A Review of Hydrogen Leak Detection Regulations and Technologies
Abstract
1. Introduction
2. Review of Codes and Standards
- Code: A code is broad in scope and is intended to carry the force of law when adopted by a provincial, territorial, or municipal authority. Codes may include references to a number of standards.
- Standard: A standard is a document that provides a set of agreed-upon rules, guidelines, or characteristics for activities or their results. Standards establish accepted practices, technical requirements, and terminologies for diverse fields.
2.1. Requirements for General Hydrogen Systems and Technologies
2.2. Sensor Testing Requirements
2.3. Specific Requirements for Hydrogen Leak-Detection Instruments
3. Review of Sensor Performance Metrics
4. Review of Hydrogen Sensing Technologies
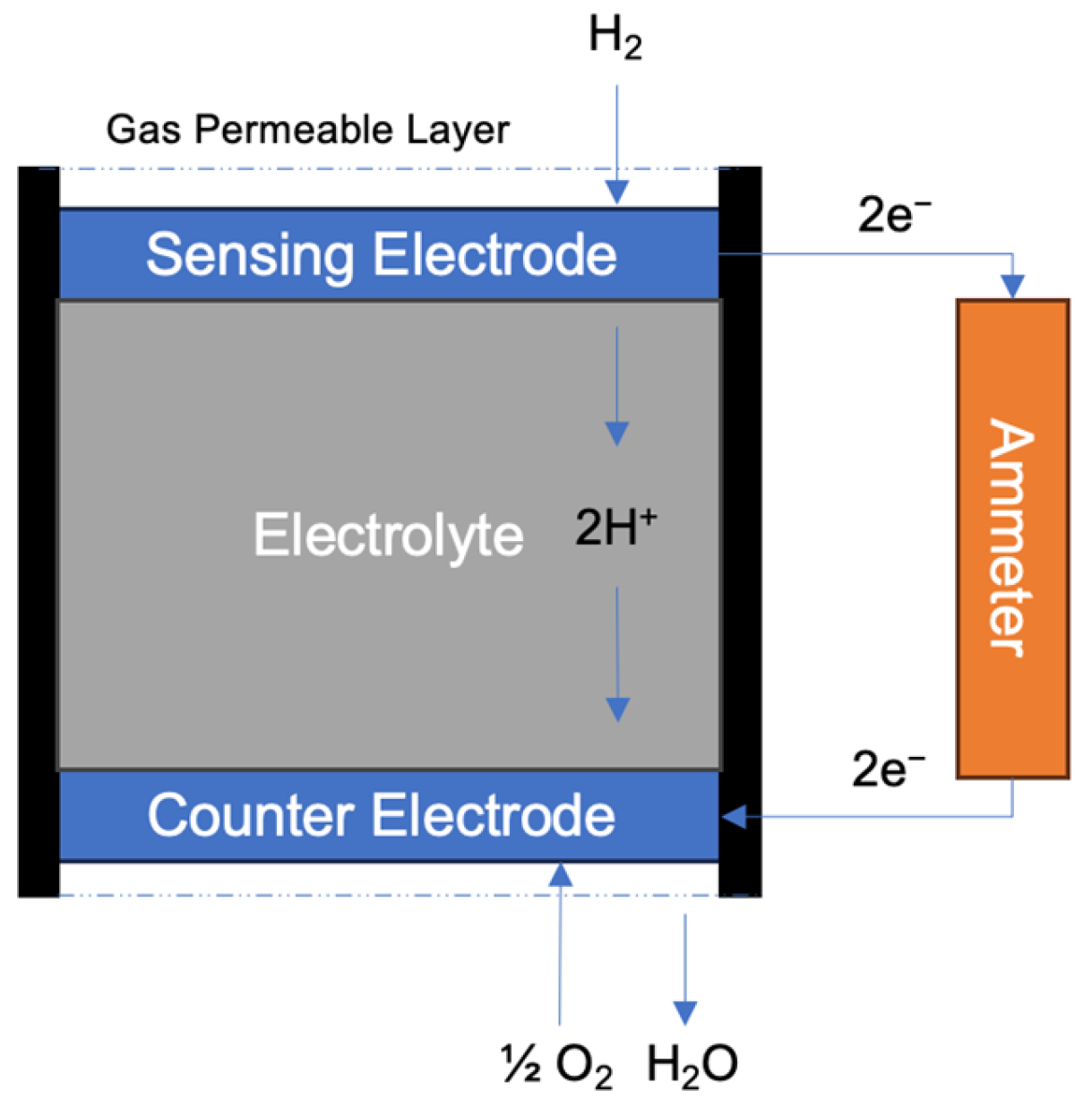
4.1. Electrochemical Sensors
4.1.1. Amperometric Type
4.1.2. Potentiometric Type
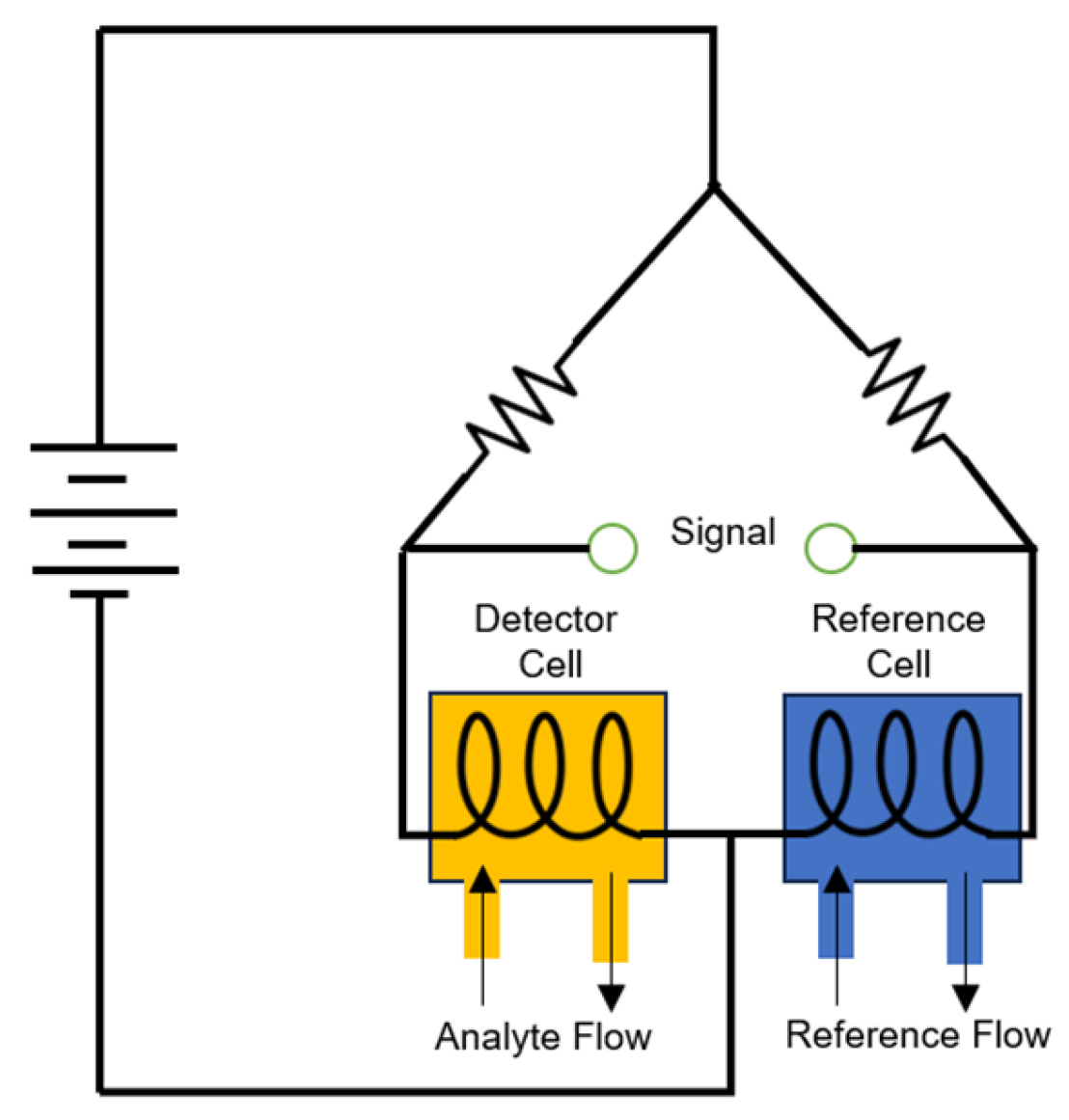
4.2. Catalytic Sensors
4.2.1. Pellistor Type
4.2.2. Thermoelectric Type
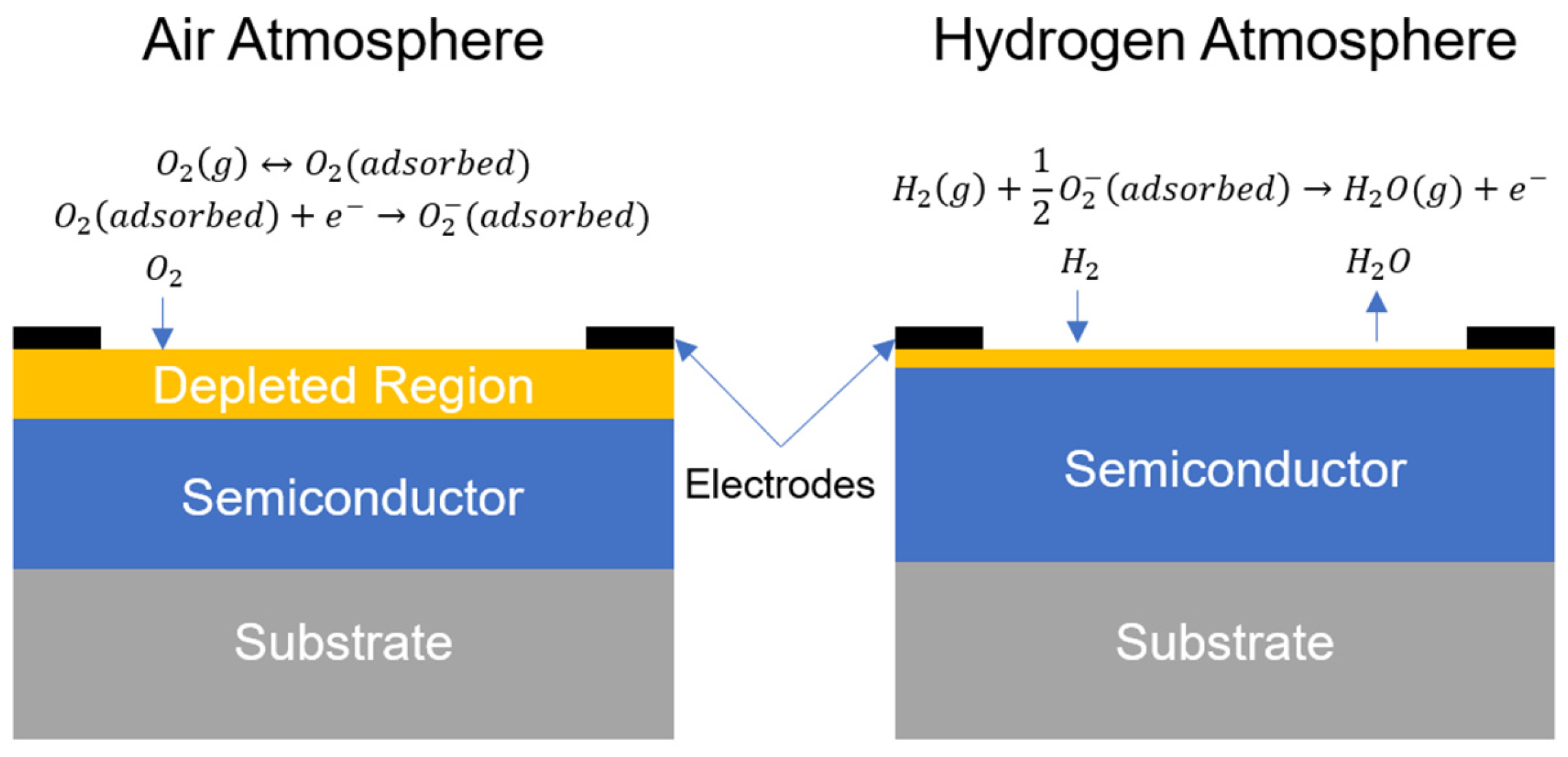
4.3. Resistance-Based Sensors
4.3.1. Semiconducting Metal-Oxide Type
