CO2 Storage in Deep Oceanic Sediments in the form of Hydrates: Energy Evaluation and Advantages Related to the Use of N2-Containing Mixtures
Abstract
1. Introduction
2. Role of Porous Sediments and Process Kinetics
- (1)
- mixed sample preparation;
- (2)
- excess gas;
- (3)
- excess water;
- (4)
- dissolved gas method.
3. Materials and Methods
3.1. Experimental Apparatus
3.2. Materials
3.3. Experimental Procedure
- -
- VPORE [cm3]: internal volume available for the production of hydrates;
- -
- Z: compressibility factor, calculated with the Peng–Robinson Equation;
- -
- -
- specific heat equal to 0.83 kJ/kgK for the porous quartz sediment;
- -
- specific heat equal to 0.86 kJ/kgK for pure carbon dioxide;
- -
- for the binary CO2/N2 mixtures, the following values were considered: 0.98 kJ/kgK for (70/30) vol%, 1.00 kJ/kgK for (50/50) vol% and 1.01 kJ/kgK for (40/60) vol%.
4. Results
4.1. Energy Spent per Unit of Hydrate Formed
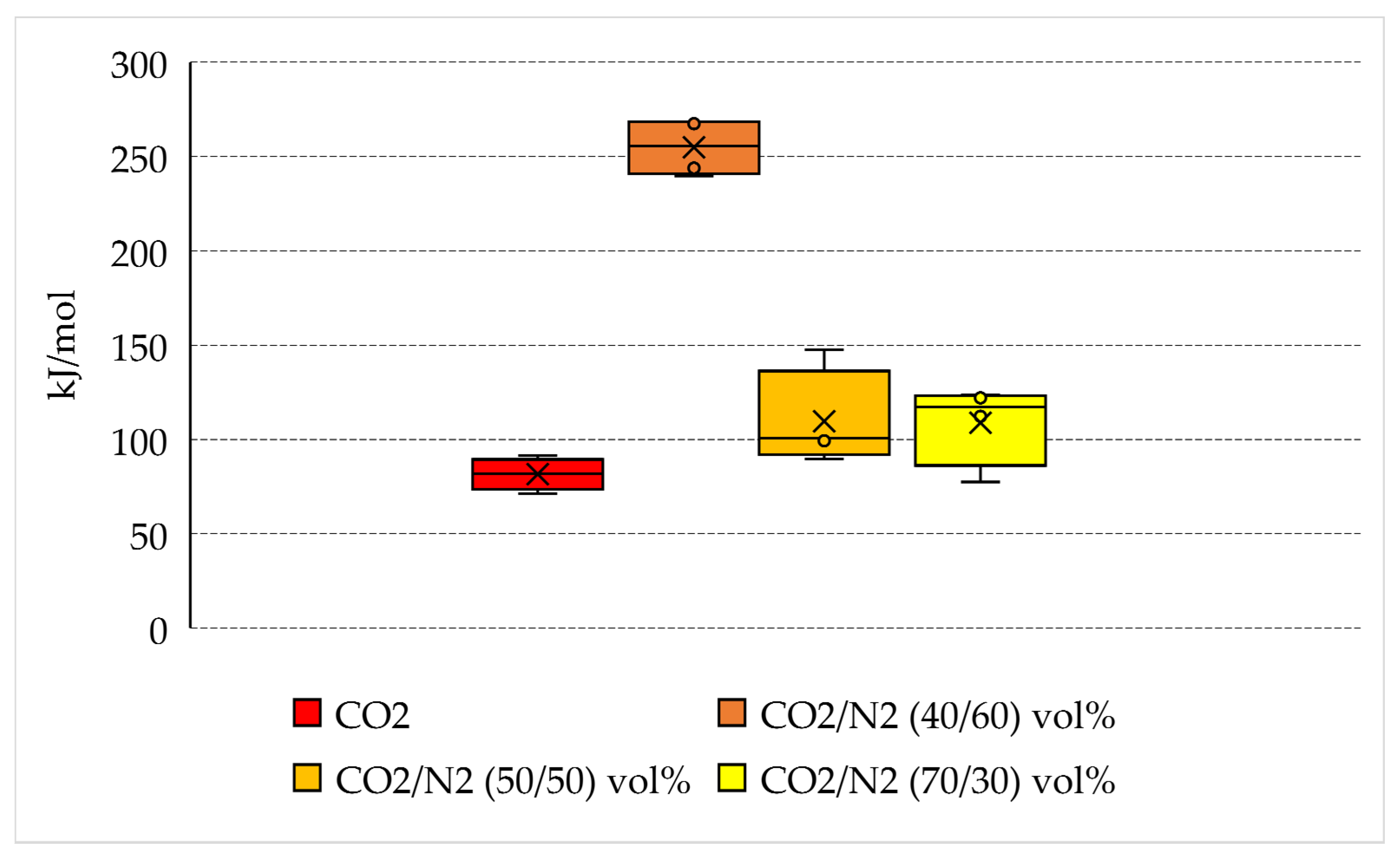
4.2. Energy Spent per Unit of CO2 Captured into Hydrates
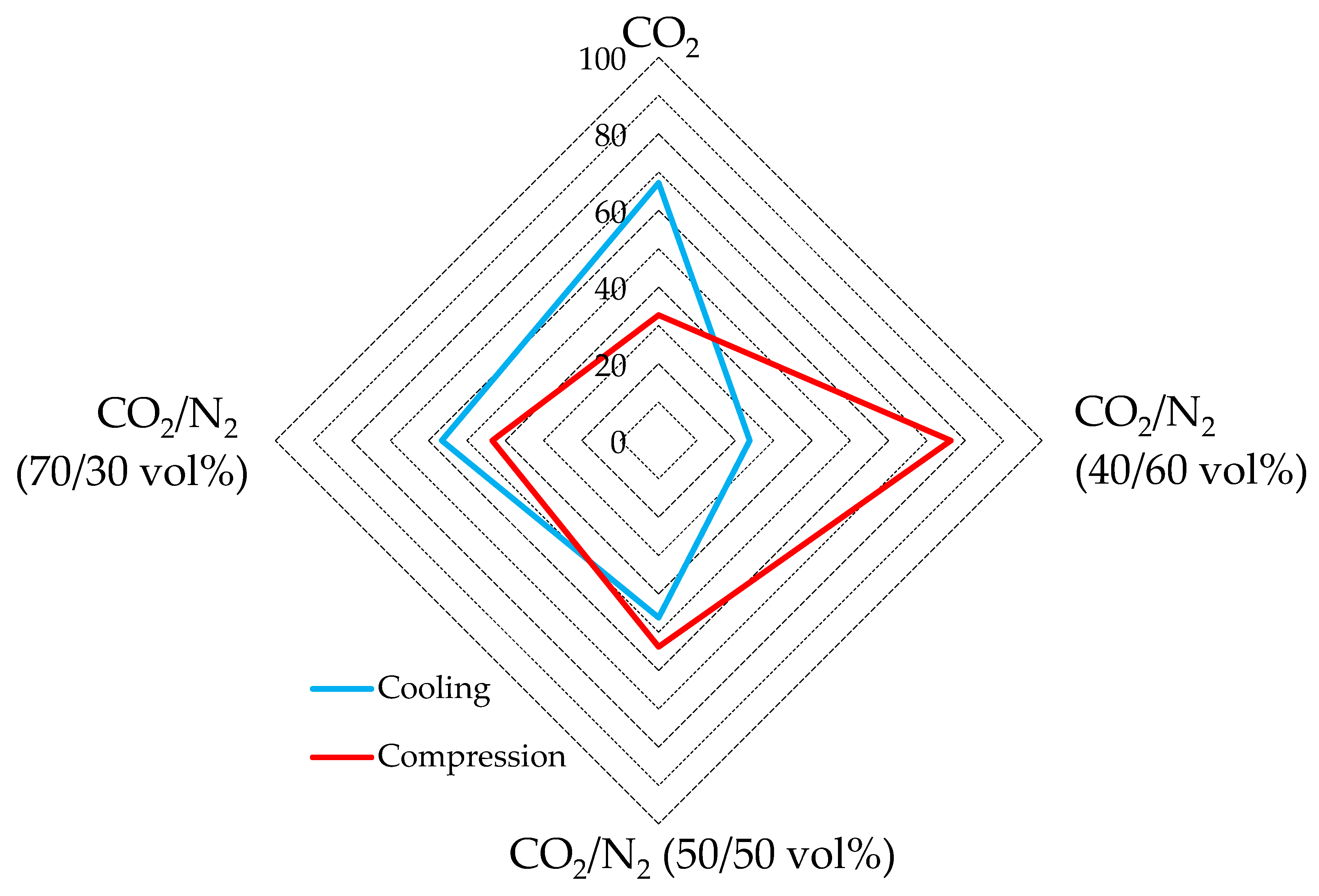
4.3. Possible Variations in Energy Spent
5. Conclusions
- (1)
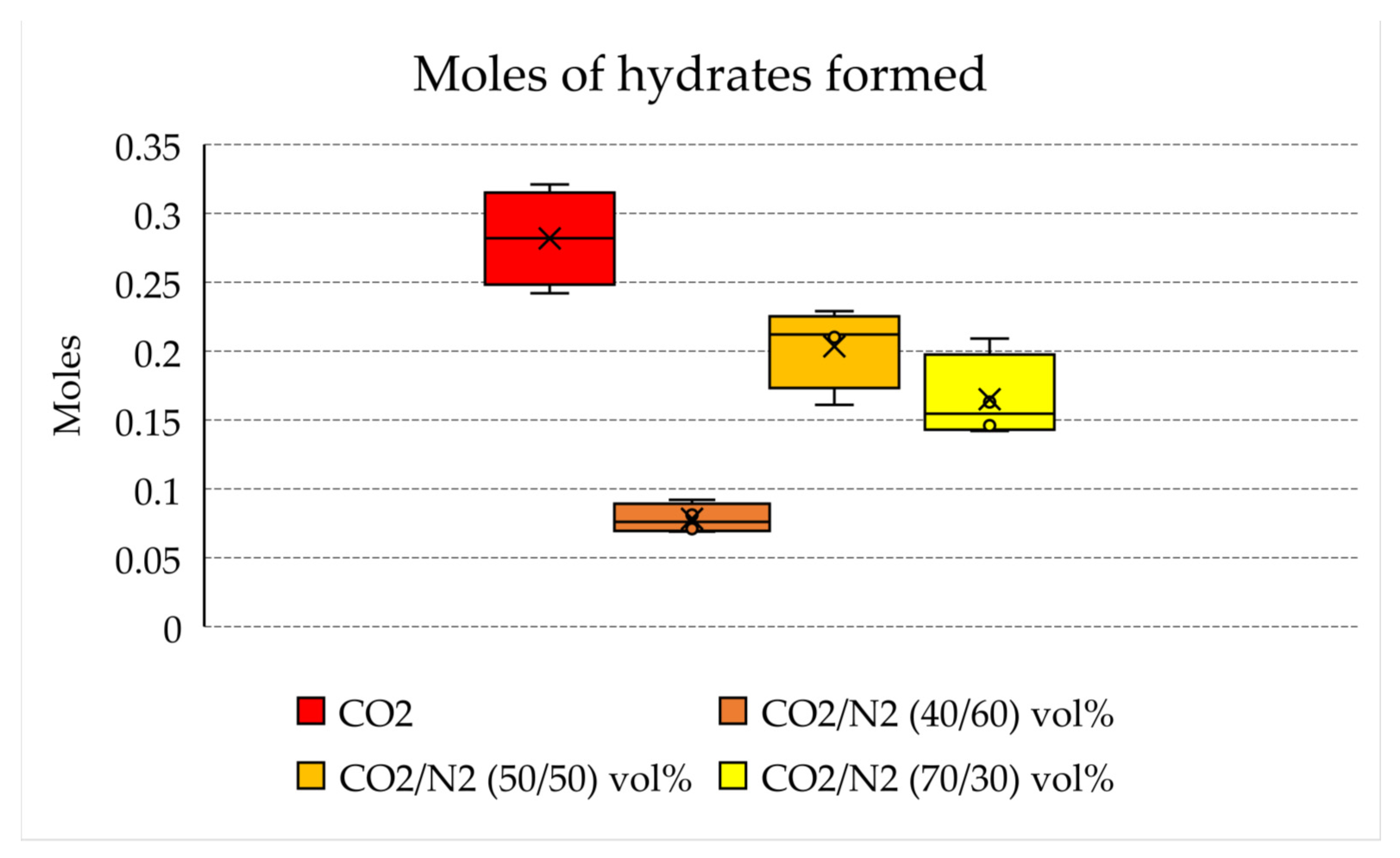
- With pure CO2, 0.24–0.32 moles of hydrates were produced, while 0.14–0.21 and 0.16–0.23 moles were produced, respectively, with 70/30 and 50/50 vol% mixtures. Conversely, the production obtained with the CO2/N2 40/60 vol% mixture was not meaningful (0.07–0.10 moles).
