Novel Design and Synthesis of Ni-Mo-Co Ternary Hydroxides Nanoflakes for Advanced Energy Storage Device Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Ni-Mo-Co Triple Hydroxide (THs) Nanoflakes
2.2. Material Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
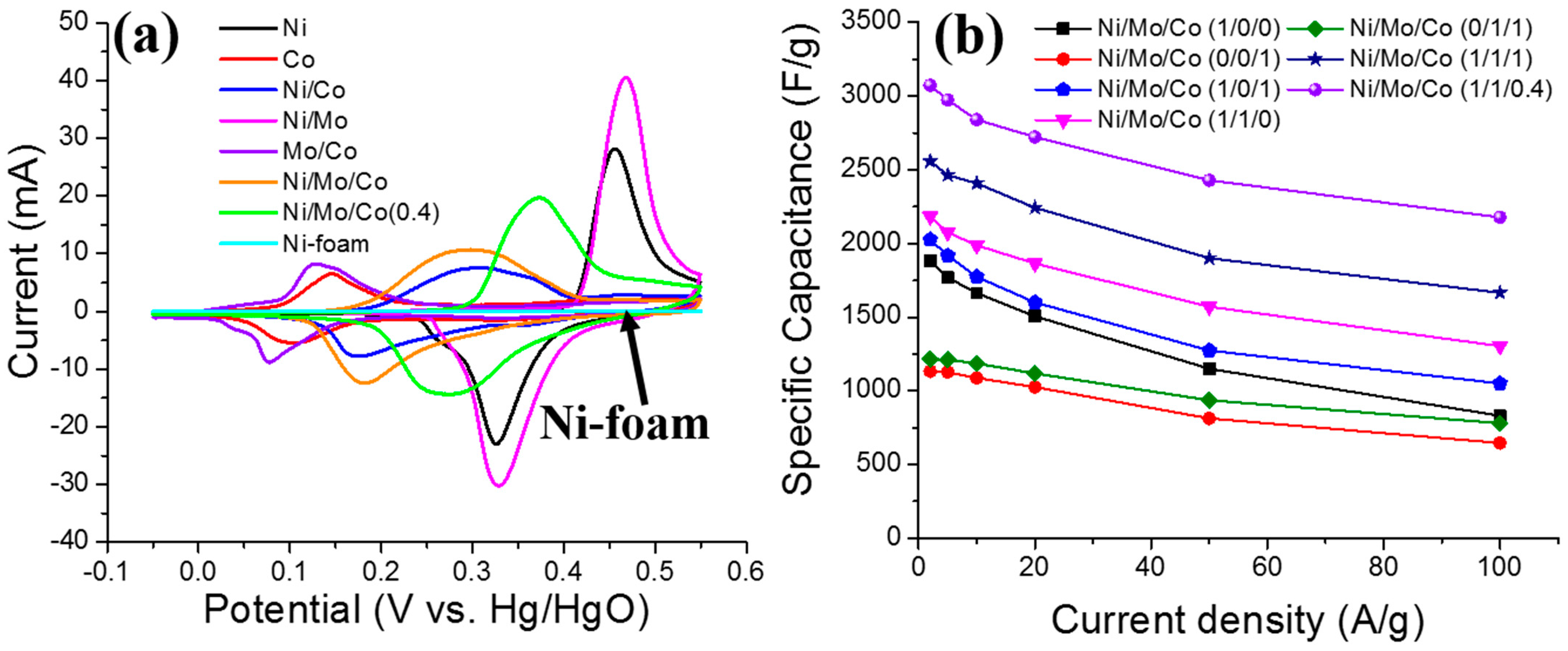
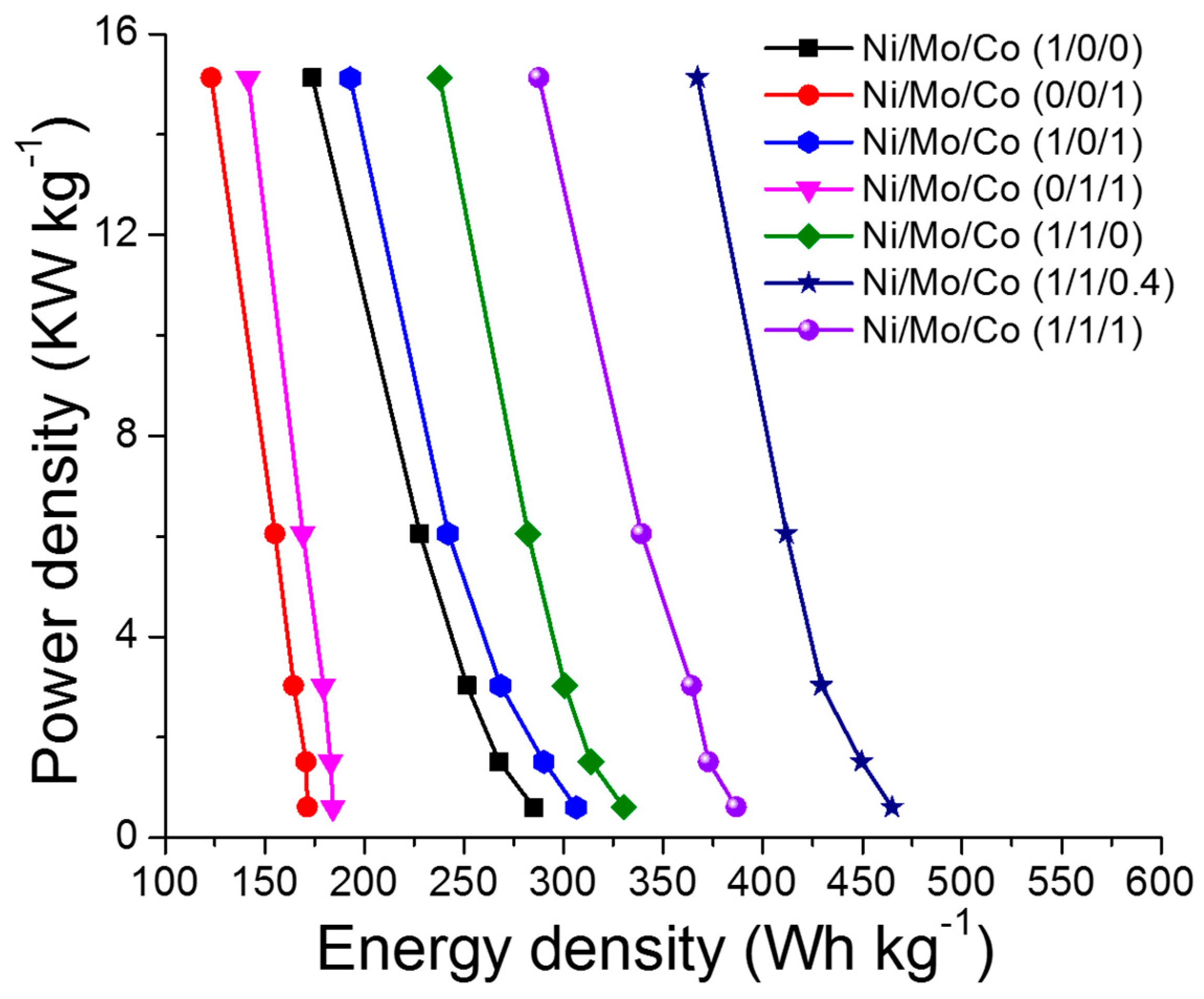
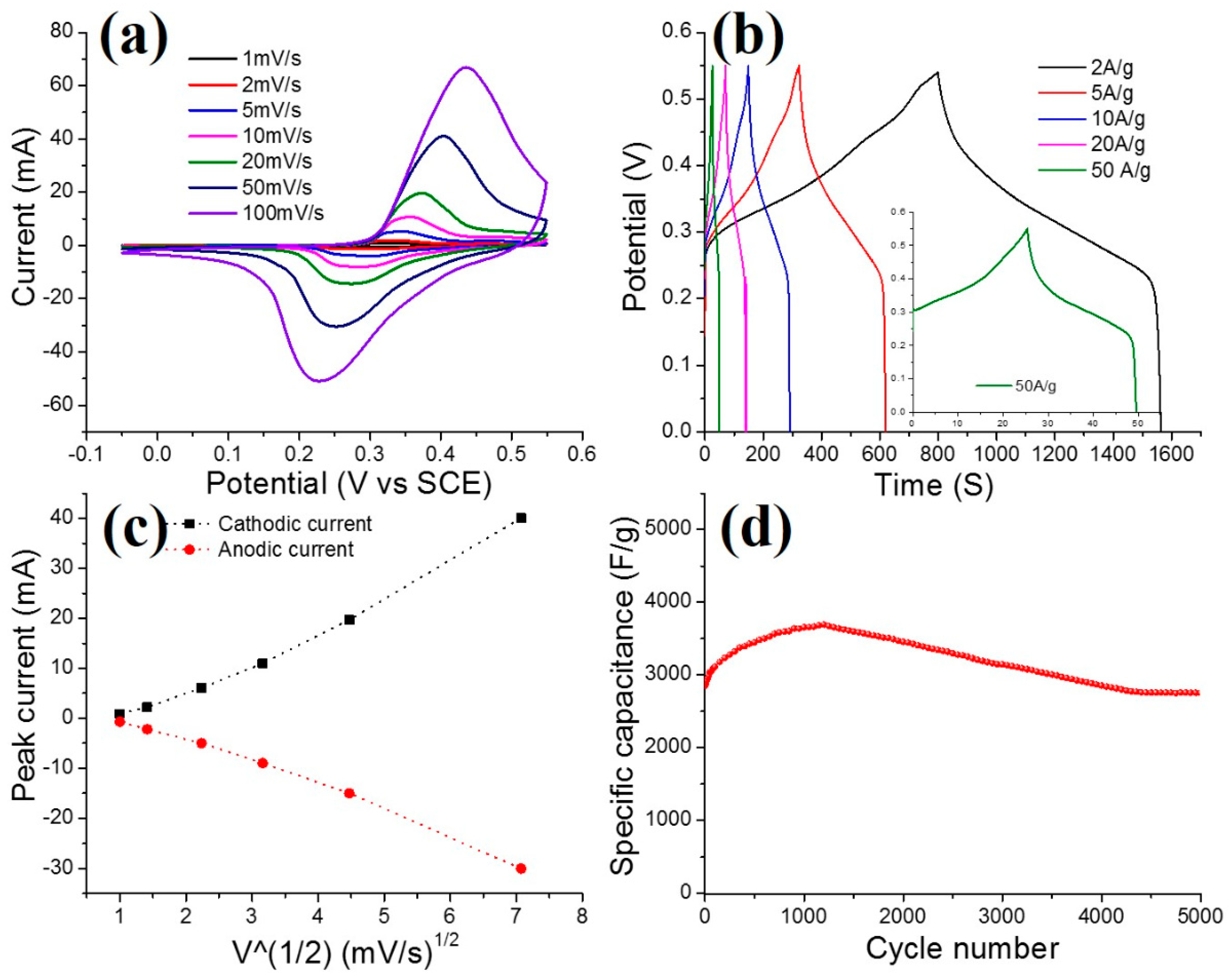
3.1. Electrochemical Performance of the Supercapacitors (SCs)
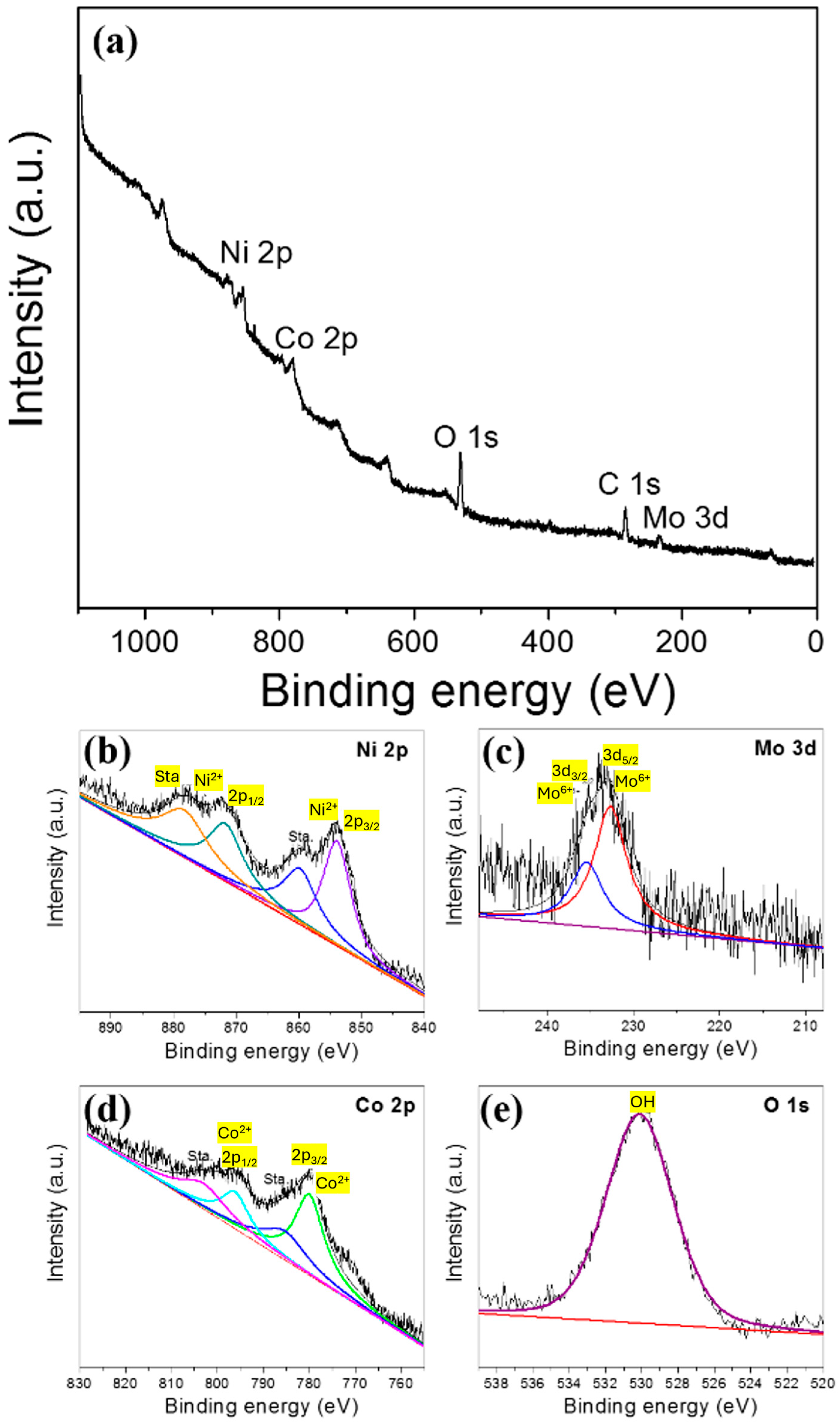
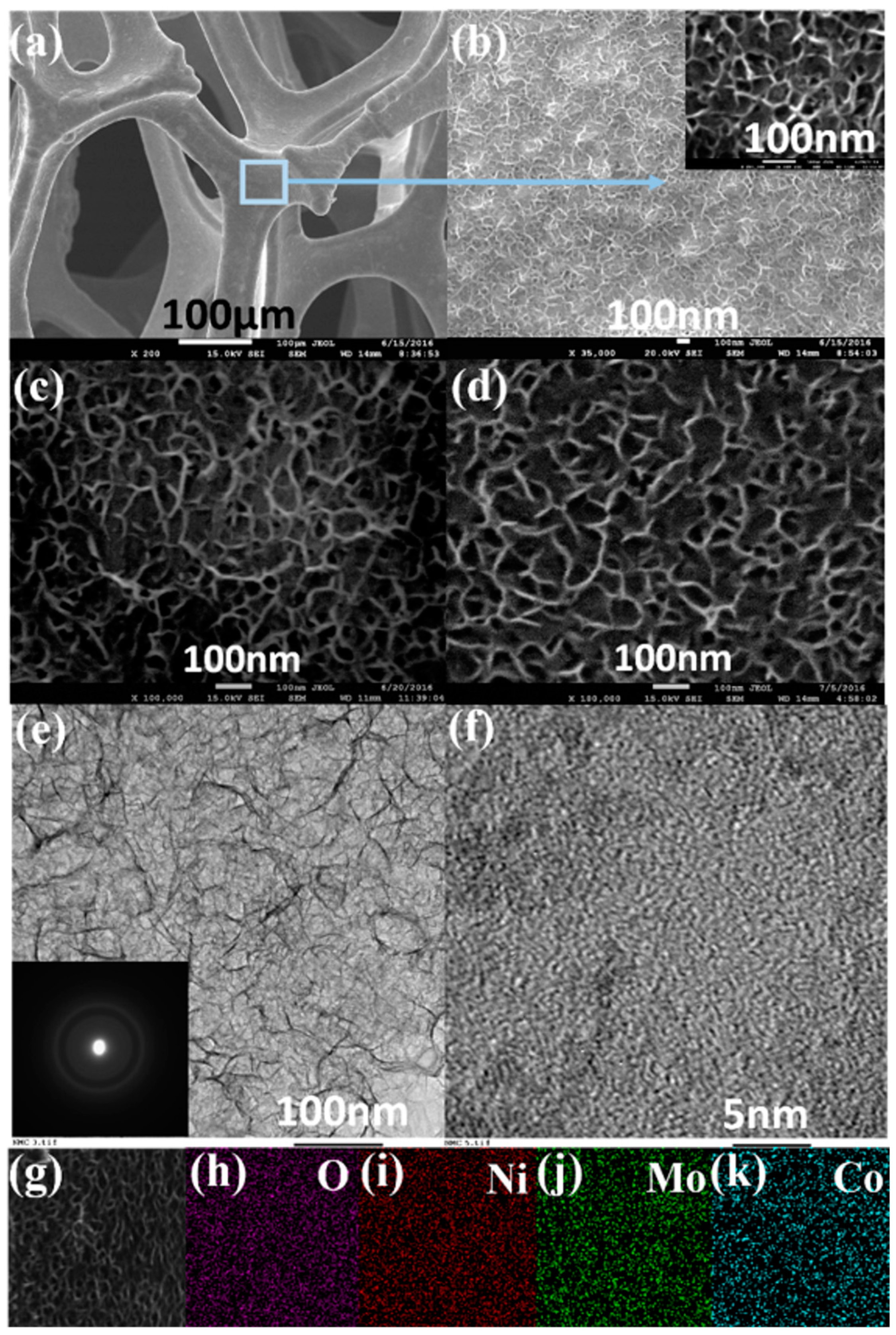
3.2. Morphology and Structural Analysis
3.3. Electrochemical Test of Ni-Mo-Co (1/1/0.4) THs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Ashritha, M.G.; Hareesh, K. Chapter 9—Electrode materials for EDLC and pseudocapacitors. In Smart Supercapacitors; Hussain, C.M., Ahamed, M.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 179–198. [Google Scholar]
- Schütter, C.; Ramirez-Castro, C.; Oljaca, M.; Passerini, S.; Winter, M.; Balducci, A. Activated Carbon, Carbon Blacks and Graphene Based Nanoplatelets as Active Materials for Electrochemical Double Layer Capacitors: A Comparative Study. J. Electrochem. Soc. 2015, 162, A44. [Google Scholar] [CrossRef]
- Sahoo, R.; Pal, A.; Pal, T. Chapter 19—Noble Metal–Transition Metal Oxides/Hydroxides: Desired Materials for Pseudocapacitor. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 395–430. [Google Scholar]
- Liu, R.; Zhou, A.; Zhang, X.; Mu, J.; Che, H.; Wang, Y.; Wang, T.-T.; Zhang, Z.; Kou, Z. Fundamentals, advances and challenges of transition metal compounds-based supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, X.; Yang, W.; Yu, Z.; Sun, X.; Zhang, Y.; Yang, X.; Kimura, H.; Hou, C.; Guo, Z.; et al. Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors. Adv. Compos. Hybrid Mater. 2021, 4, 906–924. [Google Scholar] [CrossRef]
- Xue, T.; Wang, X.; Lee, J.-M. Dual-template synthesis of Co(OH)2 with mesoporous nanowire structure and its application in supercapacitor. J. Power Sources 2012, 201, 382–386. [Google Scholar] [CrossRef]
- Hu, C.-C.; Chen, J.-C.; Chang, K.-H. Cathodic deposition of Ni(OH)2 and Co(OH)2 for asymmetric supercapacitors: Importance of the electrochemical reversibility of redox couples. J. Power Sources 2013, 221, 128–133. [Google Scholar] [CrossRef]
