Abstract
For spark ignition and compression ignition ammonia engines, a typical approach to ensure stable operation involves the blending of ammonia with hydrogen and diesel, respectively. For the ammonia/hydrogen fuel, in this study a comprehensive comparison was conducted firstly for the differences among existing chemical mechanisms according to the experimental data of ignition, oxidation, and flame propagation. The result indicates that the current reaction mechanisms for ammonia/hydrogen fuel exhibit high prediction accuracy only within limited condition ranges. Subsequently, considering the completeness of combustion reaction pathways for ammonia/hydrogen fuel, a chemical mechanism of ammonia and ammonia/hydrogen fuel was developed and optimized in this study, and the comprehensive validation demonstrates the accuracy of the developed mechanism. On this basis, the ammonia mechanism was integrated with the detailed n-heptane mechanism to derive a mechanism for ammonia/diesel fuel that includes 1351 species and 6227 reactions. The good performance of this mechanism was demonstrated in terms of the experimental data of ignition and oxidation. In addition, the ignition sensitivity and reaction pathways of ammonia/hydrogen fuel were investigated based on the constructed mechanism, and the significance of C3–C7/N reactions was also analyzed for the ammonia/diesel fuel ignition process.
1. Introduction
To address global warming and decrease greenhouse gas emissions, clean and effective alternative fuels for diesel engines have become an important solution [1]. As a zero-carbon fuel, ammonia (NH3) does not emit carbon dioxide after combustion. Storage and transport of ammonia also has obvious advantages [2]. However, ammonia faces some challenges. The auto-ignition temperature of ammonia is high, and the flame speed is slow [3]. Additionally, nitrous oxide (N2O) can be produced from the ammonia combustion, and the global climate impact of N2O is nearly 300 times greater than that of carbon dioxide over a 100-year period [4]. For the former challenge, combining NH3 with high-reactivity fuels such as diesel and hydrogen presents an effective approach to address these issues. For spark-ignition ammonia engines, the combination of ammonia and hydrogen is a primary method to accelerate ammonia combustion and improve in-cylinder combustion, allowing the engine to operate in the ammonia/hydrogen fuel mode. For compression-ignition ammonia engines, diesel is one of the primary pilot ignition fuels, which ignites ammonia after ignition itself through compression. In this case, the engine operates in an ammonia/diesel fuel mode. For the later challenge, considering the coexistence of NH3 and N2O in the ammonia engine exhaust, much attention has been paid to the simultaneous removal of NH3 and N2O through the selective catalytic reduction (SCR) [5].
To accurately predict the in-cylinder combustion process of ammonia engines, one approach is to couple the fuel reaction mechanism with multidimensional fluid dynamics, thereby establishing a numerical model for in-cylinder combustion of ammonia engines. Therefore, for both spark-ignition and compression-ignition ammonia engines, the chemical mechanisms of ammonia/hydrogen and ammonia/diesel fuel are essential for conducting accurate in-cylinder simulation. Hydrogen is an important intermediate species in the ammonia combustion, and the hydrogen sub-mechanism is one of the indispensable components of the ammonia mechanism. Theoretically, the ammonia mechanism has the capability to describe the ammonia/hydrogen fuel combustion process. However, considering the nonlinear characteristics of chemical reaction processes [6], further research is needed to demonstrate whether the existing ammonia mechanism can adequately describe the combustion process of ammonia/hydrogen fuel. At the same time, an accurate ammonia mechanism is a crucial component for the reaction mechanism for ammonia/diesel fuel. Therefore, this study focuses on the reaction mechanisms relevant to ammonia/hydrogen and ammonia/diesel fuels.
Several researches have been conducted on the chemical mechanism relevant to ammonia and ammonia/hydrogen fuels. Otomo et al. [7] developed a mechanism for ammonia oxidation with 32 species and 213 reactions, focusing on NH2, HNO, and N2H2. This mechanism accurately reproduces experimental data on laminar flame velocities (LBVs) and ignition delay times (IDTs) across various equivalence ratios (ϕ) and pressures (p), and it has been used to study the NH3/H2 fuel combustion. However, the predicted values of LBVs for NH3/H2 fuel are found to be lower than experimental values. Shrestha et al. [8] conducted experimental studies on LBVs of ammonia in the condition of oxygen-enriched air and also investigated the LBVs of ammonia/hydrogen/air mixtures at various pressures and temperatures. A chemical kinetic for ammonia was developed, comprising 125 species and 1099 reactions. Stagni et al. [9] conducted experiments to measure the mole fractions of fuel, oxidizer, and final products at 126.7 kPa. In order to investigate the oxidation characteristics of NH3/H2 at low and medium temperatures and under dilution conditions, a mechanism containing 31 species and 203 reactions was constructed. At ϕ = 0.25 and 1, Zhang et al. [10] conducted oxidation experiments on NH3/H2 fuels at hydrogen contents of 0–70% in fuels. A chemical mechanism for NH3 and NH3/H2 was established, which involves 38 species and 263 reactions. The prediction of this mechanism for the concentration of oxidation NH3/H2 fuels is in agreement with the experimental results.
The mechanism constructed by Bertolino et al. [11] is optimized based on LBVs, species concentrations, and IDTs from Stagni et al. [12] This optimization contains 31 species and 203 reactions, which has good agreement with the data over the whole range of experimental conditions. Gotama et al. [13] investigated the LBVs and Markstein length of NH3/H2/air flames under various conditions. A mechanism for NH3/H2 combustion with 26 species and 119 reactions was constructed using the measured LBVs, which allows a good simulation of the measurements from the current experiment. Han et al. [14] focused on the NH3/syngas fuel and designed a chemical mechanism for ammonia/syngas, comprising 35 species and 177 reactions. A detailed kinetic analysis of the NH3 oxidation process was performed. To enhance the accuracy in predicting the oxidation of ammonia, Zhou et al. [15] developed a reaction mechanism for ammonia, ammonia/hydrogen, ammonia/syngas, and ammonia/methane fuels based on the Aramco 3.0 mechanism [16] and the Glarborg mechanism [17]. The reactions were updated related to NH2, NNH, N2H2, and NOx. The proposed mechanism is comprised of 169 species and 1268 reactions, and it was validated extensively against the experimental data of LBVs, oxidation, and IDTs. Singh et al. [18,19] proposed a mechanism containing 32 species and 259 reactions for ammonia and ammonia/hydrogen fuels. The calculated results of this mechanism for molar fractions of NH3, O2, and H2O during the ammonia oxidation process were found to be in good agreement with the experimental data at low and medium temperatures and high pressures. For ammonia/hydrogen combustion, the mechanism is able to predict the IDTs and LBVs under a wide range of conditions. Based on current experimental data, Girhe et al. [20] performed a comprehensive evaluation of 16 current NH3 and NH3/H2 mechanisms. It was found that the prediction performance varied widely among the mechanisms, and no single mechanism was able to make comprehensive predictions under all conditions.