- (2)
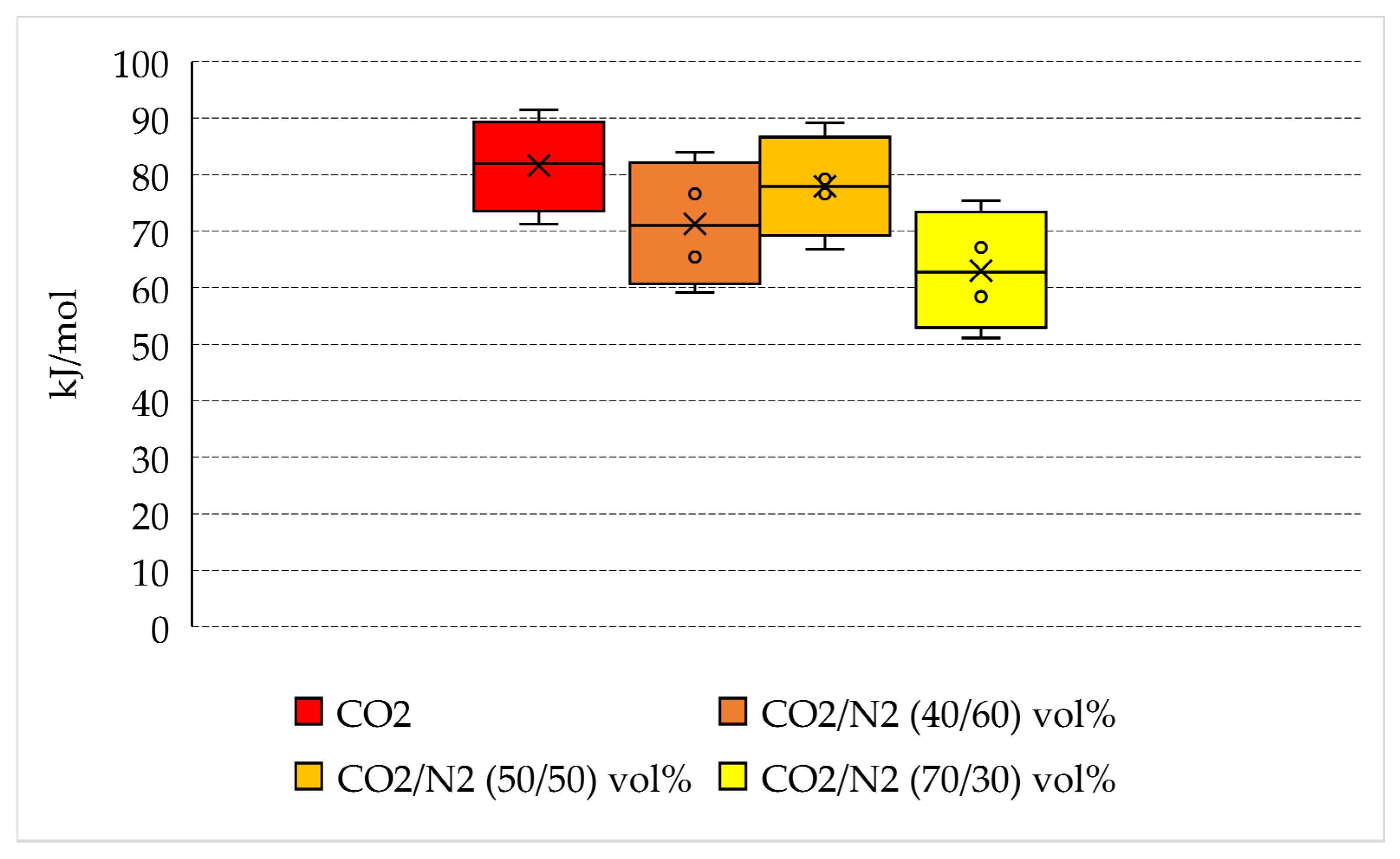
- The production of hydrates with pure carbon dioxide required more energy than N2-containing mixtures (71.2–91.4 kJ/mol). No marked differences were noticed between pure CO2 and 50/50 vol% and 40/60 vol% mixtures, whose corresponding mean energy spent per mole of hydrate formed was equal, respectively, to 77.9 and to 71.3 kJ/mol, while the 70/30 vol% mixture required a significantly lower amount of energy: 51.1–75.4 kJ/mol, with a mean value equal to 63 kJ/mol.