- Vialat, P.; Mousty, C.; Taviot-Gueho, C.; Renaudin, G.; Martinez, H.; Dupin, J.-C.; Elkaim, E.; Leroux, F. High-Performing Monometallic Cobalt Layered Double Hydroxide Supercapacitor with Defined Local Structure. Adv. Funct. Mater. 2014, 24, 4831–4842. [Google Scholar] [CrossRef]
- Huang, B.; Wang, W.; Pu, T.; Li, J.; Zhu, J.; Zhao, C.; Xie, L.; Chen, L. Two-dimensional porous (Co, Ni)-based monometallic hydroxides and bimetallic layered double hydroxides thin sheets with honeycomb-like nanostructure as positive electrode for high-performance hybrid supercapacitors. J. Colloid Interface Sci. 2018, 532, 630–640. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Hou, S.; Yang, Q.; Zhu, Z. Preparation of bulk doped NiCo2O4 bimetallic oxide supercapacitor materials by in situ growth method. Inorg. Nano-Met. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, S.; Chen, Y.; Gao, P.; Zhu, C.; Yang, P.; Qi, L. Hierarchical nanosheet-based NiMoO4 nanotubes: Synthesis and high supercapacitor performance. J. Mater. Chem. A 2015, 3, 739–745. [Google Scholar] [CrossRef]
- Yu, X.; Lu, B.; Xu, Z. Super long-life supercapacitors based on the construction of nanohoneycomb-like strongly coupled CoMoO4-3D graphene hybrid electrodes. Adv. Mater. 2014, 26, 1044–1051. [Google Scholar] [CrossRef]
- Khadka, A.; Samuel, E.; Joshi, B.; Kim, Y.I.; Aldalbahi, A.; El-Newehy, M.; Lee, H.-S.; Yoon, S.S. Bimetallic CoMoO4 Nanosheets on Freestanding Nanofiber as Wearable Supercapacitors with Long-Term Stability. Int. J. Energy Res. 2023, 2023, 2910207. [Google Scholar] [CrossRef]
- Prabakaran, P.; Arumugam, G.; Ramu, P.; Selvaraj, M.; Assiri, M.A.; Rokhum, S.L.; Arjunan, S.; Rajendran, R. Construction of hierarchical MnMoO4 nanostructures on Ni foam for high-performance asymmetric supercapacitors. Surf. Interfaces 2023, 40, 103086. [Google Scholar] [CrossRef]
- Kumcham, P.; Sreekanth, T.; Yoo, K.; Kim, J. Surfactant-assisted morphology modification of nanostructured MnMoO4 for high-performance asymmetric supercapacitors. New J. Chem. 2024, 48, 7820–7835. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.; Feng, R.; Zhu, K.; Zhao, F.; Wang, C.; Wang, J.; Wang, L.; Chu, P.K. Fabrication of Bimetallic Oxides (MCo2O4, M=Cu, Mn) on Ordered Microchannel Electro-Conductive Plate for High-Performance Hybrid Supercapacitors. Sustainability 2021, 13, 9896. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, Y.; Wan, H.; Xu, X.; Jiang, S. Enhanced cycle performance of hierarchical porous sphere MnCo2O4 for asymmetric supercapacitors. Electrochim. Acta 2019, 301, 294–303. [Google Scholar] [CrossRef]
