Influence of Microwave-Assisted Chemical Thermohydrolysis of Lignocellulosic Waste Biomass on Anaerobic Digestion Efficiency
Abstract
1. Introduction
2. Materials and Methods
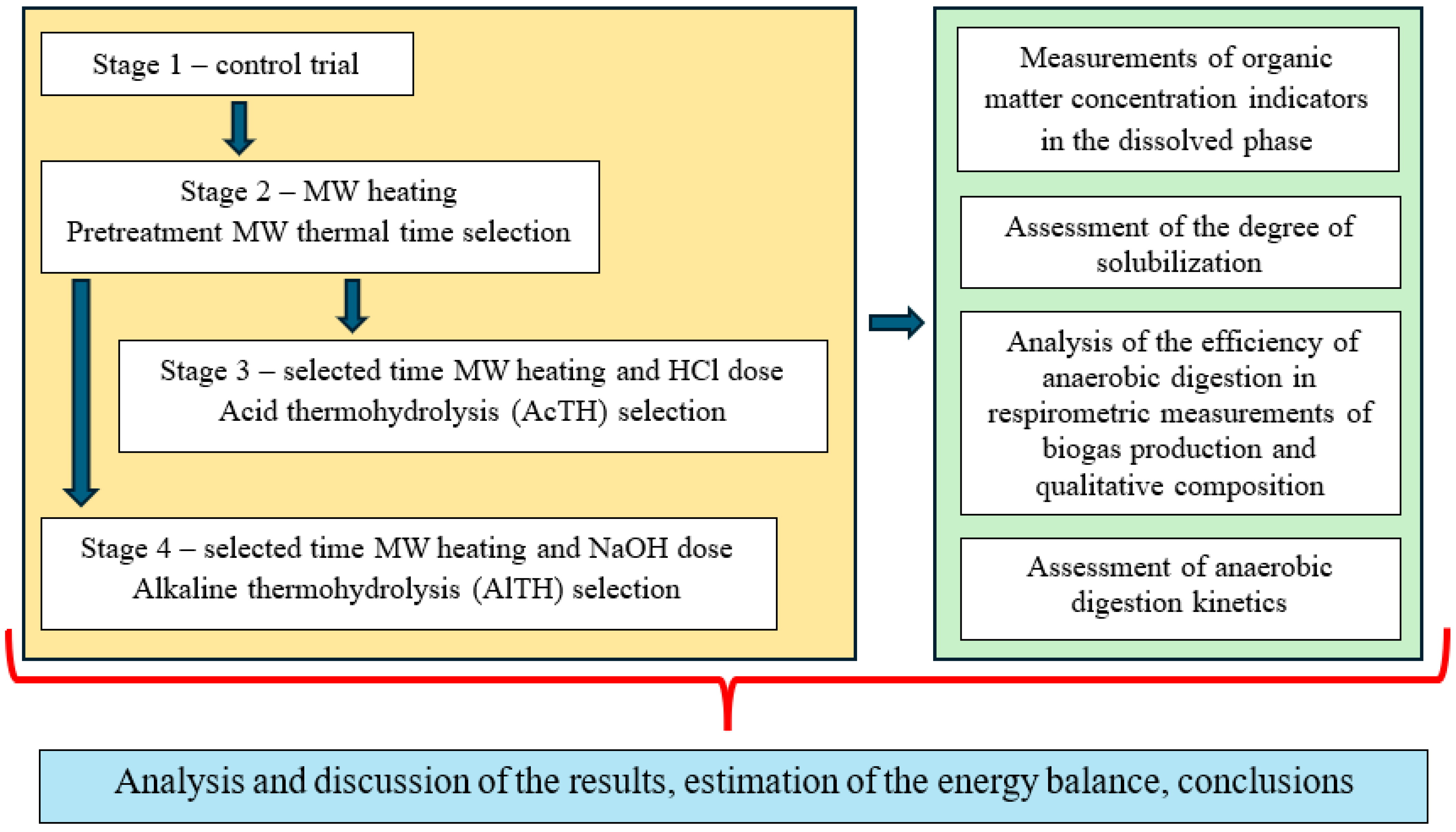
2.1. Organization of the Experiment
2.2. Materials
2.2.1. Lignocellulosic Biomass
2.2.2. Anaerobic Sludge
2.2.3. Chemical Reagents
2.3. Chemical Pretreatment
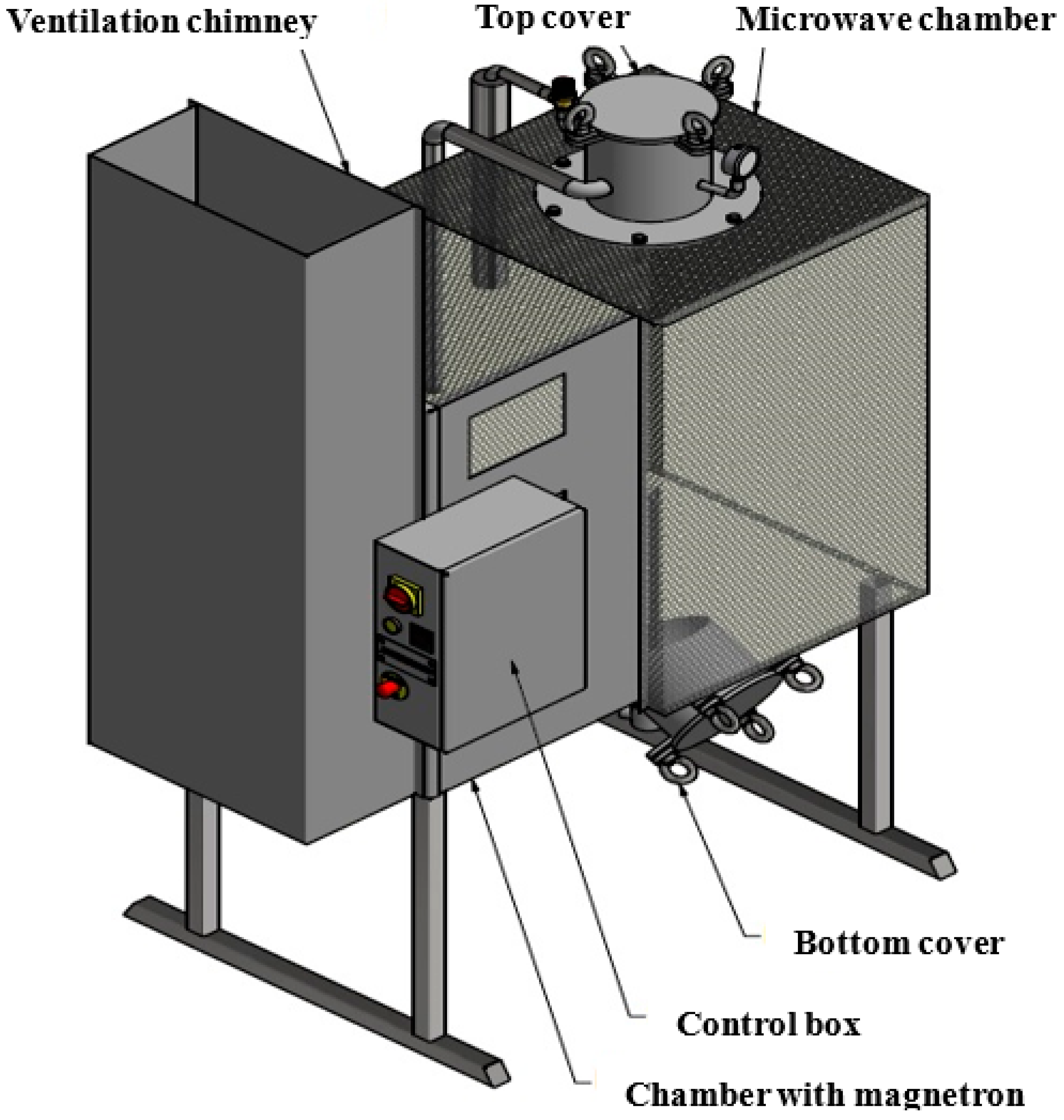
2.4. MW Hydrothermal Pretreatment Reactor
2.5. Respirometric Anaerobic Digestion
2.6. Calculation Methods
2.7. Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Concentration of Dissolved Organic Matter
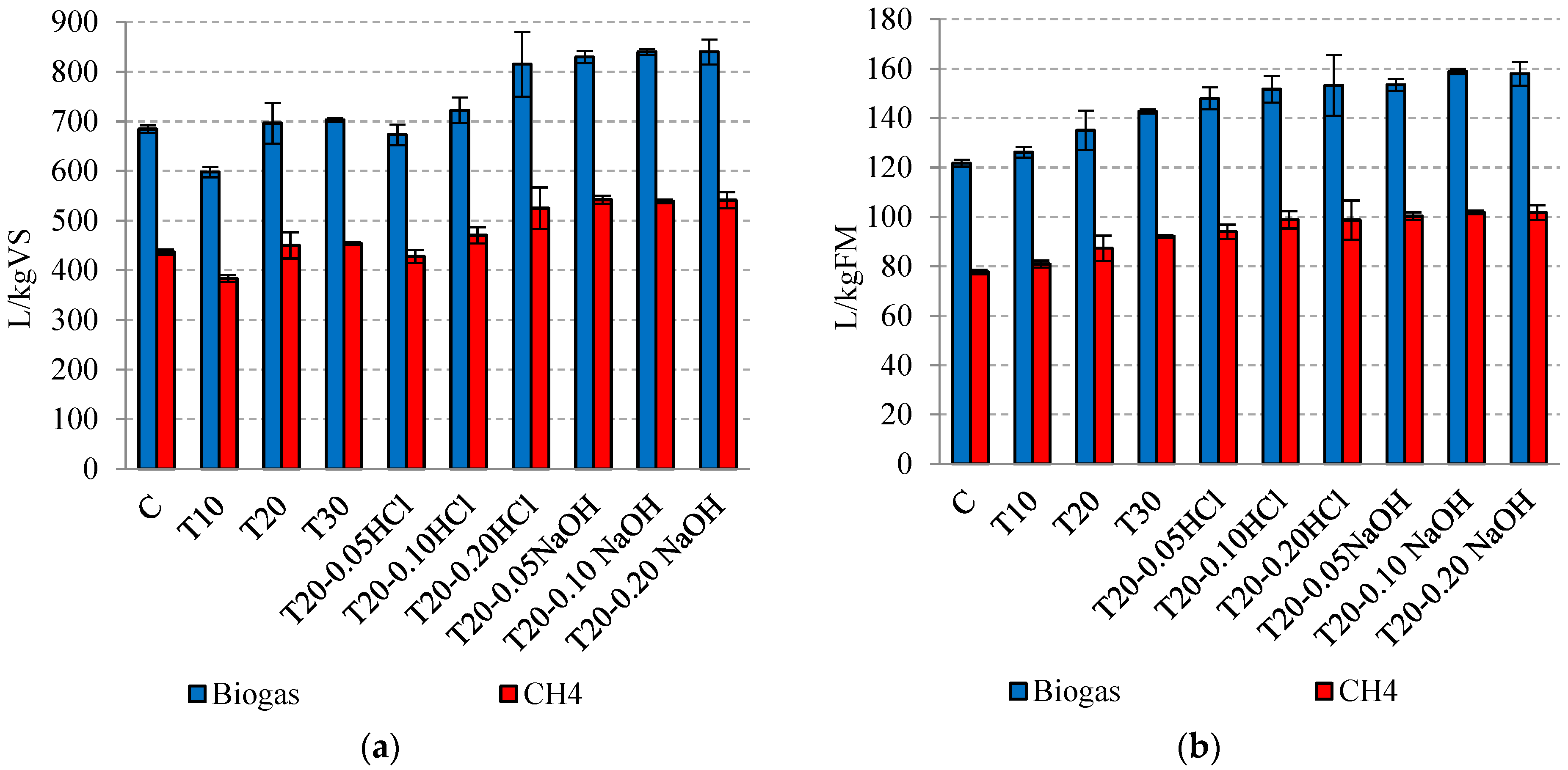
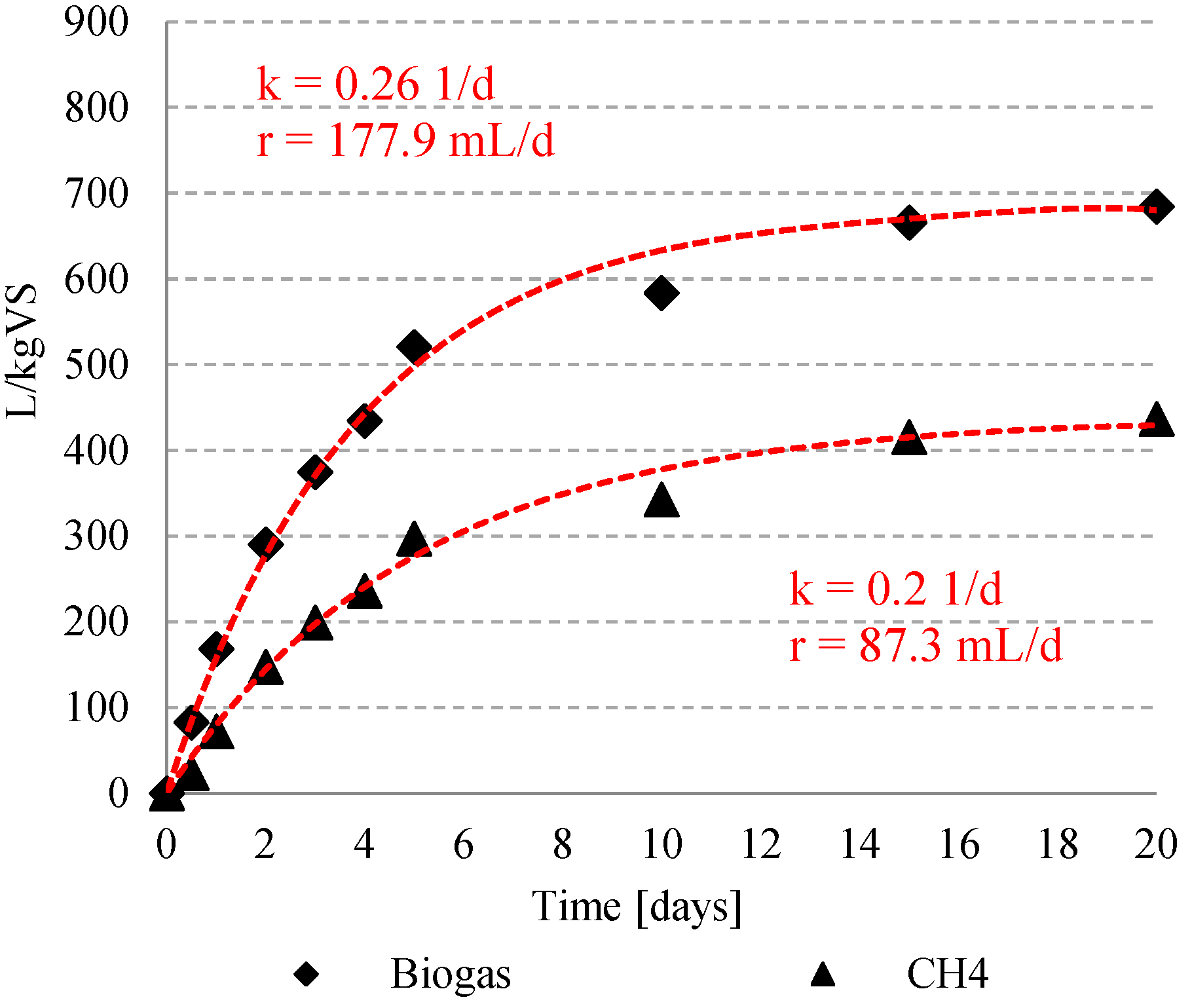
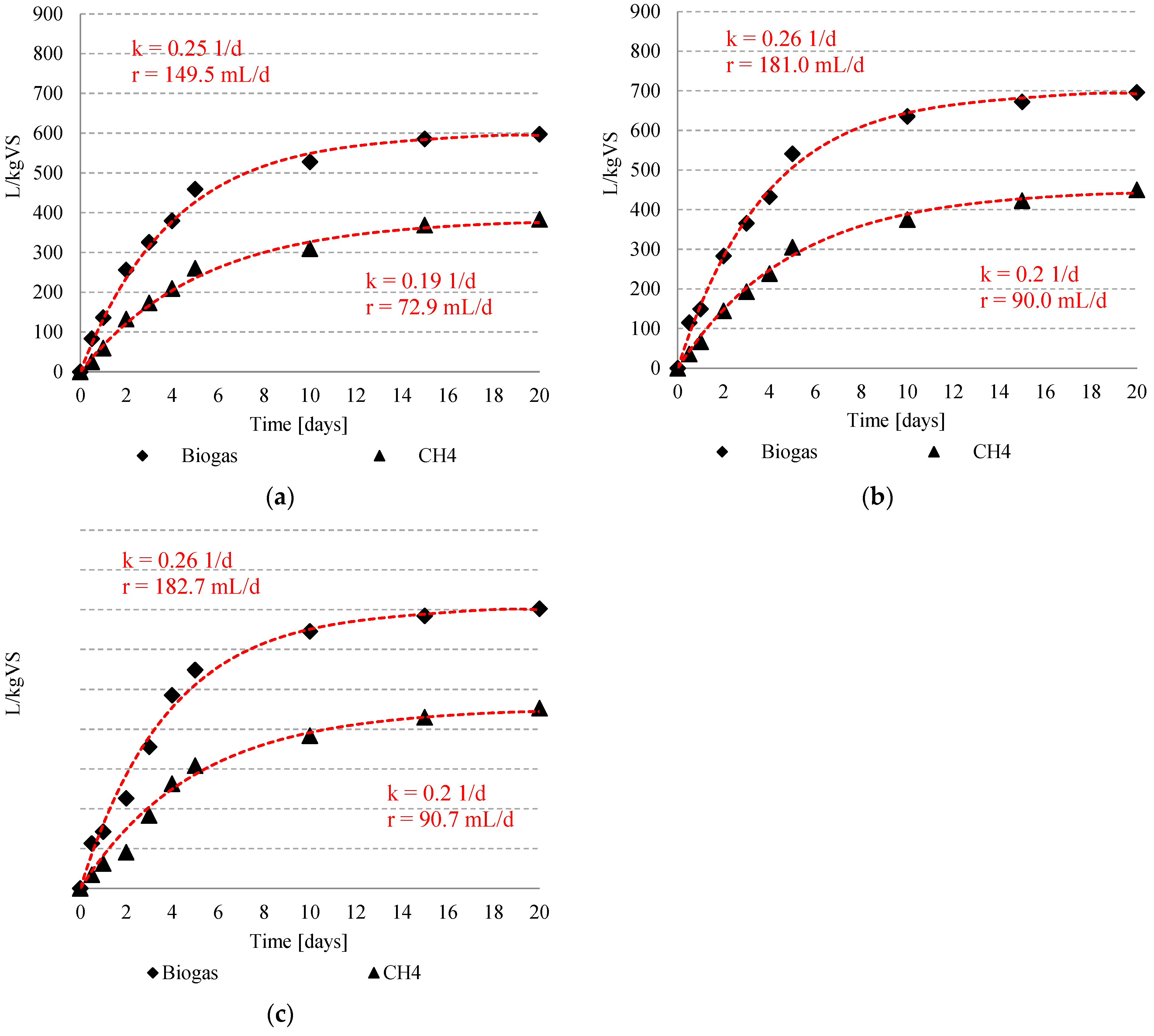
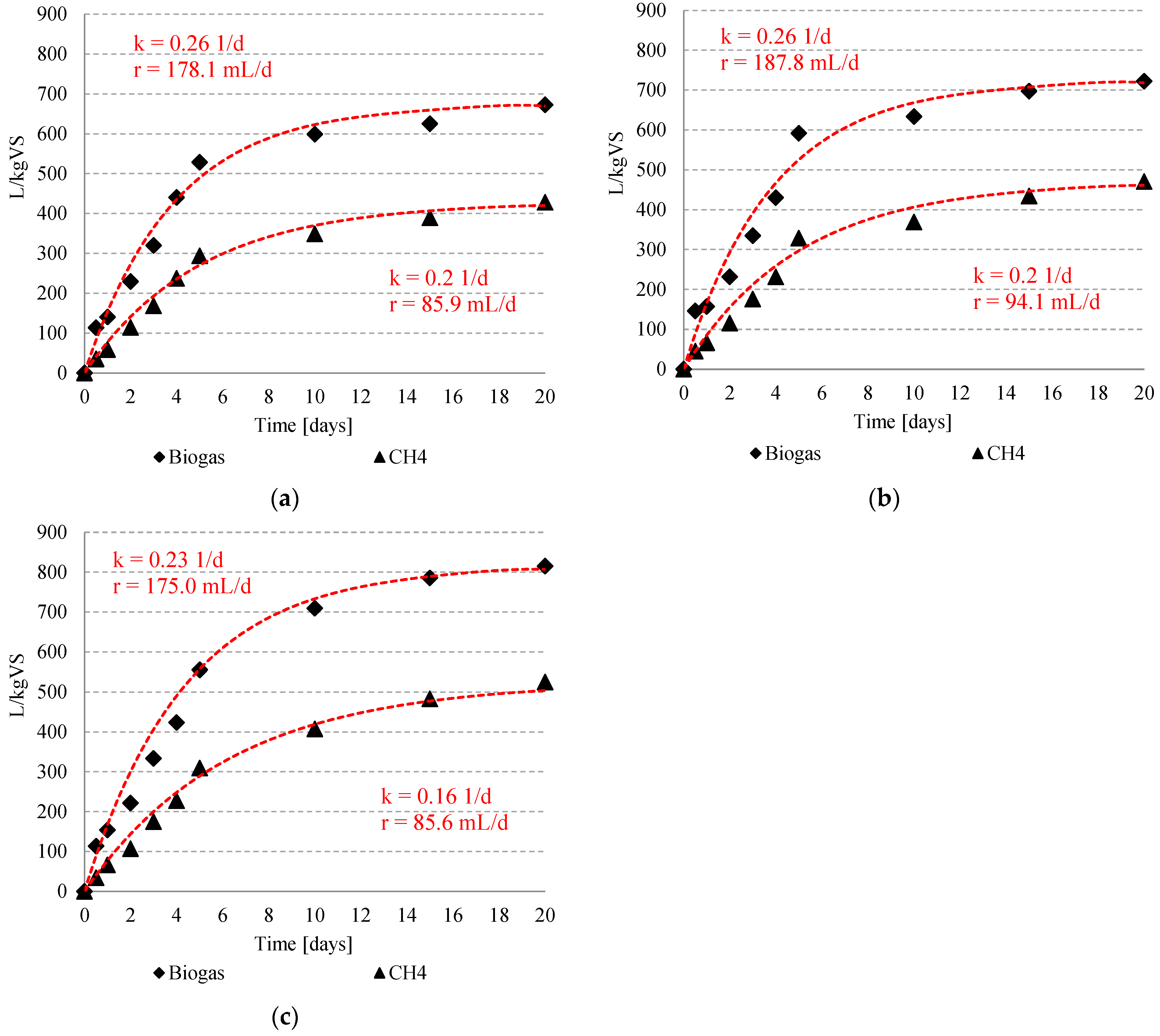
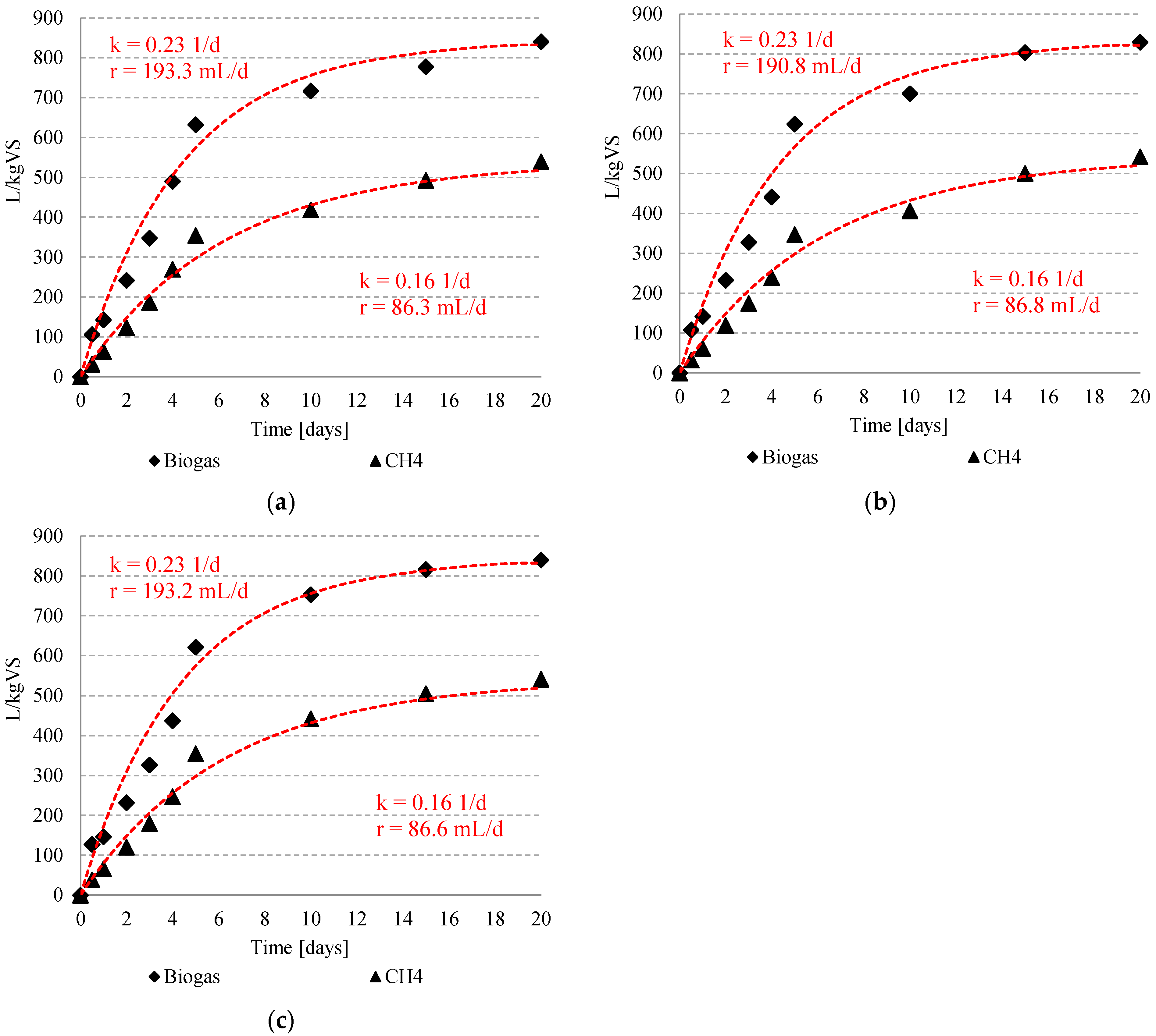
3.2. Biogas and Methane Production
3.3. Energy Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of Methane Fermentation as a Valorisation Method for Food Waste Products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Ferdeş, M.; Dincă, M.N.; Moiceanu, G.; Zabava, B.Ş.; Paraschiv, G. Microorganisms and Enzymes Used in the Biological Pretreatment of the Substrate to Enhance Biogas Production: A Review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Ahmed, B.; Kumar Tyagi, V.; Kazmi, A.A.; Khursheed, A. New Insights into Thermal-Chemical Pretreatment of Organic Fraction of Municipal Solid Waste: Solubilization Effects, Recalcitrant Formation, Biogas Yield and Energy Efficiency. Fuel 2022, 319, 123725. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Hua, D.; Zhao, Y.; Chen, L.; Zhou, L.; Chen, G. Effect of Microaerobic Microbial Pretreatment on Anaerobic Digestion of a Lignocellulosic Substrate under Controlled PH Conditions. Bioresour. Technol. 2021, 328, 124852. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Hernandez, R.; Fortela, D.L.B.; Sharp, W.; Chistoserdov, A.; Gang, D.; Revellame, E.; Holmes, W.; Zappi, M.E. A Review of Pretreatment Methods to Enhance Solids Reduction during Anaerobic Digestion of Municipal Wastewater Sludges and the Resulting Digester Performance: Implications to Future Urban Biorefineries. Appl. Sci. 2020, 10, 9141. [Google Scholar] [CrossRef]
- Kang, Y.R.; Su, Y.; Wang, J.; Chu, Y.X.; Tian, G.; He, R. Effects of Different Pretreatment Methods on Biogas Production and Microbial Community in Anaerobic Digestion of Wheat Straw. Environ. Sci. Pollut. Res. 2021, 28, 51772–51785. [Google Scholar] [CrossRef]
- Kisielewska, M.; Rusanowska, P.; Dudek, M.; Nowicka, A.; Krzywik, A.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Evaluation of Ultrasound Pretreatment for Enhanced Anaerobic Digestion of Sida Hermaphrodita. Bioenergy Res. 2020, 13, 824–832. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y. Impact of Hydrothermal Pretreatment on Anaerobic Digestion Efficiency for Lignocellulosic Biomass: Influence of Pretreatment Temperature on the Formation of Biomass-Degrading Byproducts. Chemosphere 2020, 256, 127116. [Google Scholar] [CrossRef] [PubMed]
