Abstract
A reduction in greenhouse gases has become an inescapable requirement. An effective scenario for achieving carbon neutrality is to develop a hydrogen economy. Its success, however, requires strict control of the different processes involved in planned hydrogen chains. The energy chain considered in this paper is a stationary application which involves the production of hydrogen by electrolysis (a power-to gas process) and its combustion in gas turbine combined cycles to generate electricity (a gas-to-power process). In such applications, the need is twofold: (i) to control the risk of explosive atmospheres by performing safe gas detection in the presence of hydrogen and (ii) to secure the reliability of all chain processes using hydrogen-rich gases by achieving reliable analyses of these gases. This paper is dedicated to the development of hydrogen energy to decarbonize the thermal production of electricity. We will first describe the hydrogen chain that would best suit the power generation sector. Then, we will highlight the properties of hydrogen that are critical for its reliable operation. Finally, we will review the sensing technologies suitable for hydrogen-containing fuels. This review paper was published as part of a Joint Industrial Project (JIP) aimed at enabling the safe and reliable deployment of hydrogen energy.
1. Introduction
By escaping the carbon cycle, hydrogen energy offers drastic solutions to contemporary environmental problems. Among them, the most critical is climate change caused by greenhouse gases (CO2; CH4; other light hydrocarbons; etc.). However, using hydrogen as transportation fuel would also eliminate deleterious pollution caused by carbon monoxide and sulfur oxides, as well as unburned hydrocarbons and volatile organic compounds (VOCs) which are harmful to health and precursors of noxious ozone.
The dihydrogen molecule (H2), more commonly called hydrogen, is the smallest and lightest molecule on Earth. It is a possible energy carrier (or vector) rather than a primary energy, as there are very scarce deposits thereof; these are qualified as “natural” or “white” hydrogen.
The main supply of hydrogen is currently from fossil fuels via two major routes, which are (i) the steam reforming of natural gas that yields “blue” or “grey” H2, depending on whether the resultant CO2 is captured or not, and (ii) the gasification of coal which leads to “black” (or “brown”) H2. These processes have large CO2 footprints. However, an increasing number of projects are conducted or planned to produce H2 by the electrolytic splitting of water into H2 and O2, using either solar power (“yellow” H2), wind/hydro power (“green” H2), or nuclear power generated during off-peak hours (“pink” or “purple” H2), e.g., using “Gen IV” nuclear reactors. There are currently many such power-to-gas projects implemented or under study to produce hydrogen, giving rise to what is often referred to as the “H2 rainbow” (Figure 1) [1].
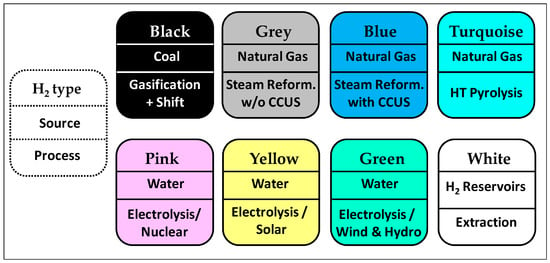
Figure 1.
The “H2 rainbow”: the various types and production routes of dihydrogen [1].
Facilities likely to use the dihydrogen generated in these projects include combustion plants, which correspond to gas-to-power systems. Since its combustion is clean and free of CO2 emissions, this unique gas could substitute for fossil fuels and offer drastic solutions to major environmental issues. At first, it would provide a radical solution to endemic urban pollution by eliminating most noxious emissions (VOCs, CO, SOx, ozone, PAH, and soot particles); but, above all, it would open the sought route towards carbon neutrality.
Although a sequence of power to gas and gas to power does not seem the best option from a simple thermodynamic point of view, this path is becoming desirable due to recent evolutions of electrical networks. Indeed, the strong development of renewable energies (PV and wind) in many world regions generates power generation peaks at certain time windows of the day. Because large production nodes (nuclear or steam power plants) are not able to shut down and restart on demand, this results in overproduction and, increasingly, to negative electricity prices. In this context, the availability of power-to-gas facilities absorbing these electricity surpluses to produce dihydrogen takes on its full meaning [2,3].
This is why initiatives devoted to the creation of hydrogen energy chains, from production, storage, and transport to end-uses, are blossoming worldwide. Figure 2 provides an overview of the number and sizes of recently launched projects around the world [4].
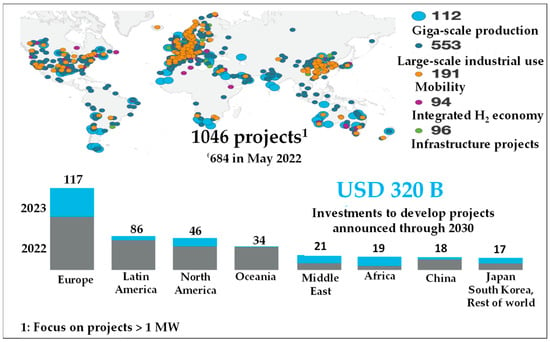
Figure 2.
Evolution of hydrogen deployment projects around the world; source: project and investment tracker, as of 31 January 2023 [4].
Now, hydrogen is considered a key priority to achieve carbon neutrality. For example, the EU environmental agenda (“REPowerEU” plan) includes the following important projects: 1—the objective of producing 6 GW of renewable hydrogen this year; 2—the perspective for 40 GW by 2030; and 3—reaching carbon neutrality by 2050.
Every hydrogen chain involves a set of very distinct links, namely the following: (i) production; (ii) compression/storage; (iii) transportation/distribution; and (iv) a final process which achieves the intended end-use. Currently, the main markets of industrial hydrogen are the chemical, oil, and petrochemical sectors (ammonia and methanol syntheses; oil and naphtha reforming/cracking; fuel desulfurization) and to a much lesser extent aerospace propulsion. But, in the future, hydrogen is expected to bring major benefits to the industry, mobility, and electricity sectors, all of which suffer from their strong “addiction” to fossil fuels.
In industry, hydrogen is going to displace fossils, for example, in the sectors of steel making and ammonia and e-fuel synthesis.
In the field of mobility, H2 can be either used directly as a fuel delivered to the combustion chambers of engines or indirectly as a primary energy for fuel cells that convert it into electricity before mechanical power is generated. There is a large corpus of literature dedicated to this important application [5,6,7].
As this paper will focus on the production of electricity, i.e., on gas-to-power projects, the end-use will be industrial combustion equipment that will be the heat source for power cycles (Brayton, Rankine, or Diesel).
Regardless of the type of end-use, the transition to hydrogen economies requires high investments to create the necessary infrastructures. In addition, it must overcome several technological obstacles. In particular, hydrogen has specific physical and chemical properties which have an impact on the management of the safety and reliability of the entire process chain and requires rigorous checking of the gas sensors. In this article, the words “sensing” and “sensor” applied to pure hydrogen and hydrogen–hydrocarbon mixtures will be used in their broad senses and will cover the needs for both operation safety and process control. Operation safety requires monitoring the concentrations in the air of flammable gases in relation to their explosive limits, a duty which is traditionally called “gas detection”. In this article, the notion of “process control” is understood as the means for correctly monitoring the composition of the gas, which is essential for the correct operation of the different links in the chain, namely electrolysis, transport, and combustion. Process control requires access to the concentrations of all critical constituents of the processed gas, including here hydrogen, which practically means a full compositional analysis. In this regard, it must be noted that most energy transition scenarios involve mixing hydrogen with natural gas and that the fast and accurate analysis of hydrogen–hydrocarbon mixtures remains a delicate metrology application. This important point will be discussed in Section 3.
The main structure of this article will be as follows:
- -
- The stakes of replacing fossil fuels with hydrogen in the power generation sector, which is the focus of this article, will be highlighted in Section 2.
- -
- Section 3 will present the safety and reliability aspects of processes involving hydrogen.
- -
- The review of sensing techniques will be the subject of Section 4, in which we will evaluate the performances of the sensor candidates with the greatest potential, based on industrial metrological standards, namely in terms of reproducibility, response/recovery times, and reliability.
2. Electric Power Generation: A Key Sector to Implement Energy Chains
Apart from nuclear plants, most thermal power units run on fossil fuels. The power generation sector thus represents a space of choice for the deployment of hydrogen energy chains.
2.1. Deployment Strategies
The generally favored strategy for displacing fossil fuels is to mix H2 with natural gas (“NG”), leading to so-called “H2NG blends” (abbreviated to “H2NG”) that will fuel new or old power plants, which are based on gas turbines, boilers, or even gas engines [8,9,10,11,12,13].
A particularly interesting scenario, as illustrated by Figure 3 [10], lies in the joint deployment of (i) electrolysis units for the production of (green, yellow or pink) hydrogen, (ii) its transportation using existing networks, and (iii) its combustion in power plants, especially in gas turbine combined cycles (GTCCs) or cogeneration units, as these two types of installation today offer the best energy conversion efficiencies.
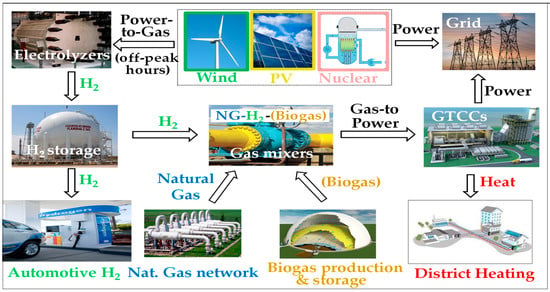
Figure 3.
Projection of possible production/transportation/use H2 chains within a power-to-gas/gas-to-power scenario.
2.2. Main Components of a Hydrogen-Based Energy Chain Intended for Power Generation
The different equipment pieces of a hydrogen-based power chain have distinct safety and reliability needs.
2.2.1. The Electrolyzer
Electrolysis is a process that splits water into dihydrogen and dioxygen using a direct electrical current. The main components of an electrolyzer are (i) a pair of electrodes consisting of a cathode and an anode on which H2 and O2 are, respectively, produced through electrochemical redox reactions, (ii) an electrolyte which contains ions and carries the electric current between the electrodes, and (iii) an external electrical source which powers the electrochemical reactions [14,15,16].
Today, there are several industrial processes, based namely on alkaline media, proton exchange membranes (PEMs), anion exchange membranes (AEMs), and solid oxides. This article will focus on alkaline electrolysis using, e.g., potassium hydroxide (KOH) as the electrolyte, which is currently the most widespread technology.
The dihydrogen and dioxygen molecules are released as gases from their respective electrodes, being separated by a diaphragm; they are then compressed and stored. Highly efficient separation is mandatory to obtain the purest possible H2 and O2 products. However, they can be contaminated by traces of water and consequently by traces of electrolyte, resulting in potentially alkaline and therefore corrosive gas streams.
In safety terms, it is necessary to have H2 sensors capable of detecting leaks at the level of 200 ppm and above 4% v/v which is the Lower Explosive Limit (LEL) of H2, with response/recovery times faster than 10 s. In terms of product quality (and therefore of process control), the manufacturers and users of electrolyzers must also tightly control the purity, i.e., the composition of the H2 and O2 products. More specifically, the control of the commercial purity of H2 requires a detection limit better than 10 ppm of non-hydrogen species, with a precision of 5–10 ppm.
2.2.2. The Transportation Networks
The transport of hydrogen through pipeline networks gives rise to large-scale industrial plans, such as the European Hydrogen Backbone (EHB) initiative [17] and the National Clean Hydrogen Strategy of the US DOE [18].
Three options exist: (1) to create new pipeline networks dedicated to hydrogen; (2) to convert existing NG pipelines to transport pure H2; and (3) to adapt existing networks to transport H2NG streams, which is by far the most feasible, economical, and rapid strategy [8,19,20,21]. Noteworthily, as an indicator of feasibility, about 2600 km of hydrogen pipelines are already in operation in the US [22].