Attention has also been paid to the chemical mechanisms of ammonia/diesel fuels. Considering the similarity of cetane number between n-heptane(nC7H16) and diesel, existing studies have used nC7H16 as a single-component representative fuel for diesel. On this basis, the detailed mechanism of NH3/nC7H16 fuel has been studied by several researchers, including Yu et al. [21], Dong et al. [22], Thorsen et al. [23], and Pan et al. [24]. The mechanisms for NH3 and nC7H16 used in these studies were identical and proposed by Glarborg et al. [17] and Zhang et al. [25], respectively. Specifically, the IDTs of NH3/nC7H16 were investigated by Yu et al. [21] with the ammonia fractions of 0–40% at T = 635–945 K. A detailed mechanism of NH3/nC7H16 was constructed, showing the capability to capture variations in IDTs at concerned initial conditions. Dong et al. [22] optimized the rate constants for several reactions in the constructed NH3/nC7H16 detail mechanism. The study shows that this mechanism can accurately predict the IDTs and LBVs of NH3/nC7H16 under various experimental conditions. The oxidation mole fraction of NH3/nC7H16 was measured by Thorsen et al. [23] at 100 atm pressure and T = 400–900 K. Based on these measurements, an NH3/nC7H16 mechanism was developed, which incorporated C3-C7/N reactions and updated the HO2 reactions. The study indicated that it is crucial to update the reaction rates of nC7H16-NH2 and NH2-HO2, as this will result in the development of improved predictive mechanisms for the ignition and oxidation processes of NH3/nC7H16 fuel. Pan et al. [24] measured the mole fraction distribution of key intermediate species involved in the NH3/nC7H16 oxidation process over a T = 500–1200 K. Based on these measurements, a detailed NH3/nC7H16 mechanism was developed. Then an analysis was conducted on the interaction between nC7H16 and ammonia under low to moderate temperatures. Additionally, Wang et al. [26] and Xu et al. [27] conducted studies on reduced mechanisms for NH3/nC7H16. Wang et al. [26] integrated the nC7H16 skeletal mechanism proposed by Chang et al. [28] with the ammonia mechanism developed by Dong et al. [22]. This resulted in the construction of a comprehensive NH3/nC7H16 mechanism. Further simplification of this mechanism yields a simplified NH3/nC7H16 mechanism with 74 species and 495 reactions. Xu et al. [27] coupled the NH3 mechanism from Bertolino et al. [11] with the nC7H16 skeletal mechanism from Chang et al. [28], developing a NH3/nC7H16 mechanism that includes 69 species and 389 reactions. The combustion process under specified conditions can be well predicted by this mechanism while maintaining high computational efficiency.
Currently, several studies have been conducted on the reaction mechanism for ammonia/hydrogen, but there is a lack of a comprehensive comparison of different ammonia/hydrogen mechanisms based on existing experimental data. In the ammonia/n-heptane mechanisms, although multiple reactions involving C-N interactions have been coupled, the specific roles of these reactions remain unclear. Therefore, the aim of this study is to compare the currently proposed ammonia/hydrogen mechanisms based on the existing experimental data, clarifying the differences among the various ammonia/hydrogen mechanisms. Subsequently, a new ammonia/hydrogen chemical mechanism will be developed and optimized based on the existing data. Additionally, by coupling with the n-heptane reaction mechanism, a chemical mechanism for ammonia/diesel fuel will be developed along with a discussion on the importance of C-N reactions.
2. Simulation and Analysis Methods
In this study the CHEMKIN-PRO 15112 software was utilized for simulations of IDTs, LBVs, and species concentrations using the mechanism. Specifically, under constant volume adiabatic conditions, this study employs a zero-dimensional homogeneous closed reactor module to calculate IDTs. The time corresponding to the maximum concentration of OH radicals was used to define IDTs. To account for the effects of heat loss, the volume change during the compression ignition process is considered when calculating the IDTs in the rapid compression machine (RCM). Simulated LBVs were obtained with the premixed laminar flame module. The concentration of oxidizing species is calculated using the perfectly stirred reactor module, with the end time set to 10 times the residence time.
In the sensitivity analysis, this study calculates the sensitivity coefficients for IDTs through Equation (1)
where Si is the sensitivity coefficient for the i-th reaction; τ(2ki) and τ(0.5ki) denote the IDTs predicted value when pre-exponential factors of the i-th reaction is multiplied by 2 and 0.5, respectively. τ(ki) denotes the IDTs predicted by the initial mechanism. This study analyzes the global reaction pathway of ignition of the ammonia/hydrogen fuel, focusing on the reaction pathway at the point where 20% of the fuel is consumed.
3. Development of the Chemical Kinetic Models
3.1. Comparisons of Ammonia/Hydrogen Kinetic Models
The current ammonia/hydrogen reaction mechanisms primarily involve the Zhang mechanism [10], Stagni mechanism [9], Gotama mechanism [13], Otomo mechanism [7], Shrestha mechanism [8], Han mechanism [14], Zhou mechanism [15], Bertolino mechanism [11], Singh mechanism [19], as shown in Table 1. To identify the ammonia/hydrogen reaction mechanism with the best overall predictive performance, simulations of ignition, laminar flame, and oxidation processes were conducted using the nine mechanisms mentioned above. The results obtained were then compared and analyzed against the currently published experimental data.

Table 1.
Reaction mechanisms for the ammonia/hydrogen fuel.
3.1.1. Ignition Delay Times
The IDTs for each ammonia/hydrogen mechanism were derived at p = 1.2 atm and 10 atm with ϕ = 1 and 0–70% H2, and the calculated results and the experimental data of Chen et al. [29] are shown in Figure 1.