- Szaja, A.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Grządka, E.; Montusiewicz, J.; Lebiocka, M. Pre-Treatment of Spent Coffee Grounds Using Hydrodynamic Cavitation. Energies 2024, 17, 2229. [Google Scholar] [CrossRef]
- Paul Choudhury, S.; Kalamdhad, A.S. Optimization of Electrokinetic Pretreatment for Enhanced Methane Production and Toxicity Reduction from Petroleum Refinery Sludge. J. Environ. Manag. 2021, 298, 113469. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Chen, K.Q.; Zhu, R.; Sun, W.X.; Zou, H.; Guo, R.B. Improved Anaerobic Digestion Performance of Miscanthus Floridulus by Different Pretreatment Methods and Preliminary Economic Analysis. Energy Convers. Manag. 2018, 159, 121–128. [Google Scholar] [CrossRef]
- Deng, Y.; Qiu, Y.; Yao, Y.; Ayiania, M.; Davaritouchaee, M. Weak-Base Pretreatment to Increase Biomethane Production from Wheat Straw. Environ. Sci. Pollut. Res. 2020, 27, 37989–38003. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, D.; Liu, X.; Xu, Q.; Yang, Q.; Li, X.; Liu, Y.; Wang, Q.; Ni, B.J.; Li, H. The Underlying Mechanism of Calcium Peroxide Pretreatment Enhancing Methane Production from Anaerobic Digestion of Waste Activated Sludge. Water Res. 2019, 164, 114934. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Long-Term Pre-Treatment of Municipal Sewage Sludge with Solidified Carbon Dioxide (SCO2)—Effect on Anaerobic Digestion Efficiency. Appl. Sci. 2023, 13, 3075. [Google Scholar] [CrossRef]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A Comprehensive Review on the Pretreatment of Lignocellulosic Wastes for Improved Biogas Production by Anaerobic Digestion. Int. J. Environ. Sci. Technol. 2021, 19, 3429–3456. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Wang, X.; Zheng, T.; Wang, P. Preparation and performance of polyacrylonitrile fiber functionalized with iminodiacetic acid under microwave irradiation for adsorption of Cu(II) and Hg(II). Chem. Eng. J. 2015, 276, 349–357. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the Recent Advances of Microwave Pretreatment Technologies for the Conversion of Lignocellulosic Biomass into Sustainable Biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef]
- Mohan, M.; Deshavath, N.N.; Banerjee, T.; Goud, V.V.; Dasu, V.V. Ionic liquid and sulfuric acid-based pretreatment of bamboo: Biomass delignification and enzymatic hydrolysis for the production of reducing sugars. Ind. Eng. Chem. Res. 2018, 57, 10105–10117. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Peng, H.; Chen, H.; Qu, Y.; Li, H.; Xu, J. Bioconversion of different sizes of microcrystalline cellulose pretreated by microwave irradiation with/without NaOH. Appl. Energy 2014, 117, 142–148. [Google Scholar] [CrossRef]