- Bai, X.; Cao, D.; Zhang, H. Simultaneously morphology and phase controlled synthesis of cobalt manganese hydroxides/reduced graphene oxide for high performance supercapacitor electrodes. Ceram. Int. 2020, 46 Pt B, 19135–19145. [Google Scholar] [CrossRef]
- Zou, X.; Sun, Q.; Zhang, Y.; Li, G.-D.; Liu, Y.; Wu, Y.; Yang, L.; Zou, X. Ultrafast surface modification of Ni3S2 nanosheet arrays with Ni-Mn bimetallic hydroxides for high-performance supercapacitors. Sci. Rep. 2018, 8, 4478. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Zhang, H.; Yan, S.; Zhang, C.; Liu, S. One-Step Synthesis of Ultrathin NiMn Layered Double Hydroxide Nanosheets for Supercapacitors. ChemElectroChem 2019, 6, 4456–4463. [Google Scholar] [CrossRef]
- Ma, K.; Cheng, J.P.; Liu, F.; Zhang, X. Co-Fe layered double hydroxides nanosheets vertically grown on carbon fiber cloth for electrochemical capacitors. J. Alloys Compd. 2016, 679, 277–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; da Silva, M.I.; Toma, H.E.; Angnes, L.; Martins, P.R.; Araki, K. Trimetallic oxides/hydroxides as hybrid supercapacitor electrode materials: A review. J. Mater. Chem. A 2020, 8, 10534–10570. [Google Scholar] [CrossRef]
- Xing, J.; Wu, S.; Ng, K.S. Electrodeposition of ultrathin nickel–cobalt double hydroxide nanosheets on nickel foam as high-performance supercapacitor electrodes. RSC Adv. 2015, 5, 88780–88786. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Chu, W.; Wang, N. High-stable α-phase NiCo double hydroxide microspheres via microwave synthesis for supercapacitor electrode materials. Chem. Eng. J. 2017, 316, 277–287. [Google Scholar] [CrossRef]
- Wang, A.-L.; Xu, H.; Li, G.-R. NiCoFe Layered Triple Hydroxides with Porous Structures as High-Performance Electrocatalysts for Overall Water Splitting. ACS Energy Lett. 2016, 1, 445–453. [Google Scholar] [CrossRef]
- Chen-Wiegart, Y.C.K.; Liu, Z.; Faber, K.T.; Barnett, S.A.; Wang, J. 3D analysis of a LiCoO2–Li(Ni1/3Mn1/3Co1/3)O2 Li-ion battery positive electrode using x-ray nano-tomography. Electrochem. Commun. 2013, 28, 127–130. [Google Scholar] [CrossRef]
