Recent Development of Thermal Insulating Materials for Li-Ion Batteries
Abstract
1. Introduction
2. Battery Thermal Behavior
2.1. Battery Heat Generation
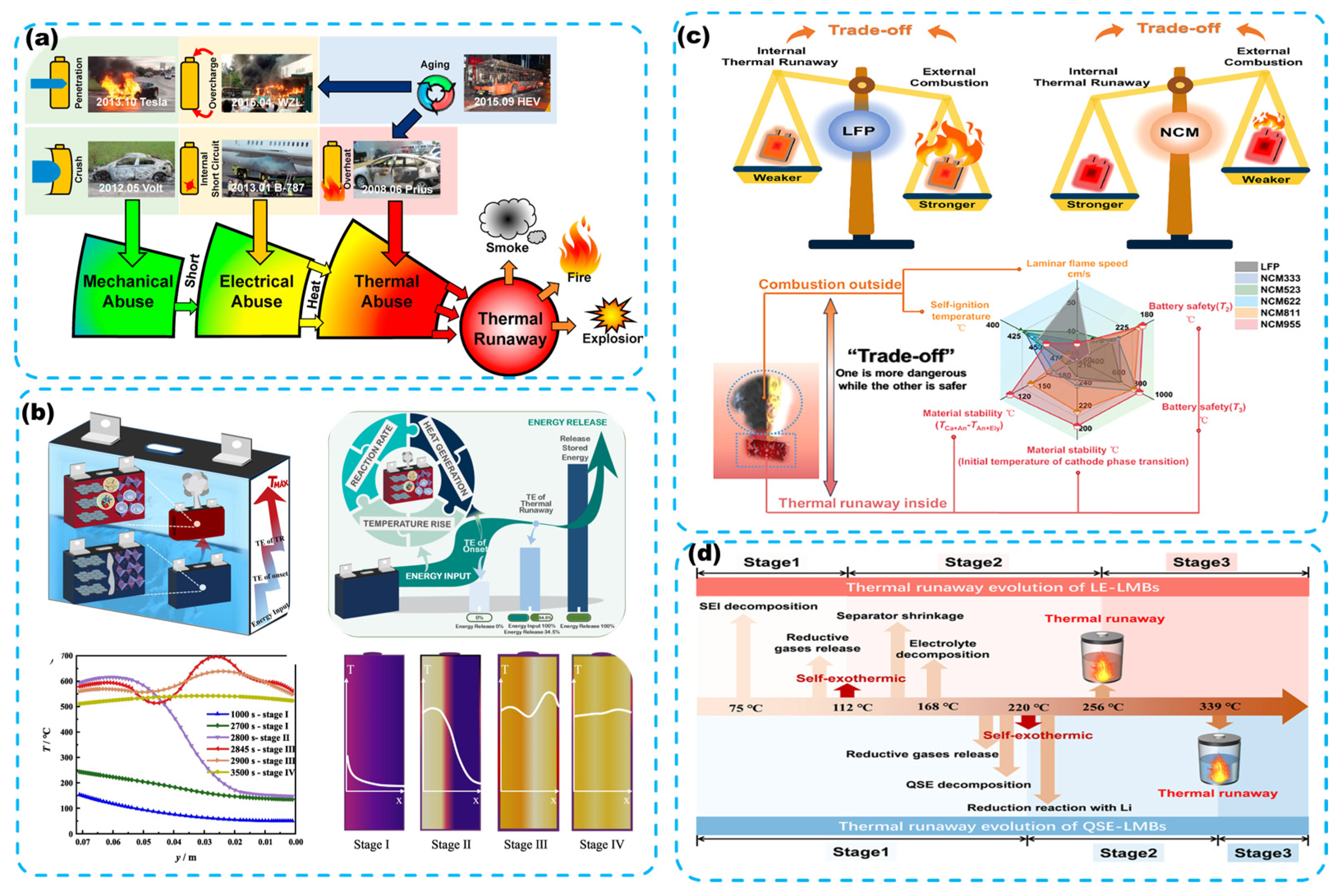
2.2. TR in LIBs
2.3. The Necessity of Thermal Management
3. Phase Change Materials
3.1. The Organic PCM
3.1.1. Paraffin
- Properties
- Synthesis and modification
3.1.2. Non-Paraffin
- Properties
- Synthesis and modification.
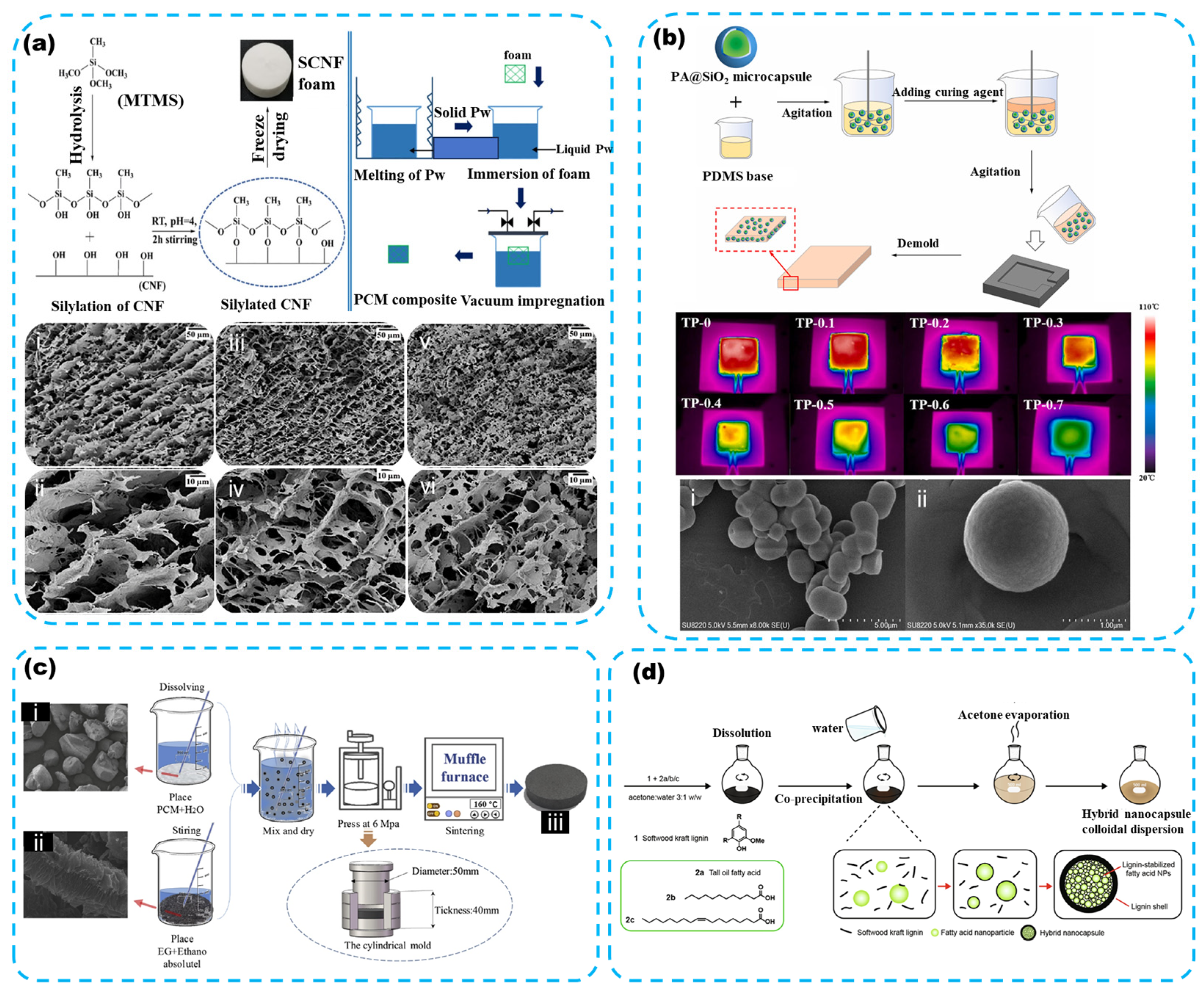
3.2. The Inorganic PCM
3.2.1. Salt and Salt Hydrates
- Properties
- Synthesis and modification
3.2.2. Metals and Alloys
3.3. The Composite PCM

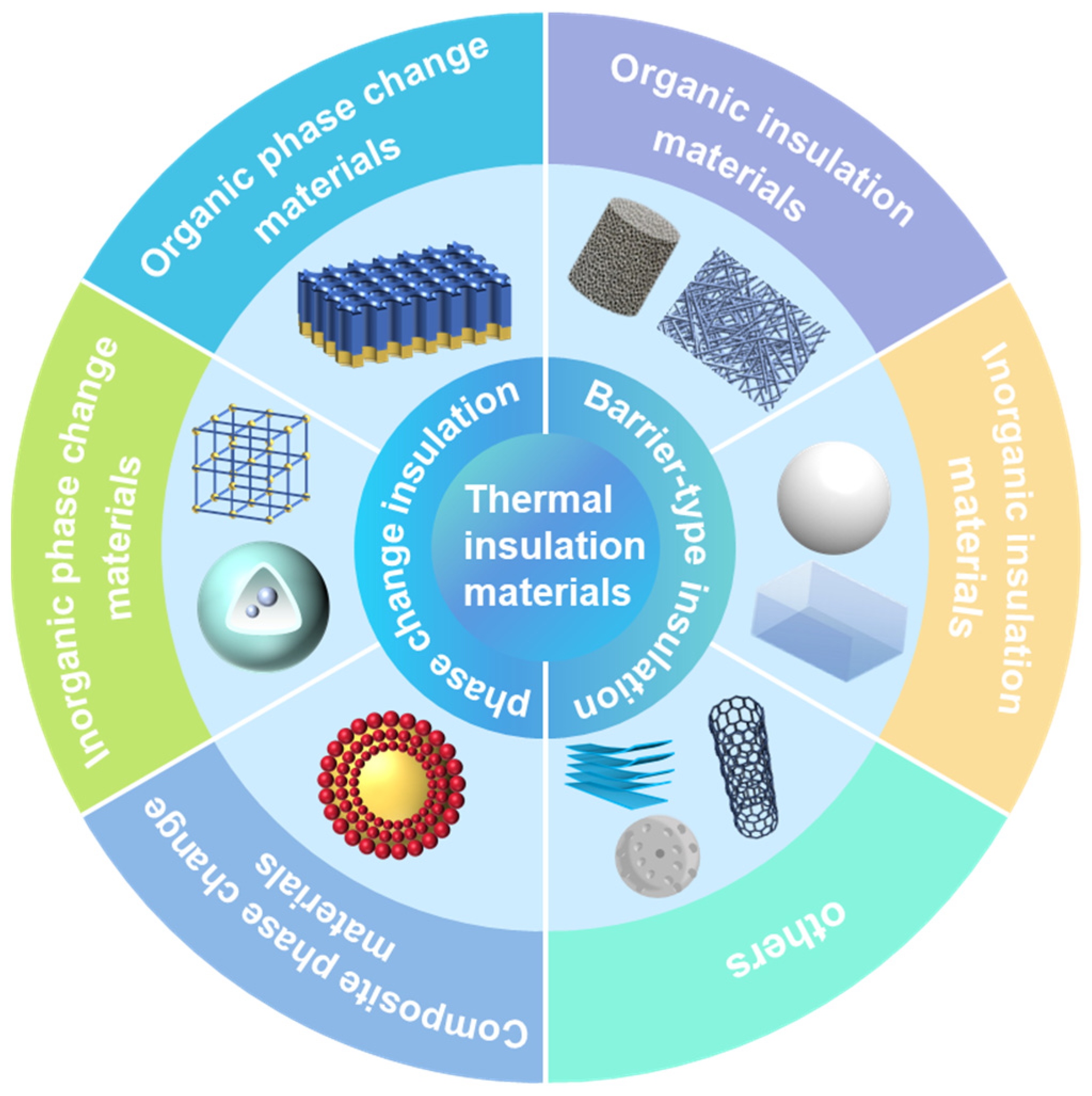
4. Barrier-Type Insulating Materials
4.1. Organic Thermal Insulating Materials
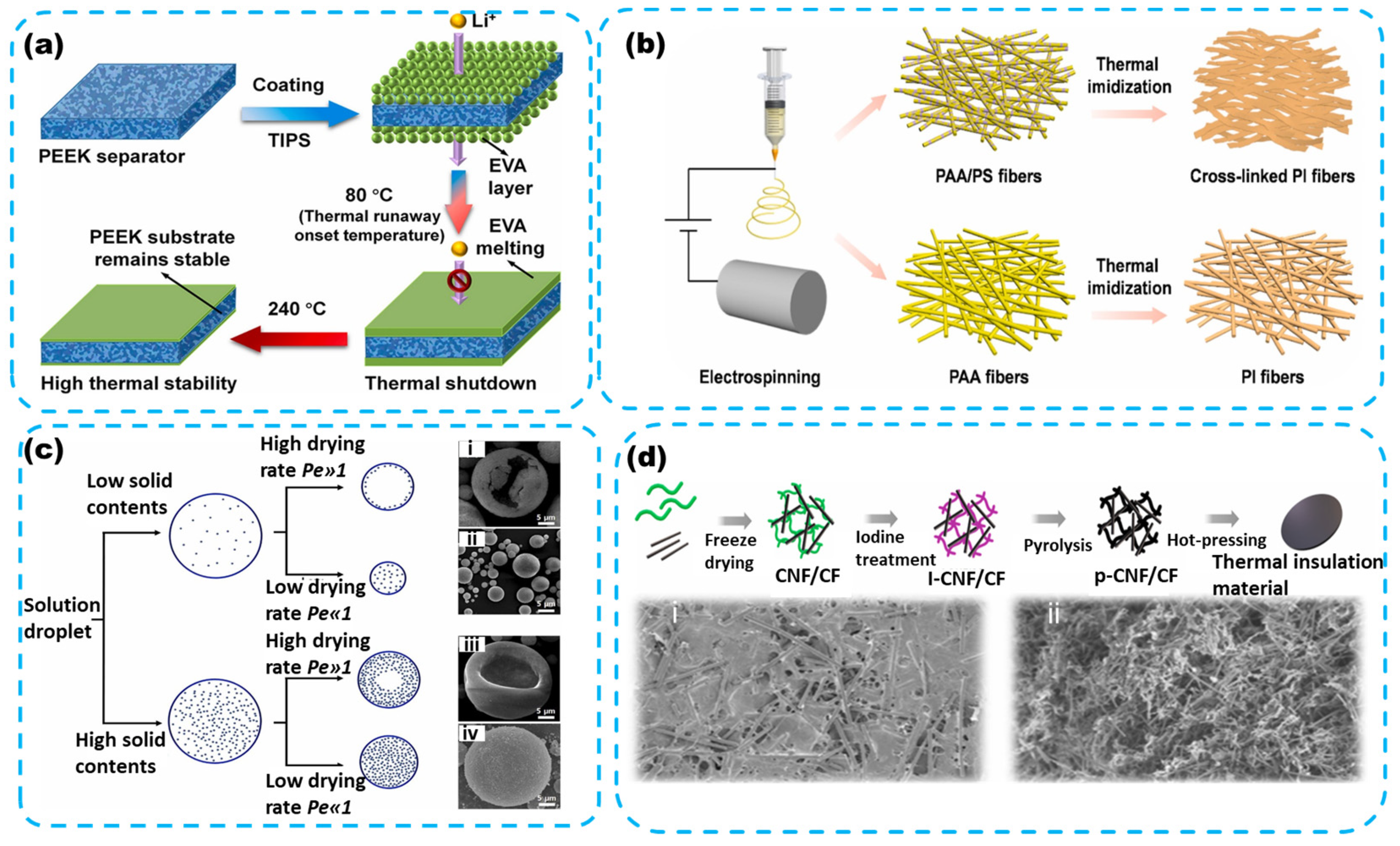
4.1.1. Polymer Thermal Insulating Film
- Properties
- Synthesis
- Modification of polymer thermal insulating film
4.1.2. Fiber and Foam Materials
- Properties
- Synthesis
- Modification of foam and fiber materials
4.2. Inorganic Thermal Insulating Materials
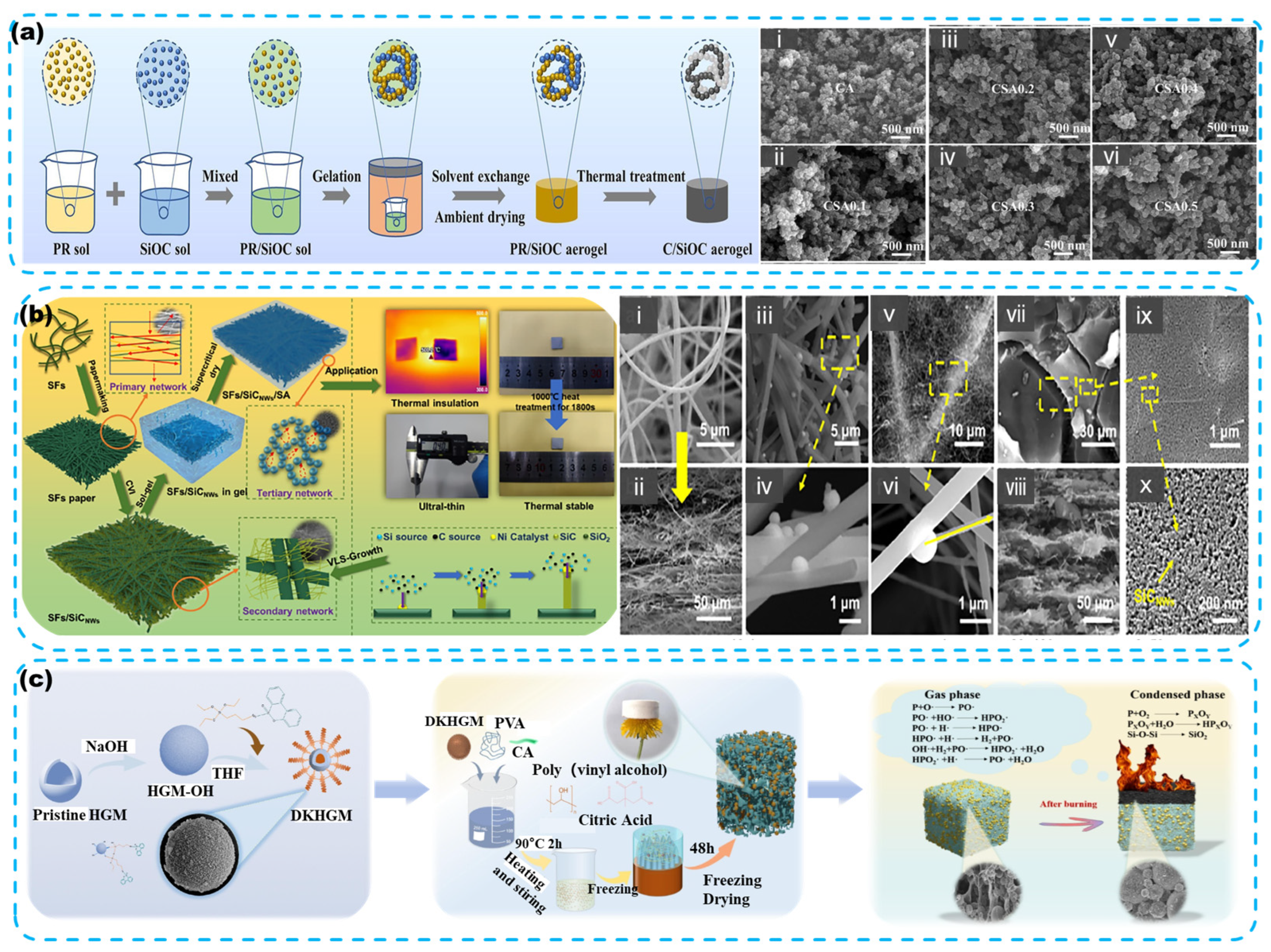
4.2.1. Aerogels
- Properties
- Synthesis
- Modification of Aerogel
4.2.2. Ceramics
- Properties
- Synthesis
5. Application of Thermal Insulating Materials in Batteries
5.1. Performance of PCM
5.2. Performance of Barrier-Type Insulation
6. Conclusions and Prospect
Funding
Conflicts of Interest
References
- Tilley, S.D. Recent advances and emerging trends in photo-electrochemical solar energy conversion. Adv. Energy Mater. 2018, 9, 1802877. [Google Scholar] [CrossRef]
- Neill, S.P.; Angeloudis, A.; Robins, P.E.; Walkington, I.; Ward, S.L.; Masters, I.; Lewis, M.J.; Piano, M.; Avdis, A.; Piggott, M.D.; et al. Tidal range energy resource and optimization–Past perspectives and future challenges. Renew. Energy 2018, 127, 763–778. [Google Scholar] [CrossRef]
- Medhaug, I.; Stolpe, M.B.; Fischer, E.M.; Knutti, R. Reconciling controversies about the ‘global warming hiatus’. Nature 2017, 545, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cano, Z.P.; Banham, D.; Ye, S.Y.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z.W. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Goodenough, J.B. Energy storage materials: A perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Song, Z.Y.; Zhang, X.B.; Li, J.Q.; Hofmann, H.; Ouyang, M.G.; Du, J.Y. Component sizing optimization of plug-in hybrid electric vehicles with the hybrid energy storage system. Energy 2018, 144, 393–403. [Google Scholar] [CrossRef]
- Saxena, A.; Gnanaseelan, N.; Kamaraj, S.; Caballero-Briones, F. Polymer electrolytes for lithium ion batteries. In Rechargeable Lithium-Ion Batteries; CRC Press: Boca Raton, FL, USA, 2020; pp. 260–288. [Google Scholar]
- Zhang, Q.-K.; Zhang, X.-Q.; Yuan, H.; Huang, J.-Q. Thermally stable and nonflammable electrolytes for lithium metal batteries: Progress and perspectives. Small Sci. 2021, 1, 2100058. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Wei, C.; Yu, C.; Chen, S.; Chen, S.; Peng, L.; Wu, Y.; Li, S.; Cheng, S.; Xie, J. Unraveling the LiNbO3 coating layer on battery performances of lithium argyrodite-based all-solid-state batteries under different cut-off voltages. Electrochim. Acta 2023, 438, 141545. [Google Scholar] [CrossRef]
- Tete, P.R.; Gupta, M.M.; Joshi, S.S. Developments in battery thermal management systems for electric vehicles: A technical review. J. Energy Storage 2021, 35, 102255. [Google Scholar] [CrossRef]
- Wu, W.X.; Wang, S.F.; Wu, W.; Chen, K.; Hong, S.H.; Lai, Y.X. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Longchamps, R.S.; Yang, X.G.; Wang, C.Y. Fundamental insights into battery thermal management and safety. ACS Energy Lett. 2022, 7, 1103–1111. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase change materials application in battery thermal management system: A Review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef] [PubMed]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.H.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Tran. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Q.; Guo, S.; Yu, L.; Hu, X. Thermoregulating separators based on phase-change materials for safe lithium-ion batteries. Adv. Mater. 2021, 33, 2008088. [Google Scholar] [CrossRef]
- Tang, S.; Guo, W.; Fu, Y. Advances in composite polymer electrolytes for lithium batteries and beyond. Adv. Energy Mater. 2021, 11, 2000802. [Google Scholar] [CrossRef]