Whatever option is chosen, the safety of this chain link requires tighter monitoring of H2 leaks that can in particular occur at the flanges between pipes, due to its high diffusivity. Additionally, since most gas pipelines are buried, sensing systems must be capable of handling gas samples originating from underground gas streams having varying humidity and temperature levels (possibly down to −20 °C in northern regions) and pressures (up to 90 bars in current NG networks). Leak detection equipment on the ground (e.g., by mobile gas chromatographs) or in the air (e.g., by LiDAR and LiDAR-DIAL systems) [23] must be adapted to hydrogen. Detailed compositional analyses that are carried out at surface stations must also be adapted to H2NG.
H2 pipelines will feed compressed hydrogen (also called “CH2”) to tank stations; remote consumers are supplied via hydrogen tanks, compressed hydrogen tube trailers, liquid hydrogen (LH2) trailers, or LH2 tank trucks. Here again, proper gas sensing will be needed.
2.2.3. The Production of Electric Power
The power generation units are the end-users of such hydrogen energy chains.
Gas turbines (GTs) can combust a wide variety of fuels, including pure and blended H2. In addition, they boast high energy effectiveness when installed in combined cycles (GTCCs) or cogeneration units, as well as fast site assembly and commissioning [10]. Therefore, GTs are placed at the forefront to promote the deployment of hydrogen energy across the power generation sector [9,24]. This is why this paper will focus on these machines as paradigmatic hydrogen end-users.
The specific properties of hydrogen largely impact the design of GTs intended to burn H2 or H2NG blends.
At first, from an energy standpoint, hydrogen is a “double-faceted” fuel. Indeed, Table 1 shows that it can be categorized either as Low Calorific Value (LCV), on a volume basis, or as High Calorific Value (HCV), on a mass basis, since its Lower Heating Value (LHV) is 120.7 MJ per kg but hardly 10.8 MJ per Nm3 due to its low density [13,25,26]. This causes a mixed HCV/LCV behavior which must be considered when designing combustion systems (piping; manifolds; combustors; etc.). These technical aspects will be covered in Section 3.

Table 1.
Low Heating Value (LHV) of H2 compared to CH4 [27].
3. Essential Safety and Reliability Considerations
3.1. Hydrogen Safety
Product and operation safety is a sine qua non condition for the success of any hydrogen chain [28,29,30,31,32,33].
This section will attempt to generically cover the safety of all processes involved in the chain. Safety guidelines are prescribed by international organizations such as the European Hydrogen Safety Panel [34], the CHS (Center for Hydrogen Safety) [35], and, more particularly, by the ISO 21789:2022 standard for gas turbines [36]. The essential duty is to avoid uncontrolled inflammation events, which requires special attention due to the unique properties of lightness, diffusivity, inflammability, and combustion behavior of dihydrogen molecules.
In practice, operation safety is achieved by continuously controlling the concentration of the flammable gas so that it is substantially lower than its Lower Explosive Limit (LEL). For this, there are two approaches:
- -
- One can perform a compositional analysis of the gas, i.e., determine the concentrations of all its flammable constituents and use the so-called “Le Chatelier’s rule” to calculate the resulting LEL of the mixture; this rule is set out in Appendix A attached to this paper.
- -
- Alternatively, one can use catalytic detector devices (see Section 4.5.2 below), which directly give the LEL of the gas without the need for compositional analysis.
Turning now to applications involving hydrogen, Table 2 shows the striking properties of the H2 molecule that need to be carefully considered, the CH4 molecule being taken as a reference:
- -
- Hydrogen density is nearly eight times lower, and its diffusivity in the air is about three times larger; both properties induce a risk of forming H2 clouds in potential stagnation zones located at height.
- -
- It has an exceptionally large explosivity range (from 4 to 75% in the air) and a low ignition energy (0.018 mJ), two factors which increase the inflammation risk in the case of leaks.
- -
- Hydrogen flames are not visible because they do not emit in the visible spectrum, which poses a particular risk during fire interventions but prevents the spread of fire by radiant heating.
- -
- Finally, as regards the combustion process in gas turbines, the very high flame speed of hydrogen tends to generate “flashbacks”, i.e., flame retro-propagation inside the combustors, with the risk of inflicting severe damage to machine components. It should be noted that, although laminar flames behave very differently from the highly turbulent flames that prevail in gas turbines, their speed gives a reasonable idea of the reactivity of hydrogen in GT combustors, in comparison with methane.

Table 2.
Compared combustion properties of H2 and CH4 [37,38].
Table 2.
Compared combustion properties of H2 and CH4 [37,38].
| Property − Fuel | Spec. Gravity /Air [-] | Fick Diffusion Coeff. in Air; 1 atm; 25 °C [cm2·s−1] | LEL–UEL Low/Up. Expl. Lim. [% vol] | Min. Ignition Energy [mJ] | Lamin. Flame Speed [cm·s−1] |
|---|---|---|---|---|---|
| H2 | 0.07 | 0.75 | 4.0–75 | 0.018 | 265 |
| CH4 | 0.55 | 0.25 | 5.0–15 | 0.033 | 33 |
| H2/CH4 | 7.9 | 3 | 7.1 * | ≈0.5 | 8 |
* Ratio between the flammability ranges (UEL–LEL).
Additional features contribute to the special sensitivity of hydrogen handling:
- -
- Its high propensity to develop deflagration-to-detonation transitions.
- -
- The high and fast pressure rises during explosions, which are consequently very difficult to dampen by venting. Indeed, H2 has the highest rate of pressure increase in a closed volume:[dP/dt]max = Kgas V−1/3 (KH2 >> KCH4)
- -
- The high mobility of hydrogen molecules not only in gaseous media but also through sealing defects and even through solid walls. In fact, regarding the modes of transport of hydrogen in different media, three distinct types of processes must be distinguished:
- Transport process in the air: H2 is about fifteen times lighter and three times more diffusive than air; it therefore has a strong propensity for buoyancy: in the event of a leak from an installation, H2 tends to expand rapidly in the air and, if this installation is confined in a closed volume, to accumulate under the roofs of machine rooms. Whenever the process allows it, outdoor installation is preferable since it allows a fast dilution of H2 leaks in open air and avoids the formation of potentially explosive H2 clouds.
- Effusion through gaps: The term effusion describes the situation of a confined gas escaping through a tiny orifice or gap. Such gas leaks behave like laminar flows. H2 and CH4 have similar dynamic viscosities (≈10 µPa.s) but hydrogen has an eight times lower density; its kinematic viscosity is therefore also eight times lower. This explains why H2 easily escapes through very small gaps (sealing defects), which increases the criticality of tightly fastening flanges and fittings and requires increased precautions during maintenance and assembly procedures.
- Diffusion across metallic materials: Graham’s law, which describes the transport of a gas across a solid, states that the rate of this process is inversely proportional to the square root of the molecular mass. Therefore, hydrogen diffuses much faster than hydrocarbons. Additionally, H2 molecules tend to dissociate into H atoms in the lattices of transition metals: these H atoms can then diffuse through the alloys of industrial components; their recombination at the level of alloy defects (typically at voids or inclusions) can cause material embrittlement and “blistering”. These effects are magnified by pressure and temperature. The compatibility of hydrogen with structural alloys must therefore be checked.
Finally, it should be emphasized that fruitful lessons have been learned from the nuclear accident that occurred in 1979 at the “Three Miles Island” power plant [39] as well as from the experience accumulated over about of six decades by the aerospace industry; these lessons are very important to define the best practices for the safe storage and handling of hydrogen.
3.2. Process Control
- 1.
- The proper control of electricity production, which constitutes the last link in the hydrogen energy chain, calls for specific considerations.
At first, as regards the reliable combustion of gases in industrial and domestic equipment, including gas turbines, an essential parameter of any gaseous fuel is its “Wobbe Index” and, more precisely, its “Modified Wobbe Index”, which govern the sizing of the gas fuel system as well as the interchangeability of different gas fuels [40,41]. These indices are defined as follows:
where LHV is the Lower Heating Value of the fuel (in kJ/Nm3), SG is its specific gravity (a dimensionless property, independent of the temperature), and T is its absolute temperature (in K).
WI = LHV/SG0.5 and MWI = LHV/(SG0.5 × T0.5)
Setting the MWI around a defined value and controlling the gas pressure keeps the flame at a suitable position inside the combustors. The MWI also conditions both the aerothermal patterns and the heat release process; it consequently plays a role in flame stability and the avoidance of thermoacoustic activity [42,43].
- 2.
- Now, since the Wobbe Index depends both on the LHV and specific gravity, the hydrogen content of the gas fuel must be continuously monitored to make possible the proper tuning of the GT combustion process.
As regards the environmental performance of gas turbines, the use of hydrogen poses an operational constraint tied to the control of NOx emissions.
Indeed, H2 flames are very hot and would generate more NOx than CH4 if combustion would be performed using traditional diffusion flames. Dedicated, sophisticated “Dry Low NOx” combustors are therefore required to cleanly burn H2 and H2NG.
This requirement posed by the combustion process is in addition to the normal safety duty of monitoring potential fuel leaks.
In summary, the GT operator who must burn H2NG and, even more so, pure H2 must carry out a continuous analysis of the incoming gas mixture to perform proper combustion tuning and ensure the safety and reliability of their plant.
These important considerations will be integrated in the evaluation of H2 sensor candidates, which will be covered in Section 4.
4. Sensing Technologies and Devices
4.1. Metrological Criteria
The evaluation of the available techniques and detection devices likely to respond to hydrogen applications calls upon a certain number of metrological standards which precisely define their performances [27,28]:
- The sensitivity of a sensor defines the variation in its response relative to the variation in the concentration of the target gas. In other words, the more a sensor can detect small changes in the concentration (ΔC) of a gas with a significant variation in its response (ΔR), the more sensitive it is. Equation (1) makes it possible to quantify the sensitivity Sb of a sensor.
Based on the sensitivity of a sensor, it is also possible to define the lower and upper detection limits, i.e., respectively, the lowest concentration and the highest concentration it can detect.
- The selectivity is its ability to provide a response solely based on the target gas among the rest of the gas matrix. In a binary system, the value of the selectivity St of a sensor is expressed by the ratio of the sensitivities of two gaseous components, Sbgas 1 and Sbgas 2, as expressed by Equation (2):
The higher this ratio, the more selective the sensor will be with respect to component 1. Conversely, the lower this ratio, the more selective it will be with respect to component 2.
- The response time “tx” is the time required to obtain 15% (t15), 50% (t50), or 90% (t90) of its final response. In this paper, we will consider the response time t90.
- The recovery time is defined in the same way as the response time: the 90% recovery time is the time required to return to 10% of the previously measured concentration signal. The ability of a sensor to permanently return to its base line value is called reversibility.
- The stability represents its ability to provide a response without drift over time for the same measured concentration.
In addition to meeting suitable sensing performance criteria, other practical aspects must be considered when choosing a sensing technology. Among them, the most relevant are the calibration frequency, robustness, price, maintainability, and possibly compatibility with the hosting facility, e.g., in terms of safety and possibly installation feasibility (size; complexity).
The next paragraphs of this section will be devoted to the presentation and evaluation of the most widespread sensing techniques based on their sensing performances. We will also examine their potential advantages and disadvantages in relation to the different uses in the hydrogen value chain.
4.2. Gas Chromatography: A Separative Method
Gas chromatography (“GC”) performs the separation and sensing of gas mixtures based on the process of diffusion within a stationary phase which is generally packed in the form of a “chromatography column”.
The operating principle of chromatography is presented in Figure 4.
