Abstract
Significant concerns over energy security and environmental impact reduction will drive all stakeholders to generate proper alternative energies. Biodiesel is a prospective cleaner-burning biofuel that can contribute on addressing these concerns globally. Presently, pure biodiesel (B100) application is still facing several obstacles, principally in terms of its cold flow properties. Improvement in cold flow behavior parameters is the solution to promoting biodiesel implementation at a higher percentage and wider environmental temperature range. This study provides a detailed review of several improvement methods, both physical, chemical, and biological, from various scientific sources, to elevate the cold fluidity characteristics of biodiesel. The investigated methods convincingly offer proper enhancement in the cold flow properties of biodiesel. Mostly, this improvement is accompanied by an alleviation in oxidation stability, cetane number, and/or viscosity. However, the skeletal isomerization method presents promising cold fluidity refinement with minimal reduction in other physical properties. Therefore, the continuous development of these methods promises global sustainable application of high-quality biodiesel.
1. Introduction
1.1. Global Energy Outlook
Global energy consumption is forecasted to rise continuously until 2050 due to world economic growth and rapid population increase [1]. This sharp rise will be led by the industrial sector and followed by the transportation sector. The high demand for industrial products and the energy efficiency of industrial processes at a low–medium level primarily determine the multiplication of energy utilization in the industrial sector. In the transportation sector, the better economic condition of society requires a more convenient transportation mode. Consequently, its energy consumption will be less efficient than that of the conventional mode. On the other side, the crisis of fossil fuel availability threatens all countries in the world and makes its price unaffordable [2,3]. This situation cannot be separated from the heavy dependence on fossil fuels, such as natural gas, petroleum, and coal, to meet their energy needs [4].
Furthermore, the excessive use of fossil fuel will promote adverse impacts on humans and the environment [5]. Emissions from this fossil fuel combustion consist of carbon dioxide (CO2), carbon monoxide (CO), hydrocarbon (HC), sulfur oxide (SOx), nitrogen oxide (NOx), and particulate matter (PM). These components cannot be completely degraded by the environment and, consequently, they will be accumulated in the atmosphere. The excessive appearance of CO2 causes the greenhouse gas (GHG) effect, leading to global warming and climate change phenomena [6]. The high concentration of CO2 and other released hazardous compounds (CO, HC, and PM) in the atmosphere also causes several health problems in humans [5]. Moreover, SOx and NOx emissions can trigger acid rain, which has damaging effects on the existing ecosystem [7].
To anticipate those problems and provide energy security, all countries in the world have made efforts to meet their energy requirement with alternative energy for sustainable application. The share of non-fossil fuel utilization in all primary energy sources was reported at 20% in 2022 and predicted to be in the range of 25–35% in 2050 via various schemes [1]. The growth in alternative energy implementation is accompanied by the expansion in energy demand, which is estimated to increase by 30–50% in 2050 [1]. In line with this action, global researchers have also been continuously developing alternative energy on an industrial scale as a substitute for fossil fuels. Their works can be seen from the significant increase in scientific publications in this area, presented in Figure 1, in the last decade. No less than three thousand scientific articles on alternative energy have been published in this period. In fact, the number of related publications in 2023 reached more than 47,000 articles. Based on these articles, the most expected characteristics of the alternative energy are renewability, biodegradability, lower toxicity, less pollutant production, and being economically competitive [8,9,10]. Additionally, the compatibility of the investigated fuels and targeted machines must be examined and assessed to guarantee safety in their application [3].
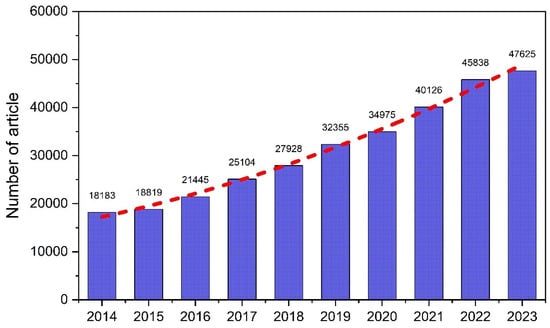
Figure 1.
Number of published scientific articles on the alternative and renewable energy sector in the period of 2014–2023 (from Scopus accessed on 26 July 2024).
In addition to technical and environmental aspects, the increasing call for alternative energy for sustainable application is also driven by social, political, and economical aspects. In social aspects, the generation of alternative fuels can expand the employment opportunities and develop the advanced agricultural ecosystem [7,11]. Then, the political aspect considers the strengthening of energy supply security and the affirmation of geographically based political independence as the basis for enforcing the sustainable energy application [7,11]. Meanwhile, the economical point of view emphasizes the reason for energy import reduction and economic growth to functionalize self-produced environmentally friendly energy [3,12]. All considerations in every aspect are summarized in the diagram shown in Figure 2.
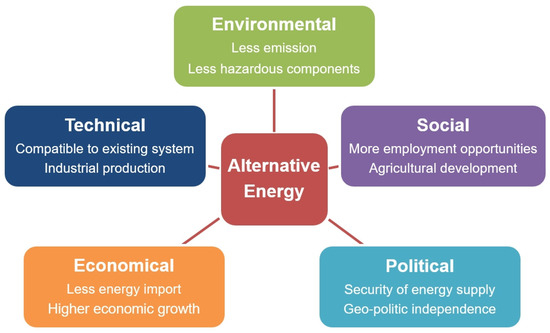
Figure 2.
Primary considerations of alternative energy application. Adapted and modified from Anwar and Garforth [7].
Based on the description above, the implementation of alternative energy has a very important role in the success of the 2030 Agenda for Sustainable Development. It greatly supports the achievement of several Sustainable Development Goals (SDGs). Mainly, this activity directly influences the realization of Affordable and Clean Energy (SDG-7), which targets the increasing use of new and renewable energies to substitute for fossil energy. Secondly, utilization of alternative energy provides cleaner emissions, which assists the goals of Climate Change (SDG-13), Good Health and Well-being (SDG-3), Responsible Consumption and Production (SDG-12), and Life on Land (SDG-15). Then, its massive production is required to be efficient in terms of land use. This point understandably contributes to SDG-12 and SDG-15. Afterward, it also correlates with the purposes of Decent Work and Economic Growth (SDG-8) and No Poverty (SDG-1). Those goals are reflected in the impact of alternative energy usage. Its attractiveness sufficiently drives economic growth, encourages expansion of decent work opportunities, and is highly expected to reduces poverty rates.
1.2. Biodiesel as an Alternative Energy Source
By observing the global map for alternative fuel development to date, biodiesel is a prospective cleaner-burning fuel to be widely implemented as a petro-diesel replacement fuel around the world. Biodiesel, for instance, shares several typical properties with petro-diesel, as shown in Table 1, although their chemical components are totally different. Petro-diesel is dominantly composed of long-chain alkanes and a small number of alkenes [12,13]. Meanwhile, biodiesel consists of long-chain hydrocarbons with ester functional groups [12,13]. However, both are insoluble in water (organic phase) [13]. Moreover, they are similar in some physical properties, such as kinematic viscosity, density, cetane number, flash point, and distillation temperature. Thus, they can technically replace each other or be blended to be a diesel fuel. Apart from being able to substitute for fossil diesel, biodiesel also has some other benefits in terms of renewability, sustainability, biodegradability, flammability, explosivity, toxicity, and emission [13,14].


Table 1.
Typical property comparison of petro-diesel and biodiesel.
Table 1.
Typical property comparison of petro-diesel and biodiesel.
| Properties | Unit | Petro-Diesel | Biodiesel | ||
|---|---|---|---|---|---|
| ASTM D975 [15,16] | EN 590 [16] | ASTM D6751 [17,18,19] | EN 14214 [13,20] | ||
| Chemical component [12,13] | - | Alkanes, alkenes (C10–C21) | Fatty acid alkyl esters (C8–C24 alkyl esters) | ||
| Phase [13] | - | Organic (insoluble in water) | Organic (insoluble in water) | ||
| Kinematic viscosity, at 40 °C | mm2/s | 1.9–4.1 | 2.0–4.5 | 1.9–6.0 | 3.5–5.0 |
| Density, at 15 °C | kg/m3 | n.s. *) | 820–845 | n.s. *) | 860–900 |
| Cetane number | - | ≥40 | ≥51 | ≥47 | ≥51 |
| Flash point | °C | ≥52 | ≥55 | ≥93 | ≥101 |
| Distillation temperature, at 90% | °C | 282–338 | n.s. *) | ≤360 | n.s. *) |
Note: *) n.s. = not specified.
Generally, biodiesel is synthesized by transesterification or esterification processes. The former process generates biodiesel from triglyceride-rich feedstocks (such as vegetable oils, fats, and used cooking oils) and short-chain alcohols using a basic or acidic catalyst [21]. In this process, the glyceride-attached fatty acids are taken out, promoting alkyl ester monomers, and replaced by an alcohol functional group to form glycerol as a by-product. The general reaction formula of transesterification is presented in Figure 3. In the other method, the esterification process requires free fatty acid-rich feedstocks (such as fatty acid distillates) and short-chain alcohols to produce biodiesel in the existence of an acidic catalyst [22]. In this reaction, the activated methoxy component attacks the carboxylic acid functional group in the free fatty acid to produce a mono-alkyl ester and water as by-products. The appropriate formula of the esterification reaction is shown in Figure 4.
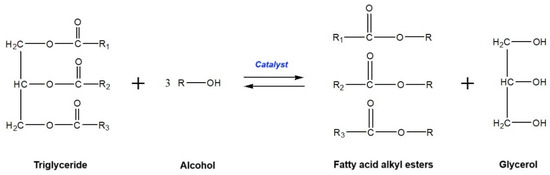
Figure 3.
General reaction formula of transesterification.
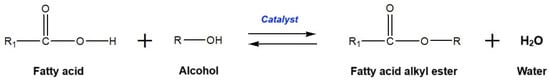
Figure 4.
General reaction formula of esterification.
Since its discovery in 1937 [7], biodiesel has been made from several categories of feedstocks, which were divided into four generations. The first-generation biodiesel was obtained from vegetable oil transesterification [23]. Palm [24], soybean [25], rapeseed [26], and sunflower oil [27] were the main oil-based crops used in this generation. However, the utilization of these feedstocks, with their future projection, potentially causes fundamental problems. Due to the use of edible materials, conflicts over their utilization in food and energy sector will occur to secure their supply in each sector [28]. The direct impact is higher commodity prices; hence, sectors with smaller purchasing power will experience an allocation shortage [29]. The global situation becomes even more dangerous when the food supply is obstructed. This will seriously threaten human life. Apart from economic and human life points of view, potential threats also arise from the environmental side in which the higher oil demand will require a larger plantation area [30].
The second-generation biodiesel was sourced from non-edible oils, animal fats, and wastes [28]. The primary non-edible sources used to produce biodiesel worldwide were jatropha [31], karanja [32], and jojoba oil [5,33]. Animal fat feedstocks used, for instance, were usually chicken fat [34] and beef tallow [35]. Then, the waste sources for biodiesel production were used cooking oils (UCOs) [36] and fatty acid distillates [14,37]. These low-cost materials were expected to overcome the problems in the first-generation feedstocks. However, the higher content of free fatty acid (FFA) and impurities in these sources generated fatty acid alkyl esters in a lower yield [38,39]. Moreover, the esters required more physical and chemical treatments to meet the biodiesel standard [12]. Consequently, the manufacturing cost of biodiesel was higher than that of the older generation [29].
The third-generation biodiesel feedstocks were microorganism-based oil or lipid. The prominent lipid-rich microorganism candidate was microalgae [29]. Microalgae are high-efficiency photosynthetic microorganisms containing a lot of essential components, such as carbohydrates, protein, lipids, vitamins, and bio-active compounds [40,41]. Compared to conventional oil crops, the main benefits of microalgae were a faster growth rate and higher lipid productivity [42,43,44]. The previous study by Chisti [45] reported that open microalgae cultivation per plantation area could potentially produce in the range of five to twenty times more oil than oil palm. Moreover, the environment-related superiorities of microalgae were high CO2 capture capability and adaptability in low-grade cultivation media [46,47]. Microalgae have been successfully cultivated with a CO2 supply from CO2-rich industrial flue gas [48]. Additionally, they could be cultured in various waste media, such as glycerol wastewater [49], palm oil mill effluent (POME) [17], waste molasses [50], and mining wastewater [51]. All the microalgae benefits stated above were expected to supply the energy requirement while minimizing impacts on the environment. Nevertheless, high FFA and fuel-based contamination contents in microalgal oil were challenges of using microalgae as biodiesel feedstocks [12].
Furthermore, the fourth-generation biodiesel feedstocks were proposed by genetically engineering the microorganisms used in the third-generation materials [28]. The functions of genetic modification were to improve the biomass growth rate, oil content, and adaptability to the culture medium [12]. The development of this generation, however, is still in the preliminary stage [3,28]. Thus, it requires further study to determine the optimum fuel-supporting genetic formula [12,28]. The classification of all biodiesel feedstocks discussed above is summarized in Table 2.


Table 2.
Summary of biodiesel feedstock classification and its distinctive characteristics.
Table 2.
Summary of biodiesel feedstock classification and its distinctive characteristics.
| Generation | Category | Feedstocks | Distinctive Characteristics | |
|---|---|---|---|---|
| First generation | Edible oils |
|
|
|
| Second generation | Non-edible oils |
|
|
|
| Animal fats |
|
| ||
| Wastes |
|
| ||
| Third generation | Microalgae |
|
|
|
| Fourth generation | Genetically modified microorganism |
|
| |
In the past decade, alkyl ester-based biofuel production has grown continuously to meet the increasing demand for diesel fuel, especially in the transportation sector [164]. Figure 5A demonstrates worldwide biodiesel production in 2014–2023. In this period, the quantity of commercial biodiesel manufacturing continued to increase significantly, reaching 58.59 billion liters in 2023 [165]. It was prominently produced from vegetable oils, with a percentage of ~70%-v [164]. The three largest shares were palm (29%-v), soybean (23%-v), and rapeseed biodiesel (14%-v) [164]. The remaining percentage was yielded from UCOs and animal fats [164].
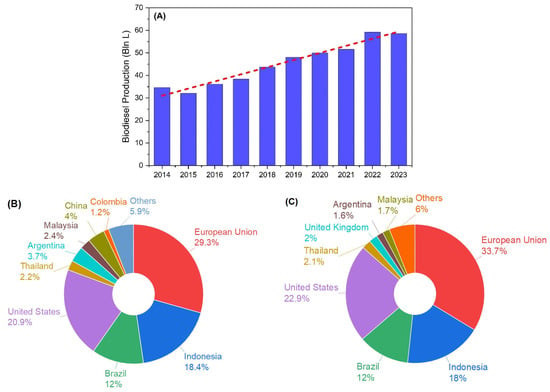
Figure 5.
Current situation of biodiesel: (A) global biodiesel production in 2014–2023 (red line is production trendline); (B) distribution of biodiesel producers in 2023; (C) distribution of biodiesel consumers in 2023 (data were obtained from [164,165]).
As shown in Figure 5B, the big four vegetable biodiesel producers in 2023 were the European Union (EU), United States (US), Indonesia, and Brazil. They dominated global vegetable oil-based biodiesel production, with a combined share of 80.6%-v. They were also declared as the four largest biodiesel consuming countries/regions in the world, as presented in Figure 5C. As the biggest country/region for biodiesel generation and utilization in 2023, the European Union, nowadays, relies on rapeseed oil supply as the main biodiesel feedstock [164]. Nevertheless, the biodiesel production and consumption in the EU are predicted to decrease in the future because of a palm biodiesel reduction and an electrification policy in the transportation sector [164]. In this situation, Indonesia took advantage of its excess palm oil availability to generate palm biodiesel [164]. The increasing palm biodiesel production was used by the Indonesian Government to support the implementation of B30 (blend of biodiesel 30%-v and petro-diesel 70%-v) in 2020 [164,166] and B35 (blend of biodiesel 35%-v and petro-diesel 65%-v) in 2023 [167]. Moreover, it has proposed to drive biodiesel applications at a higher level for the next few years [168]. Then, Brazil and the United States of America implemented biodiesel synthesis primarily from soybean oil [164]. Brazil is forecasted to face a multiplication in biodiesel demand in line with the growth in diesel vehicles in the coming years [164]. Meanwhile, the future outlook for biodiesel application in USA is mostly like that of the EU, with biodiesel production declining due to decreased consumption in fueled transportation [164].
2. Physicochemical Properties of Biodiesel
2.1. Physical Properties of Biodiesel
Worldwide biodiesel production is supplied with numerous types of feedstocks, which have different components and composition. This fact leads to the distinction in biodiesel characteristics depending on the raw material. However, the primary application of biodiesel as a petro-diesel replacement fuel in common diesel machines requires it to be standardized. The standardization of biodiesel quality serves to guarantee its safe operation and normal emissions [7]. Currently, there are two main standards of biodiesel specifications that are used as references throughout the world. These are ASTM D6751, defined by the American standardization institution, and EN 14214, issued by the European standardization committee [7]. The specification details of both reference standards and other standards in several biodiesel producer countries (Indonesia, Malaysia, Brazil, and India) are demonstrated in Table 3. Although referring to the international standards, each country or relevant authority can adjust the value of crucial parameters to suit their purposes and needs of biodiesel. For example, Indonesia has applied a tighter standard for the cold filter plugging point, monoglyceride content, and water content than the international standards. This regulation is set to support a higher biodiesel volumetric percentage in diesel fuel for domestic consumption and wider utilization in low-temperature areas.
2.2. Chemical Properties of Biodiesel
Generally, biodiesel feedstocks can be obtained from various resources depending on their abundance in a region or country. The amazing diversity in biodiesel feedstocks can be seen in Table 2, which is divided into several generation and categories. Each feedstock has a specific fatty acid profile and these differ from each other [7], as shown in Table 4. These fatty acids can be classified into two categories, namely saturated fatty acid (SFA) and unsaturated fatty acid (UFA) [169]. SFA is defined as a fatty acid component without a double bond in the carbon chain. It is generally symbolized with Cx:0 (x denotes the number of carbon chains and 0 indicates there is no double bond in the carbon chain). The major SFAs found in vegetable oils are palmitic acid (C16:0) and stearic acid (C18:0). Furthermore, UFA represents a double-bond fatty acid component and its acronym is Cx:y (y indicates the number of double bonds in the carbon chain). Then, UFA is divided again into two sub-categories, mono-unsaturated fatty acid (MUFA) for a single double-bond fatty acid component and poly-unsaturated fatty acid (PUFA) for more than one double bond in the fatty acid carbon chain [169]. The examples of MUFA compounds in biodiesel feedstocks are oleic acid (C18:1) and palmitoleic acid (C16:1). Meanwhile, linoleic acid (C18:2) and linolenic acid (C18:3) are the top two PUFA components mostly found in the fatty acid ester feedstocks.



Table 3.
Physical properties of biodiesel based on American Standard (ASTM D6751), European Standard (EN 14214), Indonesian Standard (KEPDIRJEN 195.K/EK.05/DJE/2022), Malaysian Standard (MS 2008:2014), Brazilian Standard (ANP N° 920/2023), and Indian Standard (IS 15607).
Table 3.