- (3)
- The opposite trend was observed when the energy consumption was normalized as a function of the quantity of CO2 stored. With pure carbon dioxide, a mean of 81.6 kJ/mol was required. The mixtures containing 30 vol% and 50 vol% N2, led to similar results, with a mean consumption equal to 108.8 kJ/mol and 109.7 kJ/mol. Conversely, the CO2/N2 40/60 vol% mixture required 254.9 kJ/mol.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hansen, A.D.; Kuramochi, T.; Wicke, B. The status of corporate greenhouse gas emissions reporting in the food sector: An evaluation of food and beverage manufactures. J. Clean. Prod. 2022, 361, 132279. [Google Scholar] [CrossRef]
- Vasquez, L.; Iriarte, A.; Almeida, M.; Villalobos, P. Evaluation of greenhouse gas emissions and proposals for their reduction at a university campus in Chile. J. Clean. Prod. 2015, 108, 924–930. [Google Scholar] [CrossRef]
- Bello, A.; Ivanova, A.; Cheremisin, A. A comprehensive review of the role of CO2 foam EOR in the reduction of carbon footprint in the petroleum industry. Energies 2023, 16, 1167. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.T.; Park, Y.K. A comparative study on the reduction effect in greenhouse gas emissions between the combined heat and power plant and boiler. Sustainability 2020, 12, 5144. [Google Scholar] [CrossRef]
- Cozzi, L.; Gould, T.; Bouckart, S.; Crow, D.; Kim, T.Y.; McGlade, C.; Olejarnik, P.; Wanner, B.; Wetzel, D. World Energy Outlook 2020; International Energy Agency: Paris, France, 2020; pp. 1–461. [Google Scholar]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernàndez, M.C.; Gross, R.; Hallett, J.P. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 sequestration in deep sedimentary formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Bashir, A.; Ali, M.; Patil, S.; Aljawad, M.S.; Mahmoud, M.; Al-Shehri, D.; Hoteit, H.; Kamal, M.S. Comprehensive review of CO2 geological storage: Exploring principles, mechanisms, and prospects. Earth Sci. Rev. 2024, 249, 104672. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, W.; Xu, T.; Zhang, Y.; Zhang, X.; Xing, Y.; Feng, B.; Xia, Y. Seismicity induced by geological CO2 storage: A review. Earth Sci. Rev. 2023, 239, 104369. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Gunter, W.D.; Wiwehar, B.; Perkins, E.H. Aquifer disposal of CO2-rich greenhouse gases: Extension of the time scale of experiments for CO2-sequestering reactions by geochemical modelling. Mineral. Petrol. 1997, 59, 121. [Google Scholar] [CrossRef]
- Le Gallo, Y.; Couillens, P.; Manai, T. CO2 sequestration in depleted oil of gas reservoirs. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Kuala Lumpur, Malaysia, 20–22 March 2002; p. 74104. [Google Scholar]
- Agartan, E.; Gaddipati, M.; Yip, Y.; Savage, B.; Oxgen, C. CO2 storage in depleted oil and gas fields in the Gulf of Mexico. Int. J. Greenh. Gas Control 2018, 72, 38–48. [Google Scholar] [CrossRef]
- Talapatra, A. A study on the carbon dioxide injection into coal seam aiming at enhancing coal bed methane (ECBM) recovery. J. Pet. Explor. Prod. Technol. 2020, 10, 1965–1981. [Google Scholar] [CrossRef]
- Shi, J.Q.; Durucan, S. CO2 storage in deep unmineable coal seams. Oil Gas Sci. Technol. 2005, 60, 547–558. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Carbon storage in basalt. Science 2014, 344, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.E.; Caldeira, K. Ocean storage of CO2. Elements 2008, 4, 319–324. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, F.; Ning, X.; Chuanbao, Z.; Zhang, J.; Zhang, C. Factor analysis and mechanism disclosure of supercritical CO2 filtration behavior in tight shale reservoirs. Environ. Sci. Pollut. Res. 2022, 29, 17682–17694. [Google Scholar] [CrossRef]