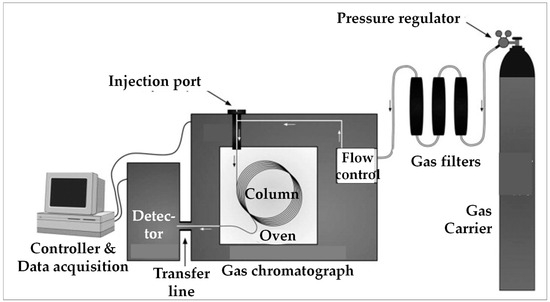
Figure 4.
A typical gas chromatograph and its main components [44].
The gas sample is injected into the column and transported through it by an appropriate gaseous carrier (Ar; He; N2). During its transit in the column, the gas mixture is “eluted”, i.e., is separated into its molecular components which thus reach, at different “retention times”, a detector installed downstream of the column. Each component thus has a characteristic retention time [44,45,46,47]. At each passage of a molecule, the detector generates an electrical current that is recorded as a peak, the area of which is proportional to the concentration of the detected components. The set of peaks forms a chromatogram, its base line corresponding to the carrier gas alone.
The type of the detector depends on the nature and physical or chemical properties of the components [46,47,48,49]:
- Catharometric detectors, or thermal conductivity detectors (TCDs), are used for species having much higher thermal conductivities than the carrier gas (see Section 4.4.3).
- Flame ionization detectors (FIDs) are for combustible components: these are burned in a flame, generating ions that are collected and quantified [50].
- Electron capture detectors (ECDs) are used for electroactive molecules such as halogenated compounds.
- Flame photometry detectors (FPDs) use the photons emitted during the combustion of certain compounds (containing, e.g., sulfur or phosphorus).
- Atomic emission detectors (AEDs), based on argon or helium plasmas, are versatile detectors able to detect many species; these are atomized and ionized in the plasma, passing from their ground state to excited states; their relaxation process emits electromagnetic waves that are detected using optical devices (see Section 4.4.2) [49,51,52].
- Mass spectrometry is a widely used detection mode [53,54,55]; it measures the abundance of fragmented and ionized molecules whose signals appear on the mass spectrum in an order defined by their (m/z) ratio (m for mass, z for ionic charge) [54].
In addition, some specific detectors exist for compounds containing nitrogen or phosphorus.
The performance of GC depends both on the quality of separation of the column and that of the detection technology.
Advantages: Thanks to its separation function and multiple detection devices, gas chromatography is a versatile, sensitive, and selective analysis method offering low detection limits. GC coupled with TCD is widely used in industry to analyze process gas mixtures [56,57,58] with a sensitivity of up to 0.5 ppm and relatively short response times [59]. Since H2 has a very high thermal conductivity, it can be analyzed in many matrices including in air (safety applications), in oxygen (electrolysis applications; [50]), and in natural gas mixtures (upstream of combustion equipment) [60,61]. It has been qualified by the International Organization for Standardization (ISO) [56]. The recalibrations of gas chromatographs are relatively spaced out over time (every three months [58]).
Disadvantages: The duration of the elution process prevents very short response times, and moreover, the measurements are sequential and not continuous, which is unfavorable for measurements in the air to detect leaks. The recalibration is not easy to make automatic; however, research is being carried out to obtain continuous measurement [47] and to produce small-sized devices [57,62].
In summary, GC is a very useful technique for processes requiring extensive compositional analyses of gas mixtures.
4.3. Spectrometric Method: Raman Spectrometry
Raman spectrometry (RS) is based on the vibration of molecule bonds. But unlike infrared (IR) spectrometry, it measures the intensity of a very intense monochromatic electromagnetic wave, which is scattered inelastically by the analyte. The latter can be a solid, vitreous, liquid, or gaseous material. The molecules of the material change polarization, absorb a small portion of this wave, and scatter both elastically and inelastically the remaining portion. The analysis of the inelastically scattered wave makes it possible to identify and quantify the target component.
Advantages: RS has been successfully applied to NG components [63,64] and is promising for H2-containing matrices [65,66] including H2-NG mixtures [67,68]; the detection limit of H2 in N2 is about 2 ppm [69].
Disadvantages: Like GC, RS is not very suitable for continuous measurements, but here again, research is being conducted to meet this capability. About 30 s are necessary to obtain a spectrum, which is a relatively short time but is considered too long for safety applications [63].
Furthermore, like for GC, the cost and size of the Raman spectrometry devices are not propitious for multipoint analysis applications.
Besides the sophisticated GC and RS techniques, it is interesting to explore alternative detection techniques involving equipment of a smaller size and which is easier to configure and implement. Therefore, we shall focus on two categories of devices:
Physical sensors, including acoustic, optical, and thermal conductivity sensing techniques.
Chemical sensors, including the catalytic, electrochemical, resistive, and work function techniques.
4.4. Physical Sensors
4.4.1. Acoustic Sensors
Acoustic sensors, sometimes also called “mechanical sensors”, rely on the detection of modulated acoustic waves that result from the adsorption of a gas on a reactive surface. This adsorption causes either a temperature change, a piezoelectric signal, or a change in the propagation of the acoustic waves inside the material. Three types of sensing devices are used: a quartz crystal microbalance; surface acoustic wave sensor; and ultrasonic sensor (Figure 5):
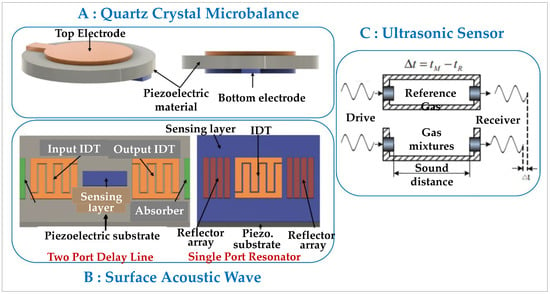
Figure 5.
Schematic representation of the various techniques for detecting acoustic sensors [70].
- -
- The quartz crystal microbalance (QCM), sketched in item A of Figure 5, is historically the first acoustic sensing device. The piezoelectric quartz crystal is compressed between two electrodes. Its resonance at a known frequency creates an acoustic wave that is confined within the crystal. When the gaseous analyte adsorbs on the surface of the latter, the resonance frequency varies due to the change in mass. This results in a modulation of the acoustic wave inside the material. This modulation is a function of the mass of the adsorbed gas analyte [71,72,73]. As it stands, the quartz crystal is unable to differentiate between a gas and a mixture of gases, so to achieve a certain selectivity, a catalytic material (metal or polymer) is added to the quartz [74,75]. This provides an additional response component to mass variation. Hydrogen undergoes catalytic oxidation on the surface of the material, inducing a variation in the material’s surface temperature.
- -
- The sensing based on surface acoustic waves (SAWs; item B of Figure 5) uses a principle similar to that of QCMs by the use of a piezoelectric material. The difference is that two interdigitated transducers (IDTs) placed on either side of the piezoelectric material are used (1) to cause the latter to resonate, thereby creating a Rayleigh acoustic wave from an electrical signal, and (2) to convert the acoustic wave having passed through the material into an electrical signal. One measures the variation in the amplitude of acoustic waves generated at the surface due to the presence of the adsorbed gas [76,77,78,79].
- -
- The ultrasound (US) measurement technique or “ultrasonic sensor” (item C of Figure 5) operates like a sonar. One irradiates a material using a laser, thus generating US waves that are reflected by the surface of the material. The propagation of these waves is a function of the nature and concentration of the gas. A US wave receiver analyzes the change in the reception time of the reflected waves, i.e., the change in their propagation speed caused by the adsorbed gas [80].
Advantages: A considerable advantage of these three sensing techniques is their ability to operate in oxygen-free gas matrices, which is the case when dealing with H2-NG applications. It is difficult to quantify the sensitivity of acoustic sensors using Equation (1) (see Section 4.1), as sensitivities are expressed either in Hz, in Hz/vol, or in Hz/ppm. However, the relevant information can be drawn by analyzing the variations in the signal [70]. Both the QCM and SAW detection methods have relatively good sensitivities for the detection of light gases: with SAW sensors, variations of 2–3% v/v of H2 contents can be detected [77,79].
Disadvantages: The responses are very dependent on environmental parameters such as the humidity and temperature [70,81]. Furthermore, the mechanism of sensing requires a specific material to be found to selectively respond to the given gas analyte [70]. US sensing allows for the correction of this lack of selectivity owing to its operating principle. This mechanical analysis process secures a good selectivity which is enhanced by the proper choice of material [82].
Note: US sensing is compatible with real-time operation and has response and recovery times in the order of a second, which is not the case with the QCM and SAW techniques. US sensing seems suitable for applications such as leak detection and the analysis of H2/NG gas mixtures.
Table 3 shows some examples of materials used for the three acoustic detection sensing types.

Table 3.
Examples of acoustic sensors.
4.4.2. Optical Sensors
Optical sensors are very popular devices. They are based on spectroscopic techniques, namely infrared, UV–visible, and Raman (reviewed above), which perform the identification of chemical groups. The coupling with GC-MS makes it possible to identify and quantify molecules in the gas phase.
Since the expansion of fiber optics technologies, many efficient sensing devices have emerged.
An optical fiber is a narrow, flexible glass or plastic tube that transmits light from one end to the other: the wall (the “sheath”) allows a total internal reflection of the light transported in the “core”, thus acting as a waveguide. The notion of “mode” defines the electromagnetic mechanism according to which the light is transmitted: there are “monomode” or “multimode” fibers. Five types of devices are commonly used [83]; they involve (i) reflectivity on micro-mirrors; (ii) Bragg network fibers; (iii) interferometry; (iv) surface plasmon resonance; and (v) the interaction with an evanescent field. Figure 6 sketches these technique variants that use different detection methods:
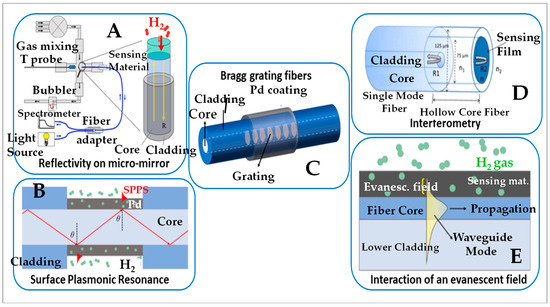
Figure 6.
Schematic representation of the various sensors of optical type [83].
- -
- The technology of reflectivity on micro-mirrors or RMM (item A of Figure 6) measures the variation in light intensity reflected by thin layers of a sensitive material (most often palladium), also called micro-mirrors, placed inside of the optical cladding. This variation in luminous intensity is characteristic of the gas present and proportional to its content [86].
- -
- The surface plasmon resonance (SPR) technology (item B of Figure 6) is based on the generation of surface plasmons [87], which are electromagnetic waves whose propagation on a metal surface depends on the superficial physicochemical state of that metal and changes when the gaseous analyte is adsorbed. The plasmons generated are excited using prismatic connector mechanisms, optical fibers, or even waveguides. One measures the change in the resonance wavelength of these plasmons, which depends on the nature of the gas adsorbed on the metal.
- -
- Fiber Bragg Gratings (FBGs; item C of Figure 6) are optical fibers in which a network of two alternating layers of materials with different refractive indices has been specifically etched at the core of the optical fiber according to Bragg’s law; this allows the periodic modulation of the refractive index throughout the propagation path. The etched networks are covered with a material sensitive to the gas to be detected and whose refractive index is different from that of the material of the fiber core. Thus, the signal obtained by this method is the variation in the Bragg wavelength corresponding to the maximum difference in reflectivity between the gas-sensitive materials and the core material. The nature and concentration of the gaseous analyte modify the reflectivity of the sensitive material, which allows for the analysis of that gas [88].
