Abstract
The anaerobic digestion (AD) of livestock blood represents a sustainable solution for the management of waste generated by the meat processing industry while simultaneously generating renewable energy. The improper treatment of livestock blood, which is rich in organic matter and nutrients, can result in environmental risks such as water pollution, soil degradation, and greenhouse gas emissions. This review examines a range of AD strategies, with a particular focus on technological advances in reactor design, pretreatment, and co-digestion, with the aim of optimizing process efficiency. While the high protein content of blood has the potential to enhance biogas production, challenges such as ammonia inhibition and process instability must be addressed. Innovations such as bio-carriers, thermal pretreatment, and co-digestion with carbon-rich substrates have demonstrated efficacy in addressing these challenges, resulting in stable operation and enhanced methane yields. The advancement of AD technologies is intended to mitigate the environmental impact of livestock blood waste and facilitate the development of a circular bioeconomy. Furthermore, the possibility of utilizing slaughterhouse blood for the recovery of valuable products, including proteins, heme iron, and bioactive peptides, was evaluated with a view to their potential applications in the pharmaceutical and food industries. Furthermore, the potential of utilizing protein-rich blood as a substrate for mixed culture fermentation in volatile fatty acid (VFA) biorefineries was explored, illustrating its viability in biotechnological applications.
1. Introduction
Livestock blood waste, a byproduct of slaughterhouses, presents a considerable environmental hazard due to its elevated organic and nutrient content. In light of the considerable global meat production that generates millions of tons of blood waste annually, the development of sustainable waste management solutions is of paramount importance.
Livestock blood waste is often reused as animal or poultry feed [1], representing a potential for sustainable energy production during anaerobic digestion (AD). Compared to its use in the production of animal feed, AD has the potential to convert nutrient-rich waste into biogas, creating a more environmentally friendly energy source and solving several problems related to the disposal of blood waste [2]. The high organic load of blood waste poses a significant environmental risk if not handled properly, including the potential for water pollution [3], soil degradation [4], and greenhouse gas emissions [5]. However, AD can capture these emissions in the form of biogas, primarily methane, which reduces pollution and contributes to the supply of renewable energy. In addition, the protein-rich composition of blood waste improves the efficiency of biogas production and often results in higher methane output than other organic substrates [6]. This transition in utilization is in line with the circular bioeconomy model, where waste is converted into energy and by-products such as volatile fatty acids (VFAs), which have additional applications in biotechnology [7]. Furthermore, AD reduces the risk of contamination with pathogens, which is a major problem when blood is used directly in animal feed [8]. Consequently, the use of blood waste for biogas production not only offers a high-quality, sustainable alternative to conventional animal feed but also covers environmental and energy goals, making it a key component of sustainable waste management strategies in the meat processing industry.
This review’s novelty lies in its comprehensive assessment of the latest technological advances designed to optimize the AD of livestock blood. The paper not only assesses strategies to enhance biogas production and process stability but also investigates the potential for valorizing blood waste through the recovery of high-value compounds, such as bioactive peptides and VFAs, with applications in diverse industries. This approach facilitates the development of a circular bioeconomy by transforming waste into valuable resources.
2. Overview of Blood Waste from Livestock Slaughtering
The generation of blood waste is an inherent aspect of livestock slaughter and represents a significant environmental and management challenge. The volume of blood waste is considerable, and it is also rich in organic matter and nutrients. If not properly managed, this waste can pose serious environmental risks [9].
Blood constitutes approximately 3–4% of the live weight of common livestock animals such as cattle, pigs, and sheep [10]. Regarding the considerable magnitude of global meat production, this percentage equates to a considerable quantity of livestock blood waste on an annual basis. The average adult cow, weighing approximately 600 kg, can produce up to 18 L of blood at the time of slaughter. A pig weighing approximately 110 kg has been observed to produce approximately 4–5 L of blood. A sheep weighing approximately 45 kg can produce approximately three liters of blood.
Globally, the Food and Agriculture Organization (FAO) reported that over 70 billion land animals were slaughtered for meat in 2020 [11]. This considerable quantity of blood waste produces millions of tons each year. As the world’s largest pork producer, China annually slaughters over 700 million pigs, generating an estimated 2.8 million tons of pig blood waste. In the United States, approximately 130 million pigs are processed on an annual basis, resulting in the generation of 520,000 tons of pig blood waste. In 2022, the global cattle industry is estimated to have produced approximately 9.77 million tons of blood waste (Figure 1). This considerable quantity of cattle blood waste is indicative of the substantial volume of livestock processed for meat in a range of geographical regions. The distribution of blood waste generation varies significantly across different regions, as the regional data show. Approximately 2.4 million tons of cattle blood waste were generated in Africa, while the Americas, driven by major cattle-producing countries such as the United States and Brazil, generated approximately 3.46 million tons. In Asia, which includes major cattle producers, the total volume of cattle blood waste reached approximately 2.98 million tons. In comparison, Europe and Oceania, which have comparatively smaller cattle industries, contributed 0.71 million tons and 0.22 million tons of cattle blood waste, respectively [12].
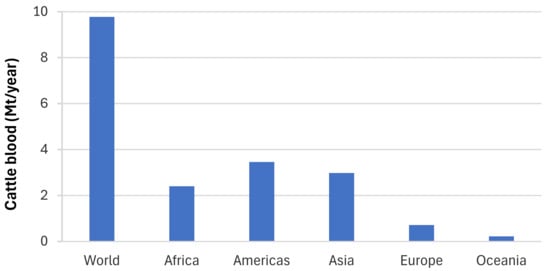
Figure 1.
Regional cattle blood waste production in 2022 (in megatons).
The generation of blood waste in slaughterhouses can be attributed to several key processes within the meat production chain. The most significant source of blood waste is the process of bleeding, also known as exsanguination [13]. The preliminary phase of the slaughtering process entails the severing of major blood vessels, which serve to drain the livestock’s blood. This is performed to ensure the meat’s quality and safety by reducing bacterial growth and facilitating subsequent processing steps. The blood collected at this stage is frequently not fully utilized due to its rapid coagulation, contamination, or the absence of suitable collection systems, resulting in considerable waste [14]. After the initial bleeding stage, additional blood waste is generated as knives, saws, conveyors, and other processing equipment accumulate blood residues that require frequent cleaning, resulting in blood-contaminated wastewater [15]. The regular cleaning of slaughter areas is essential for maintaining hygiene standards. However, this process inevitably results in the generation of wash water that is contaminated with blood, fats, and other organic matter. In the event of inefficient handling or mechanical failure, there is a possibility of unintentional spillage of blood onto floors and surfaces, which would consequently contribute to the waste stream. The residuals contribute to a diluted form of blood waste that still presents environmental risks due to its high biochemical oxygen demand (BOD) and nutrient levels [16].
It is a common consequence of traditional slaughter methods that there is an increased volume of blood waste due to the less efficient bleeding and collection practices that are employed. These methods typically entail manual bleeding techniques without specialized equipment, resulting in incomplete exsanguination and a considerable amount of blood spillage onto floors or down drains. This makes it challenging to collect and utilize the blood [17]. Furthermore, this results in an increased environmental impact due to the elevated organic load in waste streams. Additionally, it represents a lost opportunity to recover valuable resources from the blood. In contrast, contemporary facilities utilize sophisticated techniques and equipment that enhance blood recovery rates, reducing waste. Innovations such as pre-slaughter stunning minimize animal movement, thereby facilitating more controlled and efficient bleeding. Precise cutting tools and methods ensure rapid exsanguination, while specialized equipment such as bleeding troughs, vacuum systems, and closed collection containers facilitate efficient blood collection and prevent contamination [18]. Anticoagulants and rapid cooling prevent blood coagulation, thereby extending the time available for processing the blood into valuable byproducts such as blood meal or plasma products. These practices not only reduce the environmental impact of the meat processing industry but also improve the utilization of resources and contribute to the sustainability of the industry.
The generation of blood waste has a considerable environmental impact. The discharge of untreated blood waste into waterways has the potential to result in eutrophication, which can lead to a depletion of dissolved oxygen and subsequent negative impact on aquatic ecosystems [19]. Inappropriate land disposal can result in alterations to soil chemistry, which may affect microbial activity and plant growth and potentially lead to contamination of groundwater resources [20]. The decomposition of blood waste produces foul odors and greenhouse gases, including methane and carbon dioxide, which contribute to air pollution and climate change [21]. These environmental risks require effective management strategies to mitigate the impact of blood waste from livestock slaughter.
3. Chemical Composition of Livestock Blood
The blood of livestock is a complex and nutrient-rich fluid with significant potential for various applications, yet it is often underutilized. The composition of blood is subject to variation depending on several factors, including species, diet, age, and the animal’s health status. However, it can be broadly characterized as comprising water, proteins, lipids, carbohydrates, and a range of minerals [22]. In cattle, the blood contains 80.9% water, 0.62% minerals, 0.23% lipids, and 17.3% proteins by live weight [23]. In particular, the high protein content is of great interest due to the presence of essential amino acids and bioactive peptides that can be used in both food and non-food industries (Table 1).
The composition of blood can be divided into two principal components: plasma and cellular elements. Plasma, which constitutes approximately 55–60% of the total blood volume, is a straw-colored liquid that is primarily composed of water, proteins, and electrolytes [24]. Plasma serves as a medium for the transportation of nutrients, hormones, and waste products throughout the body. The principal proteins present in plasma are albumin, globulins, and fibrinogen [25]. Albumin, the most abundant protein, plays a pivotal role in maintaining blood pressure and facilitating the transportation of a range of substances, including hormones and fatty acids [26]. Globulins are involved in immune functions and the transport of metals, while fibrinogen is essential for the process of blood clotting [27].
The cellular components of blood include erythrocytes (red blood cells), leukocytes (white blood cells), and thrombocytes (platelets). Red blood cells, which constitute approximately 40–45% of the total blood volume, are primarily responsible for the transportation of oxygen and carbon dioxide [28]. These cells contain hemoglobin, a protein rich in heme iron, which makes it a highly bioavailable source of this essential nutrient. In addition to its function in oxygen transport, hemoglobin plays a critical role in maintaining the body’s iron balance. White blood cells constitute less than 1% of the blood volume and are vital for the body’s defense against infection. They are composed of various types, including lymphocytes, neutrophils, and macrophages. Platelets are small, disk-shaped fragments of cells that are essential for the processes of blood clotting and wound healing [29]. The functional properties of blood-derived proteins and peptides, including emulsifying, gelling, and water-binding capabilities, offer potential benefits in food processing applications [30].
The chemical composition of blood exhibits considerable interspecies variability. For example, bovine blood typically contains approximately 17% protein and less than 1% minerals, with particularly high levels of heme iron, rendering it a valuable source for nutritional supplements and fortification [31]. In contrast, porcine blood may exhibit slightly higher protein levels but a distinct mineral profile, including higher concentrations of magnesium and zinc [32]. Despite its lower overall protein content, poultry blood remains a rich source of essential amino acids and bioactive peptides, which have been linked to various health benefits, including antioxidant and antihypertensive activities [33].
Despite its high nutritional and functional value, livestock blood is frequently discarded as waste in the meat industry, which presents a significant environmental challenge. The BOD of blood waste is exceedingly high, typically ranging from 150,000 to 200,000 mg/L [34], and its chemical oxygen demand (COD) often exceeds 300,000 mg/L, making it a major pollutant if not properly managed. The improper disposal of blood can lead to the proliferation of pathogens such as Bacillus [35], Brucella [36], Salmonella [37], and Mycobacterium [38], posing serious health risks.