- Tian, X.L.; Yi, Y.K.; Fang, B.R.; Yang, P.; Wang, T.; Liu, P.; Qu, L.; Li, M.T.; Zhang, S.Q. Design strategies of safe electrolytes for preventing thermal runaway in lithium ion batteries. Chem. Mater. 2020, 32, 9821–9848. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Lu, Y. Designing safer lithium-based batteries with nonflammable electrolytes: A review. eScience 2021, 1, 163–177. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Li, L.; Chen, R.; Guo, S. Ionogel electrolytes for high-performance lithium batteries: A review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Wang, H.C.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X.M. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Hubble, D.; Brown, D.E.; Zhao, Y.Z.; Fang, C.; Lau, J.; McCloskey, B.D.; Liu, G. Liquid electrolyte development for low-temperature lithium-ion batteries. Energy Environ. Sci. 2022, 15, 550–578. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium batteries and the solid electrolyte interphase (SEI)—Progress and outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.S.; Hsu, H.J.; Feng, X.N.; Xu, G.L.; Zhuang, M.H.; Gao, H.; Lu, L.G.; Han, X.B.; Chu, Z.Y.; et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Palacín, M.R.; de Guibert, A. Why do batteries fail? Science 2016, 351, 1253292. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wei, Z.B.; He, W.D.; Zhao, J.Y. Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar] [CrossRef]
- Ren, D.S.; Feng, X.N.; Liu, L.S.; Hsu, H.J.; Lu, L.G.; Wang, L.; He, X.M.; Ouyang, M.G. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Wang, L.; Feng, X.N.; He, X.M. Probing the heat sources during thermal runaway process by thermal analysis of different battery chemistries. J. Power Sources 2018, 378, 527–536. [Google Scholar] [CrossRef]
- Feng, X.N.; Zheng, S.Q.; Ren, D.S.; He, X.M.; Wang, L.; Cui, H.; Liu, X.; Jin, C.Y.; Zhang, F.S.; Xu, C.S.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Mao, B.B.; Huang, P.F.; Chen, H.D.; Wang, Q.S.; Sun, J.H. Self-heating reaction and thermal runaway criticality of the lithium ion battery. Int. J. Heat Mass Tran. 2020, 149, 119178. [Google Scholar] [CrossRef]
- Liu, J.L.; Huang, Z.H.; Sun, J.H.; Wang, Q.S. Heat generation and thermal runaway of lithium-ion battery induced by slight overcharging cycling. J. Power Sources 2022, 526, 231136. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Qu, Z.G.; Zhang, J.F.; Rao, Z.H. Rapid prediction method for thermal runaway propagation in battery pack based on lumped thermal resistance network and electric circuit analogy. Appl. Energy 2020, 268, 115007. [Google Scholar] [CrossRef]
- Hong, J.C.; Wang, Z.P.; Qu, C.H.; Zhou, Y.J.; Shan, T.X.; Zhang, J.H.; Hou, Y.K. Investigation on overcharge-caused thermal runaway of lithium-ion batteries in real-world electric vehicles. Appl. Energy 2022, 321, 119229. [Google Scholar] [CrossRef]
- Ren, D.S.; Feng, X.N.; Lu, L.G.; He, X.M.; Ouyang, M.G. Overcharge behaviors and failure mechanism of lithium-ion batteries under different test conditions. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Yuan, Q.F.; Zhao, F.G.; Wang, W.D.; Zhao, Y.M.; Liang, Z.Y.; Yan, D.L. Overcharge failure investigation of lithium-ion batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Liu, J.; Wei, R.; Weng, J.; Wang, J. Investigation of a commercial lithium-ion battery under overcharge/over-discharge failure conditions. RSC Adv. 2018, 8, 33414–33424. [Google Scholar] [CrossRef]
- Ouyang, D.X.; He, Y.P.; Chen, M.Y.; Liu, J.H.; Wang, J. Experimental study on the thermal behaviors of lithium-ion batteries under discharge and overcharge conditions. J. Therm. Anal. Calorim. 2018, 132, 65–75. [Google Scholar] [CrossRef]
- Liang, J.L.; Gan, Y.H.; Li, Y. Investigation on the thermal performance of a battery thermal management system using heat pipe under different ambient temperatures. Energy Convers. Manag. 2018, 155, 1–9. [Google Scholar] [CrossRef]
- Kvasha, A.; Gutiérrez, C.; Osa, U.; de Meatza, I.; Blazquez, J.A.; Macicior, H.; Urdampilleta, I. A comparative study of thermal runaway of commercial lithium ion cells. Energy 2018, 159, 547–557. [Google Scholar] [CrossRef]
- Feng, X.N.; Ouyang, M.G.; Liu, X.; Lu, L.G.; Xia, Y.; He, X.M. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Wang, Z.P.; Yuan, J.; Zhu, X.Q.; Wang, H.; Huang, W.; Wang, Y.T.; Xu, S.Q. Overcharge-to-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Chen, R.S.; Nolan, A.M.; Lu, J.Z.; Wang, J.Y.; Yu, X.Q.; Mo, Y.F.; Chen, L.Q.; Huang, X.J.; Li, H. The thermal stability of lithium solid electrolytes with metallic lithium. Joule 2020, 4, 812–821. [Google Scholar] [CrossRef]