- Ecker, M.; Nieto, N.; Käbitz, S.; Schmalstieg, J.; Blanke, H.; Warnecke, A.; Sauer, D.U. Calendar and cycle life study of Li(NiMnCo)O2-based 18,650 lithium-ion batteries. J. Power Sources 2014, 248, 839–851. [Google Scholar] [CrossRef]
- Li, P. Novel Design and Synthesis of Transition Metal Hydroxides and Oxides for Energy Storage Device Applications; Wayne State University: Detroit, MI, USA, 2016. [Google Scholar]
- Li, H.; Gao, Y.; Wang, C.; Yang, G. A Simple Electrochemical Route to Access Amorphous Mixed-Metal Hydroxides for Supercapacitor Electrode Materials. Adv. Energy Mater. 2015, 5, 1401767. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Zheng, S.; Guo, X.; Xue, H.; Pang, H. Recent Progress in Some Amorphous Materials for Supercapacitors. Small 2018, 14, 1800426. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Yang, G. Amorphous nickel oxide and crystalline manganese oxide nanocomposite electrode for transparent and flexible supercapacitor. Chem. Eng. J. 2018, 347, 101–110. [Google Scholar] [CrossRef]
- Cui, L.-F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-Amorphous Core–Shell Silicon Nanowires for High Capacity and High Current Battery Electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef]
- Niu, L.; Li, Z.; Xu, Y.; Sun, J.; Hong, W.; Liu, X.; Wang, J.; Yang, S. Simple Synthesis of Amorphous NiWO4 Nanostructure and Its Application as a Novel Cathode Material for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 8044–8052. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General Solution Growth of Mesoporous NiCo2O4 Nanosheets on Various Conductive Substrates as High-Performance Electrodes for Supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Wang, Z.; Li, X.; Guo, H.; Wang, J.; Zhang, D. Facile construction of Co(OH)2@Ni(OH)2 core-shell nanosheets on nickel foam as three dimensional free-standing electrode for supercapacitors. Electrochim. Acta 2019, 293, 40–46. [Google Scholar] [CrossRef]