- Li, C.; Cheng, Y.; Li, Q.; Ansari, U.; Liu, Y.; Yan, C.; Lei, C. Development and verification of the comprehensive model for physical properties of hydrate sediment. Arab. J. Geosci. 2018, 11, 325. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Castellani, B.; Rossi, A.; Minicucci, M.; Zannotti, M.; Li, Y.; Rossi, F. May sediments affect the inhibiting properties of NaCl on CH4 and CO2 hydrates formation? An experimental report. J. Mol. Liq. 2022, 359, 119300. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Review on the usage of small-chain hydrocarbons (C2-C4) as aid gases for improving the efficiency of hydrate-based technologies. Energies 2023, 16, 3576. [Google Scholar] [CrossRef]
- Ito, K.; Sawano, Y.; Tada, K.; Sato, T. Numerical simulation of enhanced gas recovery from methane hydrate using the heat of CO2 hydrate formation under specific geological conditions. Gas Sci. Eng. 2024, 126, 205325. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Li, Y.; Rossi, F. Influence of different proportion of CO2/N2 binary gas mixture on methane recovery through replacement processes in natural gas hydrates. Chem. Eng. Process. 2022, 175, 108932. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of the Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Cao, X.; Wang, H.; Yang, K.; Wu, S.; Chen, Q.; Bian, J. Hydrate-based CO2 sequestration technology: Feasibilities, mechanisms, influencing factors, and applications. J. Pet. Sci. Eng. 2022, 219, 111121. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation. Energies 2022, 15, 6540. [Google Scholar] [CrossRef]
- Teng, Y.H.; Zhang, D.X. long-term viability of carbon sequestration in deep-sea sediments. Sci. Adv. 2018, 4, 6588. [Google Scholar] [CrossRef]
- Song, C.; Pan, W.; Srimat, T.S.; Zheng, J.; Li, Y.; Wang, Y.H.; Xu, B.Q.; Zhu, Q.M. Trireforming of methane over Ni catalysts for CO2 conversion to syngas with desired H2/CO ratios using flue gas of power plants without CO2 separation. Stud. Surf. Sci. Catal. 2004, 153, 315–322. [Google Scholar]
- Kacem, M.; Pellerano, M.; Delebarre, A. Pressure swing adsorption for CO2/N2 and CO2/CH4 separation: Comparison between activated carbons and zeolites performances. Fuel Process. Technol. 2015, 138, 271–283. [Google Scholar] [CrossRef]
- Zueco, J.; Lòpez-Asensio, D.; Fernàndez, F.J.; Lòpez-Gonzàlez, L.M. Exergy analysis of a steam-turbine power plant using thermocombustion. Appl. Therm. Eng. 2020, 180, 115812. [Google Scholar] [CrossRef]
- Lehmkuhler, F.; Paulus, M.; Sternemann, C.; Lietz, D.; Venturini, F.; Gutt, C.; Tolan, M. The carbon dioxide-water interface at conditions of gas hydrate formation. J. Am. Chem. Soc. 2009, 131, 585–589. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Trout, B.I. A new approach for studying nucleation phenomena using molecular simulations: Application to CO2 hydrate clathrates. J. Chem. Phys. 2002, 117, 1786–1796. [Google Scholar] [CrossRef]
- Wilder, J.W.; Sheshardi, K.; Smith, D.H. Resolving apparent contradictions in equilibrium measurements for clathrate hydrates in porous media. J. Phys. Chem. B 2001, 105, 9970–9972. [Google Scholar] [CrossRef]
- Anderson, R.; Llamendo, M.; Tohidi, B.; Burgass, R.W. Experimental measurement of methane and carbon dioxide clathrate hydrate equilibria in mesoporous silica. J. Phys. Chem. B 2003, 107, 3507–3514. [Google Scholar] [CrossRef]
- Lee, S.; Seo, Y. Experimental measurement and thermodynamic modelling of the mixed CH4+C3H8 clathrate hydrate equilibria in silica gel pores: Effects of pore size and salinity. Langmuir 2010, 26, 9742–9748. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, J.W.; Ryu, H.J. Phase behavior of methane and carbon dioxide hydrates in meso- and micro-sized porous media. Fluid Phase Equilib. 2008, 274, 68–72. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, S.; Guo, P.; Fan, S.; Zhang, S. Understanding the characteristic of methane hydrate equilibrium in materials and its potential application. Chem. Eng. J. 2018, 349, 775–781. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Liang, J.; Li, S.; Zeng, X.; Li, W. Depositional characteristics and accumulation model of gas hydrates in northern South China Sea. Mar. Pet. Geol. 2014, 56, 74–86. [Google Scholar] [CrossRef]
