Synergy Effect of High K-Low Ca-High Si Biomass Ash Model System on Syngas Production and Reactivity Characteristics during Petroleum Coke Steam Gasification
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
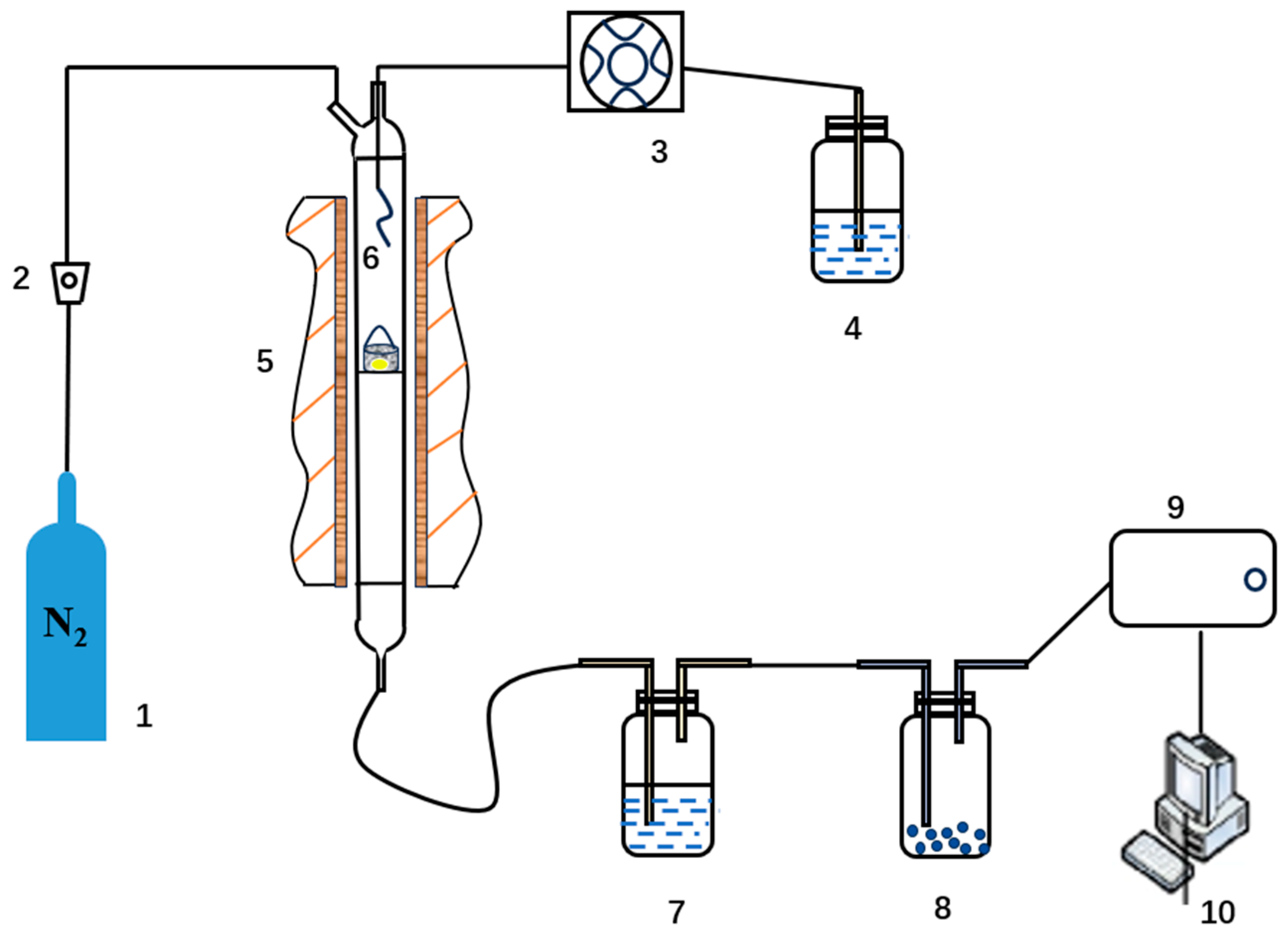
2.2. Steam Gasification Experiments
2.3. Semi-Char Structure Analysis
3. Results and Discussion
3.1. Influence and Synergy Effect of High K-Low Ca-High Si Model System on Gas Release Characteristics of PC Steam Gasification Process
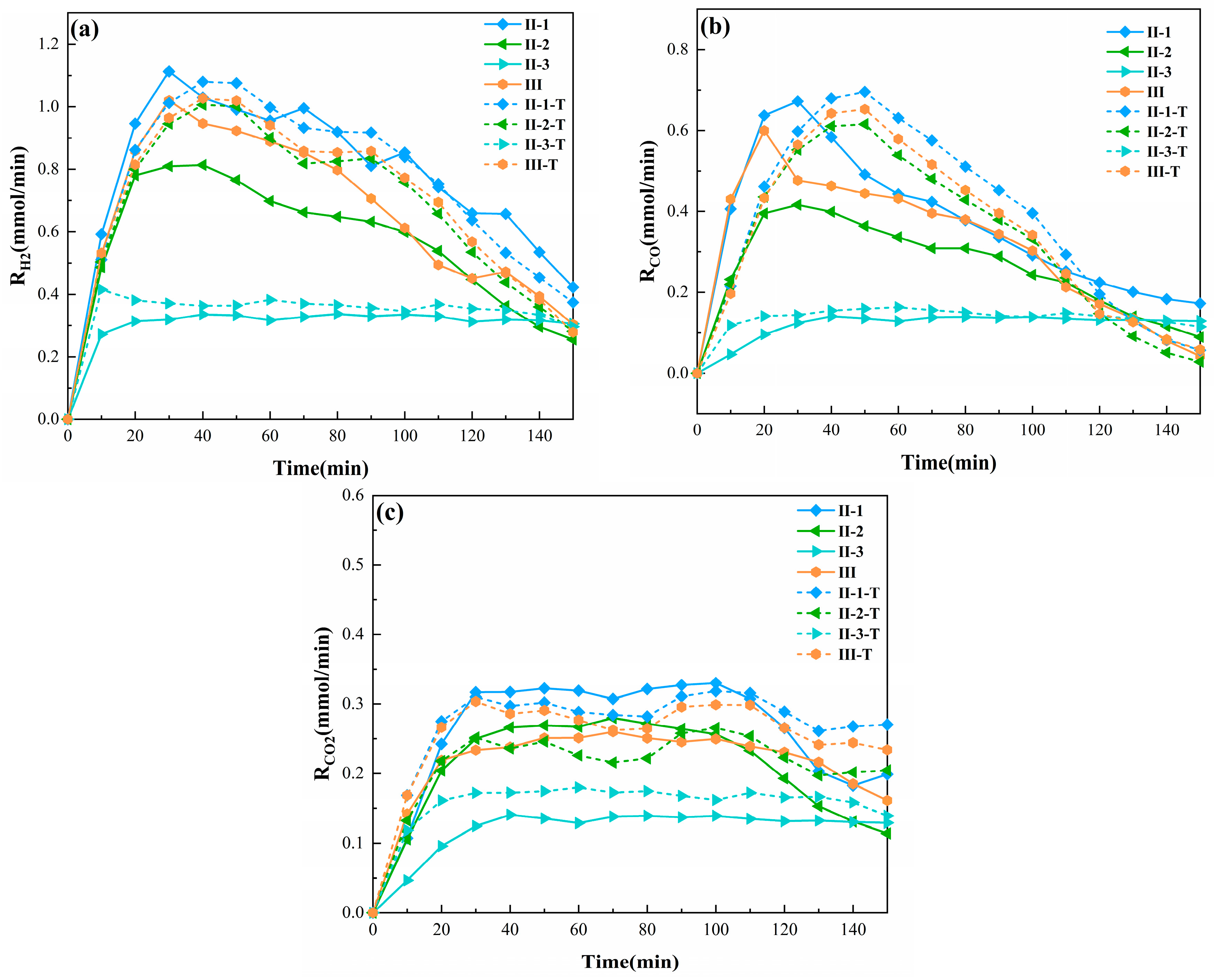
3.1.1. Syngas Release Characteristics
3.1.2. Synergy Effect of BAMS on Syngas Release
3.2. Influence and Synergy Effect of High K-Low Ca-High Si Model System on Syngas Yield in PC Steam Gasification Process
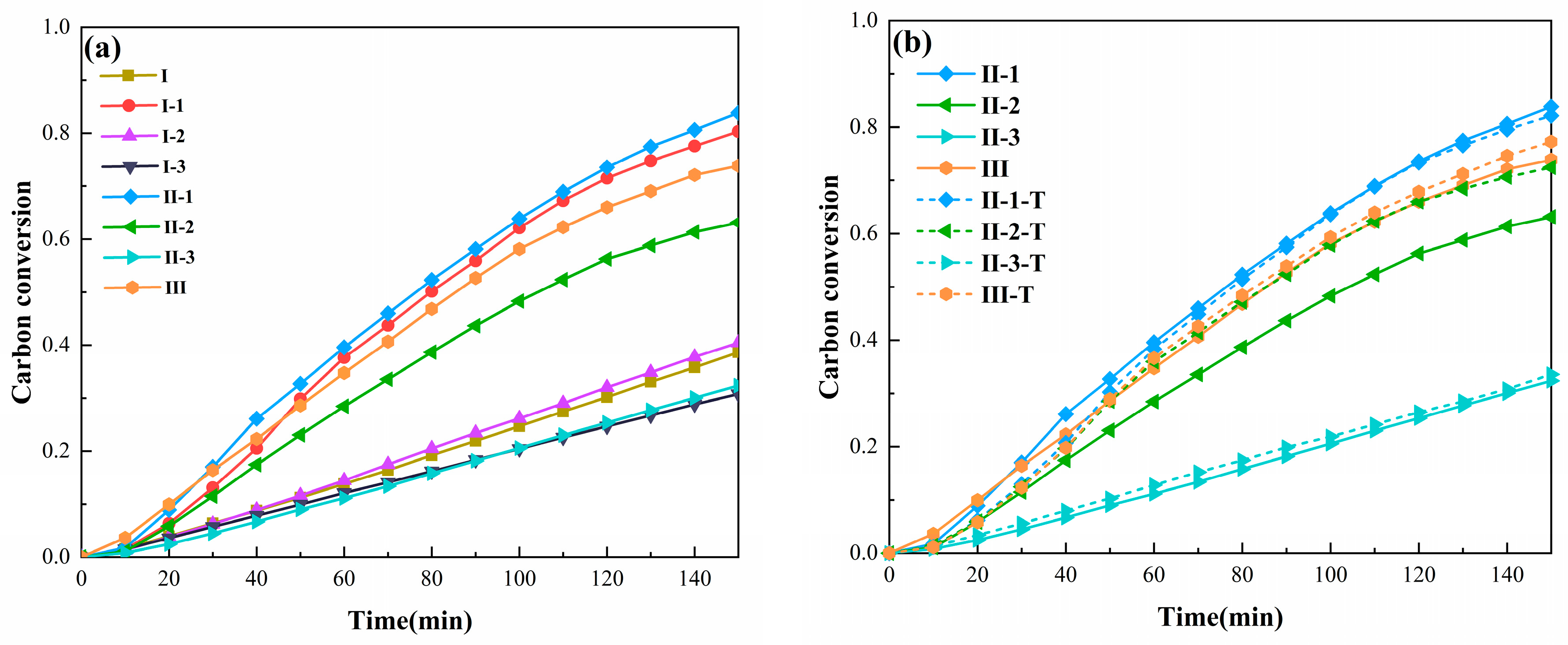
3.2.1. Syngas Yield of the PC Gasification Process
3.2.2. Synergy Effect of Syngas Yield in PC Steam Gasification Process
3.3. Influence and Synergy Effect of High K-Low Ca-High Si Model System on Gasification Reaction Characteristics of PC
3.3.1. Influence of Gasification Reaction Characteristics of PC
3.3.2. Synergy Effect of Gasification Reaction Characteristics of PC
3.4. Correlation between Structural Evolution and Reaction Synergy Behavior of PC Steam Gasification in High K-Low Ca-High Si Model Systems
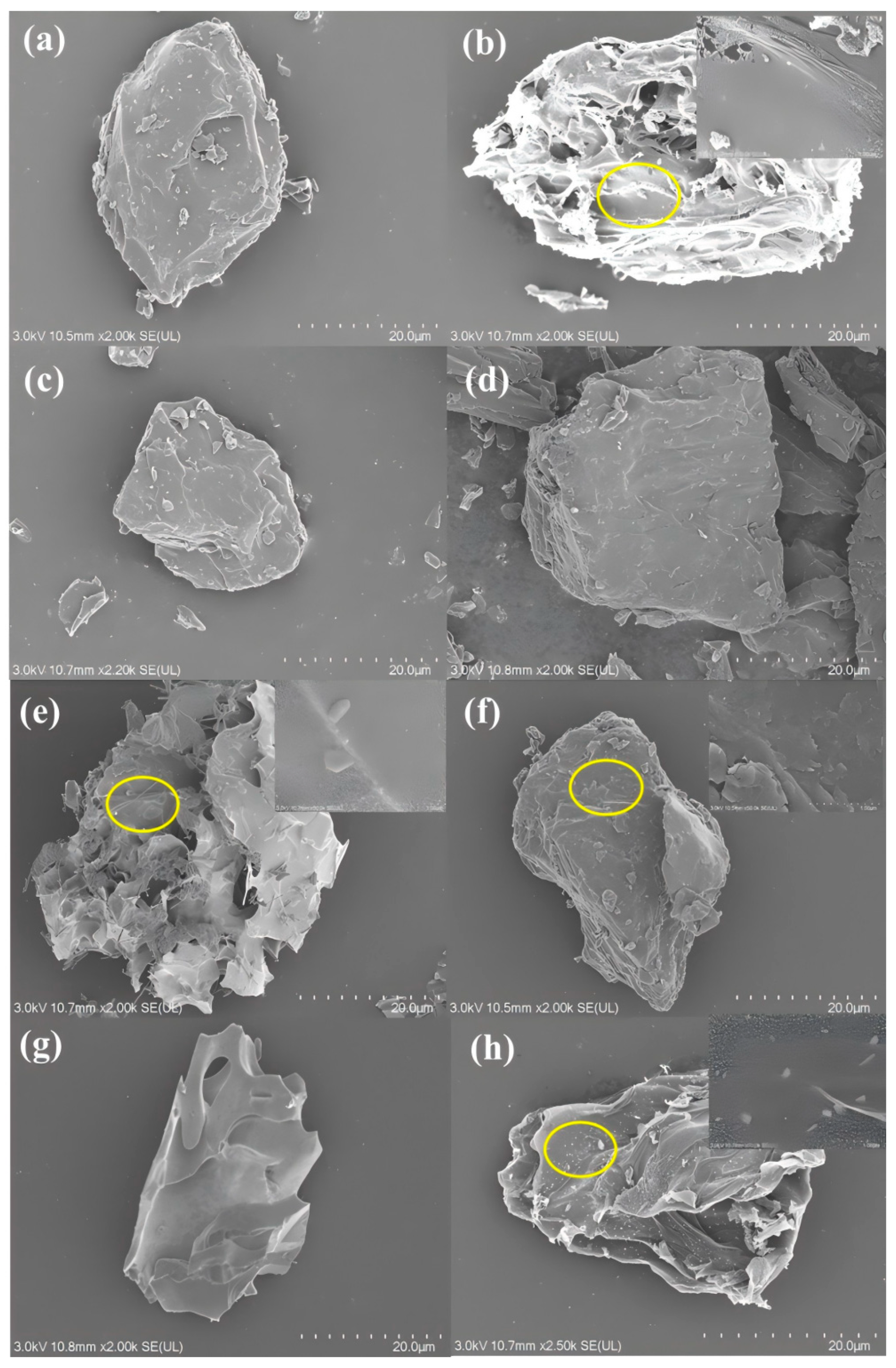
3.4.1. Evolution of Surface Microstructure
3.4.2. Evolution of Carbon Structure
3.5. Synergy Mechanism of BAMS with High K-Low Ca-High Si
3.5.1. Synergistic Catalysis Mechanism of K-Ca Binary System
3.5.2. Mechanism of Catalytic Inhibition of SiO2 in the K-Si/Ca-Si Binary System
3.5.3. Synergistic Catalysis Mechanism of K-Ca-Si Ternary System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics, and synergy behavior. Fuel 2021, 304, 121517. [Google Scholar] [CrossRef]