- Hou, J.X.; Lu, L.G.; Wang, L.; Ohma, A.; Ren, D.S.; Feng, X.N.; Li, Y.; Li, Y.L.; Ootani, I.; Han, X.B.; et al. Thermal runaway of Lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes. Nat. Commun. 2020, 11, 5100. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, Y.; Sun, P.; Zheng, B. Effects of thermal insulation layer material on thermal runaway of energy storage lithium battery pack. J. Energy Storage 2024, 76, 109812. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.F.; Yang, N.; Wang, F.Q.; Chen, Y.P.; Lu, D.C.; Liu, H.; Du, Q.; Ren, X.T.; Shi, M.Y. Experimental study on the alleviation of thermal runaway propagation from an overcharged lithium-ion battery module using different thermal insulation layers. Energy 2022, 257, 124768. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Tian, J.; Mei, W.; Jiang, L.; Sun, J.; Wang, Q. Modeling the propagation of internal thermal runaway in lithium-ion battery. Appl. Energy 2024, 362, 123004. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, J.; Feng, X.; Xu, J.; Xu, C.; Wang, H.; Liu, H.; Hou, B.; Rui, X.; Gu, Y.; et al. The trade-off characteristic between battery thermal runaway and combustion. Energy Storage Mater. 2024, 69, 103380. [Google Scholar] [CrossRef]
- Chen, S.; Peng, Q.; Wei, Z.; Li, Y.; Yue, Y.; Zhang, Y.; Zeng, W.; Jin, K.; Jiang, L.; Wang, Q. Revealing the quasi-solid-state electrolyte role on the thermal runaway behavior of lithium metal battery. Energy Storage Mater. 2024, 70, 103481. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, Y.; Zhang, C.; Xu, H.; Zhou, Q.; Wang, C. A novel hybrid battery thermal management system for prevention of thermal runaway propagation. IEEE Trans. Transp. Electrif. 2022, 9, 5028–5038. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef]
- Raoux, S. Phase change materials. Annu. Rev. Mater. Res. 2009, 39, 25–48. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Phase change materials and their basic properties. In Thermal Energy Storage for Sustainable Energy Consumption: Fundamentals, Case Studies and Design; Springer: Berlin/Heidelberg, Germany, 2007; pp. 257–277. [Google Scholar]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Cao, X.; Du, Y.; Zhang, Z.; Gui, Y. Latent heat thermal energy storage systems with solid–liquid phase change materials: A review. Adv. Eng. Mater. 2018, 20, 1700753. [Google Scholar] [CrossRef]
- Sharma, R.; Ganesan, P.; Tyagi, V.; Metselaar, H.; Sandaran, S. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Gulfam, R.; Zhang, P.; Meng, Z. Advanced thermal systems driven by paraffin-based phase change materials–A review. Appl. Energy 2019, 238, 582–611. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, N.; Peng, J.; Fang, X.M.; Gao, X.N.; Fang, Y.T. Preparation and thermal energy storage properties of paraffin/expanded graphite composite phase change material. Appl. Energy 2012, 91, 426–431. [Google Scholar] [CrossRef]
- Hong, Y.; Xin-Shi, G. Preparation of polyethylene–paraffin compound as a form-stable solid-liquid phase change material. Sol. Energy Mater. Sol. Cells 2000, 64, 37–44. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhang, X.L.; Hua, W.S. Review of preparation technologies of organic composite phase change materials in energy storage. J. Mol. Liq. 2021, 336, 115923. [Google Scholar] [CrossRef]
- Li, W.; Dong, Y.; Zhang, X.; Liu, X.L. Preparation and Performance Analysis of Graphite Additive/Paraffin Composite Phase Change Materials. Processes 2019, 7, 447. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, B.; Zhang, G.; Xiao, C.; Yang, X. Preparation of Quasi-Thermoplastic Phase Change Polymer with Intrinsic Antileakage Performance for Battery Thermal Management. Adv. Mater. Interfaces 2021, 8, 2100807. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R.Z. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]
- Zhao, X.W.; Liu, P.F.; Ye, L. Synthesis, structure and phase transition property of acrylic acid grafted paraffin. J. Mol. Struct. 2014, 1064, 37–43. [Google Scholar] [CrossRef]