- Ha, S.H.; Mai, N.L.; An, G.; Koo, Y.-M. Microwave-assisted pretreatment of cellulose in ionic liquid for accelerated enzymatic hydrolysis. Bioresour. Technol. 2011, 102, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-H.; Yue, Z.-B.; Yu, H.-Q.; Liu, S.-Y.; Harada, H.; Li, Y.-Y. Mechanisms of microwave irradiation pretreatment for enhancing anaerobic digestion of cattail by rumen microorganisms. Appl. Energy 2012, 93, 229–236. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Amini, N.; Haritos, V.S.; Tanksale, A. Microwave Assisted Pretreatment of Eucalyptus Sawdust Enhances Enzymatic Saccharification and Maximizes Fermentable Sugar Yield. Renew. Energy 2018, 127, 653–660. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M. Microwave support of the alcoholic fermentation process of cyanobacteria Arthrospira platensis. Environ. Sci. Pollut. Res. 2020, 27, 118–124. [Google Scholar] [CrossRef]
- Balat, M. Production of Bioethanol from Lignocellulosic Materials via the Biochemical Pathway: A Review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Tung, T.T.; Pereira, A.L.C.; Poloni, E.; Dang, M.N.; Wang, J.; Le, T.S.D.; Kim, Y.J.; Pho, Q.H.; Nine, M.J.; Shearer, C.J.; et al. Irradiation Methods for Engineering of Graphene Related Two-Dimensional Materials. Appl. Phys. Rev. 2023, 10, 031309. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M.; Rusanowska, P. Progress in the production of biogas from Virginia mallow after alkaline-heat pretreatment. Biomass Bioenergy 2019, 126, 174–180. [Google Scholar] [CrossRef]
- Abdel-Hay, M.M. Principles of Microwave Heating for the Food Industry. Emerg. Therm. Process. Food Ind. 2023, 95–117. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, J.; Wang, K.; Cai, C.; Xu, D. Modeling and Analysis of the Friction in a Non-Linear Sliding-Mode Triboelectric Energy Harvester. Acta Mech. Sin. Xuebao 2022, 38, 521330. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild Microwaves, Ultrasonic and Alkaline Pretreatments for Improving Methane Production: Impact on Biochemical and Structural Properties of Olive Pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Z.; Xiouras, C.; Stefanidis, G.D.; Li, X.; Gao, X. Fundamentals and Applications of Microwave Heating to Chemicals Separation Processes. Renew. Sustain. Energy Rev. 2019, 114, 109316. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Chen, C.; Kline, D.J.; Giroux, M.; Pei, Y.; Jiao, M.; Liu, D.; Mi, R.; Xie, H.; et al. Synthesis of Metal Oxide Nanoparticles by Rapid, High-Temperature 3D Microwave Heating. Adv. Funct. Mater. 2019, 29, 1904282. [Google Scholar] [CrossRef]
- Chhanwal, N.; Bhushette, P.R.; Anandharamakrishnan, C. Current Perspectives on Non-Conventional Heating Ovens for Baking Process—A Review. Food Bioprocess. Technol. 2019, 12, 1–15. [Google Scholar] [CrossRef]