4.3.2. Metallic-Resistor Type (Thin-Film Resistor)
4.4. Thermal Conductivity Type
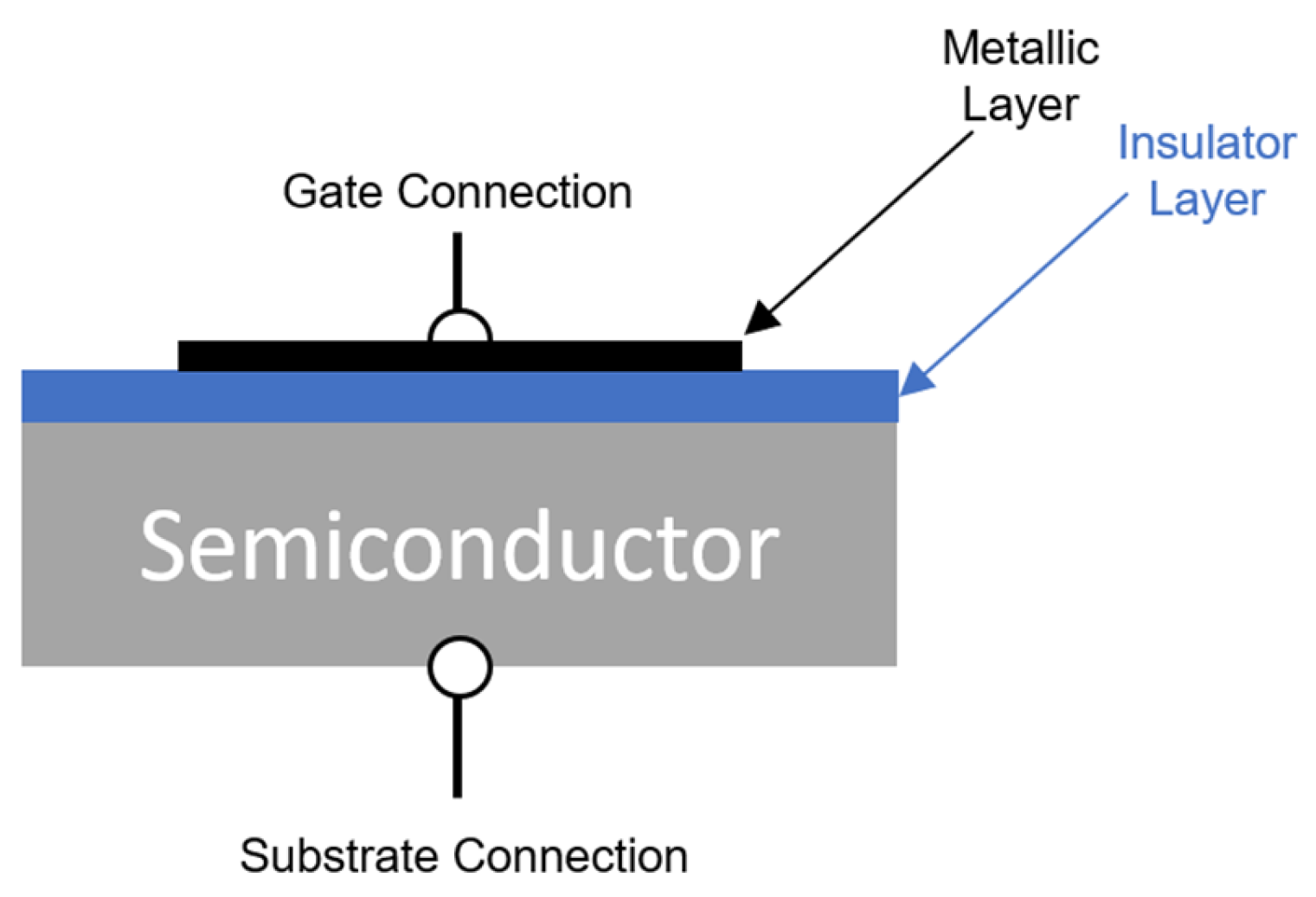
4.5. Work Function Sensors
4.5.1. Metal–Semiconductor Diodes (Schottky Type)
4.5.2. Metal–Insulator–Semiconductor Transistors (MOSFET Type)
4.5.3. Metal–Insulator–Semiconductor Capacitors
4.6. Optical Sensors
4.6.1. Grating-Based Sensors
4.6.2. Plasmonic Sensors
4.6.3. Evanescent Field-Based Sensors
4.7. Comparison between Sensor Types
5. Commercially Available Hydrogen Sensors
5.1. Electrochemical Sensors
5.2. Catalytic Sensors
5.3. Resistance-Based Sensors
| Sensor Type | Sensor Make and Model | Price Range |
|---|---|---|
| Electrochemical | Honeywell Sensepoint XCD | $$$ |
| Honeywell XNX Universal Transmitter | $$$ | |
| ATO-GAS-H2 | $$ | |
| ATO-GAS-H2-A | $$ | |
| IGD TOC-750X-H2 | $$ | |
| Dräger Polytron 6100 EC WL | $$$ | |
| Catalytic | Dräger Polytron 8200 CAT | $$$ |
| Sensitron S2157H2 | $$ | |
| Resistance-Based | Hanwei MQ-8 | $ |
| H2scan HY-ALERTA 600B | $$$ |
6. The Future of Hydrogen Detection
Author Contributions
Funding
Conflicts of Interest
References
- Demirbas, A. Future Hydrogen Economy and Policy. Energy Sources Part B Econ. Plan. Policy 2017, 12, 172–181. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Service Canada. Net-Zero Emissions by 2050. Available online: https://www.canada.ca/en/services/environment/weather/climatechange/climate-plan/net-zero-emissions-2050.html (accessed on 28 November 2023).
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Chapter 7-Hydrogen Logistics: Safety and Risks Issues. In Hydrogen Infrastructure for Energy Applications: Production, Storage, Distribution and Safety; Academic Press: Cambridge, MA, USA, 2018; pp. 127–148. ISBN 9780128120354. [Google Scholar]
- Standards Council of Canada. Types of Standards. Available online: https://scc.ca/en/types-standards (accessed on 2 February 2023).
- The American Society of Mechanical Engineers. ASME B31.12-Hydrogen Piping & Pipelines; The American Society of Mechanical Engineers: New York, NY, USA, 2023. [Google Scholar]
- ISO 26142:2010; Hydrogen Detection Apparatus—Stationary Applications. International Organization for Standardization: Geneva, Switzerland, 2010.
- SAE J3089; Characterization of On-Board Vehicular Hydrogen Sensors. SAE International: Warrendale, PA, USA, 2018.