- He, Q.; Yu, J.; Song, X.; Ding, L.; Wei, J.; Yu, G. Utilization of biomass ash for upgrading petroleum coke gasification: Effect of soluble and insoluble components. Energy 2020, 192, 116642. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Ma, C.; Yang, W.; Pudasainee, D.; Gupta, R.; Sun, L. Synergistic effect on the co-gasification of petroleum coke and carbon-based feedstocks: A state-of-the-art review. J. Energy Inst. 2022, 102, 1–13. [Google Scholar] [CrossRef]
- Dutta, S.; Wen, C.Y.; Belt, R.J. Reactivity of coal and char. 1. In carbon dioxide atmosphere. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 20–30. [Google Scholar] [CrossRef]
- Mao, L.; Liu, T.; Zhao, Y.; Zheng, M. Synergism between lignite and high-sulfur petroleum coke in CO2 gasification. Green Process. Synth. 2023, 12, 20228143. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Y.; Cai, Z.; Zhang, J.; Yu, G. The synergistic effecting mechanisms of biomass pyrolysis, biomass char gasification, and biomass ash on CO2 co-gasification of biomass and high-sulfur petroleum coke. Fuel 2024, 365, 131203. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, T.; Ge, L.; Li, Q.; Zhao, C.; Huang, J.; Wang, Y. Study on the preparation of high value-added activated carbon from petroleum coke: Comparison between one-and two-step methods for carbonization and activation. Energy 2024, 292, 130570. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Z.; Yoo, C.G.; Shen, X.; Hou, Q.; Boudesocque-Delaye, L. Editorial: The application of green chemistry in biomass valorization: Green route, green catalyst and green solvent. Front. Chem. 2023, 11, 1277256. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, W.; Shen, Y.; Luo, S.; Ren, D. Progress in the change of ash melting behavior and slagging characteristics of co-gasification of biomass and coal: A review. J. Energy Inst. 2023, 111, 101414. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, W.; Wu, S.; Zeng, X.; Cui, P. High quality syngas production from catalytic steam gasification of biomass with calcium-rich construction waste. J. Energy Inst. 2023, 111, 101433. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Tan, H.; Du, W.; Qu, X. Extraction and quantitation of various potassium salts in straw ash. Environ. Prog. Sustain. Energy 2015, 34, 333–338. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.-C.; Li, W.-Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Q.; Gong, Y.; Ding, L.; Yu, G. Effect of biomass leachates on structure evolution and reactivity characteristic of petroleum coke gasification. Renew. Energy 2020, 155, 111–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Ashizawa, M.; Kajitani, S.; Hara, S. A new approach to catalytic coal gasification: The recovery and reuse of calcium using biomass derived crude vinegars. Fuel 2010, 89, 417–422. [Google Scholar] [CrossRef]
- Kang, S.; Li, J.; Zheng, Y.; Zhu, X.; Bi, J. Reaction kinetics study on the char-steam catalytic gasification under pressurized conditions. Coal Convers. 2011, 34, 31–35. [Google Scholar]
- Zhang, Y.; Hara, S.; Kajitani, S.; Ashizawa, M. Modeling of catalytic gasification kinetics of coal char and carbon. Fuel 2010, 89, 152–157. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, H.; Zhu, Z.; Lv, Q. CO2 gasification reactivity of brown coal char. Coal Convers. 2012, 38, 314–320. [Google Scholar]
- Zhao, H.; Wu, J.; Xiao, W.; Cao, K. Study on catalytic gasification of Yining long flame coal char. Technol. Dev. Chem. Ind. 2011, 40, 33–35. [Google Scholar]