Table 1.
Characteristic of livestock blood waste.
Table 1.
Characteristic of livestock blood waste.
| Parameter | Unit | Value | References |
|---|---|---|---|
| Water | % | 80.9 | [29] |
| Lipids | % | 0.23 | [30] |
| Proteins | % | 17.3 | [30] |
| BOD | mg/L | 150,000–200,000 | [39] |
| COD | mg/L | >300,000 | [39] |
| Calcium | mg/dL | 8–11 | [40] |
| Phosphorus | mg/dL | 4.5–8.0 | [40] |
| Magnesium | mg/dL | 2.0–3.5 | [40] |
| Copper | μg/mL | 0.6–1.5 | [40] |
| Zinc | μg/mL | 0.8–1.4 | [40] |
4. Environmental Impact of Livestock Blood Waste
The improper management of blood waste, which is rich in organic matter and nutrients, can result in significant ecological harm. Discharging untreated blood waste into water or soil can result in various environmental issues, including water contamination, soil degradation, and deterioration of air quality [21] (Figure 2).

Figure 2.
Environmental impacts of blood waste.
The release of blood into water systems without adequate treatment can reduce oxygen levels in the water. This phenomenon occurs due to microorganisms’ consumption of organic matter, which requires a significant amount of oxygen. Consequently, the oxygen concentration in the water is significantly reduced, which can result in the death of aquatic organisms due to anoxia [39]. The high levels of nutrients present in the blood, particularly nitrogen and phosphorus, can also contribute to the process of eutrophication, whereby water bodies become over-enriched with nutrients [19]. This results in the excessive growth of algae and other aquatic plants, which can subsequently deplete oxygen levels and disrupt the equilibrium of aquatic ecosystems.
Furthermore, the presence of blood in water bodies can markedly alter the physical and chemical properties of the water, rendering it unsuitable for human consumption, irrigation, and recreational purposes [41]. Blood waste can also facilitate the introduction of pathogens, including Salmonella, Escherichia coli, and other bacteria, into water sources, posing significant health risks to both humans and animals [42]. The environmental and health risks are particularly acute in many developing countries, where blood waste is often discharged directly into rivers and streams.
The improper disposal of blood waste on land can result in soil degradation [23]. The elevated nitrogen content of blood can disrupt the equilibrium of soil nutrients, resulting in excess of certain nutrients while depleting others. Such contamination can impair soil fertility and impede plant growth [43]. Furthermore, the surplus of organic matter derived from blood waste can result in anaerobic conditions within the soil, thereby fostering the proliferation of deleterious microorganisms while impeding beneficial soil organisms’ growth. Such alterations in the soil microbiota can diminish soil quality and its capacity to sustain plant life [16]. In addition, the high salt content of blood can also result in soil salinization, particularly in arid and semi-arid regions where natural leaching processes are constrained. Salinization can result in a reduction in soil permeability, a decline in its water-holding capacity, and an alteration of the soil’s chemical composition, rendering it less hospitable to a diverse range of plant species. Such circumstances may result in a decline in agricultural productivity and, in extreme cases, render the land entirely unsuitable for agricultural use [44].
In addition, the improper disposal of blood waste can also affect air quality. As blood decomposes, it releases a variety of gases, including ammonia, methane, and hydrogen sulfide, all of which contribute to air pollution [45]. Ammonia and hydrogen sulfide are especially problematic due to their potent and unpleasant odors, which can be detrimental to human health when present in high concentrations [46]. Methane, a potent greenhouse gas, contributes to global warming and climate change [5]. In addition to the gases, the decomposition of blood can also result in the production of volatile organic compounds (VOCs). These compounds have the potential to react with other atmospheric pollutants, leading to the formation of smog and ground-level ozone. The release of these gases is frequently exacerbated by open-air disposal practices that are prevalent in numerous rural and developing regions, where blood waste is permitted to decompose on the ground or in open pits. This creates a public nuisance due to the foul odors and poses direct health risks to nearby communities.
5. Recovery of Valuable Components (Other than Biogas) from Livestock Blood
5.1. Recovery of Bioactive Peptides
Approximately 70% of the blood from slaughterhouses is discarded as waste or used as livestock feed or fertilizer. This has the potential to enrich the soil, given that blood is recognized as the greatest natural source of nitrogen. However, fertilizer and feed are regarded as relatively low-value products. Despite its reputation as a problematic waste product in the meat industry due to its capacity to impede floc formation, blood can also possess functional value. This is because the iron present in the hemoglobin of red blood cells is more bioavailable than inorganic iron, such as that derived from plants [47]. Additionally, the nutritional value of blood is attributed to its dry organic matter, which is primarily composed of proteins (17–19%), with albumins and globulins being the predominant constituents [48]. Blood cells contain hemoglobin, while blood plasma is composed of proteins such as albumin, globulin, and fibrinogen. Blood cells contain a greater proportion of proteins than blood plasma. The recovery and utilization of these proteins represent an opportunity to enhance profitability while mitigating the risk of environmental contamination. Precursor proteins can source bioactive peptides and other compounds, including heme iron, plasma proteins, hemoglobin, immunoglobulins, and superoxide dismutase [49]. Hemoglobin, which constitutes up to 95% of the total protein content of blood, is a significant component of the blood protein complex. It is regarded as a source of bioactive compounds, including heme iron polypeptides, angiotensin-converting enzyme (ACE) inhibitors, antioxidant and antimicrobial peptides, and opioids [50]. Bioactive peptides have been demonstrated to possess antioxidant properties, thereby conferring a protective effect against degenerative diseases [51]. It is of the utmost importance to extract valuable compounds as rapidly as possible, without diluting blood, as removing moisture is a costly process [41].
Bioactive peptides are not active in proteins; they require appropriate processing to be activated [51]. The valorization of blood proteins to obtain blood peptides and other bioactive products, including heme iron, can be achieved through a variety of techniques, including enzymatic hydrolysis, microbial fermentation, and non-enzymatic processes [49].
To obtain bioactive peptides, hydrolysis is performed with digestive enzymes (such as peptidases, trypsin, chymotrypsin, and pepsin) or with specific or non-specific proteases (such as alkaline phosphatase) [49]. Enzymatic hydrolysis allows the solubilization of blood proteins; this releases heme peptides, improving heme iron assimilation [52]. The enzymes used for protein hydrolysis are derived from papaya or pineapple or from Bacillus thermoproteolyticus, B. amyloliquefaciens, B. licheniformis, and Streptomyces griseus [53]. For example, the hydrolysis of pig blood revealed 26 bioactive peptides with antihypertensive properties [51]. The hydrolysis of hemoglobin and plasma with pepsin, trypsin, and alkaline conditions resulted in the generation of numerous bioactive peptides with antimicrobial, antibacterial, and antioxidant properties [53]. The hydrolysis of hemoglobin yielded the bioactive α137-141 peptides, which were subsequently enriched by electrodialysis with an ultrafiltration membrane [54]. At a peptide concentration of 8% in the feed, the concentration of bioactive peptides increased approximately 4 times with relatively low energy consumption. Of the six plant, animal, and bacterial proteases (papain, ficin, trypsin, a mixture of trypsin and chymotrypsin, subtilisin, and Protamex), hydrolysis with subtilisin and trypsin yielded a recovery of 73–79% of heme iron in the form of soluble peptides, with a degree of hydrolysis of 23.92 and 16.36%, respectively [55]. The quantity of iron recovered exhibited a significant correlation with the degree of hydrolysis. The proteolysis that occurred with plant enzymes was less extensive. The hydrolysates obtained with Protamex exhibited the highest in vitro antioxidant activities, including free radical scavenging. The trypsin hydrolysates demonstrated the most pronounced metal chelating properties. The advantages of hydrolysis include high specificity, a controlled process level, and mild reaction conditions [49]. The disadvantages of this process include low efficiency and a lengthy duration. In addition to enzymatic hydrolysis, chemical hydrolysis has also been employed to obtain bioactive compounds from blood. The two methods yielded hydrolysates from red blood cells with comparable levels of ACE activity. The activity was found to be 18.7–49.4% following chemical hydrolysis under acidic conditions (0.03 N HCl, 32 h, 50 °C) and 14.2–47.7% following enzymatic hydrolysis (serine protease; 2.5%, 2 h, 60 °C) [56].
In microbial fermentation, certain bacteria, fungi, and yeasts produce protein hydrolases that they utilize to hydrolyze proteins, producing short peptides as the final product. The activity of these enzymes is influenced by various factors, including temperature, acidity, and humidity [49]. For example, a mixture of slaughterhouse blood, molasses, and an inoculum (a pure culture of Lactobacillus acidophilus or whey) was fermented at 35 °C for four days, resulting in the recovery of proteins, amino acids, and lipids [57]. The fermentation of blood with whey resulted in a greater yield of amino acids than that observed with the use of L. acidophilus. Compared to enzymatic hydrolysis, microbial fermentation is a more cost-effective process, has a shorter production cycle, and allows for the extraction of proteins of varying sequences and sizes through a diverse range of enzymes [49].
Non-enzymatic methods, which include ultrasound and microwave-assisted extractions, subcritical water extraction, chemical precipitation, and adsorption (using adsorbents such as activated carbon, silicic acid, carboxymethylcellulose, or magnesium dioxide), are primarily employed as a preliminary treatment step prior to hydrolysis [47,49]. Given that blood cells contain a greater concentration of proteins than blood plasma, the effective recovery of these cells is primarily supported by ultrasonic solubilization, which is regarded as a pivotal technology for protein recycling. In the context of blood waste, the highest solubilization rate (96.01%) of proteins and their conversion into low-molecular-weight products were achieved at an ultrasonic frequency of 20 kHz, an irradiation density of 0.5 W/mL, and a sonication time of 30 min [58].
The valorization of blood proteins produces peptides that can exhibit a range of biological activities and enhance the bioavailability of heme iron. The resulting products have a variety of potential applications, including use in the food, pharmaceutical, and paper industries.
In the food industry, blood proteins can replace chemical preservatives, functioning as antimicrobial components and antioxidants to preserve food. For example, some antioxidant peptides, including EVGK, RCLQ, LDGP, and TGVGTK [59] or α137-141 peptide (a component of hemoglobin) [60], have been employed as biopreservatives due to their ability to reduce lipid oxidation significantly. Furthermore, blood proteins can substitute for low-molecular-weight synthetic surfactants and function as natural stabilizers in food emulsions, reducing the formation of lipid oxidation products and increasing the viscosity and ζ-potential, thereby enhancing the stability of emulsions [47,61]. In addition, blood proteins can be utilized as biomarkers for in vivo quality assessment of meat products [62] and as binders in the meat industry, enhancing the sensory characteristics of meat products [2].
The advantageous activity of blood protein hydrolysates includes the provision of dietary supplementation with heme iron [55]. Despite the enhanced bioavailability of heme iron relative to its inorganic form, its incorporation into food products is constrained by its pro-oxidant propensity. Both hemoglobin and its hydrolysates are susceptible to oxidative discoloration [50]. Nevertheless, the considerable advantages associated with the antioxidant activity of blood protein hydrolysates may offset the pro-oxidant characteristics of heme iron [55]. In addition, the presence of bound iron in hemoglobin can result in a metallic taste, which may limit the use of this bioactive product in the food industry. To capitalize on the exceptional nutritional value of hemoglobin and its hydrolysates, the removal of heme groups from hemoglobin has been conducted, and the following methods have been developed: enzymatic hydrolysis, chemical oxidative destruction, extraction with organic solvents, modification with carbon monoxide, treatment with carbon dioxide in a controlled atmosphere, or subcritical hydrolysis with water in an inert atmosphere [63].