Physical properties of biodiesel based on American Standard (ASTM D6751), European Standard (EN 14214), Indonesian Standard (KEPDIRJEN 195.K/EK.05/DJE/2022), Malaysian Standard (MS 2008:2014), Brazilian Standard (ANP N° 920/2023), and Indian Standard (IS 15607).
| Properties | Unit | Method | American Standard (ASTM D6751) [7,17,18,19] | European Standard (EN 14214) [7,13,20] | Indonesian Standard (195.K/EK.05/DJE/2022) [170] | Malaysian Standard (MS 2008:2014) [17,171] | Brazilian Standard (ANP N° 920/2023) [172] | Indian Standard (IS 15607) [173] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |||
| Density at 15 °C | kg/m3 | EN 12185/ ISO 3675 | - | - | 860 | 900 | - | - | 860 | 900 | 850 a | 900 a | 860 | 900 |
| Density at 40 °C | kg/m3 | D4052 | - | - | - | - | 850 | 890 | - | - | - | - | - | - |
| Kinematic viscosity at 40 °C | mm2/s | D445/ EN ISO 3104 | 1.9 | 6.0 | 3.5 | 5.0 | 2.3 | 6.0 | 3.5 | 5.0 | 3.0 | 5.0 | 3.0 | 6.0 |
| Cetane number | - | D613/ EN ISO 5165 | 47 | - | 51 | - | 51 | - | 51 | - | report | - | 51 | - |
| Flash point (close cup) | °C | D93/ EN ISO 3679 | 93 | - | 101 | - | 130 | - | 120 | - | 100 | - | 120 | - |
| Cloud point | °C | D2500 | −3 | 12 | - | - | - | - | - | - | - | - | - | - |
| Pour point | °C | D97/ISO 3016 | −15 | 10 | - | - | - | - | - | - | - | - | - | - |
| Cold filter plugging point | °C | D6371/EN 116 | - | - | - | report | - | 15 | - | 15 | - | report | - | - |
Carbon residue
| wt% | D4530 D4530/ EN ISO 10370 | - - | 0.05 - | - - | - 0.3 | - - | 0.05 0.3 | - - | 0.05 0.3 | - - | - - | - - | 0.05 - |
| Copper strip corrosion, 3 h at 50 °C | Rating | D130/ EN ISO 2160 | - | No. 3 | Class 1 | Class 1 | Class 1 | Class 1 | Class 1 | |||||
| Distillation temperature, at 90% | °C | D1160 | - | 360 | - | - | - | 360 | - | - | - | - | - | - |
| Acid number | mg KOH/g | D664/ EN14104 | - | 0.5 | - | 0.5 | - | 0.4 | - | 0.5 | - | 0.5 | - | 0.5 |
| Iodine number | g I /100 g | EN14111/ AOCS Cd 1-25 | - | - | - | 120 | - | 115 | - | 110 | - | - | - | report |
| Oxidation stability, at 110 °C | h | EN14112/ EN15751 | 3 | - | 8 | - | 11 | - | 10 | - | 13 | - | 6 | - |
| Ester content | wt% | EN14103/ SNI 7182:2015 | - | - | 96.5 | - | 96.5 | - | 96.5 | - | 96.5 | - | 96.5 | - |
| Linolenic acid methyl ester | wt% | EN14103 | - | - | - | 12 | - | - | - | 12 | - | - | - | - |
| Polyunsaturated (≥4 double bonds) methyl esters | wt% | EN14103 | - | - | - | 1 | - | - | - | 1 | - | - | - | - |
| Methanol content | wt% | EN14110 | - | 0.2 | - | 0.2 | - | - | - | 0.2 | - | 0.2 | - | - |
| Monoglyceride content | wt% | D6584/ EN14105 | - | - | - | 0.7 | - | 0.525 | - | 0.7 | - | 0.5 | - | - |
| Diglyceride content | wt% | EN14105 | - | - | - | 0.2 | - | - | - | 0.2 | - | 0.2 | - | - |
| Triglyceride content | wt% | EN14105 | - | - | - | 0.2 | - | - | - | 0.2 | - | 0.2 | - | - |
| Free glycerol | wt% | D6584/ EN14105 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 |
| Total glycerol | wt% | D6584/ EN14105 | - | 0.24 | - | 0.25 | - | 0.24 | - | 0.25 | - | 0.20 | - | 0.25 |
| Alkaline metals (Na + K) | ppm | EN 14538 | - | 5 | - | 5 | - | 5 | - | 5 | - | 2.5 | - | report |
| Alkaline earth metals (Ca + Mg) | ppm | EN 14538 | - | 5 | - | 5 | - | 5 | - | 5 | - | 2.5 | - | report |
| Sulphur content | ppm | D5453/ EN ISO 20846 | - | - | - | 10 | - | 10 | - | 10 | - | 10 | - | 50 |
| Sulphur (S 15 Grade) | ppm | D5463 | - | 15 | - | - | - | - | - | - | - | - | - | - |
| Sulphur (S 500 Grade) | ppm | D5463 | - | 500 | - | - | - | - | - | - | - | - | - | - |
| Sulphated ash content | wt% | D874/ ISO 3987 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 |
| Phosphorus content | ppm | D4951/EN14107/AOCS Ca 12-55 | - | 10 | - | 4 | - | 4 | - | 4 | - | 3.0 | - | 10 |
| Water and sediment | %-v | D2709 | - | 0.05 | - | - | - | - | - | - | - | - | - | - |
| Water content | ppm | D6304/ EN ISO 12937 | - | - | - | 500 | - | 340 | - | 500 | - | 200 | - | 500 |
| Total contamination | ppm | D6217/EN12662 | - | - | - | 24 | - | 20 b | - | 24 | - | 24 | - | 24 |
| Cold soak filterability | s | Annex to D6751 | - | 360 | - | - | - | - | - | - | - | - | - | - |
Note: a measured at 20 °C; b unit: mg/L.

Table 4.
Profiles of fatty acids in several fatty acid-rich feedstocks (wt%) and its influence on selected biodiesel physical properties.
Table 4.
Profiles of fatty acids in several fatty acid-rich feedstocks (wt%) and its influence on selected biodiesel physical properties.
| Fatty Acids | Composition (wt%) and Physical Property Value | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | R | SN | SF | CN | CS | C | J | JB | K | CI | UC | CF | BT | MS | MC | MT | ||
| Caprylic | C8:0 | - | - | - | - | - | 6.5 | - | - | - | - | - | - | - | - | - | - | - | - |
| Capric | C10:0 | - | - | - | - | - | 5.6 | - | - | - | - | - | - | - | - | 0.1 | - | - | - |
| Lauric | C12:0 | 0.2 | - | - | - | - | 46.9 | - | - | - | - | - | - | 0.4 | 0.1 | 0.2 | - | 9.8 | 3.1 |
| Myristic | C14:0 | 1.0 | 0.1 | - | 0.1 | - | 18.7 | 0.7 | - | 0.1 | 1.1 | - | - | 0.9 | 0.7 | 2.4 | 3.6 | - | - |
| Palmitic | C16:0 | 43.0 | 10.9 | 2.8 | 7.0 | 6.1 | 9.7 | 25.9 | 11.8 | 17.7 | 2.6 | 9.7 | 14.7 | 36.6 | 24.1 | 24.4 | 14.9 | 7.2 | 1.6 |
| Palmitoleic | C16:1 | - | 0.1 | 0.3 | 0.1 | - | 0.1 | 0.3 | 0.1 | 0.8 | - | - | 0.3 | 0.2 | 5.7 | 2.7 | 1.0 | 24.5 | 10.1 |
| Margaric | C17:0 | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | 0.2 | 0.9 | - | - | - |
| Stearic | C18:0 | 4.6 | 4.2 | 1.3 | 4.5 | 2.5 | 2.8 | 1.7 | 2.1 | 6.4 | 5.1 | 7.1 | 13.2 | 4.2 | 6.4 | 19.1 | 28.1 | 9.5 | 4.3 |
| Oleic | C18:1 | 40.0 | 25.0 | 64.4 | 18.7 | 29.6 | 6.8 | 15.9 | 27.3 | 41.8 | 45.8 | 52.4 | 46.1 | 42.4 | 41.4 | 41.7 | 5.4 | 11.6 | 16.8 |
| Linoleic | C18:2 | 10.1 | 52.7 | 22.3 | 67.5 | 59.2 | 2.2 | 55.1 | 57.6 | 32.9 | 31.5 | 16.5 | 24.7 | 9.9 | 18.8 | 5.9 | 1.1 | 3.2 | 1.2 |
| Linolenic | C18:3 | 0.3 | 6.2 | 7.3 | 0.8 | 0.7 | - | 0.2 | 0.6 | 0.2 | 6.1 | 5.2 | 0.2 | 0.2 | 1.1 | 0.7 | - | 0.7 | 1.1 |
| Arachidic | C20:0 | 0.4 | 0.3 | 0.6 | 0.4 | - | 0.1 | 0.2 | 0.3 | 0.1 | - | - | 0.8 | 0.3 | 0.1 | 0.4 | 32.4 | - | - |
| Eicosenoic | C20:1 | - | 0.1 | 1.0 | 0.1 | - | - | 0.1 | 0.3 | - | - | 3.5 | - | 0.2 | 0.4 | 0.5 | 1.9 | - | - |
| Eicosadienoic | C20:2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.0 | - | - |
| Eicosatetraenoic | C20:4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.9 | 0.6 |
| Eicosapentaenoic | C20:5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.3 | 1.7 |
| Behenic | C22:0 | - | 0.3 | - | 0.7 | - | - | 0.1 | - | - | - | 4.2 | - | - | 0.3 | 0.03 | 6.7 | - | - |
| Erucic | C22:1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.4 | 0.06 | - | - | - |
| Docosapentaenoic | C22:5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 8.1 | 0.9 |
| Docosahexaenoic | C22:6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6.2 | 0.7 |
| Total saturated (wt%) | 49.2 | 15.9 | 4.7 | 12.7 | 8.6 | 90.4 | 28.6 | 14.2 | 24.3 | 8.9 | 22.4 | 28.7 | 42.4 | 32.1 | 47.7 | 89.6 | 26.5 | 8.9 | |
| Total unsaturated (wt%) | 50.4 | 84.1 | 95.3 | 87.2 | 89.5 | 9.2 | 71.5 | 85.8 | 75.7 | 83.5 | 77.6 | 71.3 | 52.8 | 67.8 | 51.5 | 9.4 | 61.5 | 33.1 | |
| Total mono-unsaturated (wt%) | 40.0 | 25.2 | 65.7 | 18.9 | 29.6 | 6.9 | 16.3 | 27.6 | 42.6 | 45.8 | 55.9 | 46.4 | 42.7 | 47.9 | 44.8 | 8.3 | 36.1 | 26.9 | |
| Total poly-unsaturated (wt%) | 10.4 | 58.9 | 29.6 | 68.3 | 59.9 | 2.2 | 55.2 | 58.2 | 33.1 | 37.6 | 21.8 | 24.9 | 10.1 | 19.9 | 6.6 | 1.1 | 25.4 | 6.2 | |
| Pour point (°C) | 6 | −3.2 | −11 | −2 | −12 | −3.8 | −0.2 | −5.1 | 2 | −6.2 | 1 | 13 | −6.5 | 4.4 | 10 | −9 | n.a. | n.a. | |
| Cloud point (°C) | 6 | 0 | −3.5 | 1.3 | n.a. | −1.2 | 1.2 | −3 | 3 | 5.8 | 6 | 12 | n.a. | 7.5 | 13.8 | −3 | 6.2 | 10.6 | |
| CFPP (°C) | 4 | −2 | −11.3 | −2 | −11 | −4.7 | 4.8 | −7.5 | n.a. | n.a. | −7 | 11 | n.a. | 2.7 | 12.2 | n.a. | n.a. | n.a. | |
| Oxidation stability (h) | 20.3 | 4.08 | 7.4 | 1.3 | 1.4 | 11 | 1.8 | 3 | 3.1 | 15.5 | 4.1 | 6.1 | 3 | 8.7 | 0.45 | n.a. | n.a. | n.a. | |
| Cetane number | 61.2 | 51.8 | 54.1 | 51.9 | 54.8 | 61 | 53.3 | 52.5 | 53.5 | 54.4 | 55.4 | n.a. | 56.2 | 57 | 60.9 | 70 | 46.2 | 46.2 | |
| Reference | [54,69] | [13,69] | [13,69] | [13,69] | [64] | [69] | [69] | [69] | [85] | [33] | [32,69] | [91] | [69,129] | [69] | [69] | [146] | [145] | [145] | |
Notes: P—palm; S—soybean; R—rapeseed; SN—sunflower; SF—safflower; CN—coconut; CS—cottonseed; C—corn; J—jathropha; JB—jojoba; K—karanja; CI—polanga (Calophyllum inophyllum); UC—used cooking oil; CF—chicken fat; BT—beef tallow; MS—microalgae Spirulina platensis; MC—microalgae Chlorella vulgaris; MT—microalgae Tetraselmis chuii; n.a.—not available.
3. Cold Flow Properties of Biodiesel
3.1. Parameters of Cold Flow Properties
Cold flow properties (CFPs) of biodiesel are defined as behaviors or characteristics of biodiesel flow in low-temperature condition [174]. These properties are commonly indicated by the parameters of pour point, cloud point, and cold filter plugging point. Pour point (PP) is a physical property used to measure the lowest temperature at which the fuel can still flow from one point to another [175]. Starting at this point and lower, the crystalline solids are agglomerated to form a gel phase; thus, the fuel loses its fluidity and stops flowing [13,69]. It is commonly related to the freezing or melting point of a fluid mixture [7]. The international standard testing procedures of the pour point parameter are ASTM D97 and EN ISO 3016 [13,20].
Cloud point (CP) is the temperature at which the formation of a crystalline solid starts to show a cloudy appearance after being exposed to low temperature [175]. In other words, the wax crystals formed by low temperature exposure begin to be visible at this point [13]. Below this point, molecule solidification will be greater and more crystals are created. Consequently, it increases fuel viscosity and significantly reduces its pumping ability [175]. The CP parameter can be determined using ASTM D2500 as a standard testing procedure [17,18,19].
Cold filter plugging point (CFPP) is a cold flow property used to indicate the lowest temperature at which a certain volume of biodiesel (20 mL) successfully flows through a standard filter (4.5 × 10−2 mm) in a specified flow time (≤60 s) [13]. An operating temperature below this point causes clogging in filtration devices. This parameter is widely used to accurately evaluate the low-temperature flow performance of biodiesel [7]. The CFPP parameter can be quantified using ASTM D6371 and EN 116 as international standard testing procedures [174].
3.2. Effect of Fatty Acid Profiles on Cold Flow Properties
The variety of fatty acid profiles in the parent oil feedstocks experimentally has effects on the low-temperature flow behavior of biodiesel. As shown in Table 4, the biodiesel with a high SFA content has problems regarding the cold flow properties. The values of PP, CP, and/or CFPP are too high. This correlation can be seen in palm biodiesel (P), polanga biodiesel (CI), chicken fat biodiesel (CF), beef tallow biodiesel (BT), microalgae Chlorella vulgaris biodiesel (MC), and microalgae Tetraselmis chuii biodiesel (MT). On the other hand, biodiesel having a high UFA content provides better cold flow behavior, such as soybean biodiesel (S), rapeseed biodiesel (R), sunflower biodiesel (SN), safflower biodiesel (SF), corn biodiesel (C), and jojoba biodiesel (JB). To ensure this correlation, the melting data comparison of primary saturated and unsaturated fatty acid methyl esters (FAMEs) is shown in Table 5. As previously discussed, the pour point of a component can refer to its melting point [7]. For the same carbon chain, the unsaturated FAME component has a significantly lower melting point than the saturated component. Moreover, the greater number of double bonds also provides a lower melting point or better cold flow properties. Therefore, the higher SFA content promotes poor cold flow properties, and vice versa.


Table 5.
Data tabulation of additive effects on the biodiesel cold flow properties.
Table 5.
Data tabulation of additive effects on the biodiesel cold flow properties.
| Biodiesel | Additives | PP (°C) | CP (°C) | CFPP (°C) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||
| Palm biodiesel | B100 | Diethyl ether (5 vol%) | 16 | 10 | - | - | - | - | [176] |
| Ethanol (5 vol%) | 16 | 11 | - | - | - | - | |||
| n-butanol (5 vol%) | 16 | 12 | - | - | - | - | |||
| Palm biodiesel | B100 | Glycerol ketals (5 vol%) | 14 | 9 | 16 | 11 | - | - | [177] |
| Glycerol acetates (5 vol%) | 14 | 9 | 16 | 11 | - | - | |||
| Palm fatty acid isopropyl esters (5 vol%) | 14 | 10 | 16 | 11 | - | - | |||
| Palm fatty acid isobutyl esters (5 vol%) | 14 | 10 | 16 | 12 | - | - | |||
| Palm fatty acid 2-butyl esters (5 vol%) | 14 | 8 | 16 | 10 | - | - | |||
| Palm biodiesel | B100 | Ozonized palm oil (1 wt%) | 12 | 10 | 18 | 12 | - | - | [178] |
| Ozonized sunflower oil (1 wt%) | 12 | 11 | 18 | 13 | - | - | |||
| Ozonized soybean oil (1 wt%) | 12 | 13 | 18 | 13 | - | - | |||
| Ozonized rapeseed oil (1 wt%) | 12 | 13 | 18 | 13 | - | - | |||
| Soybean biodiesel | B100 | Oleyl alcohol (10 wt%) | - | - | 0 | −4 | - | - | [179] |
| Tert-butyl alcohol (10 wt%) | - | - | 0 | −4 | - | - | |||
| Soybean biodiesel | B10 | Tetradecyl methacrylate-citric anhydride-styrene co-polymer (0.15 wt%) | −13 | −24 | 0 | −3 | −1 | −8 | [180] |
| B20 | Tetradecyl methacrylate-citric anhydride-styrene co-polymer (0.15 wt%) | −13 | −23 | 1 | −3 | −1 | −9 | [180] | |
| B30 | Tetradecyl methacrylate-citric anhydride-styrene co-polymer (0.15 wt%) | −12 | −22 | 1 | −3 | 0 | −10 | ||
| Soybean biodiesel | B100 | Ozonized palm oil (1 wt%) | −2 | −10 | 1 | −2 | - | - | [178] |
| Ozonized sunflower oil (1 wt%) | −2 | −9 | 1 | 1 | - | - | |||
| Ozonized soybean oil (1 wt%) | −2 | −12 | 1 | 1 | - | - | |||
| Ozonized rapeseed oil (1 wt%) | −2 | −11 | 1 | −1 | - | - | |||
| Rapeseed biodiesel | B100 | Ozonized palm oil (1 wt%) | −13 | −10 | −4 | −6 | - | - | [178] |
| Ozonized sunflower oil (1 wt%) | −13 | −30 | −4 | −6 | - | - | |||
| Ozonized soybean oil (1 wt%) | −13 | −28 | −4 | −4 | - | - | |||
| Ozonized rapeseed oil (1 wt%) | −13 | −30 | −4 | −3 | - | - | |||
| Canola biodiesel | B100 | Oleyl alcohol (10 wt%) | - | - | −13 | −18 | - | - | [179] |
| Tert-butyl alcohol (10 wt%) | - | - | −13 | −16 | - | - | |||
| Sunflower biodiesel | B100 | Ozonized palm oil (1 wt%) | −5 | −15 | 1 | −2 | - | - | [178] |
| Ozonized sunflower oil (1 wt%) | −5 | −24 | 1 | 0 | - | - | |||
| Ozonized soybean oil (1 wt%) | −5 | −22 | 1 | 0 | - | - | |||
| Ozonized rapeseed oil (1 wt%) | −5 | −20 | 1 | 1 | - | - | |||
| Coconut biodiesel | B10 | Methyl acrylate polymer (0.03 wt%) | −13 | −21 | −3 | −11 | −5 | −16 | [181] |
| B20 | Methyl acrylate polymer (0.03 wt%) | −13 | −22 | −3 | −11 | −4 | −16 | ||
| B30 | Methyl acrylate polymer (0.03 wt%) | −10 | −12 | −3 | −7 | −4 | −9 | ||
| Polanga (Calophyllum inophyllum) biodiesel | B10 | Methyl acrylate polymer (0.03 wt%) | −2 | −9 | −1 | −5 | 0 | −8 | [181] |
| B20 | Methyl acrylate polymer (0.03 wt%) | 1 | −4 | 4 | −2 | 5 | −1 | ||
| B30 | Methyl acrylate polymer (0.03 wt%) | 4 | −3 | 7 | 1 | 5 | −2 | ||
| Used cooking biodiesel | B100 | Methyl acrylate polymer (0.04 wt%) | −11 | −19 | −8 | −9 | −9 | −15 | [182] |
| Ethylene vynil acetate co-polymer (0.04 wt%) | −11 | −17 | −8 | −8 | −9 | −11 | |||
| α-olefin polymer (0.04 wt%) | −11 | −14 | −8 | −9 | −9 | −10 | |||
| Maleic anhydride polymer (0.08 wt%) | −11 | −11 | −8 | −9 | −9 | −10 | |||
| Used cooking biodiesel | B100 | α-olefin polymer (0.06 wt%) | 5 | 2 | 7 | 5 | 6 | 3 | [183] |
| B20 | α-olefin polymer (0.04 wt%) | −10 | −17 | −2 | −9 | −1 | −11 | ||
Nevertheless, the fatty acid composition inversely influences the oxidation stability and cetane number properties. Oxidation stability is a major physical property that indicates the potential reactivity with oxygen, which can change the fuel properties [91]. Meanwhile, the cetane number is used to identify the auto-ignition and ignition delay of the fuel [69]. According to the data in Figure 6, biodiesel having a higher SFA content leads to longer oxidation stability and a higher cetane number. In comparison, biodiesel dominated by UFA content has lower oxidation stability and a lower cetane number. The higher unsaturation degree will also cause lower oxidation stability and a lower cetane number [7]. The summary of fatty acid composition effects on biodiesel cold flow properties, oxidation stability, and cetane number is visualized in Figure 7.
