 MJ/kg. |
- -
- HydroProcessing: An SCWO system implemented in the Harlingen WWTP in Texas, USA, in 2001. Some operating parameters of this system included a 150 ton/day capacity, 592 °C temperature, 24.5 MPa pressure, 20–90 s reaction time, and 6–9% solid content. The consumption of heater, oxygen, and pumps were 4.1 MWh/dry ton sludge, 1.5 ton/dry ton sludge, and 0.55 MWh/dry ton sludge, respectively. The capital and operating costs of the project were about 3 million USD and 100 USD/dry ton of sludge, respectively.
- -
- Chematur AB: Two SCWO systems were developed: (i) first system included a 250 kg/h capacity for demonstration purposes, and (ii) second had a capacity of 1.1 ton/h, built with a plan to treat the sewage sludge of Kobe, Japan. The operational parameters of the second system included a 25 MPa pressure, 30–90 s reaction time, 400–600 °C, and operation at 15% dry solids. The consumption of natural gas, oxygen, cooling water, and electricity was around 21.9 Nm3/dry ton sludge, 1.05 ton/dry ton sludge, 100 m3/dry ton sludge, and 228.6 kWh/dry ton sludge, respectively. The capital and operating cost of the project were about 5 million GBP and 70 GBP/dry ton sludge, respectively.
- -
- SuperWater solution: It was implemented in the Iron Bridge Regional Water Reclamation facility in Orlando, USA. Tested between 2009 and 2011, the SCWO system had a capacity of 5 t/d. The capital and operating costs were around 268 USD/dry ton sludge and 33.7 million USD, respectively. System’s parameters included 35 dry ton sludge/d capacity, a 600 °C operating temperature, 26 MPa pressure, a 30–60 s reaction time, and 10% dry sludge.
3.1.3. Transesterification
| Ref. | Sludge Collection | Sewage Sludge Properties | Parameters | Gas Production |
|---|---|---|---|---|
| [138] | WWTP in Jiangsu, China. | 73.5–88.5 wt% MC. UA: 7.6–31% , 2.3–6.2% , 0.4–3.5% , 20.2–34.1% , 0.9–3.3% . Up to 13.7 MJ/kg HHV | 316 L batch reactor under 400 °C, 1 h reaction time. | Total gas production ranged from 10.7 to 43.3 mol/kg (mean gas production of 18.9 mol/kg). |
| [139] | WWTP in Shaanxi, China. | 84 wt% MC. UA: 38.2% , 2.4% , 4.7% , 23.7% , 1% . A total of 14.6 MJ/kg LHV | Heating rate reactor (70 °C/min) under 550–750 °C and 30 MPa | production up 18.9 mol/kg under 750 °C and 20 min |
| [140] | Municipal WWTP in Zhengzhou, China. | 79 wt% MC, 65 wt% OM. UA: 7.4% , 15.5% , 1.3% , 55.7% , 3.8% , TOC of 0.88 g/L | 0.6 L reactor, 27 MPa, 6 min retention time and 380–460 °C temperature | production ranged from 2.5 mol/kg (380 °C) to 19.9 mol/kg (460 °C), and production ranged from 1.8 mol/kg (380 °C) to 8.2 mol/kg (460 °C). |
| [141] | Municipal WWTP in Jiangsu, China. | 77–88.5 wt% MC. UA: 7.6–27.5% , 2.1–5.2% , 0.4–3.8% , 12.9–34.1% , 0.9–2.5% . Up to 12.6 MJ/kg HHV. | 316 L batch reactor under 400 °C, 10 min reaction time and 24 Mpa. | yield was 1.06 mol/kg (control). With 5 wt% of and , it was 2.7 and 3.6 mol/kg, respectively. For a mix of 3.3 wt% Ni and 1.67 wt% NaOH, 4.8 mol/kg, |
| [142] | Municipal WWTP in Nanjing, China. | 83.2 wt% MC, organic matter of 45.1 wt%. UA: 19.5% , 3.7% , 3.2% , 18.5% , 0.2% , 8.7 MJ/kg HHV | 316 L batch reactor under 400 °C, 1 h reaction time and 22.1 MPa. | With of 2–10 wt%, yield was 0.6–1.05 mol/kg. With , yield decreased from 0.31 mol/kg (2 wt% ) to almost 0 mol/kg (10 wt% ). |
| [143] | WWTP in Jiangsu, China. | 73.9 wt% MC, 26.2 wt% OM. UA: 12.9% , 2.1% , 1.93% , 4.24% , 1.01% . 4.8 MJ/kg HHV. | 316 L batch reactor under 400 °C, 0.5 h reaction. | With no FA, yield was 0.16 mol/kg. With FA of 1, 2, 4 and 6 wt%, yield was 0.52, 1.2, 3.47 and 10.07 mol/kg, respectively. |
| [144] | WWTP in Higahashi-Hiroshima, Japan. | 79.16 wt% MC. UA: 43.1% , 6.6% , 4.4% , 25.9% , 2.4% on db. | 316 L reactor under 500–600 °C, 25 MPa and 5–60 s reaction time. | At 550 °C and 600 °C, composition was about 38.5–39.4 vol%. At 500 °C, composition was 49.5 vol%. |
| [145] | Domestic WWTP in Japan. | 78.8 wt% VM. UA: 38.3% , 5.9% , 7.9% , 33% , 1% on db. | Bench-scale reactor under 600 °C, 23 MPa and 1 h reaction time. | Total gas yield of 9.8 mol/kg with a composition of 60% of . |
| [146] | WWTP in Nanjing, China. | 75 wt% MC, 40.8 wt% OM. UA: 19.5% , 3.7% , 3.18% , 14.25% , 0.17% on db. A total of 9.45 MJ/kg HHV. | Batch reactor under 23 MPa pressure, 400 °C for 10 min. | yield was 0.12 mol/kg without catalyst, and 0.47 mol/kg with 1 cycle of . yield decreased with more cycles. |
| [147] | WWTP in Hangzhou, China. | 35.14 wt% VM, 57.4 wt% OM. UA: 18.94% , 2.21% , 2.89% , 12.79% , 0.6% on db. A total of 5.89 MJ/kg LHV. | 0.5 L batch reactor under 26–28 MPa, 1 h reaction and 380–460 °C temperature. | yield was 6.44 mol/kg with 38.4% of composition. |
| [148] | Paşaköy WWTP in Istanbul, Turkey | 57.4 wt% VM. UA: 29.4% , 4.4% , 17.9% , 5.29% , 0.47% on db. pH of 5.84. | 3.12 L reaction (20 L feed tank), operated with a 25 mL/min flow. | production increase from 3.4 L/h at 500 °C to almost 4.5 L/h at 650 °C. |
| [149] | Xi’an WWTP in Shaanxi, China. | 87 wt% MC, 51.12 wt% VM. UA: 37.58% , 4.4% , 5.72% , 24.44% , 0.84% . 9.64 MJ/kg HHV. | Batch reactor under 25 MPa pressure and 20 min reaction time. | The yield was 0.66, 1.93, 3.95, 7.44 and 11.81 mol/kg for 400 °C, 450 °C, 500 °C, 550 °C and 600 °C, respectively. |
| Ref. | Type | Sludge Collection | Parameters | Gas Production |
|---|---|---|---|---|
| [136] | T | Municipal WWTP located in Tamil Nadu, India. | Lipid extraction performed for 6 h using 50 mL of chloroform methanol (2:1 ratio), diethyl ether, n-hexane and ethanol. Samples heated (50 °C for 0.5 h), and removed solvent | Total lipids extracted from PSS and SSS were between 3 and 6.5 g and 3.3 and 4.9 g using different solvents with concentration of 32.5% (PSS) and 24.5% (SSS). The generated biodiesel included: 89.2–91.2%, ester content, of 40.6–42.9 MJ/kg HHV, 65–72.6 cetane and saponification value of 131–162 mg of |
| [150] | SS collected from two WWTPs in Beijing, China. | SS heated (45–75 °C), stirred at 300 rpm, added to methanol, and hexane solutions for 8 h. and hexane added, and the final mixture was centrifuged (3000 rpm) for 5 min, and filtrated. | Average biodiesel yield was 14.9% and 3.7% for A2/O and MBR processes. Maximum biodiesel yield of 16.6% for A2/O treatment was obtained using methanol:SS ratio of 10, 60 °C, and 5% con-centration. For the MBR process, the maximum value of 4.2% used ratio of 8, 50 °C, and 5% . | |
| [151] | WWTP at Universi-dad Rey Juan Car-los, Madrid, Spain. | Used methanol and n-hexane for extraction and reaction, and Zr-SBA-15 as catalyst. FAME producted in a 25 mL reactor at 209 °C, 2000 rpm, 50:1 methanol ratio and 12.5 wt% catalyst. | 2 approaches used. (i) 1-step direct conversion: Overall weight FAME yield for PSS and SSS were about 15.5 wt% and 10 wt% (based on dried sludge mass), respectively. (ii) 2-step process: FAME yield was lower than 6% for PSS and almost negligible for SSS. | |
| [152] | WWTP in Osong City, Korea. | 1 L flask using a mixture of dewatered SS, methanol and n-hexane, and as catalyst, and stirred at 100 rpm. Reaction time varied from 1 to 8 h, and temperature of 55 °C (n-hexane) and 105 °C (xylene). | Maximum biodiesel yield generated from in situ transesterification were between 8.04% and 9.68% using n-hexane (solvent) and 10 mL/g methanol:SS ratio, and for a mix of PSS and SSS using 2 mL/g methanol:sludge ratio, it was 3.28% (n-hexane) and 8.12% (xylene) | |
| [153] | T | WWTP in Villapérez-Oviedo (Asturias, Spain). | Hexane and methanol as solvents and extraction used 1:2 SS:hexane ratio. Solvent removed at 70 °C, and samples stored after 1 h drying at 105 °C. For solid liquid extraction, 1:10 SS:hexane ratio for 4 h. | Maximum production of 0.4 g FAME/100 g dry SS was achieved (26.8% of the total lipid extracted) for 24 h reaction. Total lipids extraction was 9% (1.75 g lipids/100 g dry SS) using hexane. For methanol (4% v/v), a 2.1% FAME content was achieved, whereas 0.4% was obtained for solid–liquid procedure. |
| [154] | Municipal WWTP in Oviedo, Spain | Reactor at 60 °C for 24 h, and methanol. NaOH (catalyst) at 4 ratios of methanol: 4%, 30%, 50% and 70%. SS:methanol ratio of 1:10. Used also in methanol (4% v/v) and mixed with SS in a 20:1 ratio. Microwave, sonication and particle sieving used for lipid extraction. | Maximum biodiesel yield of 14.3% (mass of FAME/lipid content). After 5 h reaction, biodiesel yield using 0.4% transesterification reached 22.2%, whereas the biodiesel yield using between 30% to 70% reached between 7 and 14.5%. Using microwave applied to dried SS for 4 min increased the FAMEs production by 110% (from 22.1 to 46.7%), whereas sonication improved by up to 42%. | |
| [155] | PSS and SS from the Gangneung WWTP, in Korea. | Parameters used: 0.08% (w/w) alkaline/acidic catalysts with 40 mL/g-lipid methanol, 20 mL/g-lipid n-hexane at 50 °C for 24 h. Biodiesel extracted with n-hexane, centrifuged, separated, and dried for 24 h. | The contents of carbohydrate, crude lipid, ash, crude protein, and other elements were 8.9%, 14.5%, 17.8%, 42.8% and 16%, respectively. Lipid content organic solvents ranged from 2.9% to 5.7%, and using treatment methods (i.e., BDM, microwave, autoclave and ultrasonication), was about 10–14.5%. | |
| [156] | WWTP in Beijing, China. | , and as catalysts. Optimised reaction for used 1:10 SS:methanol ratio at 60 °C, 300 rpm, and 8 h extraction. For and , extraction time was 5 h. | The biodiesel yield was 1.2%, 6% and 6.8% using KOH, KOH/CaO and KOHAC as catalysts, respectively. The results showed that these are not good catalysts for biodiesel production from SS when compared with | |
| [157] | WWTP in Beijing, China. | / catalysts prepared by 79 wt% of Dry SS (10 g) added to n-hexane and ethanol (0.2 L each), and extraction at 80 °C for 10 h. Catalysts (0.4–1.2 g), n-hexane (50 mL) and methanol (128 mL) used to extract crude fat and reacted for 0.5–6 h, 130–170 °C. | Biodiesel yields of 57%, 50.3% and 50.5% were achieved for temperatures of 130 °C, 150 °C and 170 °C, respectively. The biodiesel yield increased from 33.7% to 73.3% from 0.5 h to 4 h reaction time, but for 6 h reaction time, the biodiesel yield was 72.1%. The highest FAME yield (73.3%) was achieved at 130 °C, 4 h reaction time, using 10 g of dry SS and 0.8 g catalyst. | |
| [137] | P | WWTP in Pavia, Italy. | Microwave system at different operating conditions, including temperature of 180–650 °C and reaction times of about 1–28 min. | Highest value of oil to sludge was about 25% under 280 °C and 8 min reaction time, and the lowest value (7%) was at 180 °C at 50 min reaction |
| [158] | WWTP in Texas, USA. | Bench-scale fluidized bed reactor, 150–300 °C, ethanol:bio-oil ratio (w/w) between 1 and 3, 2–4 hs reaction, and as catalyst. | Generated bio-oil upgraded to biodiesel. Bio-oil and biodiesel HHV were 36.43 MJ/kg and 39.97 MJ/kg. SS-derived biodiesel yield was 89.33% (max) at 150 °C, 3 h reaction time, and ethanol:oil ratio of 2. | |
| [159] | T and P | Jungnang WWTP in Seongdong-Gu, Seoul city, Korea. | Dried SS (10 g) 0.2 L of solvent (hexane) used to extract the lipids at 80 °C for 24 h. To separate solvent, used an evaporator at 80 °C for 3 h. SSRB produced by pyrolysis. A total of 20 g of SSR used at 600 °C for 4 h. | Highest biodiesel yield (33.5 wt.%) at 305 °C via thermally induced transesterification in 1 min using the SS biochar. Biodiesel yield from (trans)esterification was less than 1% with 5 wt.% . Kinetics (<1 min) of thermally induced transesterification was faster than normal transesterification (3–24 h). |