In the pharmaceutical industry, blood proteins serve as a source of iron for the treatment of anemia and as a source of immunoglobulins, thrombin, or superoxide dismutase, which offer a range of health benefits [49]. In other industries, blood-derived products are employed in the production of adhesives (e.g., the paper industry) and bioplastics following the blending of the former with a plasticizer or superabsorbent materials [64].
The abundance of proteins has led to blood being considered a valuable product in the context of animal feed production and agriculture. For instance, the simultaneous production of dried protein feed and liquid amino acid fertilizer on a large scale (5 tons/day) was optimized by processing blood containing 76.11% moisture and 18.23% protein [65]. The processing unit included a series of steps, beginning with ultrasonic pretreatment to solubilize the proteins and followed by enzymatic degradation of the proteins to amino acids. The resulting amino acids were then separated from the solution using a membrane technique, producing a high-purity liquid fertilizer. Finally, the protein feed was subjected to microwave drying, resulting in a dry product. The optimal conditions for the ultrasonic pretreatment were identified as a processing time of 10 min per batch, an ultrasonic power of 0.5 W/mL, and a frequency of 20 kHz. The protein solubility rate was 97.72% under the optimal pretreatment conditions. The hemoglobin concentration in plasma was 15.35% for the treated blood and 0.03% for the untreated blood. The enzymatic degradation process was optimized under conditions of 2 h of operation at 60 °C, with a combination of commercial exo-type (1 h treatment) and endo-type (1 h treatment) proteases. The enzymatic reaction resulted in a reduction in the protein content to approximately 5.8 g/L, while the concentration of amino acids reached 77.8 g/L. The combination of ultrasonic pretreatment and enzymatic digestion enabled the conversion of 70% of the proteins into amino acids. Coagulation with chitosan (0.05%, w/v) was employed to enhance the efficiency of solid–liquid separation through the membrane filter. The non-degraded proteins and free amino acids were separated from the enzymatic degradation product on a selective basis. The yield of amino acid liquid fertilizer (comprising over 20% amino acids) was approximately 61%, while the dewatered cake constituted approximately 39% of the total output. In the final stage of microwave drying, the optimal duration was determined to be 60 min, with a maximum thickness of the dewatered cake of 2 cm. This process yielded a protein feed with a protein content of approximately 78% and a moisture content of less than 7%.
5.2. Extraction of VFAs
The anaerobic processing of slaughterhouse blood is regarded as the primary source of VFAs as renewable bulk chemicals derived from agro-industrial wastes. The results of the mixed anaerobic fermentation process yielded a VFA concentration of 70–100 g/L, primarily comprising n-butyric acid (25–30 g/L), acetic acid (25–30 g/L), and isovaleric acid (15–30 g/L) [66]. The presence of VFAs as ammonium carboxylates is a consequence of elevated ammonia concentrations (up to 12 g/L) at pH levels between 6.5 and 8. The purification of ammonium carboxylates is therefore a requisite step. The downstream processing required to recover VFAs represents a significant technological challenge. Ion exchange, liquid–liquid extraction, filtration, membrane techniques (ultrafiltration, nanofiltration, pertraction, and pervaporation), electrodialysis, or precipitation, as well as esterification and membrane-based solvent extraction (MBSE) have been proposed as potential techniques for the development of a carboxylate-based biorefinery for VFA recovery using blood as a protein-rich substrate [67]. In the esterification study, a range of carboxylic acids were recovered as methyl esters, including acetic, butyric, isobutyric, propionic, valeric, and isovaleric acids. Additionally, ammonium sulfate was identified as a valuable byproduct of this process. The esters are employed in the fragrance industry, while the ammonium sulfate can be utilized as a fertilizer. In the context of carboxylate-based biorefineries for VFA recovery, the use of octanol/trioctylamine as an extractant and a hydrophobic membrane has been demonstrated to be an effective approach for the separation of isovaleric and butyric acids, with a preference for acetic acid [67]. Both methods demonstrated effective recovery. In the esterification study, 60–83% (methyl esters) and 50% (NH4)2SO4 were recovered, while in the MBSE study, >80% recovery was achieved. The ratio of octanol to trioctylamine was 80/20.
The elevated concentration of ammonia in slaughterhouse blood is a contributing factor to the inhibition of methanogenesis during AD. Concurrently with the generation of ammonia as a result of protein consumption, the accumulation of VFAs can reach a notable degree during acidogenesis. The formation of VFAs is enhanced by the introduction of blood into the fermenters. For instance, the supplementation of the organic fraction of municipal solid waste with sheep blood (80:20, based on volatile solids (VS)) resulted in the production of up to 15 g/L of VFA [67]. The high ammonia concentration resulting from the presence of blood in the fermentation broth led to the production of up to 100 g VFA/L, with the participation of microorganisms belonging to the order Clostridiales (70–90%) [7]. In the absence of buffers or other methanogenesis inhibitors, the predominant acids were n-butyric, iso-butyric, isovaleric, and propionic.
The production of VFAs using slaughterhouse blood was investigated in semi-continuous, batch, and fed-batch reactors [7]. The results demonstrated that low blood loadings (1–5%, v/v) did not inhibit methanogenic activity and no accumulation of VFAs was observed. At a loading of 15–50%, the accumulation of VFAs was observed until day 7, whereas at a loading of 75–90%, the accumulation was observed until day 13. The highest yield of VFAs (up to 100 g/L with a conversion rate of 0.82 g VFA/g VS) was obtained in batch and fed-batch fermenters at an initial blood loading of 122 g VS/L. This yield can be attributed to the duration of the process, which lasted between 41 and 45 days. It was higher than that achieved in the semi-continuous process with a hydraulic retention time (HRT) of 7 days. In addition to the blood loading, the inoculum acclimatization also had an impact on the VFA production. The yield of VFAs was 70% that obtained with the acclimatized inoculum. The enzymatic hydrolysis of blood as a pretreatment step did not affect the final concentration of VFAs. However, it did improve the initial production rate and influence the VFA profile [68]. Following the pretreatment stage, the predominant acids were acetic acid and n-butyric acid. In the absence of a pretreatment, n-butyric acid displaced acetic acid. The application of an enzymatic hydrolysis process resulted in a notable inhibition of VFA production within the semi-continuous reactors.
6. AD as a Sustainable Treatment Solution of Livestock Blood Waste
AD is a widely utilized and sustainable technology for the treatment of organic waste, including that derived from the slaughtering of livestock [69]. This process not only assists in the management of the considerable quantities of waste produced in slaughterhouses, but it also offers the additional benefits of biogas production and nutrient recovery.
6.1. Mono-Digestion of Livestock Blood Waste
Mono-digestion is defined as the process of AD of a single organic substrate, resulting in the production of biogas and other by-products, including digestate. Blood waste represents a particularly promising substrate for AD, given its high nitrogen content, protein richness, and high organic load [70]. This presents a potential feedstock for biogas production, yet also poses unique challenges related to process stability, ammonia inhibition, and the necessity for specialized reactor conditions [71]. The breakdown of proteins in the AD process results in the release of ammonia as a byproduct. While ammonia serves as a nitrogen source for the growth of methanogenic bacteria, excessive concentrations of ammonia can cause toxicity, inhibit microbial activity, and potentially result in complete process failure [72]. The inhibition threshold for ammonia is typically above 1.5 g/L for ammonia nitrogen, although this can vary depending on the microbial community and operating conditions [73].
A further considerable challenge is the risk of rapid acidification resulting from the high protein content of blood. The breakdown of proteins produces VFAs, which can accumulate and result in a reduction in the pH of the digester [74]. If the pH level declines to an insufficient degree, it can impede the final stage of AD, namely methanogenesis, which is responsible for the production of methane.
The study by Ortner et al. [75] demonstrated that by regulating ammonia levels and supplementing with trace elements, stable AD of nitrogen-rich slaughterhouse waste can be accomplished even under conditions of elevated ammonia. The authors investigated the anaerobic mono-digestion of nitrogen-rich slaughterhouse waste (with a blood content of 29%) under varying conditions of ammonia concentration, temperature, and addition of trace elements. The objective of the research was to ascertain the impact of elevated total Kjeldahl nitrogen (TKN) levels (approximately 11 g/kg) on process stability and biogas production. The study revealed that ammonia concentrations within the range of 7.7 to 9.1 g/kg could result in the accumulation of VFAs and a 25% reduction in methane yield, with a critical threshold for NH4-N of 830–1060 mg/L. However, the addition of trace elements led to a notable improvement in process stability, a reduction in VFA concentrations, and the maintenance of high methane yields of approximately 280 Nm3/t COD at an organic loading rate (OLR) of 2.5 kg VS/(m3·d) under mesophilic conditions (37 °C). Furthermore, the research investigated psychrophilic conditions (25 °C), wherein it was discovered that ammonia inhibition occurred at lower concentrations (140 mg/L). However, stable operation was only feasible at an OLR of 0.4 kg VS/(m3·d), which was considerably lower than the previously discussed results.
Furthermore, the high viscosity of blood waste can complicate the mixing and pumping of the substrate within the digester [76]. Insufficient mixing can result in stratification and localized inhibition, which in turn reduces the efficiency of the digestion process [77].
The study conducted by Adou et al. [78] on the anaerobic mono-digestion of slaughterhouse wastewater in Côte d’Ivoire offers significant insights into overcoming these challenges. The research demonstrated that slaughterhouse wastewater containing blood and other organic materials could be anaerobically digested under controlled conditions, yielding consistent results. The study was conducted under mesophilic conditions in a batch reactor, resulting in the production of 20,870 mL of biogas, which demonstrates the viability of blood-containing waste as a substrate for AD. The maintenance of a volatile fatty acid-to-total alkalinity ratio below 0.4 and ammonia nitrogen levels that, while high (ranging from 684 to 1239 mg/L), did not inhibit methanogenesis, proved to be crucial for achieving stability. Furthermore, the removal efficiencies for organic matter were notably high, with reductions in total chemical oxygen demand (tCOD), soluble chemical oxygen demand (sCOD), and BOD of 49.93%, 65.85%, and 82.22%, respectively.
To successfully mono-digest blood waste and mitigate the challenges associated with high ammonia levels and acidification, a common approach is to carefully control the OLR. By incrementally increasing the quantity of blood waste introduced to the digester, the microbial community can evolve to accommodate the elevated nitrogen levels, thereby mitigating the risk of ammonia inhibition [79]. This gradual increase in OLR allows the microbial consortia to gradually adapt to the distinctive characteristics of blood waste.
To further address the challenges of stabilizing mono-digestion, particularly in managing ammonia inhibition and ensuring consistent biogas production, additional strategies have been explored. These include the use of additives to improve process stability and optimize methane yields. The research conducted by Bayr et al. [80] offers valuable insights into the optimization of anaerobic mono-digestion processes, with a particular emphasis on the utilization of swine slaughterhouse waste. The objective of the study was to ascertain whether the addition of specific additives could serve to stabilize the digestion process and enhance biogas production. Two additives were subjected to testing: one comprising iron (Fe), hydrochloric acid (HCl), and trace elements, and another containing solely Fe and HCl. The findings demonstrated that the additive comprising trace elements enabled the system to accommodate a higher OLR of 2.25 kg VS/(m3·d) in comparison to reactors lacking additives, which necessitated a lower OLR due to instability. Methane production remained at a high level, approximately 700 dm3 CH4/kg VS, under stable conditions. Moreover, the utilization of additives led to a notable reduction in hydrogen sulfide (H2S) levels in the biogas, which is of paramount importance for enhancing biogas quality. The findings of this study illustrate that the integration of additives, particularly those comprising trace elements, can enhance process stability and methane yields, thereby offering a promising approach for addressing the challenges associated with ammonia inhibition and process instability in slaughterhouse waste digestion.