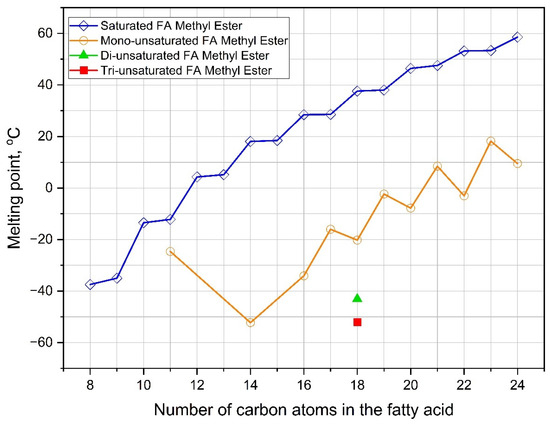
Figure 6.
Melting point comparison of major saturated and unsaturated FAMEs from vegetable oils [7,184].
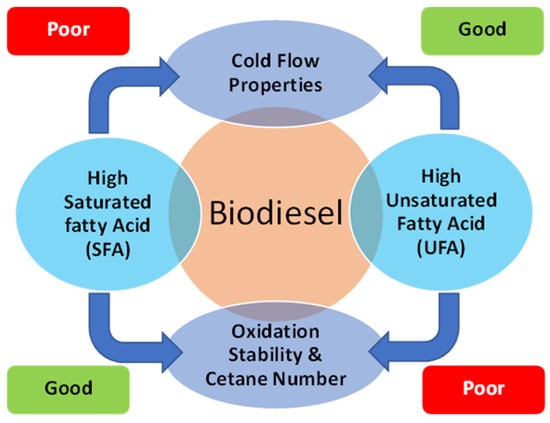
Figure 7.
Effect of fatty acid composition on biodiesel cold flow properties, oxidation stability, and cetane number (adapted from [185]).
3.3. Influence of Cold Flow Properties on Biodiesel Application
Cold flow properties (CFPs) have a prominent meaning for biodiesel application. These properties provide normal diesel fuel combustion, especially in low-temperature areas [169]. The utilization of low-CFP biodiesel, more SFA content, and less UFA content, in a low-temperature environment, will promote some operability problems in terms of molecule crystallization, causing blockages in the fuel flow line, filter, or injector [13]. It also becomes thicker and loses its flow characteristic [186]. This situation inhibits fuel supply into the combustion chamber, causing significant losses in power output, fuel consumption efficiency, and initial engine starting ability in low-temperature conditions [13]. For long-term application, it causes wear in and even damage to the engine [186].
However, the low-CFP biodiesel with a high SFA content has good oxidation stability and cetane number properties [7]. High oxidation stability provides more stable fuel and prevents property changes during distribution, storage, and use [169]. Fuel with a high cetane number ensures normal ignition time (the required time to start the combustion after fuel injection) [69]. Often, a high cetane number is correlated with the lower NOx emissions [69,169].
4. Methods for Improving Cold Flow Properties
The biodiesel that has problems associated with cold flow properties requires treatments to improve its quality and enable its application in a higher percentage of commercial fuel. As mentioned in the previous section, the main reason for this is the high SFA content in biodiesel products. Generally, the proposed methods for improving cold flow properties can be classified into three categories: (i) physical methods; (ii) chemical methods; and (iii) biological methods. Then, these categories can be divided into several sub-categories based on the process. The detailed classification chart of methods used to improve cold flow properties is depicted in Figure 8.
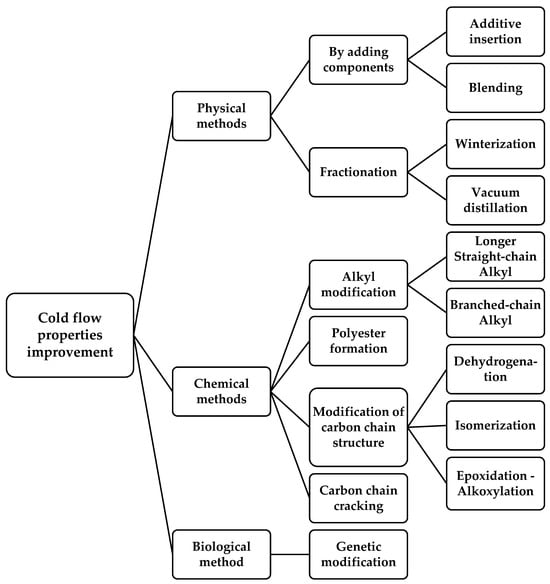
Figure 8.
Classification chart of methods used to improve cold flow properties.
4.1. By Adding Components
4.1.1. Additive Insertion
This method is implemented by adding chemical compounds in small amounts to biodiesel to prevent the plugging phenomenon in the diesel engine system. The selection stage of additives also needs to consider the negative effects on the other physical parameters, such as fuel kinematic viscosity and density, to maintain biodiesel quality [13,174]. There are two types of additives proposed for improving cold flow properties: pour point depressant and wax crystalline modifier [187]. The first mentioned additive category has the function of inhibiting crystal enlargement at low temperatures, thereby preventing agglomerate formation [174]. The second additive type is mixed with biodiesel to obstruct the organization of formed crystals at low temperatures [13]. In other words, this compound becomes a shield between crystalline solids, avoiding gel formation and maintaining the flow ability of the fuel.
In this method, the additives commonly used to improve cold flow properties are non-aromatic chemicals with a low freezing point and low molecular weight [13,179]. Based on these characteristics, numerous potential chemical groups have been evaluated as additives: short-chain alcohols [179], low-molecular-weight co-polymers [180,181,182], glycerol derivatives [177], fatty acid branched-chain alkyl esters [177], branched fatty acid alkyl esters [188], diethyl ether [176], and ozonized vegetable oils [178]. The effects of these proposed additives on the cold flow properties of various fatty esters are tabulated in Table 5. According to Table 5, low-molecular-weight polymers and co-polymers show the best performance in terms of improving biodiesel cold flow properties. The addition of a small amount of these components, in the range of 0.03–0.15 wt%, significantly decreases PP, CP, and CFPP of biodiesel. Meanwhile, short-chain alcohols and diethyl ether must be mixed with biodiesel in an amount of at least 5 wt% to achieve a remarkable impact.
4.1.2. Blending
Blending is proposed to mix poor-CFP biodiesel with good-CFP components in a certain ratio. It is the simplest method used to elevate cold flow properties of biodiesel. Primarily, the blending component is a biodiesel with a high UFA content [189]. The utilization of high-UFA biodiesel as a blending agent is not without problems. Based on the reciprocal effect of SFA and UFA components, mixing poor- and good-CFP biodiesel needs optimization to control the negative elevation of oxidation stability and the cetane number [13]. Besides that, biodiesel cold flow properties can also be lifted using fossil diesel or kerosene; however, this reduces the sustainability and environmentally friendly aspect of biodiesel products [190]. Then, other chemicals used for blending agents are short-chain fatty acid methyl ester [174], branched-chain fatty acid methyl ester [188], fatty acid longer-chain alkyl ester [184], and short-chain alcohol [191]. The effects of these proposed blending agents on the cold flow properties of various fatty esters are presented in Table 6. According to Table 6, the utilization of blending agents successfully decreases the value of PP, CP, and/or CFPP with various degrees of effectiveness. Different from others, the application of branched-chain saturated fatty acid methyl ester shows minimum effects on the other physical properties. This is because branched-chain saturated fatty acid methyl ester provides better cold flow properties than its parent compound, but it presents an insignificant change in oxidation stability and the cetane number [188]. However, the commercial production of this component still requires further technological development and feasibility evaluation.


Table 6.
Data tabulation of blending agent effects on the biodiesel cold flow properties.
Table 6.
Data tabulation of blending agent effects on the biodiesel cold flow properties.
| Biodiesel | Blending Agent | PP (°C) | CP (°C) | CFPP (°C) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||
| Palm biodiesel | Soybean biodiesel (20 wt%) | - | - | - | - | 10 | 8 | [192] |
| Soybean biodiesel (40 wt%) | - | - | - | - | 10 | 5 | ||
| Soybean biodiesel (60 wt%) | - | - | - | - | 10 | 0 | ||
| Rapeseed biodiesel (20 wt%) | - | - | - | - | 10 | 7 | ||
| Rapeseed biodiesel (40 wt%) | - | - | - | - | 10 | 2 | ||
| Rapeseed biodiesel (60 wt%) | - | - | - | - | 10 | −3 | ||
| Soybean biodiesel (20 wt%) Rapeseed biodiesel (20 wt%) | - | - | - | - | 10 | 3 | ||
| Soybean biodiesel (40 wt%) Rapeseed biodiesel (20 wt%) | - | - | - | - | 10 | −2 | ||
| Soybean biodiesel (20 wt%) Rapeseed biodiesel (40 wt%) | - | - | - | - | 10 | −3 | ||
| Palm biodiesel | Methyl iso-stearat (25 wt%) | 16.7 | 10.3 | 14.2 | 8.6 | - | - | [188] |
| Methyl iso-stearat (50.1 wt%) | 16.7 | 4 | 14.2 | 3.1 | - | - | ||
| Methyl iso-oleat (25 wt%) | 16.7 | 11 | 14.2 | 8.7 | - | - | ||
| Methyl iso-oleat (49.8 wt%) | 16.7 | 4 | 14.2 | 2.2 | - | - | ||
| Soybean biodiesel | Rapeseed biodiesel (20 wt%) | - | - | - | - | −3 | −2 | [192] |
| Rapeseed biodiesel (40 wt%) | - | - | - | - | −3 | −4 | ||
| Rapeseed biodiesel (60 wt%) | - | - | - | - | −3 | −8 | ||
| Soybean biodiesel | Methyl iso-stearat (25 wt%) | 0.3 | −4.3 | 0.6 | −3.5 | - | - | [188] |
| Methyl iso-stearat (50 wt%) | 0.3 | −10 | 0.6 | −7.3 | - | - | ||
| Methyl iso-oleat (25 wt%) | 0.3 | −4 | 0.6 | −3.4 | - | - | ||
| Methyl iso-oleat (49.8 wt%) | 0.3 | −9.7 | 0.6 | −7.3 | - | - | ||
| Canola biodiesel | Methyl iso-stearat (24.7 wt%) | −10.7 | −14.7 | −2.5 | −5.7 | - | - | [188] |
| Methyl iso-stearat (49.8 wt%) | −10.7 | −17 | −2.5 | −10.1 | - | - | ||
| Methyl iso-oleat (24.9 wt%) | −10.7 | −18 | −2.5 | −5.7 | - | - | ||
| Methyl iso-oleat (48.5 wt%) | −10.7 | −27 | −2.5 | −8.7 | - | - | ||
| Rapeseed biodiesel | N-butanol (10 vol%) | - | - | −6 | −7 | −10 | −11 | [191] |
| N-butanol (20 vol%) | - | - | −6 | −8 | −10 | −13 | ||
| N-butanol (50 vol%) | - | - | −6 | −9 | −10 | −17 | ||
| Rapeseed biodiesel (butyl esters) | N-butanol (10 vol%) | - | - | −7 | −8 | −14 | −16 | [191] |
| N-butanol (20 vol%) | - | - | −7 | −8 | −14 | −16 | ||
| N-butanol (50 vol%) | - | - | −7 | −9 | −14 | −17 | ||
| Jatropha biodiesel | Petro-diesel (20 vol%) | 18 | 16.5 | 20.2 | 17 | - | - | [193] |
| Petro-diesel (40 vol%) | 18 | 15.5 | 20.2 | 17 | - | - | ||
| Petro-diesel (60 vol%) | 18 | 15 | 20.2 | 15.5 | - | - | ||
| Kerosene (20 vol%) | 18 | 14 | 20.2 | 15 | - | - | ||
| Kerosene (40 vol%) | 18 | 10 | 20.2 | 12 | - | - | ||
| Kerosene (60 vol%) | 18 | 7 | 20.2 | 8 | - | - | ||
| Used cooking palm biodiesel | Used cooking coconut biodiesel (50 vol%) | 12 | 4 | 13 | 5 | - | - | [194] |
| Used cooking sunflower biodiesel (50 vol%) | 12 | 3 | 13 | 4 | - | - | ||
4.2. Fractionation
4.2.1. Cold Fractionation (Winterization)
Cold fractionation, or commonly called winterization, is a method used to separate components in a mixture based on the melting point differences of its components [195]. This process is carried out by cooling biodiesel slowly until reaching a desired temperature (crystallization temperature) and filtering it into split solid and liquid phases [13]. The solid phase consists of higher-melting-point or poor-CFP components, which are associated with saturated fatty esters [174]. In contrast, the liquid phase is dominated by lower-melting-point or good-CFP components, which are identical to unsaturated fatty acid alkyl esters [196]. By removing saturated fatty esters, the biodiesel product is expected to have better cold flow properties than the crude product. However, the main adverse effects of winterization are loss in biodiesel yield, reduction in oxidation stability, and downgrade in the cetane number [13,193]. The latter effect directly corresponds to diesel engine performance in terms of poorer ignitability and higher NOx emissions [169,187].
Biodiesel winterization can be conducted with or without additional chemicals. Dry winterization is operated naturally without additional chemicals. This process typically requires a longer time and produces a lower yield [187]. The other methods are detergent and solvent winterization [174,197]. They use detergent and solvent, respectively, as additional chemicals to enhance the winterization process. Both chemicals play roles in separating the trapped liquid from solids and elevating liquid-phase recovery in low-temperature exposure [198]. The detailed effects of the winterization process on improving biodiesel cold flow properties are summarized in Table 7.


Table 7.
Data tabulation of winterization effects on the biodiesel cold flow properties.
Table 7.
Data tabulation of winterization effects on the biodiesel cold flow properties.
| Biodiesel | Winterization Agent | PP (°C) | CP (°C) | CFPP (°C) | Biodiesel Yield (wt%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||
| Palm biodiesel | No agent | 7 | 5.5 | 7.5 | 6.5 | - | - | 65.5 | [199] |
| Palm biodiesel | Sorbitan palmitate (0.5 wt%) | - | - | 16 | 8.5 | - | - | 74.4 | [200] |
| Palm biodiesel-like mixture | Sorbitan palmitate (1 wt%) | - | - | 17 | 10 | - | - | 41.6 | [198] |
| Sorbitan monostearate (0.75%) | - | - | 17 | 12 | - | - | 73.4 | ||
| Sorbitan tristearate (1 wt%) | - | - | 17 | 11 | - | - | 50.5 | ||
| L-ascorbyl palmitate (1 wt%) | - | - | 17 | 14 | - | - | 42.9 | ||
| L-ascorbyl di-palmitate (1 wt%) | - | - | 17 | 16 | - | - | 35.6 | ||
| Cholesterol palmitate (1 wt%) | - | - | 17 | 16 | - | - | 56.9 | ||
| Soybean biodiesel | No agent | −2 | −21 | −0.3 | −20 | - | - | 33.4 | [201] |
| Isopropanol (0.2 wt%) | −2 | −9 | −0.3 | −8.7 | - | - | 86.0 | ||
| n-hexane (0.2 wt%) | −2 | −11.3 | −0.3 | −10.3 | - | - | 78.4 | ||
| Soybean biodiesel | Sorbitan palmitate (0.5 wt%) | - | - | −1.5 | −4.5 | - | - | 76.6 | [200] |
| Rice biodiesel | Sorbitan palmitate (0.5 wt%) | - | - | 1 | 0 | - | - | 23.1 | [200] |
| Cottonseed biodiesel | Sorbitan palmitate (0.5 wt%) | - | - | 1.5 | −4 | - | - | 46.0 | [200] |
| Jatropha biodiesel | No agent | 18 | 16.5 | 20.2 | 17.8 | - | - | - | [193] |
| Karanja biodiesel | No agent | 9 | 3 | 16 | 9 | - | - | 91.33 | [196] |
| Peanut biodiesel | No agent (one stage) | 15 | −3 | 20 | 12 | 17 | 6 | 91.46 | [202] |
| No agent (three stages) | 15 | −3 | 20 | 2 | 17 | −3 | 90.51 | ||
| Methanol (mass ratio 4:1; one stage) | 15 | −6 | 20 | 0 | 17 | −8 | 91.07 | ||
| Used cooking biodiesel | No agent | 13.7 | 10.6 | 14.5 | 11.5 | - | - | - | [193] |
| Used cooking biodiesel | Sodium dodecylsulfate (0.3 wt%) Magnesium sulfate (1 wt%) | - | - | - | - | −10 | −17 | 73.1 | [197] |
4.2.2. Vacuum Distillation
Distillation is a separation method used to purify products from impurities by utilizing vapor pressure (corresponds to volatility) or boiling point differences of components [203]. This method can be implemented to modify fatty ester composition in biodiesel and obtain better cold flow properties [204]. The desired components are unsaturated fatty esters, which have a lower melting point and higher boiling point than the unfavorable saturated fatty esters [205]. However, the characteristics of these unsaturated components are lower oxidation stability and a lower cetane number than in their saturated form. Hence, implementation of this process requires optimization of saturated fatty ester removal.
Application of this process at atmospheric pressure is hampered by the high boiling point of fatty esters, rendering high energy consumption and low energy efficiency. Besides that, high temperature distillation above 200 °C potentially degrades fatty esters before thermal separation occurs, causing a significant alteration in the physical properties of biodiesel [205,206,207]. To overcome these problems, vacuum distillation is then proposed to provide a lower operating temperature. The lower temperature condition is associated with a reduction in energy consumption, but it is not linear. This process still requires additional energy for negative-pressure conditioning, but its energy requirement is relatively smaller than that required for high-temperature heating. Moreover, the application of vacuum distillation can also minimize fatty ester decomposition [205]. This ensures that there are no drastic changes in the physical properties of biodiesel.
Finally, this method is reported to be able to improve biodiesel CFP. As presented in Table 8, vacuum application in the distillation is verified to decrease PP, CP, and/or CFPP. Yeong et al. proved that vacuum distillation alleviated PP, CP, and CFPP of palm fatty acid distillate biodiesel by 6–8 °C [205]. Iakovlieva et al. applied vacuum conditions to distill rapeseed biodiesel with PP depression up to 2 °C [207]. Furthermore, Table 8 also shows that the negative-pressure implementation reduces the operating temperature to the range of 165–259 °C. Normally, biodiesel distillation is carried out at temperature above 332 °C to separate methyl palmitate from biodiesel [206], whereas methyl stearate removal requires a distillation temperature of more than 353 °C at atmospheric pressure. In the future, temperature and pressure optimization will be two major topics for further development of vacuum distillation for biodiesel CFP refinement [206].


Table 8.
Results of vacuum distillation applications for improving cold flow properties.
Table 8.
