Optimization of Cellulose Recovery Using Deep Eutectic Solvent Fractionation: A Response Surface Method Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Fractionation from Sugarcane Bagasse Using DES Fractionation
2.3. Statistical Design and Optimization of Cellulose Fractionation Using RSM
+ β13X1X3 + β23X2X3 + β11X21 + β22X22 + β33X23
2.4. Quantitative Analysis of Sugars and Their Breakdown Products in the Liquid Fraction
2.5. Analysis of the Cellulose Component
3. Results and Discussion
3.1. Chemical Composition of Raw Sugarcane Bagasse
3.2. Effects of Different DESs on the Fraction of Cellulose
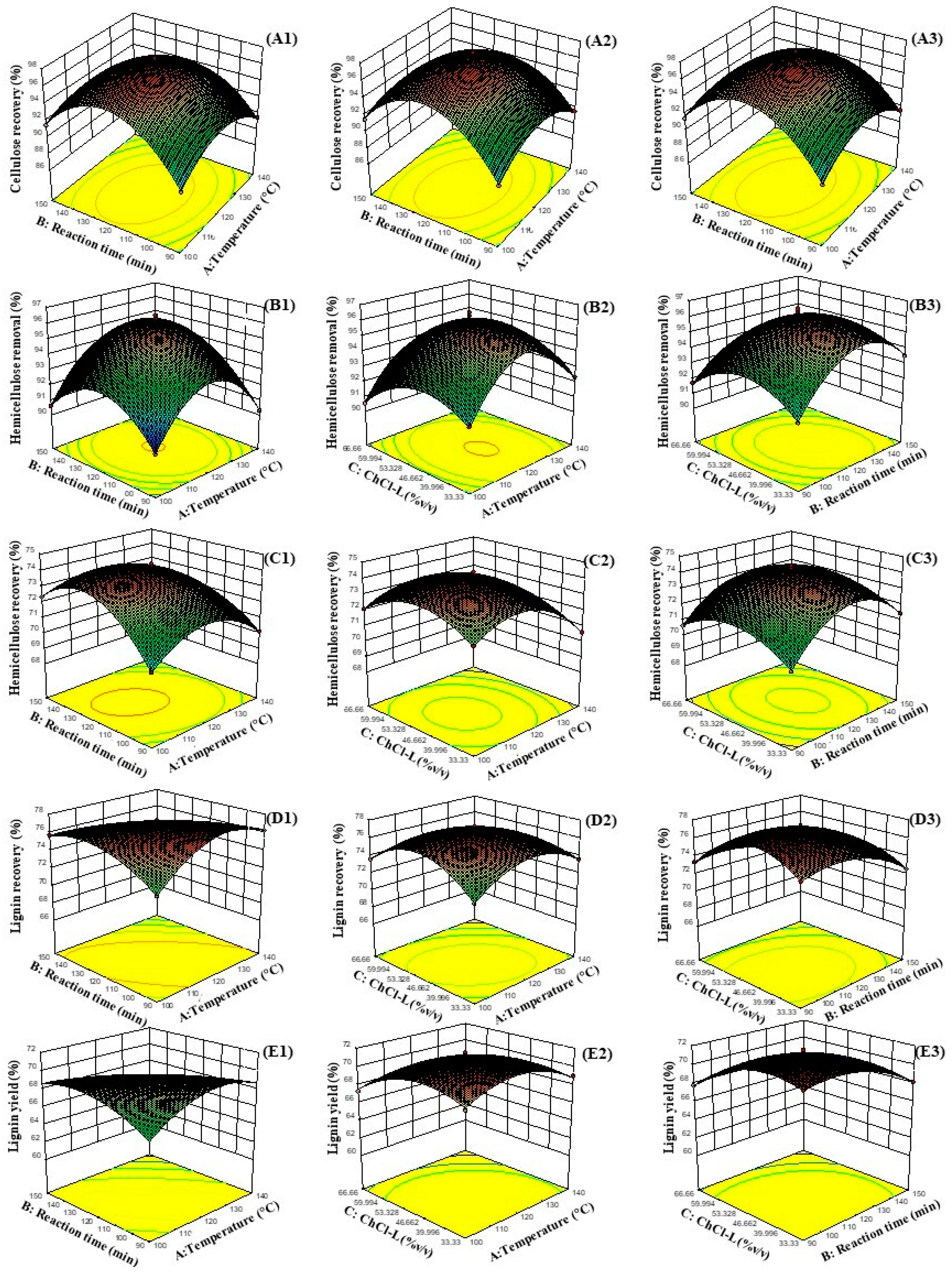
3.3. Influence of Independent Factors on Cellulose Recovery, Hemicellulose Removal, Hemicellulose Recovery, Lignin Recovery, and Lignin Yield
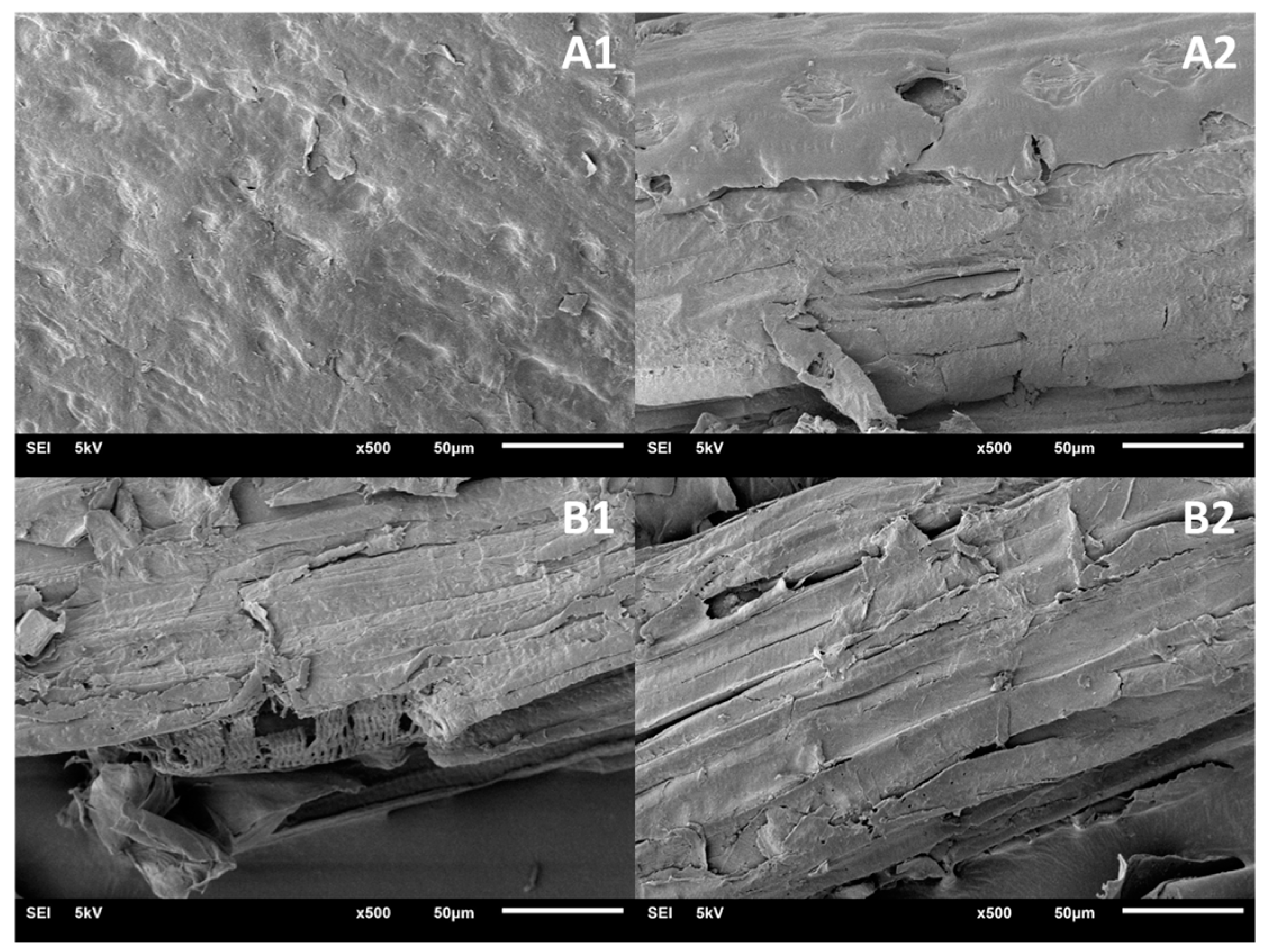
3.4. Characterization of Raw Materials and Fractionated Sugarcane Bagasse after DES Fractionation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Yıldız, İ. Energy and Water Pollution. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Oxford, UK, 2018; pp. 950–979. [Google Scholar]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Padhye, L.P.; Jasemizad, T.; Govarthanan, M.; Karmegam, N.; Wijesekara, H.; Amarasiri, D.; Hou, D.; Zhou, P.; Biswal, B.K.; et al. Impacts of climate change on the fate of contaminants through extreme weather events. Sci. Total Environ. 2024, 909, 168388. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.W.; Sinha, A.; Ahmed, Z.; Qin, Q.; Zaidi, S.A.H. Effects of biomass energy consumption on environmental quality: The role of education and technology in Asia-Pacific Economic Cooperation countries. Renew. Sustain. Energy Rev. 2021, 142, 110868. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Mikula, K.; Samoraj, M.; Gil, F.; Taf, R.; Moustakas, K.; Chojnacka, K. Lignocellulosic biomass fertilizers: Production, characterization, and agri-applications. Sci. Total Environ. 2024, 923, 171343. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, V.; Tsai, M.-L.; Chen, C.-W.; Sun, P.-P.; Nargotra, P.; Wang, J.-X.; Dong, C.-D. Development of lignocellulosic biorefineries for the sustainable production of biofuels: Towards circular bioeconomy. Bioresour. Technol. 2023, 381, 129145. [Google Scholar] [CrossRef]
- Tan, Y.T.; Chua, A.S.M.; Ngoh, G.C. Deep eutectic solvent for lignocellulosic biomass fractionation and the subsequent conversion to bio-based products—A review. Bioresour. Technol. 2020, 297, 122522. [Google Scholar] [CrossRef]
- Kiran Kumar, A.; Sharma, S.; Dixit, G.; Shah, E.; Patel, A.; Boczkaj, G. Techno-economic evaluation of a natural deep eutectic solvent-based biorefinery: Exploring different design scenarios. Biofuels Bioprod. Biorefin. 2020, 14, 746–763. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.d.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Tantayotai, P.; Panakkal, E.J.; Chuetor, S.; Kirdponpattara, S.; Thomas, A.S.S.; Sharma, B.K.; Sriariyanun, M. Hydrothermal pretreatment optimization and deep eutectic solvent pretreatment of lignocellulosic biomass: An integrated approach. Bioresour. Technol. Rep. 2022, 17, 100957. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.; Deng, S.; Deng, O.; Zhou, W.; et al. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Patil, S.; Kumar, R.; Ha, G.S.; Park, Y.K.; Ali Khan, M.; Kumar Yadav, K.; Fallatah, A.M.; Jeon, B.H. Integrated hydrothermal and deep eutectic solvent-mediated fractionation of lignocellulosic biocomponents for enhanced accessibility and efficient conversion in anaerobic digestion. Bioresour. Technol. 2022, 351, 127034. [Google Scholar] [CrossRef]
- Thi, S.; Lee, K.M. Comparison of deep eutectic solvents (DES) on pretreatment of oil palm empty fruit bunch (OPEFB): Cellulose digestibility, structural and morphology changes. Bioresour. Technol. 2019, 282, 525–529. [Google Scholar] [CrossRef]
- Lin, W.; Xing, S.; Jin, Y.; Lu, X.; Huang, C.; Yong, Q. Insight into understanding the performance of deep eutectic solvent pretreatment on improving enzymatic digestibility of bamboo residues. Bioresour. Technol. 2020, 306, 123163. [Google Scholar] [CrossRef]
- Hong, S.; Song, Y.; Yuan, Y.; Lian, H.; Liimatainen, H. Production and characterization of lignin containing nanocellulose from luffa through an acidic deep eutectic solvent treatment and systematic fractionation. Ind. Crops Prod. 2020, 143, 111913. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, K.; Xu, Q.; Zhao, Y.; Li, J.; Yang, G.; Zhang, Y.; Wang, Y.; Zhang, L.; Wang, B.; et al. Microwave-assisted choline chloride/lactic acid pretreatment for high-efficiency separation and value-added utilization of Sugarcane bagasse components. Ind. Crops Prod. 2024, 220, 119251. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Che, X.; Liu, S.; Tian, W. Multivariate analysis of the process of deep eutectic solvent pretreatment of lignocellulosic biomass. Ind. Crops Prod. 2020, 150, 112363. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Denver, CO, USA, 2008. [Google Scholar]