- -
- Interferometry (item D of Figure 6) measures the interference between several coherent waves; the wavelength of constructive interferences depends on the minute change in size of the sensitive material used as the detector, this size change resulting from the adsorption of a gaseous analyte. In particular, hydrogen has the ability to diffuse inside some metals, causing their expansion. One measures the change in the constructive wavelength which is associated with the gaseous analyte.
- -
- The evanescent-field detection method (item E of Figure 6) refers to the loss of light intensity caused by the interaction between the gaseous analyte and an electromagnetic wave crossing a modified optical fiber; the modification consists in replacing a portion of the cladding material with another one which is sensitive to the analyte and through which the wave passes; this creates an “evanescent field”. At the surface of the sensitive material, the gas induces a variation in the light intensity of the evanescent field that is proportional to its superficial concentration. The amplitude of the wave decreases exponentially when the distance from the source increases [89]. The sensitive material can be coated with specific adsorbing substances to enhance the sensitivity [81].
Advantages: Overall, these five optical methods exhibit excellent gas sensitivity while enabling operation in various environments, not necessarily requiring the presence of oxygen.
Disadvantages: Their major drawbacks are (i) a high production cost, (ii) bulky devices, and (iii) a high sensitivity to external conditions, which make them difficult to implement and tune in industrial environments [83,90].
A recent study carried out in underground environments, namely in mines, has revealed major issues in terms of the response and recovery times, which depend on the chosen sensitive material performance and the thickness of the coating [91]. Although this study showed that this type of sensor can operate in real time when using reduced thicknesses of appropriate materials, the sensitivity and stability of the sensing increases with the thickness of the sensitive material. It is therefore difficult to obtain a good compromise between the sensitivity, stability, and response and recovery times.
In conclusion, optical sensors, especially the SPR ones, seem suitable to detect hydrogen leakages in an ambient environment exempt from major temperature and humidity changes.
When the temperature, pressure, and humidity can be well controlled, it should be possible to perform H2-NG analyses with good sensitivity and response/recovery times.
Table 4 shows examples of H2-sensitive materials used in the five acoustic detection techniques.

Table 4.
Examples of optical sensor materials.
4.4.3. Catharometric Sensors
Catharometry, or “thermal conductivity detection (TCD)” can be used to quantify a gaseous analyte by measuring its thermal conductivity. Because H2 has one of the highest thermal conductivities (about 10 times greater than that of CH4), it is possible to detect this gas in a mixture of several gases, e.g., hydrocarbons, by using a differential method.
Figure 7 illustrates the principle of this technique.
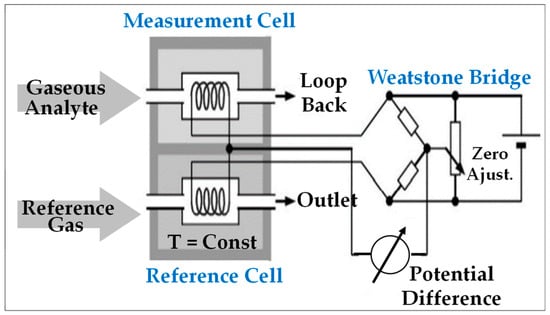
Figure 7.
Schematic representation of catharometric sensing device [81].
The sensing material (in the form of winding) is heated to a fixed temperature. When the gaseous analyte passes through it, the material loses heat in the function of the thermal conductivity of the target gas and its speed, which must be kept constant.
One can use two different types of devices based on this detection principle:
- -
- The first device, the so-called Pellistor configuration (Figure 5), comprises two cells: (i) a measuring cell in which an electrically heated thermistor (usually based on Pt or Pd) is swept by the surrounding gas and (ii) a reference cell in which another thermistor is swept by a reference gas (air or natural gas, for example).
The temperature variation resulting from the difference in thermal conductivity between the analyte and the reference gas results in an imbalance of the Wheatstone electrical bridge, allowing for the quantification of the target gas concentration by reading the electrical signal resulting from this imbalance.
- -
- Another simpler configuration involves two thermistors maintained at a constant temperature difference. As it passes through the hotter thermistor, the gaseous analyte withdraws a quantity of heat according to its thermal conductivity: the current or voltage required to maintain the temperature difference between the two thermistors allows for the deduction of the thermal conductivity of the gas stream and, therefore, its concentration in the air or the concentration of the gas analyte inside the gas mixture.
Advantages: This sensing technique has the advantage of being robust in terms of the stability of the response over the lifespan of the sensitive material. The choice of palladium, platinum, or gold [98] as a sensing material provides the best sensitivity, which makes the detection of hydrogen leaks and the analysis of H2-NG mixtures feasible [99].
Disadvantages: This technique applies only to individual gaseous analytes having a thermal conductivity much higher than that of the other components of the gas matrix (hydrocarbons; air), which is the case for hydrogen. Moreover, it is necessary to control the surrounding conditions, especially the temperature and pressure. Finally, even for H2, low concentration changes may not be detectable, which limits the use of this type of sensor. In particular, the catharometric technique does not seem suitable to analyze the purity of hydrogen at the outlet of electrolyzers due to the very low O2 contents (several ppm) that would not entail detectable changes in the thermal conductivity.
4.5. Chemical Sensors
4.5.1. Electrochemical Sensors
Electrochemical sensors are among the most popular sensing technologies thanks to a potentially good selectivity for different gas analytes of interest and their good sensitivity (a few ppm). The electrochemical sensors do not need to be heated, which induces a low power consumption for their operation. They are widely used to measure leaks. These sensors are composed of two to three electrodes (working, auxiliary, and possibly a reference) that are immersed in an electrolyte which surrounds the electrodes; the electrochemical cell formed by the electrodes and the electrolyte is separated from the gas matrix by a membrane which is permeable to the gaseous analyte. Two detection devices are commonly used:
- -
- Amperometric devices operate at a constant potential. The gaseous analyte reacts at the working electrode (at the anode in the case of H2; at the cathode in the case of O2); one measures here the resulting current between the working and auxiliary electrode; Faraday’s law allows for the correlation of this current to the concentration of the target gas [100].
- -
- In a Potentiometric device, the electrochemical cell operates at zero current. One measures the potential difference between the working and the reference electrode, which is related through Nernst’s law to the gas concentration and the standard potential of the redox couple which is formed by the analyte and the species it produces through its reaction at the working electrode.
Figure 8 illustrates the operating principle of an amperometric sensor used for H2; the commonly used electrolyte is a protonated liquid (H+ donor).
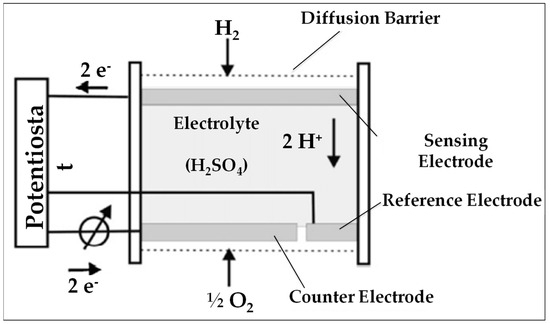
Figure 8.
Sketch of an electrochemical sensor of the amperometric type [81].
Advantages: Thanks to their moderate cost, good sensitivity, and simplicity of use, electrochemical sensors are suitable for the detection of hydrogen leakage in the air despite response times in the order of one minute. They are also suitable to monitor the purity of H2 as it is also possible to detect oxygen (by cathodic reduction) as well as other substances at some concentration levels using appropriate electrolyte compositions [101].
Disadvantages: Regular calibrations are necessary. In addition, the need to isolate the electrochemical cell by a membrane creates a diffusion delay of the gaseous analyte towards the working electrode, resulting in increased response times. These also depend on the partial pressure of the analyte, i.e., its concentration in the gas matrix. The typical response time is approximately 50 s for 1% H2 using a platinum electrode, a Nafion [37] or Teflon [38] membrane, and 0.1 M concentrated Pt salts as the electrolyte solution. Therefore, this technique is unsuitable when average response times are required and for long time measurements (such as H2-NG analyses) due to the consumption of the electrochemical reagent, which causes progressive measurement drifts.
4.5.2. Catalytic Sensors
These sensors involve catalytic reactions between two reagents in contact with a catalyst which accelerates these reactions. In many cases, the reaction is oxidation involving a reducing species and atmospheric O2. The principle consists in measuring the heat generated by the reaction.
Figure 9 shows the basic configuration adopted for this detection technique.
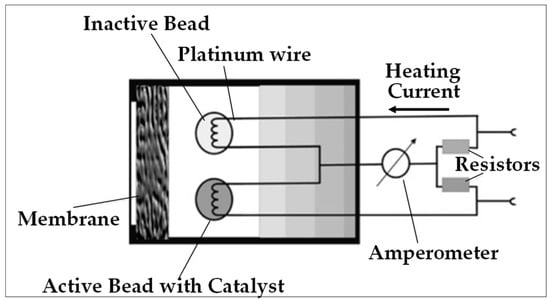
Figure 9.
Schematic representation of catalytic detection technique: the two beads and two resistors form a Wheatstone bridge.
There are two types of configurations used:
- -
- The Pellistor device uses two electrically heated coils which also serve as thermistors. These coils heat porous beads called “Pellistor beads” in which they are embedded. One of these beads is a catalyst which constitutes the active component used for detection, while the other is inactive and serves as a reference to counteract external parasitic effects.
The coils are mounted in a Wheatstone bridge that can measure small changes in the electrical resistances. The heat generated by the catalytic oxidation of H2 on the active bead causes a variation in its electrical resistance, which is translated into the concentration of the target gas.
- -
- The thermoelectric sensing mode, based on the Seebeck effect, relies on the temperature difference that exists between two different materials that have conducting or semiconducting properties and are connected together at two junction points, one of them being a catalyst sensitive to the gaseous analyte. The exothermic reaction between H2 and O2 (from the air) at the surface of the sensitive material creates a temperature difference between both junctions, resulting in a potential difference, which is translated into the concentration of the analyte.
Since these sensors need oxygen to carry out the oxidation reaction, they are suitable for gas detection in air but not to analyze H2-NG mixtures. The selectivity depends on the nature of the sensitive material used, which must selectively respond to the analyte.
Disadvantages: Because most combustible species (especially CH4) are reactive towards the usual catalytic beads (Pt or Pd), the selectivity is poor. Another drawback is that the active beads are easily deactivated by halogenated and siloxane species that are often used for cleaning during maintenance procedures, hence the need to periodically check their operability and their calibration.
Advantages: Both Pellistor and thermoelectric devices are available at moderate costs and are widely and successfully used for the detection of combustible gases in air [102,103].
Additionally, catalytic sensors are of high interest for monitoring gas leaks; indeed, not only do they give a signal that accounts for all the combustible species contained in the fuel, but this signal is also simply proportional to the molar concentration of the overall gas mixture in the air.
This is of primary interest from a safety point of view because it is not necessary to assess the composition of the fuel to control its LEL.
This important theoretical point as well as the demonstration of Le Chatelier’s law are developed in Appendix B attached to this paper.
4.5.3. Resistive Sensors
Resistive sensors (Figure 10) rely on the variation in the electrical resistance of a semiconducting material when it adsorbs the gaseous analyte. This type of sensor is composed of a heating resistor which is placed on a substrate and is most often made of a ceramic. This heating resistor is in direct contact with an insulating layer, which is itself in contact with a layer of the sensitive material. Depending on the voltage applied, the heating resistor rises in temperature and heats the layer of sensitive material, which allows the gas to be adsorbed on the surface of said layer.