- Gao, Z.; Hu, J.; Guo, Z.; Wang, X.; Wu, X. Investigation on the co-gasification of coal char with biomass char and the distributed activation energy. Proc. Csee 2011, 31, 51–57. [Google Scholar]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Polikarpova, S.B.; Ponomarev, V.E.; Murashova, E.V.; Sokolovskiy, P.V.; Kustov, L.M. A synergistic effect of potassium and transition metal compounds on the catalytic behaviour of hydrolysis lignin in CO2-assisted gasification. Energies 2023, 16, 4335. [Google Scholar] [CrossRef]
- Ding, X.; Jiao, W.; Liu, Y.; Wang, Z.; Fang, Y. Methane production from high-pressure catalytic steam hydrogasification of sawdust char on K-modified transition metal composite catalysts. Fuel 2024, 363, 131000. [Google Scholar]
- Jiang, M.-Q.; Zhou, R.; Hu, J.; Wang, F.-C.; Wang, J. Calcium-promoted catalytic activity of potassium carbonate for steam gasification of coal char: Influences of calcium species. Fuel 2012, 99, 64–71. [Google Scholar] [CrossRef]
- Monterroso, R.; Fan, M.; Zhang, F.; Gao, Y.; Popa, T.; Argyle, M.D.; Towler, B.; Sun, Q. Effects of an environmentally-friendly, inexpensive composite iron–sodium catalyst on coal gasification. Fuel 2014, 116, 341–349. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Xu, D.; Zhang, S.; Lv, P.; Jiang, Y.; Song, X.; Kontchouo, F.M.; Jiao, Y.; Li, B.; et al. Synergistic effect mechanism of biomass ash-derived K-Ca-Si catalytic system on syngas production and reactivity characteristics of high-sulfur petroleum coke gasification. Fuel 2024, 365, 131224. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Wu, S.; Huang, S.; Gao, J. Potassium-catalyzed steam gasification of petroleum coke for H2 production: Reactivity, selectivity and gas release. Fuel Process. Technol. 2011, 92, 523–530. [Google Scholar] [CrossRef]
