Optimization of Combined Hydrothermal and Mechanical Refining Pretreatment of Forest Residue Biomass for Maximum Sugar Release during Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition Analysis
2.3. Hydrothermal and Mechanical Refining Pretreatment
2.4. Enzymatic Hydrolysis of Pretreated Biomass
2.5. Sugar Concentrations, Conversion Calculation
2.6. Model Development, Statistical Analysis, and Optimization
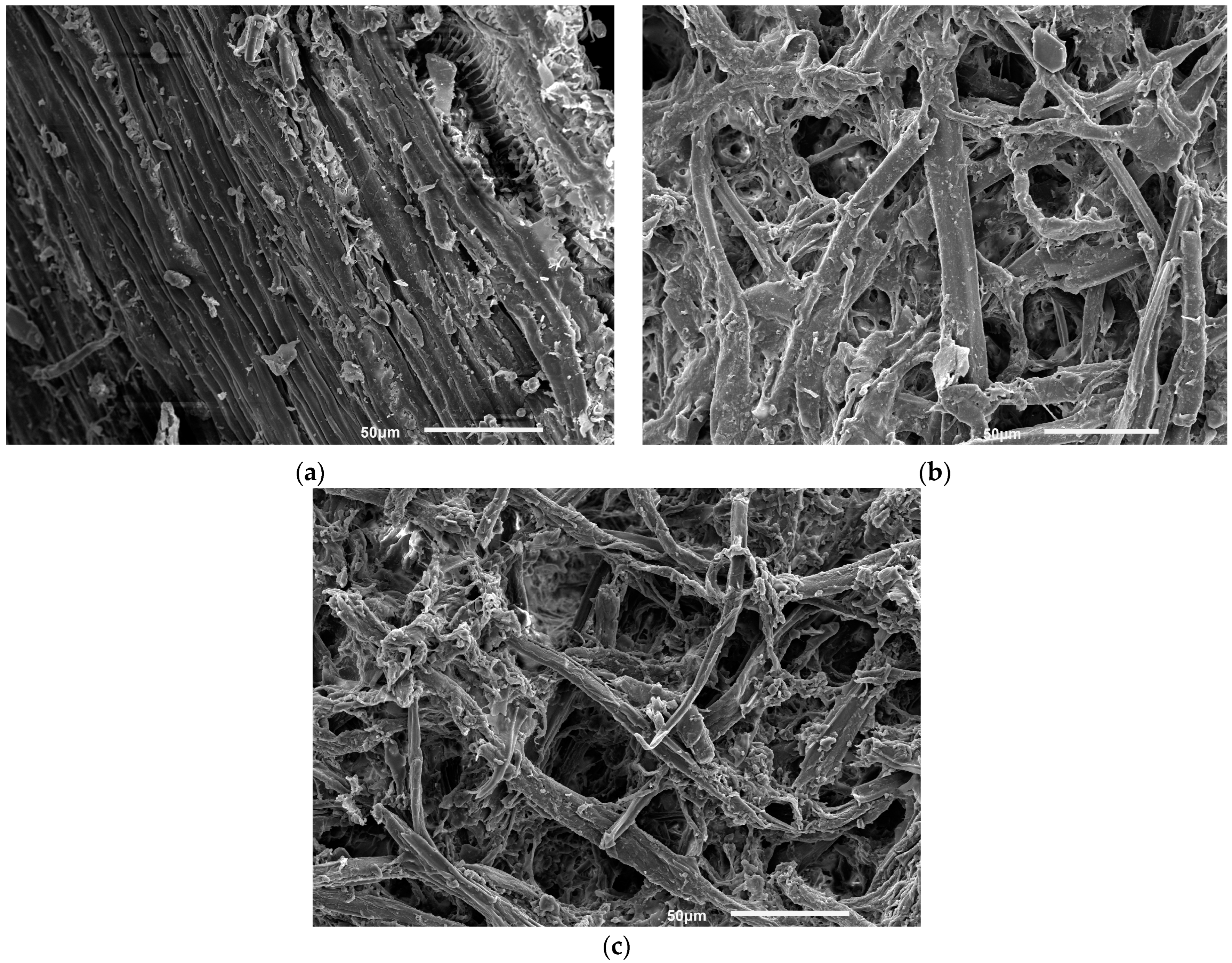
2.7. Scanning Electron Microscopy (SEM) Analysis
3. Results and Discussion
3.1. Composition of Biomass
3.2. Response Surface Model Based on CCD Evaluation
3.3. Effects of Pretreatment Process Parameters on Glucan Conversion
3.4. Effects of Pretreatment Process Parameters on Xylan Conversion
3.5. Effects of Pretreatment Process Parameters on Overall Conversion
3.6. Response Surface Model Optimization
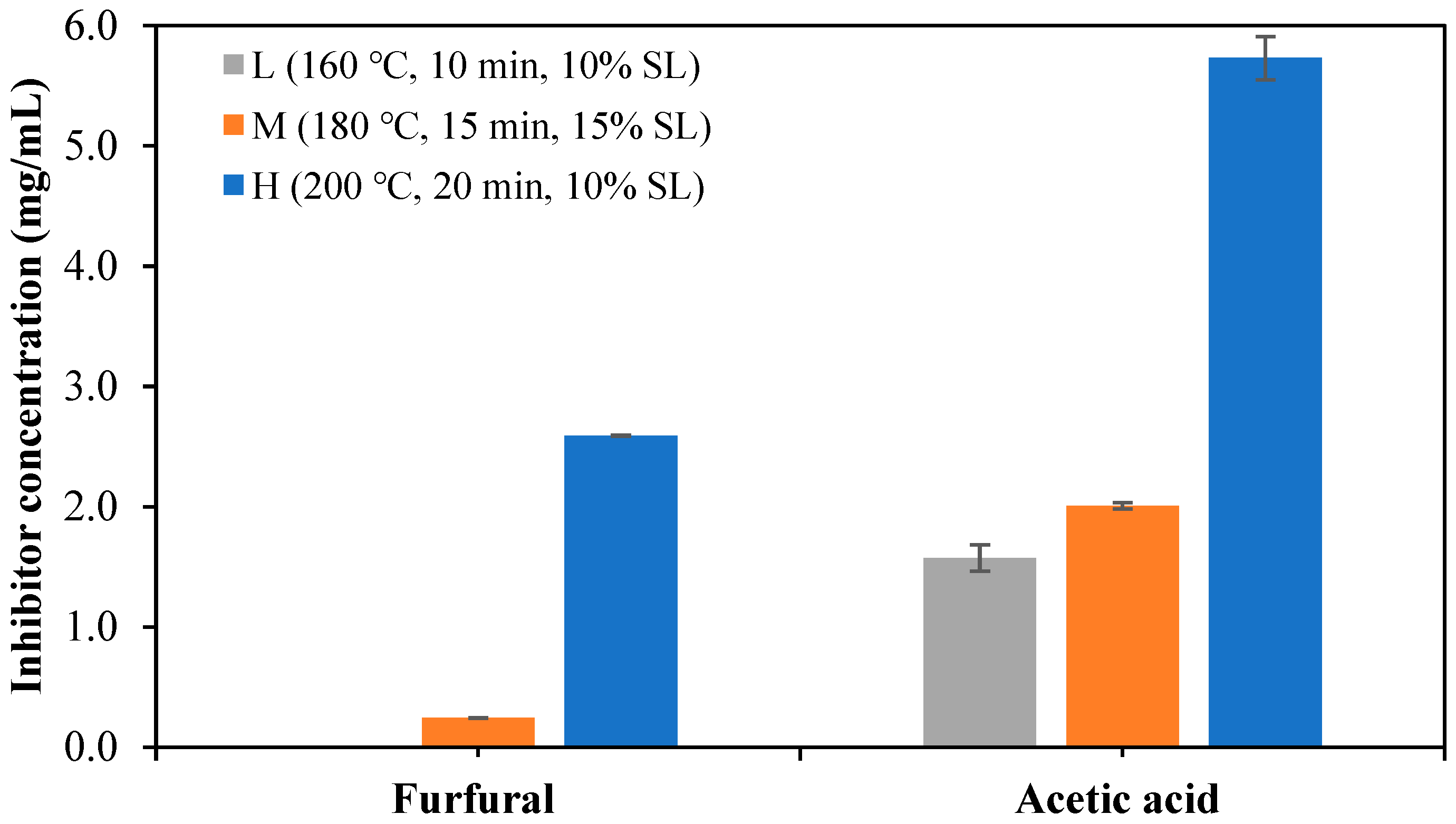
3.7. Inhibitor Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| FRB | Forest residue biomass |
| RSM | Response surface methodology |
| CCD | Central composite design |
| LAPs | Laboratory Analytical Procedures |
| NREL | National Renewable Energy Laboratory |
| HPLC | High-performance liquid chromatography |
| UV-Vis spectroscopy | Ultraviolet–visible (UV-Vis) spectroscopy |
| SEM | Scanning electron microscopy |
| SED | Secondary electron detector |
| WD | Working distance |
| PC | Probe current |
| ANOVA | Analysis of variance |
| VIF | Variance inflation factor |
| CV | Coefficient of variance |
| L | Low |
| M | Medium |
| H | High |
| HMF | 5-hydroxymethylfurfural |
| TEA | Techno-economic analysis |
| LCA | Life-cycle assessment |
References
- Zhang, Y.; Ding, Z.; Hossain, S.; Maurya, R.; Yang, Y.; Singh, V.; Kumar, D.; Salama, E.-S.; Sun, X.; Sindhu, R.; et al. Recent advances in lignocellulosic and algal biomass pretreatment and its biorefinery approaches for biochemicals and bioenergy conversion. Bioresour. Technol. 2023, 367, 128281. [Google Scholar] [CrossRef] [PubMed]
- Thanigaivel, S.; Priya, A.; Dutta, K.; Rajendran, S.; Vasseghian, Y. Engineering strategies and opportunities of next generation biofuel from micro-algae: A perspective review on the potential bioenergy feedstock. Fuel 2022, 312, 122827. [Google Scholar] [CrossRef]
- Hossain, S.; Theodoropoulos, C.; Yousuf, A. Techno-economic evaluation of heat integrated second generation bioethanol and furfural coproduction. Biochem. Eng. J. 2019, 144, 89–103. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Adhya, T.K.; Pattnaik, R.; Mishra, S. A critical review on prospects and challenges in production of biomethanol from lignocellulose biomass. Biomass Convers. Biorefin. 2022, 12, 1835–1849. [Google Scholar] [CrossRef]
- Boro, M.; Verma, A.K.; Chettri, D.; Yata, V.K. Strategies involved in biofuel production from agro-based lignocellulose biomass. Environ. Technol. Innov. 2022, 28, 102679. [Google Scholar] [CrossRef]