- Shen, Z.; Kwon, S.; Lee, H.L.; Toivakka, M.; Oh, K. Cellulose nanofibril/carbon nanotube composite foam-stabilized paraffin phase change material for thermal energy storage and conversion. Carbohydr. Polym. 2021, 273, 118585. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zhao, Y.; Ling, Z.; Zhang, Z.; Fang, X. Compatible paraffin@ SiO2 microcapsules/polydimethylsiloxane composites with heat storage capacity and enhanced thermal conductivity for thermal management. Compos. Sci. Technol. 2022, 218, 109192. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.J.; Chiu, J.N.; Martin, V. Polyols as phase change materials for surplus thermal energy storage. Appl. Energy 2016, 162, 1439–1452. [Google Scholar] [CrossRef]
- Tao, J.L.; Luan, J.D.; Liu, Y.; Qu, D.Y.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Tomassetti, S.; Aquilanti, A.; Muciaccia, P.F.; Coccia, G.; Mankel, C.; Koenders, E.A.B.; Di Nicola, G. A review on thermophysical properties and thermal stability of sugar alcohols as phase change materials. J. Energy Storage 2022, 55, 105456. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.Y.; Luo, J.P.; Sun, K.Y.; Zhang, J.; Tan, Z.C.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Tariq, S.L.; Ali, H.M.; Akram, M.A.; Janjua, M.M.; Ahmadlouydarab, M. Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 2020, 176, 115305. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Henn, A.; Penttilä, P.; Österberg, M. Lignin-fatty acid hybrid nanocapsules for scalable thermal energy storage in phase-change materials. Chem. Eng. J. 2020, 393, 124711. [Google Scholar] [CrossRef]
- Abdulmunem, A.R.; Hamed, H.M.; Samin, P.M.; Mazali, I.I.; Sopian, K. Thermal management of lithium-ion batteries using palm fatty acid distillate as a sustainable bio-phase change material. J. Energy Storage 2023, 73, 109187. [Google Scholar] [CrossRef]
- Nazari, M.; Jebrane, M.; Terziev, N. Multicomponent bio-based fatty acids system as phase change material for low temperature energy storage. J. Energy Storage 2021, 39, 102645. [Google Scholar] [CrossRef]
- Yuan, M.; Ren, Y.; Xu, C.; Ye, F.; Du, X. Characterization and stability study of a form-stable erythritol/expanded graphite composite phase change material for thermal energy storage. Renew. Energy 2019, 136, 211–222. [Google Scholar] [CrossRef]
- Yan, K.; Feng, Y.; Qiu, L. Thermal and photo/electro-thermal conversion characteristics of high energy storage density expanded graphite/polyethylene glycol shaped composite phase change materials. Sol. Energy 2024, 272, 112477. [Google Scholar] [CrossRef]
- Qiu, L.; Yan, K.; Feng, Y.; Liu, X. Nano additives-enhanced PEG /AlN composites with high cycle stability to improve thermal and heat storage properties. Energy 2023, 278, 127794. [Google Scholar] [CrossRef]
- Oya, T.; Nomura, T.; Okinaka, N.; Akiyama, T. Phase change composite based on porous nickel and erythritol. Appl. Therm. Eng. 2012, 40, 373–377. [Google Scholar] [CrossRef]
- Yu, K.Y.; Liu, Y.S.; Yang, Y.Z. Review on form-stable inorganic hydrated salt phase change materials: Preparation, characterization and effect on the thermophysical properties. Appl. Energy 2021, 292, 116845. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, C.C.; Lin, N.Z.; Xie, B.S.; Zhang, D.Y.; Chen, J. Review on tailored phase change behavior of hydrated salt as phase change materials for energy storage. Mater. Today Energy 2021, 22, 100866. [Google Scholar] [CrossRef]
- Man, X.; Lu, H.; Xu, Q.; Wang, C.; Ling, Z. Review on the thermal property enhancement of inorganic salt hydrate phase change materials. J. Energy Storage 2023, 72, 108699. [Google Scholar] [CrossRef]
- Huang, J.; Dai, J.; Peng, S.; Wang, T.; Hong, S. Modification on hydrated salt-based phase change composites with carbon fillers for electronic thermal management. Int. J. Energy Res. 2019, 43, 3550–3560. [Google Scholar] [CrossRef]
- Mombeki Pea, H.J.; An, Z.; Du, X.; Hou, W.; Zhang, D.; Liu, X. Experimental and analytical studies on latent heat of hydrated salt/modified EG-based form-stable composite PCMs for energy storage application. Renew. Energy 2024, 222, 119978. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Deng, Y.; Nian, H.; Jiang, H. Supercooling suppression and thermal conductivity enhancement of Na2HPO4·12H2O/expanded vermiculite form-stable composite phase change materials with alumina for heat storage. ACS Sustain. Chem. Eng. 2018, 6, 6792–6801. [Google Scholar] [CrossRef]
- Lu, J.; Deng, Y.; Luo, D.; Wu, F.; Dai, X. Preparation and performance enhancement of MXene/Na2HPO4· 12H2O@ SiO2 phase change microcapsule. J. Energy Storage 2024, 91, 112079. [Google Scholar] [CrossRef]
