Investigation of a Fuel-Flexible Diffusion Swirl Burner Fired with NH3 and Natural Gas Mixtures
Abstract
1. Introduction
2. Numerical Model
2.1. Governing Equations for Reactive Fluid Flow
2.2. Turbulent Closure-Realizable k- Model
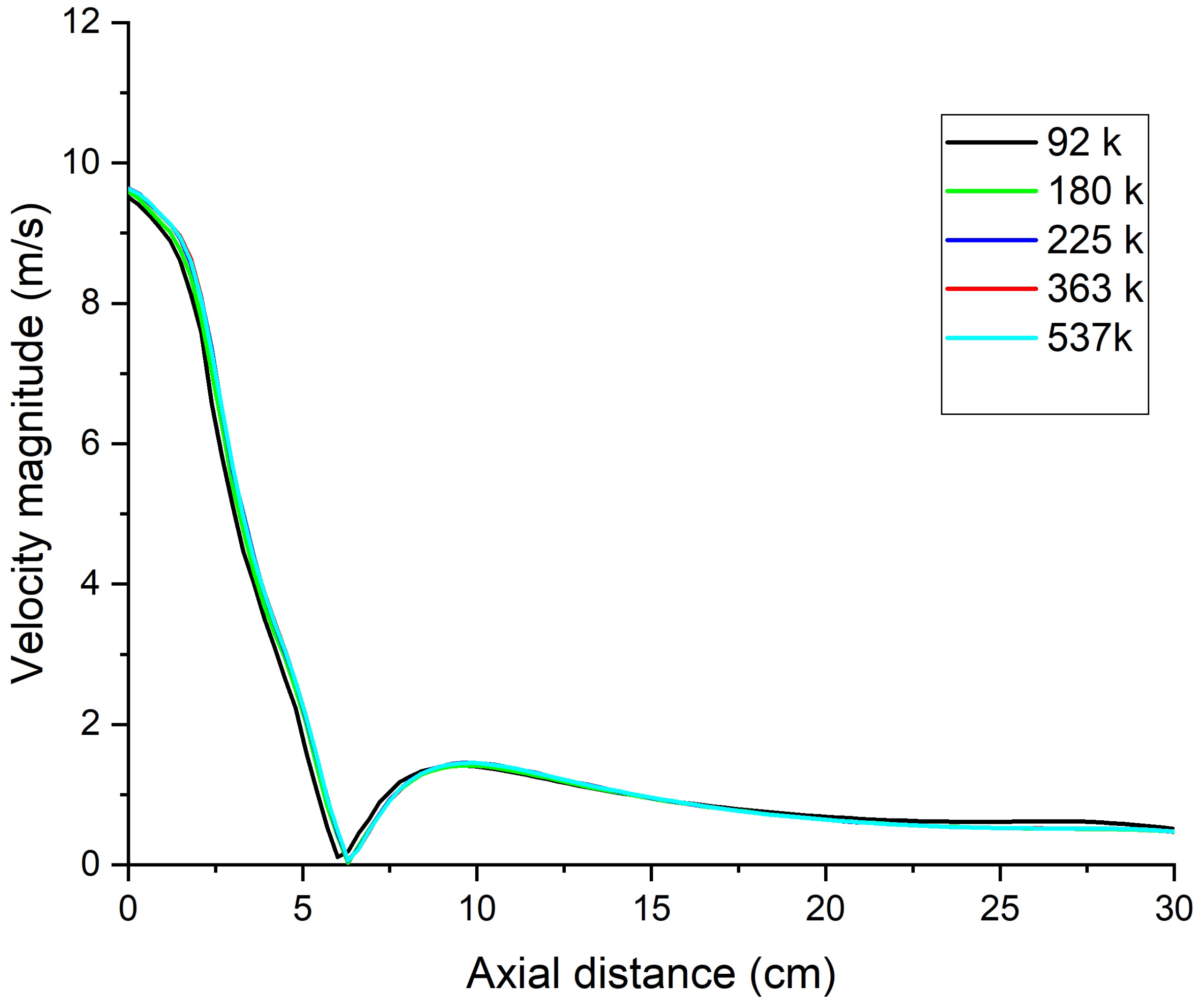
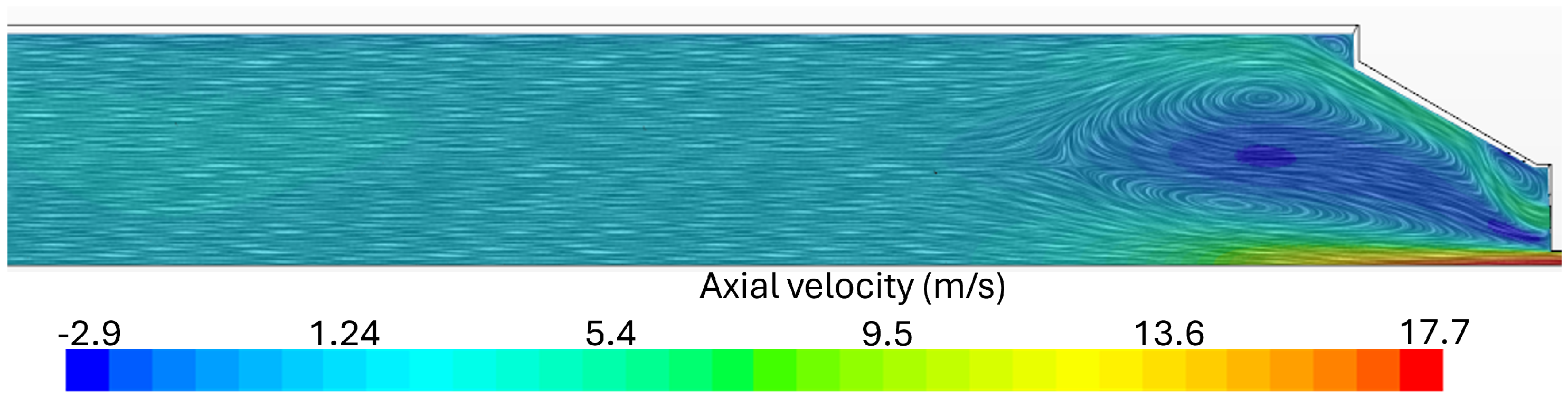
2.3. Mesh Details
2.4. Chemical Kinetic Mechanism
2.5. Radiation Model
2.6. Modelling Combustion
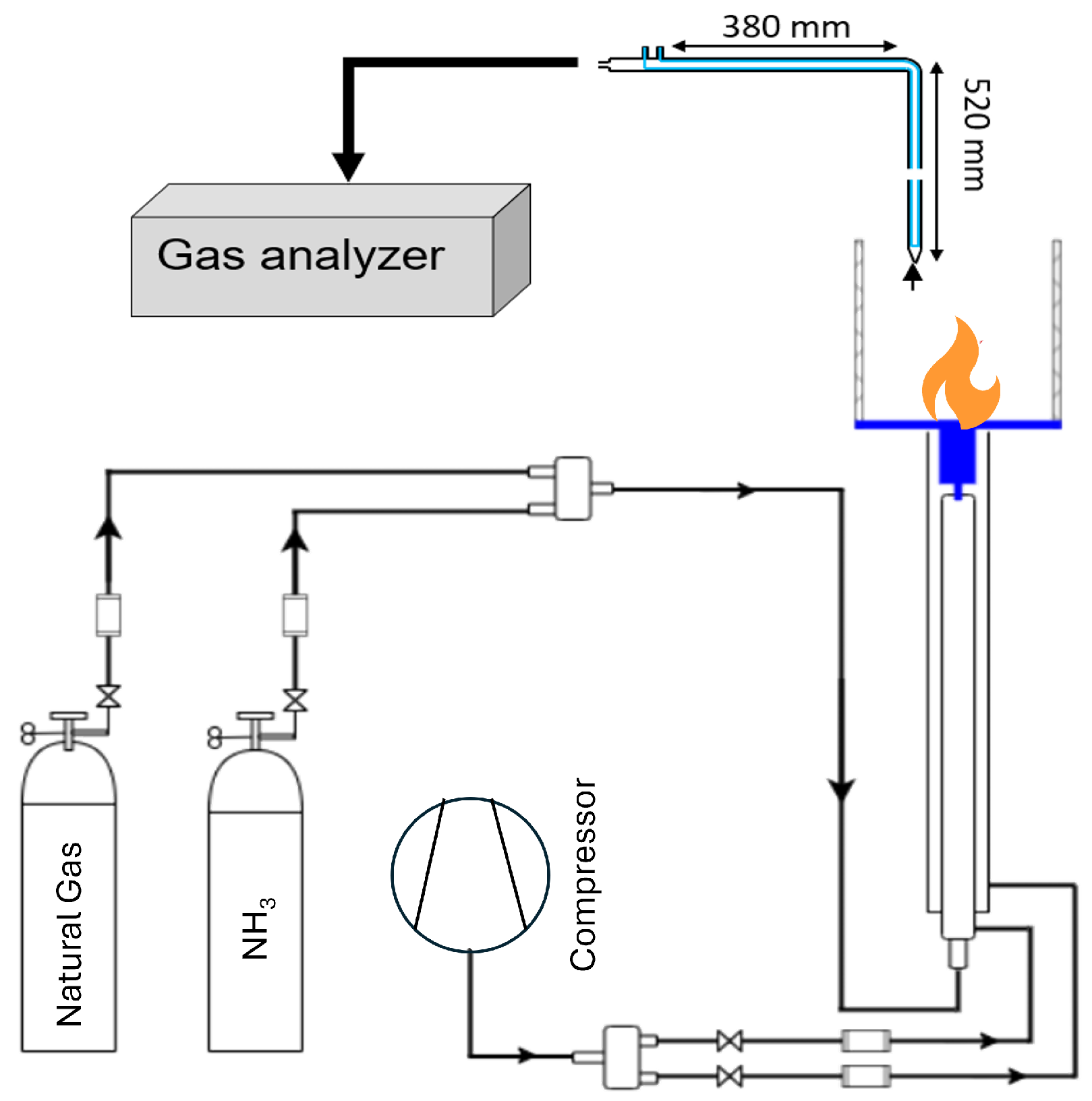
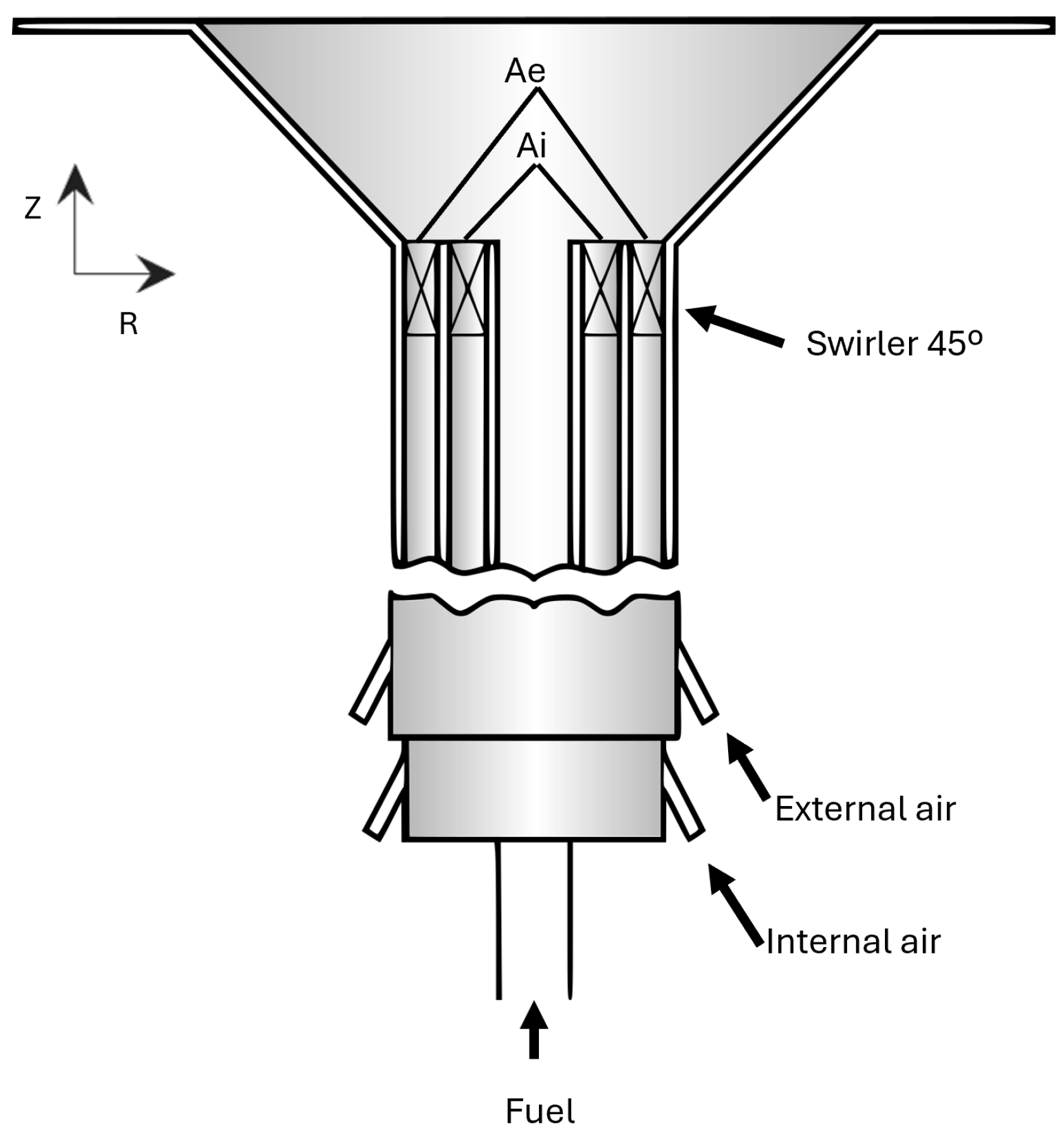
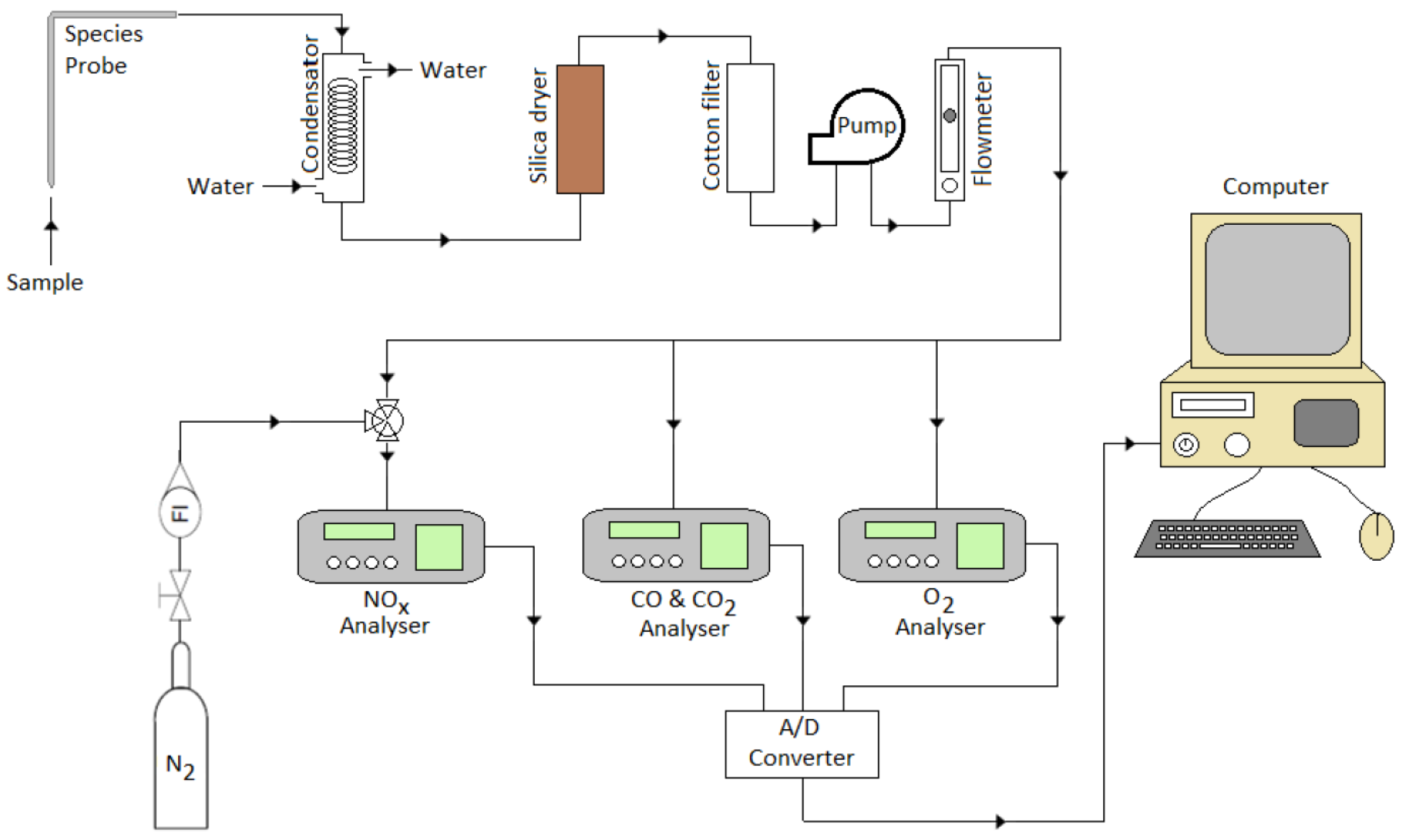
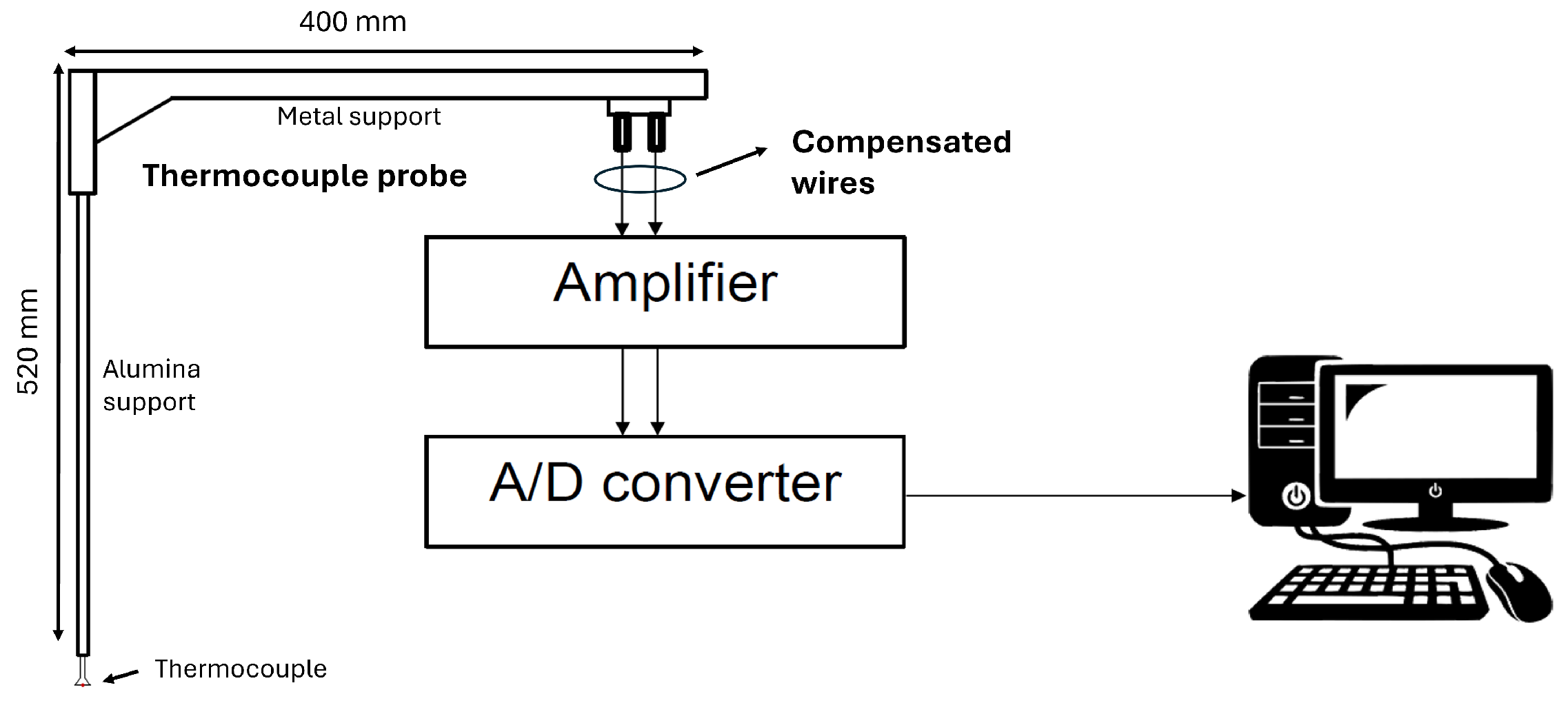
3. Experimental Setup
4. Experimental Results
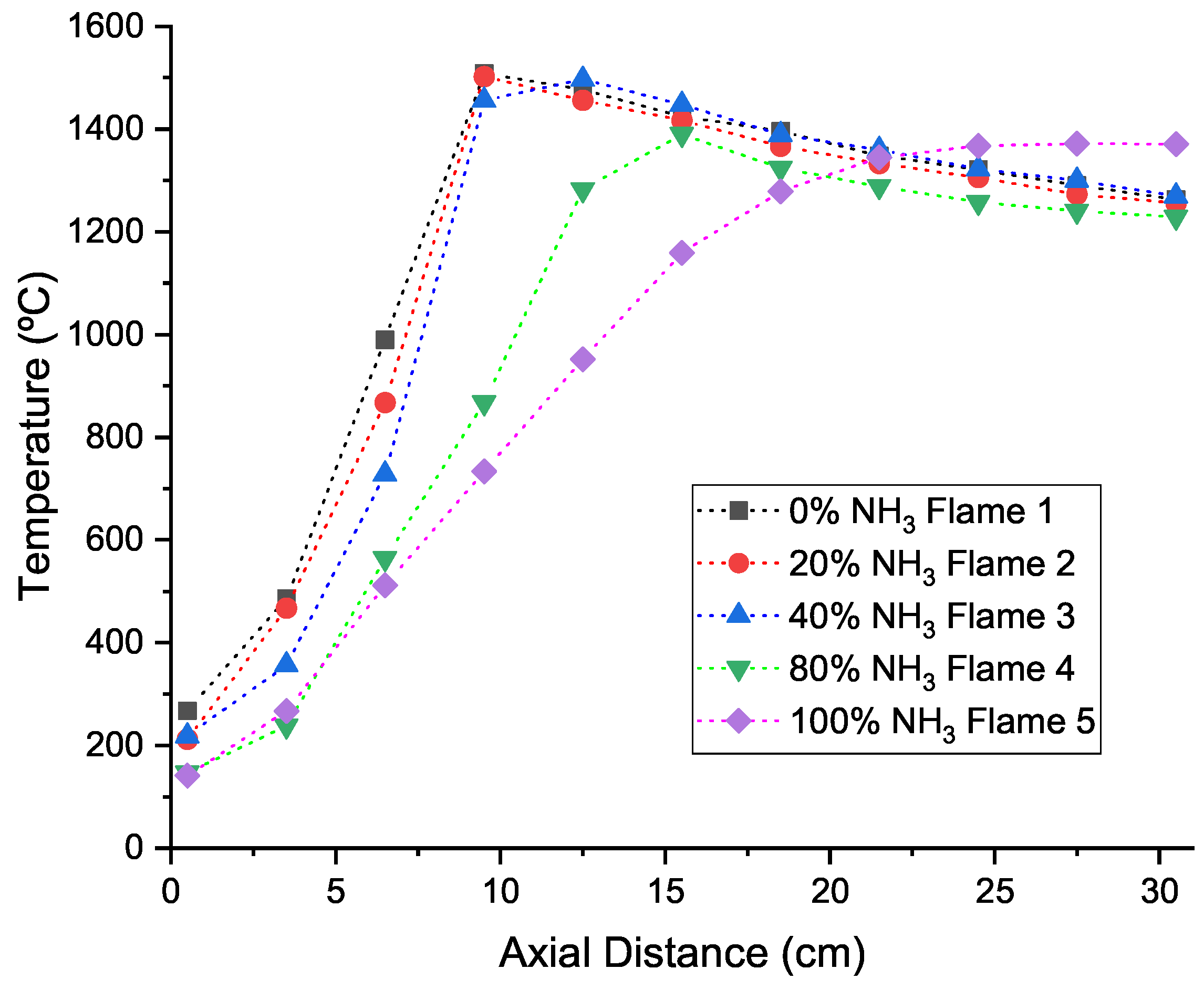
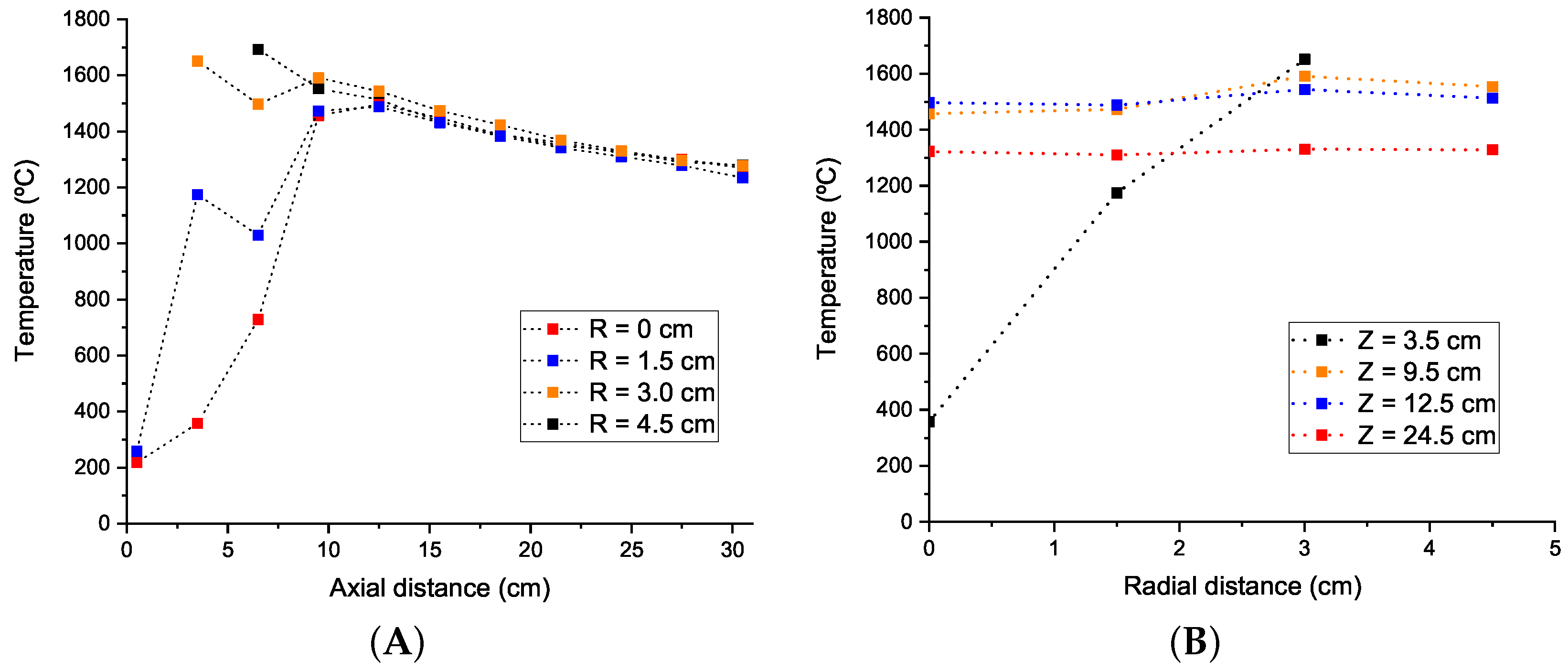
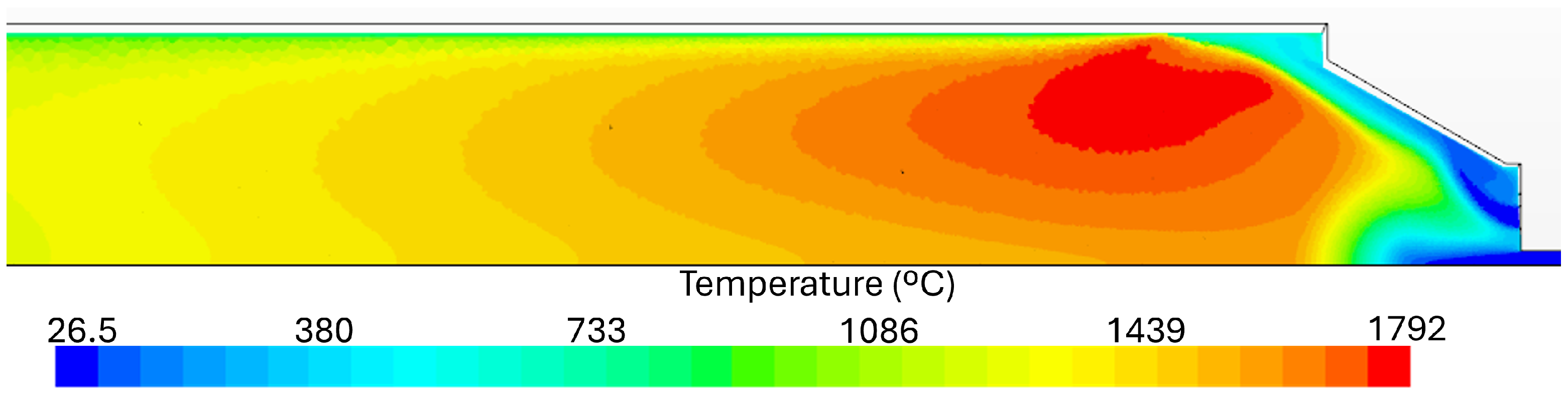
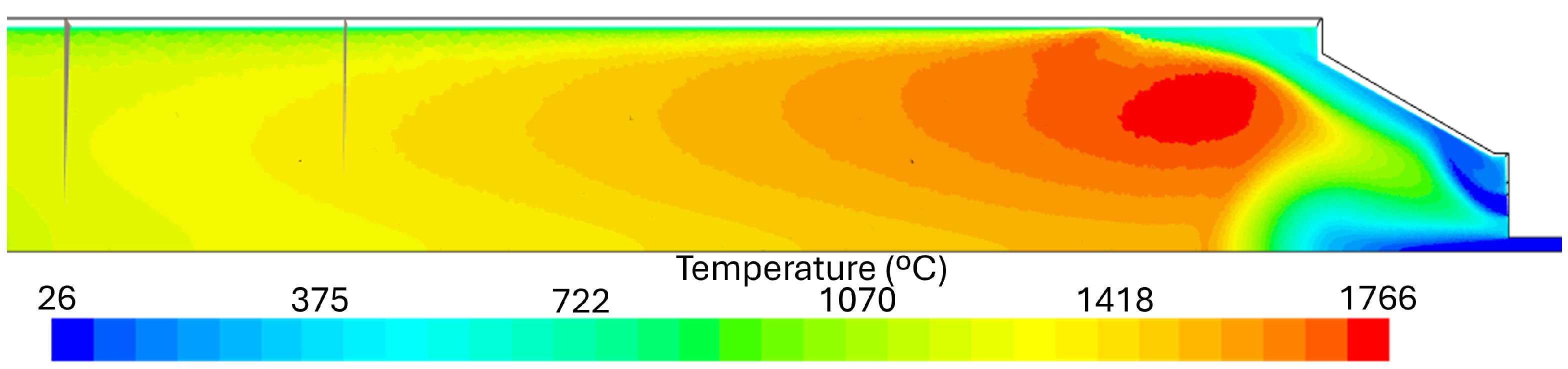
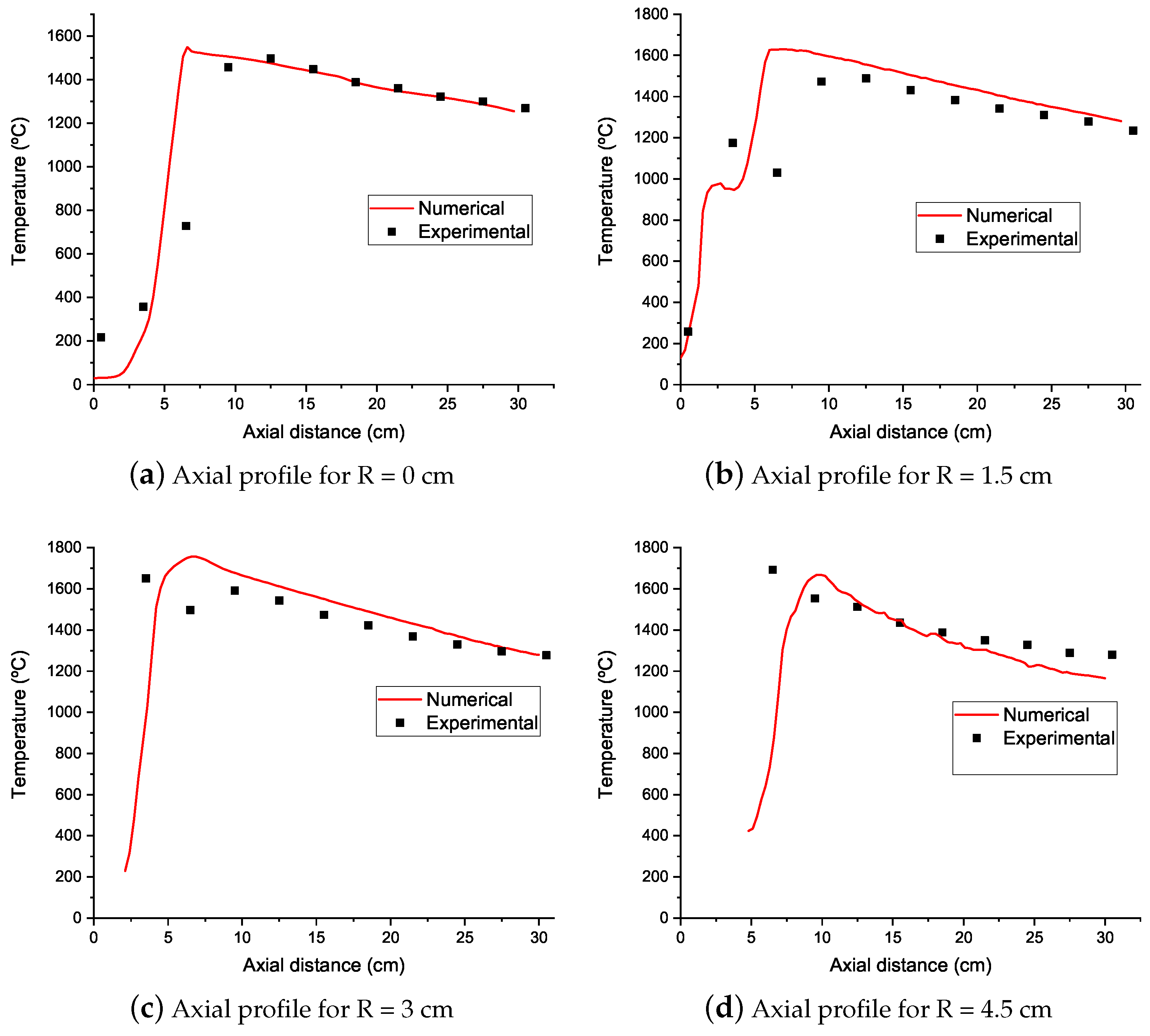
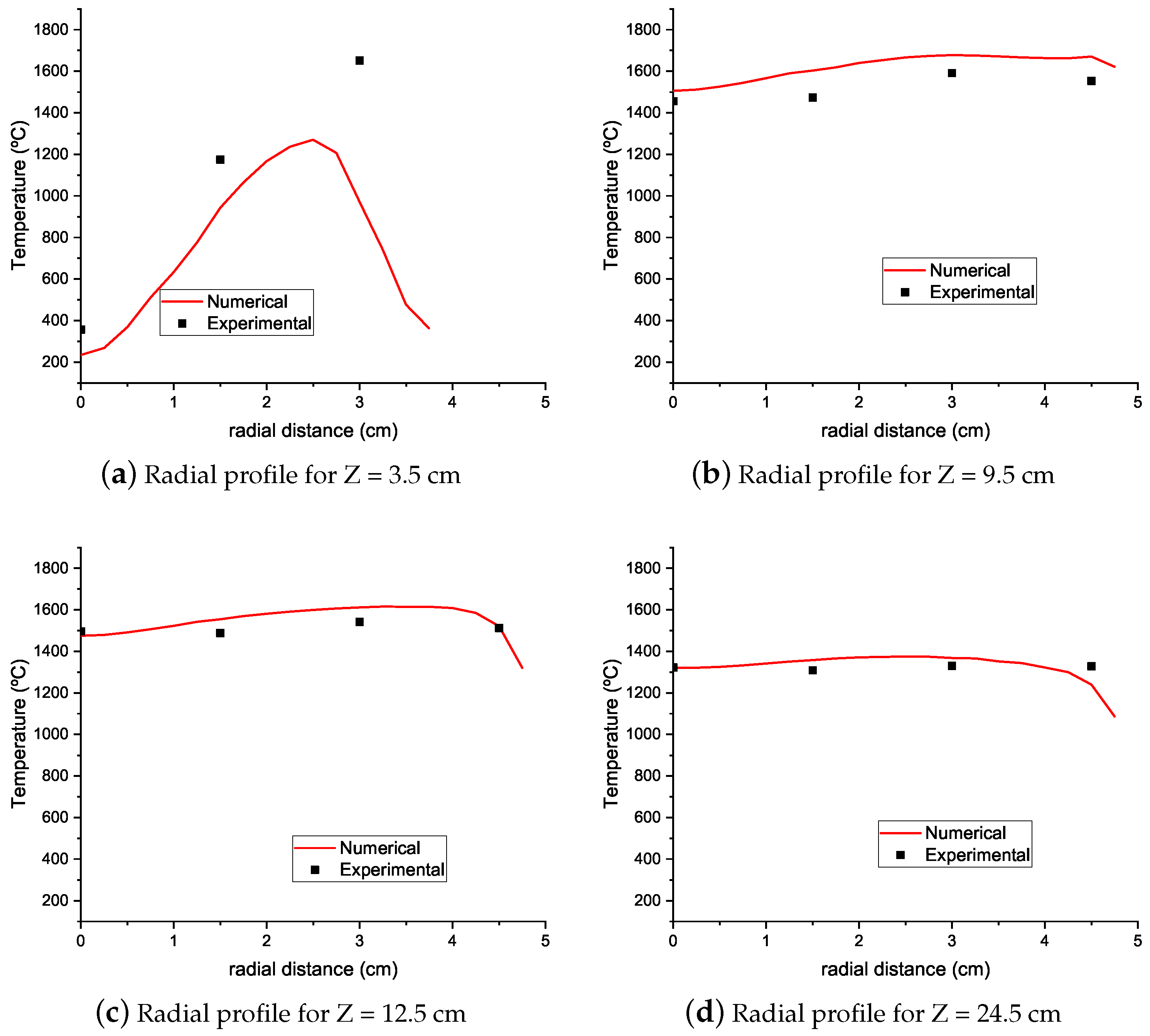
4.1. Temperature Results