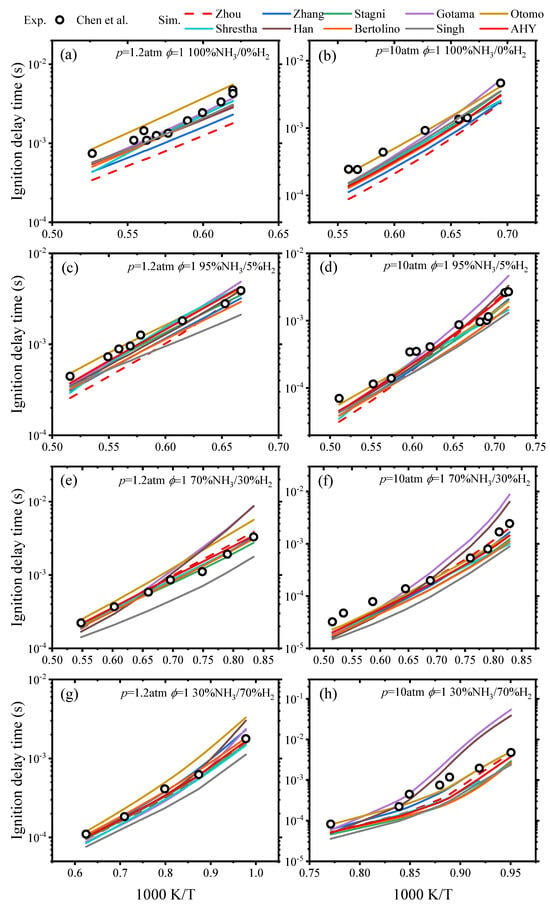
Figure 1.
Comparisons of predicted IDTs from various ammonia/hydrogen mechanisms with experimental values at different pressures and H2 fractions [29].
As shown in Figure 1, the IDTs of NH3/H2 blends reduce by increasing H2 fraction. This indicates that under the current conditions, the addition of hydrogen can promote the ignition of ammonia. The Otomo mechanism can effectively predict the IDTs at 5–70% H2 under p = 1.2 atm and 10 atm. However, within the T = 1175–1425 K, the Otomo mechanism slightly overestimates the IDTs when the H2 is 30% and p = 1.2 atm. This indicates that the improved kinetics of NH2, HNO, and N2H2 by the Otomo mechanism are justified. The Gotama mechanism and Han mechanism slightly overestimate the IDTs within the low-temperature range under conditions of 30% H2 and 70% H2 at 10 atm while providing accurate predictions under other conditions. The Zhou mechanism underpredicts the IDTs in the high-temperature range for pure ammonia and 5% H2 at 1.2 atm while providing good predictions under other conditions. The Zhang mechanism generally underpredicts the IDTs for pure ammonia conditions. Under the condition of 5% H2 at 1.2 atm, the simulated IDTs of the Zhang mechanism are lower than the experimental value. However, at 10 atm, the simulated values of the Zhang mechanism agree well with the experimental results. The Stagni mechanism underpredicts the IDTs for 0–70% H2 at 10 atm. The Shrestha mechanism and Bertolino mechanism both underpredict the IDTs at 70% H2 and p = 10 atm while providing good predictions under other conditions. In accordance with the predictions of the Singh mechanism, the IDTs is accurately estimated in pure ammonia conditions. However, in the H2 fraction over 30%, the predicted values are much lower.
3.1.2. Species Concentrations in Oxidation Process
The molar fractions of H2O, NH3, NO, and N2O under various temperatures for ϕ = 0.25 and 1 and different H2 contents were simulated using each ammonia/hydrogen mechanism, where the residence time was 1 s and p = 1 atm. The simulated results are shown in Figure 2 and Figures S1–S8 in the Supplementary Material, compared with the experimental values from Zhang et al. [10].
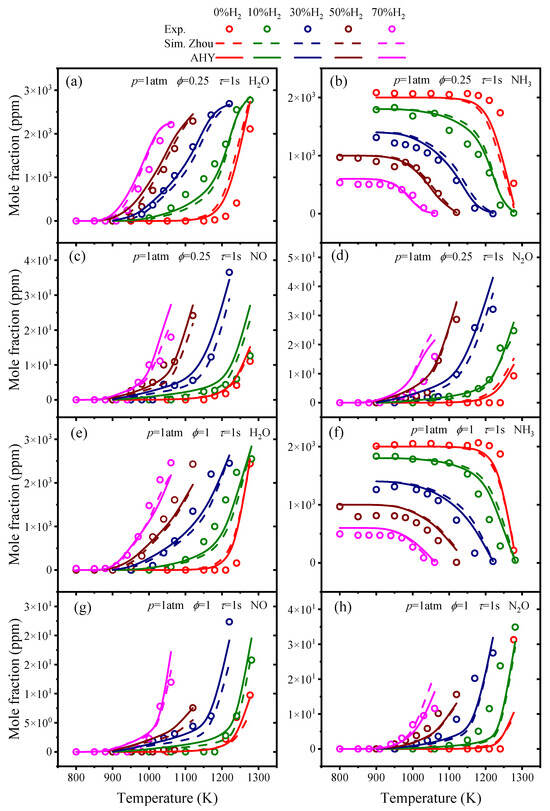
Figure 2.
Comparisons of experimental values and predicted oxidation concentrations of species from the Zhou mechanism and AHY mechanism.
From Figure 2 and the Supplemental Figures S1–S8, it can be observed that the nine NH3/H2 mechanisms mentioned show good predictive capability for the oxidation concentrations of H2O and NH3. The Bertolino mechanism, Singh mechanism, Shrestha mechanism, Han mechanism, Gotama mechanism, and Otomo mechanism exhibit significant prediction deviations for NO and N2O. The Zhang mechanism, Zhou mechanism, and Stagni mechanism provide accurate predictions for the oxidation of ammonia/hydrogen fuel.
3.1.3. Laminar Burning Velocity
The laminar burning velocity of ammonia/hydrogen fuel was modeled at different equivalence ratios using various ammonia/hydrogen reaction mechanisms with H2 fractions from 0% to 60% under conditions of 298 K and 1 atm. The simulation results are compared with experimental data [13,15,30,31,32,33,34,35,36,37], as shown in Figure 3.