- El Khaled, D.; Novas, N.; Gazquez, J.A.; Manzano-Agugliaro, F. Microwave Dielectric Heating: Applications on Metals Processing. Renew. Sustain. Energy Rev. 2018, 82, 2880–2892. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Bhaskar, T.; Bhavya, B.; Singh, R.; Naik, D.V.; Kumar, A.; Goyal, H.B. Thermochemical Conversion of Biomass to Biofuels. In Biofuels Alternative Feedstocks and Conversion Processes; Academic Press: Cambridge, MA, USA, 2011; pp. 51–77. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Rusanowska, P.; Kazimierowicz, J. Influence of Microwave Radiation on Pollutant Removal and Biomethane Production Efficiency in Anaerobic Treatment of High-Load Poultry Wastewater. Appl. Sci. 2023, 13, 3553. [Google Scholar] [CrossRef]
- Komarov, V.V. A Review of Radio Frequency and Microwave Sustainability-Oriented Technologies. Sustain. Mater. Technol. 2021, 28, e00234. [Google Scholar] [CrossRef]
- Demianchuk, B.; Huliiev, S.; Ugol’nikov, A.; Kliat, Y.; Kosenko, A. Development of Microwave Technology of Selective Heating the Components of Heterogeneous Media. East.-Eur. J. Enterp. Technol. 2022, 1, 43–52. [Google Scholar] [CrossRef]
- Yue, L.; Cheng, J.; Tang, S.; An, X.; Hua, J.; Dong, H.; Zhou, J. Ultrasound and Microwave Pretreatments Promote Methane Production Potential and Energy Conversion during Anaerobic Digestion of Lipid and Food Wastes. Energy 2021, 228, 120525. [Google Scholar] [CrossRef]
- Zou, X.; Yang, R.; Zhou, X.; Cao, G.; Zhu, R.; Ouyang, F. Effects of Mixed Alkali-Thermal Pretreatment on Anaerobic Digestion Performance of Waste Activated Sludge. J. Clean. Prod. 2020, 259, 120940. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Nowicka, A.; Dudek, M. Application of Hydrodynamic Cavitation in the Disintegration of Aerobic Granular Sludge—Evaluation of Pretreatment Time on Biomass Properties, Anaerobic Digestion Efficiency and Energy Balance. Energies 2024, 17, 335. [Google Scholar] [CrossRef]
- Klein, M.; Griess, O.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Bioethanol production from Ficus religiosa leaves using microwave irradiation. J. Environ. Manag. 2016, 177, 20–25. [Google Scholar] [CrossRef]
- Maceiras, R.; Alfonsín, V.; Seguí, L.; González, J.F. Microwave Assisted Alkaline Pretreatment of Algae Waste in the Production of Cellulosic Bioethanol. Energies 2021, 14, 5891. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Liang, T.; Elmaadawy, K.; Liu, B.; Hu, J.; Hou, H.; Yang, J. Anaerobic Fermentation of Waste Activated Sludge for Volatile Fatty Acid Production: Recent Updates of Pretreatment Methods and the Potential Effect of Humic and Nutrients Substances. Process Saf. Environ. Prot. 2021, 145, 321–339. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Girón-Bol, L.M.; Gómez-Muñoz, D.F.; Marmolejo-Rebellón, L.F.; Torres-Lozada, P. Thermal Pre-Treatment as a Tool for Energy Recovery from Food Waste through Anaerobic Digestion. Effect on Kinetic and Physicochemical Characteristics of the Substrate. Environ. Technol. Innov. 2021, 21, 101262. [Google Scholar] [CrossRef]