- Dutta, N.; Usman, M.; Luo, G.; Zhang, S. An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustain. Chem. 2022, 3, 35–55. [Google Scholar] [CrossRef]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. U.S. Department of Energy. 2016. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016. [Google Scholar]
- Casau, M.; Dias, M.F.; Matias, J.C.O.; Nunes, L.J.R. Residual Biomass: A Comprehensive Review on the Importance, Uses and Potential in a circular bioeconomy approach. Resources 2022, 2022, 35. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Monteiro, A.; Valente, I.M.; Sousa, C.; Miranda, C.; Castro, C.; Cortez, P.P.; Cabrita, A.R.J.; Trindade, H.; Fonseca, A.J.M. Upcycling post-harvest biomass residues from native European Lupinus species: From straws and pod shells production to nutritive value and alkaloids content for ruminant animals. Front. Nutr. 2023, 10, 1195015. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Passarini, L. Valorization of Biomass Residues from Forest Operations and Wood Manufacturing Presents a Wide Range of Sustainable and Innovative Possibilities. In Current Forestry Reports 6; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemi-cellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; Tanobe, V.O.d.A.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Qiao, J.; Cui, H.; Wang, M.; Fu, X.; Wang, X.; Li, X.; Huang, H. Integrated biorefinery approaches for the industrialization of cellulosic ethanol fuel. Bioresour. Technol. 2022, 360, 127516. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Kumar, D.; Rajendran, K.; Mittal, A. Pretreatment Technologies for Lignocellulosic Biomass Refineries. In Advances Lignocellulosic Biofuel Production Systems; Woodhead Publishing: Cambridge, UK, 2023; pp. 81–106. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.G.; Ashokkumar, V.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour. Technol. 2023, 369, 128328. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment Friendly Pretreatment Approaches for the Bioconversion of Ligno-cellulosic Biomass into Biofuels and Value-Added Products. Environments 2022, 10, 6. [Google Scholar] [CrossRef]
- Zhang, T.; Kumar, R.; Wyman, C.E. Sugar yields from dilute oxalic acid pretreatment of maple wood compared to those with other dilute acids and hot water. Carbohydr. Polym. 2013, 92, 334–344. [Google Scholar] [CrossRef]
- Brodeur-Campbell, M.; Klinger, J.; Shonnard, D. Feedstock mixture effects on sugar monomer recovery following dilute acid pretreatment and enzymatic hydrolysis. Bioresour. Technol. 2012, 116, 320–326. [Google Scholar] [CrossRef]