- Mohammadi, M.; Khorrami, M.K.; Vatanparast, H.; Karimi, A.; Sadrara, M. Classification and determination of sulfur content in crude oil samples by infrared spectrometry. Infrared Phys. Technol. 2022, 127, 104382. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Bao, Z.; Lu, Z.; Chen, J.; Cai, J.; Guo, S.; Yao, S. Relationships between char reactivity and char structure from a suite of organic model compounds. Fuel Process. Technol. 2023, 249, 107852. [Google Scholar] [CrossRef]
- He, J.; Zou, C.; Zhao, J.; Xi, J.; She, Y.; Ren, M.; Xu, Y. Influence of Raman spectroscopy test conditions on the results of carbon chemical structure of chars. Energies 2022, 15, 5627. [Google Scholar] [CrossRef]
- Ban, Y.; Liu, Q.; Zhou, H.; Li, N.; Zhao, B.; Shi, S.; He, R.; Zhi, K. Catalytic effect of representative calcium salts on the steam gasification of a Shengli lignite. Fuel 2019, 255, 115832. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Jiang, Y.; Guo, F.; Zhao, X.; Wu, J. The mechanism of the ash fusion characteristics of gasification coke affected by SiO2/Al2O3 ratio and CaO content in blending coals. Int. J. Coal Prep. Util. 2022, 42, 941–959. [Google Scholar] [CrossRef]
- Ge, Z.; Cao, X.; Zha, Z.; Ma, Y.; Zeng, M.; Wu, K.; Chu, S.; Tao, Y.; Zhang, H. The mineral transformation and molten behaviors of biomass waste ashes in gasification-melting process. Fuel Process. Technol. 2022, 226, 107095. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhu, L.; Zhang, Z.; Xiao, H. Experimental study on co-pyrolysis of petroleum coke and coals: Synergy effects and co-gasification reactivity. Fuel 2020, 279, 118368. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; He, Q.; Gong, Y.; Yu, G. Analysis of coal gasification reactivity, kinetics, and mechanism with iron-based catalyst from coal liquefaction. ACS Omega 2021, 6, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Cui, M.; Hu, Y.; Wang, J. Calcium-promoted catalytic activity of potassium carbonate for steam gasification of coal char: Transformations of sulfur. Fuel 2013, 112, 687–694. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Catalytic steam gasification of coal char with alkali carbonates: A study on their synergic effects with calcium hydroxide. Fuel Process. Technol. 2016, 142, 34–41. [Google Scholar] [CrossRef]
- Bai, Y.; Lv, P.; Li, F.; Song, X.; Su, W.; Yu, G. Investigation into Ca/Na compounds catalyzed coal pyrolysis and char gasification with steam. Energy Convers. Manag. 2019, 184, 172–179. [Google Scholar] [CrossRef]


| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| FC | VM | Ash | C | H | S | N | O | |
| PC | 86.73 | 10.97 | 2.3 | 91.82 | 0.980 | 5.517 | 1.357 | 0.326 |
| Samples | KCl | CaCO3 | SiO2 |
|---|---|---|---|
| I | 0 | 0 | 0 |
| I-1 | 0.15 g | 0 | 0 |
| I-2 | 0 | 0.045 g | 0 |
| I-3 | 0 | 0 | 0.105 g |
| II-1 | 0.15 g | 0.045 g | 0 |
| II-2 | 0.15 g | 0 | 0.105 g |
| II-3 | 0 | 0.045 g | 0.105 g |
| III | 0.15 g | 0.045 g | 0.105 g |
| Samples | Tm (min) (Theoretical Value) | Rm (mmol/min) (Theoretical Value) | ||||
|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2 | CO | CO2 | |
| I | 10 | 10 | 70 | 0.39 | 0.14 | 0.15 |
| I-1 | 20 | 50 | 50 | 1.06 | 0.63 | 0.36 |
| I-2 | 70 | 70 | 70 | 0.44 | 0.14 | 0.19 |
| I-3 | 10 | 20 | 40 | 0.38 | 0.14 | 0.14 |
| II-1 | 30 (40) | 30 (40) | 100 (100) | 1.11 (1.08) | 0.67 (0.68) | 0.33 (0.32) |
| II-2 | 30 (40) | 30 (50) | 70 (100) | 0.81 (1.01) | 0.42 (0.62) | 0.28 (0.26) |
| II-3 | 10 (40) | 40 (60) | 40 (60) | 0.33 (0.41) | 0.14 (0.16) | 0.14 (0.18) |
| III | 30 (40) | 20 (50) | 70 (110) | 1.02 (1.03) | 0.60 (0.65) | 0.26 (0.30) |
| Samples | I | I-1 | I-2 | I-3 | II-1 | II-2 | II-3 | III |
|---|---|---|---|---|---|---|---|---|