- Ji, Q.; Yu, X.; Wu, P.; Yagoub, A.E.-G.A.; Chen, L.; Abdullateef Taiye, M.; Zhou, C. Pretreatment of sugarcane bagasse with deep eutectic solvents affect the structure and morphology of lignin. Ind. Crops Prod. 2021, 173, 114108. [Google Scholar] [CrossRef]
- Chourasia, V.R.; Pandey, A.; Pant, K.K.; Henry, R.J. Improving enzymatic digestibility of sugarcane bagasse from different varieties of sugarcane using deep eutectic solvent pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Xu, L.H.; Zhang, C.; Guo, K.N.; Yuan, T.Q.; Wen, J.L. A synergistic hydrothermal-deep eutectic solvent (DES) pretreatment for rapid fractionation and targeted valorization of hemicelluloses and cellulose from poplar wood. Bioresour. Technol. 2021, 341, 125828. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Sun, Y.; Yang, Q.; Zhang, F.; Fang, G.; Zhu, H.; Liu, Y. Selective degradation of hemicellulose and lignin for improving enzymolysis efficiency via pretreatment using deep eutectic solvents. Bioresour. Technol. 2023, 376, 128937. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, Fat Mass and Immune System: Role for Leptin. Front. Physiol. 2018, 1, 640. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Lusi, A.; Wan, C. High-Solid Lignocellulose Processing Enabled by Natural Deep Eutectic Solvent for Lignin Extraction and Industrially Relevant Production of Renewable Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 12205–12216. [Google Scholar] [CrossRef]