- Meng, X.; Deng, D. Bio-inspired synthesis of α-Ni(OH)2 nanobristles on various substrates and their applications. J. Mater. Chem. A 2016, 4, 6919–6925. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Chen, C.-H.; Chien, H.-C.; Lu, S.-Y.; Hu, C.-C. A Cost-Effective Supercapacitor Material of Ultrahigh Specific Capacitances: Spinel Nickel Cobaltite Aerogels from an Epoxide-Driven Sol–Gel Process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232. [Google Scholar] [CrossRef]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D Graphene–Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Xia, X.; Tu, J.; Zhang, Y.; Wang, X.; Gu, C.; Zhao, X.B.; Fan, H.J. High-Quality Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef]
- Pu, J.; Tong, Y.; Wang, S.; Sheng, E.; Wang, Z. Nickel–cobalt hydroxide nanosheets arrays on Ni foam for pseudocapacitor applications. J. Power Sources 2014, 250, 250–256. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–Cobalt Layered Double Hydroxide Nanosheets for High-performance Supercapacitor Electrode Materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Guo, D.; Luo, Y.; Yu, X.; Li, Q.; Wang, T. High performance NiMoO4 nanowires supported on carbon cloth as advanced electrodes for symmetric supercapacitors. Nano Energy 2014, 8, 174–182. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, Y.; Luo, Y.; Fang, C.; Xiong, J. Growth of Ultrathin Mesoporous Ni-Mo Oxide Nanosheet Arrays on Ni Foam for High-performance Supercapacitor Electrodes. Electrochim. Acta 2015, 176, 1343–1351. [Google Scholar] [CrossRef]
- Xiao, K.; Xia, L.; Liu, G.; Wang, S.; Ding, L.-X.; Wang, H. Honeycomb-like NiMoO4 ultrathin nanosheet arrays for high-performance electrochemical energy storage. J. Mater. Chem. A 2015, 3, 6128–6135. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Zhang, G.; Xu, M.; Lu, W.; Zhou, L.; Huang, H. Design of Hierarchical Ni-Co@Ni-Co Layered Double Hydroxide Core–Shell Structured Nanotube Array for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors. Adv. Funct. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Wu, N.; Low, J.; Liu, T.; Yu, J.; Cao, S. Hierarchical hollow cages of Mn-Co layered double hydroxide as supercapacitor electrode materials. Appl. Surf. Sci. 2017, 413, 35–40. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Su, D.; Tang, Z.; Zhang, J.; Liu, Y.; Yuan, A.; Kong, Q. Ultrathin Ni-Al layered double hydroxide nanosheets with enhanced supercapacitor performance. Ceram. Int. 2017, 43, 14395–14400. [Google Scholar] [CrossRef]