| Xf (theoretical) | 0.39 | 0.80 | 0.40 | 0.31 | 0.84 (0.82) | 0.63 (0.72) | 0.32 (0.34) | 0.74 (0.77) |
| Samples | IG/Iall (AD) (TD) | ID3/IG (AD) (TD) |
|---|---|---|
| I | 0.107 | 1.01 |
| I-1 | 0.092 (−0.015) | 1.18 (0.17) |
| I-2 | 0.100 (−0.007) | 1.03 (0.02) |
| I-3 | 0.118 (0.011) | 0.75 (−0.26) |
| II-1 | 0.076 (−0.031) | 1.56 (0.55) |
| II-2 | 0.095 (−0.012) | 1.16 (0.15) |
| II-3 | 0.109 (0.002) | 0.87 (−0.14) |
| III | 0.083 (−0.024) | 1.46 (0.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Tian, L.; Sun, J.; Ding, K.; Li, B.; Bai, Y.; Rout, L.; Liu, X.; Xu, G.; Yu, G. Synergy Effect of High K-Low Ca-High Si Biomass Ash Model System on Syngas Production and Reactivity Characteristics during Petroleum Coke Steam Gasification. Energies 2024, 17, 4650. https://doi.org/10.3390/en17184650
Wei J, Tian L, Sun J, Ding K, Li B, Bai Y, Rout L, Liu X, Xu G, Yu G. Synergy Effect of High K-Low Ca-High Si Biomass Ash Model System on Syngas Production and Reactivity Characteristics during Petroleum Coke Steam Gasification. Energies. 2024; 17(18):4650. https://doi.org/10.3390/en17184650
Chicago/Turabian StyleWei, Juntao, Lina Tian, Jiawei Sun, Kuan Ding, Bin Li, Yonghui Bai, Lipeeka Rout, Xia Liu, Guangyu Xu, and Guangsuo Yu. 2024. "Synergy Effect of High K-Low Ca-High Si Biomass Ash Model System on Syngas Production and Reactivity Characteristics during Petroleum Coke Steam Gasification" Energies 17, no. 18: 4650. https://doi.org/10.3390/en17184650
APA StyleWei, J., Tian, L., Sun, J., Ding, K., Li, B., Bai, Y., Rout, L., Liu, X., Xu, G., & Yu, G. (2024). Synergy Effect of High K-Low Ca-High Si Biomass Ash Model System on Syngas Production and Reactivity Characteristics during Petroleum Coke Steam Gasification. Energies, 17(18), 4650. https://doi.org/10.3390/en17184650