Results of vacuum distillation applications for improving cold flow properties.
| Biodiesel | Pressure (kPa) | Temperature (°C) | PP (°C) | CP (°C) | CFPP (°C) | Biodiesel Yield (wt%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||||
| Rapeseed biodiesel (methyl ester) | 0.4–0.7 | 165–215 | −16 | −18.5 | - | - | - | - | - | [207] |
| Rapeseed biodiesel (ethyl ester) | 0.4–0.7 | 165–215 | −17 | −19 | - | - | - | - | - | [207] |
| Palm fatty acid distillate (PFAD) biodiesel | 1 | 194.8 | 15 | 9 | 20 | 13 | 19 | 11 | 59.6 | [205] |
| Microalgae Nannochloropsis oceania biodiesel | 5 | 255–259 | - | - | - | - | - | 5 | 57.8 | [204] |
4.3. Alkyl Modification
Generally, a fatty ester molecule is composed of a fatty acid and short-chain alkyl from methyl or ethyl alcohol. The application of this fatty ester typically does not cause any problems in a small-percentage mixture and a room-temperature environment. However, it will be a serious obstacle when running with a high-level content and in cold conditions [13]. One solution is to replace methanol or ethanol as transesterification reactants with longer straight- or branched-chain alcohols [174]. Some of the proposed longer straight-chain alcohols are 1-propanol and 1-butanol. According to Figure 9 and Table 9, fatty ester products from these alcohols have a lower melting point than methyl or ethyl esters. Meanwhile, the limit of the alcohol carbon chain length is four atoms, because alcohols with more carbon atoms potentially promote fatty esters having a higher pour point [169]. This phenomenon, in which n-butyl ester has a lower PP than n-pentyl ester, is also presented in Table 9. Other benefits of using longer carbon chain alcohols are improving oxidation stability [208], elevating fuel ignitability [174], reducing heat emission [169], and suppressing smoke generation [169]. However, the weaknesses of using these alcohols are greater expense [13], higher ester viscosity [209,210], lower reactivity during transesterification [174], and more coke formation in the fuel injector [169]. The third weakness correlates with increasing concerns regarding monoglycerol content in the biodiesel product [209].
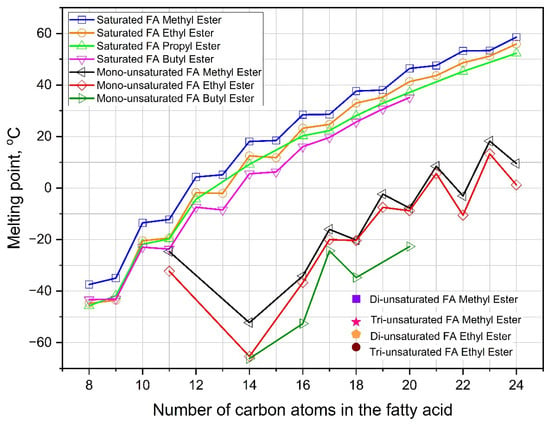
Figure 9.
Melting points of various fatty acid straight-chain alkyl esters [7,184].
Furthermore, the prospective reactants from branched-chain alcohol moieties are isopropanol, 2-butanol, isobutanol, and isopentanol. As demonstrated in Table 9, the utilization of these alcohols as reactants in biodiesel production successfully decreases PP, CP, and/or CFPP. In other words, this alcohol group renders better low-temperature fluidity than methanol and ethanol. Nonetheless, it potentially deteriorates oxidation stability and cetane number [211]. Other potential frailties of branched-chain alcohols are the same as those of the straight-chain moiety in terms of commodity price [13], viscosity of ester products [209,210], reactivity [174], and coke formation [169]. Even though they both have shortcomings in terms of reactivity, the branched-chain alcohol is still better than the straight-chain alcohols [174], while the other comparable weaknesses are almost the same for both alcohols.


Table 9.
Results of alkyl modification for improving cold flow properties.
Table 9.
Results of alkyl modification for improving cold flow properties.
| Feedstock | Alcohol | Alkyl Ester | PP (°C) | CP (°C) | CFPP (°C) | Ref. |
|---|---|---|---|---|---|---|
| Palm oil | Methanol | Methyl ester | 14 | - | - | [209] |
| Ethanol | Ethyl ester | 8 | - | - | ||
| n-propanol | n-propyl ester | 6 | - | - | ||
| n-butanol | n-butyl ester | 4 | - | - | ||
| Isobutanol | Isobutyl ester | 0 | - | - | ||
| n-pentanol | n-pentyl ester | 4 | - | - | ||
| Isopentanol | Isopentyl ester | 2 | - | - | ||
| n-hexanol | n-hexyl ester | 6 | - | - | ||
| n-heptanol | n-heptyl ester | 10 | - | - | ||
| n-octanol | noctyl ester | 12 | - | - | ||
| Palm oil | Methanol | Methyl ester | 12 | 16 | - | [212] |
| Isopropanol | Isopropyl ester | −3 | 6 | - | ||
| 2-butanol | 2-butyl ester | −6 | 7 | - | ||
| Isobutanol | Isobutyl ester | −3 | 7 | - | ||
| Isopentanol | Isopentyl ester | −9 | 8 | - | ||
| Soybean oil | Methanol | Methyl ester | - | 0 | - | [213] |
| Isopropanol | Isopropyl ester | - | −10 | - | ||
| Soybean oil | Methanol | Methyl ester | −3 | −2 | - | [214] |
| Ethanol | Ethyl ester | −6 | −2 | |||
| Isopropanol | Isopropyl ester | −12 | −9 | - | ||
| 2-butanol | 2-butyl ester | −15 | −12 | |||
| Soybean oil | Methanol | Methyl ester | −2 | 0 | - | [187] |
| Ethanol | Ethyl ester | −4 | 1 | - | ||
| Isopropanol | Isopropyl ester | −12 | −9 | - | ||
| n-butanol | n-butyl ester | −7 | −3 | - | ||
| 2-butanol | 2-butyl ester | −15 | −12 | - | ||
| Rapeseed oil | Methanol | Methyl ester | −9 | −4 | −14 | [211] |
| Ethanol | Ethyl ester | −15 | −1 | −6 | ||
| Biobutanol | Butyl ester | −18 | −8 | −21 | ||
| Canola oil | Methanol | Methyl ester | −9 | 1 | - | [187] |
| Ethanol | Ethyl ester | −6 | −1 | - | ||
| Isopropanol | Isopropyl ester | −12 | 7 | - | ||
| n-butanol | n-butyl ester | −16 | −6 | - | ||
| Safflower oil | Methanol | Methyl ester | −6 | - | - | [187] |
| Ethanol | Ethyl ester | −6 | −6 | - | ||
| Sunflower oil | Methanol | Methyl ester | −3 | 2 | - | [187] |
| Ethanol | Ethyl ester | −5 | −1 | - | ||
| Beef tallow | Methanol | Methyl ester | 15 | 17 | - | [187] |
| Ethanol | Ethyl ester | 12 | 15 | - | ||
| n-propanol | n-propyl ester | 9 | 12 | - | ||
| Isopropanol | Isopropyl ester | 0 | 8 | - | ||
| n-butanol | n-butyl ester | 6 | 9 | - | ||
| Isobutanol | Isobutyl ester | 3 | 8 | - | ||
| Waste cooking oil | Methanol | Methyl ester | 2 | −3 | −3 | [211] |
| Ethanol | Ethyl ester | −0.5 | −3 | −1 | ||
| Biobutanol | Butyl ester | −8 | −3 | −9 |
4.4. Polyester Formation
Polyester formation is a transesterification process used to transform monoester biodiesel into polyester biodiesel by using polyol as an additional reactant [215]. This method is the opposite to the common transesterification process used for biodiesel production. In general transesterification, triglyceride (polyester) reacts with alcohol to produce monoester as the main product and glycerol (polyol) as a by-product, whereas this process converts monoester and other polyols into polyester as the main product and alcohol as a by-product. Fundamentally, polyol is defined as a chemical compound that has more than one hydroxyl group (–OH), such as glycerol [174]. In this case, the desired polyols are glycerol analog compounds that have three hydroxyl groups, but they do not have β-hydrogen attached to the middle carbon of glycerol [216]. The absence of β-hydrogen elevates both molecule and thermal stability during oxidation [215]. Notably, thermal devastation due to the presence of β-hydrogen will potentially eliminate the ester group in the polyester, generating a free fatty acid and undesired ester (enol acetate) [216].
Figure 10 shows the overall reversible reaction formula of polyester formation from mono-fatty esters and polyol. In detail, the process of inserting the monoester into the polyol backbone takes place gradually, one-by-one. The appearance of alkyl (R4) instead of β-hydrogen in the β position is proven to enhance biodiesel cold flow properties. The evidence of the polyester effect on biodiesel cold fluidity is demonstrated in Table 10. Of all data available in Table 10, trymethylolpropane (TMP) is a polyol widely used for polyester production. This compound has an ethyl group attached to the middle polyol backbone [215]. The formation of trymethylolpropane ester (TMPE) from TMP and monoesters reduces the PP of biodiesel to below 0 °C. Other polyols that can be used to create polyester are pentaerythritil monomethyl ether, pentaerythritil monoethyl ether, pentaerythritil monobutyl ether, pentaerythritil monophenyl ether, and 3,3-bis(hydroxymethyl)-oxetane.
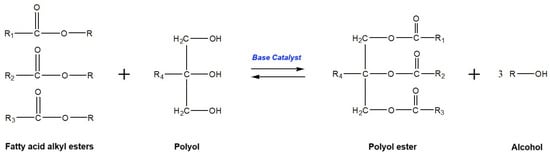
Figure 10.
Reaction scheme of polyester formation (adapted and modified from [174]).
Besides the astonishing results in pour point reduction and the benefits in terms of oxidation and thermal stability, polyester also presents a remarkable increase in kinematic viscosity [216]. According to Table 10, kinematic viscosities of polyester biodiesel exhibit an increase of up to ten times compared to those of the monoester biodiesel. Therefore, the polyester yield must be limited to a low value to minimize its negative impact on biodiesel quality. Another way is to use polyester as an additive or blending agent of biodiesel to refine cold flow properties.


Table 10.
Results of polyester formation for improving cold flow properties.
Table 10.
Results of polyester formation for improving cold flow properties.
| Feedstock | Polyol | PP (°C) | CP (°C) | Kinematic Viscosity at 40 °C (cSt) | Ref. |
|---|---|---|---|---|---|
| Palm oil | Trymethylolpropane | −1 | - | 49.7 | [217] |
| High oleic palm oil | Trymethylolpropane | −9–−37 | - | 45.5–50.7 | [217] |
| Canola oil | Trymethylolpropane | −66 | −27 | 40.5 | [218] |
| Palm kernel oil | Di-trymethylolpropane | −6 | - | 111.7 | [219] |
| Used cooking oil | Trymethylolpropane | −8 | - | 38.6 | [220] |
| Oleic acid | Trymethylolpropane | −51 | −21 | 48.9 | [218] |
| Oleic acid | Trymethylolpropane | −35 a | - | 47.4 | [216] |
| Pentaerythritil monophenyl ether | −19 a | - | 67.9 | ||
| Pentaerythritil monomethyl ether | −15 a | - | 47.8 | ||
| Pentaerythritil monoethyl ether | −35 a | - | 46.3 | ||
| Pentaerythritil monobutyl ether | −33 a | - | 45.9 | ||
| 3,3-bis(hydroxymethyl)-oxetane | −28 a | - | 35.4 |
Note: a melting point.
4.5. Modification of Carbon Chain Structure
4.5.1. Dehydrogenation
Dehydrogenation, usually called desaturation, is a hydrogen removal process used to create a double bond in the carbon chain or generate hydrocarbons having a greater unsaturation degree [7]. This process is specifically considered to lift biodiesel cold flow properties by transforming saturated fatty esters into unsaturated components, as visualized in Figure 11. Based on this chart, components having a greater unsaturation degree are favorable to meet a high level of cold flow properties. However, a higher unsaturated fatty ester content in biodiesel will contribute to a remarkable alleviation in the oxidation stability index (OSI) and cetane number (CN). Therefore, application of fatty ester desaturation to enhance biodiesel cold flow properties must be followed by refinement actions for both properties. Consequently, this will multiply production cost.
Based on the authors’ best effort, the reports on fatty ester dehydrogenation are inadequate. Macias et al. [221] conducted a catalytic reaction of methyl oleate using a mesoporous gallium-niodium oxide solid catalyst. This catalyst has driven isomerization, cracking, dehydrogenation, and dimerization reactions for a wide range of selectivity. The dehydrogenation reaction was detected at a reaction temperature of 220 °C, based on the presence of methyl linoleate and linolenate in the product. This was also indicated by positive iodine value differences between the products and feedstock. In this case, observational dehydrogenation successfully reduced the pour point of biodiesel. Additionally, this paper also concluded that dehydrogenation of biodiesel was inversely correlated with the acidity strength and the amount of impregnated gallium in the acid catalyst. The lower acidity strength and doped-gallium content in the catalyst presented a higher catalyst performance in the dehydrogenation reaction.
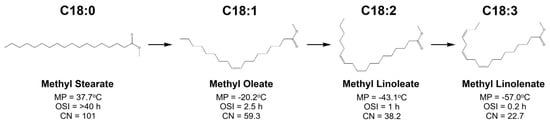

Figure 11.
Desaturation steps of C18 fatty ester with melting point (MP), oxidation stability index (OSI), and cetane number (CN) characteristics (adapted and modified from [7,184,222]).
4.5.2. Isomerization
Isomerization is a process that modifies the carbon chain structure in a hydrocarbon molecule, thereby producing an isomeric product. In this method, the structure of the carbon chain is rearranged to create a new structure, but its chemical formula is still the same as that of the parent component [7]. There are five types of isomerization: skeletal, positional, functional, geometric, and optical isomerization [223]. For the first type, the isomeric product has a different chain structure than the feed, such as straight-chain and branched-chain hydrocarbon. Functional isomerization will change the position of the double-bond, triple-bond, or functional group in the hydrocarbon, for example, 1-butene and 2-butene. Functional isomerization generates an isomeric product with a different functional group, for example, 1-hexene and cyclo-hexane. The last two isomerization categories are specified as stereo isomerization, which produces different geometric (cis- and trans-) and spatial arrangements of the asymmetric carbon atom (D- and L-), respectively.
Regarding biodiesel, the discussion is focused on skeletal isomerization to generate fatty esters having a lower melting point, which correlates with better cold flow properties. In other words, this section specifically talks about the transformation process of natural straight-chain fatty esters into branched-chain fatty esters as isomeric products. The primary targeted components are saturated straight-chain fatty esters, such as palmitate ester and stearate ester. Figure 12 shows that methyl palmitate and methyl stearate can be commonly isomerized into methyl iso-palmitate and methyl iso-stearate, respectively. These isomeric products provide a lower melting point (MP) than the natural products. As illustrated in Figure 12, the branching of linear-chain fatty esters preferably occurs at the second-last carbon (iso-branch form), opposing the ester functional group. Branching may also take place at the third-last carbon (anteiso- branch form) or closer to the middle carbon, which generally results in a lower melting point than the iso- branching [7]. However, its formation is difficult due to the effects of the ester group molecule. To see more details, the tabulation of biodiesel isomerization results for CFP improvement is presented in Table 11.
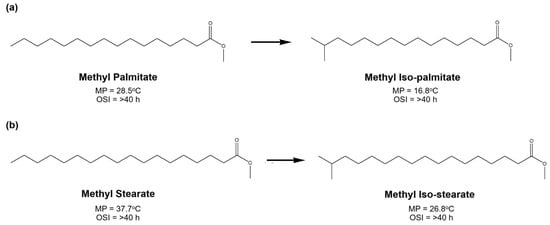
Figure 12.
Skeletal isomerization of saturated straight-chain fatty esters: (a) methyl palmitate; and (b) methyl stearate (adapted and modified from [7,184]).
For other fuel properties, the appearance of branched-chain fatty esters in the biodiesel has insignificant effects on the oxidation stability. Based on the data available in Figure 12, the oxidation stability index (OSI) of branched-chain fatty acids in the saturated form is still higher than forty hours, while for the cetane number, the existence of isomerized fatty esters has a slight consequence. Although they both have a saturated structure, the linear-chain ester has a slightly better cetane number that the branched form [224]. Therefore, the effect of branched-chain ester components on the biodiesel cetane number also needs to be considered for commercial application.


Table 11.
Results of fatty ester isomerization for improving cold flow properties.
Table 11.
Results of fatty ester isomerization for improving cold flow properties.
| Biodiesel | Catalyst | PP (°C) | CP (°C) | Ref. | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Palm biodiesel | Pt/β-zeolite | 15 | 9 | 17.5 | 12.8 | [225] |
| Coconut biodiesel | Pt/β-zeolite | 9 | −3 | 14.2 | −2.3 | [225] |
| Soybean biodiesel | SO42−/ZrO2 | - | - | 5.2 | −1.5 | [226] |
| H-mordenite zeolite | - | - | 5.2 | 0 | ||
| Methyl palmitate | Pt/β-zeolite | - | - | 30 | 20 | [227] |
| Methyl stearate | H-ferrierite zeolite | 37.7 a | −25 | n.a. | −13.9 | [188] |
| Methyl oleate | H-ferrierite zeolite | −20.2 a | −34 | n.a. | −18.5 | [188] |
| Methyl oleate | H-ferrierite zeolite | −20.2 a | −34 | n.a. | −16 | [228] |
| Methyl oleate | β-zeolite | −15.6 | −15.2 | [229] | ||
| Methyl palmitate-methyl oleate mixture | Pt/β-zeolite | - | - | 17 | 9.5 | [230] |
Note: a approximated by melting point.
The isomerization reaction for saturated hydrocarbon branching generally occurs through a five-step process, as illustrated in Figure 13. The reaction mechanism is explained as follows [7,225]:
- -
- Dehydrogenation of saturated linear-chain fatty ester using a metal catalyst to generate an unsaturated fatty ester. In this step, no branching is formed.
- -
- Attachment of protons (protonation) to the unsaturated linear-chain fatty ester to produce fatty ester carbocation. This step requires an acid-based catalyst to accelerate protonation.
- -
- Fatty ester carbocation is transformed into intermediate carbocation, and the carbon chain structure is then rearranged to create a new structure with branching (saturated branched-chain fatty ester carbocation). In this stage, the type of acid catalyst determines the reaction rate and product selectivity.
- -
- Release of protons (deprotonation) from branched-chain fatty ester carbocation to establish an unsaturated branched-chain fatty ester.
- -
- Hydrogenation of the unsaturated branched-chain fatty ester to promote the saturated form of the branched-chain fatty ester.
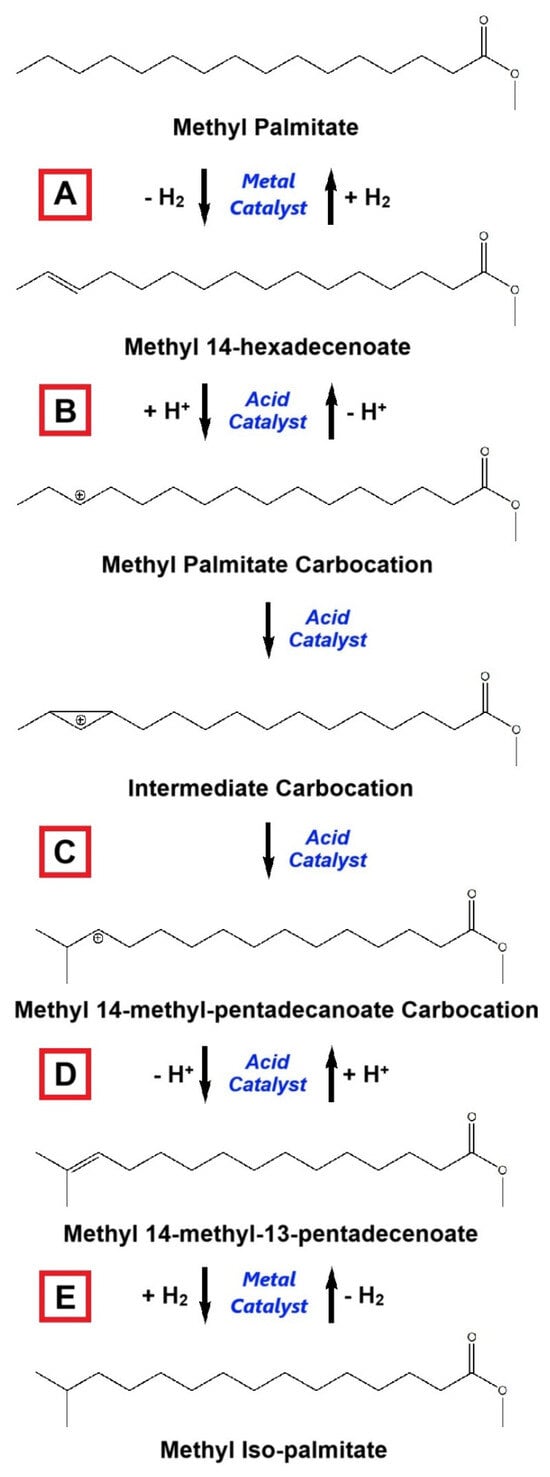
Figure 13.