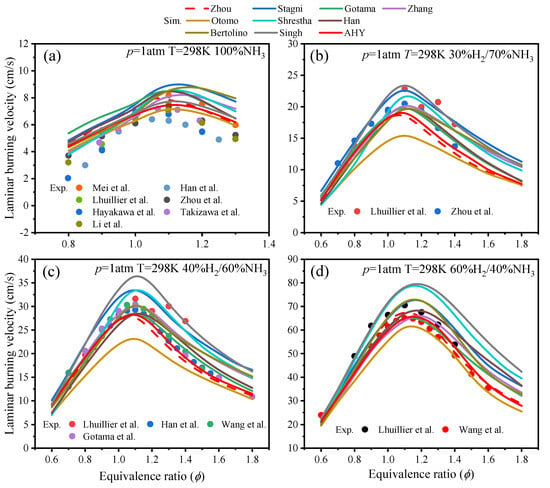
Figure 3.
Comparisons of experimental values and the simulated values of LBVs from various ammonia/hydrogen mechanisms at different temperatures and H2 fractions [13,15,30,31,32,33,34,35,36,37].
Figure 3 indicates that under pure ammonia conditions, the Otomo mechanism predicts accurately. However, it shows lower predictions for LBVs with a 15–40% hydrogen fraction. The Shrestha mechanism and Stagni mechanism both exhibit overpredictions of LBVs under all conditions. The Gotama mechanism shows overpredictions of LBVs for H2 fraction from 0% to 25%. Additionally, at 35% H2 and ϕ > 1, it also overpredicts LBVs. The Zhang mechanism and Bertolino mechanism both exhibit overpredictions of LBVs for H2 fraction from 0% to 25% under conditions with ϕ > 1. At H2 fractions of 30%, 40%, and 60%, the Zhou mechanism predicts LBVs for NH3/H2 mixtures with good accuracy. However, for pure ammonia, the LBVs prediction is high. The Singh mechanism is effective in predicting LBVs in pure ammonia conditions; however, the predicted LBVs are significantly higher with a hydrogen fraction greater than 40%.
From the above, it can be seen that the nine ammonia/hydrogen mechanisms mentioned in this study cannot provide consistent predictions for IDTs, species concentrations, and LBVs for ammonia/hydrogen, indicating discrepancies in the understanding of the NH3/H2 fuel combustion process. In summary, although the Zhou mechanism underpredicts IDTs under pure ammonia conditions, it provides relatively accurate predictions for LBVs and species concentrations of NH3/H2. Therefore, the Zhou mechanism is selected for the update of the ammonia/hydrogen mechanism.
3.2. Development of Ammonia/Hydrogen Kinetic Models
Considering the behaviors of the ammonia/hydrogen mechanisms mentioned above, in the present study a new NH3/H2 reaction mechanism was constructed. Regarding the understanding of the ammonia combustion process, Thorsen et al. [23] identified additional reactions involving HO2 + HO2, NH2 + HO2 (M), NH2 + HO2, and amine + NO2. Dong et al. [22] also emphasized the significance of NH2 + HO2 reaction. To ensure accuracy, therefore, this study integrates the mechanisms of Thorsen et al. [23] and Dong et al. [22] with the Zhou mechanism [15], resulting in a more comprehensive framework focusing on ammonia and ammonia/hydrogen fuel. Reactions affecting the IDTs were identified on the basis of sensitivity analyses. The reaction rate constant of a reaction is calculated through the Arrhenius equation k = ATnexp(−E/RT), where k, A, n, and E are the rate constant, pre-exponential factor, temperature dependence factor, and activation energy, respectively. Based on the uncertainty of the reaction rate constants, the pre-exponential factors of these reactions were adjusted to improve the prediction performance of the mechanism. The lower limit of the tuned A is 0.5 times the original value of A, and the upper limit of the tuned A is 2 times the original value. The specific reactions added into the Zhou mechanism and the tuned values of rate constants of related reactions are presented in Table 2. In this study, the optimized mechanism for ammonia/hydrogen fuel is designated as the AHY mechanism. As shown in Figure 1, Figure 2 and Figure 3, the optimized mechanism shows good prediction performance for ignition, oxidation, and flame propagation processes of ammonia and ammonia/hydrogen fuel. A detailed discussion is provided in Section 4.1.

Table 2.
Rate constants of important reactions. Units: mol, cm, s, cal.
3.3. Development of Ammonia/n-Heptane Kinetic Model
In the reaction mechanism of n-heptane, the Zhang mechanism [25] has been extensively validated and effectively captures the IDTs and oxidation component concentrations of n-heptane. Therefore, this study selects the Zhang mechanism [25] to represent the diesel mechanism.
On this basis, the current ammonia/diesel mechanism is primarily derived from the integration of the AHY mechanism and the nC7H16 mechanism of Zhang et al. [25]. In such a process, priority is given to the reactions from the n-heptane mechanism for any overlapping reactions present in both mechanisms. For the NH3/nC7H16 mechanism, the assessment of interactions between C and N represents a crucial aspect [23]. The C/N mechanism of the ammonia/hydrogen/diesel mechanism in this study is primarily divided into two parts: C1–C2/N and C3–C7/N. The AHY mechanism includes reactions associated with the C1–C2/N interactions from the Glarborg mechanism [17]. For the reactions involving C3–C7/N, the mechanism employs the C3–C7 and N reactions from both the Dong mechanism [22] and the Thorsen mechanism [23]. The C3–C7/N reactions are categorized as follows: (1) RH + NH2 = R + NH3; (2) R + NO2 = RO + NO; (3) RH + NO2 = R + HONO; (4) R + HNO = RH + NO; (5) RO2 + NO = RO + NO2. In the above reactions, R, RH, and RO2 are defined as shown in Table 3. The final integrated ammonia/diesel mechanism contains 1351 species and 6227 reactions. In this study, the developed reaction mechanism of ammonia/diesel fuel is designated as the AHP mechanism. It should be pointed out that considering the large number of species and reactions and the limited number of experimental values, there are still uncertainties for the rate constants of reactions in the proposed reaction mechanism [38].

Table 3.
Specific components of R, RH, and RO2.