3.1.4. Microbial Electrolysis Cell
3.1.5. Microbial Fuel Cell
| Ref. | Feedstock | Parameters | Gas Production |
|---|---|---|---|
| [163] | Wastewater collected prior to primary clarification from the Howdon WWTP, in Newcastle, UK. | 100 L dual-chamber MEC with 6-cell cassettes (88 L working volume). Anode and cathode electrodes surface area of 16.4 and 3.4 m2/m3 (anode-to-cathode ratio of 5:1), respectively. | continuously generated for 1 year with average production rate of 7 L/m3.d. Average energy recovery and coulombic efficient were 48.7% and 41.2%, respectively. |
| [164] | Different types of wastewater, including urban wastewater from Rubi WWTP, in Barcelona, Spain. | 130 L dual-chamber MEC with 10-cell cassettes. Anode-to-cathode ratio volume of 3.5:1, and used an anionic exchange membrane (AEM) to separate the chambers. | The average production was 32 L/m3.d with 95% of purity (5% was methane). The OM removal efficiency was around 25% for a 2-day retention time and OLR of 0.25 gCOD/L.d. |
| [165] | Wastewater obtained from a domestic WWTP in England. | 175 L dual-chamber MEC with 13 m2/m3 cathode specific area and 34 m2/m3 anode surface area-to-volume ratio under 5 h HRT. | The average production was 5.2 L/m3.d with 93% purity, and the COD removal was 63.5%. |
| [166] | Primary sludge collected from the Gold Bar WWTP in Alberta, Canada. | Dual-chamber MEC. Anode and cathode about 0.42 and 0.17 L, respectively. A 40 cm2 membrane to separate the chambers. Semi- continuous fed mode (45 mL/d) and residence time of 8 days. | The production rate found was 145 L/m3.d, and the COD removal efficiency was up to 73%. |
| [167] | Raw sludge obtained from a WWTP in Jinju, Republic of Korea. | 2.5 L reactor with 16 mm anode-cathode electrode. Reactors under 30, 35 and 40 °C for 6 days and stirred at 100 rpm. Operated in fed-batch mode. Raw and seed sludge was mixed under 7:3 ratio. | Maximum production was 111 L/m3 under 35 °C, whereas under 30 °C and 40 °C, the production was 85 L/m3 and 98 L/m3, respectively. The yield at 30 °C and 40 °C was 82.1 L/kgCOD and 77.1 L/kgCOD, respectively. |
| [168] | Wastewater collected from the municipal WWTP of Leon, in Spain. | Single-chamber 3 L membrane less MEC operated under batch and continuous mode at 21 °C with 4 h, 8 h, 12 h and 24 h HRTs | At batch mode, production rate was 1.3–1.4 L/m3.d and 54% COD removal efficiency. The energy and net-energy consumptions were 2.92 and 2.14 kWh/kgCOD. |
| Ref. | Feedstock | COD on Wastewater | MFC System | COD Removal Rate | Maximum Power Density | Max Energy Recovery | Coulombic Efficiency | Operation Time/HRT |
|---|---|---|---|---|---|---|---|---|
| [161] | Wastewater collected from the Pepper’s Ferry Regional WWTP, in the USA. | 155 mg/L. | 96 tubular MFC modules (2 L liquid each) | 76.8% | - | 0.006 kWh/m3 | - | 1 year and 18 h HRT. |
| [162] | Effluent from the primary clarifier of a WWTP, in Switzerland. | Up to 130 mg/L. | 45 L (4 units with 11.2 L each) used in a full-scale WWTP. | 13.5–67% | 73 4, 82 5, 80 6 mW/m2 | 0.012 kWh/m3 | 24.8% | 9 months and 12–44 h HRT |
| [169] | Municipal wastewater from 2 WWTPs (Xiao Jiahe and Yong Feng), in China. | 60–100 mg/L (Xiao Jiahe), 200–400 mg/L (Yong Feng). | 1000 L system (50 stacked modules, 20 L each). | 70–80% | 60 W/m3 | 0.033 kWh/m3 | 41–75% | 100 days to 1 year, 2 h HRT. |
| [170] | Primary effluent wastewater from Pennsylvania State University WWTP, in US. | 376–428 mg/L. | 3 cell sizes: 0.028 2 L, 0.22 3 L and 85 1 L. CSA: 15 m2/m3 (small) and 7.3 m2/m3 (big) | 75–80% | 83 1 W/m3/3042 W/m3 | - | 13–27% | - |
| [171] | Primary effluent wastewater from the Pennsylvania State University WWTP, in US. | 480–1010 mg/L | 2 L reactor (1.4 L liquid vol.). A total of 0.86 L anode volume and CSA of 29 m2/m3. | 57% | 22 W/m3 | - | 4.4% (min) and 42% (max) | 8 h HRT |
| [172] | Effluent from the primary clarifier from the Haeundae domestic WWTP, in Korea. | 144 mg/L. | 5 MFC units (150 mL each) in series. CSA: 400 m2/m3 | 34% | 16.7 W/m3 | - | 12% | 8 months 2.5 h HRT |
| [173] | Wastewater from primary clarifier from a municipal WWTP, in Switzerland. | 200–450 mg/L | 1000 L system (64 MFC × 16.25 L each) into 4-quadruple stacks. | 34.4–95.4% | - | 0.015–0.060 kWh/m3 | 4.7–14.9% (25% max) | 18 months |
| [174] | Effluent from the primary clarifier of the Mumbai municipal WWTP, in India. | 1650 mg/L | 0.7 L system. | 68% | 621 mW/m2 | - | 47–48%. | - |
| [175] | Wastewater collected from Al Gabal Al Asfar WWTP, in Egypt. | 92–350 mg/L | Double chamber MFC with 2 × 0.3 L (anode and cathode) | Up to 72.85%. | 209 7 mW/m2/117 8 mW/m2 | - | - | 24 h HRT |
| [176] | Effluent from primary clarifier from the Taiping municipal WWTP, in China | 200–350 mg/L | 1.5 m3 system | 63% (92% max) | 406 mW/m3 | 0.0034 kWh/m3 | - | 5 h HRT |
3.1.6. Hydropower
3.1.7. Water Electrolysis
| Ref. | Study Aims | Main Findings |
|---|---|---|
| [177] | Investigate the hydro potential in a WWTP in Wisconsin, in the USA. |
|
| [178] | Assess the potential of hydropower technology in WWTPs |
|
| [181] | Investigate the benefits/feasibility of a hydropower system in Zeekoegat WW-TP, in South Africa (60 mL/d capacity). |
|
| [182] | Evaluate the energy recovery potential and economic viability of different WWTPs in Ireland and UK. |
|
| [183] | Study the hydro potential of treated wastewater discharged from the Torun WWTP, in Poland. |
|
| [184] | Study the implementation of micro hydropower turbines in 4 different WWTPs, in Ireland. |
|
| [185] | Feasibility study of hydropower in Kiheung Respia WWTP, South Korea. |
|
| [186] | Hydro system a WWTP, in Pakistan. |
|
| [187] | Investigate the performance of propeller turbines in recovering energy in the aeration tank of a WWTP. |
|
| [188] | Find the optimal design and best hydropower technology for Tatlar WWTP, in Turkey. |
|
| [189] | System at Clarkson WWTP (Canada). |
|
| WWTP | Location | Turbine Type | Total Installed Power (kW) | Flow (m3/s) | H (m) |
|---|---|---|---|---|---|
| North Head, Sydney | Australia | Kaplan | 4500 | 3.5 | 60 |
| Emmerich | Germany | Archimedes | 13 | 0.4 | 3.8 |
| As Samra | Jordan | Pelton | 1600 | 3.2 | 104 |
| Francis | 1680 | 41 | |||
| Aïre, Geneva | Switzerland | Kaplan | 200 | 3.2 | 5 |
| Engelberg | Pelton | 50 | 0.16 | 54.4 | |
| Grächen | 262 | 0.09 | 365 | ||
| La Douve I, Leysin | 430 | 0.08 | 545 | ||
| La Douve II, Leysin | 75 | 0.108 | 83 | ||
| Morgental, St. Gallen | 1350 | 0.84 | 190 | ||
| Profay, Le Chable | 350 | 0.1 | 449 | ||
| La Asse, Nyon | Pump as turbine | 220 | 0.293 | 94.3 | |
| Elsholt | UK | Archimedes | 180 | 2.6 | - |
| Deer Island, Boston | USA | Kaplan | 2000 | 13.1 | 8.8 |
| Point Loma, San Diego | Francis | 1350 | 7.6 | 27 | |
| Hsinchu | Taiwan | - | 11 | - | - |
| Taichung | - | 68 | - | - |
3.2. Non-Site-Specific Sources
3.2.1. Solar Energy
3.2.2. Wind
3.2.3. Hybrid System
4. Grid Services and Energy Market Participation of WWTPs
| Ref. | Type | Study Objective | Main Findings |
|---|---|---|---|
| [196] | Solar thermal | Optimise a solar dryer in a WWTP in Morocco. |
|
| [197] | Design a system for drying SS from Antalya Metropolitan Municipality WWTP, in Turkey. |
| |
| [198] | Proposed a thin layer sandwich-like chamber for SS drying |
| |
| [199] | Investigated the SS drying system for a WWTP in Beijing, China, |
| |
| [200] | Studied the potential of a solar air heater for drying SS. |
| |
| [23] | Solar PV | Assess the status of solar PV in 105 WWTPs in California, USA, to evaluate its usage in WWTPs. |
|
| [201] | Study the benefits of a PV system in a WWTP, in Romania. |
| |
| [202] | Assess the SBBGR in a WWTP using a solar system. |
| |
| [203] | PV-Battery system | Integration of PV-battery system for 2 small-scale decentralised WWTPs, in the Netherlands. |
|
| [204] | Wind | Assess the benefits of the 100 kW wind turbine to supply electricity for a WWTP located in Texas, US. |
|
| [205] | PV and Biogas | Study the potential of anaerobic co-digestion and solar PV in a WWTP in Loures, Portugal. |
|
| [206] | Solar thermal/biomass | Propose a 20 MW solar/biomass system to supply a WWTP in Spain. |
|
| [207] | PV, battery and diesel generator | Analyse the potential of using a hybrid system to reduce consum-ption in a WWTP, in Romania. |
|
| [208] | PV, heat pump, water electrolyser) | Investigate the economic benefits for implementing a hybrid generation in a WWTP. |
|
| [209] | MHP and MEC | Integration of MEC and MHP to assess potential benefits for a WWTP. |
|
| [210] | FC and Solar thermal | Conduct an economic analysis for a hybrid system in Collegno WWTP, in Italy, which serves serves 270,000 PE and treats 38,500 m3/day. |
|
| [211] | SOFC, solar thermal, and GT | Evaluate the potential benefits of a hybrid system in a WWTP, in Italy. |
|
| Ref. | WWTP | Location | Technology/ System | Findings |
|---|---|---|---|---|
| [212] | ACUA WWTP | New Jersey, US | 7.5 MW Wind |
|
| [213] | Field’s Point WWTP | Providence, US. | 4.5 MW Wind |
|
| [214] | JRDWRF | City of Pueblo, Colorado, US | 309 kW solar PV |
|
| [195] | EMWD | California, US. | PV solar, FC, and turbines |
|
| [215] | PLWWTP | San Diego, USA | Biogas upgrading |
|
| [216] | Werdhölzli | Switzerland | Sludge incineration |
|
| [217] | Deer Island WWTP | Boston, US | Solar PV, wind, and hydro |
|
Challenges and Opportunities of Using RES and Market Strategies in WWTPs
| Ref. | Organisation | Technology/System | Details |
|---|---|---|---|
| [222] | Hunter Water | 6 MW solar PV |
|
| [223] | Icon Water | 720 kW solar PV and 1.23 MW Hydro |
|
| [224] | QST, GoR, and Melb W | Hydrothermal liquefaction |
|
| [225] | Loganholme WWTP | Gasification |
|
| [226] | Melb W | 25 MW biogas, 25 MW hydro and 24 MW Solar |
|
| [227] | SA Water | 154 MW PV and 34 MWh battery |
|
| [228] | Sydney water | PV, biogas, hydro |
|
| [229] | WC | PV, wind, biogas, hydro |
|
| Ref. | Study Aim | Main Findings |
|---|---|---|
| [18] | Investigate the electricity spot price behaviour for SA Water in Australia |
|
| [194] | Investigated the Laguna WWTP in California, USA, participating in a DR scheme. |
|
| [230] | WWTPs, aggregated as VPP, to provide services. |
|
| [231] | Control strategy for WWTPs for a short-term demand side |
|
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Maktabifard, M.; Zaborowska, E.; Makinia, J. Energy neutrality versus carbon footprint minimization in municipal wastewater treatment plants. Bioresour. Technol. 2020, 300, 122647. [Google Scholar] [CrossRef] [PubMed]
- BECA. Opportunities for Renewable Energy in the Australian Water Sector. ARENA. 2015. Available online: https://arena.gov.au/assets/2016/01/Opportunities-for-renewable-energy-in-the-Australian-water-sector.pdf (accessed on 13 September 2022).
- Government, N. Energy Efficiency Opportunities in Wastewater Treatment Facilities. 2019. Available online: https://www.environment.nsw.gov.au/resources/business/wastewater-treatment-facilities-energy-efficiency-opportunities-190114.pdf (accessed on 10 February 2022).