- Singh, A.; Tsai, M.-L.; Chen, C.-W.; Singhania, R.R.; Patel, A.K.; Tambat, V.; Dong, C.-D. Role of hydrothermal pretreatment towards sustainable biorefinery. Bioresour. Technol. 2023, 367, 128271. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Sganzerla, W.G.; Larnaudie, V.; Veersma, R.J.; van Erven, G.; Ríos-González, L.J.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Ferrari, M.D.; Kabel, M.A.; et al. Advances in process design, techno-economic assessment and environmental aspects for hydrothermal pretreatment in the fractionation of biomass under biorefinery concept. Bioresour. Technol. 2023, 369, 128469. [Google Scholar] [CrossRef]
- Tan, L.; Zhao, J.; Qu, Y. Efficient Physical and Chemical Pretreatment of Lignocellulosic Biomass. In Biorefinery of Oil Producing Plants for Value-Added Products; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.; Singh, V. Fermentation of undetoxified sugarcane bagasse hydrolyzates using a two stage hydrothermal and mechanical refining pretreatment. Bioresour. Technol. 2018, 261, 313–321. [Google Scholar] [CrossRef]
- Paul, A.; Jia, L.; Majumder, E.L.-W.; Yoo, C.G.; Rajendran, K.; Villarreal, E.; Kumar, D. Poly(3-hydroxybuyrate) production from industrial hemp waste pretreated with a chemical-free hydrothermal process. Bioresour. Technol. 2023, 381, 129161. [Google Scholar] [CrossRef]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and Limitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Arce, C.; Kratky, L. Mechanical pretreatment of lignocellulosic biomass toward enzymatic/fermentative valorization. IScience 2022, 25, 104610. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jiang, K.; Liu, X.; Han, D.; Zhang, Q. Review on development of ionic liquids in lignocellulosic biomass refining. J. Mol. Liq. 2022, 359, 119326. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Singh, V. Promise of Combined Hydrothermal/Chemical and Mechanical Refining for Pretreatment of Woody and Herbaceous Biomass. In Biotechnol Biofuels 9; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Paudel, S.R. Multi-stage pre-treatment of lignocellulosic biomass for multi-product biorefinery: A review. Sustain. Energy Technol. Assess. 2022, 49, 101702. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chang, F.; Inoue, S.; Endo, T. Increase in enzyme accessibility by generation of nanospace in cell wall supramolecular structure. Bioresour. Technol. 2010, 101, 7218–7223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—A review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Phojaroen, J.; Jiradechakorn, T.; Kirdponpattara, S.; Sriariyanun, M.; Junthip, J.; Chuetor, S. Performance Evaluation of Combined Hydrothermal-Mechanical Pretreatment of Lignocellulosic Biomass for Enzymatic Enhancement. Polymers 2022, 14, 2313. [Google Scholar] [CrossRef]