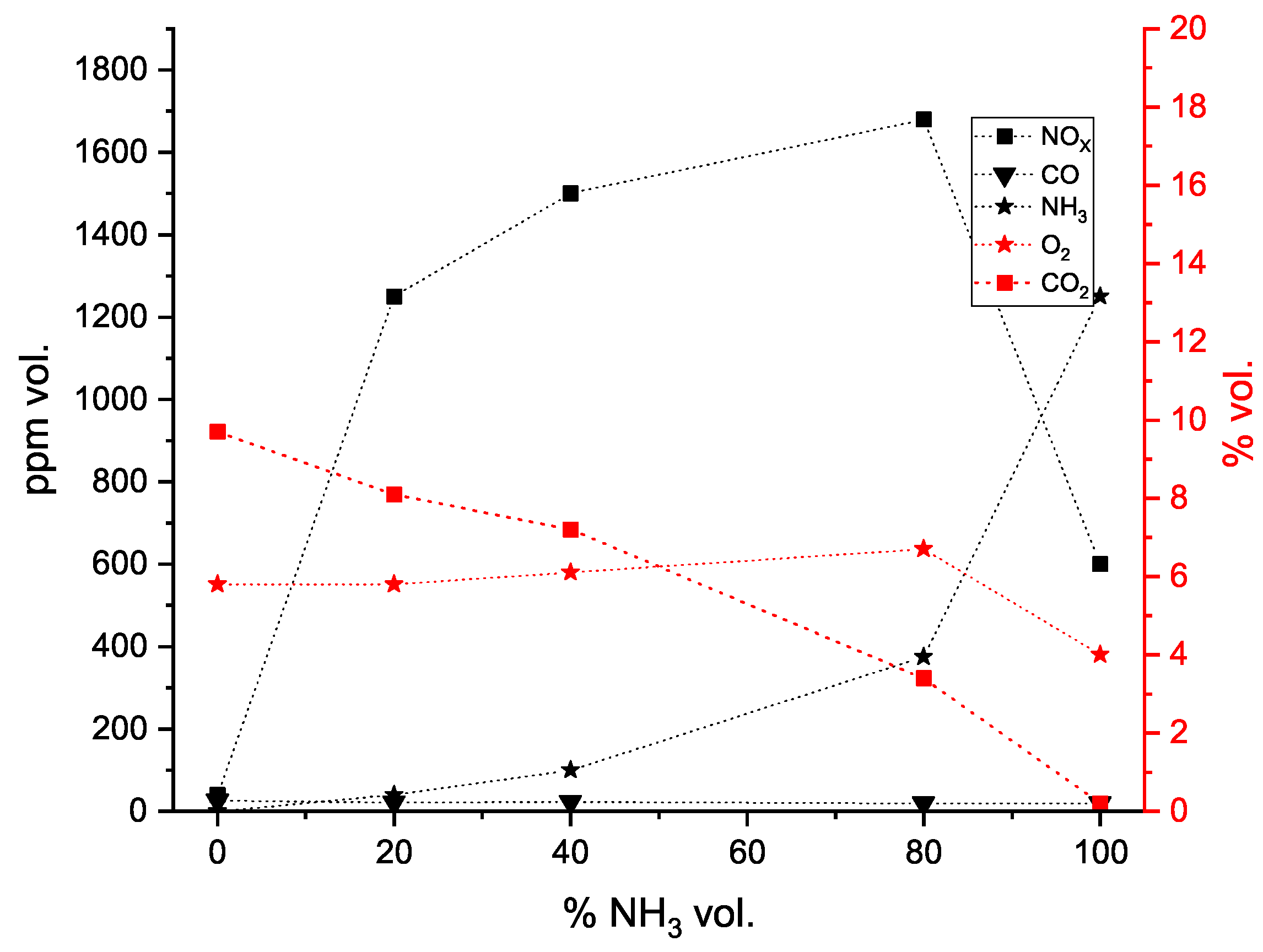
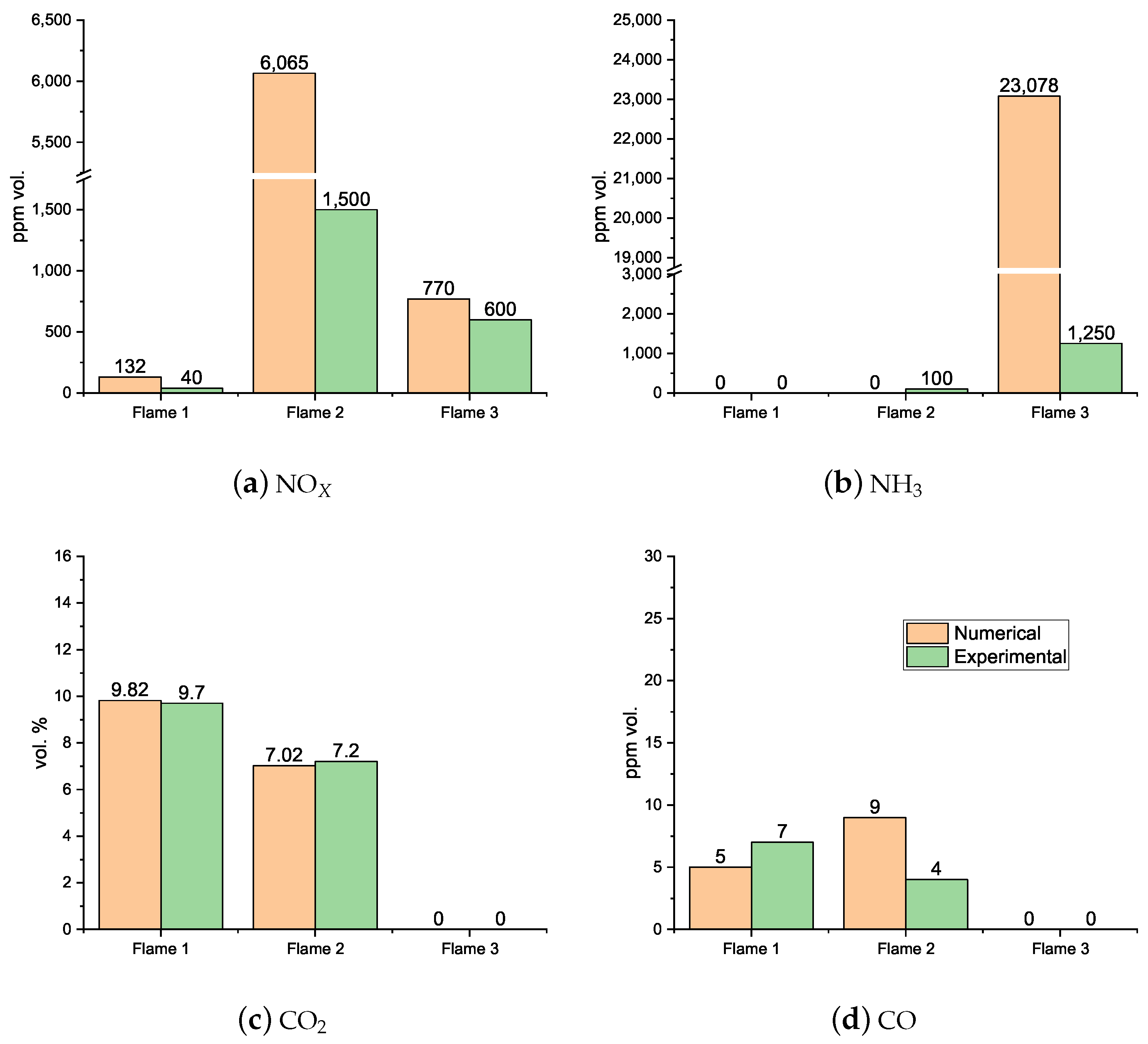
4.2. Flue Gas Emissions
5. Numerical Results
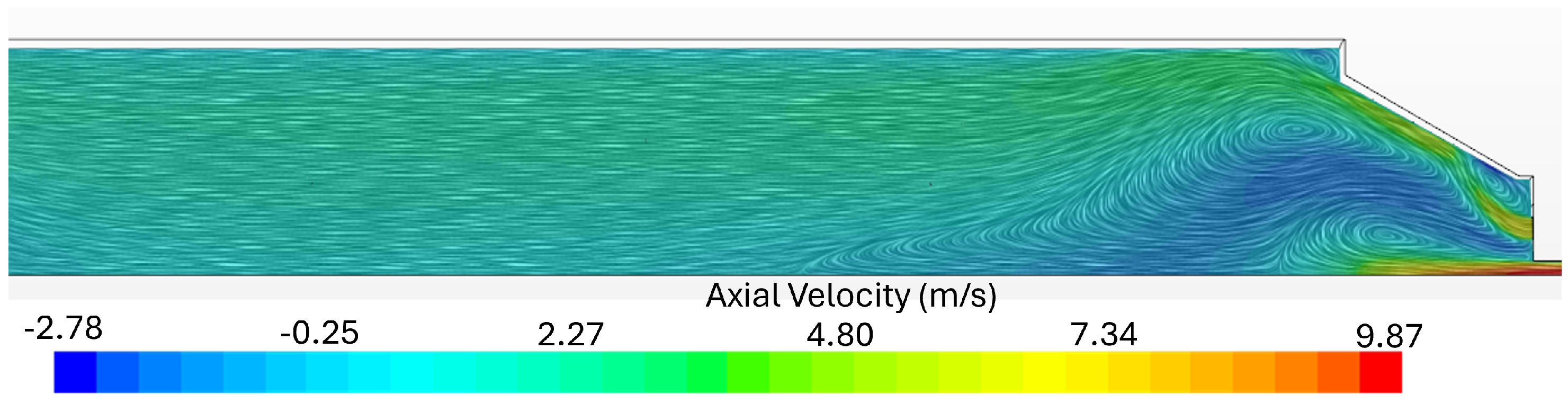
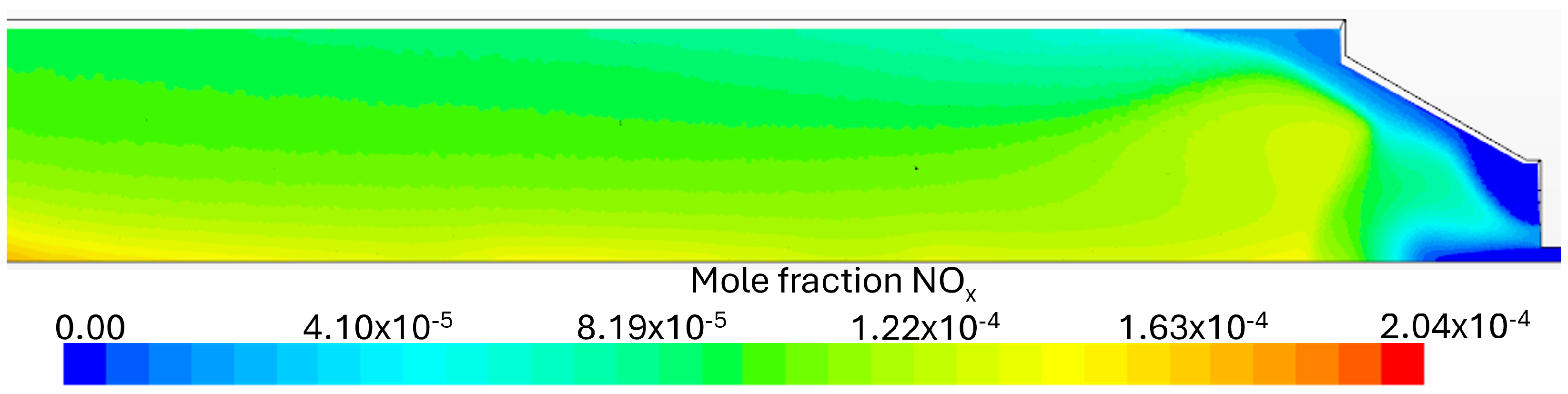
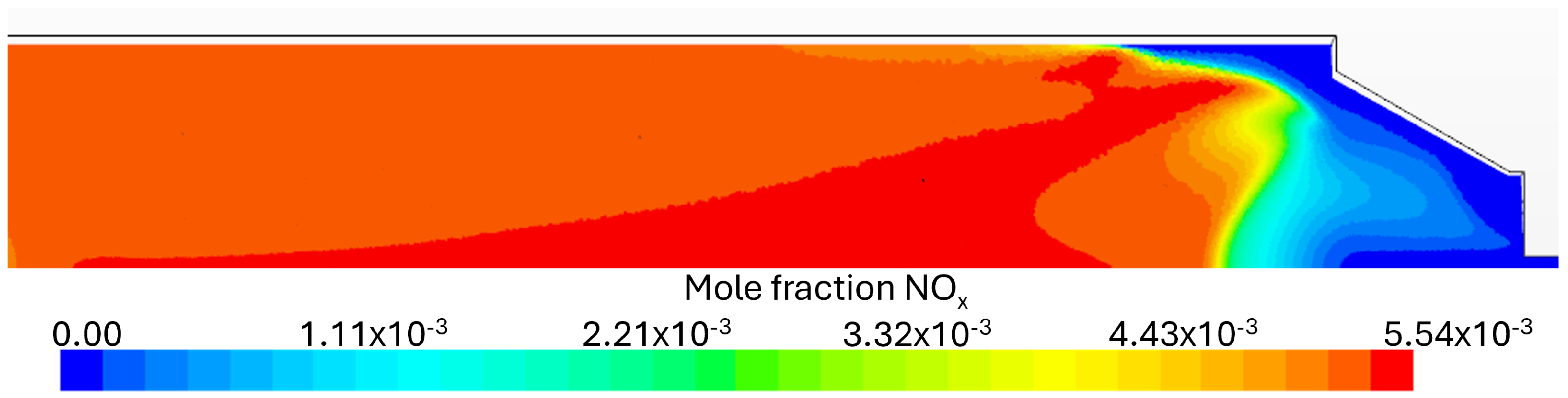
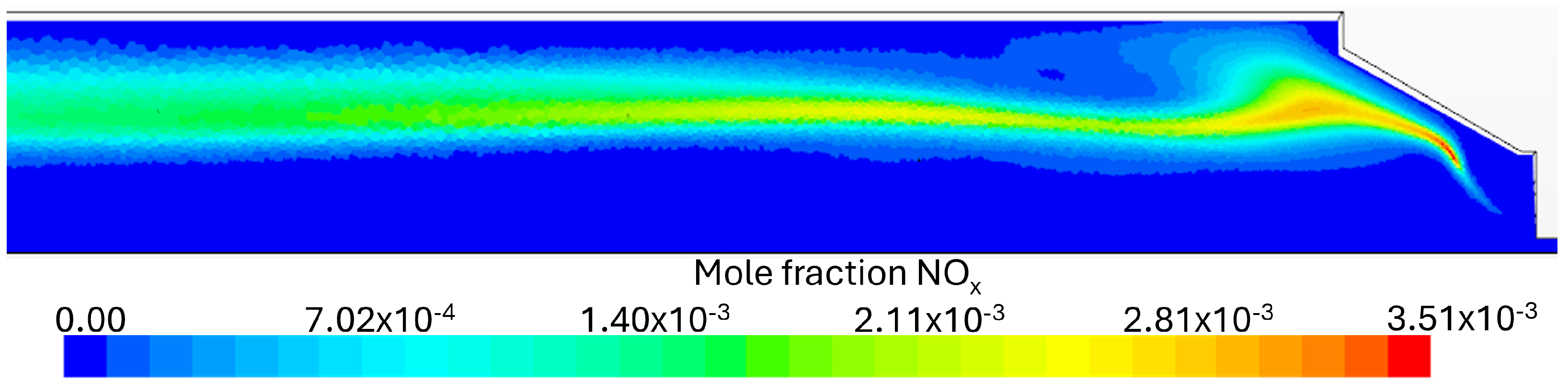
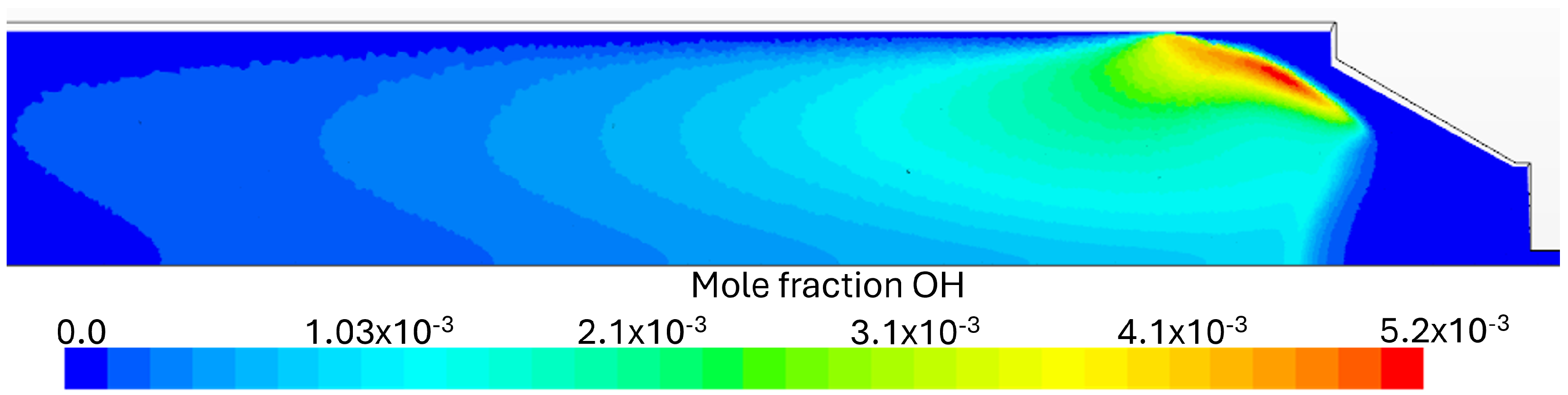
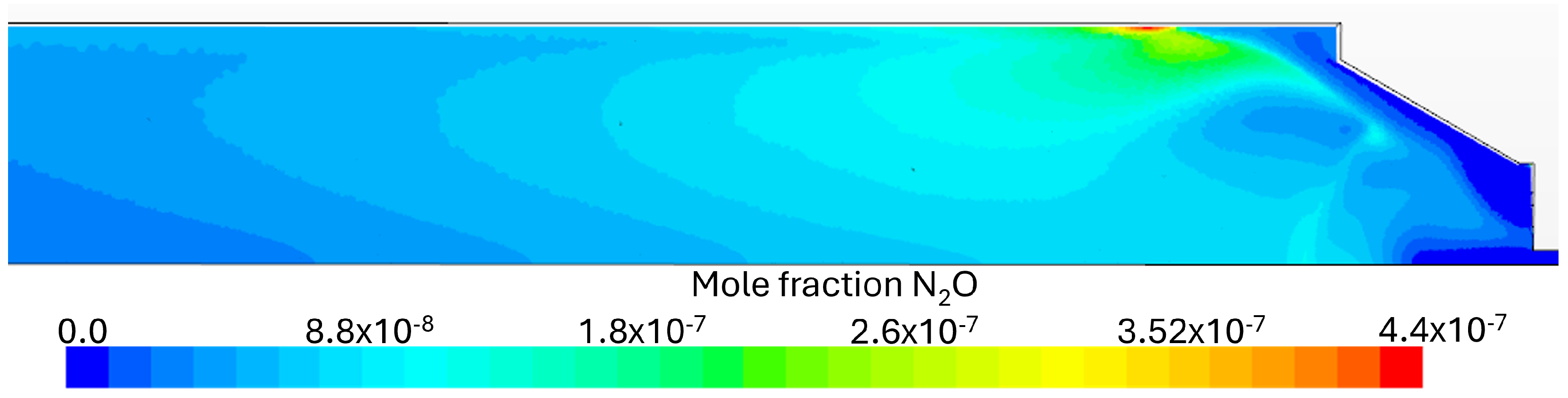
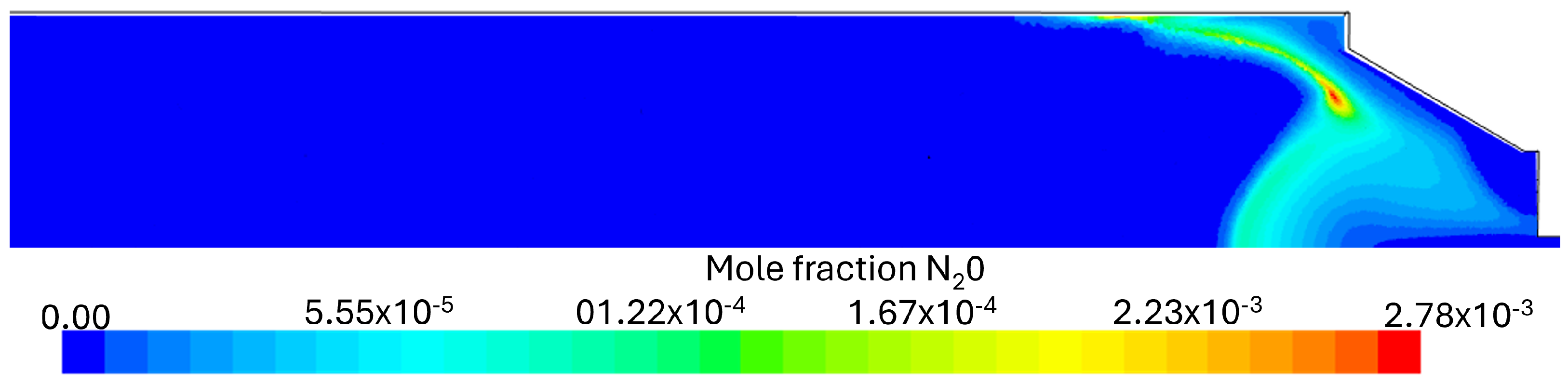
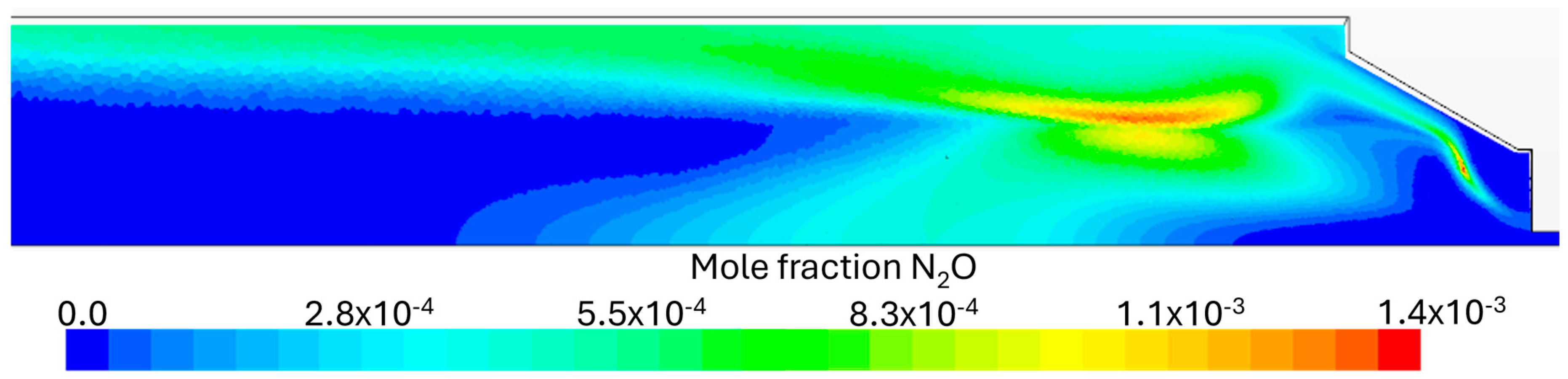
Flue Gas Emissions
6. Conclusions
- Regarding the industrial application, there are several relevant implications that can be drawn from this study. First, it was experimentally demonstrated that a conventional gas burner, typically used in the industry, can operate stably using pure NG and its blends with up to 100% vol. of NH3. It was verified that the flame stability was maintained with the addition of NH3. This implies that, in an industrial