- Topare, N.S.; Attar, S.; Manfe, M.M. Sewage/wastewater treatment technologies: A review. Sci. Revs. Chem. Commun. 2011, 1, 18–24. [Google Scholar]
- Gandiglio, M.; Lanzini, A.; Soto, A.; Leone, P.; Santarelli, M. Enhancing the energy efficiency of wastewater treatment plants through co-digestion and fuel cell systems. Front. Environ. Sci. 2017, 5, 70. [Google Scholar] [CrossRef]
- Kirchem, D.; Lynch, M.; Bertsch, V.; Casey, E. Market effects of industrial demand response and flexibility potential from wastewater treatment facilities. In Proceedings of the 2018 15th International Conference on the European Energy Market (EEM), Lodz, Poland, 27–29 June 2018; pp. 1–6. [Google Scholar]
- Lima, D.; Appleby, G.; Li, L. A Scoping Review of Options for Increasing Biogas Production from Sewage Sludge: Challenges and Opportunities for Enhancing Energy Self-Sufficiency in Wastewater Treatment Plants. Energies 2023, 16, 2369. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thattai, A.T.; Fan, L.; Lindeboom, R.E.; Spanjers, H.; Aravind, P. Solid Oxide Fuel Cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Riley, D.M.; Tian, J.; Güngör-Demirci, G.; Phelan, P.; Villalobos, J.R.; Milcarek, R.J. Techno-economic assessment of chp systems in wastewater treatment plants. Environments 2020, 7, 74. [Google Scholar] [CrossRef]
- Ibrahim, A.; Akilli, H. Supercritical water gasification of wastewater sludge for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 10328–10349. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of sewage sludge for biofuel application: A review on fundamentals, current challenges and strategies. Biomass Bioenergy 2022, 165, 106570. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Ghoneim, W.; Helal, A.; Wahab, M.A. Renewable energy resources and recovery opportunities in wastewater treatment plants. In Proceedings of the 2016 3rd International Conference on Renewable Energies for Developing Countries (REDEC), Zouk Mosbeh, Lebanon, 13–15 July 2016; pp. 1–8. [Google Scholar]
- Islam, A.K.; Dunlop, P.S.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-hydrogen production from wastewater: A comparative study of low energy intensive production processes. Clean Technol. 2021, 3, 156–182. [Google Scholar] [CrossRef]
- Escapa, A.; Mateos, R.; Martínez, E.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- AlSayed, A.; Soliman, M.; Eldyasti, A. Microbial fuel cells for municipal wastewater treatment: From technology fundamentals to full-scale development. Renew. Sustain. Energy Rev. 2020, 134, 110367. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, F.; Zhang, R.; Zhao, L.; Qi, J. Recent progress on biodiesel production from municipal sewage sludge. Renew. Sustain. Energy Rev. 2021, 135, 110260. [Google Scholar] [CrossRef]
- Do, P.; Chow, C.W.; Rameezdeen, R.; Gorjian, N. Understanding the impact of spot market electricity price on wastewater asset management strategy. Water Conserv. Sci. Eng. 2022, 7, 101–117. [Google Scholar] [CrossRef]
- Riffat, R.; Husnain, T. Fundamentals of Wastewater Treatment and Engineering; Crc Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal; IWA Publishing: London, UK, 2007. [Google Scholar]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199. [Google Scholar]
- Strazzabosco, A.; Kenway, S.J.; Lant, P.A. Solar PV adoption in wastewater treatment plants: A review of practice in California. J. Environ. Manag. 2019, 248, 109337. [Google Scholar] [CrossRef]
- Patrick Dube, S.C.; Collins, D.; Deslauriers, S.; Fillmore, L.; McFadden, L.; Moss, L.; Serfass, P.; Stone, L.; Turgeon, J. Accelerating Resource Recovery: Biosolids Innovations and Opportunities; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Mintz, M.M.; Snyder, S.W. An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: Challenges and opportunities towards energy-neutral WWTPs. Renew. Sustain. Energy Rev. 2015, 50, 346–362. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kumar, J.; Vu, M.T.; Mohammed, J.A.; Pathak, N.; Commault, A.S.; Sutherland, D.; Zdarta, J.; Tyagi, V.K.; Nghiem, L.D. Biomethane production from anaerobic co-digestion at wastewater treatment plants: A critical review on development and innovations in biogas upgrading techniques. Sci. Total Environ. 2021, 765, 142753. [Google Scholar] [CrossRef]
- Kuo-Dahab, W.C.; Amirhor, P.; Zona, M.; Duest, D.; Park, C. Investigating anaerobic co-digestion of sewage sludge and food waste using a bench-scale pilot study. In Proceedings of the WEFTEC 2014, New Orleans, LA, USA, 28 September–1 October 2014; Water Environment Federation: Alexandria, VA, USA, 2014. [Google Scholar]
- Azarmanesh, R.; Zonoozi, M.H.; Ghiasinejad, H. Characterization of food waste and sewage sludge mesophilic anaerobic co-digestion under different mixing ratios of primary sludge, secondary sludge and food waste. Biomass Bioenergy 2020, 139, 105610. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Mutnuri, S. Anaerobic co-digestion of sewage sludge and food waste. Waste Manag. Res. 2016, 34, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.; Ruiz, L.; Pascual, A.; Ruiz, B. Co-digestion of used oils and urban landfill leachates with sewage sludge and the effect on the biogas production. Appl. Energy 2013, 107, 438–445. [Google Scholar] [CrossRef]
- Di Maria, F.; Sordi, A.; Cirulli, G.; Micale, C. Amount of energy recoverable from an existing sludge digester with the co-digestion with fruit and vegetable waste at reduced retention time. Appl. Energy 2015, 150, 9–14. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E.; Singh, B.; Almås, Å.; Brattebø, H.; Kacprzak, M. Anaerobic digestion of sewage sludge with grease trap sludge and municipal solid waste as co-substrates. Environ. Res. 2017, 155, 249–260. [Google Scholar] [CrossRef]
- Abudi, Z.N.; Hu, Z.; Xiao, B.; Abood, A.R.; Rajaa, N.; Laghari, M. Effects of pretreatments on thickened waste activated sludge and rice straw co-digestion: Experimental and modeling study. J. Environ. Manag. 2016, 177, 213–222. [Google Scholar] [CrossRef]
- Chu, X.; Wu, G.; Wang, J.; Hu, Z.-H. Dry co-digestion of sewage sludge and rice straw under mesophilic and thermophilic anaerobic conditions. Environ. Sci. Pollut. Res. 2015, 22, 20143–20153. [Google Scholar] [CrossRef]
- Elsayed, M.; Andres, Y.; Blel, W.; Gad, A. Methane production by anaerobic co-digestion of sewage sludge and wheat straw under mesophilic conditions. Int. J. Sci. Res. Eng. Technol. (IJSET) 2015, 4, 1–6. [Google Scholar]
- Potdukhe, R.M.; Sahu, N.; Kapley, A.; Kumar, R. Co-digestion of waste activated sludge and agricultural straw waste for enhanced biogas production. Bioresour. Technol. Rep. 2021, 15, 100769. [Google Scholar] [CrossRef]
- Yalcinkaya, S.; Malina Jr, J.F. Model development and evaluation of methane potential from anaerobic co-digestion of municipal wastewater sludge and un-dewatered grease trap waste. Waste Manag. 2015, 40, 53–62. [Google Scholar] [CrossRef]
- Yalcinkaya, S.; Malina, J.F. Anaerobic co-digestion of municipal wastewater sludge and un-dewatered grease trap waste for assessing direct feed of grease trap waste in municipal digesters. Int. Biodeterior. Biodegrad. 2015, 104, 490–497. [Google Scholar] [CrossRef]
- Alqaralleh, R.M.; Kennedy, K.; Delatolla, R.; Sartaj, M. Thermophilic and hyper-thermophilic co-digestion of waste activated sludge and fat, oil and grease: Evaluating and modeling methane production. J. Environ. Manag. 2016, 183, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, M.; Pavlostathis, S.G. Anaerobic co-digestion of municipal sludge with fat-oil-grease (FOG) enhances the destruction of sludge solids. Chemosphere 2022, 292, 133530. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.R.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Investigating the effect of crude glycerol from biodiesel industry on the anaerobic co-digestion of sewage sludge and food waste in ternary mixtures. Energy 2022, 241, 122818. [Google Scholar] [CrossRef]
- Silvestre, G.; Fernández, B.; Bonmatí, A. Addition of crude glycerine as strategy to balance the C/N ratio on sewage sludge thermophilic and mesophilic anaerobic co-digestion. Bioresour. Technol. 2015, 193, 377–385. [Google Scholar] [CrossRef]
- Bai, X.; Chen, Y.-C. Synergistic effect and supernatant nitrogen reduction from anaerobic co-digestion of sewage sludge and pig manure. Bioresour. Technol. Rep. 2020, 10, 100424. [Google Scholar] [CrossRef]
- Borowski, S.; Domański, J.; Weatherley, L. Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Waste Manag. 2014, 34, 513–521. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Improvement of the anaerobic digestion of sewage sludge by co-digestion with wine vinasse and poultry manure: Effect of different hydraulic retention times. Fuel 2022, 321, 124104. [Google Scholar] [CrossRef]
- Alkhrissat, T.; Kassab, G.; Abdel-Jaber, M.t. Impact of Iron Oxide Nanoparticles on Anaerobic Co-Digestion of Cow Manure and Sewage Sludge. Energies 2023, 16, 5844. [Google Scholar] [CrossRef]
- Avila, R.; Justo, Á.; Carrero, E.; Crivillés, E.; Vicent, T.; Blánquez, P. Water resource recovery coupling microalgae wastewater treatment and sludge co-digestion for bio-wastes valorisation at industrial pilot-scale. Bioresour. Technol. 2022, 343, 126080. [Google Scholar] [CrossRef]
- Beltrán, C.; Jeison, D.; Fermoso, F.G.; Borja, R. Batch anaerobic co-digestion of waste activated sludge and microalgae (Chlorella sorokiniana) at mesophilic temperature. J. Environ. Sci. Health Part A 2016, 51, 847–850. [Google Scholar] [CrossRef]
- Ortega-Martinez, E.; Sapkaite, I.; Fdz-Polanco, F.; Donoso-Bravo, A. From pre-treatment toward inter-treatment. Getting some clues from sewage sludge biomethanation. Bioresour. Technol. 2016, 212, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-M.; Han, S.-K.; Lee, C.-Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Q.; Wang, D.; Zhao, J.; Wu, Y.; Liu, Y.; Ni, B.-J.; Wang, Q.; Zeng, G.; Li, X. Improved methane production from waste activated sludge by combining free ammonia with heat pretreatment: Performance, mechanisms and applications. Bioresour. Technol. 2018, 268, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Trzcinski, A.P.; Tian, X.; Wang, C.; Lin, L.L.; Ng, W.J. Combined ultrasonication and thermal pre-treatment of sewage sludge for increasing methane production. J. Environ. Sci. Health Part A 2015, 50, 213–223. [Google Scholar] [CrossRef][Green Version]
- Serrano, A.; Siles, J.Á.; Gutiérrez, M.d.C.; Martín, M.d.l.Á. Comparison of Pre-treatment Technologies to Improve Sewage Sludge Biomethanization. Appl. Biochem. Biotechnol. 2021, 193, 777–790. [Google Scholar] [CrossRef]
- Martínez, E.; Gil, M.; Rosas, J.; Moreno, R.; Mateos, R.; Morán, A.; Gómez, X. Application of thermal analysis for evaluating the digestion of microwave pre-treated sewage sludge. J. Therm. Anal. Calorim. 2017, 127, 1209–1219. [Google Scholar] [CrossRef]
- Serrano, A.; Siles, J.; Martín, M.; Chica, A.; Estévez-Pastor, F.; Toro-Baptista, E. Improvement of anaerobic digestion of sewage sludge through microwave pre-treatment. J. Environ. Manag. 2016, 177, 231–239. [Google Scholar] [CrossRef]
- Liu, J.; Yang, M.; Zhang, J.; Zheng, J.; Xu, H.; Wang, Y.; Wei, Y. A comprehensive insight into the effects of microwave-H2O2 pretreatment on concentrated sewage sludge anaerobic digestion based on semi-continuous operation. Bioresour. Technol. 2018, 256, 118–127. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Herrera, R.R.; Pedreguera, A.Z.; Espinoza, J.E.R. Effects of ultrasonic pretreatment on the solubilization and kinetic study of biogas production from anaerobic digestion of waste activated sludge. Int. Biodeterior. Biodegrad. 2017, 123, 1–9. [Google Scholar] [CrossRef]
- Neumann, P.; González, Z.; Vidal, G. Sequential ultrasound and low-temperature thermal pretreatment: Process optimization and influence on sewage sludge solubilization, enzyme activity and anaerobic digestion. Bioresour. Technol. 2017, 234, 178–187. [Google Scholar] [CrossRef]
- Houtmeyers, S.; Degrève, J.; Willems, K.; Dewil, R.; Appels, L. Comparing the influence of low power ultrasonic and microwave pre-treatments on the solubilisation and semi-continuous anaerobic digestion of waste activated sludge. Bioresour. Technol. 2014, 171, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, C.; Trzcinski, A.P.; Lin, L.; Ng, W.J. Interpreting the synergistic effect in combined ultrasonication–ozonation sewage sludge pre-treatment. Chemosphere 2015, 140, 63–71. [Google Scholar] [CrossRef] [PubMed]
- BioCNG. Fact Sheet: City of Janesville Wastewater Treatment Plant. 2015. Available online: https://biocng.us/wp-content/uploads/2015/06/Janesville-Fact-Sheet-2015.pdf (accessed on 11 June 2024).
- Socalgas. Point Loma Wastewater Treatment Plant. Available online: https://www.sandiego.gov/public-utilities/water-quality/water-wastewater-facilities/point-loma (accessed on 15 June 2024).
- Amaresco, I. Case Study—San Antionio Water System, TX. 2017. Available online: https://www.ameresco.com/wp-content/uploads/2017/11/san-antonio-water-system-tx.pdf (accessed on 17 July 2024).
- EPA. An Overview of Renewable Natural Gas from Biogas. 2020. Available online: https://www.epa.gov/sites/default/files/2020-07/documents/lmop_rng_document.pdf (accessed on 11 August 2024).