Table 2 presents a range of biogas and methane yields obtained from the AD of various types of blood and protein waste under different conditions. These studies elucidate the impact of various factors, including feedstock characteristics, operating temperatures, OLRs, and pretreatment methods, on the efficacy of biogas production. The yields are presented in terms of the volume of biogas or methane produced per unit of total solids (TS), VS, COD, or protein content. This provides insight into the potential for optimizing biogas recovery from livestock blood waste. The considerable diversity of conditions and results highlights the necessity of adapting AD processes to align with the distinctive characteristics of the waste material and the desired outcomes.

Table 2.
The yields of biogas/methane obtained during mono-digestion of livestock blood.
6.2. Co-Digestion of Livestock Blood Waste
Co-digestion is the AD of two or more distinct types of organic substrates. For the digestion of blood waste, co-digestion represents a practical solution to the challenges of ammonia inhibition, rapid acidification, and process instability. The process benefits from a more balanced carbon-to-nitrogen (C/N) ratio, improved microbial diversity, and enhanced biogas production when blood waste is mixed with other substrates, such as agricultural residues, food waste, or manure [82]. This method not only optimizes the AD process but also provides an effective means of managing multiple organic waste streams. The optimal ratio for AD is typically between 20:1 and 30:1. However, the C/N ratio for blood waste is considerably lower (below 10:1) due to its high nitrogen and low carbon content. The introduction of carbon-rich substrates, such as plant residues, food waste, or dairy manure, through co-digestion helps to balance the C/N ratio, thereby reducing the risk of ammonia inhibition and enhancing the stability of the digestion process.
The selection of suitable co-substrates is of significant importance for the optimization of the co-digestion process. Cuetos et al. [90] demonstrated that anaerobic co-digestion of poultry blood with the organic fraction of municipal solid waste (OFMSW) can mitigate nitrogen inhibition and improve methane production. The authors investigated the AD of poultry blood, which is rich in nitrogen and can cause digestion inhibition when treated alone. The objective was to dilute the nitrogen content and improve process stability by co-digesting it with OFMSW. The experiment was conducted in a semi-continuous mesophilic anaerobic digester with an HRT of 36 days. The process initially exhibited an imbalance due to an increased OLR; however, reducing the OLR to its initial level enabled the system to return to a stable state. The co-digestion process yielded a methane production of 0.20 m3/kg VS, and the final gas production was stable with low ammonia concentrations. In subsequent studies, Cuetos et al. [91] investigated the efficacy of anaerobic co-digestion of poultry blood with corn residues as a means of mitigating nitrogen inhibition and enhancing biogas production. In the batch tests, an increase in corn concentration from 15% to 70% (on a VS basis) resulted in a significant enhancement in biogas production, with methane yields rising from 130 L CH4/kg VS to 188 L CH4/kg VS. In the semi-continuous digester, the biogas yield was 165 L CH4/kg VS, although some VFA accumulation was observed. A comparable rationale was investigated in the co-digestion of Euphorbia tirucalli with porcine blood [92]. By combining a high-protein substrate, such as pig blood, with the carbon-rich Euphorbia, the researchers observed a notable enhancement in acetate production. This synergy demonstrates the advantages of substrate balancing to enhance process efficiency, irrespective of the objective or whether methane production or VFA production is being performed.
Anaerobic co-digestion was found to significantly improve both process stability and the rate of reaching a steady state in AD. Mozhiarasi et al. [93] the co-digestion of slaughterhouse waste (SHW) with vegetable, fruit, and flower market waste (VMW) was compared to mono-digestion. Co-digestion demonstrated a more rapid stabilization and a greater degree of predictability in digestion outcomes. This improvement is attributed to the complementary properties of the two types of waste. The presence of proteins and fats in SHW can result in the accumulation of ammonia and the formation of long-chain fatty acids, which can impede the digestion process when digested alone. However, when combined with the carbon-rich and easily degradable VMW, the nutrient profile becomes more balanced, thereby preventing these problems. During the process of mono-digestion of SHW, the system often exhibits a lag phase or slower degradation rate as a result of the inhibitory effects of ammonia and the high concentration of fatty acids, which extend the time required to achieve a stable methane production rate. In contrast, co-digestion mixtures, especially those with an optimal ratio of 1:3 SHW to VMW, achieved faster stabilization by diluting inhibitory compounds and promoting more efficient microbial activity. The co-digestion mixtures reached a steady state within 20 days, in contrast to the over 25 days required for the mono-digestion of VMW or SHW. Furthermore, co-digestion enhances buffering capacity and preserves a more optimal pH range for methanogenic bacteria, which is vital for effective methane production. This accelerated stabilization leads to enhanced biogas yields, diminished reactor downtime, and a more sustainable digestion process, collectively making co-digestion a beneficial approach for waste management and energy production.
One of the primary challenges associated with AD, particularly in the context of carbohydrate-rich substrates such as vegetable waste, is the rapid accumulation of VFAs. This can result in a decline in pH and subsequent inhibition of methanogens, the microorganisms responsible for methane production. Miramontes-Martínez et al. [94] demonstrated that SHW can play a pivotal role in enhancing the stability of the AD process by forming a humidification system known as the carbonate-acetic buffer. The SMW is rich in proteins, which are broken down into ammonia (NH3) during the AD process. The ammonia produced from protein degradation reacts with water to form NH4⁺, which is essential for maintaining a stable pH within the digester. The high protein content of SHW, which constitutes approximately 49.04% of the VS, contributes a substantial quantity of ammonia to the digestion system. The ammonia generated from the decomposition of SHW engages in a chemical reaction with carbonates (which are frequently present in the digestion medium) to form ammonium bicarbonate (NH4HCO3). This compound is highly effective at buffering the digester, thereby preventing abrupt pH shifts that could disrupt microbial activity. The carbonate-acetic acid buffering system thus serves to maintain a stable environment for methanogenic bacteria, which are highly sensitive to fluctuations in acidity. This buffering capacity serves to mitigate the acidifying effects of VFAs produced during the decomposition of organic matter, particularly from carbohydrate-rich feedstocks such as FVW. The study demonstrated a direct correlation between the formation of the carbonate acetic buffer and enhanced process stability and methane yield. In the co-digestion of FVW and SHW, the buffer system effectively mitigated pH fluctuations, resulting in enhanced methane yields (up to 1352 mL CH4/g VS in a 1:2 FVW ratio) and a more resilient digestion process, even when confronted with disturbances such as alterations in feedstock composition or organic loading rates.
Another advantage of slaughterhouse waste is that it can correct nutrient imbalances and improve the biodegradability of agricultural residues, which in turn leads to improved methane production and more efficient AD. Meneses-Quelal et al. [95] investigated the anaerobic co-digestion of SHW with agricultural residues (amaranth, quinoa, and wheat straw) with the objective of optimizing methane production and improving waste management. The research, conducted at the laboratory scale under mesophilic conditions, revealed that the co-digestion of SHW with quinoa at a 25:75 ratio yielded the highest methane production, reaching 407 mL CH4/g VS. The incorporation of SHW proved effective in rectifying nutrient imbalances by establishing equilibrium in the C/N ratio, thereby enhancing the biodegradability of agricultural residues. This, in turn, facilitated accelerated fermentation and augmented biogas production.
The study by Jo et al. [96] clearly demonstrated the synergistic effect of SHW co-digested with cattle manure (CM) and pig manure (PM). The synergy index (SI) values were the highest when SHW was mixed with CM, indicating that the combination of SHW and CM resulted in a greater methane yield than expected based on the individual yields. The optimal synergy was observed at a mixing ratio of CM/PM/SHW = 0.27:0.31:0.42, resulting in the highest SI value of 1.18. This synergy is largely attributed to the buffering capacity of CM and PM, which helped neutralize the high ammonia concentrations generated by protein degradation in SHW. Furthermore, the addition of SHW resulted in a reduction in the lag phase of methane production. As the quantity of SHW in the mixture increased, the duration of the lag phase decreased. For instance, reactors with elevated levels of SHW exhibited a more rapid attainment of a stable methane production rate, accompanied by a notable reduction in the lag phase duration, reaching a minimum of 3.9 days in reactors with the highest SHW concentrations. Furthermore, SHW, with a high VS/TS ratio of 96.11%, is more readily degradable than CM (30.8%) and PM (61.6%). The co-digestion of SHW with CM or PM resulted in enhanced organic matter degradation and accelerated methane production. Reactors containing higher proportions of SHW demonstrated biodegradability levels, reaching 87.9%.
Table 3 presents a summary of the biogas and methane yields obtained from a range of co-digestion processes involving slaughterhouse blood. Co-digestion involves the combination of blood with other organic materials, including manure, rumen content, vegetative waste, municipal waste, and agricultural residues, with the objective of enhancing the efficiency of AD. The results demonstrate the impact of varying operational parameters, including feedstock composition, OLR, HRT, and reactor type, on biogas and methane production. These variations underscore the potential for optimizing co-digestion processes to enhance energy recovery from SHW while addressing environmental concerns associated with waste management in the meat processing industry.

Table 3.
The yields of biogas/methane obtained during co-digestion slaughtered blood.
7. Technological Innovations in Livestock Blood Waste AD
The conversion of livestock blood waste into renewable energy and valuable co-products represents a promising solution to the challenge of transforming a problematic by-product of the meat industry. Nevertheless, the process encounters a number of technological obstacles that have the potential to impact its overall efficiency and sustainability. These challenges include issues related to ammonia inhibition, process stability, high organic loading rates, and effluent management. Recent innovations in reactor design, pretreatment methods, and microbial engineering have sought to surmount these obstacles, thereby enhancing the feasibility and effectiveness of AD for livestock blood waste.
Innovations in AD of Livestock Blood Waste
The implementation of two-stage AD systems with biocarriers has markedly advanced the treatment of high-strength organic waste, including livestock blood. This technology employs a two-stage digestion process, comprising hydrolysis and acidogenesis in the initial stage, followed by methanogenesis in the subsequent stage. Such an arrangement allows for optimized conditions in each phase, thereby improving the overall stability of the system. The immobilization of microorganisms within the reactor by biocarriers enhances microbial retention and activity. For example, microorganisms such as Methanobrevibacter and Methanobacterium beijingense, which are critical for methane production, demonstrate enhanced growth and activity on these carriers. The typical operating conditions include mesophilic temperatures (30–40 °C) and HRTs that are tailored to each stage. These systems have demonstrated methane yields of up to 384 mL CH4/g COD when treated with low OLR and COD removal efficiencies of up to 68.5% when utilized for the processing of poultry blood waste [88]. Biocarriers enhance cell residence time and facilitate contact between microorganisms and organic matter, thereby facilitating more efficient digestion and methane production.