General mechanism of hydro-isomerization for saturated fatty esters, represented by methyl palmitate: (A) Dehydrogenation; (B) Protonation; (C) Isomerization; (D) Deprotonation; and (E) Hydrogenation (adapted and modified from [7,231]).
This mechanism is also called hydro-isomerization because of the involvement of the hydrogenation–dehydrogenation process [174]. The branching process of unsaturated hydrocarbon at the location of the double bond does not involve a dehydrogenation (A) and hydrogenation step (E), producing branched-chain unsaturated hydrocarbon. Hereinafter, when the process implicates dehydrogenation–hydrogenation, the unsaturated hydrocarbon will be branched at the single-bonded carbon atom.
4.5.3. Epoxidation–Alkoxylation
Epoxidation–alkoxylation is a combination process proffered to bring down cold fluidity parameters (PP, CP, and/or CFPP) of biodiesel by reducing the double bond in the fatty ester and converting it into an alkoxy product [232]. Different from other methods, the targeted components in this method are unsaturated fatty esters [233]. Although the unsaturated fatty ester has good low-temperature flow behavior, its cetane number and oxidation stability are not as expected [174,234]. Therefore, the combination of epoxidation and alkoxylation is offered to improve oxidation stability, cetane number, and also cold fluidity characteristics [235]. This method will change the carbon chain pattern of fatty ester from an unsaturated-linear pattern into a saturated-branched pattern a with lower PP and/or CP [174].
Generally, the reaction scheme of epoxidation–alkoxylation for fatty ester branching is illustrated in Figure 14. Initially, the double bond in the unsaturated fatty ester molecule is epoxidized using peroxide and formic acid to create an oxirane ring or cyclic ether functional group. The first process is called epoxidation (A). Afterward, the oxirane ring is opened by a strong acid catalyst and it will bind an alkoxy molecule [174]. The alkoxy molecule originates from alcohol, which is activated by a strong acid catalyst, such as sulfuric acid and boron trifluoride [236,237]. The process integrating ring opening and alkoxy attachment in the epoxidized fatty ester is well known as alkoxylation (B). Ultimately, three products result from the alkoxylation stage: (i) alkoxy-hydroxy saturated fatty ester (main product); (ii) dihydroxy saturated fatty ester; and (iii) keto saturated fatty ester.
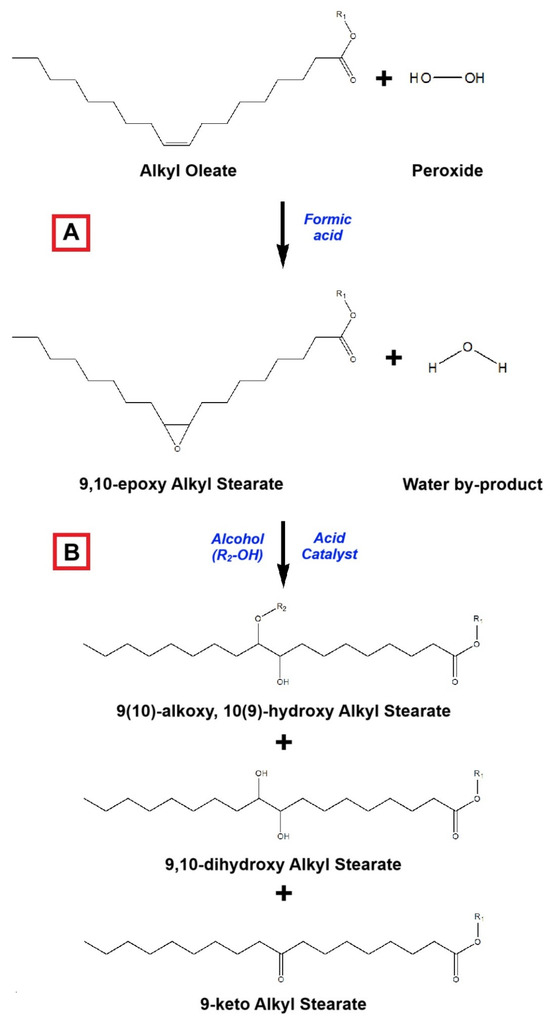
Figure 14.
Reaction scheme of epoxidation–alkoxylation, represented by methyl oleate: (A) Epoxidation; and (B) Alkoxylation (adapted and modified from [232,235,236,237]).
The results of fatty ester epoxidation–alkoxylation have been previously reported for improving cold flow properties, as presented in Table 12. Of all data introduced in Table 12, the longer carbon chain or higher molecular weight of alkoxy will reduce the PP and/or CP of biodiesel. This phenomenon has been explicitly explained by Smith et. al. [236] as the bigger molecule of alkoxy hindering the formation of tidy and compact fatty ester molecules. It will bend the primary carbon chain and interrupt crystal formation, thus definitely decreasing the melting point. This reason also clarifies the remarkable decline in the melting point from high- to low-saturation-degree fatty esters. In addition to the superiorities stated above, the alkoxylated fatty esters also have some drawbacks: (i) higher kinematic viscosity; (ii) slightly lower higher-heating value (HHV); and (iii) higher corrosive properties than the normal fatty esters [232,234,235,237]. The last two weaknesses are specifically due to the higher presence of oxygen in the alkoxylated fatty esters. Hence, the implementation of this treatment for biodiesel CFP improvement must be properly controlled to optimize alkoxylation conversion and achieve the desired quality.


Table 12.
Results of fatty ester epoxidation–alkoxylation for improving cold flow properties.
Table 12.
Results of fatty ester epoxidation–alkoxylation for improving cold flow properties.
| Biodiesel | Alkoxy | PP (°C) | CP (°C) | Ref. | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Canola butyl ester | Methoxy | - | - | −3 | −4 | [236] |
| Canola butyl ester | n-buthoxy | - | - | −3 | −5 | [233] |
| Canola butyl ester | n-pentoxy | −12 | −12 | −3 | −5 | [235] |
| n-hexoxy | −12 | −12 | −3 | −5 | ||
| n-octoxy | −12 | −12 | −3 | −5 | ||
| 2-ethylhexoxy | −12 | −12 | −3 | −6 | ||
| Poultry fat methyl ester | Tert-butoxy | −3 | −3 | 2 | 3 | [234] |
| Used cooking methyl ester | 2-ethylhexoxy | 10 | −14 | - | - | [238] |
| High oleic acid methyl ester | Methoxy | 0 | −1 | 2 | 3 | [237] |
| Ethoxy | 0 | −4 | 2 | −2 | ||
| n-propoxy | 0 | −6 | 2 | −3 | ||
| Isopropoxy | 0 | −10 | 2 | −3 | ||
| n-butoxy | 0 | −6 | 2 | −4 | ||
| Isobutoxy | 0 | −8 | 2 | −4 | ||
| n-hexoxy | 0 | −10 | 2 | −7 | ||
| n-octoxy | 0 | −10 | 2 | −7 | ||
| n-decoxy | 0 | −14 | 2 | −11 | ||
| High oleic acid isopropyl ester | n-butoxy | −12 a | −22 | −9 b | −21 | [239] |
| Isobutoxy | −12 a | −23 | −9 b | −21 | ||
| n-hexoxy | −12 a | −23 | −9 b | −23 | ||
| n-octoxy | −12 a | −23 | −9 b | −23 | ||
| 2-ethylhexoxy | −12 a | −24 | −9 b | −23 | ||
| n-decoxy | −12 a | −24 | −9 b | −23 | ||
Note: a pour point of soybean isopropyl ester. b cloud point of soybean isopropyl ester.
4.6. Carbon Chain Cracking
Carbon chain cracking, usually called hydrocracking, is a thermal process used to break carbon chains into two or more shorter carbon chain molecules. In this topic, hydrocracking is proposed to particularly break down the carbon chain saturated fatty esters, hence probably producing lighter fatty esters and alkane hydrocarbons [7]. As depicted in Figure 15, both probable products show considerable melting point (MP) reduction compared to their parent fatty esters. The positive point is that their oxidation stability index (OSI) can be maintained at high values. However, these desired products have problems in terms of the cetane number, which proportionally corresponds to fuel ignitability in diesel engines [230]. Therefore, the optimum composition of basic fatty esters and cracked products should be considered in the final product to maintain the quality of biodiesel.
Reviewing further, the general hydrocracking mechanism of saturated hydrocarbon is described as follows [7,240]:
- -
- Dehydrogenation of saturated hydrocarbon to promote double-bond-containing hydrocarbon;
- -
- Protonation of unsaturated hydrocarbon to stimulate carbenium ion formation;
- -
- Carbenium ions are dissociated into shorter carbon chain carbocation and unsaturated hydrocarbon;
- -
- Deprotonation of carbocation to generate unsaturated hydrocarbon;
- -
- Unsaturated molecules are then hydrogenated to become saturated hydrocarbons.
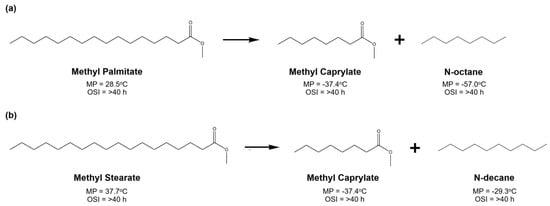
Figure 15.
Hydrocracking (expected) of saturated straight-chain fatty esters: (a) methyl palmitate; and (b) methyl stearate (adapted and modified from [7,184,241]).
This five-stage hydrocracking mechanism is illustrated in Figure 16. This mechanism is almost the same as the hydro-isomerization reaction scheme. Moreover, the required catalyst also has similarities with hydro-isomerization: (i) a metal catalyst for hydrogenation–dehydrogenation stage; and (ii) an acid catalyst for protonation–deprotonation and dissociation. Although active for both reactions, the required reaction temperature will be used as a distinguishing feature. Commonly, hydrocracking requires a higher temperature than hydro-isomerization.
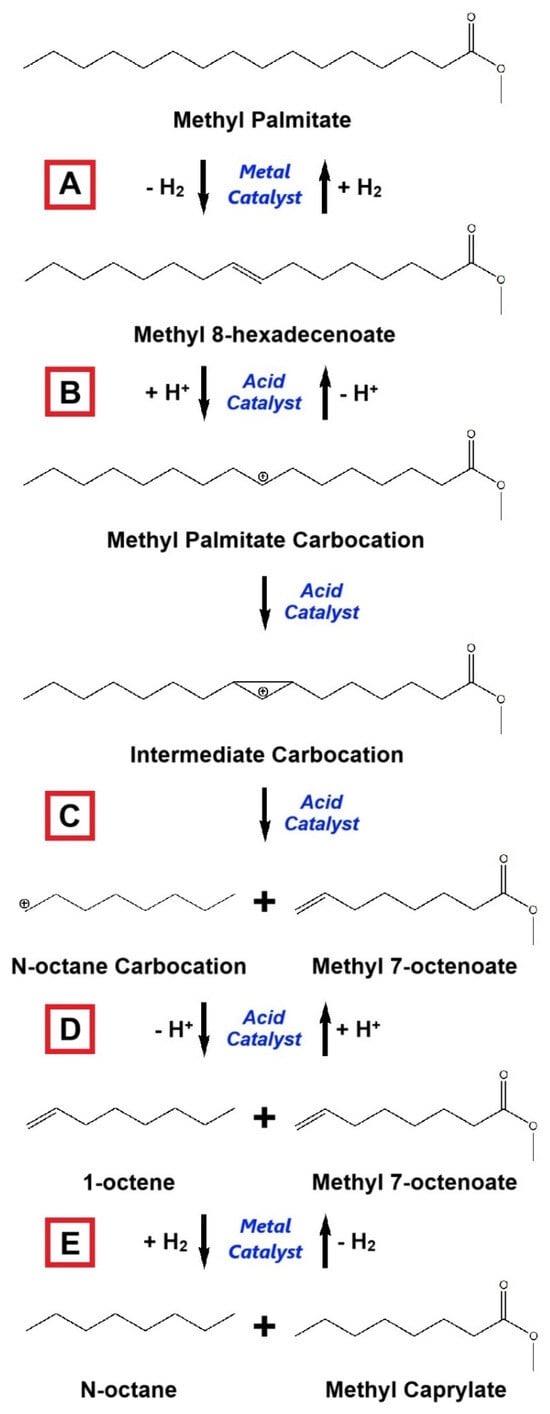
Figure 16.
General mechanism of hydrocracking (expected) for saturated fatty esters, represented by methyl palmitate: (A) Dehydrogenation; (B) Protonation; (C) Cracking; (D) Deprotonation; and (E) Hydrogenation (adapted and modified from [7]).
In addition to temperature, the selection of an appropriate catalyst also plays an important role in the hydrocracking process. This process probably generates many combinations of products in a set of hydrocracking processes [232]. Meanwhile, the desired cracking products are two medium hydrocarbons to minimize yield loss and the reduction in physical properties [7]. For example, the hydrocracking process of C16 hydrocarbon is expected to create two identical C8 hydrocarbons, as demonstrated in Figure 15a. In fact, its products vary from C3 to C13 hydrocarbons. Hence, this process needs an acid catalyst that has high selectivity towards the desired products. Several hydrocracking catalysts have been explored previously to improve the cold flow properties of biodiesel. Their performances in terms of improving cold flow properties are summarized in Table 13.


Table 13.
Results of fatty ester hydrocracking for improving cold flow properties.
Table 13.
Results of fatty ester hydrocracking for improving cold flow properties.
| Biodiesel | Catalyst | PP (°C) | CP (°C) | Ref. | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Soybean biodiesel | H-modenite zeolite | - | - | 5.2 | 0 | [226] |
| SO42−/ZrO2 | - | - | 5.2 | 0 | ||
| Soybean biodiesel | Not specified | −7 | −41 | - | - | [242] |
| Sunflower biodiesel | Not specified | −12 | −50 | - | - | [242] |
| Canola biodiesel | No catalyst | −9 | −23 | 1 | −21 | [243] |
| Canola biodiesel | No catalyst | - | - | −2 | −23 | [244] |
| Peanut biodiesel | Not specified | 7 | 1 | - | - | [242] |
| Cottonseed biodiesel | Not specified | 1 | 0 | - | - | [242] |
| Methyl oleate | SAPO-11 | 4 | −18 | - | - | [221] |
| H-ZSM-5 (30) | 4 | −15 | - | - | ||
| H-β-zeolite | 4 | −17 | - | - | ||
| H-Y-zeolite | 4 | −15 | - | - | ||
| H-MCM-22 | 4 | −17 | - | - | ||
4.7. Genetic Modification
Genetic modification is a biological treatment method implemented by oil producers, whereby they can produce oils with desirable fatty acid profiles or specific properties [169,232]. In this study, the discussion is focused on the use of genetic modification to produce biodiesel feedstock oil with superior cold fluidity characteristics. The first option is by genetically engineering oil crops. This method is intended to generate oil crops that can produce unsaturated fatty acid-rich oil, which obviously has good cold flow properties. Kraic et al. [245] succeeded in genetically engineering cereals to create polyunsaturated C18–C22 fatty acids. The creation of these bio-hydrocarbons with more than two double bonds occurred because of ω-3 desaturase and Δ6-desaturase enzymes encoded by the engineered genes.
Vollmann and Eynck [163] modified genes in the camelina plant by combining it with several other genes to produce long-chain polyunsaturated fatty acids (C20:5 and C22:6), high-oleic-acid oil, and high-linoleic-acid oil. Jafarihaghighi et al. [162] studied the fatty acid profile of oil extracted from genetically modified pennyroyal (Mentha pulegium). Based on the analysis results, this oil was classified as high-oleic-acid oil. Oleic acid was chosen as a desirable fatty acid because it had good cold flow properties. Jia et al. [161] explored Schizochytrium sp. as a subject of genetic modification for polyunsaturated C16–C20 fatty acid production.
The second preferred approach is to modify genes of oil plants to produce lower melting point fatty acids or fatty esters. Tao et al. [158] successfully engineered Escherichia coli (E. coli) microbes to synthesize fatty acid branched-chain alkyl esters in the form of fatty acid isobutyl esters and fatty acid isoamyl esters. Then, they also genetically modified Pichia pastoris (P. pastoris) microbes to produce branched-chain fatty acid isobutyl and isoamyl esters. Haushalter et al. [159] cultivated modified E. coli microbes to manipulate the features of the fatty acid and specifically promote branched-chain fatty acids in the form of iso- and anteiso- branching. Bai et al. [160] also reported the use of genetically manipulated E. coli to produce branched-chain and cyclic-chain fatty acids. Vollmann and Eynck [163] also combined genes in camelina plant with castor fatty acid hydroxylase gene, yielding hydroxy fatty acids.
Based on the current laboratory-scale reports, genetic modification is emerging with attractive results in enhancement of cold flow properties. This approach also has potential benefits in terms of growth rate, oil productivity, and adaptability in various environments [3,12,28]. However, biodiesel production with this technique is estimated to require high capital and operational expenses [28,39]. Therefore, further development is still needed to promote more effective methodologies, at both laboratory and commercial scales.
5. Conclusions
Cold flow properties, indicated by PP, CP, and/or CFPP, are prominent parameters that ensure the quality and operability of biodiesel as a fossil diesel replacement fuel. Most biodiesel experiences some problems with these properties, thus preventing its implementation at a higher percentage and wider environmental temperature range. The cold flow properties of biodiesel are primarily affected by fatty ester profiles, in which unsaturated fatty esters are preferred for their better cold fluidity. On the contrary, saturated fatty esters exhibit high oxidation stability and a high cetane number. According to these characteristics, physical, chemical, and biological methods are all offered to improve cold flow properties. Blending with fatty esters having higher cold flow abilities, winterization, vacuum distillation, branched-chain alkyl modification, dehydrogenation, and hydrocracking are proven to promote better cold flow abilities, with the effects of worsening oxidation stability and/or cetane number. Additive insertion and longer-chain alkyl modification potentially deteriorate fuel viscosity. Genetic modification is an attractive biological approach; however; it still requires further development. Meanwhile, skeletal isomerization presents proper cold fluidity elevation with minimal oxidation stability and cetane number reduction. Therefore, continuous development of these processes will bring closer the realization of high-quality biodiesel applications, providing sustainability, energy security, and environmental impact alleviation.
Author Contributions
Y.S.P.: Investigation, Data curation, Formal analysis, Visualization, Validation, Writing—original draft, Writing—review and editing. I.G.B.N.M.: Conceptualization, Methodology, Formal analysis, Validation, Supervision. A.I.: Conceptualization, Methodology, Formal analysis, Validation, Supervision, Writing—review and editing. T.P.: Formal analysis, Validation, Supervision. T.H.S.: Conceptualization, Methodology, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: (1) Indonesian Educational Scholarship (BPI), Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia [01198/BPPT/BPI.06/9/2023]; (2) Educational Fund Management Institution (LPDP), Ministry of Finance of the Republic of Indonesia [01198/BPPT/BPI.06/9/2023]; and (3) Palm Oil Fund Management Agency (BPDPKS) of the Republic of Indonesia [GRS-20210211164201].
Data Availability Statement
All datasets used in this study were public and available in the literature cited in the figures, tables, and narratives.
Acknowledgments
The authors would like to thank to Department of Chemical Engineering, Institut Teknologi Bandung and Department of Chemical Engineering, Universitas Gadjah Mada for research and publication facility support.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- United Stated Energy Information Administration. International Energy Outlook 2023 with Projections to 2050; United States Department of Energy: Washington, DC, USA, 2023.