- Hauptmeier, K.; Penkuhn, M.; Tsatsaronis, G. Economic assessment of a solid oxide fuel cell system for biogas utilization in sewage plants. Energy 2016, 117, 361–368. [Google Scholar] [CrossRef]
- Grasham, O.; Dupont, V.; Camargo-Valero, M.A.; García-Gutiérrez, P.; Cockerill, T. Combined ammonia recovery and solid oxide fuel cell use at wastewater treatment plants for energy and greenhouse gas emission improvements. Appl. Energy 2019, 240, 698–708. [Google Scholar] [CrossRef]
- Sanaye, S.; Imeni, M.; Yazdani, M. Clean production of power and heat for waste water treatment plant by integrating sewage sludge anaerobic digester and solid oxide fuel cell. Energy Convers. Manag. 2023, 288, 117136. [Google Scholar] [CrossRef]
- Basrawi, F.; Ibrahim, T.K.; Habib, K.; Yamada, T.; Idris, D.M.N.D. Techno-economic performance of biogas-fueled micro gas turbine cogeneration systems in sewage treatment plants: Effect of prime mover generation capacity. Energy 2017, 124, 238–248. [Google Scholar] [CrossRef]
- Movahed, P.; Avami, A. Techno-economic optimization of biogas-fueled micro gas turbine cogeneration systems in sewage treatment plant. Energy Convers. Manag. 2020, 218, 112965. [Google Scholar] [CrossRef]
- Chang, C.-C.; Do, M.V.; Hsu, W.-L.; Liu, B.-L.; Chang, C.-Y.; Chen, Y.-H.; Yuan, M.-H.; Lin, C.-F.; Yu, C.-P.; Chen, Y.-H. A case study on the electricity generation using a micro gas turbine fuelled by biogas from a sewage treatment plant. Energies 2019, 12, 2424. [Google Scholar] [CrossRef]
- Gholamian, E.; Mehr, A.; Yari, M.; Carton, J. Dynamic simulation and techno-economic assessment of hydrogen utilization in dual fuel (Hydrogen/biogas) micro gas turbine systems for a wastewater treatment plant. Process Saf. Environ. Prot. 2023, 169, 220–237. [Google Scholar] [CrossRef]
- Di Fraia, S.; Massarotti, N.; Vanoli, L.; Costa, M. Thermo-economic analysis of a novel cogeneration system for sewage sludge treatment. Energy 2016, 115, 1560–1571. [Google Scholar] [CrossRef]
- Silvestre, G.; Fernández, B.; Bonmatí, A. Significance of anaerobic digestion as a source of clean energy in wastewater treatment plants. Energy Convers. Manag. 2015, 101, 255–262. [Google Scholar] [CrossRef]
- Felca, A.T.A.; Barros, R.M.; Tiago Filho, G.L.; dos Santos, I.F.S.; Ribeiro, E.M. Analysis of biogas produced by the anaerobic digestion of sludge generated at wastewater treatment plants in the South of Minas Gerais, Brazil as a potential energy source. Sustain. Cities Soc. 2018, 41, 139–153. [Google Scholar] [CrossRef]
- MosayebNezhad, M.; Mehr, A.; Gandiglio, M.; Lanzini, A.; Santarelli, M. Techno-economic assessment of biogas-fed CHP hybrid systems in a real wastewater treatment plant. Appl. Therm. Eng. 2018, 129, 1263–1280. [Google Scholar] [CrossRef]
- Farahbakhsh, M.T.; Chahartaghi, M. Performance analysis and economic assessment of a combined cooling heating and power (CCHP) system in wastewater treatment plants (WWTPs). Energy Convers. Manag. 2020, 224, 113351. [Google Scholar] [CrossRef]
- De Arespacochaga, N.; Valderrama, C.; Peregrina, C.; Hornero, A.; Bouchy, L.; Cortina, J. On-site cogeneration with sewage biogas via high-temperature fuel cells: Benchmarking against other options based on industrial-scale data. Fuel Process. Technol. 2015, 138, 654–662. [Google Scholar] [CrossRef]
- Hantoko, D.; Yan, M.; Kanchanatip, E.; Adnan, M.A.; Mubeen, I.; Hamid, F.S. Supercritical water gasification of sewage sludge and combined cycle for H2 and power production–a thermodynamic study. Int. J. Hydrogen Energy 2019, 44, 24459–24470. [Google Scholar] [CrossRef]
- Hu, M.; Hu, H.; Ye, Z.; Tan, S.; Yin, K.; Chen, Z.; Guo, D.; Rong, H.; Wang, J.; Pan, Z. A review on turning sewage sludge to value-added energy and materials via thermochemical conversion towards carbon neutrality. J. Clean. Prod. 2022, 379, 134657. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Hao, B.; Liu, L.; Wang, S.; Wu, Z. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021, 311, 127811. [Google Scholar] [CrossRef]
- Adar, E.; Karatop, B.; İnce, M.; Bilgili, M.S. Comparison of methods for sustainable energy management with sewage sludge in Turkey based on SWOT-FAHP analysis. Renew. Sustain. Energy Rev. 2016, 62, 429–440. [Google Scholar] [CrossRef]
- Swann, L.; Downs, D.; Waye, M. Waste to energy solution–The sludge treatment facility in Tuen Mun, Hong Kong. Energy Procedia 2017, 143, 500–505. [Google Scholar] [CrossRef]
- Enebe, N.L.; Chigor, C.B.; Obileke, K.; Lawal, M.S.; Enebe, M.C. Biogas and syngas production from sewage sludge: A sustainable source of energy generation. Methane 2023, 2, 192–217. [Google Scholar] [CrossRef]
- Haghighat, M.; Majidian, N.; Hallajisani, A. Production of bio-oil from sewage sludge: A review on the thermal and catalytic conversion by pyrolysis. Sustain. Energy Technol. Assess. 2020, 42, 100870. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Adar, E.; İnce, M.; Bilgili, M.S. Evaluation of development in supercritical water oxidation technology. Desalin. Water Treat. 2019, 161, 243–253. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Ollero, P.; Nilsson, S. Gasification of wastes in a pilot fluidized bed gasifier. Fuel Process. Technol. 2014, 121, 63–69. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Frandsen, F.J.; Henriksen, U.B. Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 1: Process performance and gas product characterization. Waste Manag. 2017, 66, 123–133. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Lu, C.-H.; Liao, C.-K.; Ger, R.H.-R. Characteristics of hydrogen energy yield by co-gasified of sewage sludge and paper-mill sludge in a commercial scale plant. Int. J. Hydrogen Energy 2016, 41, 21641–21648. [Google Scholar] [CrossRef]
- Schmid, M.; Beirow, M.; Schweitzer, D.; Waizmann, G.; Spörl, R.; Scheffknecht, G. Product gas composition for steam-oxygen fluidized bed gasification of dried sewage sludge, straw pellets and wood pellets and the influence of limestone as bed material. Biomass Bioenergy 2018, 117, 71–77. [Google Scholar] [CrossRef]
- Ayol, A.; Yurdakos, O.T.; Gurgen, A. Investigation of municipal sludge gasification potential: Gasification characteristics of dried sludge in a pilot-scale downdraft fixed bed gasifier. Int. J. Hydrogen Energy 2019, 44, 17397–17410. [Google Scholar] [CrossRef]
- Schweitzer, D.; Gredinger, A.; Schmid, M.; Waizmann, G.; Beirow, M.; Spörl, R.; Scheffknecht, G. Steam gasification of wood pellets, sewage sludge and manure: Gasification performance and concentration of impurities. Biomass Bioenergy 2018, 111, 308–319. [Google Scholar] [CrossRef]
- Ma, J.; Chen, M.; Yang, T.; Liu, Z.; Jiao, W.; Li, D.; Gai, C. Gasification performance of the hydrochar derived from co-hydrothermal carbonization of sewage sludge and sawdust. Energy 2019, 173, 732–739. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Ko, J.-H.; Kim, J.-S. Gasification of dried sewage sludge using an innovative three-stage gasifier: Clean and H2-rich gas production using condensers as the only secondary tar removal apparatus. Fuel 2018, 216, 810–817. [Google Scholar] [CrossRef]
- Roche, E.; De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and air-steam gasification of sewage sludge. The influence of dolomite and throughput in tar production and composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Cho, M.-H.; Kim, J.-S. Steam/oxygen gasification of dried sewage sludge in a two-stage gasifier: Effects of the steam to fuel ratio and ash of the activated carbon on the production of hydrogen and tar removal. Energy 2015, 91, 160–167. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Choi, Y.-K.; Park, K.-B.; Kim, J.-S. Air co-gasification of coal and dried sewage sludge in a two-stage gasifier: Effect of blending ratio on the producer gas composition and tar removal. Energy 2019, 185, 708–716. [Google Scholar] [CrossRef]
- Mun, T.-Y.; Cho, M.-H.; Kim, J.-S. Air gasification of dried sewage sludge in a two-stage gasifier. Part 3: Application of olivine as a bed material and nickel coated distributor for the production of a clean hydrogen-rich producer gas. Int. J. Hydrogen Energy 2014, 39, 5634–5643. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Cho, M.-H.; Kim, J.-S. Air gasification of dried sewage sludge in a two-stage gasifier. Part 4: Application of additives including Ni-impregnated activated carbon for the production of a tar-free and H2-rich producer gas with a low NH3 content. Int. J. Hydrogen Energy 2016, 41, 1460–1467. [Google Scholar] [CrossRef]
- Jaramillo-Arango, A.; Fonts, I.; Chejne, F.; Arauzo, J. Product compositions from sewage sludge pyrolysis in a fluidized bed and correlations with temperature. J. Anal. Appl. Pyrolysis 2016, 121, 287–296. [Google Scholar] [CrossRef]
- Huang, F.; Yu, Y.; Huang, H. Temperature influence and distribution of bio-oil from pyrolysis of granular sewage sludge. J. Anal. Appl. Pyrolysis 2018, 130, 36–42. [Google Scholar] [CrossRef]
- Arazo, R.O.; Genuino, D.A.D.; de Luna, M.D.G.; Capareda, S.C. Bio-oil production from dry sewage sludge by fast pyrolysis in an electrically-heated fluidized bed reactor. Sustain. Environ. Res. 2017, 27, 7–14. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Barbarias, I.; Bilbao, J.; Olazar, M. Sewage sludge valorization by flash pyrolysis in a conical spouted bed reactor. Chem. Eng. J. 2015, 273, 173–183. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Xie, Q.; Peng, P.; Liu, S.; Min, M.; Cheng, Y.; Wan, Y.; Li, Y.; Lin, X.; Liu, Y.; Chen, P. Fast microwave-assisted catalytic pyrolysis of sewage sludge for bio-oil production. Bioresour. Technol. 2014, 172, 162–168. [Google Scholar] [CrossRef]
- Deng, S.; Tan, H.; Wang, X.; Yang, F.; Cao, R.; Wang, Z.; Ruan, R. Investigation on the fast co-pyrolysis of sewage sludge with biomass and the combustion reactivity of residual char. Bioresour. Technol. 2017, 239, 302–310. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Jiang, W.; Shao, L.; Zhang, L.; Feng, L. Effects of pyrolysis temperature and addition proportions of corncob on the distribution of products and potential energy recovery during the preparation of sludge activated carbon. Chemosphere 2019, 221, 175–183. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Yang, L.; Zhu, Y. High quality syngas produced from the co-pyrolysis of wet sewage sludge with sawdust. Int. J. Hydrogen Energy 2018, 43, 5463–5472. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Paz-Ferreiro, J.; Surapaneni, A.; Fouche, L.; Halder, P.; Setiawan, A.; Shah, K. Transformation of biosolids to biochar: A case study. Environ. Prog. Sustain. Energy 2019, 38, 13113. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Giannis, A.; Yang, Y.; Wang, J.-Y. Products evolution during hydrothermal conversion of dewatered sewage sludge in sub-and near-critical water: Effects of reaction conditions and calcium oxide additive. Int. J. Hydrogen Energy 2015, 40, 5776–5787. [Google Scholar] [CrossRef]
- Su, Y.; Liu, D.; Gong, M.; Zhu, W.; Yu, Y.; Gu, H. Investigation on the decomposition of chemical compositions during hydrothermal conversion of dewatered sewage sludge. Int. J. Hydrogen Energy 2019, 44, 26933–26942. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers. Manag. 2014, 78, 815–821. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Silva, R.D.V.K.; Lei, Z.; Shimizu, K.; Zhang, Z. Hydrothermal treatment of sewage sludge to produce solid biofuel: Focus on fuel characteristics. Bioresour. Technol. Rep. 2020, 11, 100453. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khoury, O.; Zohar, M.; Poverenov, E.; Darzi, R.; Laor, Y.; Posmanik, R. Hydrothermal carbonization of sewage sludge coupled with anaerobic digestion: Integrated approach for sludge management and energy recycling. Energy Convers. Manag. 2020, 224, 113353. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.; Hou, T.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z. Conversion of biomass waste to solid fuel via hydrothermal co-carbonization of distillers grains and sewage sludge. Bioresour. Technol. 2022, 345, 126545. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, D.; Lee, K.; Park, K.Y. Solid fuel production through hydrothermal carbonization of sewage sludge and microalgae Chlorella sp. from wastewater treatment plant. Chemosphere 2019, 230, 157–163. [Google Scholar] [CrossRef]
- Nazari, L.; Yuan, Z.; Ray, M.B.; Xu, C.C. Co-conversion of waste activated sludge and sawdust through hydrothermal liquefaction: Optimization of reaction parameters using response surface methodology. Appl. Energy 2017, 203, 1–10. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous hydrothermal liquefaction of biomass in a novel pilot plant with heat recovery and hydraulic oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Biller, P.; Johannsen, I.; Dos Passos, J.S.; Ottosen, L.D.M. Primary sewage sludge filtration using biomass filter aids and subsequent hydrothermal co-liquefaction. Water Res. 2018, 130, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Malins, K.; Kampars, V.; Brinks, J.; Neibolte, I.; Murnieks, R.; Kampare, R. Bio-oil from thermo-chemical hydro-liquefaction of wet sewage sludge. Bioresour. Technol. 2015, 187, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Toor, S.S.; Conti, F.; Nielsen, A.H.; Rosendahl, L.A. Hydrothermal liquefaction of high ash containing sewage sludge at sub and supercritical conditions. Biomass Bioenergy 2020, 135, 105504. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Liu, L.; Wang, Y.; Jing, Z.; Wang, S. Comprehensive evaluation on product characteristics of fast hydrothermal liquefaction of sewage sludge at different temperatures. Energy 2018, 159, 686–695. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Savage, P.E. Hydrothermal liquefaction of sewage sludge under isothermal and fast conditions. Bioresour. Technol. 2017, 232, 27–34. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Q.; Meng, H.; Han, W.; Li, J.; Zhang, J. Catalytic liquefaction of municipal sewage sludge over transition metal catalysts in ethanol-water co-solvent. Bioresour. Technol. 2018, 249, 361–367. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, H.; Xu, B.; Xiang, B.; Chen, Z.; Li, C.; Zeng, G. Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: Yield and composition of bio-oil, immobilization and risk assessment of heavy metals. Bioresour. Technol. 2014, 159, 72–79. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Waqas, M.; Easa, A.; Attia, E.S.M.; Al-Jabri, H. Energy recovery and nutrients recycling from municipal sewage sludge. Sci. Total Environ. 2020, 715, 136775. [Google Scholar] [CrossRef]