- Kainthola, J.; Shariq, M.; Kalamdhad, A.S.; Goud, V.V. Enhanced Methane Potential of Rice Straw with Microwave Assisted Pretreatment and Its Kinetic Analysis. J. Environ. Manag. 2019, 232, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short Duration Microwave Assisted Pretreatment Enhances the Enzymatic Saccharification and Fermentable Sugar Yield from Sugarcane Bagasse. Renew. Energy 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M. Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies 2021, 14, 8018. [Google Scholar] [CrossRef]
- Shah, T.A.; Ali, S.; Afzal, A.; Tabassum, R. Effect of Alkali Pretreatment on Lignocellulosic Waste Biomass for Biogas Production. Int. J. Renew. Energy Res. 2018, 8, 1318–1326. [Google Scholar] [CrossRef]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Świca, I.; Zieliński, M. Ultrasonic Disintegration to Improve Anaerobic Digestion of Microalgae with Hard Cell Walls—Scenedesmus sp. and Pinnularia sp. Plants 2023, 12, 53. [Google Scholar] [CrossRef]
- Islam, M.S.; Ranade, V. Enhancing Bmp and Digestibility of Daf Sludge via Hydrodynamic Cavitation; SSRN: Rochester, NY, USA, 2023; pp. 1–33. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Zieliński, M.; Kazimierowicz, J. The Influence of the Ultrasound Disintegration of Microalgal–Bacterial Granular Sludge on Anaerobic Digestion Efficiency. Appl. Sci. 2023, 13, 7387. [Google Scholar] [CrossRef]
- Mohammad Rahmani, A.; Gahlot, P.; Moustakas, K.; Kazmi, A.A.; Shekhar Prasad Ojha, C.; Tyagi, V.K. Pretreatment Methods to Enhance Solubilization and Anaerobic Biodegradability of Lignocellulosic Biomass (Wheat Straw): Progress and Challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and Fermentation of Lignocellulosic Biomass: Reaction Mechanisms and Process Engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Sapci, Z. The Effect of Microwave Pretreatment on Biogas Production from Agricultural Straws. Bioresour. Technol. 2013, 128, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sayara, T.; Sánchez, A. A Review on Anaerobic Digestion of Lignocellulosic Wastes: Pretreatments and Operational Conditions. Appl. Sci. 2019, 9, 4655. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.H.; Kim, D.H. Fermentative Hydrogen Production Using Lignocellulose Biomass: An Overview of Pre-Treatment Methods, Inhibitor Effects and Detoxification Experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.T.; García, A.; Teymennet-Ramírez, K.V.; Arenas-Olivares, D.Y.; Martínez-Morales, F.; Trejo-Hernández, M.R. Removal of Phenolic Inhibitors from Lignocellulose Hydrolysates Using Laccases for the Production of Fuels and Chemicals. Biotechnol. Prog. 2024, 40, e3406. [Google Scholar] [CrossRef] [PubMed]