The dual functionality of shell waste as a biocarrier and pH buffer represents another promising avenue of innovation. The presence of a high calcium carbonate (CaCO3) content in shell waste has the effect of increasing microbial retention and stabilizing the pH of the reactor during AD. As demonstrated by Wang et al. [104] the incorporation of shells into a methanogenic digester treating chicken blood resulted in sustained pH equilibrium and a notable enhancement in methane generation. The operating conditions of the study included mesophilic temperatures (26 ± 2 °C) with varying OLRs ranging from 0.4 to 1.3 kg COD/(m3·d) over a period of 400 days. The methane yield reached 244.8 mL/g COD, which was higher than that observed in conventional systems without pH buffering, at an OLR of 0.6 kg COD/(m3·d). The shells provided additional surface area for methanogens to colonize, while their buffering action prevented ammonia-induced pH imbalances that can inhibit methane production.
The pasteurization of livestock blood has been demonstrated to be an effective pretreatment for the enhancement of methane production in AD processes. In a study conducted by Zhang et al. [105], the pasteurization of blood at 70 °C for one hour resulted in a 15% increase in methane yield compared to unpasteurized blood. The digestion process was conducted in batch mode at mesophilic temperatures (36 °C) with an inoculum-to-substrate ratio of 4:1 on a VS basis. Although the pasteurization process initially resulted in a delay in methane production due to heat coagulation effects, it ultimately led to an overall increase in yield, reaching 0.479 m3 CH4/kg VS compared to 0.418 m3 CH4/kg VS for unpasteurized blood.
The integration of bioelectricity generation from biogas produced during AD represents a significant technological advancement in the management of livestock blood waste. This process captures the methane produced during AD and converts it to electricity using biogas engines, thereby rendering the facilities energy self-sufficient. The optimal conditions for biogas production entail maintaining mesophilic temperatures (approximately 35 °C) with HRTs of 8–48 h. Modifying the OLR based on the characteristics of the waste stream allows for the optimization of methane yield. For example, a typical SHW treatment facility can achieve up to 65% methane content in the biogas, which is then used to generate bioelectricity (e.g., combined heat and power systems). This integration not only offsets energy consumption but also reduces greenhouse gas emissions, thereby providing a sustainable and cost-effective waste management solution [106].
The use of activated carbon is an effective method for addressing the issues associated with the digestion of protein-rich substrates, such as blood waste, which often results in elevated levels of ammonium and VFAs. As an adsorbent, activated carbon reduces ammonium concentrations and moderates VFA accumulation, thereby protecting the microbial community. In a study conducted by Cuetos et al. [102], both granular and powdered activated carbon were tested in semi-continuous anaerobic digesters at mesophilic temperatures (37 °C) with an HRT of 36 days. The reactors were operated at an OLR of 1.15 kg VS per cubic meter per day while treating poultry blood. The incorporation of activated carbon resulted in enhanced specific methane production, reaching 216 mL CH4/g VS added, and facilitated the stabilization of the digestion process even under conditions of elevated VFA concentrations (up to 6 g/L) and ammonia levels (6–8 g/L).
The application of response surface methodology (RSM) enables the precise calibration of AD operational parameters to achieve optimal methane production. In a study by Habchi et al. [107], the effects of varying organic loading (0.5 to 2.5 g VS/L) and HRT (24–190 h) on methane yield from poultry SHW containing a significant amount of blood were evaluated using RSM. The optimal conditions were identified as an organic load of 1.6 g VS/L and an HRT of 108 h, resulting in the highest methane yield of 378 NmL/g VS and a cumulative production of 566 NmL for a load of 2 g VS/L over 140 h. This optimization technique illustrates the potential for systematic modeling to enhance process efficiencies.
The combination of thermal pretreatment and micronutrient supplementation enhances the efficiency of AD. Méndez-Contreras et al. [108] investigated the effects of low-temperature thermal pretreatment (80 °C for 1 h) and supplementation with micronutrients, including iron (Fe), cobalt (Co), nickel (Ni), and molybdenum (Mo). This approach facilitated the solubilization of proteins and lipids in the substrate, rendering them more accessible to microbes while inactivating pathogens present in blood waste. The optimal concentrations of micronutrients were determined to be 75 mg/L Fe2⁺, 10.5 mg/L Co2⁺, 15 mg/L Ni2⁺, and 0.3 mg/L Mo6⁺, which resulted in a 16.6% increase in biogas production. The combined effect of pretreatment and supplementation resulted in a notable increase in methane yields and a marked improvement in the overall stability of the AD process.
Technological advancements in livestock blood waste management demonstrate the significant potential for enhancing methane production, process efficiency, and environmental sustainability through innovative modifications to AD processes, operational conditions, and pretreatment strategies.
8. Technological Advancements in Livestock Blood Waste Management
The management of blood waste from livestock slaughterhouses has long been a significant challenge from the environmental, economic, and public health perspectives. Conventional techniques, including incineration, land application, and rendering, have demonstrated limited efficacy and the potential to result in considerable environmental contamination. However, recent technological advancements are transforming livestock blood waste into a valuable resource, thereby reducing its environmental impact and increasing its utility. These innovations can be broadly classified into three main categories: treatment technologies, resource recovery, and emerging biotechnology applications. The application of advanced technologies for the treatment of blood-rich abattoir wastewater offers a number of innovative approaches to enhance process efficiency and biogas production.
The Enlarged-Clarifier Hybrid Upflow Anaerobic Sludge Blanket (EC-HUASB) reactor, as described by Loganath and Mazumder [109], represents a significant improvement over conventional UASB reactors, increasing reactor capacity by 50.6%. This is achieved through the implementation of an innovative enlarged clarifier, which facilitates enhanced separation of suspended solids, thereby achieving total suspended solids (TSS) removal efficiencies of up to 98%. These improvements permit the reactor to operate effectively over a wide range of OLRs, with a maximum COD removal efficiency of 95.8% achieved at an OLR of 18.75 kg COD/m3/day under a 10 h HRT. The EC-HUASB’s capacity to settle finer bio-solids not only enhances effluent quality but also facilitates efficient methane recovery, achieving a production rate of 61.5 L/day. The integration of both suspended and attached biomass in the reactor design results in higher methane yields and overall stability, thereby making it a robust solution for the treatment of blood-rich wastewater.
Extending UASB reactor improvements, Musa et al. [110] have introduced the integration of synthetic grass media at the base of the reactor, with the objective of enhancing microbial retention and digestion efficiency. This approach addresses the challenge of maintaining process stability at higher OLRs, where traditional UASB reactors often encounter difficulties. The additional surface area for microbial attachment allows for the maintenance of consistent methane yields, reaching up to 0.35 m3 CH4/kg COD removed, even at elevated OLRs. This modification markedly enhances the AD of blood waste and offers a dependable approach to augmenting methane production while sustaining high COD removal efficiencies, which have reached up to 95%.
To further enhance the AD process, Marzuki et al. [111] demonstrated the advantages of utilizing acclimatized seed sludge in UASB reactors. The acclimation process, utilizing modified synthetic wastewater, facilitates the development of a more resilient microbial population, adapted to the challenging conditions inherent to slaughterhouse wastewater treatment. This approach has the additional benefit of reducing the time required for reactor start-up and increasing methane production by 47%. Specific methane production rates of 0.31 L CH4/g COD added are achieved, which is a significant improvement over the 0.21 L CH4/g COD produced by non-acclimatized reactors. The enhanced resilience at elevated OLRs underscores the significance of acclimatized seed sludge in optimizing biogas production.
Building upon the concept of hybrid systems, Hernández-Fydrych et al. [112] employed a HUASB reactor containing natural zeolite, which proved effective in the removal of nutrients, including ammonium and phosphate. The natural zeolite not only functions as a filter to retain small flocs, but also enhances the reactor’s capacity to maintain high removal efficiencies for both COD (67.7%) and VS (72.1%) at high OLRs. Furthermore, the presence of zeolite contributes to the stability of methane production, thereby supporting the sustainable generation of biogas in addition to nutrient removal.
In a study conducted by Viet et al. [113], an anaerobic membrane bioreactor (AnMBR) was employed to effectively treat high-strength slaughterhouse wastewater. The combination of AD and microfiltration membrane technology enables the effective removal of COD and suspended solids, with removal efficiencies of 90–99%. Furthermore, the use of membrane filtration ensures the production of a high-quality effluent. The AnMBR system is capable of operating with short HRTs (8 to 48 h), with methane production reaching 0.29 m3 CH4/kg COD and methane content in the biogas reaching up to 74%. This approach illustrates how the integration of membrane technology with AD can enhance biogas production and mitigate the environmental impact of slaughterhouse wastewater treatment.
Finally, the Down-flow Expanded Granular Bed Reactor (DEGBR) evaluated by Dlamini et al. [114] incorporated a biological pre-treatment stage with Eco-flush™, which targeted the degradation of fats, oils, and grease (FOG). The system demonstrated high removal efficiencies, with 92% for COD, 97% for FOG, and 94% for TSS, and supported biogas production, although with some variability due to the influence of Eco-flush™. The introduction of facultative bacteria by Eco-flush™ was found to interfere with methanogenesis, indicating the necessity for the careful selection of pretreatment agents to prevent adverse effects on methane production.
These advancements in AD technologies for treating blood-rich slaughterhouse wastewater have been demonstrated to significantly improve process stability, effluent quality, and methane yield. Each innovation contributes to the efficient recovery of biogas, thereby enhancing the effectiveness and sustainability of these systems for industrial wastewater treatment.
Enhancing the AD of Blood Waste
In summary, the AD of blood waste presents considerable challenges due to its high protein and nitrogen content, which can result in ammonia inhibition and process instability. Table 4 presents a comparative analysis of innovative strategies and technologies, emphasizing several approaches to enhance the efficiency and stability of the AD process for blood waste.

Table 4.
Enhancing the AD of blood waste.
The co-digestion of carbon-rich substrates effectively balances the carbon-to-nitrogen ratio, thereby mitigating ammonia inhibition and enhancing biogas yield. Thermal pretreatment and pasteurization enhance biodegradability by denaturing proteins and reducing pathogenic microorganisms. However, these processes require additional energy input and equipment. Chemical and enzymatic pretreatments have been demonstrated to enhance the hydrolysis of proteins, thereby improving methane production. However, such treatments may entail significant costs and potential inhibitory effects due to residual chemicals or enzyme instability.
The introduction of specialized microbial strains through bioaugmentation has been demonstrated to enhance the degradation of proteins and improve process stability. Two-stage AD has been shown to optimize environmental conditions for different microbial communities, thereby reducing ammonia inhibition and increasing methane yield. The maintenance of optimal conditions for methanogenic archaea, including the control of pH and alkalinity, is crucial for preventing process failure. The use of natural buffers such as shell waste can be an effective strategy in this regard.
The incorporation of activated carbon serves to address ammonia inhibition by adsorbing inhibitory compounds and enhancing microbial activity. In contrast, the deployment of biocarriers has the effect of increasing microbial biomass and retention time, thereby improving degradation efficiency and biogas production. Each of these strategies presents a distinct set of advantages and limitations, often involving a trade-off between operational complexity, costs, and effectiveness.
The selection of appropriate strategies for enhancing the AD of blood waste is contingent upon a number of factors, including specific operational conditions, economic considerations, and desired outcomes. The combination of multiple approaches may result in synergistic benefits; however, this requires careful optimization and management. Continued research and development are essential to refine these technologies, reduce associated costs, and facilitate their integration into industrial-scale waste management systems. By surmounting the inherent challenges associated with processing blood waste, these innovative strategies facilitate sustainable waste management and renewable energy production through enhanced AD processes.