context, fuel-flexible burners have the readiness and potential to serve as a cost-effective and efficient transitional technology for blending NH3 with conventional fossil fuels as a strategy to offset the carbon emissions of combustion systems in the near future.
- It was observed that even with the burner firing small amounts of NH3, there were still high NOx emissions, highlighting the necessity for post-combustion flue gas treatment, namely SCR (selective catalytic reduction) or staged combustion applications such as RQL (Rich–quench–Lean) for industrial applications. At higher fuel NH3 content, a substantial ammonia slip was detected in the exhaust gas emissions at 80% NH3. The presence of unburned NH3 and the formation of N2O are limiting factors in ammonia combustion applications due to their toxicity and GWP (greenhouse warming potential), respectively.
- Numerical simulations were able to qualitatively predict the axial temperature profiles of the experimental burner even though simulations with natural gas in their fuel mixture overpredicted the peak flame temperature. Downstream of this peak, the numerical simulations accurately predict the temperature profile up to the burner exit.
- It can be concluded that a cost-effective simulation can be used to qualitatively predict the experimental combustor’s flame characteristics. Complex chemistry models and detailed kinetic mechanisms were necessary to model the ammonia/natural gas multicomponent combustion process. However, simulations were not able to capture the NOx and NH3 emissions for some conditions, namely for flame 3 (40% NH3) and the NH3 slip emissions for flame 5 (100% NH3).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| CFD | Computational Fluid Dynamics |
| DOM | Discrete ordinates model |