- McGillicuddy, R.D.; Thapa, S.; Wenny, M.B.; Gonzalez, M.I.; Mason, J.A. Metal–organic phase-change materials for thermal energy storage. J. Am. Chem. Soc. 2020, 142, 19170–19180. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.Y.; Tang, Z.D.; Dong, W.J.; Li, A.; Wang, G. Optimization strategies of composite phase change materials for thermal energy storage, transfer, conversion and utilization. Energy Environ. Sci. 2020, 13, 4498–4535. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-based composite phase change materials for thermal energy storage, transfer, and conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef]
- Dai, X.; Ping, P.; Kong, D.; Gao, X.; Zhang, Y.; Wang, G.; Peng, R. Heat transfer enhanced inorganic phase change material compositing carbon nanotubes for battery thermal management and thermal runaway propagation mitigation. J. Energy Chem. 2024, 89, 226–238. [Google Scholar] [CrossRef]
- Sun, M.; Liu, T.; Li, M.; Tan, J.; Tian, P.; Wang, H.; Chen, G.; Jiang, D.; Liu, X. A deep supercooling eutectic phase change material for low-temperature battery thermal management. J. Energy Storage 2022, 50, 104240. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; He, J.; Xu, D.; Abdou, S.N.; Ibrahim, M.M.; Sun, S.; Chen, Y.; Li, H.; Xu, B.B.; et al. Experimental design of paraffin/methylated melamine-formaldehyde microencapsulated composite phase change material and the application in battery thermal management system. J. Mater. Sci. Technol. 2024, 169, 124–136. [Google Scholar] [CrossRef]
- Wen, R.; Chen, B.; Yin, L.; Yuan, X.; Ma, A. Thermal properties and non-isothermal crystallization behavior of ternary eutectic phase change material for thermal energy storage. J. Energy Storage 2024, 84, 110566. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, D.; Chen, L.; Cao, X.; Li, X.; Qu, Y. Preparation and thermal properties analysis of fatty acids/1-hexadecanol binary eutectic phase change materials reinforced with TiO2 particles. J. Energy Storage 2022, 51, 104546. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A review and evaluation of thermal insulation materials and methods for thermal energy storage systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
- Kumar, D.; Alam, M.; Zou, P.X.W.; Sanjayan, J.G.; Memon, R.A. Comparative analysis of building insulation material properties and performance. Renew. Sustain. Energy Rev. 2020, 131, 110038. [Google Scholar] [CrossRef]
- Xu, Y.; Kraemer, D.; Song, B.; Jiang, Z.; Zhou, J.; Loomis, J.; Wang, J.; Li, M.; Ghasemi, H.; Huang, X.; et al. Nanostructured polymer films with metal-like thermal conductivity. Nat. Commun. 2019, 10, 1771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Zhang, X.; Ding, X.; Zhang, P.; Shu, M.T.; Zhang, Q.; Gong, Y.; Zheng, K.; Tian, X.Y. Imidization-induced carbon nitride nanosheets orientation towards highly thermally conductive polyimide film with superior flexibility and electrical insulation. Compos. Part B Eng. 2020, 199, 108267. [Google Scholar] [CrossRef]
- Fraleoni-Morgera, A.; Chhikara, M. Polymer-based nano-composites for thermal insulation. Adv. Eng. Mater. 2019, 21, 1801162. [Google Scholar] [CrossRef]
- Wang, F.; Ke, X.; Shen, K.; Zhu, L.; Yuan, C. A critical review on materials and fabrications of thermally stable separators for lithium-ion batteries. Adv. Mater. Technol. 2022, 7, 2100772. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent development in separators for high-temperature lithium-ion batteries. Small 2019, 15, 1901689. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Wen, J.; Wang, C.; Ma, X.; Yang, Y.; Huang, G.; Ye, H.-M.; Xu, S. Research progress on high-temperature resistant polymer separators for lithium-ion batteries. Energy Storage Mater. 2022, 51, 638–659. [Google Scholar] [CrossRef]
- Ge, C.B.; Zhai, W.T. Cellular thermoplastic polyurethane thin film: Preparation, elasticity, and thermal insulation performance. Ind. Eng. Chem. Res. 2018, 57, 4688–4696. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A review on porous polymeric membrane preparation. part II: Production techniques with polyethylene, polydimethylsiloxane, polypropylene, polyimide, and polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.P.; Bouyer, D.; Drioli, E.; Kiadeh, N.T.H. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Wang, F.; Altschuh, P.; Ratke, L.; Zhang, H.; Selzer, M.; Nestler, B. Progress report on phase separation in polymer solutions. Adv. Mater. 2019, 31, e1806733. [Google Scholar] [CrossRef] [PubMed]