- Underwriters Laboratories. UL 2075: Gas and Vapor Detectors and Sensors; Underwriters Laboratories: Northbrook, IL, USA, 2013. [Google Scholar]
- CSA Group. CSA C22.2 NO. 60079-29-1:17 (R2022) Explosive Atmospheres-Part 29-1: Gas Detectors-Performance Requirements of Detectors for Flammable Gases (Adopted IEC 60079-29-1:2016, Second Edition, 2016–07, with Canadian Deviations); CSA Group: Toronto, ON, Canada, 2017. [Google Scholar]
- Milliken, J. Hydrogen, Fuel Cells & Infrastructure Technologies Program Multi-Year Research, Development and Demonstration Plan; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2007. [Google Scholar]
- Buttner, W.; Burgess, R.; Post, M.; Rivkin, C. Summary and Findings from the NREL/DOE Hydrogen Sensor Workshop; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Jayanthi, E.; Murugesan, N.; Suneesh, A.S.; Ramesh, C.; Anthonysamy, S. Sensing Behavior of Room Temperature Amperometric H2 Sensor with Pd Electrodeposited from Ionic Liquid Electrolyte as Sensing Electrode. J. Electrochem. Soc. 2017, 164, H5210. [Google Scholar] [CrossRef]
- Lin, L.; Zeng, X. Toward Continuous Amperometric Gas Sensing in Ionic Liquids: Rationalization of Signal Drift Nature and Calibration Methods. Anal. Bioanal. Chem. 2018, 410, 4587–4596. [Google Scholar] [CrossRef]
- Amperometric Sensor for Selective and Stable Hydrogen Measurement. Sens. Actuators B Chem. 2005, 106, 784–790. [CrossRef]
- Lu, X.; Wu, S.; Wang, L.; Su, Z. Solid-State Amperometric Hydrogen Sensor Based on Polymer Electrolyte Membrane Fuel Cell. Sens. Actuators B Chem. 2005, 107, 812–817. [Google Scholar] [CrossRef]
- Ramesh, C.; Murugesan, N.; Krishnaiah, M.V.; Ganesan, V.; Periaswami, G. Improved Nafion-Based Amperometric Sensor for Hydrogen in Argon. J. Solid State Electrochem. 2008, 12, 1109–1116. [Google Scholar] [CrossRef]
- Gao, W.; Zhi, Z.; Fan, S.; Hua, Z.; Li, H.; Pan, X.; Sun, W.; Gao, H. Amperometric Hydrogen Sensor Based on Solid Polymer Electrolyte and Titanium Foam Electrode. ACS Omega 2022, 7, 24895–24902. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen Sensors—A Review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Martin, L. Electrochemical Hydrogen Sensor for Safety Monitoring. Solid State Ion. 2004, 175, 527–530. [Google Scholar] [CrossRef]
- Hara, N.; Macdonald, D.D. Development of Dissolved Hydrogen Sensors Based on Yttria-stabilized Zirconia Solid Electrolyte with Noble Metal Electrodes. J. Electrochem. Soc. 1997, 144, 4152–4157. [Google Scholar] [CrossRef]
- Okuyama, Y.; Kurita, N.; Yamada, A.; Takami, H.; Ohshima, T.; Katahira, K.; Fukatsu, N. A New Type of Hydrogen Sensor for Molten Metals Usable up to 1600 K. Electrochim. Acta 2009, 55, 470–474. [Google Scholar] [CrossRef]
- Nogami, M.; Matsumura, M.; Daiko, Y. Hydrogen Sensor Prepared Using Fast Proton-Conducting Glass Films. Sens. Actuators B Chem. 2006, 120, 266–269. [Google Scholar] [CrossRef]
- Maffei, N.; Kuriakose, A.K. A Solid-State Potentiometric Sensor for Hydrogen Detection in Air. Sens. Actuators B Chem. 2004, 98, 73–76. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, H.; Zhang, Z.; Chen, D. Hierarchical Porous Hollow SnO2 Nanofiber Sensing Electrode for High Performance Potentiometric H2 Sensor. Sens. Actuators B Chem. 2018, 268, 456–464. [Google Scholar] [CrossRef]
- Schober, T. Applications of Oxidic High-Temperature Proton Conductors. Solid State Ion. 2003, 162–163, 277–281. [Google Scholar] [CrossRef]
- Tan, Y.; Tan, T.C. Sensing Behavior of an Amperometric Hydrogen Sensor: Theoretical Modeling and Experimental Verification. J. Electrochem. Soc. 1995, 142, 1923–1929. [Google Scholar] [CrossRef]
- Ivanov, I.; Baranov, A.; Mironov, S.; Akbari, S. Selective Low-Temperature Hydrogen Catalytic Sensor. IEEE Sens. Lett. 2022, 6, 1–4. [Google Scholar] [CrossRef]
- Lee, E.-B.; Hwang, I.-S.; Cha, J.-H.; Lee, H.-J.; Lee, W.-B.; Pak, J.J.; Lee, J.-H.; Ju, B.-K. Micromachined Catalytic Combustible Hydrogen Gas Sensor. Sens. Actuators B Chem. 2011, 153, 392–397. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.t.; Bourke, P.; Norris, J.O.W.; Stephan, R. Tin Dioxide Gas Sensors: Use of the Seebeck Effect. Sens. Actuators 1985, 8, 251–257. [Google Scholar] [CrossRef]
- Maekawa, S.; Tohyama, T.; Barnes, S.E.; Ishihara, S.; Koshibae, W.; Khaliullin, G. Physics of Transition Metal Oxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 9783662092989. [Google Scholar]
- Matsumiya, M.; Qiu, F.; Shin, W.; Izu, N.; Murayama, N.; Kanzaki, S. Thin-Film Li-Doped NiO for Thermoelectric Hydrogen Gas Sensor. Thin Solid Film. 2002, 419, 213–217. [Google Scholar] [CrossRef]
- Panama, G.; Lee, S.S. Catalytic Thermoelectric Hydrogen Sensor with CoSb3 Thermopile by Single-Step Deposition on Bare/Textured Glass. Surf. Interfaces 2024, 46, 104082. [Google Scholar] [CrossRef]