- Rahman, T.; Jahromi, H.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.-S. Hydrothermal liquefaction of municipal sewage sludge: Effect of red mud catalyst in ethylene and inert ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Li, R.; Li, B.; Kai, X. Hydrothermal liquefaction of sewage sludge to produce bio-oil: Effect of co-pretreatment with subcritical water and mixed surfactants. J. Supercrit. Fluids 2019, 144, 28–38. [Google Scholar] [CrossRef]
- Liu, R.; Tian, W.; Kong, S.; Meng, Y.; Wang, H.; Zhang, J. Effects of inorganic and organic acid pretreatments on the hydrothermal liquefaction of municipal secondary sludge. Energy Convers. Manag. 2018, 174, 661–667. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of municipal sewage sludge in supercritical water: A review. Water Res. 2016, 89, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, P.; Pennarasi, M. Production of biodiesel from municipal sewage sludge by transesterification process. In Biomass Valorization Bioenergy; Springer: Singapore, 2020; pp. 97–111. [Google Scholar]
- Capodaglio, A.G.; Callegari, A.; Dondi, D. Microwave-induced pyrolysis for production of sustainable biodiesel from waste sludges. Waste Biomass Valorization 2016, 7, 703–709. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Fan, Y.; Zhang, H.; Su, Y. Influence of the reactant carbon–hydrogen–oxygen composition on the key products of the direct gasification of dewatered sewage sludge in supercritical water. Bioresour. Technol. 2016, 208, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, L.; Wei, W.; Jin, H.; Guo, L. Hydrogen production by sewage sludge gasification in supercritical water with high heating rate batch reactor. Energy 2022, 238, 121740. [Google Scholar] [CrossRef]
- Weijin, G.; Zizheng, Z.; Yue, L.; Qingyu, W.; Lina, G. Hydrogen production and phosphorus recovery via supercritical water gasification of sewage sludge in a batch reactor. Waste Manag. 2019, 96, 198–205. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Zhang, H.; Ma, Q.; Su, Y.; Fan, Y. Influence of NaOH and Ni catalysts on hydrogen production from the supercritical water gasification of dewatered sewage sludge. Int. J. Hydrogen Energy 2014, 39, 19947–19954. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Gong, M.; Su, Y.; Fan, Y. Influence of H2O2 and Ni catalysts on hydrogen production and PAHs inhibition from the supercritical water gasification of dewatered sewage sludge. J. Supercrit. Fluids 2017, 130, 183–188. [Google Scholar] [CrossRef]
- Fan, Y.; Zhu, W.; Gong, M.; Su, Y.; Zhang, H.; Zeng, J. Catalytic gasification of dewatered sewage sludge in supercritical water: Influences of formic acid on hydrogen production. Int. J. Hydrogen Energy 2016, 41, 4366–4373. [Google Scholar] [CrossRef]
- Amrullah, A.; Matsumura, Y. Supercritical water gasification of sewage sludge in continuous reactor. Bioresour. Technol. 2018, 249, 276–283. [Google Scholar] [CrossRef]
- Sawai, O.; Nunoura, T.; Yamamoto, K. Supercritical water gasification of sewage sludge using bench-scale batch reactor: Advantages and drawbacks. J. Mater. Cycles Waste Manag. 2014, 16, 82–92. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Chen, C.; Zhang, H.; Lin, N.; Su, Y. Influence of reaction conditions on the catalytic activity of a nickel during the supercritical water gasification of dewatered sewage sludge. J. Supercrit. Fluids 2018, 140, 356–363. [Google Scholar] [CrossRef]
- Hantoko, D.; Kanchanatip, E.; Yan, M.; Weng, Z.; Gao, Z.; Zhong, Y. Assessment of sewage sludge gasification in supercritical water for H2-rich syngas production. Process Saf. Environ. Prot. 2019, 131, 63–72. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of sewage sludge in supercritical water and evaluation of the combined process of supercritical water gasification and oxidation. Bioresour. Technol. 2015, 176, 218–224. [Google Scholar] [CrossRef]
- Qi, J.; Zhu, F.; Wei, X.; Zhao, L.; Xiong, Y.; Wu, X.; Yan, F. Comparison of biodiesel production from sewage sludge obtained from the A2/O and MBR processes by in situ transesterification. Waste Manag. 2016, 49, 212–220. [Google Scholar] [CrossRef]
- Melero, J.; Sánchez-Vázquez, R.; Vasiliadou, I.; Castillejo, F.M.; Bautista, L.; Iglesias, J.; Morales, G.; Molina, R. Municipal sewage sludge to biodiesel by simultaneous extraction and conversion of lipids. Energy Convers. Manag. 2015, 103, 111–118. [Google Scholar] [CrossRef]
- Choi, O.; Song, J.; Cha, D.; Lee, J. Biodiesel production from wet municipal sludge: Evaluation of in situ transesterification using xylene as a cosolvent. Bioresour. Technol. 2014, 166, 51–56. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of sludge features and extraction-esterification technology on the synthesis of biodiesel from secondary wastewater treatment sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef]
- Patiño, Y.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of pretreatments and catalytic route in the quality and productivity of biodiesel obtained from secondary sludge. Biomass Bioenergy 2021, 152, 106195. [Google Scholar] [CrossRef]
- Supaporn, P.; Yeom, S.H. Optimization of a two-step biodiesel production process comprised of lipid extraction from blended sewage sludge and subsequent lipid transesterification. Biotechnol. Bioprocess Eng. 2016, 21, 551–560. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, F.; Qi, J.; Zhao, L.; Yan, F.; Li, C. Challenge of biodiesel production from sewage sludge catalyzed by KOH, KOH/activated carbon, and KOH/CaO. Front. Environ. Sci. Eng. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, F.; Dong, Y.; Wu, X.; Sun, Y.; Zhang, D.; Zhang, T.; Han, M. Function promotion of SO42−/Al2O3–SnO2 catalyst for biodiesel production from sewage sludge. Renew. Energy 2020, 147, 275–283. [Google Scholar] [CrossRef]
- Arazo, R.O.; de Luna, M.D.G.; Capareda, S.C. Assessing biodiesel production from sewage sludge-derived bio-oil. Biocatal. Agric. Biotechnol. 2017, 10, 189–196. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.; Kim, Y.-H.; Lin, K.-Y.A.; Chen, W.-H.; Tsang, Y.F.; Kwon, E.E. Use of sewage sludge biochar as a catalyst in production of biodiesel through thermally induced transesterification. Biochar 2022, 4, 67. [Google Scholar] [CrossRef]
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The role of microbial electrolysis cell in urban wastewater treatment: Integration options, challenges, and prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef]
- Hiegemann, H.; Herzer, D.; Nettmann, E.; Lübken, M.; Schulte, P.; Schmelz, K.-G.; Gredigk-Hoffmann, S.; Wichern, M. An integrated 45 L pilot microbial fuel cell system at a full-scale wastewater treatment plant. Bioresour. Technol. 2016, 218, 115–122. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Cotterill, S.; Dolfing, J.; Jones, C.; Curtis, T.; Heidrich, E. Low temperature domestic wastewater treatment in a microbial electrolysis cell with 1 m2 anodes: Towards system scale-up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Lin, L.; Dhar, B.R. Shift of biofilm and suspended bacterial communities with changes in anode potential in a microbial electrolysis cell treating primary sludge. Sci. Total Environ. 2019, 689, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Im, S.; Chung, J.-W. Optimizing the operating temperature for microbial electrolysis cell treating sewage sludge. Int. J. Hydrogen Energy 2017, 42, 27784–27791. [Google Scholar] [CrossRef]
- Moreno, R.; San-Martín, M.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Liang, P.; Duan, R.; Jiang, Y.; Zhang, X.; Qiu, Y.; Huang, X. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res. 2018, 141, 1–8. [Google Scholar] [CrossRef]
- Rossi, R.; Jones, D.; Myung, J.; Zikmund, E.; Yang, W.; Gallego, Y.A.; Pant, D.; Evans, P.J.; Page, M.A.; Cropek, D.M. Evaluating a multi-panel air cathode through electrochemical and biotic tests. Water Res. 2019, 148, 51–59. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Liu, J.; Zhu, X.; Feng, Y.; Logan, B.E. Microbial fuel cells with an integrated spacer and separate anode and cathode modules. Environ. Sci. Water Res. Technol. 2016, 2, 186–195. [Google Scholar] [CrossRef]
- Park, Y.; Park, S.; Yu, J.; Torres, C.I.; Rittmann, B.E.; Lee, T. Complete nitrogen removal by simultaneous nitrification and denitrification in flat-panel air-cathode microbial fuel cells treating domestic wastewater. Chem. Eng. J. 2017, 316, 673–679. [Google Scholar] [CrossRef]
- Blatter, M.; Delabays, L.; Furrer, C.; Huguenin, G.; Cachelin, C.P.; Fischer, F. Stretched 1000-L microbial fuel cell. J. Power Sources 2021, 483, 229130. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Marsili, E.; Ghosh, P.C. Treatment of domestic and distillery wastewater in high surface microbial fuel cells. Int. J. Hydrogen Energy 2014, 39, 21819–21827. [Google Scholar] [CrossRef]
- Ali, A.E.-H.; Gomaa, O.M.; Fathey, R.; Abd El Kareem, H.; Abou Zaid, M. Optimization of double chamber microbial fuel cell for domestic wastewater treatment and electricity production. J. Fuel Chem. Technol. 2015, 43, 1092–1099. [Google Scholar] [CrossRef]
- He, W.; Dong, Y.; Li, C.; Han, X.; Liu, G.; Liu, J.; Feng, Y. Field tests of cubic-meter scale microbial electrochemical system in a municipal wastewater treatment plant. Water Res. 2019, 155, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.I.; Qandil, M.D.; Al-Haddad, M.R.; Saravani, M.S.; Amano, R.S. Utilization of Hydroturbines in Wastewater Treatment Plants. J. Energy Resour. Technol. 2019, 141, 062011. [Google Scholar] [CrossRef]
- Llácer-Iglesias, R.M.; López-Jiménez, P.A.; Pérez-Sánchez, M. Hydropower Technology for Sustainable Energy Generation in Wastewater Systems: Learning from the Experience. Water 2021, 13, 3259. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; Di Berardino, S.; Amorim, F.; Girio, F.; Rangel, C.; de Leao, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Zawadzki, P.; Kończak, B.; Smoliński, A. Municipal wastewater reclamation: Reclaimed water for hydrogen production by electrolysis–A case study. Measurement 2023, 216, 112928. [Google Scholar] [CrossRef]
- Loots, I.; Van Dijk, M.; Barta, B.; Van Vuuren, S.; Bhagwan, J. A review of low head hydropower technologies and applications in a South African context. Renew. Sustain. Energy Rev. 2015, 50, 1254–1268. [Google Scholar] [CrossRef]
- Power, C.; McNabola, A.; Coughlan, P. Development of an evaluation method for hydropower energy recovery in wastewater treatment plants: Case studies in Ireland and the UK. Sustain. Energy Technol. Assess. 2014, 7, 166–177. [Google Scholar] [CrossRef]
- Tomczyk, P.; Mastalerek, K.; Wiatkowski, M.; Kuriqi, A.; Jurasz, J. Assessment of a Francis Micro Hydro Turbine Performance Installed in a Wastewater Treatment Plant. Energies 2023, 16, 7214. [Google Scholar] [CrossRef]
- Power, C.; Coughlan, P.; McNabola, A. Microhydropower energy recovery at wastewater-treatment plants: Turbine selection and optimization. J. Energy Eng. 2017, 143, 04016036. [Google Scholar] [CrossRef]
- Chae, K.-J.; Kim, I.-S.; Ren, X.; Cheon, K.-H. Reliable energy recovery in an existing municipal wastewater treatment plant with a flow-variable micro-hydropower system. Energy Convers. Manag. 2015, 101, 681–688. [Google Scholar] [CrossRef]
- Durrani, A.M.; Mujahid, O.; Uzair, M. Micro hydro power plant using sewage water of Hayatabad Peshawar. In Proceedings of the 2019 15th International Conference on Emerging Technologies (ICET), Peshawar, Pakistan, 2–3 December 2019; pp. 1–5. [Google Scholar]
- Hasan, A.; Salem, A.R.; Hadi, A.A.; Qandil, M.; Amano, R.S.; Alkhalidi, A. The power reclamation of utilizing micro-hydro turbines in the aeration basins of wastewater treatment plants. J. Energy Resour. Technol. 2021, 143, 081301. [Google Scholar] [CrossRef]
- Ak, M.; Kentel, E.; Kucukali, S. A fuzzy logic tool to evaluate low-head hydropower technologies at the outlet of wastewater treatment plants. Renew. Sustain. Energy Rev. 2017, 68, 727–737. [Google Scholar] [CrossRef]
- Petran, V.; Fernandes, W.; Topiwala, H.; McKeown, N.; Nardi, J.; Phillis, M.; Richardson, T.; Raza, S.; O’Sullivan, D. The Power of Wastewater-Micro Hydro Turbine at the Clarkson Wastewater Treatment Plant. Proc. Water Environ. Fed. 2015, 2015, 4271–4285. [Google Scholar] [CrossRef]
- Bousquet, C.; Samora, I.; Manso, P.; Rossi, L.; Heller, P.; Schleiss, A.J. Assessment of hydropower potential in wastewater systems and application to Switzerland. Renew. Energy 2017, 113, 64–73. [Google Scholar] [CrossRef]
- Gretzschel, O.; Schäfer, M.; Steinmetz, H.; Pick, E.; Kanitz, K.; Krieger, S. Advanced wastewater treatment to eliminate organic micropollutants in wastewater treatment plants in combination with energy-efficient electrolysis at WWTP Mainz. Energies 2020, 13, 3599. [Google Scholar] [CrossRef]
- Chauhan, D.; Ahn, Y.-H. Alkaline electrolysis of wastewater and low-quality water. J. Clean. Prod. 2023, 397, 136613. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, Y.; Pan, S.-Y.; Chiang, P.-C. Integration of green energy and advanced energy-efficient technologies for municipal wastewater treatment plants. Int. J. Environ. Res. Public Health 2019, 16, 1282. [Google Scholar] [CrossRef]
- Musabandesu, E.; Loge, F. Load shifting at wastewater treatment plants: A case study for participating as an energy demand resource. J. Clean. Prod. 2021, 282, 124454. [Google Scholar] [CrossRef]
- EMWD. Energy Efficiency Programs. EMWD—Eastern Municipal Water District. Available online: https://www.emwd.org/post/energy-efficiency-programs (accessed on 4 June 2022).