- Muche, Z.B.; Nikodimos, Y.; Tekaligne, T.M.; Merso, S.K.; Agnihotri, T.; Serbessa, G.G.; Wu, S.H.; Su, W.N.; Hwang, B.J. Thermally stable 3D cross-linked fluorinated polyimide/PVDF-HFP hybrid separator for lithium battery applications. Chem. Eng. J. 2023, 476, 146400. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Xiong, R.; Wang, X.; Shen, G.; Sun, S.; Chen, Y.; Huang, T.; Zhou, H.; Zhang, Y. High-safety poly(ethylene-co-vinyl acetate)/poly(ether ether ketone)/poly(ethylene-co-vinyl acetate) composite separator with the thermal shutdown feature for lithium-ion battery. J. Membr. Sci. 2023, 687, 122059. [Google Scholar] [CrossRef]
- Yu, J.; Dong, N.; Liu, B.; Tian, G.; Qi, S.; Wu, D. A newly-developed heat-resistance polyimide microsphere coating to enhance the thermal stability of commercial polyolefin separators for advanced lithium-ion battery. Chem. Eng. J. 2022, 442, 136314. [Google Scholar] [CrossRef]
- Hu, W.; Fu, W.; Jhulki, S.; Chen, L.; Narla, A.; Sun, Z.; Wang, F.; Magasinski, A.; Yushin, G. Heat-resistant Al2O3 nanowire-polyetherimide separator for safer and faster lithium-ion batteries. J. Mater. Sci. Technol. 2023, 142, 112–120. [Google Scholar] [CrossRef]
- Hu, F.; Wu, S.; Sun, Y. Hollow-structured materials for thermal insulation. Adv. Mater. 2019, 31, e1801001. [Google Scholar] [CrossRef]
- Doğan, B.; Tan, H. The numerical and experimental investigation of the change of the thermal conductivity of expanded polystyrene at different temperatures and densities. Int. J. Polym. Sci. 2019, 2019, 6350326. [Google Scholar] [CrossRef]
- Chung, S.Y.; Abd Elrahman, M.; Kim, J.S.; Han, T.S.; Stephan, D.; Sikora, P. Comparison of lightweight aggregate and foamed concrete with the same density level using image-based characterizations. Constr. Build. Mater. 2019, 211, 988–999. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Thermally insulating, fiber-reinforced alumina–silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 128402. [Google Scholar] [CrossRef]
- Apostolopoulou-Kalkavoura, V.; Munier, P.; Bergström, L. Thermally insulating nanocellulose-based materials. Adv. Mater. 2021, 33, 2001839. [Google Scholar] [CrossRef]
- Yang, C.G.; Zhang, Q.; Zhang, W.L.; Xia, M.; Yan, K.; Lu, J.; Wu, G.Z. High thermal insulation and compressive strength polypropylene microcellular foams with honeycomb structure. Polym. Degrad. Stab. 2021, 183, 109406. [Google Scholar] [CrossRef]
- Leng, W.Q.; Pan, B. Thermal insulating and mechanical properties of cellulose nanofibrils modified polyurethane foam composite as structural insulated material. Forests 2019, 10, 200. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.Q.; Zou, S.; Zhang, G.Q. Experimental research on a feasible rice husk/geopolymer foam building insulation material. Energ Build. 2020, 226, 110358. [Google Scholar] [CrossRef]
- Karami, P.; Khorshidi, B.; McGregor, M.; Peichel, J.T.; Soares, J.B.P.; Sadrzadeh, M. Thermally stable thin film composite polymeric membranes for water treatment: A review. J. Clean. Prod. 2020, 250, 119447. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Lee, J.W.; Sun, C.; Ma, B.S.; Kim, H.J.; Wang, C.; Ryu, J.M.; Lim, C.; Kim, T.S.; Kim, Y.H.; Kwon, S.K. Efficient, thermally stable, and mechanically robust all-polymer solar cells consisting of the same benzodithiophene unit-based polymer acceptor and donor with high molecular compatibility. Adv. Energy Mater. 2021, 11, 2003367. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wu, W.J.; Wei, J.; Bian, Y.P.; Luo, H.G. Preparation of foamed gel for preventing spontaneous combustion of coal. Fuel 2021, 300, 121024. [Google Scholar] [CrossRef]
- Wang, J.; Chai, J.; Wang, G.; Zhao, J.; Zhang, D.; Li, B.; Zhao, H.; Zhao, G. Strong and thermally insulating polylactic acid/glass fiber composite foam fabricated by supercritical carbon dioxide foaming. Int. J. Biol. Macromol. 2019, 138, 144–155. [Google Scholar] [CrossRef]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; Fu, H.; Wu, Z.; Zhang, Y.; Chao, G.; Chen, S.; Zhang, L.; Liu, T. Thermotolerant separator of cross-linked polyimide fibers with narrowed pore size for lithium-ion batteries. J. Membr. Sci. 2022, 662, 121004. [Google Scholar] [CrossRef]
- Cha, H.-A.; Jo, M.-G.; Moon, Y.K.; Hahn, B.-D.; Ahn, C.-W.; Choi, J.-J.; Kim, D.K. Highly porous YSZ ceramic foams using hollow spheres with holes in their shell for high-performance thermal insulation. J. Eur. Ceram. Soc. 2023, 43, 7041–7052. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J. Fabrication of organic based thermal resistance material using pyrolyzed cellulose nanofiber and carbon fiber for high performance thermal insulation. Mater. Today Chem. 2023, 33, 101734. [Google Scholar] [CrossRef]