| EDC | Eddy dissipation concept |
| EU | European union |
| GWP | Global warming potential |
| LFC | Laminar Flame Concept |
| Mt | Million tons [t] |
| ODE | Ordinary differential equation |
| R | Radial distance along the combustion chamber [cm] |
| RANS | Reynolds-Averaged Navier-Stokes |
| SVA | Swirl vane angle |
| TFSC | Turbulent Flame Speed Closure |
| Z | Axial distance along the combustion chamber [cm] |
| Greek symbols | |
| Turbulent Prandtl number | |
| Kinematic viscosity [m2/s] | |
| Reaction rate [Kg/(m3·s)] | |
| Reynolds stress tensor | |
| Turbulent scalar flux | |
| Density [Kg/m3] | |
| Turbulent time-scale [s] | |
| K | Thermal conductivity [W/(m·K)] |
| Dissipation rate of turbulent kinetic energy [m2/s3] | |
| Dynamic viscosity [Kg/(m·s)] | |
| Turbulent viscosity [Kg/(m·s)] | |
| Turbulent Prandtl number | |
| Equivalence ratio | |
| Other symbols | |
| Specific heat capacity at constant pressure [J/(Kg·K)] | |
| k | Turbulent kinetic energy [m2/s2] |
| p | Pressure [Pa] |
| Production term of turbulent kinetic energy [W/m3] | |
| Energy source term [W/m3] | |
| T | Temperature [K] |
| Specific time-scale [s] | |
| Large-eddy time scale [s] | |
| Mass fraction of species | |
| Species mass fraction | |
| Body forces [N/m3] | |
| Mean rate of rotation tensor | |
| Model constant | |
| Model constant | |
| Model constant | |
| Mean strain rate tensor | |
| u | Velocity vector [m/s] |
| C | Realizable k- model coefficient |
| F | Damping function |
| Diffusion flux [Kg/(m2/s)] |
References
- Commission Regulation (EU) No 813/2013. 2013. Available online: https://eur-lex.europa.eu/eli/reg/2013/813/2017-01-09 (accessed on 1 April 2024).
- Valera-Medina, A.; Syred, N.; Griffiths, A. Visualisation of isothermal large coherent structures in a swirl burner. Combust. Flame 2009, 156, 1723–1734. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. Performance assesment of hydrogen and ammonia combustion with various fuels for power generators. Int. J. Hydrogen Energy 2018, 43, 21037–21048. [Google Scholar] [CrossRef]
- American Chemical Society. Ammonia. Available online: https://www.acs.org/molecule-of-the-week/archive/a/ammonia.html (accessed on 15 February 2024).
- da Rocha, R.C.; Costa, M.; Bai, X.S. Chemical kinetic modelling of ammonia/hydrogen/air ignition, premixed flame propagation and NO emission. Fuel 2019, 246, 24–33. [Google Scholar] [CrossRef]
- Møller, M.M.K. Managing Emissions from Ammonia-Fueled Vessels. Center for Zero Carbon Shipping (MMMCZCS). Available online: https://cms.zerocarbonshipping.com/media/uploads/documents/Ammonia-emissions-reduction-position-paper_v4.pdf (accessed on 10 May 2024).