- Adnan, M.A.; Susanto, H.; Binous, H.; Muraza, O.; Hossain, M.M. Feed compositions and gasification potential of several biomasses including a microalgae: A thermodynamic modeling approach. Int. J. Hydrog. Energy 2017, 42, 17009–17019. [Google Scholar] [CrossRef]
- Riyadi, T.W.B.; Spraggon, M.; Herawan, S.G.; Idris, M.; Paristiawan, P.A.; Putra, N.R.; Faizullizam, R.M.; Silambarasan, R.; Veza, I. Biodiesel for HCCI Engine: Prospects and Challenges of Sustainability Biodiesel for Energy Transition. Results Eng. 2023, 17, 100916. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A Comprehensive Study on Prospects of Economy, Environment, and Efficiency of Palm Oil Biodiesel as a Renewable Fuel. J. Clean. Prod. 2021, 286, 124981. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Chong, J.H.; Yuniarto, A.; Hadibarata, T. The current scenario and challenges of biodiesel production in Asian countries: A review. Bioresource Technology Reports 2020, 12, 100608. [Google Scholar] [CrossRef]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. Emerging Technologies for Biodiesel Production: Processes, Challenges, and Opportunities. Biomass Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Anwar, A.; Garforth, A. Challenges and Opportunities of Enhancing Cold Flow Properties of Biodiesel via Heterogeneous Catalysis. Fuel 2016, 173, 189–208. [Google Scholar] [CrossRef]
- Shirazi, H.M.; Karimi-Sabet, J.; Ghotbi, C. Biodiesel production from Spirulina microalgae feedstock using direct transesterification near supercritical methanol condition. Bioresour. Technol. 2017, 239, 378–386. [Google Scholar] [CrossRef]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Sadewo, B.R.; Haryanto, S.A.; Sudibyo, H. Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production. Open Chem. 2021, 19, 1029–1042. [Google Scholar] [CrossRef]
- Azapagic, A.; Perdan, S.; Clift, R. Sustainable Development in Practice; Wiley: West Sussex, UK, 2004. [Google Scholar] [CrossRef]
- Pydimalla, M.; Husaini, S.; Kadire, A.; Verma, R.K. Sustainable biodiesel: A comprehensive review on feedstock, production methods, applications, challenges and opportunities. Mater. Today Proc. 2023, 92, 458–464. [Google Scholar] [CrossRef]
- Sia, C.B.; Kansedo, J.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Dewi, R.N.; Livia, K.D.; Arisa, F.; Rochmadi; Cahyono, R.B.; Budiman, A. Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction-transesterification process using palm oil as a co-solvent of methanol. Open Chem. 2020, 18, 833–842. [Google Scholar] [CrossRef]
- ASTM D975-23; Standard Specification for Diesel Fuel (D975-23). American Standard Testing and Material: West Conshohocken, PA, USA, 2023. [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Cho, H.M.; Masjuki, H.H.; Kalam, M.A.; Ong, H.C.; Gul, M.; Harith, M.H.; Yusoff, M.N.A.M. Critical Review on Sesame Seed Oil and Its Methyl Ester on Cold Flow and Oxidation Stability. Energy Rep. 2020, 6, 40–54. [Google Scholar] [CrossRef]
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Sukiman, N.L.; Chyuan, O.H.; Hassan, M.H.; Hasnul, M.H.; Zulkifli, M.S.A.; Abbas, M.M.; Zakaria, M.Z. Sustainability of Palm Biodiesel in Transportation: A Review on Biofuel Standard, Policy and International Collaboration Between Malaysia and Colombia. Bioenergy Res. 2021, 14, 43–60. [Google Scholar] [CrossRef]
- Haq, A.; Rehman, M.L.U.; Rana, Q.U.A.; Khan, A.; Sajjad, W.; Khan, H.; Khan, S.; Shah, A.A.; Hasan, F.; Ahmed, S.; et al. Production, optimization, and physicochemical characterization of biodiesel from seed oil of indigenously grown Jatropha curcas. Front. Energy Res. 2023, 11, 1225988. [Google Scholar] [CrossRef]
- Amri, K.; Kismanto, A.; Solikhah, M.D.; Pratiwi, F.T.; Arisanti, A.G. Study of biodiesel specifications for heavy-duty gas turbine application. In Journal of Physics: Conference Series; IOP Publishing Ltd.: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Masruri, W.; Azmi, F.A.; Suyono, E.A.; Sudibyo, H.; Rochmadi, R. Extractive-transesterification of Microalgae Arthrospira sp. Using Methanol-Hexane Mixture as solvent. Int. J. Renew. Energy Res. 2018, 8, 1499–1507. [Google Scholar]
- Al-Jaberi, S.H.H.; Rashid, U.; Al-Doghachi, F.A.J.; Abdulkareem-Alsultan, G.; Taufiq-Yap, Y.H. Synthesis of MnO-NiO-SO4 −2/ZrO2 solid acid catalyst for methyl ester production from palm fatty acid distillate. Energy Convers. Manag. 2017, 139, 166–174. [Google Scholar] [CrossRef]
- Roy, D.K.; Abedin, M.Z. Potentiality of Biodiesel and Bioethanol Production from Feedstock in Bangladesh: A Review. Heliyon 2022, 8, e11213. [Google Scholar] [CrossRef]
- Souza, M.F.; Hirata, G.F.; Batista, E.A.C. Evaluation of kinetics and thermodynamic parameters for simulation of palm oil biodiesel production. Fluid. Phase Equilib. 2020, 525, 112792. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Cai, R.; ChenYang, Y.; Huang, Z.; Han, B. Optimization and kinetics of biodiesel production from soybean oil using new tetraethylammonium ionic liquids with amino acid-based anions as catalysts. Fuel 2022, 324, 124510. [Google Scholar] [CrossRef]
- Meng, Y.L.; Tian, S.J.; Li, S.F.; Wang, B.Y.; Zhang, M.H. Transesterification of rapeseed oil for biodiesel production in trickle-bed reactors packed with heterogeneous Ca/Al composite oxide-based alkaline catalyst. Bioresour. Technol. 2013, 136, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Hachemane, K.; Ribeiro, A.E.; Queiroz, A.; Gomes, M.C.S.; Brito, P. Evaluation and kinetic study of alkaline ionic liquid for biodiesel production through transesterification of sunflower oil. Fuel 2022, 324, 124586. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Ziaei, S.M.; Szulczyk, K.R. Estimating the potential of algal biodiesel to improve the environment and mitigate palm oil mill effluents in Malaysia. J. Clean. Prod. 2022, 338, 130583. [Google Scholar] [CrossRef]
- Kim, T.-H.; Suh, W.I.; Yoo, G.; Mishra, S.K.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Development of direct conversion method for microalgal biodiesel production using wet biomass of Nannochloropsis salina. Bioresour. Technol. 2015, 191, 438–444. [Google Scholar] [CrossRef]
- Gutiérrez-López, A.N.; Mena-Cervantes, V.Y.; Gonzalez-Espinosa, M.A.; Sosa-Rodriguez, F.S.; Vazquez-Arenas, J.; Rodriguez-Ramirezz, R.; Hernandez-Altamirano, R. Green and fast biodiesel production at room temperature using soybean and Jatropha curcas L. oils catalyzed by potassium ferrate. J. Clean. Prod. 2022, 372, 133739. [Google Scholar] [CrossRef]
- Anwar, F.; Tariq, M.; Nisar, J.; Ali, G.; Kanwal, H. Optimization of biodiesel yield from non-food karanja seed oil: Characterization and assessment of fuel properties. Sustain. Chem. Environ. 2023, 3, 100035. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, Y.; Sharma, A. Optimization of biodiesel synthesis from Jojoba oil via supercritical methanol: A response surface methodology approach coupled with genetic algorithm. Biomass Bioenergy 2022, 156, 106332. [Google Scholar] [CrossRef]
- Asif, M.; Javed, F.; Younas, M.; Gillani, M.A.; Zimmerman, W.B.; Rehman, F. Investigating biodiesel production from Chicken fat oil using bi-functional catalysts and microbubble mediated mass transfer. Fuel 2024, 358, 130125. [Google Scholar] [CrossRef]
- Olubunmi, B.E.; Alade, A.F.; Ebhodaghe, S.O.; Oladapo, O.T. Optimization and kinetic study of biodiesel production from beef tallow using calcium oxide as a heterogeneous and recyclable catalyst. Energy Convers. Manag. X 2022, 14, 100221. [Google Scholar] [CrossRef]
- Miladinović, M.R.; Krstic, J.B.; Zdujic, M.V.; Veselinovic, L.M.; Veljovic, D.N.; Bankovic-Ilic, I.B.; Dtamenkovic, O.S.; Veljkovic, V.B. Transesterification of used cooking sunflower oil catalyzed by hazelnut shell ash. Renew. Energy 2022, 183, 103–113. [Google Scholar] [CrossRef]
- Im-Orb, K.; Arpornwichanop, A.; Simasatitkul, L. Process intensification approach for design and optimization of biodiesel production from palm fatty acid distillate. Biotechnol. Rep. 2021, 30, e00622. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xia, Q.; Wang, Y.; Shen, Z.; Huang, H.; Ge, Z.; Li, X.; He, J.; Wang, X.; Li, L.; et al. Recent Advances in Biodiesel Production Using Functional Carbon Materials as Acid/Base Catalysts. Fuel Process. Technol. 2022, 237, 107421. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Fan, J.; Han, Z. Synthesis, Catalysts and Enhancement Technologies of Biodiesel from Oil Feedstock–A Review. Sci. Total Environ. 2023, 904, 166982. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans–Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Dewati, P.R.; Rochmadi; Rohman, A.; Yuliestyan, A.; Budiman, A. Equilibrium modeling of astaxanthin extraction from haematococcus pluvialis. Indones. J. Chem. 2021, 21, 554–563. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Sudibyo, H.; Suyono, E.A.; Indarto; Budiman, A. Oil Algae Extraction of Selected Microalgae Species Grown in Monoculture and Mixed Cultures for Biodiesel Production. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 277–282. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green. Technol. Sustain. 2024, 2, 100060. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Prasakti, L.; Pratama, S.H.; Fauzi, A.; Pradana, Y.S.; Budiman, A. Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production. Open Chem. 2020, 18, 874–881. [Google Scholar] [CrossRef]
- Kholssi, R.; Ramos, P.V.; Marks, E.A.N.; Montero, O.; Rad, C. Biotechnological uses of microalgae: A review on the state of the art and challenges for the circular economy. Biocatal. Agric. Biotechnol. 2021, 36, 102114. [Google Scholar] [CrossRef]
- Malek, A.; Alamoodi, N.; Almansoori, A.S.; Daoutidis, P. Optimal dynamic operation of microalgae cultivation coupled with recovery of flue gas CO2 and waste heat. Comput. Chem. Eng. 2017, 105, 317–327. [Google Scholar] [CrossRef]
- Ren, H.; Tuo, J.; Addy, M.M.; Zhang, R.; Lu, Q.; Anderson, E.; Chen, P.; Ruan, R. Cultivation of Chlorella vulgaris in a pilot-scale photobioreactor using real centrate wastewater with waste glycerol for improving microalgae biomass production and wastewater nutrients removal. Bioresour. Technol. 2017, 245, 1130–1138. [Google Scholar] [CrossRef]
- Yew, G.Y.; Khoo, K.S.; Chia, W.Y.; Ho, Y.C.; Law, C.L.; Leong, H.Y.; Show, P.L. A novel lipids recovery strategy for biofuels generation on microalgae Chlorella cultivation with waste molasses. J. Water Process Eng. 2020, 38, 101665. [Google Scholar] [CrossRef]
- Kalin, M.; Wheeler, W.N.; Meinrath, G. The removal of uranium from mining waste water using algal/microbial biomass. J. Environ. Radioact. 2005, 78, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Farobie, O.; Sutarlan, I.F.I.; Sucahyo, L.; Bayu, A.; Hartulistiyoso, E. Biodiesel production from crude palm oil under subcritical methanol conditions: Experimental investigation and kinetic model. Bioresour. Technol. Rep. 2023, 22, 101441. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Zhang, X.; Han, K.; Lu, C. Preparation of calcium modified Zn-Ce/Al2O3 heterogeneous catalyst for biodiesel production through transesterification of palm oil with methanol optimized by response surface methodology. Fuel 2021, 284, 118986. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Kusumo, F.; Mahlia, T.M.I.; Bahar, A.H. Pilot-scale production and the physicochemical properties of palm and Calophyllum inophyllum biodiesels and their blends. J. Clean. Prod. 2016, 126, 654–666. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Fihri, A.; Zahouily, M. Sodium modified fluorapatite as a sustainable solid bi-functional catalyst for biodiesel production from rapeseed oil. Renew. Energy 2019, 133, 1295–1307. [Google Scholar] [CrossRef]
- Krstić, J.B.; Njetic, Z.B.; Kostoc, M.D.; Maric, B.D.; Simurina, O.D.; Stamenkovic, O.S.; Veljkovic, V.B. Biodiesel production from rapeseed oil over calcined waste filter cake from sugar beet processing. Process Saf. Environ. Prot. 2022, 168, 463–473. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, R.; Sosa-Rodríguez, F.S.; Vazquez-Arenas, J. Zinc oxide-co-sodium zirconate: A fast heterogeneous catalyst for biodiesel production from soybean oil. J. Environ. Chem. Eng. 2022, 10, 108191. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Oladepo, S.A.; Ganiyu, S.A. Zr-modified desilicated ZSM-5 catalysts as highly active and recyclable catalysts for production of biodiesel from soybean oil: Insight into improved catalyst properties, acidity and dispersion through desilication. Fuel 2023, 351, 128729. [Google Scholar] [CrossRef]
- Silva, A.L.; Farias, A.F.F.; Meneghetti, S.M.P.; Filho, E.A.D.S.; de Melo Costa, A.C.F. Optimization of biodiesel production via transesterification of soybean oil using α-MoO3 catalyst obtained by the combustion method. Arab. J. Chem. 2022, 15, 104012. [Google Scholar] [CrossRef]
- Cardoso, R.K.P.; Silva, G.V.A.; Alves, B.T.S.; Freire, V.A.; Alves, J.J.N.; Barbosa, B.V.S. Evaluation of the effect of Si/Mo and oil/alcohol ratios in the production of biodiesel from soybean oil. Arab. J. Chem. 2022, 15, 104074. [Google Scholar] [CrossRef]
- Elkelawy, M.; Bastawissi, H.A.E.; Esmaeil, K.K.; Rdawan, A.M.; Panchal, H.; Sadasivuni, K.K.; Suresh, M.; Israr, M. Maximization of biodiesel production from sunflower and soybean oils and prediction of diesel engine performance and emission characteristics through response surface methodology. Fuel 2020, 266, 117072. [Google Scholar] [CrossRef]
- Marinković, M.; Waisi, H.; Blagojevic, S.; Zarubica, A.; Ljupkovic, R.; Krstic, A.; Jankovic, B. The effect of process parameters and catalyst support preparation methods on the catalytic efficiency in transesterification of sunflower oil over heterogeneous KI/Al2O3-based catalysts for biodiesel production. Fuel 2022, 315, 123246. [Google Scholar] [CrossRef]
- Deviren, H.; Aydın, H. Production and physicochemical properties of safflower seed oil extracted using different methods and its conversion to biodiesel. Fuel 2023, 343, 128001. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Cortés, Á.G. High oleic safflower oil as a feedstock for stable biodiesel and biolubricant production. Ind. Crops Prod. 2021, 170, 113701. [Google Scholar] [CrossRef]
- Ejeromedoghene, O. Acid-catalyzed transesterification of Palm Kernel Oil (PKO) to biodiesel. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 1580–1583. [Google Scholar] [CrossRef]
- Kareem, S.O.; Falokun, E.I.; Balogun, S.A.; Akinloye, O.A.; Omeike, S.O. Enzymatic biodiesel production from palm oil and palm kernel oil using free lipase. Egypt. J. Pet. 2017, 26, 635–642. [Google Scholar] [CrossRef]
- Bambase, M.E.; Almazan, R.A.R.; Demafelis, R.B.; Sobremisana, M.J.; Dizon, L.S.H. Biodiesel production from refined coconut oil using hydroxide-impregnated calcium oxide by cosolvent method. Renew. Energy 2021, 163, 571–578. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Sebayang, A.H.; Kusumo, F.; Milano, J.; Shamsuddin, A.H.; Silitonga, A.S.; Ideris, F.; Siswantoro, J.; Veza, I.; Mofijur, M.; Chia, S.R. Optimization of biodiesel production from rice bran oil by ultrasound and infrared radiation using ANN-GWO. Fuel 2023, 346, 128404. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.H.; Amini, Z.; Harrison, M.D.; Wang, C.T.; Kusumo, F.; Alwi, A. Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Balamurugan, T.; Arun, A.; Sathishkumar, G.B. Biodiesel derived from corn oil–A fuel substitute for diesel. Renew. Sustain. Energy Rev. 2018, 94, 772–778. [Google Scholar] [CrossRef]
- Kaya, C.; Hamamci, C.; Baysal, A.; Akba, O.; Erdogan, S.; Saydut, A. Methyl ester of peanut (Arachis hypogea L.) seed oil as a potential feedstock for biodiesel production. Renew. Energy 2009, 34, 1257–1260. [Google Scholar] [CrossRef]
- Mahloujifar, M.; Mansournia, M. A comparative study on the catalytic performances of alkali metals-loaded KAlSiO4 for biodiesel production from sesame oil. Fuel 2021, 291, 120145. [Google Scholar] [CrossRef]
- Chizoo, E.; Okechukwu, M.F.; Dominic, O.O.; Kingsley, A.A.; Chimamkpam, A.S.; Mariagorretti, M.C.; Chinonso, E.P. Renewable diesel synthesis from sesame indicum (bene) seed oil using novel heterogeneous biocatalyst derived from the Chrysophyllum albidium seed coat. Heliyon 2023, 9, e22006. [Google Scholar] [CrossRef]
- Bilgin, A.; Gulum, M. Effects of various transesterification parameters on the some fuel properties of hazelnut oil methyl ester. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 54–62. [Google Scholar] [CrossRef]
- Saydut, A.; Erdogan, S.; Kafadar, A.B.; Kaya, C.; Aydin, F.; Hamamci, C. Process optimization for production of biodiesel from hazelnut oil, sunflower oil and their hybrid feedstock. Fuel 2016, 183, 512–517. [Google Scholar] [CrossRef]
- Khatibi, M.; Khorasheh, F.; Larimi, A. Biodiesel production via transesterification of canola oil in the presence of Na–K doped CaO derived from calcined eggshell. Renew. Energy 2021, 163, 1626–1636. [Google Scholar] [CrossRef]
- Murguía-Ortiz, D.; Cordova, I.; Manriquez, M.E.; Ortiz-Islas, E.; Cabrera-Sierra, R.; Contreras, J.L.; Alcantar-Vazquez, B.; Trejo-Rubio, M.; Vazquez-Rodriguez, J.T.; Castro, L.V. Na-CaO/MgO dolomites used as heterogeneous catalysts in canola oil transesterification for biodiesel production. Mater. Lett. 2021, 291, 129587. [Google Scholar] [CrossRef]
- Kumar, V.; Kant, P. Biodiesel production from sorghum oil by transesterification using zinc oxide as catalyst. Pet. Coal 2014, 56, 35–40. [Google Scholar]
- Lin, L.; Cunshan, Z.; Vittayapadung, S.; Xiangqian, S.; Mingdong, D. Opportunities and Challenges for Biodiesel Fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Moser, B.R. Preparation of fatty acid methyl esters from hazelnut, high-oleic peanut and walnut oils and evaluation as biodiesel. Fuel 2012, 92, 231–238. [Google Scholar] [CrossRef]
- Gui, X.; Chen, S.; Yun, Z. Continuous production of biodiesel from cottonseed oil and methanol using a column reactor packed with calcined sodium silicate base catalyst. Chin. J. Chem. Eng. 2016, 24, 499–505. [Google Scholar] [CrossRef]
- Rizal, T.A.; Khairil; Mahidin; Husin, H.; Ahmadi; Nasution, F.; Umar, H. The Experimental Study of Pangium Edule Biodiesel in a High-Speed Diesel Generator for Biopower Electricity. Energies 2022, 15, 5405. [Google Scholar] [CrossRef]
- Ruhul, M.A.; Abedin, M.J.; Rahman, S.M.A.; Masjuki, B.H.H.; Alabdulkarem, A.; Kalam, M.A.; Shancita, I. Impact of fatty acid composition and physicochemical properties of Jatropha and Alexandrian laurel biodiesel blends: An analysis of performance and emission characteristics. J. Clean. Prod. 2016, 133, 1181–1189. [Google Scholar] [CrossRef]
- Safaripour, M.; Saidi, M.; Moradi, P. Ex-situ biodiesel production from Simmondsia chinensis (Jojoba) biomass: Process evaluation and optimization. J. Ind. Eng. Chem. 2023, 124, 392–401. [Google Scholar] [CrossRef]
- Kashyap, S.S.; Gogate, P.R.; Joshi, S.M. Ultrasound assisted intensified production of biodiesel from sustainable source as karanja oil using interesterification based on heterogeneous catalyst (Γ-alumina). Chem. Eng. Process.-Process Intensif. 2019, 136, 11–16. [Google Scholar] [CrossRef]