- Djimtoingar, S.S.; Derkyi, N.S.A.; Kuranchie, F.A.; Yankyera, J.K. A review of response surface methodology for biogas process optimization PUBLIC INTEREST STATEMENT. Cogent Eng. 2022, 9, 2115283. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N. RSM approach to pre-treatment of lignocellulosic waste and a statistical methodology for optimizing bioethanol production. Waste Manag. Bull. 2024, 2, 49–66. [Google Scholar] [CrossRef]
- Yildirim, O.; Ozkaya, B.; Altinbas, M.; Demir, A. Statistical optimization of dilute acid pretreatment of lignocellulosic biomass by response surface methodology to obtain fermentable sugars for bioethanol production. Int. J. Energy Res. 2021, 45, 8882–8899. [Google Scholar] [CrossRef]
- Zaafouri, K.; Ziadi, M.; Ben Hassen-Trabelsi, A.; Mekni, S.; Aïssi, B.; Alaya, M.; Bergaoui, L.; Hamdi, M. Optimization of Hydrothermal and Diluted Acid Pretreatments of Tunisian Luffa cylindrica (L.) Fibers for 2G Bioethanol Production through the Cubic Central Composite Experimental Design CCD: Response Surface Methodology. BioMed Res. Int. 2017, 2017, 9524521. [Google Scholar] [CrossRef]
- Civitarese, V.; Spinelli, R.; Barontini, M.; Gallucci, F.; Santangelo, E.; Acampora, A.; Scarfone, A.; del Giudice, A.; Pari, L. Open-Air Drying of Cut and Windrowed Short-Rotation Poplar Stems. BioEnergy Res. 2015, 8, 1614–1620. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP): Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Fairbanks, AK, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Ash in Biomass. In NREL Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Fairbanks, AK, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.J.L.A.P. Determination of Extractives in Biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Fairbanks, AK, USA, 2005. [Google Scholar]
- Liu, W.; Wu, R.; Hu, Y.; Ren, Q.; Hou, Q.; Ni, Y. Improving enzymatic hydrolysis of mechanically refined poplar branches with assistance of hydrothermal and Fenton pretreatment. Bioresour. Technol. 2020, 316, 123920. [Google Scholar] [CrossRef]
- Cheng, M.H.; Dien, B.S.; Lee, D.K.; Singh, V. Sugar production from bioenergy sorghum by using pilot scale continuous hy-drothermal pretreatment combined with disk refining. Bioresour. Technol. 2019, 289, 121663. [Google Scholar] [CrossRef] [PubMed]
- Manmai, N.; Unpaprom, Y.; Ramaraj, R. Bioethanol Production from Sunflower Stalk: Application of Chemical and Biological Pre-Treatments by Response Surface Methodology (RSM); Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pre-treatment and disk milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef]
- Juneja, A.; Kumar, D.; Singh, V.K.; Yadvika; Singh, V. Chemical Free Two-Step Hydrothermal Pretreatment to Improve Sugar Yields from Energy Cane. Energies 2020, 13, 5805. [Google Scholar] [CrossRef]
- Resch, M.G.; Baker, J.O.; Decker, S.R. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Fairbanks, AK, USA, 2018. [Google Scholar]