- Tajima, K.; Qiu, F.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N. Micromechanical Fabrication of Low-Power Thermoelectric Hydrogen Sensor. Sens. Actuators B Chem. 2005, 108, 973–978. [Google Scholar] [CrossRef]
- Aroutiounian, V. Metal Oxide Hydrogen, Oxygen, and Carbon Monoxide Sensors for Hydrogen Setups and Cells. Int. J. Hydrog. Energy 2007, 32, 1145–1158. [Google Scholar] [CrossRef]
- Gorokh, G.; Zakhlebayeva, A.; Taratyn, I.; Lozovenko, A.; Zhylinski, V.; Iji, M.; Fedosenko, V.; Taleb, A. A Micropowered Chemoresistive Sensor Based on a Thin Alumina Nanoporous Membrane and SnxBikMoyOz Nanocomposite. Sensors 2022, 22, 3640. [Google Scholar] [CrossRef]
- Ou, L.-X.; Liu, M.-Y.; Zhu, L.-Y.; Zhang, D.W.; Lu, H.-L. Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor. Nanomicro Lett. 2022, 14, 206. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen Sensors Based on Noble Metal Doped Metal-Oxide Semiconductor: A Review. Int. J. Hydrog. Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Rashid, T.-R.; Phan, D.-T.; Chung, G.-S. A Flexible Hydrogen Sensor Based on Pd Nanoparticles Decorated ZnO Nanorods Grown on Polyimide Tape. Sens. Actuators B Chem. 2013, 185, 777–784. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive Polyaniline-Based Gas Sensors: A Mini Review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Punetha, D.; Kar, M.; Pandey, S.K. A New Type Low-Cost, Flexible and Wearable Tertiary Nanocomposite Sensor for Room Temperature Hydrogen Gas Sensing. Sci. Rep. 2020, 10, 2151. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wang, Z.; Tang, Y.; Sun, J.; Wei, X.; Zhang, Q.; Tian, G.; Wang, H. MEMS-Based Resistive Hydrogen Sensor with High Performance Using a Palladium-Gold Alloy Thin Film. J. Alloys Compd. 2023, 930, 167398. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Chen, Y.; Song, H.; Huang, P.; Dao, D.V. Hydrogen Sensor Based on Palladium-Yttrium Alloy Nanosheet. Mater. Chem. Phys. 2017, 194, 231–235. [Google Scholar] [CrossRef]
- Hassan, K.; Chung, G.-S. Fast and Reversible Hydrogen Sensing Properties of Pd-Capped Mg Ultra-Thin Films Modified by Hydrophobic Alumina Substrates. Sens. Actuators B Chem. 2017, 242, 450–460. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Kumar, A.; Chandra, R. Highly Sensitive and Selective Hydrogen Gas Sensor Using Sputtered Grown Pd Decorated MnO2 Nanowalls. Sens. Actuators B Chem. 2016, 234, 8–14. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Bhattacharya, S. Vanadium Pentoxide Nanostructures for Sensitive Detection of Hydrogen Gas at Room Temperature. J. Energy Environ. Sustain. 2017, 2, 69–74. [Google Scholar]
- Occelli, C.; Fiorido, T.; Perrin-Pellegrino, C.; Seguin, J.-L. Sensors for Anaerobic Hydrogen Measurement: A Comparative Study between a Resistive PdAu Based Sensor and a Commercial Thermal Conductivity Sensor. Int. J. Hydrog. Energy 2023, 48, 17729–17741. [Google Scholar] [CrossRef]
- Hoffheins, B.S.; Maxey, L.C.; Holmes, W., Jr.; Lauf, R.J.; Salter, C.; Walker, D. Development of Low Cost Sensors for Hydrogen Safety Applications; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1999. [Google Scholar]
- Andrews, T.; Tait, P.G.; Brown, A.C. The Scientific Papers of Thomas Andrews; Kessinger Publishing: Whitefish, MT, USA, 2010; ISBN 9781166389352. [Google Scholar]
- Daynes, H.A. Gas Analysis by Measurement of Thermal Conductivity; The University Press: Cambridge, UK, 1933. [Google Scholar]
- Berndt, D.; Muggli, J.; Wittwer, F.; Langer, C.; Heinrich, S.; Knittel, T.; Schreiner, R. MEMS-Based Thermal Conductivity Sensor for Hydrogen Gas Detection in Automotive Applications. Sens. Actuators A Phys. 2020, 305, 111670. [Google Scholar] [CrossRef]
- Harumoto, T.; Fujiki, H.; Shi, J.; Nakamura, Y. Extremely Simple Structure Hydrogen Gas Sensor Based on Single Metallic Thin-Wire under Sweep Heating. Int. J. Hydrog. Energy 2022, 47, 34291–34298. [Google Scholar] [CrossRef]
- Harumoto, T.; Shi, J.; Nakamura, Y.; Fujiki, H. Enhanced Hydrogen Gas Detectability of Sweep Heating Thin-Wire Thermal Conductivity Detector. Sens. Actuators A Phys. 2023, 358, 114446. [Google Scholar] [CrossRef]
- Potje-Kamloth, K. Semiconductor Junction Gas Sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef]
- Hölzl, J.; Schulte, F.K. Work Function of Metals. In Springer Tracts in Modern Physics; Springer Tracts in Modern Physics; Springer: Berlin/Heidelberg, Germany, 1979; pp. 1–150. ISBN 9783540092667. [Google Scholar]
- Tung, R.T. The Physics and Chemistry of the Schottky Barrier Height. Appl. Phys. Rev. 2014, 1, 011304. [Google Scholar] [CrossRef]
- Ito, K. Hydrogen-Sensitive Schottky Barrier Diodes. Surf. Sci. 1979, 86, 345–352. [Google Scholar] [CrossRef]
- Shivaraman, M.S.; Lundström, I.; Svensson, C.; Hammarsten, H. Hydrogen Sensitivity of Palladium–Thin-Oxide–Silicon Schottky Barriers. Electron. Lett. 1976, 12, 483. [Google Scholar] [CrossRef]