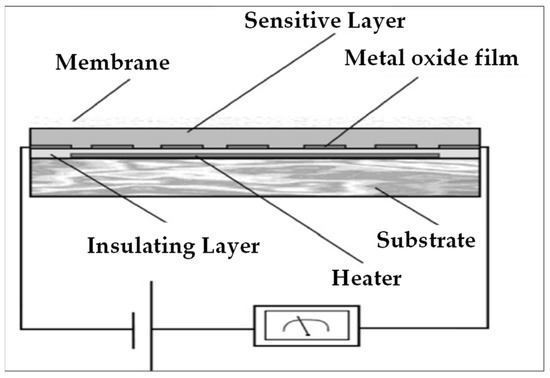
Figure 10.
Schematic representation of a resistive detection device.
When the gas is adsorbed, an electronic transfer between the gas and the surface of the semiconductor material takes place, inducing a change in the electrical resistance of the latter. This variation in the electrical resistance is proportional to the number of electrons transferred and therefore to the concentration of the adsorbed gas.
Two types of materials are commonly used for this technique:
Metal oxide semiconductors (MOSs): When a metal oxide surface is heated, the oxygen contained (e.g., in the air) is adsorbed on its surface and changes its degree of oxidation according to the temperature of the layer. When a combustible gas such as hydrogen is present, it reacts with these superficial oxygenated species, which causes in turn a variation in the electrical resistance of the MOS (Figure 11). Among the wide variety of MOS species that exist today for this technique, the most common are tin, zinc, nickel, and tungsten oxides [104,105].
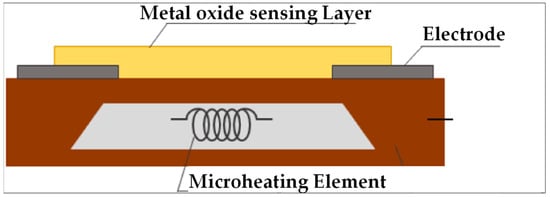
Figure 11.
Schematic representation of an MOS detection device [106].
Metallic semiconductors rely on the same principle, but unlike metal oxides, the presence of oxygen is not required to induce the electron transfer process between the metal and the sensitive layer. The materials commonly used for this technique are palladium, gold, and platinum [98,107].
Advantages: A small variation in the concentration of the reacting gas component induces a significant variation in the electrical resistance of the material. It is therefore a very sensitive technique in the range of, e.g., 20 ppm [108]. In addition, the kinetics of electronic transfer processes are very fast, and this technique allows response and recovery times in the order of a second for the most efficient sensors [109,110].
In addition, resistive sensors can selectively detect a given gas component in a mixture provided the sensitive material has the best possible affinity for that component.
Finally, depending on the materials used for the sensitive layer, resistive sensors can have large detection ranges and lifetimes ranging from 5 to 10 years in humidity-free matrices [104].
Disadvantages: To obtain short response times, it is essential to use thin layers of sensitive material, which tends to reduce the sensitivity of the resistive sensors [111]. Methane (the main component of natural gas) interferes with hydrogen, as both species are chemical reductors. Moreover, in the case of MOS, the presence of oxygen is necessary. This is why, at present, the MOS technique is unsuitable for oxygen-free applications, such as analyses of H2/NG mixtures.
Finally, both types of devices are sensitive to humidity and to poisoning by some chemical species such as sulfur compounds (namely odorants incorporated in commercial NGs), which reduce their lifespan.
4.5.4. Devices Based on the Work Function
The work function of a material represents the energy, expressed in eV, which is required to extract an electron from its surface.
Sensing devices relying on the working function are composed of a semiconductor substrate on which one places an insulating layer (often made of a metal oxide) and an upper metal layer that will constitute the sensitive system. When, e.g., H2 is adsorbed on the surface of the sensitive system, it dissociates into two hydrogen atoms that become oppositely polarized, thus creating a dipole layer on the surface. This change in the polarity of H atoms induces a modification in the energy levels at the junction between the metal and oxide layers, thus changing the working function of the material.
This variation in the work function is measured through intensity–potential measurements and makes it possible to identify, thanks to its value, the nature of the adsorbed gas and its content [112,113,114].
There are three configurations of work-function sensors, as shown in Figure 12.
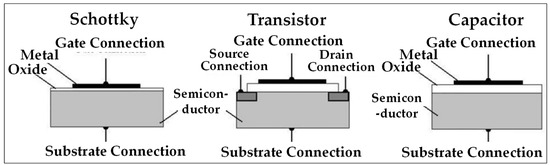
Figure 12.
Schematic representation of work-function detection technique [81,113].
Schottky diode sensors are available in two types of structures: the MS structure, where a metal is in contact with a semiconductor, and the MIS/MOS structure, where the metal is in contact with a thin insulator that is in turn in contact with a semiconductor. Commonly used metals include palladium and platinum, as well as nickel, gold, silver, and ruthenium. The choice of the semiconductor depends on the operating temperature. For instance, silicon is suitable for temperatures below 250 °C, while materials such as silicon carbide, indium phosphide, or gallium nitride are more appropriate for higher temperatures. Thin insulators are generally composed of oxides such as SiO2, Ga2O3, WO3, or HfO2.
When the metal is in contact with the semiconductor, the work function of each is modified based on the distribution of electrons according to the Fermi level. The Schottky barrier height, determined by the difference between the work functions of the metal and the semiconductor, influences the current–voltage (I-V) measurements and the sensor’s response characteristics. The measured variation in voltage, while maintaining a constant diode polarization current, is used to evaluate the hydrogen concentration.
In the Field Effect Transistor configuration, an electric field is used to modulate the conductive properties of a material and therefore its work function. To that end, a voltage is applied to the metal located at the surface, thus creating a positive polarization of the metal. This change in polarity allows the formation of a channel within the semiconductor material in which electrons will flow from a “source” point to a “drain” point. This induces an electrical signal and again allows the quantification of the gas on the surface.
The capacitor configuration is similar to the Schottky diode, except that the thickness of the insulating layer (or oxide) is much greater (<0.1 µm for the Schottky configuration against 0.1–100 µm for the capacitor configuration). Following the modification in the Fermi levels and therefore in the working functions of the metallic and semiconducting materials, electrons will accumulate within the semiconductor. This electron accumulation induces a significant modification in the electrical capacity of the sensing material (the metal). During the adsorption of a gas on the surface of the metal and following the principle of the work-function technique, the energy levels of the materials will be modified as well as the quantity of electrons accumulated and the electrical capacity of the semiconducting material. Measuring the capacity change in the material and that in the potential difference makes it possible to characterize the analyte.
Overall, working function sensors like resistive ones offer numerous possibilities for optimizing performance provided the materials have convenient sensing properties.
Advantages: They are generally very sensitive and offer a wide detection range (from 50 ppm up to 500,000 ppm at room temperature [113]). They also have the advantage of providing quick response and recovery times if the right material is chosen [112,114,115,116,117,118,119,120].
Disadvantages: A major issue faced by this technique lies in the stability and accuracy of the response, which most often undergoes irreversible drifts. This sensing technique is therefore not enough robust for the targeted applications [81,121,122,123].
5. Matrix of Sensor Performances
This paper was intended to provide a literature review of the various detection and analysis techniques for hydrogen-containing atmospheres. Table 5 summarizes the conclusions of this review in the context of power-to-gas and gas-to-power units involving electrolyzers and gas turbines, respectively.

Table 5.
Synopsis of the performances of the reviewed sensing devices.
Regarding process control, gas chromatography appears as the technique of choice for several reasons:
- -
- It fulfills the function of full compositional analysis, particularly with regard to the combustion of H2NG blends having variable hydrogen contents.
- -
- It obtains the best score in terms of sensitivity, selectivity, stability, and the ability to work in gas matrices deprived of oxygen, which are the crucial qualities expected to reach operation reliability; these analysis systems must be equipped with detectors adapted to the specific gas components being analyzed.
The main shortcomings of chromatography are its relatively long response times and the sequential nature of the measurements, which are not suited for the detection of explosive gas.
Other weak points lie in its cost and bulk; however, as already mentioned, active developments are underway to overcome these drawbacks.
Catalytic detectors appear to be an excellent safety tool because they provide reliable and continuous monitoring of explosive gas concentrations in the air. Moreover, their operation does not require any compositional analysis since, on the contrary, the signal they deliver is directly a fraction of the Lower Explosion Limit of the gas mixture, which explains their rapid response and their widespread usage.
6. Conclusions
The study reported in this review paper was carried out as part of a Joint Industrial Project dedicated to the development of the hydrogen energy chain in the electricity production sector, taking electrolyzers as hydrogen producing units and combined cycle gas turbines as power generation equipment.
Its objective was to identify and discuss the hydrogen sensing technologies likely to ensure the operational safety and reliability of the different chain processes.
After briefly recalling the prospects offered by the deployment of hydrogen as a substitute for fossil fuels, we identified the specific properties of this gas which impact the safety and reliability of industrial installations.
Then, we used as selection criteria the metrological standards applicable to sensing devices to evaluate their sensitivity, selectivity, stability, response and recovery times, etc. In doing so, we differentiated the function of safety from that of process control, as the latter requires accessing the full analysis of the process gas.
Using these criteria, we performed a comprehensive review of the possible sensing candidates. This bibliographical study highlights gas chromatography as the most capable metrological device to fulfill process control, while traditional catalytic devices are considered the technique of choice for safety purposes.
In terms of perspectives, further studies could be devoted to more precise considerations about hydrogen sensing, for instance, in terms of the required response times (routine versus emergency operational situations) and compatibility with industrial uses (equipment footprint; complexity of use; ease of calibration…).
Funding
This work was carried out as part of a PhD thesis, undertaken with a CIFRE convention No 2021/1718 (French Ministry of Research) and supported by General Electric Vernova, GRTGaz, McPhy, STEIM, and Université de Technologie de Belfort-Montbéliard.
Acknowledgments
Naguy Moussa, as doctoral student, wish to thank the companies General Electric Vernova, GRTGaz, McPhy and STEIM for funding this thesis.
Conflicts of Interest
Authors Naguy Moussa, Pierre Montagne, Pierre Biehler and Eric Impellizzeri were employed by the General Electric Vernova; Jean-Luc Fabre was employed by the GRTGaz; Alexandre Serpollier was employed by the McPhy; Térence Guillien was employed by the STEIM. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A. Theoretical Origin of Le Chatelier’s Rule
In this appendix, we will consider a gaseous fuel consisting of “n” combustible molecules mixed with air, “n” being possibly equal to 1 for a single-constituent fuel.
The symbol Xi will represent the molar fraction of molecule “i” in this fuel; therefore, Σn Xi = 1.
The overall fuel concentration in the (air/fuel) mixture will be denoted as “Yf” again in molar fraction, the air concentration therefore being Ya = 1 − Yf.
It is recalled that the LEL (and UEL) data are (traditionally) expressed in mole percentage of the gaseous fuel in the (fuel/air) mixture. For example, the LEL of methane is 5%; this means that methane can ignite if its concentration is Yf = 0.05 mole of CH4 per mole of fuel/air, i.e., in practice, 0.05 mole of CH4 per mole of air.
The asterisk (*) denotes the conditions of the fuel/air mixture at its LEL. Therefore,
Yf* = LEL/100.
The Lower and Gross Heating Values (LHV and GHV) are in J/mole of fuel.
The LEL and, similarly, the UEL of such a gaseous mixture are given by Le Chatelier’s rule which is as follows:
where LELi and UELi are the LEL and UEL of the constituent “i” of the gas.