- Ali, I.; Abdelkader, L.; El Houssayne, B.; Mohamed, K.; El Khadir, L. Solar convective drying in thin layers and modeling of municipal waste at three temperatures. Appl. Therm. Eng. 2016, 108, 41–47. [Google Scholar] [CrossRef]
- Khanlari, A.; Tuncer, A.D.; Sözen, A.; Şirin, C.; Gungor, A. Energetic, environmental and economic analysis of drying municipal sewage sludge with a modified sustainable solar drying system. Sol. Energy 2020, 208, 787–799. [Google Scholar] [CrossRef]
- Wang, P.; Mohammed, D.; Zhou, P.; Lou, Z.; Qian, P.; Zhou, Q. Roof solar drying processes for sewage sludge within sandwich-like chamber bed. Renew. Energy 2019, 136, 1071–1081. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, M.; Jia, L. Assessment on thermal behavior of municipal sewage sludge thin-layer during hot air forced convective drying. Appl. Therm. Eng. 2016, 96, 209–216. [Google Scholar] [CrossRef]
- Khanlari, A.; Sözen, A.; Afshari, F.; Şirin, C.; Tuncer, A.D.; Gungor, A. Drying municipal sewage sludge with v-groove triple-pass and quadruple-pass solar air heaters along with testing of a solar absorber drying chamber. Sci. Total Environ. 2020, 709, 136198. [Google Scholar] [CrossRef] [PubMed]
- Boncescu, C.; Robescu, L.D. The Impact of Photovoltaic Power System on Carbon Footprint in the Wastewater Treatment Plant. In Proceedings of the 2021 10th International Conference on ENERGY and ENVIRONMENT (CIEM), Bucharest, Romania, 14–15 October 2021; pp. 1–5. [Google Scholar]
- Piergrossi, V.; De Sanctis, M.; Chimienti, S.; Di Iaconi, C. Energy recovery capacity evaluation within innovative biological wastewater treatment process. Energy Convers. Manag. 2018, 172, 529–539. [Google Scholar] [CrossRef]
- Quintero Pulido, D.F.; Barreto, C.M.; Ten Kortenaar, M.V.; Balda, R.R.; Hurink, J.L.; Smit, G.J. Simulation of sizing of energy storage for off-grid decentralized wastewater treatment units: A case study in the Netherlands. Water Pract. Technol. 2018, 13, 771–779. [Google Scholar] [CrossRef]
- Mbarga, A.H.A.; Rainwater, K.; Song, L.; Cleveland, T.; Williams, W.R. Economic Analyses of the Seadrift Wind-Aided Wastewater Treatment Plant Operations. Tex. Water J. 2021, 12, 42–57. [Google Scholar] [CrossRef]
- Duarte, P.T.; Duarte, E.A.; Murta-Pina, J. Increasing self-sufficiency of a wastewater treatment plant with integrated implementation of anaerobic co-digestion and photovoltaics. In Proceedings of the 2018 International Young Engineers Forum (YEF-ECE), Costa da Caparica, Portugal, 4 May 2018; pp. 103–108. [Google Scholar]
- del Moral, A.; Petrakopoulou, F. Evaluation of the coupling of a hybrid power plant with a water generation system. Appl. Sci. 2019, 9, 4989. [Google Scholar] [CrossRef]
- Andrei, H.; Badea, C.A.; Andrei, P.; Spertino, F. Energetic-environmental-economic feasibility and impact assessment of grid-connected photovoltaic system in wastewater treatment plant: Case study. Energies 2020, 14, 100. [Google Scholar] [CrossRef]
- Jacob, R.; Short, M.; Belusko, M.; Bruno, F. Maximising renewable gas export opportunities at wastewater treatment plants through the integration of alternate energy generation and storage options. Sci. Total Environ. 2020, 742, 140580. [Google Scholar] [CrossRef]
- Islam, A.K. Hydropower coupled with hydrogen production from wastewater: Integration of micro-hydropower plant (MHP) and microbial electrolysis cell (MEC). Int. J. Hydrogen Energy 2024, 49, 1–14. [Google Scholar] [CrossRef]
- Mehr, A.; Gandiglio, M.; MosayebNezhad, M.; Lanzini, A.; Mahmoudi, S.; Yari, M.; Santarelli, M. Solar-assisted integrated biogas solid oxide fuel cell (SOFC) installation in wastewater treatment plant: Energy and economic analysis. Appl. Energy 2017, 191, 620–638. [Google Scholar] [CrossRef]
- Gandiglio, M.; Mehr, A.S.; MosayebNezhad, M.; Lanzini, A.; Santarelli, M. Solutions for improving the energy efficiency in wastewater treatment plants based on solid oxide fuel cell technology. J. Clean. Prod. 2020, 247, 119080. [Google Scholar] [CrossRef]
- ACUA. Jersey-Atlantic Wind Farm—Fact Sheet. ACUA—Atlantic City Utilities Authority. Available online: https://travel.sygic.com/en/poi/acua-wastewater-treatment-facility-poi:12844 (accessed on 26 May 2024).
- Day, D. Wind Turbines From Goldwind USA Help The Field’s Point Treatment Plant Save $1 Million A Year. Available online: https://www.tpomag.com/editorial/2015/02/wind_turbines_from_goldwind_usa_help_the_fields_point_treatment_plant_save (accessed on 28 May 2024).
- City of Pueblo (Colo.). Recognized for Using Solar Power at Wastewater Treatment Facility. US EPA—Environmental Protection Agency. Available online: https://www.environmental-expert.com/news/city-of-pueblo-colo-recognized-for-using-solar-power-at-wastewater-treatment-facility-258583 (accessed on 25 May 2024).
- Greer, D. Directed Biogas To Power Fuel Cells. BioCycle Energy. Available online: https://www.biocycle.net/directed-biogas-to-power-fuel-cells/ (accessed on 27 April 2022).
- OYJ, O. Outotec to Build the Largest and Most Advanced Sewage Sludge Thermal Treatment Plant in Switzerland. Available online: https://www.metso.com/corporate/media/news/2012/11/outotec-to-build-the-largest-and-most-advanced-sewage-sludge-thermal-treatment-plant-in-switzerland/ (accessed on 10 September 2024).
- Massachusetts Department of Energy Resources. Renewable Energy Case Study: Deer Island Wastewater Treatment Plant, Mass. Water Resources Authority. 2017. Available online: https://www.mass.gov/doc/renewable-energy-installations-at-deer-island/download (accessed on 17 July 2024).
- Enel-x. Eastern Municipal Water District Works with Enel X to Earn Payments in California Demand Response. Available online: https://www.enelx.com/n-a/en/resources/case-studies/eastern-municipal-water-district (accessed on 7 June 2024).
- Enbala. Revenue Generating Smart Grid Technology—A Pennsylvania American Water Case Study. Available online: https://www.paawwa.org/wp-content/uploads/2013/06/23Hufton.pdf (accessed on 8 June 2024).
- Generac. Puget Sound Energy: Using Real-Time Demand Response Technology to Defer Investments in Fossil Fuel; Generac: Waukesha, WI, USA, 2021. [Google Scholar]
- Abdin, I.; Zio, E. Optimal planning of electric power systems. In Optimization in Large Scale Problems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 53–65. [Google Scholar]
- Hunter Water Corporation. Renewable Energy Project. Available online: https://www.hunterwater.com.au/community/major-projects-in-your-area/renewables (accessed on 15 June 2024).
- Carbon and Energy. Icon Water. Available online: https://www.iconwater.com.au/water-education/sustainability-and-environment/sustainability-and-environment-programs/carbon-and-energy (accessed on 15 June 2024).
- ARENA. Sewage Treatment Plants Turn Sludge into Liquid Fuel. Available online: https://arena.gov.au/blog/sewage-treatment-plants-turn-sludge-into-liquid-fuel/ (accessed on 5 June 2024).
- ARENA. Sewage Treatment Plant Smells Success in Synthetic Gas Trial. Available online: https://arena.gov.au/blog/logan-gasification-sewage-treatment-plant/ (accessed on 11 June 2024).
- Melbourne Water Annual Report. Melbourne Water. 2023. Available online: http://www.melbournewater.com.au (accessed on 10 August 2024).
- Energy Management and Climate. SA Water. Available online: https://www.sawater.com.au/water-and-the-environment/recycling-and-the-environment/energy-management-and-climate (accessed on 15 June 2022).
- Energy Management and Climate Change. Sydney Water. Available online: https://www.sydneywater.com.au/water-the-environment/what-we-are-doing/energy-management-climate-change.html (accessed on 15 June 2022).
- Annual Report 2021. Water Corporation. 2021. Available online: https://pw-cdn.watercorporation.com.au/-/media/WaterCorp/Documents/About-us/Our-performance/Annual-Reports/2021-Annual-Report/Water_Corporation_Annual_Report_2021.pdf?rev=06dffd8387764bf6ad20708d7abab6ed&hash=319404485B22EBA7C62A3E80ABDBE5F1 (accessed on 11 September 2024).