- He, P.; Huang, Q.; Huang, B.; Chen, T. Controllable synthesis of Ni-Co-Mn multi-component metal oxides with various morphologies for high-performance flexible supercapacitors. RSC Adv. 2017, 7, 24353–24358. [Google Scholar] [CrossRef]
- Huang, C.; Hu, Y.; Jiang, S.; Chen, H.C. Amorphous nickel-based hydroxides with different cation substitutions for advanced hybrid supercapacitors. Electrochim. Acta 2019, 325, 134936. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Song, K.; Li, W.; Xin, J.; Zheng, Y.; Chen, X.; Yang, R.; Lv, W.; Li, Q. Hierarchical porous heterostructured Co(OH)2/CoSe2 nanoarray: A controllable design electrode for advanced asymmetrical supercapacitors. Chem. Eng. J. 2021, 419, 129435. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, G.; Wang, Z.; Xiang, G.; Hao, S.; Wang, X.; Xu, X.; Ma, W.; Zhao, G. Hollow polyhedron structure of amorphous Ni-Co-S/Co(OH)2 for high performance supercapacitors. Chin. Chem. Lett. 2021, 32, 2453–2458. [Google Scholar] [CrossRef]
- Manibalan, G.; Govindaraj, Y.; Yesuraj, J.; Kuppusami, P.; Murugadoss, G.; Murugavel, R.; Rajesh Kumar, M. Facile synthesis of NiO@Ni(OH)2-α-MoO3 nanocomposite for enhanced solid-state symmetric supercapacitor application. J. Colloid Interface Sci. 2021, 585, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhao, M.; Jiao, L.; Su, Z.; Li, M.; Song, X. Novel Mo-doped nickel sulfide thin sheets decorated with Ni–Co layered double hydroxide sheets as an advanced electrode for aqueous asymmetric super-capacitor battery. J. Power Sources 2021, 509, 230333. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Mahmoudi Najafi, S.H.; Norouzi, Z. Hierarchical NiO@Ni(OH)2 nanoarrays as high-performance supercapacitor electrode material. Electrochim. Acta 2021, 368, 137633. [Google Scholar] [CrossRef]
- Sasaki, Y.; Yamashita, T. Effect of electrolytic conditions on the deposition of nickel hydroxide. Thin Solid Film. 1998, 334, 117–119. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Choi, J.G.; Thompson, L.T. XPS study of as-prepared and reduced molybdenum oxides. Appl. Surf. Sci. 1996, 93, 143–149. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, W.; Ng, K.Y.S. Facile Synthesis of CoS Nanoparticles Anchored on the Surface of Functionalized Multiwalled Carbon Nanotubes as Cathode Materials for Advanced Li–S Batteries. Ind. Eng. Chem. Res. 2022, 61, 9322–9330. [Google Scholar] [CrossRef]