1/LELmix = Σn (xi/LELi)
1/UELmix = Σn (xi/UELi)
In a very old paper dating back to 1898 [124], Le Chatelier had experimentally shown that the LEL and GHV (Gross Heating Value) data of usual combustible molecules are directly correlated:
(LEL/100) · GHV = 50 kJ/mole (relation applicable to most organic substances and H2)
In a later paper ([125], 1911), Burgess and Wheeler found independently a similar correlation between the LEL and this time the LHV data:
(LEL/100) · LHV = KBG = 44.3 kJ/mole
The two equations are consistent because the LHV is slightly lower than the GHV, the difference being the latent heat of vaporization of water formed during combustion.
We shall see that these correlations can be established if one assumes that the combustion of a mixture occurring close to its LEL (i.e., the sustained propagation of the leanest possible flame) requires, for kinetics reasons, a minimum level of flame temperature (Tfl*) which is independent of the fuel type (a possible interpretation being that the formed organic radicals are virtually the same for all C-H fuels).
The thermal balance for such flame can be expressed by writing that the released combustion heat is transferred to both the combustion products and the excess air:
Calorific energy from combustion = Heating of combustion products + Heating of excess air
Let Cpa be the heat capacity of air. Near the LEL conditions, the first term of the right member is negligible versus the second one due to the very high excess air. Thus, considering 1 mole of (fuel/air) mixture and remembering that Yf* is the mole concentration of the fuel at the LEL, Equation (A4) is as follows:
Yf* · LHV ≈ Cpa (1 − Yf*) (Tfl* − Tamb)
Since at the LEL Yf* is much smaller than 1, the following is true:
(LEL/100) · LHV ≈ Cpa (Tfl* − Tamb)
Now, with the previous assumption that Tfl* is independent of the fuel type, one obtains the following:
(LEL/100) · LHV = Constant = k
This equation is identical to the Burgess–Wheeler correlation (A3a) that can also be written as follows:
LHV = 100 · KBG/LEL (KBG being independent of fuel type)
When the fuel is a gas mixture, the Burgess–Wheeler relation is as follows:
LHVmix = 100 KBG/LELmix
Now, the LHV of a gas mixture is a linear combination of the LHV of its constituents,
LHVmix = Σn (xi · LHVi), whereby (A3c) becomes
LHVmix = Σn (xi 100 · KBG/LELi)
Combining (A3c) and (A5a), 100 KBG / LELmix = Σn (xi 100 KBG /LELi) or
1/LELmix = Σn (xi/LELi)
This equation is nothing else than Le Chatelier’s rule (A1a) that gives the LEL of a mixture. In summary, the following can be concluded:
- -
- On the one hand, the Burgess–Wheeler rule (A3a) can be demonstrated using the concept of minimum flame propagation temperature (Tfl*).
- -
- On the other hand, Le Chatelier’s rule (A1a) can be deduced from the Burgess–Wheeler relation.
The following example shows how the Le Chatelier rule is used in practice:
The molar analysis of a fuel gas mixture “M” is 60% CH4 (LEL: 5%); 10% C4H10 (LEL: 1.8%); and 30% H2 (LEL: 4%). According to Le Chatelier’s rule, the Lower Explosive Limit of this mixture, the LEL(M), is given by
1/LEL(M) = 0.60/0.05 + 0.10/0.018 + 0.30/0.04.
Hence, the LEL(M) = 3.99%.
Appendix B. Particular Interest of Catalytic Devices in Gas Detection
The fuel here also consists of one or several combustible constituents, and the notations are the same as above, the molar fraction of the fuel in the mixture also being denoted as Yf.
Let us consider a mixture of this fuel with air interacting with a catalytic detector in which it circulates at a flowrate of Qmix (in mole/s) (Figure 9); the flow of the fuel is (Yf *Qmix), in moles per second.
A small fraction ε of that fuel flux adsorbs and reacts on the surface of the active bead of the detector and produces a quantity of heat (Hcomb) which is given by
Hcomb = ε (Yf Qmix) LHV
Note: a major interest in catalytic sensors is that such an exothermic reaction on the active bead can occur for values of Yf that are lower than the value yf* (and without any explosion risk in these conditions), Yf* being the mole fraction of the fuel corresponding to the LEL, while in absence of catalyst, the reaction would occur only if Yf ≥ Yf* and could then be explosive.
One can describe the dissipation of the heat released by the active bead using the well-known Newton law which is classically used to quantify interfacial heat transfers. Taking the inactive bead as a reference,
where Tact, Tinact, and Tmix are the temperatures of the active bead, inactive bead, and the mixture flow, respectively, h is the heat transfer coefficient between the bead and the mixture flow, and A is the surface area of the two (identical) beads.
Hcomb = h A (Tact − Tmix) ≈ h A (Tact − Tinact)
Equation (A6) becomes
Hcomb = ε Yf Qmix LHV = h A (Tact − Tinact)
The Burgess–Wheeler relation (A3a) can be rearranged as
so that Equation (A7) becomes
or
LHV = 100 KBG/LEL,
Hcomb = 100 ε Yf Qmix KBG /LEL = h A (Tact − Tinact)
(Tact − Tinact) = 100 ε Yf Qmix KBG /(LEL h A)
Now, the Wheatstone bridge associated to the bead thermistors generates an electrical signal “S” which is proportional to (Tact − Tinact) and can be written as follows:
where kel depends on the features of the thermistors and the Wheatstone bridge.
S = kel (Tact − Tinact)
Now, using (A8b),
S = 100 kel Yf [ε Qmix KBG/(h A)]/LEL ≡ 100 Yf [ε kel Qmix KBG/(h A)]/LEL
KBG is a constant and, for a given gas detection device, all factors between brackets are also constant: let Kdet be their overall product. This leads to
S = 100 Yf Kdet/LEL
In fact, a catalytic detector does not give a measurement result in terms of a molar concentration of the fuel (“Yf“) but in terms of a percentage “Cf” of its LEL.
For example, for methane that has an LEL of 5%, when a catalytic detector indicates the presence of “CCH4 = 7% of LEL”, it means that the molar CH4 concentration is YCH4 = 0.07 * 0.05 = 3.5 10−3 mole per mole of air/fuel mixture, the correspondence between Cf and Yf being as follows:
Yf = Cf/100 * LEL/100 = 10−4 Cf LEL
Therefore, instead of using “Yf”, let us use “Cf”; this changes Equation (A11):
S = 0.01 Kdet Cf
A very important point is that this result is valid whatever the composition of the fuel. In other words, thanks to its operating principle, a catalytic sensor provides access to the concentration of a single- or multi-constituent combustible gas, expressed in relation to its LEL, without having to know about its molecular composition: these sensors therefore dispense from performing compositional analyses.
This is a very interesting point from a safety standpoint, which explains the universal use of catalytic detectors to monitor explosion risks.
References
- ClimaTalk Organization Communication. The Hydrogen Rainbow—Climate Is Talking. Available online: https://climatalk.org/2022/04/18/the-hydrogen-rainbow/ (accessed on 4 July 2024).
- Li, X.; Mulder, M. Value of power-to-gas as a flexibility option in integrated electricity and hydrogen markets. Appl. Energy 2021, 304, 117863. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Mulder, M. Power-to-gas in electricity markets dominated by renewables. Appl. Energy 2018, 232, 258–272. [Google Scholar] [CrossRef]
- Hart Energy Website. Hydrogen’s Biggest Hurdles: Customers and Demand. Available online: https://www.hartenergy.com/exclusives/hydrogens-biggest-hurdles-customers-and-demand-205590 (accessed on 4 July 2024).
- Berry, G.D.; Pasternak, A.D.; Rambach, G.D.; Smith, J.R.; Schock, R.N. Hydrogen as a future transportation fuel. Energy 1996, 21, 289–303. [Google Scholar] [CrossRef]
- Basile, A.; Iulianelli, A. Hydrogen as a fuel in transportation. In Advances in Hydrogen Production, Chapter 17, Storage and Distribution; Woodhead Publishing: Walnut Street, PA, USA, 2014; pp. 499–524. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Prospects for hydrogen as a transport fuel. Int. J. Hydrogen Energy 2019, 44–31, 16029–16037. [Google Scholar] [CrossRef]
- Liye, Z. Research Progress of Natural Gas Follow-Up Hydrogen Mixing Technology. Mech. Eng. 2022, 44, 755–766. [Google Scholar] [CrossRef]
- Molière, M. A 40 MW-class Gas Turbine Unit at Daesan (Korea) burns a 95% hydrogen fuel. Mod. Power Syst. 1999, 28–29. Available online: https://www.researchgate.net/publication/349736800_A_40_MW-class_Gas_Turbine_Unit_at_Daesan_Korea_burns_a_95_hydrogen_fuel/ (accessed on 21 August 2024).
- Molière, M. The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook. Energies 2023, 16, 3962. [Google Scholar] [CrossRef]
- Das, L.M. Hydrogen-oxygen reaction mechanism and its implication to hydrogen engine combustion. Int. J. Hydrogen Energy 1996, 21, 703–715. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G.G.; Mazzocchi, L. Using Hydrogen as Gas Turbine Fuel. J. Eng. Gas Turbines Power 2005, 127, 73–80. [Google Scholar] [CrossRef]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The hydrogen-fueled internal combustion engine: A technical review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Mc Phy Brochure, Electrolyzers: The Production of Industrial Hydrogen on-Site, on Demand, According to Your Specifications. Available online: https://mcphy.com/en/equipment-services/electrolyzers/ (accessed on 4 July 2024).
- US Department of Energy Communication. Hydrogen Production: Electrolysis. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis (accessed on 4 July 2024).
- Wolf, E. Large-Scale Hydrogen Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Philadelphia, PA, USA, 2015; pp. 129–142. [Google Scholar]
- EHB Association Report, European Hydrogen Backbone. 2022. Available online: https://ehb.eu/files/downloads/ehb-report-220428-17h00-interactive-1.pdf (accessed on 4 July 2024).
- US Department of Energy Communication. DOE National Clean Hydrogen Strategy and Roadmap. 2022. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/clean-hydrogen-strategy-roadmap.pdf?Status=Master (accessed on 4 July 2024).
- Erdener, B.C.; Sergi, B.; Guerra, O.J.; Chueca, A.L.; Pambour, K.; Brancucci, C.; Hodge, B.M. A review of technical and regulatory limits for hydrogen blending in natural gas pipelines. Int. J. Hydrogen Energy 2023, 48, 5595–5617. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Yang, M.; Jia, W.; Qiu, Y.; Lan, L. From the perspective of new technology of blending hydrogen into natural gas pipelines transmission: Mechanism, experimental study, and suggestions for further work of hydrogen embrittlement in high-strength pipeline steels. Int. J. Hydrogen Energy 2022, 47, 8071–8090. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Analysis of compression and transport of the methane/hydrogen mixture in existing natural gas pipelines. Int. J. Press. Vessels Pip. 2018, 166, 24–34. [Google Scholar] [CrossRef]
- US Department of Energy Communication, Hydrogen Pipelines. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-pipelines (accessed on 4 July 2024).
- GTI Communication. Leak Detection Body of Knowledge—Recommended Practice. 2021. Available online: https://www.gti.energy/wp-content/uploads/2021/05/Leak-Detection-Body-of-Knowledge%E2%80%93Recommended-Practice-whitepaper-Jan2021-693JK31810005.pdf (accessed on 4 July 2024).
- Goldmeer, D.J. Fuel Flexible Gas Turbines as Enablers for a Low or Reduced Carbon Energy Ecosystem, GEA33861, Electrify Europe Whitepaper, Electrify Europe 2018 Congress, Vienna, Austria, 2018. Available online: https://www.gevernova.com/content/dam/gepower/global/en_US/documents/fuel-flexibility/GEA33861%20-%20Fuel%20Flexible%20Gas%20Turbines%20as%20Enablers%20for%20a%20Low%20Carbon%20Energy%20Ecosystem.pdf (accessed on 4 July 2024).