- Jilani, S.B.; Olson, D.G. Mechanism of Furfural Toxicity and Metabolic Strategies to Engineer Tolerance in Microbial Strains. Microb. Cell Factories 2023, 22, 221. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The Inhibition of Anaerobic Digestion by Model Phenolic Compounds Representative of Those from Sargassum Muticum. J. Appl. Phycol. 2019, 31, 779–786. [Google Scholar] [CrossRef]
- Ahmed, B.; Aboudi, K.; Tyagi, V.K.; álvarez-Gallego, C.J.; Fernández-Güelfo, L.A.; Romero-García, L.I.; Kazmi, A.A. Improvement of Anaerobic Digestion of Lignocellulosic Biomass by Hydrothermal Pretreatment. Appl. Sci. 2019, 9, 3853. [Google Scholar] [CrossRef]
- Barrios, J.A.; Cano, A.; Rivera, F.F.; Cisneros, M.E.; Durán, U. Efficiency of Integrated Electrooxidation and Anaerobic Digestion of Waste Activated Sludge. Biotechnol. Biofuels 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yu, L.; Huang, S.; Luo, J.; Zhuo, Y. Energy Efficiency of Pre-Treating Excess Sewage Sludge with Microwave Irradiation. Bioresour. Technol. 2010, 101, 5092–5097. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of Microwave Thermohydrolysis and Liquid Hot Water Pretreatment of Energy Crop Sida Hermaphrodita for Enhanced Methane Production. Biomass Bioenergy 2019, 128, 105324. [Google Scholar] [CrossRef]

| Stage | Variant | Mw Heating Time [min] | HCl Dose [g/gTS] | NaOH Dose [g/gTS] |
|---|---|---|---|---|
| S1 | C | - | - | - |
| S2 | T10 | 10 | - | - |
| T20 | 20 | - | - | |
| T30 | 30 | - | - | |
| S3 | T20-0.05 HCl | 20 | 0.05 | - |
| T20-0.10 HCl | 20 | 0.10 | - | |
| T20-0.20 HCl | 20 | 0.20 | - | |
| S4 | T20-0.05 NaOH | 20 | - | 0.05 |
| T20-0.10 NaOH | 20 | - | 0.10 | |
| T20-0.20 NaOH | 20 | - | 0.20 |
| Indicator | Full Name | Unit | Value | |
|---|---|---|---|---|
| Substrate | Anaerobic Sludge | |||
| TS | Total solids | mg/gFM (fresh mass) | 90 ± 15 | 86 ± 12 |
| VS | Volatile solids | mg/gFM (fresh mass) | 78 ± 6 | 65 ± 4 |
| Hydration | - | % | 91.0 ± 1.5 | 91.4 ± 1.2 |
| CODsol | Dissolved Chemical Oxygen Demand | g/L | 47.3 ± 1.2 | 41.8 ± 3.9 |
| TOCsol | Dissolved Total Organic Carbon | g/L | 14.1 ± 0.81 | 11.5 ± 0.91 |
| TCsol | Dissolved Total Carbon | g/L | 15.2 ± 0.84 | 13.7 ± 1.10 |
| TNsol | Dissolved Total Nitrogen | g/L | 3.78 ± 0.21 | 4.02 ± 0.51 |
| Sugarssol | Dissolved Sugars | g/L | 0.69 ± 0.12 | 0.12 ± 0.02 |
| Parameter | Unit | Stage/Variant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||||||
| C | T10 | T20 | T30 | T20-0.05 HCl | T20-0.10 HCl | T20-0.20 HCl | T20-0.05 NaOH | T20-0.10 NaOH | T20-0.20 NaOH | ||
| CODsol | g/L | 47.3 ±1.2 | 105 ±2.6 | 104 ±2.9 | 114 ±2.1 | 107 ±3.1 | 112 ±2.8 | 109 ±3.2 | 99 ±2.5 | 115 ±3.0 | 113 ±3.2 |
| TOCsol | g/L | 14.1 ±0.81 | 33.9 ±0.92 | 37.3 ±1.32 | 40.7 ±1.25 | 38.1 ±1.44 | 38.2 ±1.41 | 39.1 ±1.66 | 37.7 ±1.53 | 36.4 ±1.21 | 38.5 ±1.09 |
| TCsol | g/L | 15.2 ±0.84 | 35.1 ±1.01 | 41.8 ±1.25 | 38.0 ±1.33 | 37.2 ±1.14 | 39.3 ±1.13 | 36.2 ±1.24 | 39.6 ±1.55 | 37.5 ±1.18 | 38.9 ±1.25 |