- Zygmunt-Kowalska, B.; Zakrzewska, P.; Szajding, A.; Handke, B.; Kuznia, M. Polyurethane foams reinforced with microspheres-assessment of the application in construction as a thermal insulation material. Thermochim. Acta 2023, 726, 179556. [Google Scholar] [CrossRef]
- Bausch, B.; Frankl, S.; Becher, D.; Menz, F.; Baier, T.; Bauer, M.; Böse, O.; Hölzle, M. Naturally-derived thermal barrier based on fiber-reinforced hydrogel for the prevention of thermal runaway propagation in high-energetic lithium-ion battery packs. J. Energy Storage 2023, 61, 106841. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, M.; Chen, Z.; Lu, L.; Ma, Z.; Ding, Y.; Yin, L.; Liu, T.; Li, M.; Yang, L.; et al. A layered aerogel composite with silica fibers, SiC nanowires, and silica aerogels ternary networks for thermal insulation at high-temperature. J. Mater. Sci. Technol. 2025, 204, 71–80. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Duraes, L. An overview on alumina-silica-based aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef]
- Hu, L.; He, R.J.; Lei, H.S.; Fang, D.N. Carbon aerogel for insulation applications: A Review. Int. J. Thermophys. 2019, 40, 39. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Z.; Wei, Z.; Chen, S.; Huang, Z.; Fang, Z.; Li, Y.; Wang, J.; Mei, W.; Wang, Q. Enhancing battery module safety with insulation material: Hollow glass microspheres incorporating aerogel of varying particle sizes. Chem. Eng. J. 2023, 478, 147400. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv. Mater. 2021, 33, e2005569. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Li, L.; Cai, H.; Feng, J. A facile method to fabricate monolithic alumina–silica aerogels with high surface areas and good mechanical properties. J. Eur. Ceram. Soc. 2020, 40, 2480–2488. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Torres, C.E.I.; González, L.T.; Kharisov, B.I. All-carbon hybrid aerogels: Synthesis, properties, and applications. Ind. Eng. Chem. Res. 2019, 58, 16258–16286. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Z.; Jiao, R.; Chen, Y.; Cao, X.; Sun, H.; Li, J.; Li, A. Preparation of DOPO-KH550 modified hollow glass microspheres/PVA composite aerogel for thermal insulation and flame retardancy. J. Colloid Interface Sci. 2024, 654, 719–730. [Google Scholar] [CrossRef]
- Yang, D.; Dong, S.; Xin, J.; Liu, C.; Hu, P.; Xia, L.; Hong, C.; Zhang, X. Robust and thermostable C/SiOC composite aerogel for efficient microwave absorption, thermal insulation and flame retardancy. Chem. Eng. J. 2023, 469, 143851. [Google Scholar] [CrossRef]
- Yang, R.; Hu, F.; An, L.; Armstrong, J.; Hu, Y.; Li, C.; Huang, Y.; Ren, S. A hierarchical mesoporous insulation ceramic. Nano Lett. 2019, 20, 1110–1116. [Google Scholar] [CrossRef]
- Dutto, A.; Zanini, M.; Jeoffroy, E.; Tervoort, E.; Mhatre, S.A.; Seibold, Z.B.; Bechthold, M.; Studart, A.R. 3D printing of hierarchical porous ceramics for thermal insulation and evaporative cooling. Adv. Mater. Technol. 2023, 8, 2201109. [Google Scholar] [CrossRef]
- Rajan, J.T.; Jayapal, V.S.; Krishna, M.J.; Mohammed Firose, K.A.; Vaisakh, S.; John, A.K.; Suryan, A. Analysis of battery thermal management system for electric vehicles using 1-tetradecanol phase change material. Sustain. Energy Technol. Assess. 2022, 51, 101943. [Google Scholar] [CrossRef]
- Ping, P.; Dai, X.; Kong, D.; Zhang, Y.; Zhao, H.; Gao, X.; Gao, W. Experimental study on nano-encapsulated inorganic phase change material for lithium-ion battery thermal management and thermal runaway suppression. Chem. Eng. J. 2023, 463, 142401. [Google Scholar] [CrossRef]
- Zou, D.Q.; Liu, X.S.; He, R.J.; Zhu, S.X.; Bao, J.M.; Guo, J.R.; Hu, Z.G.; Wang, B.H. Preparation of a novel composite phase change material (PCM) and its locally enhanced heat transfer for power battery module. Energy Convers. Manag. 2019, 180, 1196–1202. [Google Scholar] [CrossRef]