- Utami, M.; Setiawan, P.; Falah, I.I.; Suheryanto; Shidiq, M.; Wijaya, K.; Jarin, T.; Sumathijones, C.; Abd-Elkader, O.H.; Abd-Elkader, M.O.H.; et al. Synthesis of biodiesel from castor oil catalyzed by sodium hydroxide dispersed on bentonite. Sustain. Energy Technol. Assess. 2022, 53, 102526. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Investigation of kinetics, thermodynamics, and environmental factors of biodiesel generation from sunflower and castor oil using rice husk ash/CuO/K2CO3 heterogeneous catalyst. Environ. Technol. Innov. 2023, 32, 103307. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Baskar, G. Optimization and techno-economic analysis of biodiesel production from Calophyllum inophyllum oil using heterogeneous nanocatalyst. Bioresour. Technol. 2020, 315, 123852. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; César, A.D.S. Calophyllum inophyllum L.-A Prospective Non-Edible Biodiesel Feedstock. Study of Biodiesel Production, Properties, Fatty Acid Composition, Blending and Engine Performance. Renew. Sustain. Energy Rev. 2014, 37, 644–655. [Google Scholar] [CrossRef]
- Alsaiari, R.A.; Musa, E.M.; Rizk, M.A. Biodiesel production from date seed oil using hydroxyapatite-derived catalyst from waste camel bone. Heliyon 2023, 9, e15606. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Nalla, B.T.; Choubey, G.; Mishra, R.; Vellaiyan, S. Experimental analysis of Sterculia foetida biodiesel and butanol blends as a renewable and eco-friendly fuel. Ind. Crops Prod. 2022, 178, 114612. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Yusaf, T.F. Production of biodiesel from Sterculia foetida and its process optimization. Fuel 2013, 111, 478–484. [Google Scholar] [CrossRef]
- Farokhi, G.; Saidi, M.; Najafabadi, A.T. Application of spinel Type NixZn1−xFe2O4 magnetic nanocatalysts for biodiesel production from neem seed oil: Catalytic performance evaluation and optimization. Ind. Crops Prod. 2023, 192, 116035. [Google Scholar] [CrossRef]
- Badmus, J.A.; Oyelami, K.O.; Adedeji, A.L.; Adedosu, O.T.; Bolarinwa, I.F.; Marnewick, J.L. Comparative study of physicochemical properties, fatty acid composition, antioxidant and toxicological potential of Citrullus lanatus and Citrullus colocynthis seeds oils. S. Afr. J. Bot. 2021, 142, 156–164. [Google Scholar] [CrossRef]
- Ridwan, I.; Budiastuti, H.; Indarti, R.; Wahyuni, N.L.E.; Safitri, H.M.; Ramadhan, R.L. The optimization of tetrahydrofuran as a co-solvent on biodiesel production from rubber seeds using response surface methodology. Mater. Sci. Energy Technol. 2023, 6, 15–20. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Sembiring, R.W.; Shamsuddin, A.H. Intensification of Reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2019, 133, 520–527. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Sousa, R.M.F.; De Oliveira, A.; Morais, S.A.L.; Richter, E.M.; Muñoz, R.A.A. Moringa oleifera: A potential source for production of biodiesel and antioxidant additives. Fuel 2015, 146, 75–80. [Google Scholar] [CrossRef]
- Pham, L.N.; Luu, B.V.; Phuoc, H.D.; Le, H.N.T.; Truong, H.T.; Luu, P.D.; Furuta, M.; Imamura, K.; Maeda, Y. Production of biodiesel from candlenut oil using a two-step co-solvent method and evaluation of its gaseous emissions. J. Oleo Sci. 2018, 67, 617–626. [Google Scholar] [CrossRef]
- Baskar, G.; Gurugulladevi, A.; Nishanthini, T.; Aiswarya, R.; Tamilarasan, K. Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst. Renew. Energy 2017, 103, 641–646. [Google Scholar] [CrossRef]
- Hariram, V.; Prakash, S.; Seralathan, S.; Premkumar, T.M. Data set on optimized biodiesel production and formulation of emulsified Eucalyptus teriticornisis biodiesel for usage in compression ignition engine. Data Brief. 2018, 20, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Ahmad, M.; Rehan, M.; Saeed, M.; Lam, S.S.; Nizami, A.S.; Waseem, A.; Sultana, S.; Zafar, M. Production of high quality biodiesel from novel non-edible Raphnus raphanistrum L. seed oil using copper modified montmorillonite clay catalyst. Environ. Res. 2021, 193, 110398. [Google Scholar] [CrossRef]
- Islam, M.G.U.; Jan, M.T.; Farooq, M.; Naeem, A.; Khan, I.W.; Khattak, H.U. Biodiesel production from wild olive oil using TPA decorated Cr–Al acid heterogeneous catalyst. Chem. Eng. Res. Des. 2022, 178, 540–549. [Google Scholar] [CrossRef]
- Aguila, A.F.; Carlo, J.; Arellano, M.; Dowell, K.; Panganiban, D.; Magnaye, R.C. Production and evaluation of biodiesel from rambutan (Nephelium lappaceum L.) seed oil. Int. Res. J. Innov. Eng. Sci. Technol. 2020, 6, 25–31. [Google Scholar]
- Anggono, W.; Sutrisno, S.; Suprianto, F.D.; Setiyo, M.; Wibisono, R.; Gotama, G.J. Experimental investigation of the effect of Nephelium Lappaceum seed biodiesel to the automotive diesel engine performance. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristo, UK, 2019. [Google Scholar] [CrossRef]
- Rajan, A.G.; Sivasubramanian, M.; Gowthaman, S.; Ramkumar, P. Investigation of physical and chemical properties of tobacco seed oil fatty acid methyl ester for biodiesel production. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 7670–7675. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N.; Nabi, M.D.N. The Potential of Utilising Papaya Seed Oil and Stone Fruit Kernel Oil as Non-Edible Feedstock for Biodiesel Production in Australia—A Review. Energy Rep. 2019, 5, 280–297. [Google Scholar] [CrossRef]
- Reddy, S.R.; Sarangi, S.K. Optimizing the effect of using novel hydrogen enriched nano particles added emulsified waste mango seed biodiesel in diesel engine. Fuel 2023, 342, 127783. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M.; et al. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Puad, K.; Triwahyono, S.; Jalil, A.A.; Khayoon, M.S.; Atabani, A.E.; Ramli, Z.; Majid, Z.A.; Prasetyoko, D.; Hartanto, D. Transesterification of croton megalocarpus oil to biodiesel over WO3 supported on silica mesoporous-macroparticles catalyst. Chem. Eng. J. 2017, 316, 882–892. [Google Scholar] [CrossRef]
- Renish, R.R.; Selvam, M.A.J. A critical review on production process, physicochemical properties, performance and emission characteristics of sea mango biodiesel-diesel blends. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 2600–2605. [Google Scholar] [CrossRef]
- Gunawan, S.; Wasista, H.W.; Kuswandi, K.; Widjaja, A.; Ju, Y.H. The utilization of Xylocarpus moluccensis seed oil as biodiesel feedstock in Indonesia. Ind. Crops Prod. 2014, 52, 286–291. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, S. Production and characterization of biodiesel derived from hodgsonia macrocarpa seed oil. Appl. Energy 2015, 146, 135–140. [Google Scholar] [CrossRef]
- Hasni, K.; Ilham, Z.; Dharma, S.; Varman, M. Optimization of biodiesel production from Brucea javanica seeds oil as novel non-edible feedstock using response surface methodology. Energy Convers. Manag. 2017, 149, 392–400. [Google Scholar] [CrossRef]
- Bahadorian, A.; Sadrameli, S.M.; Pahlavanzadeh, H.; Kashkouli, M.N.I. Optimization study of linseed biodiesel production via in-situ transesterification and slow pyrolysis of obtained linseed residue. Renew. Energy 2023, 203, 10–19. [Google Scholar] [CrossRef]
- Balamurugan, S.; Gokul, C.; Dheen, S.A.T.; Eashwar, S.J.; Kumar, N.A. Application of grey relational analysis in biodiesel production from linseed oil using novel eggshell catalyst. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 1962–1969. [Google Scholar] [CrossRef]
- Shobhana-Gnanaserkhar; Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Sivasangar-Seenivasagam; Izham, S.M.; Taufiq-Yap, Y.H. Biodiesel production via simultaneous esterification and transesterification of chicken fat oil by mesoporous sulfated Ce supported activated carbon. Biomass Bioenergy 2020, 141, 105714. [Google Scholar] [CrossRef]
- García-Morales, R.; Zuniga-Moreno, A.; Veronico-Sanchez, F.J.; Domenzain-Gonzalez, J.; Prez-Lopez, H.I.; Bouchot, C.; Elizalde-Solis, O. Fatty acid methyl esters from waste beef tallow using supercritical methanol transesterification. Fuel 2022, 313, 122706. [Google Scholar] [CrossRef]
- Papargyriou, D.; Broumidis, E.; Vere-Tucker, M.D.; Gavrielides, S.; Hilditch, P.; Irvine, J.T.S.; Bonaccorso, A.D. Investigation of solid base catalysts for biodiesel production from fish oil. Renew. Energy 2019, 139, 661–669. [Google Scholar] [CrossRef]
- El-Shafay, A.S.; Ağbulut, Ü.; Attia, E.A.; Touileb, K.L.; Gad, M.S. Waste to energy: Production of poultry-based fat biodiesel and experimental assessment of its usability on engine behaviors. Energy 2023, 262, 125457. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sahu, H. Intensification of biodiesel production from waste goat tallow using infrared radiation: Process evaluation through response surface methodology and artificial neural network. Appl. Energy 2014, 114, 827–836. [Google Scholar] [CrossRef]
- Faleh, N.; Khila, Z.; Wahada, Z.; Pons, M.N.; Houas, A.; Hajjaji, N. Exergo-environmental life cycle assessment of biodiesel production from mutton tallow transesterification. Renew. Energy 2018, 127, 74–83. [Google Scholar] [CrossRef]
- Mutreja, V.; Singh, S.; Ali, A. Biodiesel from mutton fat using KOH impregnated MgO as heterogeneous catalysts. Renew. Energy 2011, 36, 2253–2258. [Google Scholar] [CrossRef]
- Suresh, T.; Sivarajasekar, N.; Balasubramani, K. Enhanced ultrasonic assisted biodiesel production from meat industry waste (pig tallow) using green copper oxide nanocatalyst: Comparison of response surface and neural network modelling. Renew. Energy 2021, 164, 897–907. [Google Scholar] [CrossRef]
- CEzekannagha, B.; Ude, C.N.; Onukwuli, O.D. Optimization of the methanolysis of lard oil in the production of biodiesel with response surface methodology. Egypt. J. Pet. 2017, 26, 1001–1011. [Google Scholar] [CrossRef]
- Oza, S.; Prajapati, N.; Kodgire, P.; Kachhwaha, S.S. An ultrasound-assisted process for the optimization of biodiesel production from waste cottonseed cooking oil using response surface methodology. Water-Energy Nexus 2021, 4, 187–198. [Google Scholar] [CrossRef]
- Ameen, F.; Mathivanan, K.; Zhang, R.; Ravi, G.; Rajasekar, S. One factor at a time and two-factor optimization of transesterification parameters through central composite design (CCD) for the conversion of used peanut oil (UPNO) to biodiesel. Fuel 2023, 352, 129065. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S.; Samart, C. Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renew. Energy 2019, 134, 125–134. [Google Scholar] [CrossRef]
- Loh, J.M.; Amelia; Gourich, W.; Chew, C.L.; Song, C.P.; Chan, E.S. Improved biodiesel production from sludge palm oil catalyzed by a low-cost liquid lipase under low-input process conditions. Renew. Energy 2021, 177, 348–358. [Google Scholar] [CrossRef]
- Matinja, A.I.; Zain, N.A.M.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA-alginate-sulfate beads. Renew. Energy 2019, 135, 1178–1185. [Google Scholar] [CrossRef]
- Rajesh, K.; Natarajan, M.P.; Devan, P.K.; Ponnuvel, S. Coconut fatty acid distillate as novel feedstock for biodiesel production and its characterization as a fuel for diesel engine. Renew. Energy 2021, 164, 1424–1435. [Google Scholar] [CrossRef]
- Vernier, L.J.; Nunes, A.L.B.; Albarello, M.; de Castilhos, F. Continuous production of fatty acid methyl esters from soybean oil deodorized distillate and methyl acetate at supercritical conditions. J. Supercrit. Fluids 2022, 186, 105603. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L. Biodiesel production from rapeseed deodorizer distillate in a packed column reactor. Chem. Eng. Process. Process Intensif. 2009, 48, 1152–1156. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Bangjang, T.; Kaewchada, A.; Jaree, A. Biodiesel production from rice bran oil fatty acid distillate via supercritical hydrolysis–esterification–transesterification in a microreactor. Energy Rep. 2023, 9, 5299–5305. [Google Scholar] [CrossRef]
- Heinzl, G.C.; Mota, D.A.; Martinis, V.; Martins, A.S.; Soares, C.M.F.; Osorio, N.; Gominho, J.; Nampoothiri, K.M.; Sukumaran, R.K.; Pereira, H.; et al. Integrated bioprocess for structured lipids, emulsifiers and biodiesel production using crude acidic olive pomace oils. Bioresour. Technol. 2022, 346, 126646. [Google Scholar] [CrossRef]
- Parandi, E.; Mousavi, M.; Kiani, H.; Nodeh, H.R.; Cho, J.; Rezania, S. Optimization of microreactor-intensified transesterification reaction of sesame cake oil (sesame waste) for biodiesel production using magnetically immobilized lipase nano-biocatalyst. Energy Convers. Manag. 2023, 295, 117616. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Hai, A.; Show, P.L.; Banat, F.; Manickam, S. Enhancing biodiesel production from waste date seed oil through ultrasonic-assisted optimization: A sustainable approach using non-edible feedstocks. Chem. Eng. Process. -Process Intensif. 2023, 194, 109601. [Google Scholar] [CrossRef]
- Jain, S. Biodiesel production from food waste using insitu transesterification method. Sustain. Energy Technol. Assess. 2023, 58, 103380. [Google Scholar] [CrossRef]
- Rajendran, N.; Kang, D.; Han, J.; Gurunathan, B. Process optimization, economic and environmental analysis of biodiesel production from food waste using a citrus fruit peel biochar catalyst. J. Clean. Prod. 2022, 365, 132712. [Google Scholar] [CrossRef]
- Alonazi, M.; Al-Diahan, S.K.; Alzahrani, Z.R.A.; Bacha, A.B. Combined immobilized lipases for effective biodiesel production from spent coffee grounds. Saudi J. Biol. Sci. 2023, 30, 103772. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, F.; Zhang, R.; Zhao, L.; Qi, J. Recent Progress on Biodiesel Production from Municipal Sewage Sludge; Elsevier Ltd.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Arazo, R.O.; de Luna, M.D.G.; Capareda, S.C. Assessing biodiesel production from sewage sludge-derived bio-oil. Biocatal. Agric. Biotechnol. 2017, 10, 189–196. [Google Scholar] [CrossRef]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Angkawijaya, A.E.; Irawaty, W.; Ju, Y.H.; Tran-Nguyen, P.L.; Hartono, S.B. Utilization of waste capiz shell-Based catalyst for the conversion of leather tanning waste into biodiesel. J. Environ. Chem. Eng. 2020, 8, 104012. [Google Scholar] [CrossRef]
- Hussain, S.B.; Muhammad, S.; Shah, U.; Nosheen, A. Sustainable Biodiesel Production via Chlorella vulgaris and Tetraselmis chuii in food-based brewery Industrial Wastewater. Waste Biomass Valorization, 2023in press. [CrossRef]
- Mostafa, S.S.M.; El-Gendy, N.S. Evaluation of fuel properties for microalgae Spirulina platensis bio-diesel and its blends with Egyptian petro-diesel. Arab. J. Chem. 2017, 10, S2040–S2050. [Google Scholar] [CrossRef]
- Vinitha, V.; Meignanalakshmi, S.; Tirumurugaan, K.G.; Sarathchandra, G.; Sundaram, S.M. Enhanced lipid production and analysis of properties of biodiesel produced from freshwater microalgae Scenedesmus obtusus ON089666.1. Bioresour. Technol. Rep. 2023, 21, 101286. [Google Scholar] [CrossRef]
- Shin, H.Y.; Shim, S.H.; Ryu, Y.J.; Yang, J.H.; Lim, S.M.; Lee, C.G. Lipid Extraction from Tetraselmis sp. Microalgae for Biodiesel Production Using Hexane-based Solvent Mixtures. Biotechnol. Bioprocess. Eng. 2018, 23, 16–22. [Google Scholar] [CrossRef]
- Khoobbakht, G.; Kheiralipour, K.; Karimi, M. Optimization of Chlamydomonas alga biodiesel percentage for reducing exhaust emission of diesel engine. Process Saf. Environ. Prot. 2021, 152, 25–36. [Google Scholar] [CrossRef]
- Das, V.; Dunford, N.; Deka, D. Waste utilization and biodiesel production from Desmodesmus maximus grown in swine wastewater using waste eggshells as a catalyst. Aquac. Eng. 2022, 99, 102293. [Google Scholar] [CrossRef]
- Bibi, F.; Ali, M.I.; Ahmad, M.; Bokhari, A.; Khoo, K.S.; Zafar, M.; Asif, S.; Mubashir, M.; Han, N.; Show, P.L. Production of lipids biosynthesis from Tetradesmus nygaardii microalgae as a feedstock for biodiesel production. Fuel 2022, 326, 124985. [Google Scholar] [CrossRef]
- Patel, A.; Gami, B.; Patel, P.; Patel, B. Biodiesel production from microalgae Dunaliella tertiolecta: A study on economic feasibility on large-scale cultivation systems. Biomass Convers Biorefin. 2023, 13, 1071–1085. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Knothe, G.; Williams, J.R.; Burlow, N.P.; Reddy, C.M. Decolorization improves the fuel properties of algal biodiesel from Isochrysis sp. Fuel 2016, 179, 229–234. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Murugesan, A.G.; Ameen, F.; Maneeruttanarungroj, C.; Wang, S. Efficient bioflocculation and biodiesel production of microalgae Asterococcus limneticus on streptomyces two-stage co-cultivation strategy. Biomass Bioenergy 2023, 175, 106886. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Martin, S.S.; Agurto, C.; Santos, M.A.; Freitas, M.A.V.; Caetano, N.S. Analyzing Phaeodactylum tricornutum lipid profile for biodiesel production. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 369–373. [Google Scholar] [CrossRef]
- Chaisutyakorn, P.; Praiboon, J.; Kaewsuralikhit, C. The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. J. Appl. Phycol. 2018, 30, 37–45. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Guo, D.; Zhang, Y.; Deng, Z.; Liu, T. Metabolic engineering of microbes for branched-chain biodiesel production with low-temperature property. Biotechnol. Biofuels 2015, 8, 92. [Google Scholar] [CrossRef]
- Haushalter, R.W.; Kim, W.; Chavkin, T.A.; The, L.; Garber, M.E.; Nhan, M.; Adamns, P.D.; Petzold, C.J.; Katz, L.; Keasling, J.D. Production of anteiso-branched fatty acids in Escherichia coli; next generation biofuels with improved cold-flow properties. Metab. Eng. 2014, 26, 111–118. [Google Scholar] [CrossRef]
- Bai, W.; Anthony, W.E.; Hartline, C.J.; Wang, S.; Wang, B.; Ning, J.; Hsu, F.F.; Dantas, G.; Zhang, F. Engineering diverse fatty acid compositions of phospholipids in Escherichia coli. Metab. Eng. 2022, 74, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-L.; Wang, Y.-Z.; Nong, F.-T.; Ma, W.; Huang, P.-W.; Sun, X.-M. Identification and characterization of fatty acid desaturases in Schizochytrium sp. HX-308. Algal Res. 2022, 67, 102861. [Google Scholar] [CrossRef]
- Jafarihaghighi, F.; Bahrami, H.; Ardjmand, M.; Mirzajanzadeh, M.; Haghighi, B.J.; Mahdavi, A. Comparing among second, third, and fourth generations (genetically modified) of biodiesel feedstocks from the perspective of engine, exhaust gasses and fatty acid: Comparative assessment. Clean. Chem. Eng. 2022, 2, 100025. [Google Scholar] [CrossRef]
- Vollmann, J.; Eynck, C. Camelina as a sustainable oilseed crop: Contributions of plant breeding and genetic engineering. Biotechnol. J. 2015, 10, 525–535. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD); Food and Agriculture Organization (FAO). OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD); Food and Agriculture Organization (FAO). OECD Agriculture Statistics (database). In OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Ministry of Energy and Mineral Resources of the Republic of Indonesia. FAQ: Program Mandatori Biodiesel 30% (Mandatory Program of Biodiesel 30%). Available online: https://ebtke.esdm.go.id/post/2019/12/19/2434/faq.program.mandatori.biodiesel.30.b30?lang=en (accessed on 25 June 2024).