9. Conclusions
The disposal of livestock blood represents a significant challenge for the meat processing industry in terms of waste management. The generation of substantial quantities of blood waste during the slaughter of livestock presents a notable environmental concern, largely due to its elevated organic load and nutrient content. If not addressed, it has the potential to result in contamination of water sources and soil, as well as an increase in greenhouse gas emissions. It is therefore imperative that effective treatment strategies, such as AD, be employed in order to minimize the environmental impact and recover valuable resources, including biogas and digestate.
This review underscores the significance of AD as a sustainable methodology for the management of livestock blood. It offers the dual benefit of reducing the environmental impact of waste while simultaneously producing renewable energy. The findings indicate that while the high protein content of blood can enhance biogas yield, it also poses challenges such as ammonia inhibition and process instability. A number of technological innovations, including co-digestion, improvements to reactor design, and pretreatment methods, were identified as effective strategies for overcoming these challenges and optimizing AD performance.
The AD of blood waste offers considerable potential for high biogas yields, frequently outperforming other organic wastes in methane production due to its high protein content. However, this also results in the formation of ammonia, which has the potential to inhibit microbial activity. To address this issue, strategies such as co-digestion with carbon-rich substrates help to balance the carbon-to-nitrogen ratio, while advances in reactor design, including the use of biocarriers, multi-stage systems, and integrated filtration, improve process stability. Furthermore, pretreatment methods, including thermal processing and the use of natural adsorbents, can mitigate inhibitory effects and enhance the digestibility of blood. In conclusion, the application of AD for blood waste not only diminishes the environmental impact of the meat industry but also enhances energy autonomy.
Author Contributions
Conceptualization, M.Z.; investigation, K.B. and M.Z.; resources, K.B. and M.Z.; writing—review and editing, K.B. and M.Z.; supervision, K.B. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ofori, J.A.; Hsieh, Y.H.P. Issues Related to the Use of Blood in Food and Animal Feed. Crit. Rev. Food Sci. Nutr. 2014, 54, 687–697. [Google Scholar] [CrossRef]
- Gupta, A.K.; Fadzlillah, N.A.; Sukri, S.J.M.; Adediran, O.A.; Rather, M.A.; Naik, B.; Kumar, V.; Bekhit, A.E.D.A.; Ramli, M.A.; Jha, A.K.; et al. Slaughterhouse Blood: A State-of-the-Art Review on Transforming by-Products into Valuable Nutritional Resources and the Role of Circular Economy. Food Biosci. 2024, 61, 104644. [Google Scholar] [CrossRef]
- Aniebo, A.O.; Wekhe, S.N.; Okoli, I.C. Abattoir Blood Waste Generation in Rivers State and Its Environmental Implications in the Niger Delta. Toxicol. Environ. Chem. 2009, 91, 619–625. [Google Scholar] [CrossRef]
- Ahmad, H.R.; Aziz, T.; Zia-ur-Rehman, M.; Sabir, M.; Khalid, H. Sources and Composition of Waste Water: Threats to Plants and Soil Health. In Soil Science: Agricultural and Environmental Prospectives; Springer: Cham, Switzerland, 2016; pp. 349–370. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Uddin, M.N.; Mofijur, M.; Fattah, I.M.R.; Ong, H.C.; Lam, S.S.; Kumar, P.S.; Ahmed, S.F. Theoretical Calculation of Biogas Production and Greenhouse Gas Emission Reduction Potential of Livestock, Poultry and Slaughterhouse Waste in Bangladesh. J. Environ. Chem. Eng. 2021, 9, 105204. [Google Scholar] [CrossRef]
- Zhao, S.; Thakur, N.; Salama, E.S.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Potential Applications of Protein-Rich Waste: Progress in Energy Management and Material Recovery. Resour. Conserv. Recycl. 2022, 182, 106315. [Google Scholar] [CrossRef]
- Plácido, J.; Zhang, Y. Production of Volatile Fatty Acids from Slaughterhouse Blood by Mixed-Culture Fermentation. Biomass Convers. Biorefin. 2018, 8, 621–634. [Google Scholar] [CrossRef]
- Langone, M.; Ferrentino, R.; Freddi, F.; Andreottola, G. Anaerobic Digestion of Blood Serum Water Integrated in a Valorization Process of the Bovine Blood Treatment. Biomass Bioenergy 2019, 120, 1–8. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Salem, A.Z.M.; Reddy, P.R.K.; Elghandour, M.M.M.; Oyebamiji, K.J. Waste Recycling for the Eco-Friendly Input Use Efficiency in Agriculture and Livestock Feeding. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 1–45. [Google Scholar] [CrossRef]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- World Food and Agriculture—Statistical Yearbook 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [CrossRef]
- World Food and Agriculture—Statistical Yearbook 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [CrossRef]
- Osborne, R.C.; Harris, C.E.; Buhr, R.J.; Kiepper, B.H. Impact of Exsanguination Method on Blood Loss in Broilers. J. Appl. Poult. Res. 2023, 32, 100380. [Google Scholar] [CrossRef]
- Guillaumin, J. Blood and Blood Products. In Pharmacology in Veterinary Anesthesia and Analgesia; Wiley: Hoboken, NJ, USA, 2024; pp. 382–391. [Google Scholar] [CrossRef]
- Verma, A.K.; Umaraw, P.; Kumar, P.; Mehta, N.; Sazili, A.Q. Processing of Red Meat Carcasses. In Postharvest and Postmortem Processing of Raw Food Materials: Unit Operations and Processing Equipment in the Food Industry; Woodhead Publishing (Elsevier Inc.): Cambridge, UK, 2022; pp. 243–280. [Google Scholar] [CrossRef]
- Nauman, K.; Nauman, A.; Arshad, M. Slaughter Wastes-A Curse or Blessing: An Appraisal. In Climate Changes Mitigation and Sustainable Bioenergy Harvest Through Animal Waste; Springer: Cham, Switzerland, 2023; pp. 35–67. [Google Scholar] [CrossRef]
- Strashynskyi, I.; Pasichnyi, V.; Shevchenko, T.; Karapalov, A. Formation of Functional Indicators of Meat Slaughtered Animals. Grail Sci. 2021, 6, 125–129. [Google Scholar] [CrossRef]
- Abd El-Rahim, I.H.A.; Mashat, B.H.; Fat’hi, S.M. Effect of Halal and Stunning Slaughter Methods on Meat Quality: A Review. Int. Food Res. J. 2023, 30, 290–302. [Google Scholar] [CrossRef]
- Okpaga, F.O.; Adeolu, A.I.; Nwalo, F.N.; Okpe, A.O.; Ikpeama, C.C.; Ogwu, C.E. Safeguarding Ecosystems Using Innovative Approaches to Manage Animal Wastes. Bio-Res. 2024, 22, 2274–2291. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Ma, N.L.; Rupani, P.F.; Sultana, N.; Yaakob, M.A.; Mohamed, R.M.S.R.; Soon, C.F. Biowastes of Slaughterhouses and Wet Markets: An Overview of Waste Management for Disease Prevention. Environ. Sci. Pollut. Res. 2023, 30, 71780–71793. [Google Scholar] [CrossRef]
- Selormey, G.K.; Barnes, B.; Kemausuor, F.; Darkwah, L. A Review of Anaerobic Digestion of Slaughterhouse Waste: Effect of Selected Operational and Environmental Parameters on Anaerobic Biodegradability. Rev. Environ. Sci. Biotechnol. 2021, 20, 1073–1086. [Google Scholar] [CrossRef]
- Herdt, T.H.; Hoff, B. The Use of Blood Analysis to Evaluate Trace Mineral Status in Ruminant Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef]
- Limeneh, D.Y.; Tesfaye, T.; Ayele, M.; Husien, N.M.; Ferede, E.; Haile, A.; Mengie, W.; Abuhay, A.; Gelebo, G.G.; Gibril, M.; et al. A Comprehensive Review on Utilization of Slaughterhouse By-Product: Current Status and Prospect. Sustainability 2022, 14, 6469. [Google Scholar] [CrossRef]
- Csurka, T.; Pásztor-Huszár, K.; Tóth, A.; Németh, C.; László Friedrich, F. Animal Blood, as a Safe and Valuable Resource. J. Hyg. Eng. Des. 2021, 35, 41–47. [Google Scholar]
- Kajal; Pathania, A.R. Chemistry behind Serum Albumin: A Review. E3S Web Conf. 2021, 309, 01086. [Google Scholar] [CrossRef]
- Kianfar, E. Protein Nanoparticles in Drug Delivery: Animal Protein, Plant Proteins and Protein Cages, Albumin Nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. Ceruloplasmin and Other Copper Binding Components of Blood Plasma and Their Functions: An Update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2021, 22, 153. [Google Scholar] [CrossRef]
- Siedlinski, M.; Jozefczuk, E.; Xu, X.; Teumer, A.; Evangelou, E.; Schnabel, R.B.; Welsh, P.; Maffia, P.; Erdmann, J.; Tomaszewski, M.; et al. White Blood Cells and Blood Pressure: A Mendelian Randomization Study. Circulation 2020, 141, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food Proteins from Animals and Plants: Differences in the Nutritional and Functional Properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Eko, P.M.; Afolabi, K.D.; Mbaba, E.N.; Ekpo, U.E.; Idio, A.D. Effect of Keeping Durations Prior to Processing of Bovine Blood on Its Proximate, Gross Energy and Amino Acid Compositions. A. J. Res. Biosci. 2024, 6, 104–111. [Google Scholar]
- Zhang, H.; van der Wielen, N.; van der Hee, B.; Wang, J.; Hendriks, W.; Gilbert, M. Impact of Fermentable Protein, by Feeding High Protein Diets, on Microbial Composition, Microbial Catabolic Activity, Gut Health and beyond in Pigs. Microorganisms 2020, 8, 1735. [Google Scholar] [CrossRef]
- Saleh, A.A.; Amber, K.A.; Soliman, M.M.; Soliman, M.Y.; Morsy, W.A.; Shukry, M.; Alzawqari, M.H. Effect of Low Protein Diets with Amino Acids Supplementation on Growth Performance, Carcass Traits, Blood Parameters and Muscle Amino Acids Profile in Broiler Chickens under High Ambient Temperature. Agriculture 2021, 11, 185. [Google Scholar] [CrossRef]
- Hasan Rizvi, Z.; Goyal, B.; Misra, D.; Ruhela, M.; Singh Teotia, D.; Bhatnagar, V.; Pratap Singh, B.; Kumar, V.; Bhatnagar, S. Study of Effluent Treatment Plant at Slaughter House. Int. J. Civ. Eng. Technol. 2016, 7, 418–426. [Google Scholar]
- Enosi Tuipulotu, D.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus Cereus: Epidemiology, Virulence Factors, and Host–Pathogen Interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef] [PubMed]