- Holland, M.; Schultheiss, P.; Roberts, J.; Druce, M. Observed gas hydrate morphologies in marine sediments. In Proceedings of the International Conference on Gas Hydrates (ICGH) (6th: 2008), Vancouver, BC, Canada, 6–10 July 2008; pp. 6–10. [Google Scholar]
- Kennedy, W.J.; Garrison, R.E. Morphology and genesis of nodular chalks and hardgrounds in the Upper Cretaceous of southern England. Sedimentology 1975, 22, 311–386. [Google Scholar] [CrossRef]
- Qin, X.; Lu, J.; Lu, H.; Qiu, H.; Liang, J.; Kang, D.; Zhan, L.S.; Lu, H.F.; Kuang, Z.G. Co-existence of natural gas hydrate, free gas and water in the gas hydrate system in the Shenhu Area, South China Sea. China Geol. 2020, 3, 210–220. [Google Scholar] [CrossRef]
- Lei, L.; Seol, Y.; Choi, J.H.; Kneafsey, T.J. Pore habit of methane hydrate and its evolution in sediment matrix-laboratory visualization with phase-contrast micro-CT. Mar. Pet. Geol. 2019, 104, 451–467. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Sun, X.; Liu, W.; Song, Y. Mechanical characteristics of hydrate-bearing sediment: A review. Energy Fuel 2021, 35, 1041–1057. [Google Scholar] [CrossRef]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N.; Ebinuma, T. Basic research on the mechanical behavior of methane hydrate-sediments mixture. Soils Found. 2005, 45, 75–85. [Google Scholar]
- Luo, T.; Li, Y.; Madhusudhan, B.N.; Zhao, J.; Song, Y. Comparative analysis of the consolidation and shear behaviors of CH4 and CO2 hydrate-bearing sediments. J. Nat. Gas Sci. Eng. 2020, 75, 103157. [Google Scholar] [CrossRef]
- Luo, T.; Han, T.; Madhusudan, B.N.; Zhao, X.; Zou, D.; Song, Y. Strenght and deformation behaviors of methane hydrate-bearing marine sediments in the South China Sea during depressurization. Energy Fuel 2022, 35, 14569–14579. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A. Effects of dissociation on the shear strength and deformation behavior of methane hydrate-bearing sediments. Mar. Pet. Geol. 2014, 51, 52–62. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, T.; Zhuang, S.; Lai, Z.; Li, R. AE monitoring–based CO2 hydrate formation and its influence to hydrate-bearing sandy sediments. J. Nat. Gas Sci. Eng. 2021, 96, 104292. [Google Scholar] [CrossRef]
- Waite, W.; Bratton, P.M.; Mason, D.H. Laboratory formation of non-cementing methane hydrate-bearing sands. In Proceedings of the 7th International Conference on Gas Hydrates 2011, Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Clayton, C.R.I.; Priest, J.A.; Rees, E.V.L. The effect of hydrate cement on the stiffness of some sands. Geotechnique 2010, 60, 435–445. [Google Scholar] [CrossRef]
- Shen, S.; Li, Y.; Sun, X.; Wang, L.; Song, Y. Analysis of the mechanical properties of methane hydrate-bearing sands with various pore pressures and confining. J. Nat. Gas Sci. Eng. 2021, 87, 103786. [Google Scholar] [CrossRef]
- Shen, S.; Sun, X.; Wang, L.; Song, Y.; Li, Y. Effect of temperature on the mechanical properties of hydrate-bearing sand under different confining pressures. Energy Fuels 2021, 35, 4106–4117. [Google Scholar] [CrossRef]
- Winters, W.J.; Waite, W.F.; Mason, D.H.; Gilbert, L.Y.; Pecher, I.A. Methane gas hydrate effect on sediment acoustic and strength properties. J. Pet. Sci. Eng. 2007, 56, 127–135. [Google Scholar] [CrossRef]
- Oshima, M.; Suzuki, K.; Yoneda, J.; Kato, A.; Kida, M.; Konno, Y.; Muraoka, M.; Jin, Y.; Nagao, J.; Tenma, N. Lithological properties of natural gas hydrate-bearing sediments in pressure-cores recovered from the Krishna-Godavari Basin. Mar. Pet. Geol. 2019, 108, 439–470. [Google Scholar] [CrossRef]