4. Results and Discussion
4.1. Validation of Chemical Kinetic Models
4.1.1. Ignition, Oxidation, and Laminar Burning Velocity of Ammonia/Hydrogen Fuel
The simulation results of the AHY mechanism in this study for IDTs, species concentrations, and LBVs are shown in Figure 1, Figure 2, and Figure 3, respectively. Figure 1 demonstrates that the AHY mechanism was more accurate in the IDTs prediction at 95–100% NH3 and 1.2 and 10 atm compared to the Zhou mechanism. Under the conditions of 30% NH3 at 10 atm, the AHY mechanism shows slight improvement compared to the Zhou mechanism. Under other conditions, the predicted values of the AHY mechanism and the Zhou mechanism are in general agreement and match the experimental values. Overall, compared to other mechanisms, the AHY mechanism is able to better reproduce the effect on the IDTs of NH3/H2.
As can be seen in Figure 2, the AHY has improved accuracy in predicting LBVs for ammonia-only conditions. There is little difference in the AHY compared to the Zhou mechanism under other conditions. The AHY mechanism provides accurate predictions of LBVs for NH3/H2 fuel at H2 fractions from 0 to 60%. Figure 3 indicates that, in comparison to the Zhou mechanism, the AHY mechanism provides more accurate predictions for H2O and NH3 at ϕ = 0.25 and 1. Additionally, at ϕ = 1 and 30% H2, the AHY mechanism provides accurate predictions for NO.
Overall, the AHY mechanism effectively captures the concentrations of H2O, NH3, NO, and N2O under the current conditions for NH3/H2 fuel.
4.1.2. Ignition and Oxidation of Ammonia/n-Heptane Fuel
The IDTs of NH3/nC7H16 fuel were simulated using the AHP reaction mechanism under 20–40% NH3, ϕ = 1 and 2, and O2 concentrations of 10–18%, p = 10 and 15 bar. The simulated results were compared to the corresponding experimental values from Yu et al. [21], as illustrated in Figure 4.
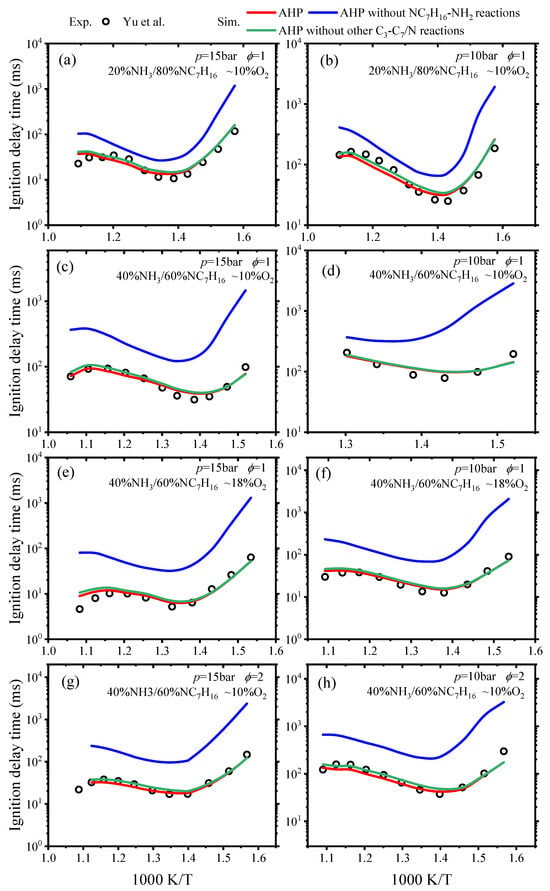
Figure 4.
Comparisons of predicted IDTs from AHP mechanism and experimental values [21].
As can be seen from the experimental values in Figure 4, the negative temperature coefficient (NTC) behavior can be seen for the fuel blend of NH3/nC7H16. There is a tendency for the IDTs to increase in line with the temperature of the fuel mixture. The comparison of simulated IDTs calculated using the AHP mechanism with experimental values demonstrates that this mechanism can effectively predict the NTC behavior of NH3/nC7H16 ignition. Figure 4a,c indicate that the increase in ammonia concentration reduces the reactivity of the NH3/nC7H16 mixture, leading to an increase in IDTs. Figure 4e,g, as well as Figure 4f,e, show that the reactivity of the NH3/nC7H16 mixture increases with higher pressure, oxygen concentration, and equivalence ratio, which could result in a decrease in IDTs. The AHP mechanism captures these phenomena over a wide temperature range. Compared to the experimental values of IDTs, the AHP mechanism slightly overestimates the IDTs in the high-temperature region under conditions of 40% NH3, p = 15 bar, ϕ = 1, and 18% O2 concentration. Overall, the AHP mechanism can accurately capture the IDTs of the NH3/nC7H16 mixture across a range of temperatures, from low to high.
In the oxidation process, Thorsen et al. [23] conducted oxidation experiments on NH3/nC7H16 at 100 atm. In experiments conducted by Pan et al. [24] at atmospheric pressure, the NH3/nC7H16 mixture consisted of 0.5% NH3, 0.5% nC7H16, 87.25% Ar, and 11.75% O2. Comparisons of predicted component concentrations from the AHP mechanism with experimental values from Thorsen et al. [23] and Pan et al. [24] are illustrated in Figure 5 and Figure 6.
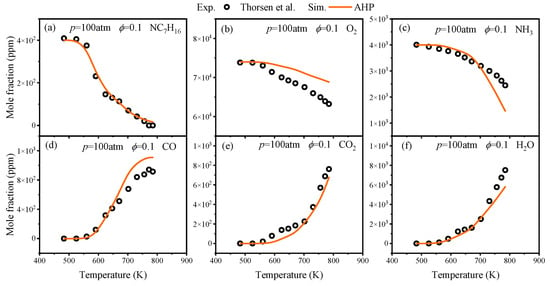
Figure 5.
Comparisons of AHP-predicted oxidation concentration values for related components with experimental values from Thorsen et al. [23].
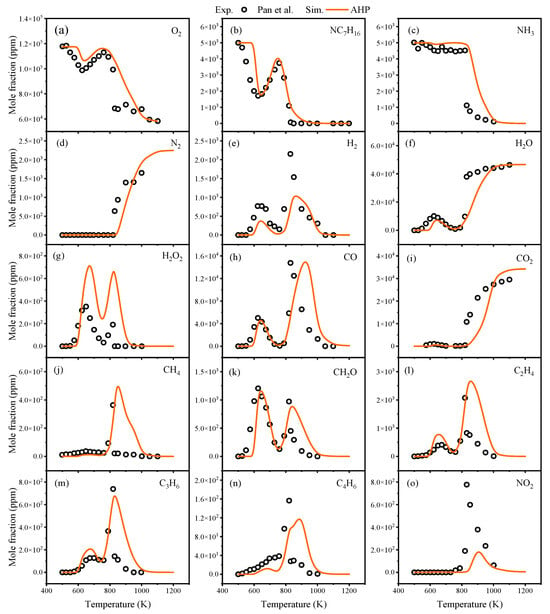
Figure 6.