- González-Espinoza, G.; Arce-Gorvel, V.; Mémet, S.; Gorvel, J.P. Brucella: Reservoirs and Niches in Animals and Humans. Pathogens 2021, 10, 186. [Google Scholar] [CrossRef]
- Robinson, K.; Assumpcao, A.L.F.V.; Arsi, K.; Erf, G.F.; Donoghue, A.; Jesudhasan, P.R.R. Effect of Salmonella Typhimurium Colonization on Microbiota Maturation and Blood Leukocyte Populations in Broiler Chickens. Animals 2022, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium Avium and Mycobacterium Abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef]
- Banerjee, P.; Garai, P.; Saha, N.C.; Saha, S.; Sharma, P.; Maiti, A.K. A Critical Review on the Effect of Nitrate Pollution in Aquatic Invertebrates and Fish. Water Air Soil. Pollut. 2023, 234, 333. [Google Scholar] [CrossRef]
- Herdt, T.H.; Rumbeiha, W.; Braselton, W.E. The Use of Blood Analyses to Evaluate Mineral Status in Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Bandaw, T.; Herago, T. Review on Abattoir Waste Management. Glob. Vet. 2017, 19, 517–524. [Google Scholar] [CrossRef]
- Aworh, M.K.; Abiodun-Adewusi, O.; Mba, N.; Helwigh, B.; Hendriksen, R.S. Prevalence and Risk Factors for Faecal Carriage of Multidrug Resistant Escherichia Coli among Slaughterhouse Workers. Sci. Rep. 2021, 11, 13362. [Google Scholar] [CrossRef] [PubMed]
- Daniel, O.; NJohn, U.; LYusuf, M.O.; Onyedikachukwu, N.B. Land Pollution Assessment from Slaughterhouses Waste Discharge in Port Harcourt. J. Eng. Res. Rep. 2021, 21, 10–28. [Google Scholar] [CrossRef]
- Boughou, N.; Qoutbane, A.; Hadji, M.; Cherkaoui, E.; Khamar, M.; Nounah, A. Treatment and Valorization of Slaughterhouse Wastewater in Agriculture. Ecol. Eng. Environ. Technol. 2024, 25, 389–398. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Witek-Krowiak, A.; Mironiuk, M.; Furman, K.; Gramza, M.; Moustakas, K.; Chojnacka, K. Valorization of Poultry Slaughterhouse Waste for Fertilizer Purposes as an Alternative for Thermal Utilization Methods. J. Hazard. Mater. 2022, 424, 127328. [Google Scholar] [CrossRef]
- Cui, G.; Bhat, S.A.; Li, W.; Ishiguro, Y.; Wei, Y.; Li, F. H2S, MeSH, and NH3 Emissions from Activated Sludge: An Insight towards Sludge Characteristics and Microbial Mechanisms. Int. Biodeterior. Biodegrad. 2022, 166, 105331. [Google Scholar] [CrossRef]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.E.; García, C.Á. Harnessing the Potential of Blood Proteins as Functional Ingredients: A Review of the State of the Art in Blood Processing. Compr. Rev. Food Sci. Food Saf. 2017, 16, 330–344. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, N.V.; Biriș, S.Ș.; Gheorghiță, N.E.; Ionescu, M.; Milea, O.E.; Dincă, M. Management of Waste and By-Products from Meat Industry. In Proceedings of the International Symposium Agricultural and Mechanical Engineering, Belgrade, Serbia, 19–21 October 2023; pp. 266–267. [Google Scholar]
- Zheng, H. Potential Food and Pharmaceutical Application of Livestock Blood Proteins. Highlights Sci. Eng. Technol. 2023, 55, 29–35. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, L.; Zhang, D.; Yu, J.; Zhu, M.; Li, C.; Zheng, X.; Blecker, C.; Li, S. Value-Added Utilization of Hemoglobin and Its Hydrolysis Products from Livestock and Poultry Blood Processing by-Products: A Review. Trends Food Sci. Technol. 2024, 151, 104645. [Google Scholar] [CrossRef]
- Chiroque, R.G.S.; Cornelio-Santiago, H.P.; Espinoza-Espinoza, L.A.; Moreno-Quispe, L.A.; Pantoja-Tirado, L.R.; Nieva-Villegas, L.M.; Nieva-Villegas, M.A. A Review of Slaughterhouse Blood and Its Compounds, Processing and Application in the Formulation of Novel Non-Meat Products. Curr. Res. Nutr. Food Sci. 2023, 11, 534–548. [Google Scholar] [CrossRef]
- He, L.; Yang, F.; Liang, Y.; Zhang, M.; Liu, X.; Zhao, S.; Jin, G. Process Optimisation of Haemoglobin Hydrolysis by Complex Proteases to Produce Haem-enriched Peptides and Its Iron Uptake Property Evaluation by Caco-2 Cell Model. Int. J. Food Sci. Technol. 2020, 55, 3412–3423. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá-Reig, F.; Reig, M.; Toldrá, F. Possible Uses of Processed Slaughter Byproducts. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–160. [Google Scholar]
- Przybylski, R.; Bazinet, L.; Firdaous, L.; Kouach, M.; Goossens, J.F.; Dhulster, P.; Nedjar, N. Harnessing Slaughterhouse By-Products: From Wastes to High-Added Value Natural Food Preservative. Food Chem. 2020, 304, 125448. [Google Scholar] [CrossRef]
- Berraquero-García, C.; Almécija, M.C.; Guadix, E.M.; Pérez-Gálvez, R. Valorisation of Blood Protein from Livestock to Produce Haem Iron-fortified Hydrolysates with Antioxidant Activity. Int. J. Food Sci. Technol. 2022, 57, 2479–2486. [Google Scholar] [CrossRef]
- Nikhita, R.; Sachindra, N.M. Optimization of Chemical and Enzymatic Hydrolysis for Production of Chicken Blood Protein Hydrolysate Rich in Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activity. Poult. Sci. 2021, 100, 101047. [Google Scholar] [CrossRef]
- Samaddar, A.; Kaviraj, A. Application of Fermentation Technology to Use Slaughterhouse Blood as Potential Protein Supplement in Fish Feed. Jordan J. Biol. Sci. 2015, 8, 23–30. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Kim, H.-J.; Shin, M.-S.; Pak, S.-H. Ultrasonic Treatment of Waste Livestock Blood for Enhancement of Solubilization. Environ. Eng. Res. 2016, 21, 22–28. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M.; Zhou, G. Purification and Identification of Antioxidant Peptides from Duck Plasma Proteins. Food Chem. 2020, 319, 126534. [Google Scholar] [CrossRef]
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an Antimicrobial Peptide Derived from Slaughterhouse By-Product and Its Potential Application on Meat as Preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Gao, M.; Zhang, H.; Kong, B.; Liu, Q. Stability of Oil-in-Water Emulsions Fortified with Enzymatic Hydrolysates from Porcine Plasma Protein. Eur. J. Lipid Sci. Technol. 2018, 120, 1700501. [Google Scholar] [CrossRef]
- Boudon, S.; Henry-Berger, J.; Cassar-Malek, I. Aggregation of Omic Data and Secretome Prediction Enable the Discovery of Candidate Plasma Biomarkers for Beef Tenderness. Int. J. Mol. Sci. 2020, 21, 664. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; de Roos, A.; Schouten, O.; Zheng, C.; Vink, C.; Vonk, B.; Kliphuis, A.; Schaap, A.; Edens, L. Properties of Hemoglobin Decolorized with a Histidine-Specific Protease. J. Food Sci. 2015, 80, 12809. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Guerrero, P.; de la Caba, K.; Bengoechea, C.; Guerrero, A. Biorefinery Concept in the Meat Industry: From Slaughterhouse Biowastes to Superaborbent Materials. Chem. Eng. J. 2023, 471, 144564. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Jeon, M.-J. Large-Scale Conversion of Livestock Blood into Amino Acid Liquid Fertilizer and Dry Protein Feedstuff: A Case Study. Processes 2024, 12, 1183. [Google Scholar] [CrossRef]
- Plácido, J.; Zhang, Y. Production and Extraction of Short Chain Carboxylic Acids from the Anaerobic-Mixed Culture Fermentation of Slaughterhouse Blood; UK AD & Biogas 2016, National Exhibition Centre (NEC): Birmingham, UK, 2016; pp. 37–41. [Google Scholar]
- Zhang, Y.; Banks, C.J. Co-Digestion of the Mechanically Recovered Organic Fraction of Municipal Solid Waste with Slaughterhouse Wastes. Biochem. Eng. J. 2012, 68, 129–137. [Google Scholar] [CrossRef]
- Plácido, J.; Zhang, Y. Evaluation of Esterification and Membrane Based Solvent Extraction as Methods for the Recovery of Short Chain Volatile Fatty Acids from Slaughterhouse Blood Anaerobic Mixed Fermentation. Waste Biomass Valor. 2018, 9, 1767–1777. [Google Scholar] [CrossRef]
- Habchi, S.; Pecha, J.; Šánek, L.; Karouach, F.; El Bari, H. Sustainable Valorization of Slaughterhouse Waste through Anaerobic Digestion: A Circular Economy Perspective. J. Environ. Manag. 2024, 366, 121920. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, N.-V.; Biriș, S.-Ș.; Gheorghiță, N.-E.; Ionescu, M.; Elena Milea, O.; Dincă, M. Management of Waste and By-Products from Meat Industry. Acta Tech. Corviniensis 2024, 17, 49–58. [Google Scholar]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.S.; Jalalah, M.; Li, X.; Salama, E.S. Synergistic Ammonia and Fatty Acids Inhibition of Microbial Communities during Slaughterhouse Waste Digestion for Biogas Production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.N.; Lendormi, T.; Drévillon, L.; Naji, A.; Louka, N.; Maroun, R.G.; Hobaika, Z.; Lanoisellé, J.L. Influence of Substrate/Inoculum Ratio, Inoculum Source and Ammonia Inhibition on Anaerobic Digestion of Poultry Waste. Environ. Technol. 2024, 45, 1894–1907. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of Ammonia Inhibition in Anaerobic Digestion of Nitrogen-Rich Substrates for Biogas Production by Ammonia Stripping: A Review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Gálvez-Martos, J.L.; Greses, S.; Magdalena, J.A.; Iribarren, D.; Tomás-Pejó, E.; González-Fernández, C. Life Cycle Assessment of Volatile Fatty Acids Production from Protein- and Carbohydrate-Rich Organic Wastes. Bioresour. Technol. 2021, 321, 124528. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.; Leitzinger, K.; Skupien, S.; Bochmann, G.; Fuchs, W. Efficient Anaerobic Mono-Digestion of N-Rich Slaughterhouse Waste: Influence of Ammonia, Temperature and Trace Elements. Bioresour. Technol. 2014, 174, 222–232. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, X.; Zhang, S.; Ye, W.; Wu, J.; Poncin, S.; Li, H.Z. Investigation of Hydrodynamics in High Solid Anaerobic Digestion by Particle Image Velocimetry and Computational Fluid Dynamics: Role of Mixing on Flow Field and Dead Zone Reduction. Bioresour. Technol. 2021, 319, 124130. [Google Scholar] [CrossRef]
- Heyer, R.; Klang, J.; Hellwig, P.; Schallert, K.; Kress, P.; Huelsemann, B.; Theuerl, S.; Reichl, U.; Benndorf, D. Impact of Feeding and Stirring Regimes on the Internal Stratification of Microbial Communities in the Fermenter of Anaerobic Digestion Plants. Bioresour. Technol. 2020, 314, 123679. [Google Scholar] [CrossRef] [PubMed]