- Maiti, M.; Bhaumik, A.K.; Mandal, A. Geological characterization of natural gas hydrate bearing sediments and their influence on hydrate formation and dissociation. J. Nat. Gas Sci. Eng. 2022, 100, 104491. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. The effect of grainsize of sediments in the CO2/CH4 replacement process within a hydrate lattice: An experimental report. Chem. Eng. Process. 2022, 181, 109149. [Google Scholar] [CrossRef]
- Gambelli, A.M. Methane replacement into hydrate reservoirs with carbon dioxide: Main limiting factors and influence of the gaseous phase composition, over hydrates, on the process. Chem. Eng. J. 2023, 478, 147247. [Google Scholar] [CrossRef]
- Kashchiev, D.; Verdoes, D.; van Rosmalen, G.M. Induction time and metastability limit in new phase formation. J. Cryst. Growth 1991, 110, 373–380. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of the difference in induction period between CH4 and CO2 hydrates: Motivations and possible consequences on the replacement process. J. Nat. Gas Sci. Eng. 2022, 108, 104848. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholabai, P.D.; Bishnoi, P.R. Kinetics of formation of methane and ethane gas hydrate. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Wang, S.R.; Yang, M.J.; Liu, W.G.; Zhao, J.F.; Song, Y.C. Investigation on the induction time of methane hydrate formation in porous media under quiescent conditions. J. Pet. Sci. Eng. 2016, 145, 565–572. [Google Scholar] [CrossRef]
- Natajaran, V.; Bishnoi, P.R.; Kalogerakis, N. Induction phenomena in gas hydrate nucleation. Chem. Eng. Sci. 1994, 49, 2075–2087. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Re-definition of the region suitable for CO2/CH4 replacement into hydrates as a function of the thermodynamic difference between CO2 hydrate formation and dissociation. Process Saf. Environ. Prot. 2023, 169, 132–141. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Rossi, A.; Castellani, B.; Minicucci, M.; Zannotti, M.; Nicolini, A.; Rossi, F. Thermodynamic assessment and microscale Raman spectroscopy of binary CO2/CH4 hydrates produced during replacement applications in natural reservoirs. J. Mol. Liq. 2022, 368, 120739. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Kinetic considerations and formation rate for carbon dioxide hydrate, formed in presence of a natural silica-based porous medium: How initial thermodynamic conditions may modify the process kinetic. Thermochim. Acta 2021, 705, 179039. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Chen, J.; Yin, Z.; Rossi, F.; Tronconi, E.; Mei, S. Experimental study on the competition between carbon dioxide hydrate and ice below the freezing point. Chem. Eng. Sci. 2023, 268, 118426. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Alavi, S. Some current challenges in clathrate hydrate science: Nucleation, decomposition and the memory effect. Curr. Opin. Solid State Mater. Sci. 2016, 20, 344–351. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Wilson, P.W.; Haymet, A.D.J. Hydrate formation and re-formation in nucleating THF/water mixtures show no evidence to support a “memory” effect. Chem. Eng. J. 2010, 161, 146–150. [Google Scholar] [CrossRef]
- Takeya, S.; Kida, M.; Minami, H.; Sakagami, H.; Hachikubo, A.; Takahashi, N.; Shoji, H.; Soloviev, V.; Wallmann, K.; Biebow, N.; et al. Structure and thermal expansion of natural gas clathrate hydrates. Chem. Eng. Sci. 2006, 61, 2670–2674. [Google Scholar] [CrossRef]
- Aregba, A.G. Gas Hydrate—Properties, Formation and Benefits. Open J. Yangtze Oil Gas 2017, 2, 27–44. [Google Scholar] [CrossRef]
- Cai, J.; Xu, C.G.; Hu, Y.F.; Ding, Y.L.; Li, X.S. Phase equilibrium and Raman spectroscopic studies of semi-clathrate hydrates for methane, nitrogen and tetra-butyl-ammonium fluoride. Fluid Phase Equilib. 2016, 413, 48–52. [Google Scholar] [CrossRef]
- Tohidi, B.; Danesh, A.; Todd, A.C.; Burgass, R.W.; Ostergaard, K.K. Equilibrium data and thermodynamic modelling of cyclopentane and neopentane hydrates. Fluid Phase Equilib. 1997, 138, 241–250. [Google Scholar] [CrossRef]