- Meng, Y. A Sustainable Approach to Fabricating Ag Nanoparticles/PVA Hybrid Nanofiber and Its Catalytic Activity. Nanomaterials 2015, 5, 1124–1135. [Google Scholar] [CrossRef]
- Vitolina, S.A.-O.; Shulga, G.; Neiberte, B.; Jaunslavietis, J.; Verovkins, A.; Betkers, T. Characteristics of the Waste Wood Biomass and Its Effect on the Properties of Wood Sanding Dust/Recycled PP Composite. Polymers 2022, 14, 468. [Google Scholar] [CrossRef]
- Kang, S.; Xiao, L.-P.; Meng, L.; Zhang, X.; Sun, R. Isolation and Structural Characterization of Lignin from Cotton Stalk Treated in an Ammonia Hydrothermal System. Int. J. Mol. Sci. 2012, 13, 15209–15226. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Alén, R.; Sahoo, G. Characterization of Hardwood Soda-AQ Lignins Precipitated from Black Liquor through Selective Acidification. BioResources 2016, 11, 9869–9879. [Google Scholar] [CrossRef]
- Atykyan, N.; Revin, V.; Shutova, V. Raman and FT-IR Spectroscopy investigation the cellulose structural differences from bacteria Gluconacetobacter sucrofermentans during the different regimes of cultivation on a molasses media. AMB Express 2020, 10, 84. [Google Scholar] [CrossRef]
- Kalembkiewicz, J.; Galas, D.; Sitarz-Palczak, E. The Physicochemical Properties and Composition of Biomass Ash and Evaluating Directions of its Applications. Pol. J. Environ. Stud. 2018, 27, 2593–2603. [Google Scholar] [CrossRef]
- Shi, J.; Xing, D.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Slathia, P.; Raina, N.; Kiran, A.; Kour, R.; Bhagat, D.; Sharma, P. Dilute acid pretreatment of pine needles of Pinus roxburghii by response surface methodology for bioethanol production by separate hydrolysis and fermentation. Biomass Convers. Biorefin. 2020, 10, 95–106. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 2018, 11, 214. [Google Scholar] [CrossRef]