- Timung, R.; Mohan, M.; Chilukoti, B.; Sasmal, S.; Banerjee, T.; Goud, V.V. Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: A comparative study. Biomass Bioenergy 2015, 81, 9–18. [Google Scholar] [CrossRef]
- Mittal, A.; Chatterjee, S.G.; Scott, G.M.; Amidon, T.E. Modeling xylan solubilization during autohydrolysis of sugar maple and aspen wood chips: Reaction kinetics and mass transfer. Chem. Eng. Sci. 2009, 64, 3031–3041. [Google Scholar] [CrossRef]
- Kaakinen, S.; Kostiainen, K.; Ek, F.; Saranpää, P.; Kubiske, M.E.; Sober, J.; Karnosky, D.F.; Vapaavuori, E. Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Glob. Chang. Biol. 2004, 10, 1513–1525. [Google Scholar] [CrossRef]
- Hamid, H.S.H.B.; Ismail, K.S.K. Optimization of enzymatic hydrolysis for acid pretreated date seeds into fermentable sugars. Biocatal. Agric. Biotechnol. 2020, 24, 101530. [Google Scholar] [CrossRef]
- Adetoyese, A.; Aransiola, E.; Ademakinwa, N.; Bada, B.; Agboola, F. Optimization study of bioethanol production from sponge gourd (Luffa cylindrica). Sci. Afr. 2020, 8, e00407. [Google Scholar] [CrossRef]
- Gopal, L.C.; Govindarajan, M.; Kavipriya, M.; Mahboob, S.; Al-Ghanim, K.A.; Virik, P.; Ahmed, Z.; Al-Mulhm, N.; Senthilkumaran, V.; Shankar, V. Optimization strategies for improved biogas production by recycling of waste through response surface methodology and artificial neural network: Sustainable energy perspective research. J. King Saud. Univ. Sci. 2021, 33, 101241. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M.; Ahmed, N.A.; Adeleke, O.; Ogunkunle, O. Biomethane yield modeling and optimization from thermally pretreated Arachis hypogea shells using response surface methodology and artificial neural network. Bioresour. Technol. Rep. 2022, 20, 101236. [Google Scholar] [CrossRef]
- Lee, I.; Yu, J.-H. Design of hydrothermal and subsequent lime pretreatment for fermentable sugar and bioethanol production from acacia wood. Renew. Energy 2021, 174, 170–177. [Google Scholar] [CrossRef]
- Lee, I.; Yu, J.-H. The production of fermentable sugar and bioethanol from acacia wood by optimizing dilute sulfuric acid pretreatment and post treatment. Fuel 2020, 275, 117943. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.-W.; Rao, J.; Tian, R.; Sun, S.-N.; Peng, F. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemi-celluloses and lignin: A review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Pihlajaniemi, V.; Pastinen, O.; Laakso, S. Reduction of surface area of lignin improves enzymatic hydrolysis of cellulose from hydrothermally pretreated wheat straw. RSC Adv. 2014, 4, 36591–36596. [Google Scholar] [CrossRef][Green Version]
- Jia, L.; Juneja, A.; Majumder, E.L.-W.; Ramarao, B.V.; Kumar, D. Efficient Enzymatic Hydrolysis and Polyhydroxybutyrate Production from Non-Recyclable Fiber Rejects from Paper Mills by Recombinant Escherichia coli. Processes 2024, 12, 1576. [Google Scholar] [CrossRef]