- Palanisamy, M.; Lin, K.W.; Lo, C.T.; Pol, V.G. In situ thermal safety aspect of the electrospun polyimide-Al2O3 separator reveals less exothermic heat energies than polypropylene at the thermal runaway event of lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 28310–28320. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, Y.; Li, X.; Zhang, L.; Yan, J. Continuous rapid fabrication of ceramic fiber sponge aerogels with high thermomechanical properties via a green and low-cost electrospinning technique. ACS Nano 2024, 18, 19054–19063. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Mu, X.; Zhu, Y.; Liao, C.; Han, L.; Wang, J.; Cai, W.; Kan, Y.; Song, L.; Hu, Y. Sandwich structured ultra-strong-heat-shielding aerogel/copper composite insulation board for safe lithium-ion batteries modules. J. Energy Chem. 2023, 76, 438–447. [Google Scholar] [CrossRef]
| Paraffin Wax | Molecular Formula | Melting Temperature (°C) | Crystallization Temperature (°C) | ΔHfus (kJ·kg−1) |
|---|---|---|---|---|
| n-Dodecane | CH3(CH2)10CH3 | −10 | −16 | 216 |
| n-Tridecane | CH3(CH2)11CH3 | −5 | −9 | 160 |
| n-Tetradecane | CH3(CH2)12CH3 | 5–6 | 0 | 227 |
| n-Pentadecane | CH3(CH2)13CH3 | 10 | 5 | 205 |
| n-Hexadecane | CH3(CH2)14CH3 | 18–19 | 17 | 237 |
| n-Heptadecane | CH3(CH2)15CH3 | 22 | 22 | 171 |
| n-Octadecane | CH3(CH2)16CH3 | 28 | 25 | 242 |
| n-Nonadecane | CH3(CH2)17CH3 | 32–33 | 27 | 222 |
| n-Eicosane | CH3(CH2)18CH3 | 36–37 | 31 | 247 |
| n-Heneicosane | CH3(CH2)19CH3 | 39–41 | 32 | 201 |
| n-Docosane | CH3(CH2)20CH3 | 42–45 | 43 | 157 |
| n-Tricosane | CH3(CH2)21CH3 | 48.9 | 51 | 142 |
| n-Tetracosane | CH3(CH2)22CH3 | 50–51 | 48–49 | 160 |
| n-Pentacosane | CH3(CH2)23CH3 | 54 | 47 | 164 |
| n-Hexacosane | CH3(CH2)24CH3 | 56 | 53–54 | 255 |
| n-Heptacosane | CH3(CH2)25CH3 | 59 | 53 | 159 |
| n-Octacosane | CH3(CH2)26CH3 | 61 | 54 | 202 |
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Physical mixing | simple operation | poor uniformity |
| Copolymerization | performance improvement | strict process |
| Melt impregnation | high production efficiency | obvious internal voids |
| Microencapsulation | high material stability | high cost |
|
Average Molar
Mass (g mol−1) |
Melting
Temperature (°C) | ΔHfus (kJ·kg−1) | Crystallization Temperature (°C) | ΔHcryst (kJ·kg−1) |
|---|---|---|---|---|
| 400 | 3.2 | 91.4 | −24 | 85–86 |
| 600 | 22.2 | 108.4 | −7 | 116 |
| 1000 | 32.0 | 149.5 | 28 | 140 |
| 1500 | 46.5 | 176.3 | 39–40 | 169 |
| 2000 | 51.0 | 181.4 | 35 | 168 |
| 3400 | 56.6 | 174.1 | 29 | 159 |
| 4000 | 59.7 | 189.7 | 22 | 167 |
| 6000 | 64.8 | 189.0 | 33 | 161 |
| 10,000 | 66.0 | 189.6 | 38 | 167 |
| 20,000 | 68.7 | 187.8 | 38 | 161 |
| Eutectic Mixture of Fatty Acids | # of C Atoms in Fatty Acids | Composition by Mass | Melting Temperature (°C) | ΔHfus (kJ·kg−1) |
|---|---|---|---|---|
| Lauric-palmitic | 12C:16C | 66:34 | 33–37 | 169 |
| Lauric-myristic | 12C:14C | 63:37 | 31–37 | 170 |
| Lauric-stearic | 12C:18C | 76:24 | 37 | 171 |
| Myristic-stearic | 14C:18C | 50:50 | 35−52 | 189 |
| Myristic-palmitic | 14C:16C | 66:34 | 44 | 181 |
| Palmitic-stearic | 16C:18C | 65:35 | 51 | 179 |
| Capric-lauric | 10C:12C | 65:35 | 13–14 | 117 |
| Capric-palmitic | 10C:16C | 75:25 | 26–33 | 142 |
| Capric-myristic | 10C:14C | 74:26 | 23 | 155 |
| Capric-stearic | 10C:18C | 87:13 | 27 | 160 |
| Compound | Latent Heat (J/g) | Thermal Conductivity (W/m·K) | Supercooling Degree (°C) | Drawback |
|---|---|---|---|---|
| LiClO3·3H2O | 253 | NR * | 8.00 | high price |
| CaCl2·6H2O | 174 | 0.550 | 47.6 | high supercooling |
| LiNO3·3H2O | 256 | NR | 10.1 | high price |
| Na2CO3·10H2O | 247 | 0.600 | 13.7 | high supercooling |
| Na2HPO4·12H2O | 280 | 0.514 | 13.0 | high supercooling |
| FeCl3·6H2O | 223 | NR | NR | NR |
| Ca(NO3)2·4H2O | 153 | 0.570 | 87.0 | high supercooling |
| Mg(NO3)2·2H2O | 142 | NR | NR | NR |
| Fe(NO3)2·9H2O | 155 | NR | NR | NR |
| MgSO4·7H2O | 202 | NR | NR | NR |
| Ca(NO3)2·3H2O | 104 | NR | NR | NR |
| Zn(NO3)2·2H2O | 68.0 | NR | NR | NR |
| FeCl3·2H2O | 90.0 | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, T.; Xia, Q.; Wei, X.; Zhu, Y. Recent Development of Thermal Insulating Materials for Li-Ion Batteries. Energies 2024, 17, 4412. https://doi.org/10.3390/en17174412
Quan T, Xia Q, Wei X, Zhu Y. Recent Development of Thermal Insulating Materials for Li-Ion Batteries. Energies. 2024; 17(17):4412. https://doi.org/10.3390/en17174412
Chicago/Turabian StyleQuan, Ting, Qi Xia, Xiaoyu Wei, and Yanli Zhu. 2024. "Recent Development of Thermal Insulating Materials for Li-Ion Batteries" Energies 17, no. 17: 4412. https://doi.org/10.3390/en17174412
APA StyleQuan, T., Xia, Q., Wei, X., & Zhu, Y. (2024). Recent Development of Thermal Insulating Materials for Li-Ion Batteries. Energies, 17(17), 4412. https://doi.org/10.3390/en17174412