- Lv, S.; Yu, Q.; Zhuang, X.; Yuan, Z.; Wang, W.; Wang, Q.; Qi, W.; Tan, X. The Influence of Hemicellulose and Lignin Removal on the Enzymatic Digestibility from Sugarcane Bagasse. BioEnergy Res. 2013, 6, 1128–1134. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, C.; Zhan, Y.; Liu, X.; Wang, J.; Meng, X.; Yoo, C.G.; Fang, G.; Ragauskas, A.J. A high-solid DES pretreatment using never-dried biomass as the starting material: Towards high-quality lignin fractionation. Green Chem. 2023, 25, 1571–1581. [Google Scholar] [CrossRef]


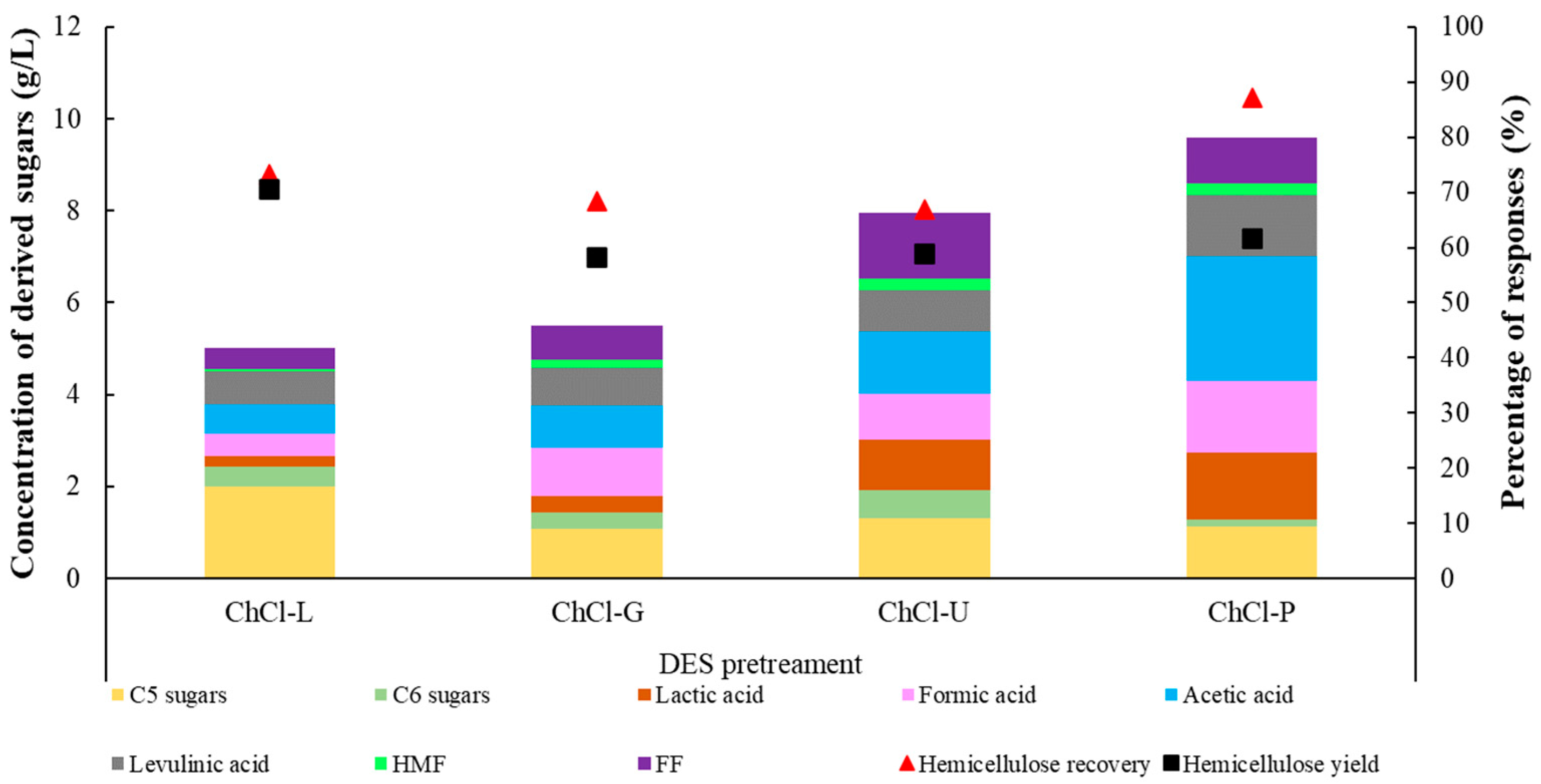
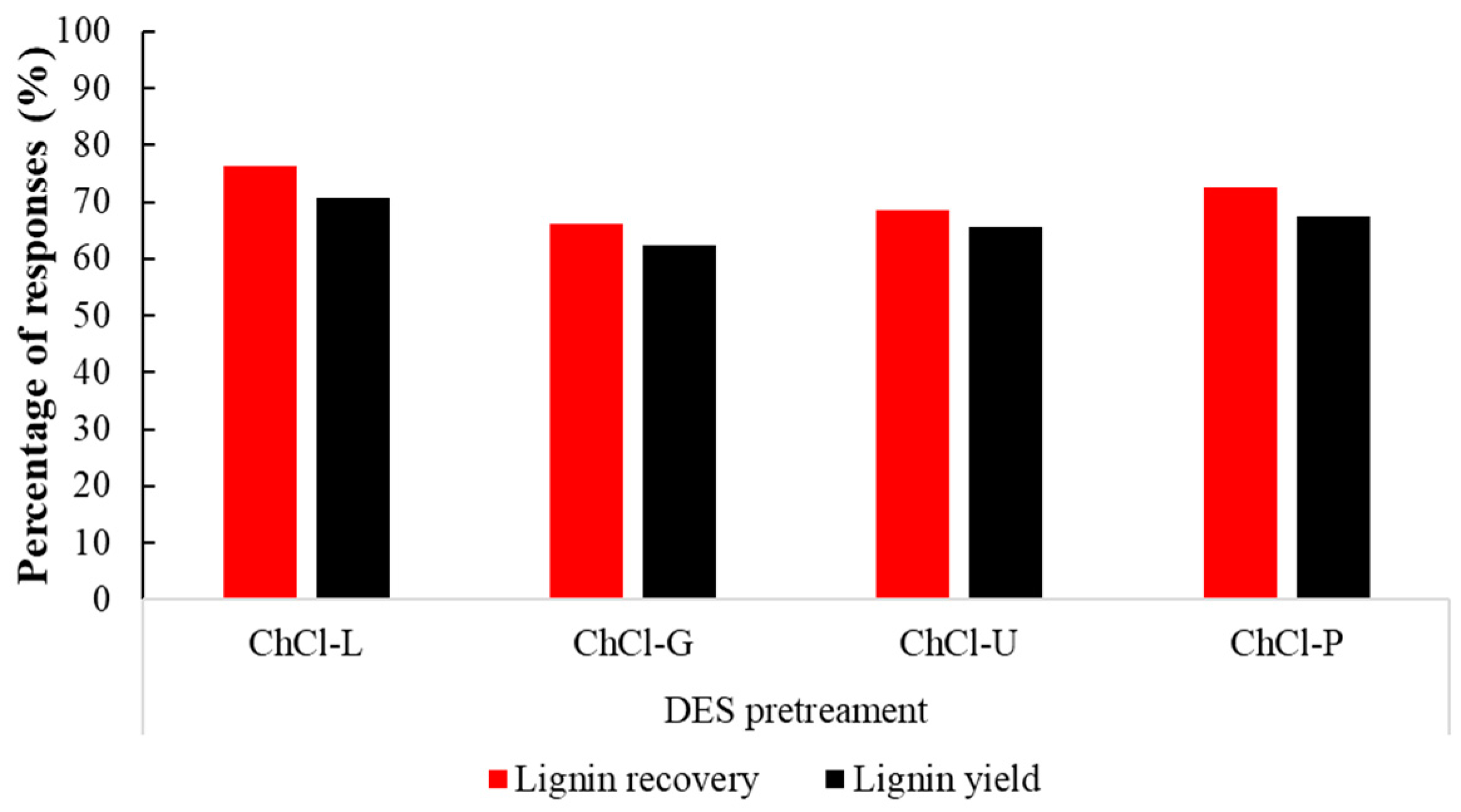

| Composition | Raw Sugarcane Bagasse |
|---|---|
| Cellulose | 35.12% ± 0.43% |
| Hemicellulose | 24.36% ± 0.91% |
| Acid-insoluble lignin | 22.78% ± 0.57% |
| Acid-soluble lignin | 5.03% ± 0.18% |
| Ash | 5.82 ± 0.22% |
| Other | 6.89 ± 0.36% |
| Factors | Responses (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Run no. | T (°C) | Time (min) | ChCl-LA (% v/v) | Cellulose Recovery (%) a | Hemicellulose Removal (%) | Hemicellulose Recovery (%) | Lignin Recovery (%) | Lignin Yield (%) |
| 1 | 120 | 120 | 49.99 | 97.35 | 96.30 | 74.3 | 77.31 | 71.28 |
| 2 | 140 | 120 | 66.66 | 90.75 | 90.76 | 70.10 | 68.27 | 64.75 |
| 3 | 120 | 150 | 66.66 | 88.05 | 91.37 | 70.71 | 69.90 | 65.09 |
| 4 | 100 | 150 | 49.99 | 91.49 | 90.62 | 72.33 | 75.69 | 70.12 |
| 5 | 120 | 90 | 33.33 | 90.64 | 91.86 | 70.40 | 75.11 | 70.88 |
| 6 | 100 | 120 | 33.33 | 94.81 | 91.78 | 72.19 | 72.74 | 69.10 |