- Schäfer, M.; Gretzschel, O.; Schmitt, T.G.; Knerr, H. Wastewater treatment plants as system service provider for renewable energy storage and control energy in virtual power plants–a potential analysis. Energy Procedia 2015, 73, 87–93. [Google Scholar] [CrossRef]
- Brok, N.B.; Munk-Nielsen, T.; Madsen, H.; Stentoft, P.A. Unlocking energy flexibility of municipal wastewater aeration using predictive control to exploit price differences in power markets. Appl. Energy 2020, 280, 115965. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Safder, U.; Nguyen, X.N.; Yoo, C. Multi-objective decision-making and optimal sizing of a hybrid renewable energy system to meet the dynamic energy demands of a wastewater treatment plant. Energy 2020, 191, 116570. [Google Scholar] [CrossRef]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants–market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef]

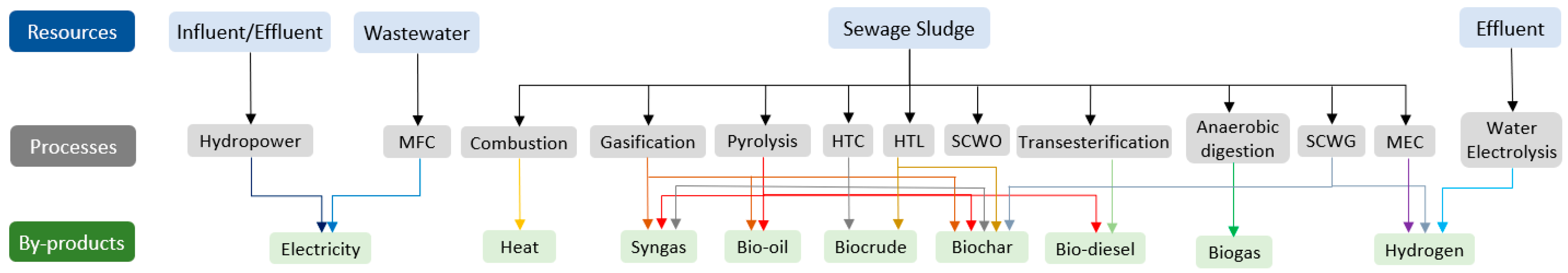
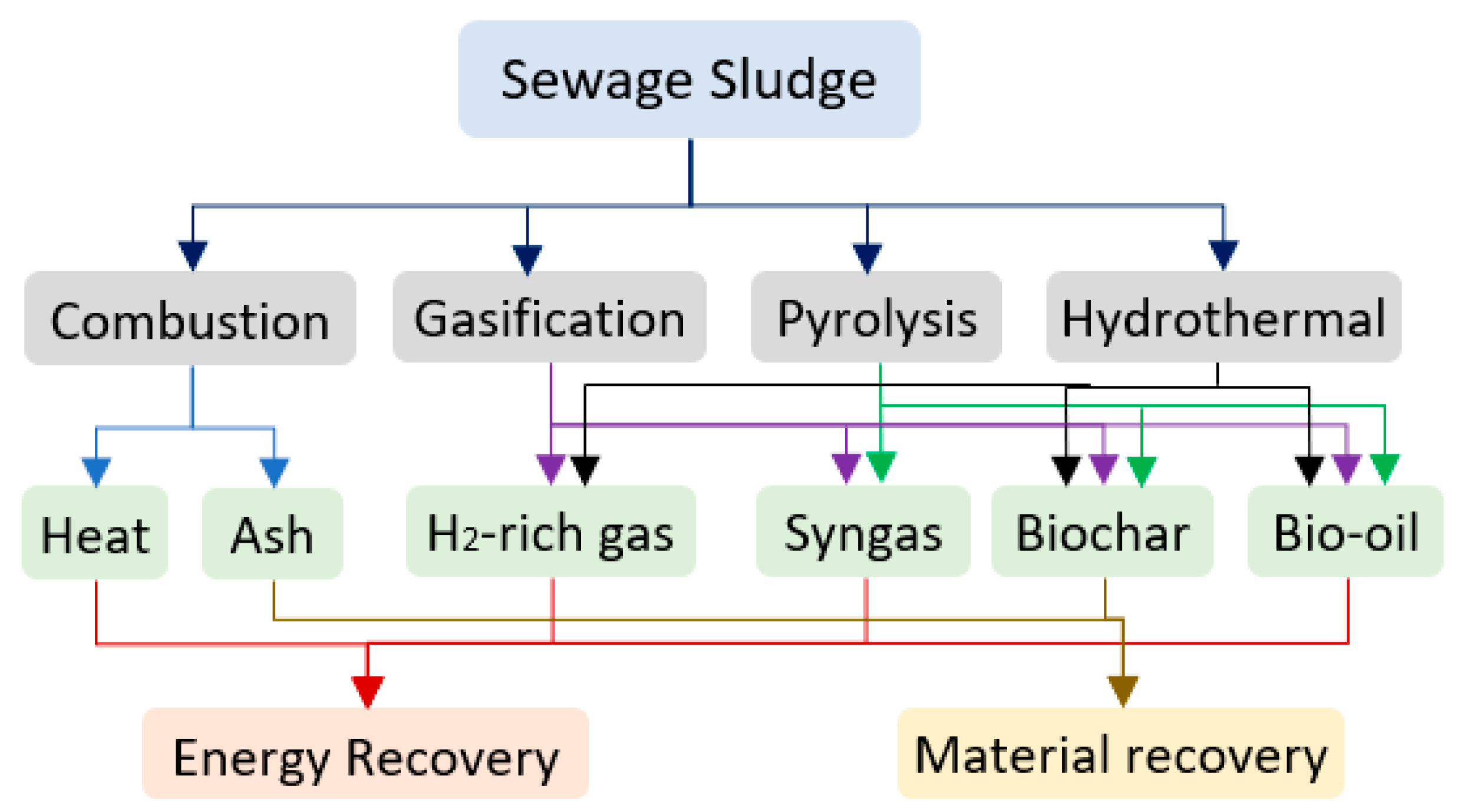
| Type | Process | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| Th | SS heated (150–200 °C) under pressure, breaking down complex OC and making SS more digestible. | - Enhance biodegradability - Reduce pathogens and OC - Increase biogas production | - High CAPEX and OPEX - High energy consumption | - Used before AD - Improve biogas yield |
| M | SS is exposed to microwave radiation to rupture cell structures of the solid particles within SS and disrupt OM, improving digestion. | - Increases production - Reduces pathogens - Improves biogas yield - Enhances dewaterability | - High CAPEX and OPEX - High energy consumption - Require controlled temperature to avoid OM degradation | - Used before AD - SS with high OC - Co-digestion processes |
| Uc | High-frequency sound waves (ultrasonication) are used to disrupt SS particles, improving degradability. | - Improve dewaterability - Increase production - Reduce need for chemicals | - High CAPEX and OPEX - Energy-intensive process - Need of special equipment | - Increase biogas yield - SS with high MC - Co-digestion processes |
| Ud | High-frequency sound waves (20 kHz–1 MHz) to disrupt SS particles to cause cavitation, improving digestibility. | - Enhance digestibility - Increase dewaterability - Reduce pathogens | - High energy consumption - Expensive equipment - Need special setup and O&M | - SS dewatering - SS with high OC - Co-digestion processes |
| O | Ozone gas is bubbled through SS, breaking down OC and killing pathogens. | - Strong pathogen control - Effective in reducing odors - Increases biodegradability | - High operational cost - Requires energy-intensive ozone generation | - Odor control - Enhance degradability - Before AD |
| FA | Apply high pressure and high temperature to SS in the presence of water or organic additives. | - Enhance biodegradability - Increase production - Reduce need for chemicals | - High CAPEX and OPEX - High energy consumption | - Enhance AD efficiency - Improve biogas yield - SS with high OC |
| Ref. | Substrate | Parameters and System Design | OLR (gVS/L.d) | VS Removal (%) | Methane Production (L/kgVS) | Methane Concentration (%) | ||
|---|---|---|---|---|---|---|---|---|
| [27] | SS and FW | 6 × 5 L reactor, 37 °C, 22 days HRT, 10–50% FW:SS ratios | Min: 2.8|Max: 4.2 | Min: 66|Max: 76 | SS: 230–280|FW: 290–330 | Co-d: 318–385 | SS: 45–57|FW: 49–57 | Co-d: 53–55.7 |
| [28] | SS and FW | 2 L reactor, 30–38 °C, 22 days HRT, ratios (1:1, 1:2 and 1:3) | NI | Min: 51.47|Max: 60.36 | SS: 356–478|FW: 511 | Co-d: 453–609 | SS: 53|FW: 50.4 | Co-d: 52–70.3 |
| [29] | SS and FW | 5 L reactor, 30–38 °C, 22 days HRT, different mixing ratios (1:1, 1.5:1, 2:1, 1:1.5 and 1:2) | 0.5–7 g VS/L | Min: 82.8 Max: 87.7 | SS: 625.4 FW: 385.9–507.5 | Co-d: 384.6–492 | SS: 55.9–58.6 FW: 58.8 | Co-d: 53–60.4 |
| [30] | SS and FW | 6 L reactor volume, 35 °C, 8–30 days HRT and three different SS:FW mixing ratio (2.4:1, 0.9:1, 0.4:1) | Min: 4.6 Max: 18.5 | Min: 39.7 Max: 70 | SS: 157–237 FW: 377–465 | Co-d: 215–400 | SS: 63–65 FW: 50–54 | Co-d: 53–61 |
| [31] | SS and FVW | 100 L reactor, 35 °C, 11–14 days HRT | Min: 1.46 Max: 2.8 | Min: 35 Max: 43 | VW: 335 SS: 84 | Co-d: 90–430 | NI | NI |
| [32] | SS, GTS, OFMSW | 2 × 6 L reactor, 38 °C, 20 days HRT and 5–30% mixing ratio | Min: 1.15 Max: 2.17 | Min: 52 Max: 65 | SS: 300|GTS: NI OFMSW: NI | Co-d: 456–547 | SS: 66|GTS: NI OFMSW: NI | Co-d: 66–69 |
| [33] | TWAS and RS | 0.25 L reactor, 37 °C, 50 days HRT, mixing ratio of 1:1 and 1:3 on volume basis | 5% TS | Min: 34.5 Max: 69.1 | RS: 216.3 TWAS: 184.6 | Co-d: 304 | NI | NI |
| [34] | SS and RS | 1.2 L reactor, 35 °C and 55 °C, between 25 (55 °C) and 75 days (35 °C) HRT, 4:1 (weight basis) mixing ratio | 20% TS | Min: 61 (35 °C) Min: 70.2 (55 °C) | RS: 222 (35 °C), 248 (55 °C) SS: 308 (35 °C), 344 (55 °C) | Co-d: 518 (35 °C), 602 (55 °C) | NI | Co-d: 36–60 |
| [35] | SS and WS | 0.5 L reactor, 37 °C, 30 days, 1.15:1.94 (VS basis) ratio | 7.73 | Min: 56.38|Max: 63.59 | SS: 136.8|WS: 243 | Co-d: 176.7–333.9 | NI | NI |
| [36] | WAS, WS, and RS | 0.15 L reactor, 30 days HRT, 1:1 mixing ratio | 4 g VS/L | NI | RS: 95|WS: 103 WAS: 87 | Co-d: 36–223 | RS: 50|WS: 48 WAS: 50 | Co-d: 30–58 |
| [37] | SS and GTW | 0.25 L reactor, 35 °C, 10–31 days HRT, 4 mixing ratios (14%, 24%, 43% and 39% GTW) | NI | Min: 31 Max: 35 | SS: 223 GTW: 606 | Co-d: 214–517 | NI | NI |
| [38] | SS and GTW | 6 L reactor, 35 °C, 15 days HRT, 1:1 mixing ration (PS and WAS) + GTW 5% VS. | 2.93 | Min: 47 Max: 59 | SS: 384 GTW: NI | Co-d: 641 | SS: 61 GTW: NI | Co-d: 69 |
| [39] | SS and FOG | For 55 °C: 10 L reactor, 20 days HRT. For 70 °C: 2 L reactor, and 2 days HRT. Mixing ratios: 20, 40, 60 and 80% FOG | NI | Min: 46.8 Max: 82 | SS: 138.3 FOG: NI | Co-d: 102–673 | SS: 61.6–62.8 FOG: NI | Co-d: 49.7–67.3 |
| [40] | SS and FOG | 1 L reactor, 35 °C, 15 days HRT, 4 mixing ratios (14%, 24%, 43% and 39% GTW) | 4 g COD/L.d | Min: 43.1 Max: 54.6 | SS: 114–128 FOG: 143–290 | Co-d: 453–609 | SS: 62.8–71.2 FOG: 63.3 | Co-d: 66.1–68.4 |
| [41] | SS, FW and G | 0.25 L reactor, 37 °C, 20 days HRT, 1:1 mixing ratio (SS:FW) with 1% and 3% glycerol. | 0.66–1.1 | Min: 8.6 Max: 17.4 | SS: 138.3 FW: NI|G: NI | Co-d: 236–526 | SS: 85.9% FW: NI|G: NI | Co-d: 77.4–79.4 |
| [42] | SS and G | 5.5 L reactor, 35 and 55 °C, 20 days HRT, 2 SS:G mixing ratios (99:1, 98.8:1.2 v/v%) | 1.2–1.6 (35 °C); 1.1–1.3 (55 °C) | Min: 36 (35 °C), 45 (55 °C) Max: 64 (35 °C), 73 (55 °C) | SS: 296 (35 °C), 354 (55 °C) G: 277–475 (35 °C), 349–490 (55 °C) | Co-d: 250–660 (35 °C) 230–530 (55 °C) | NI | Co-d: 63–72 (35 °C) 57–66 (55 °C) |
| [43] | SS and PM | 0.16 L reactor, 35 °C, 47 days HRT, different SS:PM mixing ratios (21:1, 14:1, 7:1 VS) | 0.54 (average) | Min: 56.3 Max: 71.4 | SS: 182 PM: 239 | Co-d: 190–200 | SS: 52–58 PM: 52–58 | Co-d: 52–58 |
| [44] | SS, SM PoM | 1 L (b) and 3 L (s-c) reactor, 35 °C, 15–30 days HRT, different mixing ratios | 1.27–2.86 (s-c) | 37.9–45.8 (b) 23.9–38 (s-c) | SS: 184 (b) PoM: NI|SM: NI | Co-d: 198–290 (b); 186–273 (s-c) | SS: 67–68 PoM: NI|SM: NI | Co-d: 67–68 |
| [45] | SS, WV PoM | 5 L reactor, 35 °C, 6–20 days HRT, 50:50 w/w% SS:WV mixing ratio plus 10 g/L of PoM | NI | Min: 38 Max: 58 | SS: 130 (10 days HRT) PM: NI|WV: NI | Co-d: 210–261 | NI | NI |
| [46] | SS and CM | 1 L reactor, 37 °C, 20 days HRT, 3 CM:SS mixing ratio (30:70, 50:50, 70:30 w/w%) | NI | Min: 78.4 Max: 97.3 | NI | Co-d: 335–511 | NI | NI |
| [47] | SS and M | 70 L reactor, 35–37 °C, 30 days, SS:M ratio of 0.2–1.8% VS | NI | Min: 4|Max: 35 | NI | Co-d: 226 | NI | Co-d: 79–85.5 |
| [48] | WAS and M | 0.13 L reactor, 35 °C, 25–30 days HRT, 4 WAS:M mixing ratio (3:1, 1:1, 1:3) | NI | NI | WAS: 362 M: 318 | Co-d: 354–442 | NI | NI |
| Pyrolysis Type | Residence Time | Temperature | Heating Rate | Pressure |
|---|---|---|---|---|
| Slow | >60 min | 300–700 °C | 0.1–1 °C/s | 0.1 MPa |
| Intermediate | ~10 min | 500–650 °C | 1–10 °C/s | |
| Fast | 0.5–20 s | 550–1250 °C | 10–300 °C/s | |
| Flash | <0.5 s | 800–1300 °C | >1000 °C/s | |
| Microwave | 30 min | 500–1000 °C | <5 °C/s | - |
| Vacuum | 30–120 min | 300–600 °C | 0.1–1 °C/s | 0.01–0.02 MPa |
| Hydro | 240 min | 350–600 °C | 10–300 °C/s | 5–20 MPa |
| Ref. | Type | Sludge Collection | Operating Parameters | Biogas Production/Methane Yield |
|---|---|---|---|---|
| [49] | Th | WWTP in Valladolid, Spain | System include steam boiler, 1.5 L hydrolysis reactor connected to a 5 L flash tank. Reactor loaded with 0.75 L sludge, under 110–200 °C, and 10–50 min. | yield increased up to 50% (thermal) under 30 min under 180–200 °C. production improve up to 45% using thermal compared to conventional process. |
| [50] | Th | WWTP, in Korea. | The hydrolysis thermal plant had the capacity to treat 1 ton SS/cycle. SS was dewatered before the treatment and the thermal hydrolysis process operated at 75–225 °C and 15–105 min reaction time. | Optimal parameters included 76 min reaction and 180 °C. At 150 °C and 1 h reaction, the maximum yield was 273.2 mL/g COD (40% increase compared to control). The minimum yield was 221.7 mL/g COD under 30 min and 200 °C. |
| [51] | Th and FA | WAS obtained from a WWTP in Changsha, China. | Three temperatures (35, 50 and 70 °C) were used. Reactors fed with 0.4 L of WAS, and different concentrations of ammonium stock solution was used (220–450 mg). FA concentrations between 79.7 and 163.1 mg and operate at 25 °C. | Without any treatment (control), the biochemical methane potential (BMP) was 183.4–200.6 mL/g VSS. With FA only and thermal only, the BMP was 188.1–201.8 mL/g VSS, and 184.1–196.9 mL/g VSS, respectively. With combined methods, BMP varied between 195.7 and 229.4 mL/g VSS. |
| [52] | Th and Uc | Ulu Pandan municipal WWTP, in Singapore. | Batch mode and operating temperature of 35 °C. Sewage sludge treated with ultrasonic at 5 MJ/kg TS and optimal temperature of 65 °C for thermal treatment. | Combining thermal and ultrasonic (30 s, 5000 kJ/kg TS) resulted in high COD solubilisation (760–10,200 mg/L), proteins (115–2900 mg/L) and carbohydrates (60–660 mg/L) than a single treatment. Biogas production improved by 20%. |
| [53] | Th, M, and Uc | Copero urban WWTP in Seville, Spain. | Batch mode and operating temperature of 35 °C. Thermal parameters included 75 L volume autoclave, 120 °C, 2 atm for 15 min. Microwave parameters included 100–900 W at 80 °C for 1.4 min. Ultrasonic parameters: 6 L volume 25 °C, atm pressure, 150 W power generator for 45 min. | production improved by 20%, 29%, and 95% based on microwave, thermal and sonication, respectively. The specific energy applied for the thermal, sonication and microwave was 36, 102 and 20 kJ/g TS, respectively. Sludge solubility increased by 19.2% using thermal and 83.4% using microwave. |
| [54] | M | WWTP located in the city of Leon, Spain. | Microwave oven (2450 MHz frequency) was used. SS samples were irradiated with a power output of 650–900 W. Reaction was carried out at 34 °C in 2 × 3 L reactors with mechanical stirrers. A 30:70 mixing ratio of PS:WAS was used. SS samples irradiated at energy values of 975 kJ/L. | yield without treatment was 166, 209, 213, and 226 mL/g VS considering 5, 10, 20, and 25 days, respectively. With microwave treatment, the yield increased to 214, 295, 308, and 324 mL/g VS for a 5, 10, 20, and 25 days of retention times respectively. yield increased between 29% and 43%. |