Comparisons of AHP-predicted oxidation concentration values for related components with experimental values from Pan et al. [24].
Figure 5 shows that the AHP mechanism provides accurate predictions for nC7H16, NH3, and H2O under the conditions of ϕ = 0.1 and p = 100 atm. Figure 6 indicates that due to the dominance of different branching pathways, nC7H16 exhibits two distinct oxidation phases, and the AHP mechanism is capable of capturing this phenomenon. Regarding the oxidation of NH3, the dehydrogenation of NH3 at low temperatures was found to be accelerated by the addition of nC7H16, resulting in a slight oxidation reaction of NH3 at T = 600 K. Overall, across a broad temperature range, the predictions of oxidation concentrations for most species by the AHP mechanism are generally consistent with the experimental data.
4.2. Reaction Pathway and Sensitivity Analyses of Ignition for Ammonia/Hydrogen Fuel
In order to gain further insight into the impact of varying fuel blends on ammonia, this study examined reaction pathways when the ammonia consumption reaches 20%. The specific conditions were p = 40 atm, ϕ = 1, 0% and 30% H2, and T = 1400 K. The result is shown in Figure 7 and Figures S9–S11 in the Supplementary Material.
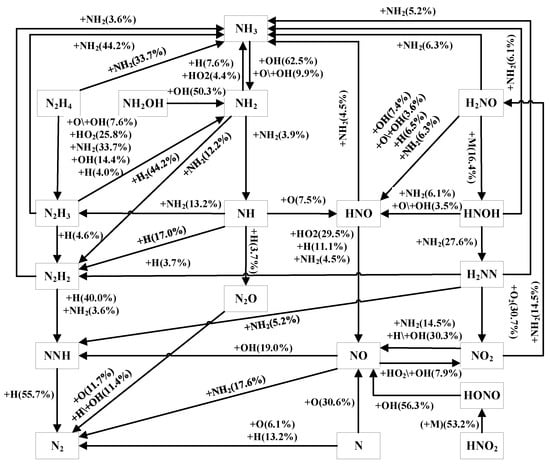
Figure 7.
Reaction pathways for the AHY mechanism at 30% H2.
From Figure 7 and Figure S9–S11, we see that for pure ammonia, compared to the Zhou mechanism, the AHY mechanism shows a reduction in the pathway flux for NH-N2H3, HNO-NH3, NO2-NH2, N2H2-N2H3, and H2NN-NH3. The oxidation pathway for NH3 remains nearly consistent. The main pathway for the oxidation of NH3 to reach N2 is NH3 → NH2 → NH → N2H2 → NNH → N2. Firstly, NH3 undergoes an abstraction reaction to generate NH2, primarily facilitated by OH radicals. The consumption pathways for NH2 primarily consist of two routes. One pathway involves the reaction of NH2 with a H radical to form N2H3. The main pathway is NH2 + NH2 → NH3 + NH, which produces the NH radical while converting NH2 into NH3. NH primarily undergoes an extraction reaction with H atoms to generate N2H2. Subsequently, N2H2 is converted to NNH, which ultimately reacts with free radical H to form N2. Under the condition of 30% H2, the AHY mechanism increases the pathway flux for N2H3 to N2H2, but decreases the pathway flux for NH to NO, NH2 to NH3, NO to N2O, and NO to N2H2.
To further investigate the AHY mechanism, the IDTs sensitivity analyses of the AHY mechanism were performed at different ammonia concentrations and temperatures. The simulation conditions were set at p = 40 atm, ϕ = 1, with an H2 fraction of 0% and 30%, and T = 1400–1800 K. The result is illustrated in Figure 8 and Figure S12.
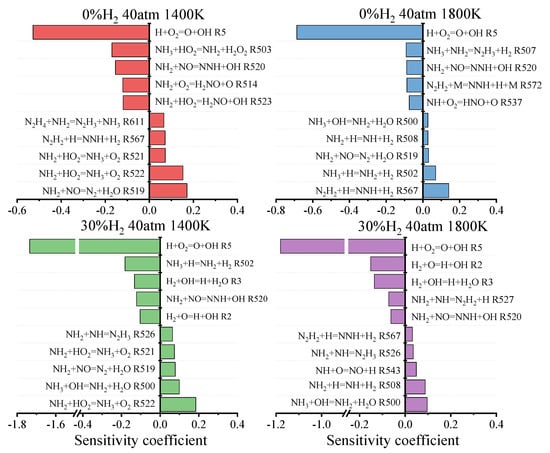
Figure 8.
Sensitivity analysis of the AHY mechanism to the IDTs under different conditions.
Figure 8 and Figure S12 in the Supplementary Material show that under the conditions of 0% H2 and 1400 K, the negative sensitivity of the reaction NH2 + HO2 = H2NO + OH increases in the AHY mechanism, while the five reactions with the maximum positive sensitivity coefficient remain unchanged compared with the Zhou mechanism. Under the conditions of 1800 K, the negative sensitivity of the reaction NH + O2 = HNO + O increases in the AHY mechanism, while the positive sensitivity of the reaction NH3 + OH = NH2 + H2O also increases. Under conditions with 30% H2, the AHY mechanism shows an increased sensitivity for the reaction NH2 + NH = N2H3 at 1400 K. At 1800 K, the negative sensitivity of the reaction NH2 + NO = NNH + OH increases, while the positive sensitivity of the reaction NH + O = NO + H also increases.