| 7 | 100 | 120 | 66.66 | 90.39 | 90.54 | 72.10 | 73.60 | 67.31 |
| 8 | 120 | 120 | 49.99 | 97.42 | 96.51 | 74.41 | 77.33 | 71.03 |
| 9 | 120 | 120 | 49.99 | 97.86 | 95.35 | 74.11 | 77.26 | 71.54 |
| 10 | 140 | 90 | 49.99 | 89.53 | 90.29 | 70.12 | 76.21 | 70.50 |
| 11 | 120 | 150 | 33.33 | 90.89 | 93.50 | 71.46 | 72.58 | 68.14 |
| 12 | 120 | 90 | 66.66 | 87.3 | 91.70 | 70.65 | 73.34 | 67.75 |
| 13 | 140 | 120 | 33.33 | 90.0 | 92.32 | 70.59 | 73.59 | 68.98 |
| 14 | 140 | 150 | 49.99 | 88.18 | 91.45 | 68.90 | 67.90 | 63.01 |
| 15 | 100 | 90 | 49.99 | 89.42 | 90.37 | 70.21 | 73.11 | 68.00 |
| Raw Sugarcane Bagasse | Fractionated Sugarcane Bagasse | Functional |
|---|---|---|
| 3419 | 3384 | O-H stretching |
| 2937 | 2924 | C–H stretching in methoxyl, methyl and methylene groups |
| 1696 | - | syringol |
| 1602 | 1604 | Aromatic ring vibrations and C=O stretching (S > G) |
| 1513 | 1514 | aromatic skeletal vibrations in lignin |
| 1462 | 1462 | CH2 bending |
| 1423 | 1424 | asymmetric stretching vibrations C=O |
| - | 1265 | C-O stretching vibration |
| 1263 | - | C-O stretching of the acetyl group |
| 1126 | 1123 | Aromatic in-plane C–H bending (typical for S units) |
| 1031 | 1032 | Holocellulose and lignin: C–O stretching |
| 835 | 833 | p-hydroxyphenyl units (H unit) |
| Biomass | BET Area (m2/g) |
|---|---|
| Raw material | 3.53 ± 0.11 |
| Fractionated sugarcane bagasse | 7.82 ± 0.08 |
| Composition | Raw Sugarcane Bagasse (%) | Fractionated Sugarcane Bagasse (%) |
|---|---|---|
| Si | 0.568 ± 0.01 | 0.45 ± 0.02 |
| Fe | 0.498 ± 0.01 | 0.17 ± 0.01 |
| Ca | 0.369 ± 0.02 | 0.16 ± 0.00 |
| Na | 0.133 ± 0.01 | - |