- Hoffrichter, A.; Miller, A.R.; Hillmansen, S.; Roberts, C. Well-to-wheel analysis for electric, diesel and hydrogen traction for railways. Transp. Res. Part D Transp. Environ. 2012, 17, 28–34. [Google Scholar] [CrossRef]
- Bossel, U.; Eliasson, B. Energy and the Hydrogen Economy. Available online: https://afdc.energy.gov/files/pdfs/hyd_economy_bossel_eliasson.pdf (accessed on 4 July 2024).
- ISO/AIWI 6976; Natural Gas—Calculation of Calorific Values, Density, Relative Density and Wobbe Indices from Composition. ISO Organization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/85277.html (accessed on 4 July 2024).
- Zhang, C.; Cao, X.; Bujlo, P.; Chen, B.; Zhang, X.; Sheng, X.; Liang, C. Review on the safety analysis and protection strategies of fast filling hydrogen storage system for fuel cell vehicle application. J. Energy Storage 2022, 45, 103451. [Google Scholar] [CrossRef]
- Fischer, M. Safety aspects of hydrogen combustion in hydrogen energy systems. Int. J. Hydrogen Energy 1986, 11–19, 593–601. [Google Scholar] [CrossRef]
- Ng, H.D.; Lee, J.H.S. Comments on explosion problems for hydrogen safety. J. Loss Prev. Process Ind. 2008, 21, 136–146. [Google Scholar] [CrossRef]
- Yang, X.; Wang, T.; Zhang, Y.; Zhang, H.; Wu, Y.; Zhang, J. Hydrogen effect on flame extinction of hydrogen-enriched methane/air premixed flames: An assessment from the combustion safety point of vie. Energy 2022, 239, 122248. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Lu, H.; Shen, H.; Zheng, T.; Zhou, W.; Ming, P.; Zhang, C. Numerical study of hydrogen leakage, diffusion, and combustion in an outdoor parking space under different parking configurations. Renew. Sustain. Energy Rev. 2023, 173, 113093. [Google Scholar] [CrossRef]
- EU Website. European Hydrogen Safety Panel. Available online: https://www.clean-hydrogen.europa.eu/get-involved/european-hydrogen-safety-panel-0_en (accessed on 4 July 2024).
- Center for Hydrogen Safety Communication. Available online: https://www.aiche.org/chs (accessed on 4 July 2024).
- ISO 21789:2022; Gas Turbine Applications—Safety. ISO Standard Organization: Geneva, Switzerland, 2022. Available online: https://www.iso.org/fr/standard/74201.html (accessed on 4 July 2024).
- Rigas, F.; Amyotte, P. Hydrogen Hazards, in Hydrogen Safety, Chapter 3; CRC Press: Boca Raton, FL, USA, 2013; pp. 27–60. [Google Scholar] [CrossRef]
- Hord, J. Is hydrogen a safe fuel? Int. J. Hydrogen Energy 1978, 3, 157–176. [Google Scholar] [CrossRef]
- Communication of the U. S. Nuclear Regulatory Commission. NUREG-0578: TMI-2 Lessons Learned Task Force Status Report and Short-Term Recommendations. Available online: https://www.nrc.gov/docs/ML0900/ML090060030.pdf (accessed on 4 July 2024).
- Meher-Homji, C.B.; Zachary, J.; Bromley, A.F. Gas Turbine Fuels-System Design, Combustion and Operability. In Proceedings of the 39th Turbomachinery Symposium; Texas A&M University: College Station, TX, USA, 2010. [Google Scholar] [CrossRef]
- Klimstra, J. Interchangeability of Gaseous Fuels—The Importance of the Wobbe-Index; SAE Technical Paper 861578; SAE International: Warrendale, PA, USA, 1986. [Google Scholar] [CrossRef]
- Abbott, D.; Bowers, J.P.; James, S.R. The Impact of Natural Gas Composition Variations on the Operation of Gas Turbines for Power Generation. Available online: https://www.semanticscholar.org/paper/THE-IMPACT-OF-NATURAL-GAS-COMPOSITION-VARIATIONS-ON-Abbott-Bowers/d56c564ba28cf8b5d2e68d10503381f91ac9912a (accessed on 4 July 2024).
- Liu, K.; Sanderson, V. The influence of changes in fuel calorific value to combustion performance for SGT-300 dry low emission combustion system. Fuel 2013, 103, 239–246. [Google Scholar] [CrossRef]
- Stauffer, E.; Dolan, J.A.; Newman, R. Gas Chromatography and Gas Chromatography—Mass Spectrometry. In Fire Debris Analysis, Chapter 8; Academic Press: Burlington, ON, Canada, 2008; pp. 235–293. [Google Scholar] [CrossRef]
- Bartle, K.D.; Myers, P. History of gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 547–557. [Google Scholar] [CrossRef]
- Grob, R.L.; Barry, E.F. Modern Practice of Gas Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Agilent Technologies Pamphlet. Fundamentals of Gas Chromatography. 2002. Available online: https://www.agilent.com/cs/library/usermanuals/public/G1176-90000_034327.pdf (accessed on 4 July 2024).
- Messer France Brochure. Gaz de Fonction Pour L’analyse. Available online: https://www.sepem-permanent.com/fichiers_produits/1331254300605messer_fiche_tech3.pdf (accessed on 4 July 2024).
- Li, C.; Long, Z.; Jiang, X.; Wu, P.; Hou, X. Atomic spectrometric detectors for gas chromatography. TrAC Trends Anal. Chem. 2016, 77, 139–155. [Google Scholar] [CrossRef]
- Kamiński, M.; Kartanowicz, R.; Jastrzębski, D.; Kamiński, M.M. Determination of carbon monoxide, methane and carbon dioxide in refinery hydrogen gases and air by gas chromatography. J. Chromatogr. A 2003, 989, 277–283. [Google Scholar] [CrossRef]
- Wylie, P.L.; Quimby, B.D. Applications of gas chromatography with an atomic emission detector. J. High Resolut. Chromatogr. 1989, 12, 813–818. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, F.; Zhu, C.; Chambers, D.M.; Hieftje, G.M. Atomic emission detector for gas chromatography and supercritical fluid chromatography. J. Anal. At. Spectrom. 1990, 5, 487–494. [Google Scholar] [CrossRef]
- Varlet, V.; Smith, F.; Augsburger, M. Indirect hydrogen analysis by gas chromatography coupled to mass spectrometry (GC–MS). J. Mass Spectrom. 2013, 48, 914–918. [Google Scholar] [CrossRef]
- Sparkman, O.D.; Penton, Z.; Kitson, F.G. The Fundamentals of GC/MS. In Gas Chromatography and Mass Spectrometry: A Practical Guide, Chapter 1; Academic Press: Cambridge, MA, USA, 2011; pp. 2–218. [Google Scholar]
- Santos, F.J.; Galceran, M.T. Modern developments in gas chromatography–mass spectrometry-based environmental analysis. J. Chromatogr. A 2003, 1000, 125–151. [Google Scholar] [CrossRef]
- Emerson Electric Co. Brochure, Green Hydrogen Producer Ensures Quality of the Network’s Gas Blend Using a Gas Chromatograph. Available online: https://www.emerson.com/documents/automation/case-study-green-hydrogen-producer-ensures-network-gas-blend-quality-using-rosemount-700xa-gas-chromatograph-en-7690954.pdf (accessed on 4 July 2024).
- Toonen, A.; van Loon, R. Hydrogen Detection with a TCD using Mixed Carrier Gas on the Agilent Micro GC. Available online: https://www.agilent.com/cs/library/applications/5991-3199EN.pdf (accessed on 4 July 2024).
- Dell’Isola, M.; Ficco, G.; Moretti, L.; Jaworski, J.; Kułaga, P.; Kukulska-Zając, K. Impact of Hydrogen Injection on Natural Gas Measurement. Energies 2021, 14, 8461. [Google Scholar] [CrossRef]
- Addach, H.; Berçot, P.; Wery, M.; Rezrazi, M. Quantitative determination of hydrogen in solids by gas chromatography. J. Chromatogr. A 2004, 1057, 219–223. [Google Scholar] [CrossRef]
- Seokyoon, M.; Yunseok, L.; Dongju, S.; Seungin, L.; Sujin, H.; Yun-Ho, A.; Youngjune, P. Critical hydrogen concentration of hydrogen-natural gas blends in clathrate hydrates for blue hydrogen storage. Renew. Sustain. Energy Rev. 2021, 141, 110789. [Google Scholar] [CrossRef]
- Castello, G.; Biagini, E.; Munari, S. The quantitative determination of hydrogen in gases, by gas chromatography with helium as the carrier gas. J. Chromatogr. A 1965, 20, 447–451. [Google Scholar] [CrossRef]
- Biswas, P.; Zhang, C.; Chen, Y.; Liu, Z.; Vaziri, S.; Zhou, W.; Sun, Y. A Portable Micro-Gas Chromatography with Integrated Photonic Crystal Slab Sensors on Chip. Biosensors 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dai, L.K.; Zhu, H.D.; Chen, Y.L.; Zhou, L. Quantitative Analysis of Main Components of Natural Gas Based on Raman Spectroscopy. Chin. J. Anal. Chem. 2019, 47, 67–76. [Google Scholar] [CrossRef]
- Petrov, D.; Matrosov, Y. Natural Gas Analysis Using Polarized Raman Spectroscopy. Anal. Chem. 2023, 95, 9409–9414. [Google Scholar] [CrossRef] [PubMed]
- Godot, A.; Coindet, G.; Hubinois, J.C. Analysis of Gases by Raman Spectroscopy: Determination of Isotopic Composition of Hydrogen Mixtures (H2, D2 and T2). Fusion Sci. Technol. 2011, 60, 998–1001. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Luan, Z.; Du, Z.; Xi, S.; Wang, B.; Cao, L.; Lian, C.; Yan, J. Raman vibrational spectral characteristics and quantitative analysis of H2 up to 400 °C and 40 MPa. J. Raman Spectrosc. 2018, 49, 1722–1731. [Google Scholar] [CrossRef]
- Endress & Hauser Brochure. Hydrogen Blending for Natural Gas-Fired Turbines. Available online: https://www.endress.com/en/endress-hauser-group/Case-studies-application-notes/hydrogen-blending-for-natural-gas-fired-turbines-casestudy (accessed on 4 July 2024).