4.3. Roles of C3–C7/N Reactions in Ammonia/n-Heptane Models
As described above, the C-N reactions for the NH3/nC7H16 mechanism involve different categories. To determine the importance of different C-N reactions, modeled results of IDTs for NH3/nC7H16 fuel from the AHP mechanism, the AHP mechanism without nC7H16-NH2, and the APH mechanism without other C3–C7/N reactions were compared, and the result is shown in Figure 4. It can be seen that the absence of the nC7H16-NH2 reaction in the C3–C7/N reactions results in the AHP mechanism overpredicting the IDTs, particularly at low temperatures where the discrepancy is more pronounced. The absence of the other C3–C7/N reactions, i.e., R + NO2 = RO + NO, RH + NO2 = R + HONO, R + HNO = RH + NO, and RO2 + NO = RO + NO2, has minimal influence on IDTs. Additionally, the reaction of C3–C6/NH2 also has a minimal effect on IDTs. This phenomenon can be attributed to the H abstraction reaction between NH2 and nC7H16, which accelerates consumption of nC7H16 in the initial phase. Therefore, it can be concluded that the nC7H16-NH2 reaction is the most significant part of the C3–C7/N reactions and also exerts a considerable influence on the IDTs of NH3/nC7H16 fuel.
5. Conclusions
In this study, the differences among the NH3/H2 mechanisms are clarified by comparing modeled values from the currently proposed NH3/H2 mechanisms with the responding available test data. Then a new reaction mechanism towards NH3 and NH3/H2 fuel was constructed. On this basis, by coupling the newly developed NH3 mechanism with the nC7H16 mechanism, an ammonia/diesel mechanism was constructed. Then ignition sensitivity and reaction pathways for ammonia/hydrogen fuel and the roles of C3–C7/N reactions in the ignition of NH3/nC7H16 fuel were discussed. The main conclusions are summarized as follows:
- (1)
- Comprehensive comparisons of modeled results of IDTs, species concentrations, and LBVs from various ammonia/hydrogen mechanisms reveal with the corresponding experimental values that the existing mechanisms exhibit high prediction accuracy only within a limited condition range.
- (2)
- The newly constructed AHY mechanism for ammonia and ammonia/hydrogen combustion incorporates essential reactions from the ammonia combustion process and adjusts the reaction rates accordingly. The consistency of this mechanism with existing IDTs, LBVs, and oxidation experimental data has been validated.
- (3)
- Based on the AHY mechanism, a new ammonia/diesel mechanism was developed by integrating the n-heptane mechanism and C1–C7/N reactions, resulting in a mechanism that includes 1351 species and 6227 reactions. Validation is conducted using experimental data, which demonstrates that the ammonia/diesel mechanism is effective in predicting ignition and oxidation across a broad range.
- (4)
- A reaction pathway and sensitivity analysis for the AHY mechanism concerning ammonia and ammonia/hydrogen fuel were conducted. The result indicates that the main oxidation reaction pathways of the mechanism remain largely consistent, although some pathway fluxes exhibit variations. Additionally, the sensitivity of reactions affecting ignition delay times is generally consistent.
- (5)
- The nC7H16-NH2 reaction was identified as a significant reaction influencing the IDTs through an investigation of the C-N reaction. In contrast, the reactions R + NO2 = RO + NO; RH + NO2 = R + HONO; R + HNO = RH + NO; RO2 + NO = RO + NO2, as well as C3–C6 + NH2, were found to have minimal impacts on IDTs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en17235956/s1, (1) Comparison of predicted values and experimental values of component concentrations in various ammonia/hydrogen mechanism oxidation. (2) Reaction pathway analysis of ammonia/hydrogen mechanism. (3) Sensitivity analysis of ammonia/hydrogen mechanism.
Author Contributions
Conceptualization, L.L. and Y.H.; methodology, J.L.; investigation, Q.C. and H.Y.; validation and data curation, H.Y. and J.L.; writing—original draft preparation, Q.C.; writing—review and editing, Y.H., J.L. and N.Z.; visualization, Q.C. and H.Y.; supervision, L.L. and Y.H.; project administration and funding acquisition, L.L. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Guangxi Yuchai Marine and Genset Power Co., Ltd. (Yulin, China), Knowledge Innovation Program of Wuhan (No. 2023020201010092), and National Natural Science Foundation of China (No. 52301382).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yapicioglu, A.; Dincer, I. A Review on Clean Ammonia as a Potential Fuel for Power Generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- IPCC. The Physical Science Basis. Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Park, Y.-K.; Kim, B.-S. Catalytic Removal of Nitrogen Oxides (NO, NO2, N2O) from Ammonia-Fueled Combustion Exhaust: A Review of Applicable Technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, P.; Chen, S. A Model for the Laminar Flame Speed of Binary Fuel Blends and Its Application to Methane/Hydrogen Mixtures. Int. J. Hydrogen Energy 2012, 37, 10390–10396. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical Kinetic Modeling of Ammonia Oxidation with Improved Reaction Mechanism for Ammonia/Air and Ammonia/Hydrogen/Air Combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An Experimental and Modeling Study of Ammonia with Enriched Oxygen Content and Ammonia/Hydrogen Laminar Flame Speed at Elevated Pressure and Temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Stagni, A.; Arunthanayothin, S.; Dehue, M.; Herbinet, O.; Battin-Leclerc, F.; Bréquigny, P.; Mounaïm-Rousselle, C.; Faravelli, T. Low- and Intermediate-Temperature Ammonia/Hydrogen Oxidation in a Flow Reactor: Experiments and a Wide-Range Kinetic Modeling. Chem. Eng. J. 2023, 471, 144577. [Google Scholar] [CrossRef]
- Zhang, X.; Moosakutty, S.P.; Rajan, R.P.; Younes, M.; Sarathy, S.M. Combustion Chemistry of Ammonia/Hydrogen Mixtures: Jet-Stirred Reactor Measurements and Comprehensive Kinetic Modeling. Combust. Flame 2021, 234, 111653. [Google Scholar] [CrossRef]