- Fan, S.; Li, S.; Wang, J.; Lang, X.; Wang, Y. Efficient capture of CO2 from simulated flue gas by formation of TBAB or TBAF semiclathrate hydrates. Energy Fuels 2009, 23, 4202–4208. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Thermodynamic and kinetic characterization of methane hydrate nucleation, growth and dissociation processes, according to the Labile Cluster Theory. Chem. Eng. J. 2021, 425, 130706. [Google Scholar] [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Aziz, F.; Bilad, M.R.; Alkahtani, M. A crucial review on the challenges and recent gas membrane development for biogas upgrading. J. Environ. Chem. Eng. 2023, 11, 110235. [Google Scholar] [CrossRef]

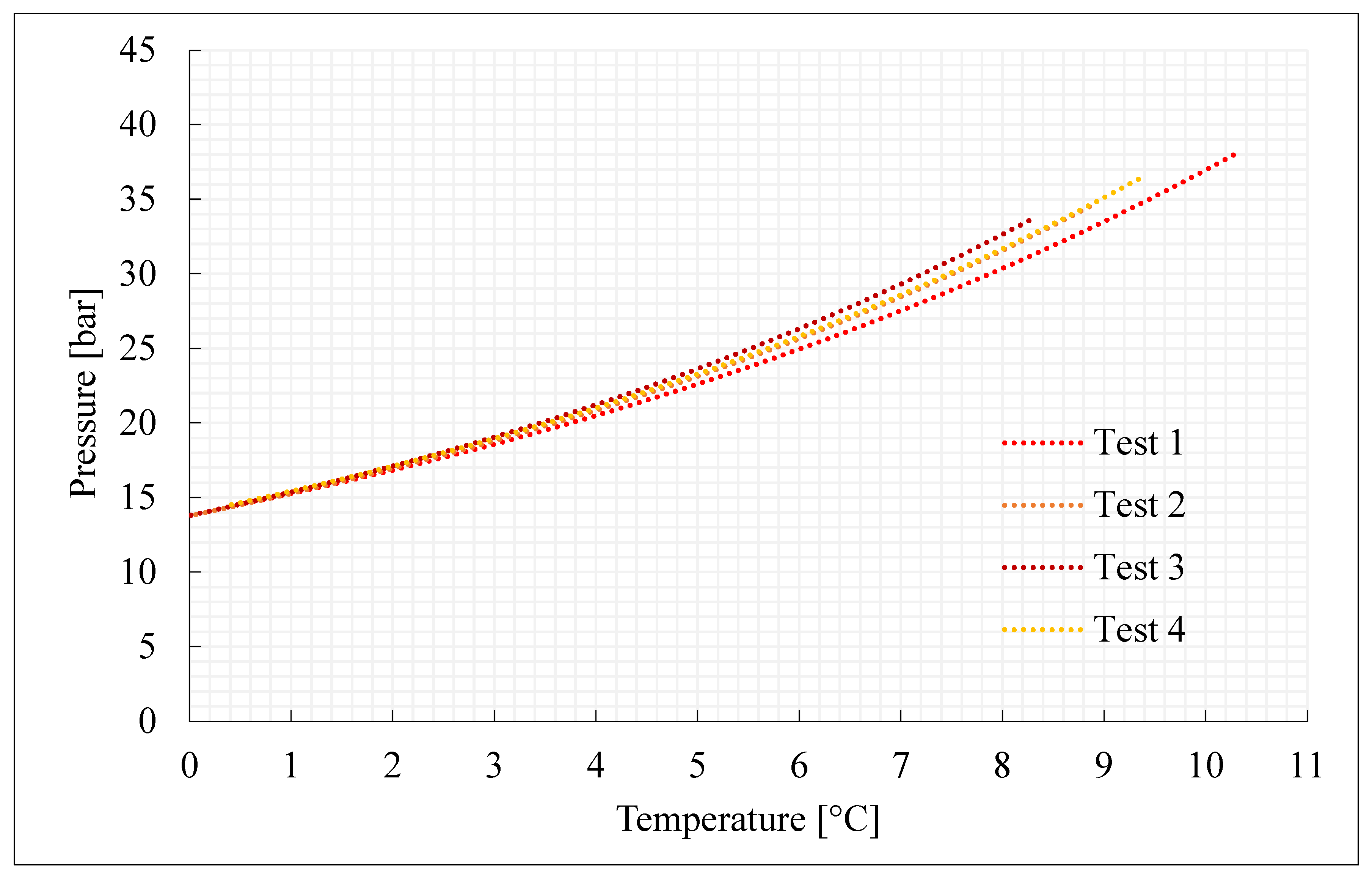


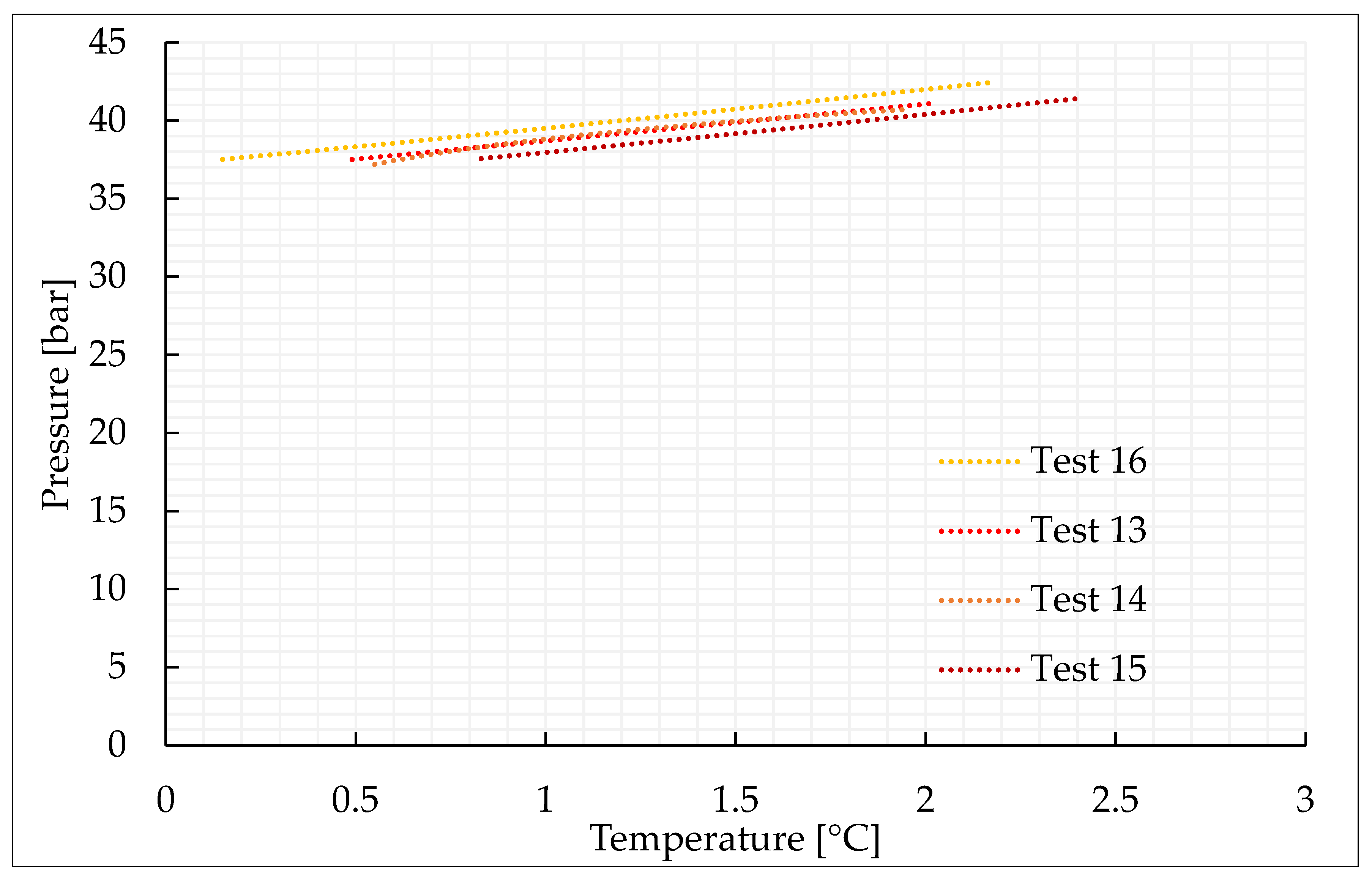

| Parameter | Unit | Pure CO2 | |||
|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | Test 4 | ||
| nCO2inj | mol | 0.45 | 0.52 | 0.41 | 0.49 |
| nHYD | mol | 0.27 | 0.32 | 0.24 | 0.30 |
| time | h | 17.6 | 19.7 | 23.1 | 17.0 |
| CO2/N2 (70/30) vol% | |||||
| Test 5 | Test 6 | Test 7 | Test 8 | ||
| nCO2/N2inj | mol | 0.36 | 0.35 | 0.40 | 0.35 |
| nHYD | mol | 0.16 | 0.15 | 0.21 | 0.14 |
| time | h | 19.4 | 18.3 | 21.1 | 18.6 |
| CO2/N2 (50/50) vol% | |||||
| Test 9 | Test 10 | Test 11 | Test 12 | ||
| nCO2/N2inj | mol | 0.41 | 0.44 | 0.44 | 0.46 |
| nHYD | mol | 0.16 | 0.21 | 0.21 | 0.23 |
| time | h | 17.8 | 19.4 | 16.7 | 22.0 |
| CO2/N2 (40/60) vol% | |||||
| Test 13 | Test 14 | Test 15 | Test 16 | ||
| nCO2/N2inj | mol | 0.50 | 0.49 | 0.48 | 0.49 |
| nHYD | mol | 0.10 | 0.07 | 0.07 | 0.08 |
| time | h | 23.3 | 24.2 | 20.8 | 22.0 |
| Mixture Tested | Energy Spent per Unit of Hydrate Formed [kJ/mol] | |||
|---|---|---|---|---|
| Pure CO2 | 91.4 | 71.2 | 83.2 | 80.6 |
| CO2/N2 (70/30) vol% | 75.4 | 51.1 | 67.1 | 58.4 |
| CO2/N2 (50/50) vol% | 89.1 | 66.8 | 79.2 | 76.6 |
| CO2/N2 (40/60) vol% | 84 | 59.1 | 76.6 | 65.4 |
| Mixture Tested | Energy Spent per Unit of CO2 Stored in Form of Hydrate [kJ/mol] | |||
|---|---|---|---|---|
| Pure CO2 | 91.4 | 71.2 | 83.2 | 80.6 |
| CO2/N2 (70/30) vol% | 123.5 | 112.3 | 77.5 | 122 |
| CO2/N2 (50/50) vol% | 147.7 | 102.1 | 89.6 | 99.3 |
| CO2/N2 (40/60) vol% | 243.8 | 267.4 | 268.5 | 239.7 |
| Mixture | Treatment | Test | [kJ/mol] | Test | [kJ/mol] | Test | [kJ/mol] | Test | [kJ/mol] |
|---|---|---|---|---|---|---|---|---|---|
| CO2 | Cool. | 1 | 17.3 | 2 | 14.5 | 3 | 13.6 | 4 | 16.1 |
| Compr. | 7.1 | 8.3 | 6.5 | 7.9 | |||||
| CO2/N2 (70/30) vol% | Cool. | 5 | 11.3 | 6 | 8.2 | 7 | 7.2 | 8 | 9.1 |
| Compr. | 6.9 | 6.6 | 7.6 | 6.6 | |||||
| CO2/N2 (50/50) vol% | Cool. | 9 | 10 | 10 | 7.5 | 11 | 5.8 | 12 | 8.1 |
| Compr. | 8.6 | 9.2 | 9.2 | 9.7 | |||||
| CO2/N2 (40/60) vol% | Cool. | 13 | 5 | 14 | 2.9 | 15 | 2.6 | 16 | 3.1 |
| Compr. | 11.2 | 10.8 | 10.7 | 10.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giuseppe, A.; Gambelli, A.M. CO2 Storage in Deep Oceanic Sediments in the form of Hydrates: Energy Evaluation and Advantages Related to the Use of N2-Containing Mixtures. Energies 2024, 17, 4102. https://doi.org/10.3390/en17164102
Di Giuseppe A, Gambelli AM. CO2 Storage in Deep Oceanic Sediments in the form of Hydrates: Energy Evaluation and Advantages Related to the Use of N2-Containing Mixtures. Energies. 2024; 17(16):4102. https://doi.org/10.3390/en17164102
Chicago/Turabian StyleDi Giuseppe, Alessia, and Alberto Maria Gambelli. 2024. "CO2 Storage in Deep Oceanic Sediments in the form of Hydrates: Energy Evaluation and Advantages Related to the Use of N2-Containing Mixtures" Energies 17, no. 16: 4102. https://doi.org/10.3390/en17164102
APA StyleDi Giuseppe, A., & Gambelli, A. M. (2024). CO2 Storage in Deep Oceanic Sediments in the form of Hydrates: Energy Evaluation and Advantages Related to the Use of N2-Containing Mixtures. Energies, 17(16), 4102. https://doi.org/10.3390/en17164102