- Khannanov, M.N. Analysis of Natural Gas Using a Portable Hollow-Core Photonic Crystal Coupled Raman Spectrometer. Appl. Spectrosc. 2020, 74, 1496–1504. [Google Scholar] [CrossRef]
- Peruski, K.M.; Vestal, B.K.; Vick, M.; Cobble, C.; Johnson, K.R.; McFarlane, J. On-Line Measurement of Hydrogen Gas Using Raman Spectroscopy for Process Gas Systems; ORNL/TM-2023/2949; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2023. [Google Scholar]
- Balasingam, J.A.; Swaminathan, S.; Nazemi, H.; Love, C.; Birjis, Y.; Emadi, A. Chemical Sensors: Gas Sensors, Acoustic Sensors. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Rosenkrantz, E.; Ferrandis, J.Y.; Lévêque, G.; Baron, D. Caractérisation d’un gaz confiné à l’aide d’un capteur acoustique—Application aux crayons combustibles nucléaires. In Technique de L’Ingenieur; Article: IN113 V1; Mesures–Analyses/Mesures Physiques: Paris, France, 2010; pp. 18–25. [Google Scholar]
- Noi, K.; Iijima, M.; Kuroda, S.; Ogi, H. Ultrahigh-sensitive wireless QCM with bio-nanocapsules. Sens. Actuators B Chem. 2019, 293, 59–62. [Google Scholar] [CrossRef]
- Zhou, L.; Kato, F.; Nakamura, F.; Oshikane, Y.; Nagakubo, A.; Ogi, H. MEMS hydrogen gas sensor with wireless quartz crystal resonator. Sens. Actuators B Chem. 2021, 334, 129651. [Google Scholar] [CrossRef]
- Christofides, C.; Mandelis, A. Operating characteristics and comparison of photopyroelectric and piezoelectric sensors for trace hydrogen gas detection. II. Piezoelectric quartz-crystal microbalance sensor. J. Appl. Phys. 1989, 66, 3986–3992. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Park, S.S.; Kim, S. High-Performance, Room Temperature Hydrogen Sensing with a Cu-BTC/Polyaniline Nanocomposite Film on a Quartz Crystal Microbalance. IEEE Sens. J. 2019, 19, 4789–4795. [Google Scholar] [CrossRef]
- Wang, W. Development of a Pd/Cu nanowires coated SAW hydrogen gas sensor with fast response and recovery. Sens. Actuators B Chem. 2019, 287, 157–164. [Google Scholar] [CrossRef]
- Tsuji, T. Highly Sensitive Ball Surface Acoustic Wave Hydrogen Sensor with Porous Pd-Alloy Film. Mater. Trans. 2014, 55, 1040–1044. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Surface Acoustic Wave Sensors for Hydrogen and Deuterium Detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef]
- Constantinoiu, L.; Viespe, C. ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Revie. Sensors 2020, 20, 5118. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Li, X.; Zhu, C.; Zhang, J. Gas concentration detection using ultrasonic based on wireless sensor networks. In Proceedings of the 2nd International Conference on Information Science and Engineering, Hangzhou, China, 4–6 December 2010; pp. 2101–2106. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yuan, D.; Zhao, Y. Review of optical hydrogen sensors based on metal hydrides: Recent developments and challenges. Opt. Laser Technol. 2021, 137, 106808. [Google Scholar] [CrossRef]
- Deng, L.; Wei, L. Fabrication and Experiment of a Hydrogen Sensor Based on Piezoelectric Qcm. In Proceedings of the 15th Symposium on Piezoelectrcity, Acoustic Waves and Device Applications (SPAWDA), Zhengzhou, China, 16–19 April 2021; pp. 526–530. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ma, R.H.; Li, L.S.; Fan, L.; Yang, Y.T.; Zhang, S.Y. A room-temperature ultrasonic hydrogen sensor based on a sensitive layer of reduced graphene oxide. Sci. Rep. 2021, 11, 2404. [Google Scholar] [CrossRef]
- Dai, J. Fiber Optical Hydrogen Sensor Based on WO3-Pd2Pt-Pt Nanocomposite Films. Nanomaterials 2021, 11, 128. [Google Scholar] [CrossRef]
- Perrotton, C. Reliable, sensitive and fast optical fiber hydrogen sensor based on surface plasmon resonance. Opt. Express 2013, 21, 382–390. [Google Scholar] [CrossRef]
- Feng, D.; Luo, X.; Liu, Y.; Ma, C.; Qiao, X. Performance improvement of FBG sensors based on the pre-stressed package technique. Opt. Fiber Technol. 2021, 65, 102623. [Google Scholar] [CrossRef]
- Vitoria, J.; Ruiz Zamarreño, C.; Ozcariz, A.; Matias, I.R. Fiber Optic Gas Sensors Based on Lossy Mode Resonances and Sensing Materials Used Therefore: A Comprehensive Review. Sensors 2021, 21, 731. [Google Scholar] [CrossRef]
- Caucheteur, C. Capteurs de gaz à fibres optiques—Prévention industrielle, détection précoce de fuites. Tech. L’ingénieur Mes. Phys. 2012; R2391 V1. [Google Scholar] [CrossRef]
- He, T. Review on Optical Fiber Sensors for Hazardous-Gas Monitoring in Mines and Tunnels. IEEE Trans. Instrum. Meas. 2023, 72, 7003722. [Google Scholar] [CrossRef]
- Zhang, C. Pd/Au nanofilms based tilted fiber Bragg grating hydrogen sensor. Opt. Commun. 2022, 502, 127424. [Google Scholar] [CrossRef]
- Nugroho, F.A. Metal–polymer hybrid nanomaterials for plasmonic ultrafast hydrogen detection. Nat. Mater. 2019, 18, 489–495. [Google Scholar] [CrossRef]
- Yahya, N.A. H2 sensor based on tapered optical fiber coated with MnO2 nanostructures. Sens. Actuators B Chem. 2017, 246, 421–427. [Google Scholar] [CrossRef]
- Luo, J. Fiber optic hydrogen sensor based on a Fabry–Perot interferometer with a fiber Bragg grating and a nanofilm. Lab Chip 2021, 21, 1752–1758. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.; Saravanan, S.P.; Skir, M.; Karthik Kannan, S. Fabrication of transition-metal (Zn, Mn, Cu)-based MOFs as efficient sensor materials for detection of H2 gas by clad modified fiber optic gas sensor technique. Opt. Fiber Technol. 2021, 65, 102614. [Google Scholar] [CrossRef]
- Wang, Y.; Mumtaz, F.; Dai, Y. Hydrogen gas sensor based on seven-core fiber interference and Pt-WO3 film. Mater. Lett. 2023, 341, 134245. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-Performance Nanostructured Palladium-Based Hydrogen Sensors—Current Limitations and Strategies for Their Mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef]
- Harumoto, T.; Shi, J.; Nakamura, Y.; Fujiki, H. Enhanced hydrogen gas detectability of sweep heating thin-wire thermal conductivity detector. Sens. Actuators Phys. 2023, 358, 114446. [Google Scholar] [CrossRef]
- Stetter, J.R.; Li, J. Amperometric Gas Sensors: A Review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef]
- Molière, M.; Cozzarin, P.; Bouchet, S.; Rech, P. Catalytic Detection of Fuel Leaks in Gas Turbines Units: Gaseous and Volatile Hydrocarbon Based Fuels. In Proceedings of the ASME Turbo Expo 2005: Power for Land, Sea, and Air, Reno, NV, USA, 6–9 June 2005; pp. 763–772. [Google Scholar] [CrossRef]
- Molière, M.; Cozzarin, P.; Bouchet, S.; Rech, P. Catalytic Detection of Fuel Leaks in Gas Turbine Units: Gas Fuels Containing Hydrogen, Carbon Monoxide and Inert. In Proceedings of the ASME Turbo Expo 2006: Power for Land, Sea, and Air, Barcelona, Spain, 8–11 May 2006; pp. 541–550. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Govardhan, K.; Grace, A.N. Metal/Metal Oxide Doped Semiconductor Based Metal Oxide Gas Sensors—A Review. Sens. Lett. 2016, 148, 741–750. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Advancements in Improving Selectivity of Metal Oxide Semiconductor Gas Sensors Opening New Perspectives for Their Application in Food Industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef]
- Occelli, C.; Fiorido, T.; Perrin-Pellegrino, C.; Seguin, J.-L. PdAu Based Resistive Hydrogen Sensor in Anaerobic Environment. In Proceedings of the Twelfth International Conference on Sensor Device Technologies and Applications, Athens, Greece, 14–18 November 2021; Available online: https://amu.hal.science/hal-03428722/document (accessed on 4 July 2024).
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Wang, Z. Improved Hydrogen Monitoring Properties Based on p-NiO/n-SnO2 Heterojunction Composite Nanofibers. J. Phys. Chem. C 2010, 114, 6100–6105. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Thin film SnO2-based gas sensors: Film thickness influence. Sens. Actuators B Chem. 2009, 142, 321–330. [Google Scholar] [CrossRef]
- Yamazaki, H.; Hayashi, Y.; Masunishi, K.; Ono, D.; Ikehashi, T. High sensitivity MEMS capacitive hydrogen sensor with inverted T-shaped electrode and ring-shaped palladium alloy for fast response and low power consumption. J. Micromechanics Microengineering 2018, 28, 094001. [Google Scholar] [CrossRef]
- Sahoo, T.; Kale, P. Work Function-Based Metal–Oxide–Semiconductor Hydrogen Sensor and Its Functionality: A Review. Adv. Mater. Interfaces 2021, 8, 2100649. [Google Scholar] [CrossRef]
- Kumar, V.; Rawal, I.; Mishra, V.N.; Dwivedi, R.; Das, R.R. Fabrication and characterization of gridded Pt/SiO2/Si MOS structure for hydrogen and hydrogen sulphide sensing. Mater. Chem. Phys. 2014, 146, 418–424. [Google Scholar] [CrossRef]
- Kim, K.S.; Chung, G.S. Fast response hydrogen sensors based on palladium and platinum/porous 3C-SiC Schottky diodes. Sens. Actuators B Chem. 2011, 1601, 1232–1236. [Google Scholar] [CrossRef]
- Hussain, M. Highly Fast Response of Pd/Ta2O5/SiC and Pd/Ta2O5/Si Schottky Diode-Based Hydrogen Sensor. Sensors 2021, 21, 1042. [Google Scholar] [CrossRef]
- Chen, Z.H.; Jie, J.S.; Luo, L.B.; Wang, H.; Lee, C.; Lee, S.T. Applications of silicon nanowires functionalized with palladium nanoparticles in hydrogen sensors. Nanotechnology 2007, 18, 45502. [Google Scholar] [CrossRef]
- Zhang, Z. Hydrogen gas sensor based on metal oxide nanoparticles decorated graphene transistor. Nanoscale 2015, 7, 10078–10084. [Google Scholar] [CrossRef]
- Kang, B.S. Hydrogen-induced reversible changes in drain current in Sc2O3/AlGaN/GaN high electron mobility transistors. Appl. Phys. Lett. 2004, 84, 4635–4637. [Google Scholar] [CrossRef]
- Hsu, C.S. On the hydrogen sensing characteristics of a Pd/AlGaN/GaN heterostructure field-effect transistor (HFET). Sens. Actuators B Chem. 2012, 165, 19–23. [Google Scholar] [CrossRef]
- Adjadj, A.; Debuy, V.; Sureau, P. Evaluation des Performances des Equipements des Systèmes de Sécurité—Etat de l’art des Déteteurs d’hydrogène. INERIS Report DRA-15-149138-06078A. 2015. Available online: https://www.ineris.fr/sites/ineris.fr/files/contribution/Documents/DRA-15-149138-06078A_DRA73.pdf (accessed on 4 July 2024).
- Hooker, S.A.; Drive, M. Nanotechnology Advantages Applied to Gas Sensor Development. In Proceedings of the Nanoparticles Conference, Puerto Rico, 21–25 April 2002; Available online: https://www.researchgate.net/publication/242483532_Nanotechnology_Advantages_Applied_to_Gas_Sensor_Development (accessed on 4 July 2024).
- Singh, A.K.; Chowdhury, N.K.; Roy, S.C.; Bhowmik, B. Review of Thin Film Transistor Gas Sensors: Comparison with Resistive and Capacitive Sensors. J. Electron. Mater. 2022, 51, 1974–2003. [Google Scholar] [CrossRef]
- Le Chatelier, H.; Boudouard, O. Sur les limites d’inflammabilité des vapeurs combustibles. Comptes Rendus. Des. Séances L’académie Des. Sci. 1898, 126, 1510–1513. [Google Scholar]
- Burgess, M.J.; Wheeler, R.V. The lower limit of inflammation of mixtures of the paraffin hydrocarbons with air. J. Chem. Soc. Trans. 1911, 99, 2013–2030. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).