- Adou, K.E.; Alle, O.A.; Kouakou, A.R.; Adouby, K.; Drogui, P.; Tyagi, R.D. Anaerobic Mono-Digestion of Wastewater from the Main Slaughterhouse in Yamoussoukro (Côte d’Ivoire): Evaluation of Biogas Potential and Removal of Organic Pollution. J. Environ. Chem. Eng. 2020, 8, 103770. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, P.; Zhang, Y.; Wang, X.; Peng, X.; Li, L. Consecutive Batch Anaerobic Digestion under Ammonia Stress: Microbial Community Assembly and Process Performance. J. Environ. Chem. Eng. 2021, 9, 106061. [Google Scholar] [CrossRef]
- Bayr, S.; Pakarinen, O.; Korppoo, A.; Liuksia, S.; Väisänen, A.; Kaparaju, P.; Rintala, J. Effect of Additives on Process Stability of Mesophilic Anaerobic Monodigestion of Pig Slaughterhouse Waste. Bioresour. Technol. 2012, 120, 106–113. [Google Scholar] [CrossRef]
- Afazeli, H.; Jafari, A.; Rafiee, S.; Nosrati, M. An Investigation of Biogas Production Potential from Livestock and Slaughterhouse Wastes. Renew. Sustain. Energy Rev. 2014, 34, 380–386. [Google Scholar] [CrossRef]
- Nazifa, T.H.; Cata Saady, N.M.; Bazan, C.; Zendehboudi, S.; Aftab, A.; Albayati, T.M. Anaerobic Digestion of Blood from Slaughtered Livestock: A Review. Energies 2021, 14, 5666. [Google Scholar] [CrossRef]
- Kim, S.-H.; Oh, S.-Y.; Kim, C.-H.; Yoon, Y.-M. Correction Method of Anaerobic Organic Biodegradability by Batch Anaerobic Digestion. K. J. Soil. Sci. Fertil. 2012, 45, 1086–1093. [Google Scholar] [CrossRef]
- Yoon, Y.-M.; Kim, S.-H.; Oh, S.-Y.; Kim, C.-H. Potential of Anaerobic Digestion for Material Recovery and Energy Production in Waste Biomass from a Poultry Slaughterhouse. Waste Manag. 2014, 34, 204–209. [Google Scholar] [CrossRef]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Biogas Production from Protein-Rich Biomass: Fed-Batch Anaerobic Fermentation of Casein and of Pig Blood and Associated Changes in Microbial Community Composition. PLoS ONE 2013, 8, e77265. [Google Scholar] [CrossRef] [PubMed]
- Hejnfelt, A.; Angelidaki, I. Anaerobic Digestion of Slaughterhouse By-Products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Otero, A.; Mendoza, M.; Carreras, R.; Fernández, B. Biogas Production from Slaughterhouse Waste: Effect of Blood Content and Fat Saponification. Waste Manag. 2021, 133, 119–126. [Google Scholar] [CrossRef]
- Wang, S.; Hawkins, G.L.; Kiepper, B.H.; Das, K.C. Treatment of Slaughterhouse Blood Waste Using Pilot Scale Two-Stage Anaerobic Digesters for Biogas Production. Renew. Energy 2018, 126, 552–562. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Sowunmi, A.; Schmidt, J.E. Valorization of Arid Region Abattoir Animal Waste: Determination of Biomethane Potential. Waste Biomass Valorization 2018, 9, 2327–2335. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Morán, A.; Otero, M.; Gómez, X. Anaerobic Co-digestion of Poultry Blood with OFMSW: FTIR and TG–DTG Study of Process Stabilization. Environ. Technol. 2009, 30, 571–582. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Martínez, E.J.; Fierro, J.; Otero, M. Feasibility of Anaerobic Co-Digestion of Poultry Blood with Maize Residues. Bioresour. Technol. 2013, 144, 513–520. [Google Scholar] [CrossRef]
- Tenci, N.A.; Ammam, F.; Huang, W.E.; Thompson, I.P. Anaerobic Co-Digestion of Euphorbia Tirucalli with Pig Blood for Volatile Fatty Acid Production. Bioresour. Technol. Rep. 2023, 21, 101333. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Speier, C.J.; Rose, P.M.B.; Weichgrebe, D.; Venkatachalam, S.S. Influence of Pre-Treatments and Anaerobic Co-Digestion of Slaughterhouse Waste with Vegetable, Fruit and Flower Market Wastes for Enhanced Methane Production. Biomass Convers. Biorefin. 2023, 13, 7079–7096. [Google Scholar] [CrossRef]
- Miramontes-Martínez, L.R.; Rivas-García, P.; Albalate-Ramírez, A.; Botello-Álvarez, J.E.; Escamilla-Alvarado, C.; Gomez-Gonzalez, R.; Alcalá-Rodríguez, M.M.; Valencia-Vázquez, R.; Santos-López, I.A. Anaerobic Co-Digestion of Fruit and Vegetable Waste: Synergy and Process Stability Analysis. J. Air Waste Manag. Assoc. 2021, 71, 620–632. [Google Scholar] [CrossRef]
- Meneses-Quelal, W.O.; Velázquez-Martí, B.; Gaibor-Chávez, J.; Niño-Ruiz, Z.; Ferrer-Gisbert, A. Anaerobic Co-Digestion of Slaughter Residues with Agricultural Waste of Amaranth Quinoa and Wheat. Bioenergy Res. 2022, 15, 1649–1663. [Google Scholar] [CrossRef]
- Jo, S.; Kadam, R.; Jang, H.; Seo, D.; Park, J. Elucidating Synergetic Effects of Anaerobic Co-Digestion of Slaughterhouse Waste with Livestock Manures. Energies 2024, 17, 3027. [Google Scholar] [CrossRef]
- Muhammad Nasir, I.; Mohd Ghazi, T.I.; Omar, R. Production of Biogas from Solid Organic Wastes through Anaerobic Digestion: A Review. Appl. Microbiol. Biotechnol. 2012, 95, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B. Evaluation of Biogas Production Yields of Different Waste Materials. Earth Sci. Res. 2012, 2, 165–174. [Google Scholar] [CrossRef][Green Version]
- López, I.; Passeggi, M.; Borzacconi, L. Co-Digestion of Ruminal Content and Blood from Slaughterhouse Industries: Influence of Solid Concentration and Ammonium Generation. Water Sci. Technol. 2006, 54, 231–236. [Google Scholar] [CrossRef]
- Banks, C.; Zhang, Y. Optimising Inputs and Outputs from Anaerobic Digestion Processes; DEFRA: London, UK, 2010. [Google Scholar]
- Salminen, E.; Rintala, J.; Lokshina, L.Y.; Vavilin, V.A. Anaerobic Batch Degradation of Solid Poultry Slaughterhouse Waste. Water Sci. Technol. 2000, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing Anaerobic Digestion of Poultry Blood Using Activated Carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef]
- Palatsi, J.; Viñas, M.; Guivernau, M.; Fernandez, B.; Flotats, X. Anaerobic Digestion of Slaughterhouse Waste: Main Process Limitations and Microbial Community Interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar] [CrossRef]
- Wang, S.; Jena, U.; Das, K.C. Long Term Performance of Pilot Methanogenic Digester Filled with Seashell Wastes Treating Slaughterhouse Wastes: Biogas Production and Environmental Impact. Biochem. Eng. J. 2022, 187, 108651. [Google Scholar] [CrossRef]
- Zhang, Y.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion. Processes 2020, 8, 1351. [Google Scholar] [CrossRef]
- Loganath, R.; Senophiyah-Mary, J. Critical Review on the Necessity of Bioelectricity Generation from Slaughterhouse Industry Waste and Wastewater Using Different Anaerobic Digestion Reactors. Renew. Sustain. Energy Rev. 2020, 134, 110360. [Google Scholar] [CrossRef]
- Habchi, S.; Lahboubi, N.; Sallek, B.; El Bari, H. Response Surface Methodology for Anaerobic Digestion of Waste from Poultry Slaughterhouse: Optimization of Load and Hydraulic Retention Time. Results Eng. 2023, 18, 101215. [Google Scholar] [CrossRef]
- Méndez-Contreras, J.M.; Atenodoro-Alonso, J.; López-Escobar, L.A.; Nava-Valente, N. Enhanced Biogas Production from Thermophilic Anaerobic Digestion of Poultry Slaughterhouse Sludge: Effect of Thermal Pretreatment and Micronutrients Supplementation. Waste Biomass Valorization 2024, 15, 2201–2214. [Google Scholar] [CrossRef]
- Loganath, R.; Mazumder, D. Performance Study on Enlarged-Clarifier Hybrid Upflow Anaerobic Sludge Blanket Reactor for Treating the Slaughterhouse Wastewater. Water Environ. J. 2020, 34, 516–528. [Google Scholar] [CrossRef]
- Musa, M.A.; Idrus, S.; Harun, M.R.; Marzuki, T.F.T.M.; Wahab, A.M.A. A Comparative Study of Biogas Production from Cattle Slaughterhouse Wastewater Using Conventional and Modified Upflow Anaerobic Sludge Blanket (UASB) Reactors. Int. J. Environ. Res. Public Health 2019, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, T.N.T.M.; Idrus, S.; Musa, M.A.; Wahab, A.M.A.; Jamali, N.S.; Man, H.C.; Ng, S.N.M. Enhancement of Bioreactor Performance Using Acclimatised Seed Sludge in Anaerobic Treatment of Chicken Slaughterhouse Wastewater: Laboratory Achievement, Energy Recovery, and Its Commercial-Scale Potential. Animals 2021, 11, 3313. [Google Scholar] [CrossRef]
- Hernández-Fydrych, V.C.; del Carmen Fajardo-Ortíz, M.; Salazar-Peláez, M.L. Performance Evaluation and Kinetics Modeling of a Hybrid UASB Reactor Treating Bovine Slaughterhouse Wastewater. Environ. Sci. Pollut. Res. 2022, 29, 80994–81005. [Google Scholar] [CrossRef]
- Tran Thi Viet, N.; Vu, D.C.; Duong, T.H. Effect of Hydraulic Retention Time on Performance of Anaerobic Membrane Bioreactor Treating Slaughterhouse Wastewater. Environ. Res. 2023, 233, 116522. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, D.N.; Basitere, M.; Njoya, M.; Ntwampe, S.K.O.; Kaskote, E. Performance Evaluation of a Biological Pre-Treatment Coupled with the Down-Flow Expanded Granular Bed Reactor (DEGBR) for Treatment of Poultry Slaughterhouse Wastewater. Appl. Sci. 2021, 11, 6536. [Google Scholar] [CrossRef]
- Salminen, E.; Rintala, J. Anaerobic Digestion of Organic Solid Poultry Slaughterhouse Waste—A Review. Bioresour. Technol. 2002, 83, 13–26. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Otero, M.; Morán, A. Anaerobic Digestion of Solid Slaughterhouse Waste: Study of Biological Stabilization by Fourier Transform Infrared Spectroscopy and Thermogravimetry Combined with Mass Spectrometry. Biodegradation 2010, 21, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Chesshire, M.; Heaven, S.; Arnold, R. Anaerobic Digestion of Source-Segregated Domestic Food Waste: Performance Assessment by Mass and Energy Balance. Bioresour. Technol. 2011, 102, 612–620. [Google Scholar] [CrossRef]
- Silvestre, G.; Fernández, B.; Bonmatí, A. Addition of Crude Glycerine as Strategy to Balance the C/N Ratio on Sewage Sludge Thermophilic and Mesophilic Anaerobic Co-Digestion. Bioresour. Technol. 2015, 193, 377–385. [Google Scholar] [CrossRef]
- García-Gen, S.; Sousbie, P.; Rangaraj, G.; Lema, J.M.; Rodríguez, J.; Steyer, J.P.; Torrijos, M. Kinetic Modelling of Anaerobic Hydrolysis of Solid Wastes, Including Disintegration Processes. Waste Manag. 2015, 35, 96–104. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).