| TNsol | mg/L | 3.78 ±0.21 | 3.82 ±0.26 | 3.98 ±0.23 | 3.70 ±0.19 | 3.51 ±0.22 | 3.40 ±0.24 | 3.09 ±0.16 | 3.81 ±0.26 | 3.72 ±0.24 | 3.48 ±0.21 |
| Sugarsol | g/L | 0.69 ±0.12 | 20.0 ±0.85 | 23.6 ±0.93 | 30.0 ±0.87 | 18.9 ±0.65 | 23.7 ±0.74 | 30.2 ±0.98 | 19.6 ±0.54 | 19.8 ±0.63 | 28.1 ±0.66 |
| COD solubilization degree | % | - | 23.48 ±1.55 | 23.08 ±1.42 | 27.13 ±1.65 | 24.29 ±1.32 | 26.32 ±1.54 | 25.10 ±1.61 | 21.05 ±1.75 | 27.53 ±1.72 | 26.72 ±1.51 |
| TOC solubilization degree | % | - | 20.06 ±1.31 | 23.50 ±1.36 | 26.91 ±1.53 | 24.25 ±1.37 | 24.33 ±1.48 | 25.24 ±1.66 | 23.88 ±1.52 | 22.54 ±1.48 | 24.68 ±1.38 |
| Time (Day) | Biogas L | %CH4 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |||||||||||||
| C | T10 | T20 | T30 | T20-0.05 HCl | T20-0.10 HCl | T20-0.20 HCl | T20-0.05 NaOH | T20-0.10 NaOH | T20-0.20 NaOH | C | T10 | T20 | T30 | T20-0.05 HCl | T20-0.10 HCl | T20-0.20 HCl | T20-0.05 NaOH | T20-0.10 NaOH | T20-0.20 NaOH | |
| 0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.00 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 | 0.0 ±0 |
| 0.5 | 0.22 ±0.01 | 0.25 ±0.03 | 0.29 ±0.02 | 0.30 ±0.01 | 0.30 ±0.01 | 0.38 ±0.02 | 0.28 ±0.02 | 0.27 ±0.01 | 0.27 ±0.01 | 0.31 ±0.01 | 28.5 ±4.0 | 30.5 ±2.27 | 31.3 ±1.20 | 30.6 ±2.52 | 31.1 ±2.15 | 31.1 ±2.15 | 30.1 ±1.87 | 30.2 ±0.85 | 30.1 ±1.15 | 30.1 ±0.17 |
| 1 | 0.46 ±0.02 | 0.45 ±0.01 | 0.45 ±0.01 | 0.45 ±0.01 | 0.44 ±0.02 | 0.49 ±0.01 | 0.45 ±0.04 | 0.42 ±0.02 | 0.43 ±0.02 | 0.44 ±0.02 | 42.8 ±1.58 | 43.8 ±2.08 | 45.0 ±0.57 | 44.0 ±3.14 | 42.2 ±1.73 | 42.2 ± | 43.1 ±1.39 | 43.4 ±1.16 | 44.6 ±0.51 | 45.2 ±1.10 |
| 2 | 0.74 ±0.06 | 0.76 ±0.01 | 0.77 ±0.01 | 0.68 ±0.01 | 0.68 ±0.02 | 0.71 ±0.02 | 0.64 ±0.02 | 0.65 ±0.01 | 0.68 ±0.01 | 0.66 ±0.02 | 50.8 ±2.14 | 51.8 ±2.47 | 51.2 ±2.73 | 40.2 ±0.91 | 49.9 ±1.25 | 49.9 ±1.25 | 48.3 ±4.24 | 51.3 ±1.03 | 51.1 ±1.84 | 52.0 ±2.25 |
| 3 | 0.98 ±0.01 | 1.00 ±0.02 | 1.02 ±0.01 | 1.03 ±0.03 | 0.96 ±0.08 | 1.01 ±0.03 | 0.94 ±0.03 | 0.92 ±0.02 | 0.97 ±0.01 | 0.92 ±0.02 | 53.2 ±2.16 | 53.2 ±1.42 | 52.8 ±2.27 | 51.5 ±1.25 | 52.5 ±1.15 | 52.5 ±1.15 | 52.6 ±1.17 | 53.3 ±1.15 | 53.8 ±0.45 | 55.2 ±1.21 |
| 4 | 1.15 ±0.03 | 1.18 ±0.04 | 1.22 ±0.01 | 1.37 ±0.06 | 1.27 ±0.14 | 1.28 ±0.03 | 1.18 ±0.02 | 1.20 ±0.02 | 1.31 ±0.02 | 1.20 ±0.01 | 54.3 ±2.57 | 55.2 ±2.38 | 55.2 ±0.87 | 54.2 ±1.25 | 53.9 ±1.42 | 53.9 ±1.42 | 53.7 ±0.68 | 54.2 ±1.01 | 55.0 ±0.51 | 56.4 ±0.72 |
| 5 | 1.34 ±0.04 | 1.38 ±0.06 | 1.46 ±0.01 | 1.52 ±0.04 | 1.48 ±0.07 | 1.65 ±0.03 | 1.45 ±0.04 | 1.56 ±0.11 | 1.61 ±0.04 | 1.58 ±0.04 | 57.1 ±1.95 | 56.8 ±1.44 | 56.4 ±0.20 | 56.2 ±0.85 | 55.5 ±0.66 | 55.5 ±0.66 | 55.7 ±1.10 | 55.7 ±0.21 | 56.1 ±0.53 | 57.1 ±1.64 |
| 10 | 1.54 ±0.03 | 1.61 ±0.08 | 1.73 ±0.05 | 1.81 ±0.01 | 1.62 ±0.02 | 1.83 ±0.04 | 2.23 ±0.58 | 1.80 ±0.02 | 1.86 ±0.03 | 1.92 ±0.04 | 58.7 ±1.46 | 58.7 ±1.80 | 59.0 ±1.96 | 59.5 ±1.97 | 58.2 ±0.70 | 58.2 ±0.70 | 57.4 ±0.71 | 58.0 ±1.07 | 58.4 ±1.48 | 58.8 ±0.75 |