- Bertolino, A.; Fürst, M.; Stagni, A.; Frassoldati, A.; Pelucchi, M.; Cavallotti, C.; Faravelli, T.; Parente, A. An Evolutionary, Data-Driven Approach for Mechanism Optimization: Theory and Application to Ammonia Combustion. Combust. Flame 2021, 229, 111366. [Google Scholar] [CrossRef]
- Stagni, A.; Cavallotti, C.; Arunthanayothin, S.; Song, Y.; Herbinet, O.; Battin-Leclerc, F.; Faravelli, T. An Experimental, Theoretical and Kinetic-Modeling Study of the Gas-Phase Oxidation of Ammonia. React. Chem. Eng. 2020, 5, 696–711. [Google Scholar] [CrossRef]
- Gotama, G.J.; Hayakawa, A.; Okafor, E.C.; Kanoshima, R.; Hayashi, M.; Kudo, T.; Kobayashi, H. Measurement of the Laminar Burning Velocity and Kinetics Study of the Importance of the Hydrogen Recovery Mechanism of Ammonia/Hydrogen/Air Premixed Flames. Combust. Flame 2022, 236, 111753. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and Kinetic Modeling Study of Laminar Burning Velocities of NH3/Syngas/Air Premixed Flames. Combust. Flame 2020, 213, 1–13. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, B.; Yang, W.; Tan, H.; Wang, J.; Dai, H.; Li, L.; ur Rahman, Z.; Wang, X.; Deng, S.; et al. An Experimental and Kinetic Modeling Study on NH3/Air, NH3/H2/Air, NH3/CO/Air, and NH3/CH4/Air Premixed Laminar Flames at Elevated Temperature. Combust. Flame 2023, 248, 112536. [Google Scholar] [CrossRef]
- Zhou, C.-W.; Li, Y.; Burke, U.; Banyon, C.; Somers, K.P.; Ding, S.; Khan, S.; Hargis, J.W.; Sikes, T.; Mathieu, O.; et al. An Experimental and Chemical Kinetic Modeling Study of 1,3-Butadiene Combustion: Ignition Delay Time and Laminar Flame Speed Measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Singh, A.S.; Mohapatra, S.; Boyapati, R.; Elbaz, A.M.; Dash, S.K.; Roberts, W.L.; Reddy, V.M. Chemical Kinetic Modeling of the Autoignition Properties of Ammonia at Low–Intermediate Temperature and High Pressure Using a Newly Proposed Reaction Mechanism. Energy Fuels 2021, 35, 13506–13522. [Google Scholar] [CrossRef]
- Singh, A.S.; Dash, S.K.; Reddy, V.M. Chemical Kinetic Analysis on Influence of Hydrogen Enrichment on the Combustion Characteristics of Ammonia Air Using Newly Proposed Reaction Model. Int. J. Energy Res. 2022, 46, 6144–6163. [Google Scholar] [CrossRef]
- Girhe, S.; Snackers, A.; Lehmann, T.; Langer, R.; Loffredo, F.; Glaznev, R.; Beeckmann, J.; Pitsch, H. Ammonia and Ammonia/Hydrogen Combustion: Comprehensive Quantitative Assessment of Kinetic Models and Examination of Critical Parameters. Combust. Flame 2024, 267, 113560. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Feng, Y.; Wang, W.; Zhu, J.; Qian, Y.; Lu, X. The Effect of Ammonia Addition on the Low-Temperature Autoignition of n-Heptane: An Experimental and Modeling Study. Combust. Flame 2020, 217, 4–11. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Jiang, Z.; Li, Y.; Gao, W.; Wang, Z.; Cheng, X.; Curran, H.J. An Experimental and Kinetic Modeling Study of Ammonia/n-Heptane Blends. Combust. Flame 2022, 246, 112428. [Google Scholar] [CrossRef]
- Thorsen, L.S.; Jensen, M.S.T.; Pullich, M.S.; Christensen, J.M.; Hashemi, H.; Glarborg, P.; Alekseev, V.A.; Nilsson, E.J.K.; Wang, Z.; Mei, B.; et al. High Pressure Oxidation of NH3/n-Heptane Mixtures. Combust. Flame 2023, 254, 112785. [Google Scholar] [CrossRef]
- Pan, J.; Tang, R.; Wang, Z.; Gao, J.; Xu, Q.; Shu, G.; Wei, H. An Experimental and Modeling Study on the Oxidation of Ammonia and N-Heptane with JSR. Proc. Combust. Inst. 2023, 39, 477–485. [Google Scholar] [CrossRef]
- Zhang, K.; Banyon, C.; Bugler, J.; Curran, H.J.; Rodriguez, A.; Herbinet, O.; Battin-Leclerc, F.; B’Chir, C.; Heufer, K.A. An Updated Experimental and Kinetic Modeling Study of N-Heptane Oxidation. Combust. Flame 2016, 172, 116–135. [Google Scholar] [CrossRef]
- Wang, B.; Dong, S.; Jiang, Z.; Gao, W.; Wang, Z.; Li, J.; Yang, C.; Wang, Z.; Cheng, X. Development of a Reduced Chemical Mechanism for Ammonia/n-Heptane Blends. Fuel 2023, 338, 127358. [Google Scholar] [CrossRef]
- Xu, L.; Chang, Y.; Treacy, M.; Zhou, Y.; Jia, M.; Bai, X.-S. A Skeletal Chemical Kinetic Mechanism for Ammonia/n-Heptane Combustion. Fuel 2023, 331, 125830. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Wang, P.; Niu, B.; Liu, J. Construction and Derivation of a Series of Skeletal Chemical Mechanisms for N-Alkanes with Uniform and Decoupling Structure Based on Reaction Rate Rules. Combust. Flame 2022, 236, 111785. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Qin, X.; Huang, Z. Effect of Hydrogen Blending on the High Temperature Auto-Ignition of Ammonia at Elevated Pressure. Fuel 2021, 287, 119563. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and Kinetic Modeling Investigation on the Laminar Flame Propagation of Ammonia under Oxygen Enrichment and Elevated Pressure Conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and Kinetic Modeling Study of Laminar Burning Velocities of NH3/Air, NH3/H2/Air, NH3/CO/Air and NH3/CH4/Air Premixed Flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental Investigation on Laminar Burning Velocities of Ammonia/Hydrogen/Air Mixtures at Elevated Temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; He, Y.; Liu, Y.; Zhu, Y.; Konnov, A.A. The Temperature Dependence of the Laminar Burning Velocity and Superadiabatic Flame Temperature Phenomenon for NH3/Air Flames. Combust. Flame 2020, 217, 314–320. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Air Premixed Flames at Various Pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning Velocity Measurements of Nitrogen-Containing Compounds. J. Hazard. Mater. 2008, 155, 144–152. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Li, B.; Gao, W. Explosion Behaviors of Ammonia–Air Mixtures. Combust. Sci. Technol. 2018, 190, 1804–1816. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental Study and Kinetic Analysis of the Laminar Burning Velocity of NH3/Syngas/Air, NH3/CO/Air and NH3/H2/Air Premixed Flames at Elevated Pressures. Combust. Flame 2020, 221, 270–287. [Google Scholar] [CrossRef]
- Octave Levenspiel. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).