- Lechuga, L.M.; Calle, A.; Golmayo, D.; Briones, F. Hydrogen Sensor Based on a Pt/GaAs Schottky Diode. Sens. Actuators B Chem. 1991, 4, 515–518. [Google Scholar] [CrossRef]
- Lu, C.-T.; Lin, K.-W.; Chen, H.-I.; Chuang, H.-M.; Chen, C.-Y.; Liu, W.-C. A New Pd-Oxide-Al/Sub 0.3/Ga/Sub 0.7/As MOS Hydrogen Sensor. IEEE Electron. Device Lett. 2003, 24, 390–392. [Google Scholar] [CrossRef]
- Cho, M.; Yun, J.; Kwon, D.; Kim, K.; Park, I. High-Sensitivity and Low-Power Flexible Schottky Hydrogen Sensor Based on Silicon Nanomembrane. ACS Appl. Mater. Interfaces 2018, 10, 12870–12877. [Google Scholar] [CrossRef]
- Chen, W.-C.; Niu, J.-S.; Liu, I.-P.; Chen, H.-Y.; Cheng, S.-Y.; Lin, K.-W.; Liu, W.-C. Hydrogen Sensing Properties of a Novel GaN/AlGaN Schottky Diode Decorated with Palladium Nanoparticles and a Platinum Thin Film. Sens. Actuators B Chem. 2021, 330, 129339. [Google Scholar] [CrossRef]
- Lundström, I.; Shivaraman, S.; Svensson, C.; Lundkvist, L. A Hydrogen−sensitive MOS Field−effect Transistor. Appl. Phys. Lett. 1975, 26, 55–57. [Google Scholar] [CrossRef]
- Briand, D.; Wingbrant, H.; Sundgren, H.; van der Schoot, B.; Ekedahl, L.-G.; Lundström, I.; de Rooij, N.F. Modulated Operating Temperature for MOSFET Gas Sensors: Hydrogen Recovery Time Reduction and Gas Discrimination. Sens. Actuators B Chem. 2003, 93, 276–285. [Google Scholar] [CrossRef]
- Yokosawa, K.; Saitoh, K.; Nakano, S.; Goto, Y.; Tsukada, K. FET Hydrogen-Gas Sensor with Direct Heating of Catalytic Metal. Sens. Actuators B Chem. 2008, 130, 94–99. [Google Scholar] [CrossRef]
- Scharnagl, K.; Karthigeyan, A.; Burgmair, M.; Zimmer, M.; Doll, T.; Eisele, I. Low Temperature Hydrogen Detection at High Concentrations: Comparison of Platinum and Iridium. Sens. Actuators B Chem. 2001, 80, 163–168. [Google Scholar] [CrossRef]
- Kim, B.-J.; Yoon, J.-H.; Kim, J.-S. Gas Sensing Characteristics of Low-Powered Dual MOSFET Hydrogen Sensors. Mater. Chem. Phys. 2013, 142, 594–599. [Google Scholar] [CrossRef]
- Steele, M.C.; Hile, J.W.; MacIver, B.A. Hydrogen-Sensitive Palladium Gate MOS Capacitors. J. Appl. Phys. 1976, 47, 2537–2538. [Google Scholar] [CrossRef]
- Armgarth, M.; Söderberg, D.; Lundström, I. Palladium and Platinum Gate Metal-Oxide-Semiconductor Capacitors in Hydrogen and Oxygen Mixtures. Appl. Phys. Lett. 1982, 41, 654–655. [Google Scholar] [CrossRef]
- Aval, L.F.; Elahi, S.M.; Darabi, E.; Sebt, S.A. Comparison of the MOS Capacitor Hydrogen Sensors with Different SiO2 Film Thicknesses and a Ni-Gate Film in a 4% Hydrogen–Nitrogen Mixture. Sens. Actuators B Chem. 2015, 216, 367–373. [Google Scholar] [CrossRef]
- Ratan, S.; Kumar, C.; Kumar, A.; Jarwal, D.K.; Mishra, A.K.; Upadhyay, R.K.; Singh, A.P.; Jit, S. Room Temperature High Hydrogen Gas Response in Pd/TiO2/Si/Al Capacitive Sensor. Micro Nano Lett. 2020, 15, 632–635. [Google Scholar] [CrossRef]
- Butler, M.A. Optical Fiber Hydrogen Sensor. Appl. Phys. Lett. 1984, 45, 1007–1009. [Google Scholar] [CrossRef]
- Ito, K.; Ohgami, T.; Nakazawa, T. Effect of Water on Hydrogen-Sensitive Tungsten Oxide Films. Sens. Actuators B Chem. 1993, 12, 161–167. [Google Scholar] [CrossRef]
- Zhou, J.; Jokerst, J.V. Photoacoustic Imaging with Fiber Optic Technology: A Review. Photoacoustics 2020, 20, 100211. [Google Scholar] [CrossRef] [PubMed]
- Sutapun, B. Pd-Coated Elastooptic Fiber Optic Bragg Grating Sensors for Multiplexed Hydrogen Sensing. Sens. Actuators B Chem. 1999, 60, 27–34. [Google Scholar] [CrossRef]
- Wang, G.; Yang, M.; Dai, J.; Cheng, C.; Yuan, Y. Microfiber Bragg Grating Hydrogen Sensor Base on Co-Sputtered Pd/Ni Composite Film. In Proceedings of the Fifth Asia-Pacific Optical Sensors Conference, Jeju, Republic of Korea, 20–22 May 2015. [Google Scholar]
- Zhou, X.; Dai, Y.; Zou, M.; Karanja, J.M.; Yang, M. FBG Hydrogen Sensor Based on Spiral Microstructure Ablated by Femtosecond Laser. Sens. Actuators B Chem. 2016, 236, 392–398. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, M.; Ming, X.-Z.; Wu, G.-X.; Zhang, W.; Dai, Y.-T. Hydrogen Sensing Characteristics of Pd-Ag Composite Film Micro-Structured Grating Fiber with Different Silver Content. Acta Photonica Sin. 2019, 48, 806004. [Google Scholar] [CrossRef]
- Zhou, X.; Karanja, J.M.; Yang, M.; Zhou, F.; Liu, K.; Ming, X.; Dai, Y. FBG Hydrogen Sensor Based on Pd87–Ni13/Pd4–Ag1 Thin Film and Femtosecond Laser Ablation. Integr. Ferroelectr. 2021, 221, 1–11. [Google Scholar] [CrossRef]