| 15 | 1.70 ±0.02 | 1.76 ±0.02 | 1.82 ±0.06 | 1.91 ±0.01 | 1.71 ±0.01 | 1.98 ±0.02 | 2.48 ±0.83 | 2.01 ±0.04 | 1.99 ±0.05 | 2.05 ±0.02 | 62.4 ±1.18 | 63.0 ±0.66 | 62.9 ±2.38 | 62.9 ±1.80 | 62.3 ±1.05 | 62.3 ±1.05 | 61.5 ±1.99 | 62.3 ±2.50 | 63.3 ±0.12 | 61.9 ±0.96 |
| 20 | 1.75 ± 0.01 | 1.79 ±0.42 | 1.88 ±0.05 | 1.96 ±0.01 | 1.77 ±0.01 | 2.05 ±0.01 | 2.55 ±0.84 | 2.06 ±0.03 | 2.12 ±0.03 | 2.11 ±0.01 | 63.8 ±0.62 | 64.2 ±0.42 | 64.7 ±2.06 | 64.5 ±0.89 | 63.6 ±0.21 | 65.1 ±0.35 | 64.4 ±1.10 | 65.4 ±1.07 | 64.2 ±0.60 | 64.4 ±1.17 |
| Stage | Variant | Amount of CH4 [dm3/kgVS] | Amount of CH4 [dm3/kgFM] | FM of the Substrate Introduced into the MW Reactor [kgFM] | Total CH4 Production from the Substrate Feed to the MW Reactor [m3] | Energy Value CH4 [kWh/m3] | The Gross Energy Output (EGout) [kWh/gVS] | The Gross Energy Output (EGout) [kWh] | Specific Energy Input (Es) [kWh] | The Net Energy Output (ENout) [kWh] | The Net Energy Gain (Enet) [kWh] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | C | 437 | 78 | - | 3.90 | 9.17 | 4.00 × 10−2 | 35.76 | 0.0 | 35.76 | 0 |
| S2 | T10 | 384 | 81 | 50 | 4.05 | 3.52 × 10−2 | 37.14 | 7.3 | 29.84 | −5.92 | |
| T20 | 450 | 87 | 4.35 | 4.12 × 10−2 | 39.89 | 7.5 | 32.39 | −3.37 | |||
| T30 | 453 | 92 | 4.60 | 4.15 × 10−2 | 42.18 | 7.8 | 34.38 | −1.38 | |||
| S3 | T20-0.05 HCl | 428 | 94 | 4.70 | 3.92 × 10−2 | 43.01 | 7.5 | 35.51 | −0.25 | ||
| T20-0.10 HCl | 470 | 99 | 4.95 | 4.31 × 10−2 | 45.39 | 37.89 | 2.13 | ||||
| T20-0.20 HCl | 525 | 99 | 4.95 | 4.81 × 10−2 | 45.39 | 37.89 | 2.13 | ||||
| S4 | T20-0.05 NaOH | 542 | 100 | 5.00 | 4.97 × 10−2 | 45.85 | 38.35 | 2.59 | |||
| T20-0.10 NaOH | 539 | 102 | 5.10 | 4.94 × 10−2 | 46.77 | 39.27 | 3.51 | ||||
| T20-0.20 NaOH | 541 | 102 | 5.10 | 4.96 × 10−2 | 46.77 | 39.27 | 3.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Zieliński, M.; Nowicka, A.; Kazimierowicz, J. Influence of Microwave-Assisted Chemical Thermohydrolysis of Lignocellulosic Waste Biomass on Anaerobic Digestion Efficiency. Energies 2024, 17, 4207. https://doi.org/10.3390/en17174207
Dębowski M, Zieliński M, Nowicka A, Kazimierowicz J. Influence of Microwave-Assisted Chemical Thermohydrolysis of Lignocellulosic Waste Biomass on Anaerobic Digestion Efficiency. Energies. 2024; 17(17):4207. https://doi.org/10.3390/en17174207
Chicago/Turabian StyleDębowski, Marcin, Marcin Zieliński, Anna Nowicka, and Joanna Kazimierowicz. 2024. "Influence of Microwave-Assisted Chemical Thermohydrolysis of Lignocellulosic Waste Biomass on Anaerobic Digestion Efficiency" Energies 17, no. 17: 4207. https://doi.org/10.3390/en17174207
APA StyleDębowski, M., Zieliński, M., Nowicka, A., & Kazimierowicz, J. (2024). Influence of Microwave-Assisted Chemical Thermohydrolysis of Lignocellulosic Waste Biomass on Anaerobic Digestion Efficiency. Energies, 17(17), 4207. https://doi.org/10.3390/en17174207