- Sun, R.C.; Xiao, L.P.; Song, G.Y. Effect of Hydrothermal Processing on Hemicellulose Structure. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hy-drothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef]
- Chen, W.-H.; Nižetić, S.; Sirohi, R.; Huang, Z.; Luque, R.; M.Papadopoulos, A.; Sakthivel, R.; Nguyen, X.P.; Hoang, A.T. Liquid hot water as sustainable biomass pretreatment technique for bioenergy production: A review. Bioresour. Technol. 2022, 344, 126207. [Google Scholar] [CrossRef]
- Kang, X.; Wang, Y.-Y.; Wang, S.; Song, X. Xylan and xylose decomposition during hot water pre-extraction: A pH-regulated hydrolysis. Carbohydr. Polym. 2021, 255, 117391. [Google Scholar] [CrossRef]
- Yusof, S.J.H.M.; Roslan, A.M.; Zakaria, M.R.; Hassan, M.A.; Shirai, Y. Kinetics of Xylan Autohydrolysis During Subcritical Hydrothermal Pretreatment of Oil Palm Frond Pressed Fiber. BioEnergy Res. 2021, 15, 439–453. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocel-lulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.; Boyce, A.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.K.; Padhi, D.; Mohanty, M.K.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass-Convers. Biorefin. 2021, 13, 1503–1527. [Google Scholar] [CrossRef]
- Wells, J.M.; Drielak, E.; Surendra, K.C.; Khanal, S.K. Hot water pretreatment of lignocellulosic biomass: Modeling the effects of temperature, enzyme and biomass loadings on sugar yield. Bioresour. Technol. 2020, 300, 122593. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Helle, S.; Cameron, D.; Lam, J.; White, B.; Duff, S. Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of S. cerevisiae. Enzyme Microb. Technol. 2003, 33, 786–792. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Eklund, R.; Gustafsson, L.; Niklasson, C.; Lidén, G. Characterization and Fermentation of Dilute-Acid Hydrolyzates from Wood. Ind. Eng. Chem. Res. 1997, 36, 4659–4665. [Google Scholar] [CrossRef]
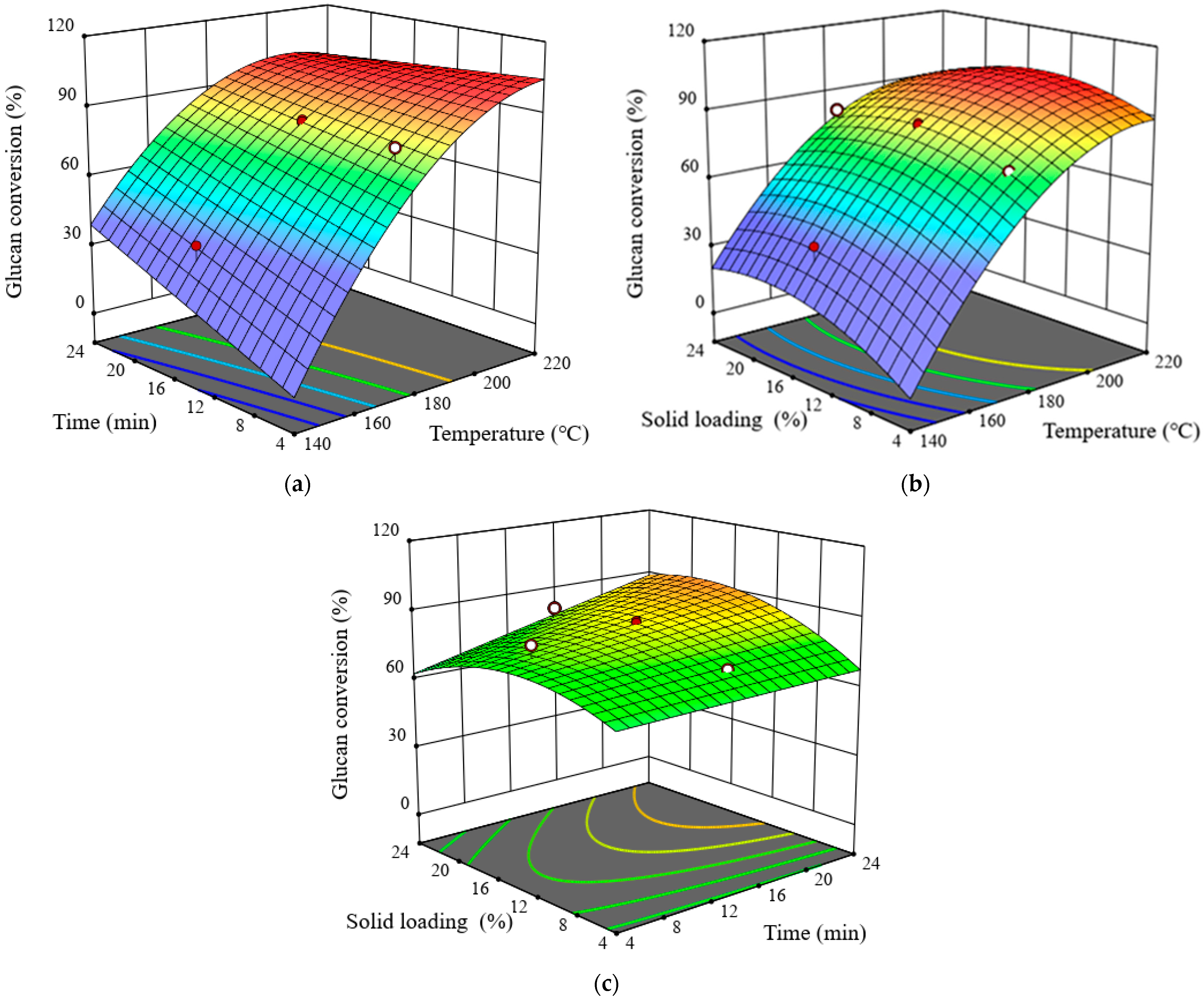
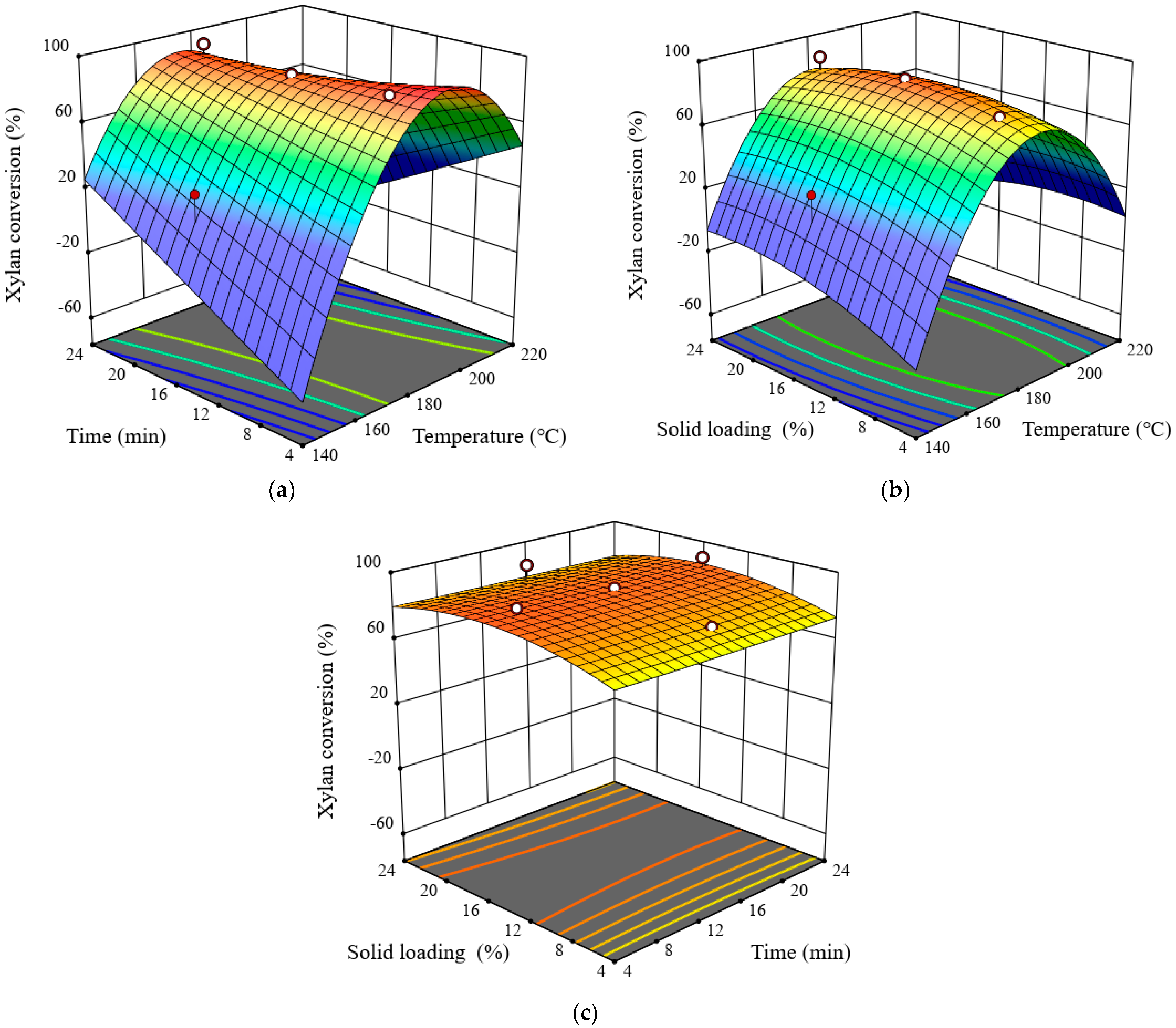
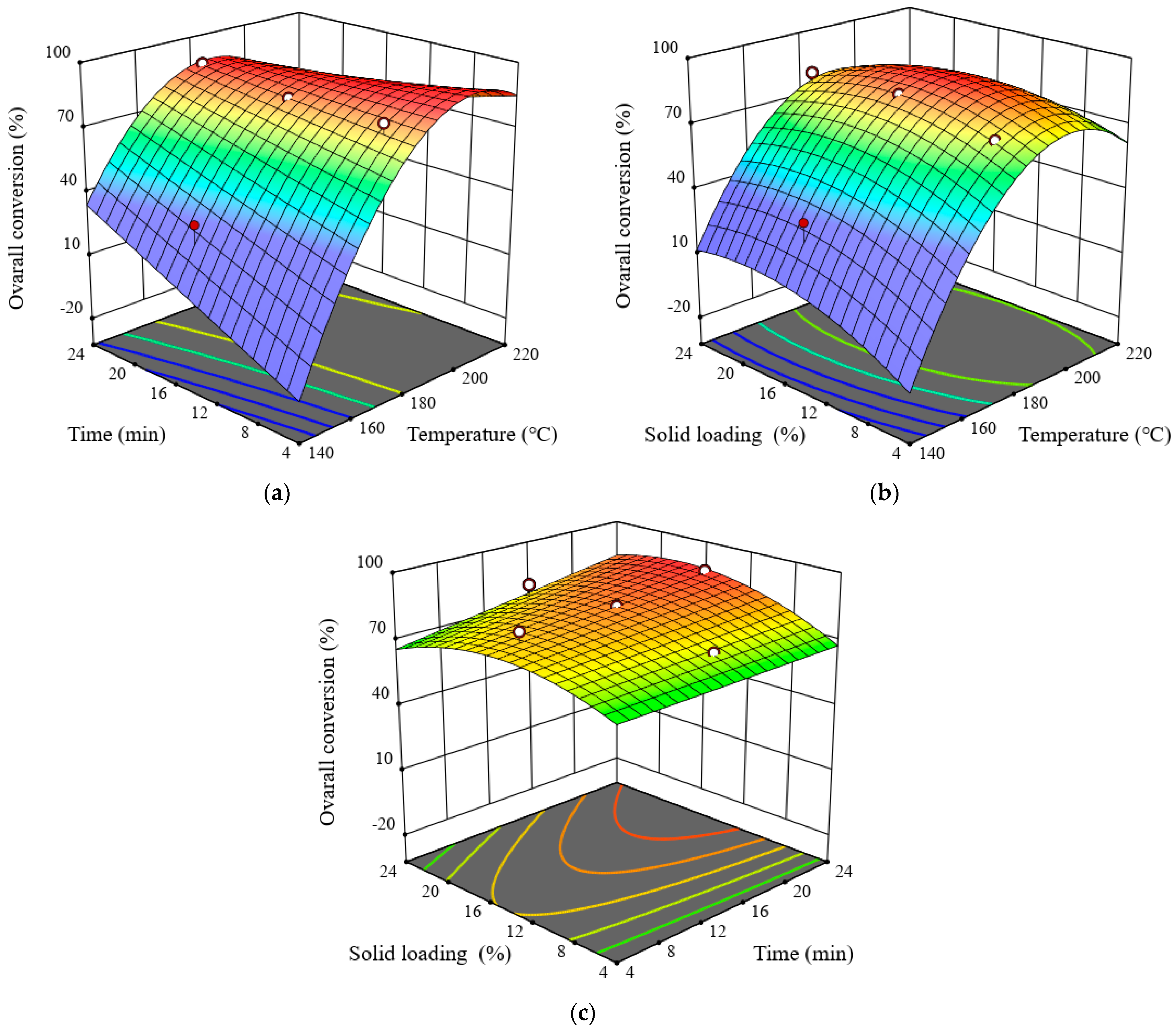
| Space Type | RSM Experiment Design | Glucan Conversion (%) | Xylan Conversion (%) | Overall Conversion (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Solid Loading (%) | Exp. 1 | Pred. 2 | Resl. 3 | Exp. 1 | Pred. 2 | Resl. 3 | Exp. 1 | Pred. 2 | Resl. 3 | |
| Factorial points | −1(160) | −1(10) | −1(10) | 46.33 | 48.23 | −1.90 | 41.23 | 44.81 | −3.58 | 43.66 | 46.00 | −2.34 |
| +1(200) | −1(10) | −1(10) | 92.72 | 95.62 | −2.90 | 77.46 | 77.40 | 0.06 | 85.90 | 87.86 | −1.96 | |
| +1(200) | −1(10) | +1(20) | 93.24 | 92.81 | 0.43 | 75.07 | 71.54 | 3.53 | 85.56 | 84.23 | 1.33 | |
| −1(160) | −1(10) | +1(20) | 43.02 | 50.57 | −7.55 | 40.28 | 52.35 | −12.07 | 41.12 | 49.80 | −8.68 | |
| −1(160) | +1(20) | −1(10) | 55.00 | 58.53 | −3.53 | 54.46 | 64.80 | −10.34 | 53.44 | 58.88 | −5.44 | |