- Silva, S.; Coelho, L.; Almeida, J.M.; Frazao, O.; Santos, J.L.; Malcata, F.X.; Becker, M.; Rothhardt, M.; Bartelt, H. H2 Sensing Based on a Pd-Coated Tapered-FBG Fabricated by DUV Femtosecond Laser Technique. IEEE Photonics Technol. Lett. 2013, 25, 401–403. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Wang, C.; Peng, G.-D.; Rao, Y.-J.; Gong, Y. Highly Sensitive Fiber Grating Hydrogen Sensor Based on Hydrogen-Doped Pt/WO3. Sens. Actuators B Chem. 2024, 404, 135250. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, M.-J.; Park, M.-S.; Jang, J.-H.; Lee, B.-H.; Kim, K.-T. Hydrogen Sensor Based on A Palladium-Coated Long-Period Fiber Grating Pair. J. Opt. Soc. Korea 2008, 12, 221–225. [Google Scholar] [CrossRef]
- Trouillet, A.; Marin, E.; Veillas, C. Fibre Gratings for Hydrogen Sensing. Meas. Sci. Technol. 2006, 17, 1124–1128. [Google Scholar] [CrossRef]
- Ai, B.; Sun, Y.; Zhao, Y. Plasmonic Hydrogen Sensors. Small 2022, 18, e2107882. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, A.; Nishiyama, M.; Igawa, H.; Seki, A.; Watanabe, K. A Hydrogen Curing Effect on Surface Plasmon Resonance Fiber Optic Hydrogen Sensors Using an Annealed Au/Ta2O5/Pd Multi-Layers Film. Opt. Express 2014, 22, 18556–18563. [Google Scholar] [CrossRef]
- Katsuki, A.; Fukui, K. H2 Selective Gas Sensor Based on SnO2. Sens. Actuators B Chem. 1998, 52, 30–37. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Kandasamy, S.; Kalantar-zadeh, K.; Wlodarski, W. Hydrogen Sensing Characteristics of WO3 Thin Film Conductometric Sensors Activated by Pt and Au Catalysts. Sens. Actuators B Chem. 2005, 108, 154–158. [Google Scholar] [CrossRef]
- Matushko, I.P.; Yatsimirskii, V.K.; Maksimovich, N.P.; Nikitina, N.V.; Silenko, P.M.; Ruchko, V.P.; Ishchenko, V.B. Sensitivity to Hydrogen of Sensor Materials Based on SnO2 Promoted with 3d Metals. Theor. Exp. Chem. 2008, 44, 128–133. [Google Scholar] [CrossRef]
- Tournier, G.; Pijolat, C. Selective Filter for SnO-Based Gas Sensor: Application to Hydrogen Trace Detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Lee, A. Temperature Modulation in Semiconductor Gas Sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Boon-Brett, L.; Black, G.; Moretto, P.; Bousek, J. A Comparison of Test Methods for the Measurement of Hydrogen Sensor Response and Recovery Times. Int. J. Hydrog. Energy 2010, 35, 7652–7663. [Google Scholar] [CrossRef]
- Hughes, R.C.; Schubert, W.K. Thin Films of Pd/Ni Alloys for Detection of High Hydrogen Concentrations. J. Appl. Phys. 1992, 71, 542–544. [Google Scholar] [CrossRef]

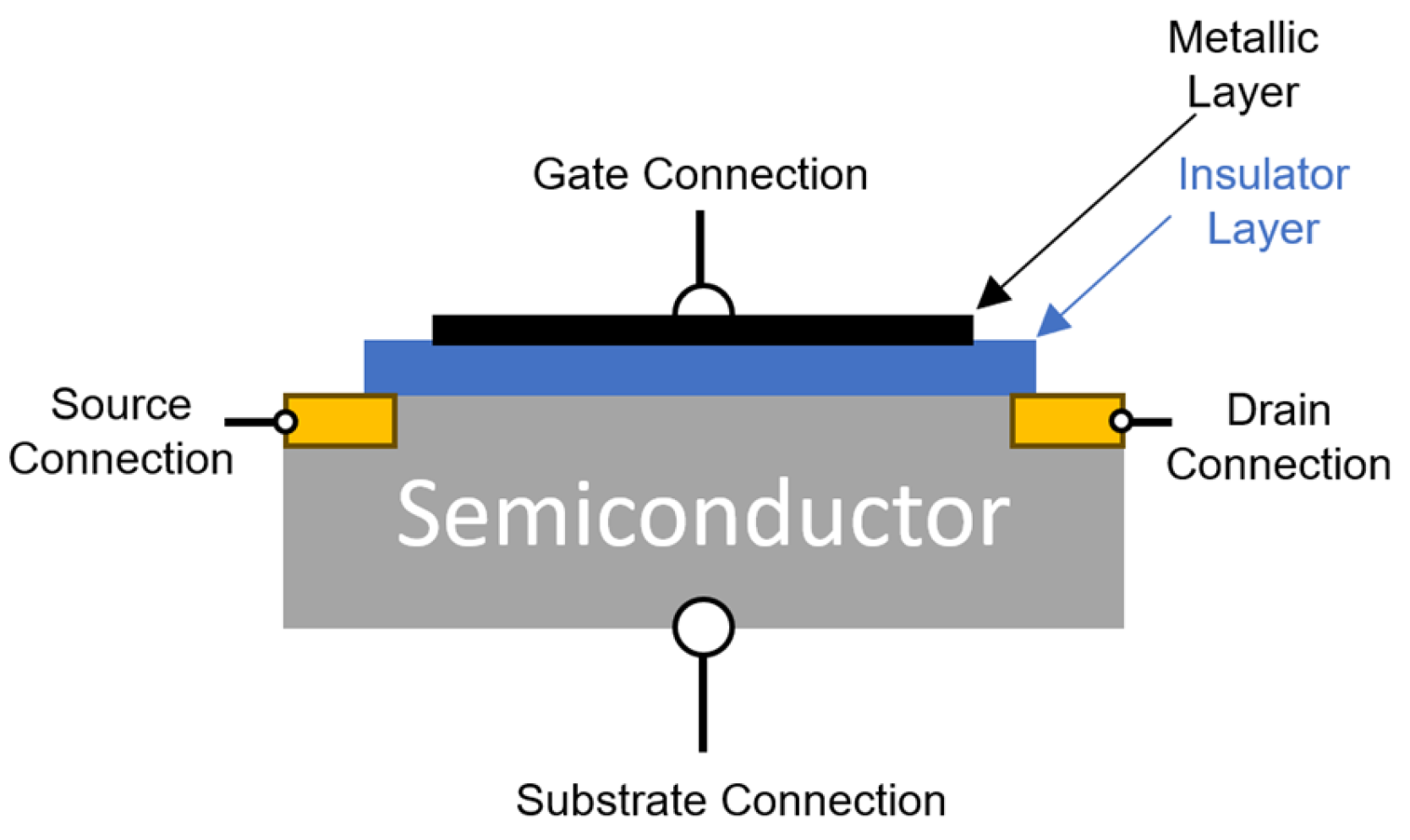
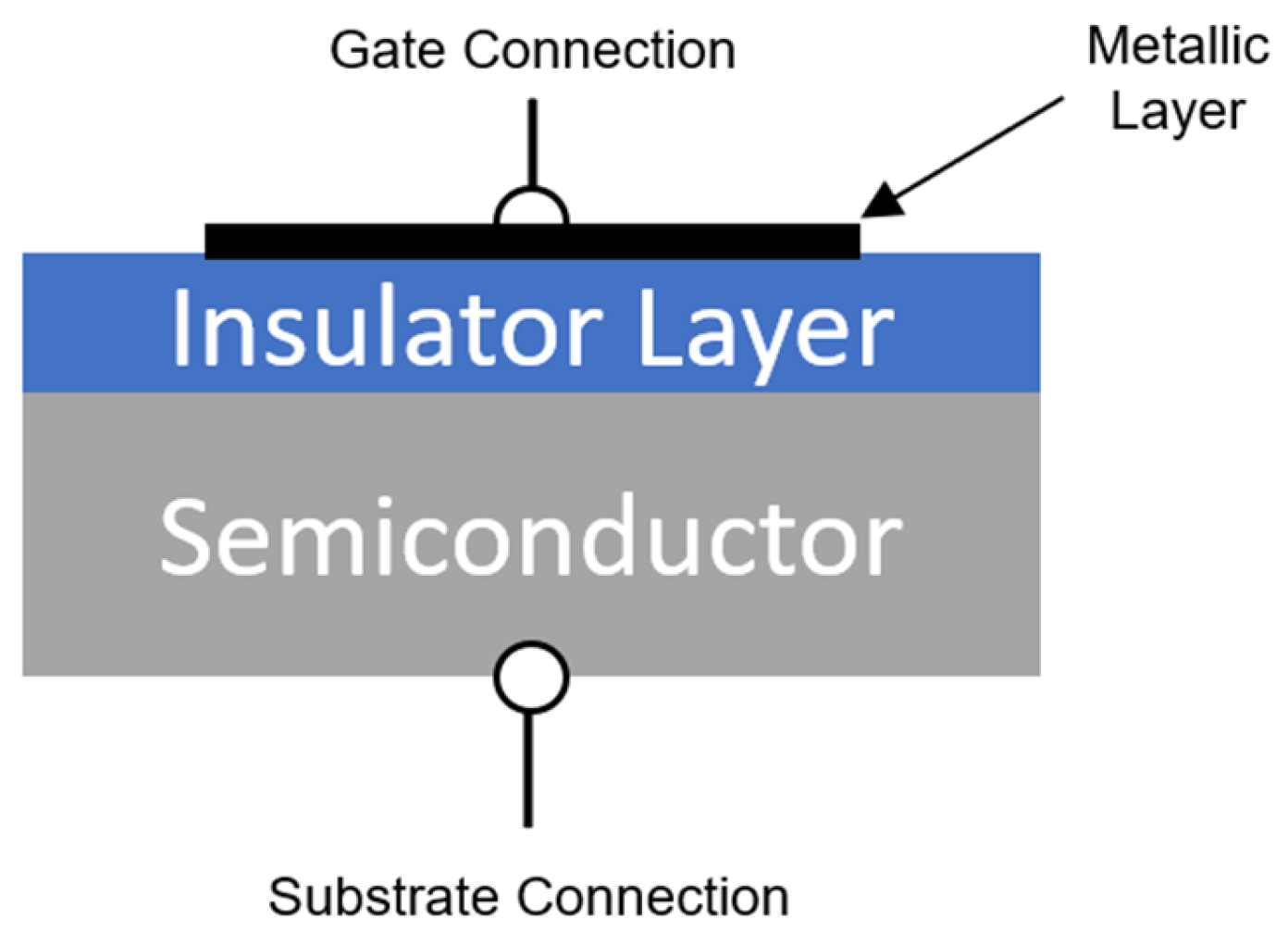
| Designation | Title | Publication Date |
|---|---|---|
| ISO/TR 15916 | Basic considerations for the safety of hydrogen systems | 2015 |
| CAN/BNQ 1784-000 | Canadian Hydrogen Installation Code | 2022 |
| ASME B31.12 | Hydrogen Piping and Pipelines | 2019 |
| NFPA 2 | Hydrogen Technologies Code | 2023 |
| Leak Grade | Readings | Examples |
|---|---|---|
| Grade 1 |
|
|
| Grade 2 |
|
|
| Grade 3 |
|
|
| Designation | Title | Publication Date |
|---|---|---|
| ISO 26142 | Hydrogen detection apparatus—Stationary applications | 2010 |
| CSA C22.2 No. 60079-29-1:17 | Explosive atmospheres—Part 29-1: Gas detectors—Performance requirements of detectors for flammable gases | 2017 |
| UL 2075 | Gas and Vapor Detectors and Sensors | 2013 |
| SAE J3089 | Characterization of On-Board Vehicular Hydrogen Sensors | 2018 |
| Metric | ISO 26142 | UL 2075 | CSA C22.2 No. 60079-29-1:17 |
|---|---|---|---|
| Measurement range | At least 1 order of magnitude | N/A | According to manufacturer |
| Temperature range | 15 °C to 25 °C, ±2 °C variation | −40 °C to 66 °C | 15 °C to 25 °C, ±2 °C variation |
| Relative humidity range | 20% to 80%, ±10% variation | 7.5% ± 0.5% to 95% ± 4% | 20% to 80%, ±10% variation |
| Response time | <30 s | N/A | According to manufacturer |
| Accuracy | According to manufacturer | N/A | According to manufacturer |
| Lifetime | N/A | Tested for a min of 1 year | N/A |
| Metric | Target Performance |
|---|---|
| Measurement range | 0.1–10% |
| Operating temperature | −30 °C to 80 °C |
| Relative humidity range | 10% to 98% |
| Response time | <1 s |
| Accuracy | 5% of full scale |
| Lifetime | 10 years |
| Selectivity | Interference resistant (e.g., hydrocarbons) |
| Metric Type | Metric | Information |
|---|---|---|
| Analytical metrics | Selectivity | The ability of the sensor to specifically detect and respond to a particular target substance or stimulus while minimizing interference from other substances or factors present in the environment. |
| Response time | The time taken by a gas sensor to reach a specified percentage of its final output after exposure to the target gas. | |
| Recovery time | The time required for a gas sensor to return to its baseline measurement after exposure to the target gas is removed. | |
| Lower detection limit (LDL) | The minimum concentration of the target gas that a gas sensor can reliably detect and quantify. | |
| Reversibility | The capability of a gas sensor to return to its initial state after exposure to the target gas is ceased. | |