| [55] | M | Copero urban WWTP in Spain. | Pre-treatment applied (400 W and 700 W), and the specific energy used was up to 30 kJ/g TS and maximum temperature of 100 °C. | yield for raw SS was 111 mL/g VS. Using microwave pre-treatment, yield increased to 118–130 mL/g VS. |
| [56] | M | Qinghe WWTP in Beijing, China | 2 L lab-scale reactors with 112 rpm and 20 days HRT. OLR of 2.92 g VS/L.d at 37 °C. Microwave- pre-treatment was used. A 2-stage reactor was set up with a 0.65 L bottle (the first stage reactor) in series with a 2 L reactor. | production improved from 215.5 mL/g VS (control) to 258.4 mL/g VS (microwaved) for a 1-stage reactor. For 2-stage, yield increased from 258.4 mL/g VS (control) to 288.3 mL/g VS (microwaved). The 2-stage was 11.6% higher than 1-stage. |
| [57] | Uc | Full-scale WWTP located in Antwerp-South, Belgium. | Ultrasound reactor at 10 L/min fixed flow rate and 25 kHz frequency applied. Power up to 1 kW was used. The AD happened at 37 °C and retention time of 3 weeks in a 1 L reactor filled with 0.5 L WAS. | production increased up to 20% using the pre-treatment. For a 23 day reaction, production was 126 mL/g DS, and 1.014 kWh/kg DS energy value. Energetic content of the surplus biogas by ultrasonic was 0.195 kWh/kg DS. |
| [58] | Th and Ud | Biobío WWTP, in Concepción, Chile. | Ud and low-thermal (55 °C) pre-treatment were conducted. Ud using specific energies of 0.5, 15.5 and 30.5 MJ/kg TS. Th treatment with retention times of 3, 8 and 13 h were also performed. | production ranged between 472 and 611 mL/g V based on 0.5–30.5 MJ/kg TS (Ultrasound) at for 3–13 h reaction, The yield increased between 16% and 50% using the methods. |
| [59] | M and Uc | WWTP in of Mechelen-Noord, Belgium. | 0.6 L reactor used with 500 g SS. Applied 100 W power for 8 min, and 800 W power for 1 min. Total energy of 96 kJ/kg SS to both treatments. | Biogas production increased to 0.26–0.28 L/g VS compared to 0.22 L/g VS (control case). Biogas improved by 27% and 20% based on Uc and M treatments, respectively. Both methods were considered not cost feasible. |
| [60] | U and O | Municipal WWTP, in Singapore. | 130 W and 20 kHz (ultrasound) and 180 W (ozonation) applied to a 0.2 L of sewage sludge. | Biogas production improved by 11–15.4%. The maximum production rate increased from 3.53 (control) to 4.32–4.54 mL /d. |
| FC | Micro GT | GT | ICE | |
|---|---|---|---|---|
| Technology status | Emerging | Mature | Mature | Mature |
| Capacity (kW) | 200–1200 | 30–250 | 1200–4700 | 110–3700 |
| Electrical Efficiency (%) | 36–45 | 26–30 | 26–37 | 30–42 |
| Thermal Efficiency (%) | 30–40 | 30–37 | 30–52 | 35–49 |
| CH4 minimum level (%) | 85 | 40 | 30 | 60 |
| Emissions | Extremely low | Very low | Low | Medium/High |
| Capital costs (USD/kW) | 3800~5280 | 800~1650 | 1100~2000 | 465~1600 |
| O&M costs (USD/kWh) | 0.004~0.019 | 0.012~0.025 | 0.008~0.014 | 0.01~0.025 |
| Ref. | Type | Study Aim | Operating Parameters |
|---|---|---|---|
| [5] | FC | Study the Castiglione WWTP in Italy to achieve self-sufficiency based on co-digestion and SOFC system. |
|
| [65] | FC | Assess the economic benefits of using SOFC in a WWTP. |
|
| [66] | FC | Study a SOFC system in a WWTP in the UK. WWTP serves 750,000 PE and 105,000 m3/day inflow. |
|
| [67] | FC | Integration of a SOFC in Parand WWTP to eva-luate economic benefits. Two objective functions were optimised. |
|
| [68] | GT | Investigate the optimum size of a gas turbine system in a sewage Wastewater plant which serves about 100,000 people. |
|
| [69] | GT | Study the feasibility of a GT system to supply energy in a real WWTP, in Iran, which treats around 74.2 mL of sewage daily. |
|
| [70] | GT | Study a GT system in the Bali WWTP in Taiwan that has a daily treatment capacity of 1320 mL. |
|
| [71] | GT | Investigates the injection of hydrogen into biogas to generate electricity in a WWTP. |
|
| [72] | ICE | Investigate the combined utilisation of syngas and biogas to power a CHP system in a WWTP. |
|
| [73] | ICE | Study the potential of a biogas-fuelled system to supply electricity to 5 WWTPs, in Spain. |
|
| [74] | ICE | Investigated the biogas energy potential in 4 WWTPs in Brazil. |
|
| [75] | FC and GT | Investigate a system of SOFC and GT in Collegno WWTP, in Italy, which serves around 270,000 PE. |
|
| [76] | GT, ORC, chiller | Investigate the benefits of a CHP system in WWTPs. |
|
| [77] | FC, ICE and GT | Study the opportunities and challenges of using FC, ICE and GT on 6 WWTPs. |
|
| [78] | SCWG and CC | Study an integrated system for production and power generation. |
|
| Type | Details | Efficiency | Op. Costs | Scale | Benefits | Limitations |
|---|---|---|---|---|---|---|
| FiBR | Stationary bed, batch or continuous operation | Low to moderate (15–25%) | Moderate (USD 30~80/ ton SS) | Small | - Simple design - Low capital cost - Easy to operate - Well-established | - Lower throughput - Low heat transfer - Energy efficiency |
| FBR | Granular material, heated by upward gas flow, continuous operation | Moderate to high (25–40%) | Moderate (USD 50~120/ ton SS) | Large | - High heat transfer efficiency - High throughput - Uniform heating - Well-established | - Complex design - Requires filtration - Higher capital cost |
| RKR | Rotating drum, externally heated, continuous operation | Moderate (20–35%) | High (USD 70~150/ ton SS) | Large | - High throughput - Suitable for high moisture content - Well-established | - High energy consumption - Expensive maintenance |
| AR | Screw conveyor moves SS through a heated zone continuously | Moderate to high (20–35%) | Moderate (USD 40~90/ ton SS) | Small/ Medium | - Continuous operation - Easy to control - Low maintenance | - Limited scalability - Needs specialized equipment - Less mature |
| MR | Direct heating using microwave radiation | High (50–70%) | High (USD 90~150/ ton SS) | Small | - Fast heating - High energy efficiency - Smaller footprint - Uniform heating | - Limited scalability - High capital cost - Complex control systems - Emerging (pilot-scale) |
| VR | Pyrolysis under reduced pressure, improving bio-oil quality and volatile product yields. | Moderate to high (30–50%) | High (USD 70~130/ ton of SS) | Small/ Medium | - High-quality bio-oil - Improved energy recovery - Better volatile product yields | - Requires vacuum pumps - High capital cost - Complex design - Emerging (limited use) |
| CSR | Conical reactor with particles suspended by gas flow. SS is fed in from the top and heated through gas flow. | Moderate (25–35%) | Moderate (USD 50~100/ ton of SS) | Medium/ Large | - Efficient heat transfer - Can handle particles of varying sizes - Uniform heating | - Complex design - Requires precise flow control - Less scalable than fluidized beds - Less mature |
| BFBR | Reactor with a bubbling action caused by gas flow and typically used for lower-density feedstocks. | Moderate (20–35%) | Moderate (USD 50~120/ ton of SS) | Small/ Medium | - Simple design - Moderate heat transfer - Suitable for moderate-sized sludge feedstocks | - Lower efficient heat transfer - Requires gas–solid separation - Mature (widely used) |
| CFBR | Fluidised bed with a continuous loop of particles in motion and continuous operation | High (30–50%) | High (USD 60~150/ ton of SS) | Large | - High heat transfer efficiency - Suitable for large-scale use - Uniform heating - Well-established | - Complex design - High energy consumption - Expensive maintenance |
| PR | Pyrolysis using high-temperature plasma arc | Very high (60–80%) | Very high (USD 150~300/ ton of SS) | Small/ Medium | - Very high temperatures (up to 10,000 °C) - Capable of decomposing almost all feedstocks | - Extremely high capital and operating cost - Need of special equipment - High energy use - Emerging (high-tech) |
| SR | Uses concentrated solar energy to heat the sludge for pyrolysis. | Moderate to high (20–40%) | Low (USD 80~150/ ton of SS) | Small/ Medium | - Renewable energy source - Sustainable | - Dependent on sunlight - Requires large infrastructure - Expensive setup |
| Type | Temperature (°C) | Pressure (MPa) | Water State | Generated Products | Advantages |
|---|---|---|---|---|---|
| HTC | 150–280 | 0.1–11 | Subcritical | Solid (char), small amount of liquid (biocrude) and gas | Sludge stabilisation, volume reduction, fertiliser |
| HTL | 280–375 | 8–22 | Sub/near critical | Liquid (biocrude), small amount of solid (char), water-soluble fractions | Use wet sludge without the need for drying |
| HTG | >375 | >22.1 | Super-critical | Gas (Syngas), small solid amount (char), and water-soluble fractions | Produce high concentrations of |
| Ref. | Plant | Study Aim | Parameters/Methods | Gas Production and Utilisation |
|---|---|---|---|---|
| [191] | The WWTP Mainz, in Germany. Wastewater inflow up to 55 mL/d (peaks of 6.300 mL/h. | Studied a 1.25 MW Electrolyser system in a WWTP. PV and CHP system to power the electrolyser. could be injected into the gas network or power FC buses. O2 could generate ozone to be used in the advanced WW treatment | WWTP power consumption of 8200 MW/y, PV generation of 227 MWh/y, CHP generation (using biogas) of 6173 MWh/y and 1800 MWh/y power bought from the grid. | and production was about 2975 MWh/year and 600 ton/year, respectively. Assuming 2% vol of feed-in limit, 1240 MWh of could be fed into the gas network per year. The remaining 1735 MWh of could be used in fuel cell buses in public transport. |
| [192] | RWW, PE, SE, TE, and SW collected from a WWTP in Gyeongsan city, South Korea. | Generate from low-grade wastewater using alkaline water splitting technology. Based on this investigation, the potential applicability for achieving energy independence in municipal WWTP is planned | WW filtered through UF membrane to produce ATW. After treatment, COD, TN, TDS in the treated effluent were around 2.8–37.9 mg/L, 0.9–28.7 mg/L and 44–377 mg/L, respectively. | production based on low-grade water was between 19.2 and 22.8 mL/h.L, whereas based on a UF treatment, it was between 20.4 and 23.4 mL/h.L for the different WW samples. For a deionised and tap waters (control), production was 23.6–26.6 mL/h.L. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, D.; Li, L.; Appleby, G. A Review of Renewable Energy Technologies in Municipal Wastewater Treatment Plants (WWTPs). Energies 2024, 17, 6084. https://doi.org/10.3390/en17236084
Lima D, Li L, Appleby G. A Review of Renewable Energy Technologies in Municipal Wastewater Treatment Plants (WWTPs). Energies. 2024; 17(23):6084. https://doi.org/10.3390/en17236084
Chicago/Turabian StyleLima, Derick, Li Li, and Gregory Appleby. 2024. "A Review of Renewable Energy Technologies in Municipal Wastewater Treatment Plants (WWTPs)" Energies 17, no. 23: 6084. https://doi.org/10.3390/en17236084
APA StyleLima, D., Li, L., & Appleby, G. (2024). A Review of Renewable Energy Technologies in Municipal Wastewater Treatment Plants (WWTPs). Energies, 17(23), 6084. https://doi.org/10.3390/en17236084