- Ministry of Energy and Mineral Resources of the Republic of Indonesia. Program Bahan Bakar Nabati B35 Siap Implementasi Mulai 1 Februari 2023 (Program of Biofuel B35 is Ready for Implementation Starting 1st February 2023). Available online: https://ebtke.esdm.go.id/post/2023/01/09/3395/program.bahan.bakar.nabati.b35.siap.implementasi.mulai.1.februari.2023 (accessed on 25 June 2024).
- Ministry of Energy and Mineral Resources of the Republic of Indonesia. Persiapan Pelaksanaan Mandatori Biodiesel B40 (Preparation for Mandatory Implementation of Biodiesel B40). Available online: https://www.esdm.go.id/id/berita-unit/direktorat-jenderal-ebtke/persiapan-pelaksanaan-mandatori-biodiesel-b40 (accessed on 25 June 2024).
- Lanjekar, R.D.; Deshmukh, D. A Review of the Effect of the Composition of Biodiesel on NOx Emission, Oxidative Stability and Cold Flow Properties; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Directorate General of New Renewable Energy and Energy Conservation, Ministry of Energy and Mineral Resources of the Republic of Indonesia. Standar dan Mutu (Spesifikasi) Bahan Bakar Nabati Jenis Biodiesel Sebagai bahan Bakar Lain yang Dipasarkan di Dalam Negeri. Available online: https://ebtke.esdm.go.id/post/2022/12/15/3376/telah.terbit.keputusan.dirjen.ebtke.nomor.195.kek.05dje2022.tentang.standard.dan.mutu.spesifikasi.bahan.bakar.nabati.jenis.biodiesel.sebagai.bahan.bakar.lain.yang.dipasarkan.di.dalam.negeri (accessed on 22 June 2024).
- Wen, P.Y. Process Synthesis and Optimisation for Multi-Biodiesel Mixture Production. Ph.D. Thesis, UTAR, Petaling Jaya, Malaysia, 2020. [Google Scholar]
- Ministry of Agriculture Livestock and Food Supply. Uses for Biodiesel in Brazil and Globally, 1st ed.; Livestock and Food Supply of Brazil; Ministry of Agriculture: Brasilia, Brazil, 2015. [Google Scholar]
- Lakshmi, G.; Rao, N.; Ramadhas, A.S.; Nallusamy, N.; Sakthivel, P. Relationships among the physical properties of biodiesel and engine fuel system design requirement. Int. J. Energy Environ. 2010, 1, 919–926. [Google Scholar]
- Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A. Methods for Improving the Cold Flow Properties of Biodiesel with High Saturated Fatty Acids Content: A Review. Renew. Sustain. Energy Rev. 2017, 54, 1401–1411. [Google Scholar] [CrossRef]
- Rasimoglu, N.; Temur, H. Cold flow properties of biodiesel obtained from corn oil. Energy 2014, 68, 57–60. [Google Scholar] [CrossRef]
- Ali, O.; Yusaf, T.; Mamat, R.; Abdullah, N.; Abdullah, A. Influence of Chemical Blends on Palm Oil Methyl Esters’ Cold Flow Properties and Fuel Characteristics. Energies 2014, 7, 4364–4380. [Google Scholar] [CrossRef]
- Giraldo, S.Y.; Rios, L.A.; Suárez, N. Comparison of glycerol ketals, glycerol acetates and branched alcohol-derived fatty esters as cold-flow improvers for palm biodiesel. Fuel 2013, 108, 709–714. [Google Scholar] [CrossRef]
- Soriano, N.U.; Migo, V.P.; Matsumura, M. Ozonized vegetable oil as pour point depressant for neat biodiesel. Fuel 2006, 85, 25–31. [Google Scholar] [CrossRef]
- Abe, M.; Hirata, S.; Komatsu, H.; Yamagiwa, K.; Tajima, H. Thermodynamic selection of effective additives to improve the cloud point of biodiesel fuels. Fuel 2016, 171, 94–100. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Wei, Z.; Dai, B.; Han, S. Synthesis and evaluation of bifunctional polymeric agent for improving cold flow properties and oxidation stability of diesel-biodiesel blends. Renew. Energy 2022, 196, 737–748. [Google Scholar] [CrossRef]
- Islam, M.M.; Hassan, M.H.; Kalam, M.A.; Zulkifli, N.W.B.M.; Habibullah, M.; Hossain, M.M. Improvement of cold flow properties of Cocos nucifera and Calophyllum inophyllum biodiesel blends using polymethyl acrylate additive. J. Clean. Prod. 2016, 137, 322–329. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Han, S. Effect of polymeric cold flow improvers on flow properties of biodiesel from waste cooking oil. Fuel 2014, 117, 876–881. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Z.; Xu, G.; Lian, X.; Yang, C.; Zhao, W.; Ma, P.; Lin, H.; Han, S. Effect of poly-alpha-olefin pour point depressant on cold flow properties of waste cooking oil biodiesel blends. Fuel 2016, 184, 110–117. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. A Comprehensive Evaluation of the Melting Points of Fatty Acids and Esters Determined by Differential Scanning Calorimetry. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Amran, N.A.; Bello, U.; Ruslan, M.S.H. The Role of Antioxidants in Improving Biodiesel’s Oxidative Stability, Poor Cold Flow Properties, and the Effects of the duo on Engine Performance: A Review. Heliyon 2022, 8, e09846. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Impact of Cold Flow Properties of Biodiesel on Engine Performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Smith, P.C.; Ngothai, Y.; Nguyen, Q.D.; O’Neill, B.K. Improving the low-temperature properties of biodiesel: Methods and consequences. Renew. Energy 2010, 35, 1145–1151. [Google Scholar] [CrossRef]
- Dunn, R.O.; Ngo, H.L.; Haas, M.J. Branched-chain fatty acid methyl esters as cold flow improvers for biodiesel. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 853–869. [Google Scholar] [CrossRef]
- Moser, B.R. Fuel property enhancement of biodiesel fuels from common and alternative feedstocks via complementary blending. Renew. Energy 2016, 85, 819–825. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef]
- Makarevičienė, V.; Kazancev, K.; Kazanceva, I. Possibilities for improving the cold flow properties of biodiesel fuel by blending with butanol. Renew. Energy 2015, 75, 805–807. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-P.; Park, S.-C.; Kim, Y.-J.; Lee, J.-S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef]
- Nainwal, S.; Sharma, N.; Sharma, A.S.; Jain, S.; Jain, S. Cold flow properties improvement of Jatropha curcas biodiesel and waste cooking oil biodiesel using winterization and blending. Energy 2015, 89, 702–707. [Google Scholar] [CrossRef]
- Niyas, M.M.; Shaija, A. Performance evaluation of diesel engine using biodiesels from waste coconut, sunflower, and palm cooking oils, and their hybrids. Sustain. Energy Technol. Assess. 2022, 53, 102681. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Env. Sci. 2009, 2, 759. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Effect of winterization and plant phenolic-additives on the cold-flow properties and oxidative stability of Karanja biodiesel. Fuel 2020, 262, 116631. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Zhao, M.; Kuang, L.; Nie, J.; Riley, W.W. Improving the cold flow properties of biodiesel from waste cooking oil by surfactants and detergent fractionation. Fuel 2011, 90, 1036–1040. [Google Scholar] [CrossRef]
- Abe, M.; Nakamura, R.; Komatsu, H.; Yamagiwa, K.; Tajima, H. Effect of additive structure on the performance of biodiesel fuel winterization. Fuel 2021, 289, 119747. [Google Scholar] [CrossRef]
- Min, H.Y.; Amran, N.A.; Samsuri, S. Winterization of biodiesel through progressive freezing for cold flow properties improvement. IOP Conf. Ser. Earth Env. Sci. 2019, 268, 012096. [Google Scholar] [CrossRef]
- Tajima, H.; Abe, M.; Komatsu, H.; Yamagiwa, K. Feasibility of additive winterization of biodiesel fuel derived from various eatable oils and fat. Fuel 2021, 305, 121479. [Google Scholar] [CrossRef]
- Dunn, R.O.; Shockley, M.W.; Bagby, M.O. Winterized Methyl Esters from Soybean Oil: An Alternative Diesel Fuel With Improved Low-Temperature Flow Properties. SAE Trans. 1997, 106, 640–649. [Google Scholar]
- Pérez, Á.; Casas, A.; Fernández, C.M.; Ramos, M.J.; Rodríguez, L. Winterization of peanut biodiesel to improve the cold flow properties. Bioresour. Technol. 2010, 101, 7375–7381. [Google Scholar] [CrossRef] [PubMed]
- Dimian, A.C.; Omota, F.; Kiss, A.A. Process for Fatty Acid Methyl Esters by Dual Reactive Distillation. Comput. Aided Chem. Eng. 2007, 24, 1307–1312. [Google Scholar] [CrossRef]
- Huang, R.; Cheng, J.; Song, W.; Qiu, Y.; Guo, H.; Yang, W. Physicochemical characterizations of microalgal methyl esters extracted with hexane and refined by vacuum distillation at different temperatures. Fuel 2021, 297, 120779. [Google Scholar] [CrossRef]
- Yeong, S.P.; Chan, Y.S.; Law, M.C.; Ling, J.K.U. Improving cold flow properties of palm fatty acid distillate biodiesel through vacuum distillation. J. Bioresour. Bioprod. 2022, 7, 43–51. [Google Scholar] [CrossRef]
- Niculescu, R.; Năstase, M.; Clenci, A. On the determination of the distillation curve of fatty acid methyl esters by gas chromatography. Fuel 2022, 314, 123143. [Google Scholar] [CrossRef]
- Iakovlieva, A.; Boichenko, S.; Lejda, K.; Vovk, O.; Shkilniuk, I. Vacuum Distillation of Rapeseed Oil Esters for Production of Jet Fuel Bio-Additives. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 363–370. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Li, Y. Effect of biodiesel ester structure optimization on low temperature performance and oxidation stability. J. Mater. Res. Technol. 2020, 9, 2727–2736. [Google Scholar] [CrossRef]
- Kinoshita, E.; Ueda, Y.; Takata, S. Combustion Characteristics of a DI Diesel Engine with Palm Oil Butyl and Isobutyl Esters. In SAE Technical Paper; SAE: Warrendale, PA, USA, 2011; p. 1937. [Google Scholar] [CrossRef]
- Yao, L.; Hammond, E.; Wang, T. Melting Points and Viscosities of Fatty Acid Esters that are Potential Targets for Engineered Oilseed. J. Am. Oil Chem. Soc. 2008, 85, 77–82. [Google Scholar] [CrossRef]
- Bouaid, A.; El boulifi, N.; Hahati, K.; Martinez, M.; Aracil, J. Biodiesel production from biobutanol. Improvement of cold flow properties. Chem. Eng. J. 2014, 238, 234–241. [Google Scholar] [CrossRef]
- Benjumea, P.N.; Agudelo, J.R.; Ríos, L.A. Cold flow properties of palm oil biodiesel. Rev. Fac. De. Ing. Univ. De. Antioq. 2014, 42, 94–104. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Tongcumpou, C.; Wiesenborn, D. Optimization of alcoholic soybean oil extraction as a step towards developing in-situ transesterification for fatty acid isopropyl esters. Ind. Crops Prod. 2016, 94, 189–196. [Google Scholar] [CrossRef]
- Lee, I.; Johnson, L.A.; Hammond, E.G. Use of branched-chain esters to reduce the crystallization temperature of biodiesel. J. Am. Oil Chem. Soc. 1995, 72, 1155–1160. [Google Scholar] [CrossRef]
- Nie, J.; Shen, J.; Shim, Y.Y.; Tse, T.J.; Reaney, M.J.T. Synthesis of Trimethylolpropane Esters by Base-Catalyzed Transesterification. Eur. J. Lipid Sci. Technol. 2020, 122, 1900207. [Google Scholar] [CrossRef]
- Cavalcante, I.M.; Rocha, N.R.D.C.; Maier, M.E.; de Lima, A.P.D.; Neto, D.M.A.; de Brito, D.H.A.; Petzhold, C.L.; Schanz, M.T.G.F.; Ricardo, N.M.P.S. Synthesis and characterization of new esters of oleic acid and glycerol analogues as potential lubricants. Ind. Crops Prod. 2014, 62, 453–459. [Google Scholar] [CrossRef]
- Yunus, R.; Fakhru’l-Razi, A.; Ooi, T.L.; Omar, R.; Idris, A. Synthesis of palm oil based trimethylolpropane esters with improved pour points. Ind. Eng. Chem. Res. 2005, 44, 8178–8183. [Google Scholar] [CrossRef]
- Sripada, P.K.; Sharma, R.V.; Dalai, A.K. Comparative study of tribological properties of trimethylolpropane-based biolubricants derived from methyl oleate and canola biodiesel. Ind. Crops Prod. 2013, 50, 95–103. [Google Scholar] [CrossRef]
- Bahadi, M.; Salimon, J.; Derawi, D. Synthesis of di-trimethylolpropane tetraester-based biolubricant from Elaeis guineensis kernel oil via homogeneous acid-catalyzed transesterification. Renew. Energy 2021, 171, 981–993. [Google Scholar] [CrossRef]
- Wang, E.; Ma, X.; Tang, S.; Yan, R.; Wang, Y.; Riley, W.W.; Reaney, M.J.T. Synthesis and oxidative stability of trimethylolpropane fatty acid triester as a biolubricant base oil from waste cooking oil. Biomass Bioenergy 2014, 66, 371–378. [Google Scholar] [CrossRef]
- Macias, E.E.; Deshmane, C.A.; Jasinski, J.B.; Carreon, M.A.; Ratnasamy, P. Catalytic transformations of methyl oleate and biodiesel over mesoporous gallium–niobium oxides. Catal. Commun. 2011, 12, 644–650. [Google Scholar] [CrossRef]
- Knothe, G. A comprehensive evaluation of the cetane numbers of fatty acid methyl esters. Fuel 2014, 119, 6–13. [Google Scholar] [CrossRef]
- Maghrebi, R.; Buffi, M.; Bondioli, P.; Chiaramonti, D. Isomerization of long-chain fatty acids and long-chain hydrocarbons: A review. Renew. Sustain. Energy Rev. 2021, 149, 111264. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Smith, J.C.; Wilson, J.R.; Salley, S.O.; Ng, K.Y.S. Investigation of the Parameters Affecting the Cetane Number of Biodiesel. J. Am. Oil Chem. Soc. 2008, 85, 1073–1081. [Google Scholar] [CrossRef]
- Reaume, S.; Ellis, N. Use of Isomerization and Hydroisomerization Reactions to Improve the Cold Flow Properties of Vegetable Oil Based Biodiesel. Energies 2013, 6, 619–633. [Google Scholar] [CrossRef]
- Yori, J.C.; D’Amato, M.A.; Grau, J.M.; Pieck, C.L.; Vera, C.R. Depression of the Cloud Point of Biodiesel by Reaction over Solid Acids. Energy Fuels 2006, 20, 2721–2726. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Use of hydroisomerization to reduce the cloud point of saturated fatty acids and methyl esters used in biodiesel production. Biomass Bioenergy 2013, 49, 188–196. [Google Scholar] [CrossRef]
- Ngo, H.L.; Dunn, R.O.; Hoh, E. C18-unsaturated branched-chain fatty acid isomers: Characterization and physical properties. Eur. J. Lipid Sci. Technol. 2013, 115, 676–683. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Optimizing Reaction Conditions for the Isomerization of Fatty Acids and Fatty Acid Methyl Esters to Their Branch Chain Products. J. Am. Oil Chem. Soc. 2011, 88, 661–671. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Synergistic Effects of Skeletal Isomerization on Oleic and Palmitic Acid Mixtures for the Reduction in Cloud Point of Their Methyl Esters. Energy Fuels 2012, 26, 4514–4520. [Google Scholar] [CrossRef]
- Misra, P.; Alvarez-Majmutov, A.; Chen, J. Isomerization catalysts and technologies for biorefining: Opportunities for producing sustainable aviation fuels. Fuel 2023, 351, 128994. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Li, H.; Jiang, S.; Zhou, W. Cold Flow Properties of Biodiesel and the Improvement Methods: A Review. Energy Fuels 2020, 34, 10364–10383. [Google Scholar] [CrossRef]
- Smith, P.C.; O’Neill, B.K.; Ngothai, Y.; Nguyen, Q.D. Butoxylation of Butyl Biodiesel: Reaction Conditions and Cloud Point Impact. Energy Fuels 2009, 23, 3798–3803. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Salley, S.O.; Ng, K.Y.S. Effects of partial hydrogenation, epoxidation, and hydroxylation on the fuel properties of fatty acid methyl esters. Fuel Process. Technol. 2009, 90, 1292–1299. [Google Scholar] [CrossRef]
- Smith, P.C.; Ngothai, Y.; Nguyen, Q.D.; O’Neill, B.K. The addition of alkoxy side-chains to biodiesel and the impact on flow properties. Fuel 2010, 89, 3517–3522. [Google Scholar] [CrossRef]
- Smith, P.C.; Ngothai, Y.; Nguyen, Q.D.; O’Neill, B.K. Alkoxylation of biodiesel and its impact on low-temperature properties. Fuel 2009, 88, 605–612. [Google Scholar] [CrossRef]
- Mushtaq, M.; Tan, I.M.; Nadeem, M.; Devi, C.; Lee, S.Y.C.; Sagir, M. A Convenient Route For The Alkoxylation Of Biodiesel And Its Influence On Cold Flow Properties. Int. J. Green. Energy 2014, 11, 267–279. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Hydroxylation and hexanoylation of epoxidized waste cooking oil and epoxidized waste cooking oil methyl esters: Process optimization and physico-chemical characterization. Ind. Crops Prod. 2019, 133, 151–159. [Google Scholar] [CrossRef]
- Moser, B.R.; Erhan, S.Z. πSynthesis and evaluation of a series of α-hydroxy ethers derived from isopropyl oleate. J. Am. Oil Chem. Soc. 2006, 83, 959–963. [Google Scholar] [CrossRef]
- Weitkamp, J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem 2012, 4, 292–306. [Google Scholar] [CrossRef]
- Burch, K.J.; Whitehead, E.G. Melting-Point Models of Alkanes. J. Chem. Eng. Data 2004, 49, 858–863. [Google Scholar] [CrossRef]
- Li, H.; Shen, B.X.; Yu, P.H. The Cold Temperature Fluidities of Biodiesel Prepared from Vegetable Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1195–1200. [Google Scholar] [CrossRef]
- Seames, W.; Luo, Y.; Ahmed, I.; Aulich, T.; Kubatova, A.; Stavova, J.; Kozliak, E. The thermal cracking of canola and soybean methyl esters: Improvement of cold flow properties. Biomass Bioenergy 2010, 34, 939–946. [Google Scholar] [CrossRef]
- Luo, Y.; Ahmed, I.; Kubatova, A.; Stavova, J.; Aulich, T.; Sadrameli, S.M.; Seames, W.S. The thermal cracking of soybean/canola oils and their methyl esters. Fuel Process. Technol. 2010, 91, 613–617. [Google Scholar] [CrossRef]
- Kraic, J.; Mihalik, D.; Klcova, L.; Gubisova, M.; Klempova, T.; Hudcovicova, M.; Ondreickova, K.; Mrkvova, M.; Havrlentova, M.; Gubis, J.; et al. Progress in the genetic engineering of cereals to produce essential polyunsaturated fatty acids. J. Biotechnol. 2018, 284, 115–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).