| AI | 0.077 ± 0.01 | 0.059 ± 0.01 |
| Mn | 0.042 ± 0.00 | - |
| Zn | 0.037 ± 0.01 | 0.032 ± 0.00 |
| Cu | 0.036 ± 0.01 | 0.036 ± 0.00 |
| Ti | 0.035 ± 0.00 | - |
| Mg | 0.030 ± 0.00 | 0.015 ± 0.00 |
| K | 0.006 ± 0.01 | 0.050 ± 0.00 |
| Compton | 0.03 | 0.02 |
| Rayleigh | - | - |
| Sum (%) | 2.08 | 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyachai, N.; Khongchamnan, P.; Laosiripojana, N.; Kreetachat, T.; Wongcharee, S.; Sakulthaew, C.; Chokejaroenrat, C.; Imman, S. Optimization of Cellulose Recovery Using Deep Eutectic Solvent Fractionation: A Response Surface Method Approach. Energies 2024, 17, 4257. https://doi.org/10.3390/en17174257
Suriyachai N, Khongchamnan P, Laosiripojana N, Kreetachat T, Wongcharee S, Sakulthaew C, Chokejaroenrat C, Imman S. Optimization of Cellulose Recovery Using Deep Eutectic Solvent Fractionation: A Response Surface Method Approach. Energies. 2024; 17(17):4257. https://doi.org/10.3390/en17174257
Chicago/Turabian StyleSuriyachai, Nopparat, Punjarat Khongchamnan, Navadol Laosiripojana, Torpong Kreetachat, Surachai Wongcharee, Chainarong Sakulthaew, Chanat Chokejaroenrat, and Saksit Imman. 2024. "Optimization of Cellulose Recovery Using Deep Eutectic Solvent Fractionation: A Response Surface Method Approach" Energies 17, no. 17: 4257. https://doi.org/10.3390/en17174257
APA StyleSuriyachai, N., Khongchamnan, P., Laosiripojana, N., Kreetachat, T., Wongcharee, S., Sakulthaew, C., Chokejaroenrat, C., & Imman, S. (2024). Optimization of Cellulose Recovery Using Deep Eutectic Solvent Fractionation: A Response Surface Method Approach. Energies, 17(17), 4257. https://doi.org/10.3390/en17174257