- Gao, P.; Zeng, Y.; Tang, P.; Wang, Z.; Yang, J.; Hu, A.; Liu, J. Understanding the Synergistic Effects and Structural Evolution of Co(OH)2 and Co3O4 toward Boosting Electrochemical Charge Storage. Adv. Funct. Mater. 2022, 32, 2108644. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Xie, K.; Yu, F.; Huang, H. A One-Step and Binder-Free Method to Fabricate Hierarchical Nickel-Based Supercapacitor Electrodes with Excellent Performance. Adv. Funct. Mater. 2013, 23, 3675–3681. [Google Scholar] [CrossRef]
- Yan, S.; Abhilash, K.P.; Tang, L.; Yang, M.; Ma, Y.; Xia, Q.; Guo, Q.; Xia, H. Research Advances of Amorphous Metal Oxides in Electrochemical Energy Storage and Conversion. Small 2019, 15, 1804371. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Mei, Y.; Yuan, S.; Mei, H.; Xu, B.; Bao, Y.; Fan, L.; Kang, W.; Dai, F.; Wang, R.; et al. Controlled Hydrolysis of Metal–Organic Frameworks: Hierarchical Ni/Co-Layered Double Hydroxide Microspheres for High-Performance Supercapacitors. ACS Nano 2019, 13, 7024–7030. [Google Scholar] [CrossRef]
- Lu, X.; Liu, T.; Zhai, T.; Wang, G.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Improving the Cycling Stability of Metal–Nitride Supercapacitor Electrodes with a Thin Carbon Shell. Adv. Energy Mater. 2014, 4, 1300994. [Google Scholar] [CrossRef]
- Wu, H.B.; Pang, H.; Lou, X.W. Facile synthesis of mesoporous Ni0.3Co2.7O4 hierarchical structures for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 3619–3626. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Wu, H.B.; Hoster, H.E.; Chan-Park, M.B.; Lou, X.W. Single-crystalline NiCo2O4 nanoneedle arrays grown on conductive substrates as binder-free electrodes for high-performance supercapacitors. Energy Environ. Sci. 2012, 5, 9453–9456. [Google Scholar] [CrossRef]
| Year | Chemical Composition | Morphology | Synthesis Method | Capacitance (F g−1) and Current Density | Decay% (after No. of Cycles) | Ref. |
|---|---|---|---|---|---|---|
| 2019 | NiCo2O4 aerogels | Aerogel | Epoxide addition procedure | 1400 | Excellent stability (2000) | [43] |
| 2010 | Gold/MnO2 | Nanoporous | Conventional chemical method | ~1145 | N/A | [44] |