| Repeatability | The ability of a gas sensor to produce consistent results when exposed to the same concentration of the target gas under the same conditions. | |
| Analytical resolution | The smallest detectable change in concentration that a gas sensor can discern. | |
| Environmental effects | The influence of external factors (e.g., temperature, humidity, and pressure) on the performance of a gas sensor. | |
| Signal drift | The gradual change in the gas sensor’s output over time, leading to a shift in measurements. | |
| Linear and dynamic range | The range of gas concentrations over which a gas detector/sensor provides accurate and proportional measurements. | |
| Limits of quantitation | The lowest and highest concentrations of the target gas that a gas sensor can measure with acceptable precision and accuracy. | |
| Logistic–operational parameters | Operational lifetime | The duration for which a gas sensor can reliably function before it needs replacement. |
| Calibration and maintenance requirements | The regular procedures needed to ensure the accuracy and proper functioning of the gas sensor. | |
| Orientation effects | The impact of the gas sensor’s positioning or orientation on its performance or operation. | |
| Warm-up time | The time needed for the gas sensor to stabilize and provide accurate readings after being turned on. | |
| Signal management | The processing and handling of the signal generated by the gas sensor. | |
| Matrix requirements | Considerations for the type of sample or environment in which the gas sensor will be used. | |
| Sample size | The volume or amount of the gas sample required for analysis. | |
| consumables | Additional consumable materials or components needed for the gas sensor to function (e.g., calibration gases and filters). | |
| Logistic–deployment parameters | Capital cost | The initial cost of purchasing the gas-sensor equipment. |
| Installation cost | The expenses associated with setting up and integrating the gas sensor into a system or environment. | |
| Physical size | The dimensions and form factor of the gas sensor. | |
| Power requirements | The energy demands of the gas sensor for its operation. | |
| Shelf life | The duration for which the gas sensor can be stored and remain functional before it needs to be used or replaced. | |
| Placement | The optimal location or positioning of the gas sensor for effective gas monitoring. | |
| Electronic interface | The means of communication and data transfer between the gas sensor and external devices or systems. | |
| Control circuitry | The internal circuitry responsible for managing the gas detector/sensor’s operation and output. | |
| Pneumatic connections | The connections used to transport gas to the gas sensor for analysis. | |
| Government regulations | The compliance requirements and standards set by regulatory bodies for gas detectors/sensors. | |
| Maturity/availability | The level of development and accessibility of the gas detector/sensor technology in the market. |
| Advantages | Disadvantages |
|---|---|
|
|
| Advantages | Disadvantages |
|---|---|
| Advantages | Disadvantages |
|---|---|
|
|
| Advantages | Disadvantages |
|---|---|
|
|
| Advantages | Disadvantages |
|---|---|
|
|
| Advantages | Disadvantages |
|---|---|
|
| Advantages | Disadvantages |
|---|---|
|
| Advantages | Disadvantages |
|---|---|
|
|
| Advantages | Disadvantages |
|---|---|
|
|
| Metric | Electrochemical | Catalytic | Resistance Based | Thermal Conductivity | Work Function | Optical |
|---|---|---|---|---|---|---|
| Selectivity | Acceptable | Acceptable | Acceptable | Poor | Good | Good |
| Response time | Acceptable | Good | Acceptable | Good | Acceptable | Good |
| Detection range | Acceptable | Acceptable | Acceptable | Good | Good | Poor |
| Lower detection limit | Good | Good | Good | Poor | Good | Good |
| Environmental sensitivity | Acceptable | Poor | Poor | Poor | Acceptable | Acceptable |
| Market availability | Good | Good | Acceptable | Acceptable | Poor | Poor |
| Operational lifetime | Acceptable | Poor | Acceptable | Good | Good | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qanbar, M.W.; Hong, Z. A Review of Hydrogen Leak Detection Regulations and Technologies. Energies 2024, 17, 4059. https://doi.org/10.3390/en17164059
Qanbar MW, Hong Z. A Review of Hydrogen Leak Detection Regulations and Technologies. Energies. 2024; 17(16):4059. https://doi.org/10.3390/en17164059
Chicago/Turabian StyleQanbar, Mohammed W., and Zekai Hong. 2024. "A Review of Hydrogen Leak Detection Regulations and Technologies" Energies 17, no. 16: 4059. https://doi.org/10.3390/en17164059
APA StyleQanbar, M. W., & Hong, Z. (2024). A Review of Hydrogen Leak Detection Regulations and Technologies. Energies, 17(16), 4059. https://doi.org/10.3390/en17164059