| +1(200) | +1(20) | −1(10) | 98.92 | 94.47 | 4.45 | 62.53 | 57.27 | 5.26 | 85.76 | 81.19 | 4.57 | |
| +1(200) | +1(20) | +1(20) | 96.56 | 97.76 | −1.20 | 47.48 | 50.71 | −3.23 | 79.76 | 81.52 | −1.76 | |
| −1(160) | +1(20) | +1(20) | 66.78 | 66.98 | −0.20 | 64.76 | 71.63 | −6.87 | 64.49 | 66.64 | −2.15 | |
| Axial points | −α(146.36) | 0(15) | 0(15) | 41.57 | 35.22 | 6.35 | 34.14 | 17.89 | 16.25 | 38.34 | 29.26 | 9.08 |
| +α(213.64) | 0(15) | 0(15) | 99.00 | 100.96 | −1.96 | 21.08 | 27.70 | −6.62 | 73.70 | 76.97 | −3.27 | |
| 0(180) | −α(6.59) | 0(15) | 83.27 | 77.67 | 5.60 | 91.76 | 86.87 | 4.89 | 83.34 | 78.39 | 4.95 | |
| 0(180) | +α(23.41) | 0(15) | 89.29 | 90.50 | −1.21 | 90.92 | 86.17 | 4.75 | 87.79 | 86.93 | 0.86 | |
| 0(180) | 0(15) | −α(6.59) | 74.61 | 73.79 | 0.82 | 80.32 | 78.49 | 1.83 | 74.38 | 73.29 | 1.09 | |
| 0(180) | 0(15) | +α(23.41) | 82.10 | 78.53 | 3.57 | 87.12 | 79.31 | 7.81 | 81.48 | 76.76 | 4.72 | |
| Center points | 0(180) | 0(15) | 0(15) | 83.63 | 84.17 | −0.54 | 87.64 | 86.22 | 1.42 | 82.68 | 82.63 | 0.05 |
| 0(180) | 0(15) | 0(15) | 82.40 | 84.17 | −1.77 | 85.06 | 86.22 | −1.16 | 81.08 | 82.63 | −1.55 | |
| 0(180) | 0(15) | 0(15) | 85.30 | 84.17 | 1.13 | 85.87 | 86.22 | −0.35 | 83.30 | 82.63 | 0.67 | |
| 0(180) | 0(15) | 0(15) | 84.49 | 84.17 | 0.32 | 86.28 | 86.22 | 0.06 | 82.87 | 82.63 | 0.24 | |
| 0(180) | 0(15) | 0(15) | 84.20 | 84.17 | 0.03 | 86.09 | 86.22 | −0.13 | 82.61 | 82.63 | −0.02 | |
| 0(180) | 0(15) | 0(15) | 84.25 | 84.17 | 0.08 | 84.75 | 86.22 | −1.47 | 82.26 | 82.63 | −0.37 | |
| Factor | Glucan Conversion | Xylan Conversion | Overall Conversion | |||
|---|---|---|---|---|---|---|
| Coefficient | VIF | Coefficient | VIF | Coefficient | VIF | |
| Intercept | 84.17 | - | 86.22 | - | 82.63 | - |
| A—Temperature | 19.54 | 1.00 | 2.92 | 1.00 | 14.19 | 1.00 |
| B—Time | 3.81 | 1.00 | −0.2094 | 1.00 | 2.54 | 1.00 |
| C—Solid loading | 1.41 | 1.00 | 0.2450 | 1.00 | 1.03 | 1.00 |
| AB | −2.86 | 1.00 | −10.03 | 1.00 | −4.89 | 1.00 |
| AC | −1.29 | 1.00 | −3.35 | 1.00 | −1.86 | 1.00 |
| BC | 1.53 | 1.00 | −0.1763 | 1.00 | 0.9913 | 1.00 |
| A² | −5.69 | 1.02 | −22.43 | 1.02 | −10.44 | 1.02 |
| B² | −0.0301 | 1.02 | 0.1057 | 1.02 | 0.0094 | 1.02 |
| C² | −2.83 | 1.02 | −2.59 | 1.02 | −2.69 | 1.02 |
| R2 | 0.97 | - | 0.91 | - | 0.94 | - |
| CV (%) | 5.68 | - | 12.48 | - | 7.30 | - |
| Adequate precision | 20.88 | - | 10.98 | - | 15.40 | - |
| Source | Glucan Conversion | Xylan Conversion | Overall Conversion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F Value | Sum of Squares | df | Mean Square | F Value | Sum of Squares | df | Mean Square | F Value | |
| Model | 6087.74 | 9 | 676.42 | 34.12 | 8344.19 | 9 | 927.13 | 11.75 | 4707.95 | 9 | 523.11 | 18.06 |
| X1—Temperature | 5215.93 | 1 | 5215.93 | 263.14 | 116.26 | 1 | 116.26 | 1.47 | 2748.40 | 1 | 2748.40 | 94.89 |