| 2012 | 3D graphene/Co3O4 | Nanowire | Chemical vapor deposition | ~1100 at 10 A g−1 | N/A | [45] |
| 2012 | Co3O4/NiO | Nanowires | Two-step solution-based method | 853 at 2 A g−1 | Excellent stability (6000) | [46] |
| 2013 | Ni-Co hydroxide | Nanosheets | Hydrothermal | 1734 at 6 A g−1 | 14% (1000) | [47] |
| 2014 | Ni-Co hydroxide | Nanosheets | One-step process | 2682 at 3 A g−1 | 18% (5000) | [48] |
| 2014 | NiMoO4 | Nanowires | Facile hydrothermal method | 1517 at 1.2 A g−1 | 4.7% (1000) | [49] |
| 2015 | Ni-Mo metal oxide | Ultrathin mesoporous | Conventional chemical method | 954 at 2 A g−1 | 22.3% (5000) | [50] |
| 2015 | NiMoO4 | Ultrathin nanosheets | Electrodeposition | 1694 at 1 A g−1 | 7.2% (9000) | [51] |
| 2017 | Ni-Co@Ni-Co layered double hydroxide | Core–shell structured nanotube | Conventional chemical method | 319 at 2 A g−1 | 3.1% (3000) | [52] |
| 2017 | Mn-Co layered double hydroxide | Hierarchical hollow cages | Conventional chemical method | 511 at 2 A g−1 | 10% (2000) | [53] |
| 2017 | Ni-Al nanosheets | Nanosheets | Conventional chemical method | 1919 at 2 A g−1 | Recover to 433 after 3000 | [54] |
| 2017 | NiCoMn2 metal oxide | Various morphologies | Hydrothermal | 1434.2 at 2 mA cm−2 | 5.7% (3000) | [55] |
| Now | Ni-Mo-Co ternary hydroxide | Nanosheets | One-step electrodeposition | 3074 at 2 A g−1 | 3.37% (5000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, P.; Tang, Z.; Ng, K.Y.S. Novel Design and Synthesis of Ni-Mo-Co Ternary Hydroxides Nanoflakes for Advanced Energy Storage Device Applications. Energies 2024, 17, 3881. https://doi.org/10.3390/en17163881
Wang Z, Li P, Tang Z, Ng KYS. Novel Design and Synthesis of Ni-Mo-Co Ternary Hydroxides Nanoflakes for Advanced Energy Storage Device Applications. Energies. 2024; 17(16):3881. https://doi.org/10.3390/en17163881
Chicago/Turabian StyleWang, Zhao, Peifeng Li, Zhuolun Tang, and Ka Yuen Simon Ng. 2024. "Novel Design and Synthesis of Ni-Mo-Co Ternary Hydroxides Nanoflakes for Advanced Energy Storage Device Applications" Energies 17, no. 16: 3881. https://doi.org/10.3390/en17163881
APA StyleWang, Z., Li, P., Tang, Z., & Ng, K. Y. S. (2024). Novel Design and Synthesis of Ni-Mo-Co Ternary Hydroxides Nanoflakes for Advanced Energy Storage Device Applications. Energies, 17(16), 3881. https://doi.org/10.3390/en17163881