- Okafor, E.C.; Tsukamoto, M.; Hayakawa, A.; Somarathne, K.A.; Kudo, T.; Tsujimura, T.; Kobayashi, H. Influence of wall heat loss on the emission characteristics of premixed ammonia-air swirling flames interacting with the combustor wall. Proc. Combust. Inst. 2021, 38, 5139–5146. [Google Scholar] [CrossRef]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2019, 37, 5401–5409. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Manna, M.V.; Sabia, P.; Raffaele, R.; Mara, d.J. NH3 effect on CH4 oxidation regimes and chemical kinetics. In Proceedings of the European Combustion Meeting, Rouen, France, 26–28 April 2023; pp. 760–765. [Google Scholar]
- Cheng, J.; Zhang, B. Analysis of explosion and laminar combustion characteristics of premixed ammonia-air/oxygen mixtures. Fuel 2023, 351, 128860. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, B. Experimental study on the explosion characteristics of ammonia-hydrogen-air mixtures. Fuel 2024, 363, 131046. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Zhao, N.; Chen, M.; Zheng, H. Numerically study of CH4/NH3 combustion characteristics in an industrial gas turbine combustor based on a reduced mechanism. Fuel 2022, 327, 124897. [Google Scholar] [CrossRef]
- Mashruk, S.; Vigueras-Zuniga, M.; del Cueto, M.T.; Xiao, H.; Yu, C.; Maas, U.; Valera-Medina, A. Combustion features of CH4/NH3/H2 ternary blends. Int. J. Hydrogen Energy 2022, 47, 30315–30327. [Google Scholar] [CrossRef]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A New k-ϵ Eddy Viscosity Model for High Reynolds Number Turbulent Flows—Model Development and Validation. Comput. Fluids 1995, 24, 227–238. [Google Scholar] [CrossRef]
- Spalart, P.R.; Rumsey, C.L. Effective Inflow Conditions for Turbulence Models in Aerodynamic Calculations. AIAA J. 2007, 45, 2544–2553. [Google Scholar] [CrossRef]
- Siemens Industries Digital Software Realizable K-epsilon model. In SIEMENS STAR-CCM+ Documentation; Siemens: Munich, Germany, 2020.
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 5 August 2024).
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Siemens Industries Digital Software Participating Media Radiation (DOM). In SIEMENS STAR-CCM+ Documentation; Siemens: Munich, Germany, 2020.
- Modest, M. Narrow-band and full-spectrum k-distributions for radiative heat transfer—Correlated-k vs. scaling approximation. J. Quant. Spectrosc. Radiat. Transf. 2003, 76, 69–83. [Google Scholar] [CrossRef]
- Tarek Echekki, E.M. (Ed.) Turbulent Combustion Modeling; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Siemens Industries Digital Software Reacting Species Transport. In SIEMENS STAR-CCM+ Documentation; Siemens: Munich, Germany, 2020.
- Stiehl, B.; Worbington, T.; Miegel, A.; Martin, S.; Velez, C.; Ahmed, K. Combustion and Emission Characteristics of a Lean Axial-Stage Combustor. In Proceedings of the ASME Turbo Expo 2019: Turbomachinery Technical Conference and Exposition, Phoenix, AZ, USA, 17–21 June 2019. Volume 4B: Combustion, Fuels, and Emissions, Turbo Expo: Power for Land, Sea, and Air. [Google Scholar] [CrossRef]
- Stiehl, B.; Genova, T.; Otero, M.; Reyes, J.; Ahmed, K.A.; Martin, S.M. NOx Emission of an Axial-Staged Combustor at High-Pressure. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Virtual, 24–28 August 2020. [Google Scholar] [CrossRef]
- Bösenhofer, M.; Wartha, E.M.; Jordan, C.; Harasek, M. The Eddy Dissipation Concept—Analysis of Different Fine Structure Treatments for Classical Combustion. Energies 2018, 11, 1902. [Google Scholar] [CrossRef]
- Halouane, Y.; Dehbi, A. CFD simulations of premixed hydrogen combustion using the Eddy Dissipation and the Turbulent Flame Closure models. Int. J. Hydrogen Energy 2017, 42, 21990–22004. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Sotoudeh, F.; Jun, D.; Lee, B.J.; Esfahani, J.A.; Karimi, N. On the effects of NH3 addition to a reacting mixture of H2/CH4 under MILD combustion regime: Numerical modeling with a modified EDC combustion model. Fuel 2022, 326, 125096. [Google Scholar] [CrossRef]
- Mansouri, Z.; Boushaki, T. Experimental and numerical investigation of turbulent isothermal and reacting flows in a non-premixed swirl burner. Int. J. Heat Fluid Flow 2018, 72, 200–213. [Google Scholar] [CrossRef]
- Lorena, M. Fuel-Flexible Hydrogen Burner for Industrial Furnace Systems; Instituto Superior Técnico: Lisboa, Portugal, 2023. [Google Scholar]
- Pacheco, G.P.; Rocha, R.C.; Franco, M.C.; Mendes, M.A.A.; Fernandes, E.C.; Coelho, P.J.; Bai, X.S. Experimental and Kinetic Investigation of Stoichiometric to Rich NH3/H2/Air Flames in a Swirl and Bluff-Body Stabilized Burner. Energy Fuels 2021, 35, 7201–7216. [Google Scholar] [CrossRef]
- Franco, M.C.; Rocha, R.C.; Costa, M.; Yehia, M. Characteristics of NH3/H2/air flames in a combustor fired by a swirl and bluff-body stabilized burner. Proc. Combust. Inst. 2021, 38, 5129–5138. [Google Scholar] [CrossRef]
- Ramos, C.F.; Rocha, R.C.; Oliveira, P.M.; Costa, M.; Bai, X.S. Experimental and kinetic modelling investigation on NO, CO and NH3 emissions from NH3/CH4/air premixed flames. Fuel 2019, 254, 115693. [Google Scholar] [CrossRef]
- Rocha, R.C.; Ramos, C.F.; Costa, M.; Bai, X.S. Combustion of NH3/CH4/Air and NH3/H2/Air Mixtures in a Porous Burner: Experiments and Kinetic Modeling. Energy Fuels 2019, 33, 12767–12780. [Google Scholar] [CrossRef]
- De, D. Measurement of flame temperature with a multielement thermocouple. J. Inst. Energy (UK) 1981, 54, 113–116. [Google Scholar]
- Hindasageri, V.; Vedula, R.P.; Prabhu, S.V. Thermocouple error correction for measuring the flame temperature with determination of emissivity and heat transfer coefficient. Rev. Sci. Instrum. 2013, 84, 024902. [Google Scholar] [CrossRef]
- IPCC. Annex V11: Glossary; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Al-Abdeli, Y.M.; Masri, A.R. Recirculation and flowfield regimes of unconfined non-reacting swirling flows. Exp. Therm. Fluid Sci. 2003, 27, 655–665. [Google Scholar] [CrossRef]
- Mashruk, S.; Okafor, E.; Kovaleva, M.; Alnasif, A.; Pugh, D.; Hayakawa, A.; Valera-Medina, A. Evolution of N2O production at lean combustion condition in NH3/H2/air premixed swirling flames. Combust. Flame 2022, 244, 112299. [Google Scholar] [CrossRef]
- Barreto, T.A. Numerical Validation of an Open Flame, Swirl-Stabilized, Pure Hydrogen Burner; Instituto Superior Técnico: Lisbon, Portugal, 2023. [Google Scholar]
- Zhang, M.; An, Z.; Wei, X.; Wang, J.; Huang, Z.; Tan, H. Emission analysis of the CH4/NH3/air co-firing fuels in a model combustor. Fuel 2021, 291, 120135. [Google Scholar] [CrossRef]








| Fuel | Methane | Ammonia |
|---|---|---|
| Density (kg/m3) | 0.66 | 0.73 |
| Laminar burning velocity (m/s) | 0.38 | 0.07 |
| Auto-ignition temperature (K) | 859 | 930 |
| Low heating value (MJ/kg) | 50.05 | 18.8 |
| Adiabatic flame temperature (with air) (K) | 2223 | 1850 |
| Primary Air | Fuel Inlet | |
|---|---|---|
| Model/Brand | MCR–100 SLPM® | MCR–250 SLPM® |
| Control Range | 0.025–120 lpm | 0.025–250 lpm |
| Standard Accuracy | ±0.8% of reading ±0.2% full scale | ±0.8% of reading ±0.2% full scale |
| Gas Species | Model/Brand | Analysis Method | Value Range |
|---|---|---|---|
| NOx | Horiba pg-250 | Chemiluminescence | 0–2500 ppm vol. |
| O2 | Horiba CMA-331 A | Paramagnetism | 0–10% vol. |
| CO | Horiba CMA-331 A | Nondispersive | 0–5000 ppm vol. |
| CO2 | Horiba CMA-331 A | Nondispersive | 0–50% vol. |
| Flame | Power (kW) | Fuel (lpm) | Air (lpm) | Equivalence Ratio | Fuel Mixture / % |
|---|---|---|---|---|---|
| 1 | 5 | 8.8 | 105.5 | 0.8 | 0% NH3 |
| 2 | 5 | 10.13 | 105.5 | 0.8 | 20% NH3 |
| 3 | 5 | 11.79 | 105.5 | 0.8 | 40% NH3 |
| 4 | 5 | 17.52 | 105.5 | 0.8 | 80% NH3 |
| 5 | 5 | 23.15 | 105.5 | 0.8 | 100% NH3 |
| Boundary | Momentum Equation | Energy Equation |
|---|---|---|
| Inlet-fuel | mass flow = kg/s | |
| Inlet-air | mass flow = kg/s | |
| Outlet | Pressure Outlet | |
| Wall |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, G.; Pereira, J.; Mendes, M.; Coelho, P. Investigation of a Fuel-Flexible Diffusion Swirl Burner Fired with NH3 and Natural Gas Mixtures. Energies 2024, 17, 4206. https://doi.org/10.3390/en17174206
Pacheco G, Pereira J, Mendes M, Coelho P. Investigation of a Fuel-Flexible Diffusion Swirl Burner Fired with NH3 and Natural Gas Mixtures. Energies. 2024; 17(17):4206. https://doi.org/10.3390/en17174206
Chicago/Turabian StylePacheco, Gonçalo, José Pereira, Miguel Mendes, and Pedro Coelho. 2024. "Investigation of a Fuel-Flexible Diffusion Swirl Burner Fired with NH3 and Natural Gas Mixtures" Energies 17, no. 17: 4206. https://doi.org/10.3390/en17174206
APA StylePacheco, G., Pereira, J., Mendes, M., & Coelho, P. (2024). Investigation of a Fuel-Flexible Diffusion Swirl Burner Fired with NH3 and Natural Gas Mixtures. Energies, 17(17), 4206. https://doi.org/10.3390/en17174206