| X2—Time | 198.56 | 1 | 198.56 | 10.02 | 0.5986 | 1 | 0.5986 | 0.0076 | 88.14 | 1 | 88.14 | 3.04 |
| X3—Solid loading | 27.07 | 1 | 27.07 | 1.37 | 0.8199 | 1 | 0.8199 | 0.0104 | 14.58 | 1 | 14.58 | 0.5034 |
| X1X2 | 65.61 | 1 | 65.61 | 3.31 | 804.61 | 1 | 804.61 | 10.19 | 191.00 | 1 | 191.00 | 6.59 |
| X1X3 | 13.29 | 1 | 13.29 | 0.6703 | 89.71 | 1 | 89.71 | 1.14 | 27.57 | 1 | 27.57 | 0.95 |
| X2X3 | 18.64 | 1 | 18.64 | 0.9402 | 0.2485 | 1 | 0.2485 | 0.0031 | 7.86 | 1 | 7.86 | 0.2714 |
| X1² | 465.80 | 1 | 465.80 | 23.50 | 7247.95 | 1 | 7247.95 | 91.84 | 1569.64 | 1 | 1569.64 | 54.19 |
| X2² | 0.0131 | 1 | 0.0131 | 0.0007 | 0.1611 | 1 | 0.1611 | 0.0020 | 0.0013 | 1 | 0.0013 | 0.0000 |
| X3² | 115.58 | 1 | 115.58 | 5.83 | 96.55 | 1 | 96.55 | 1.22 | 104.28 | 1 | 104.28 | 3.60 |
| Residual | 198.22 | 10 | 19.82 | 789.22 | 10 | 78.92 | 289.65 | 10 | 28.96 | |||
| Lack of Fit | 193.50 | 5 | 38.70 | 41.02 | 784.00 | 5 | 156.80 | 150.10 | 286.76 | 5 | 57.35 | 99.27 |
| Pure Error | 4.72 | 5 | 0.9435 | 5.22 | 5 | 1.04 | 2.89 | 5 | 0.5777 | |||
| Total | 6285.96 | 19 | 9133.41 | 19 | 4997.60 | 19 | ||||||
| Parameters and Responses | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| Temperature (°C) | In range | 146 | 214 |
| Time (min) | In range | 6 | 24 |
| Solid loading (%) | In range | 6 | 24 |
| Glucan conversion (%) | Maximize | 41.57 | 99.0 |
| Xylan conversion (%) | Maximize | 21.08 | 91.92 |
| Overall conversion (%) | Maximize | 38.34 | 87.79 |
| Glucan Conversion (%) | Xylan Conversion (%) | Overall Conversion (%) | |
|---|---|---|---|
| Model predicted values | 94.57 | 79.78 | 87.84 |
| Experimental values | 92.43 | 79.07 | 86.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.S.; Therasme, O.; Volk, T.A.; Kumar, V.; Kumar, D. Optimization of Combined Hydrothermal and Mechanical Refining Pretreatment of Forest Residue Biomass for Maximum Sugar Release during Enzymatic Hydrolysis. Energies 2024, 17, 4929. https://doi.org/10.3390/en17194929
Hossain MS, Therasme O, Volk TA, Kumar V, Kumar D. Optimization of Combined Hydrothermal and Mechanical Refining Pretreatment of Forest Residue Biomass for Maximum Sugar Release during Enzymatic Hydrolysis. Energies. 2024; 17(19):4929. https://doi.org/10.3390/en17194929
Chicago/Turabian StyleHossain, Md Shahadat, Obste Therasme, Timothy A. Volk, Vinod Kumar, and Deepak Kumar. 2024. "Optimization of Combined Hydrothermal and Mechanical Refining Pretreatment of Forest Residue Biomass for Maximum Sugar Release during Enzymatic Hydrolysis" Energies 17, no. 19: 4929. https://doi.org/10.3390/en17194929
APA StyleHossain, M. S., Therasme, O., Volk, T. A., Kumar, V., & Kumar, D. (2024). Optimization of Combined Hydrothermal and Mechanical Refining Pretreatment of Forest Residue Biomass for Maximum Sugar Release during Enzymatic Hydrolysis. Energies, 17(19), 4929. https://doi.org/10.3390/en17194929










