Analysis of Multi-Biofuel Production during Cultivation of the Green Microalga Tetraselmis subscordiformis
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisation of the Experiment
2.2. Materials
2.2.1. Microalgae Biomass
2.2.2. Medium Used in S1 (Biomass Production)
2.2.3. Medium Used in S2 (Hydrogen Production)
2.2.4. Anaerobic Sludge Used in S4–S6 (Methane Production)
2.3. Research Stations
2.3.1. Photobioreactor Used in S1 (Biomass Production)
2.3.2. Photobioreactor Used in S2 (Hydrogen Production)
2.3.3. Bioreactor Used in S4 to S6 (Methane Production)
2.4. Analytical Methods
2.5. Procedures Used in Individual Stages
2.5.1. S1 (Biomass Production)
2.5.2. S2 (Hydrogen Production)
2.5.3. S3 (Bio-Oil Production)
2.5.4. S4 to S6 (Methane Production)
2.6. Statistical Analysis
3. Results and Discussion
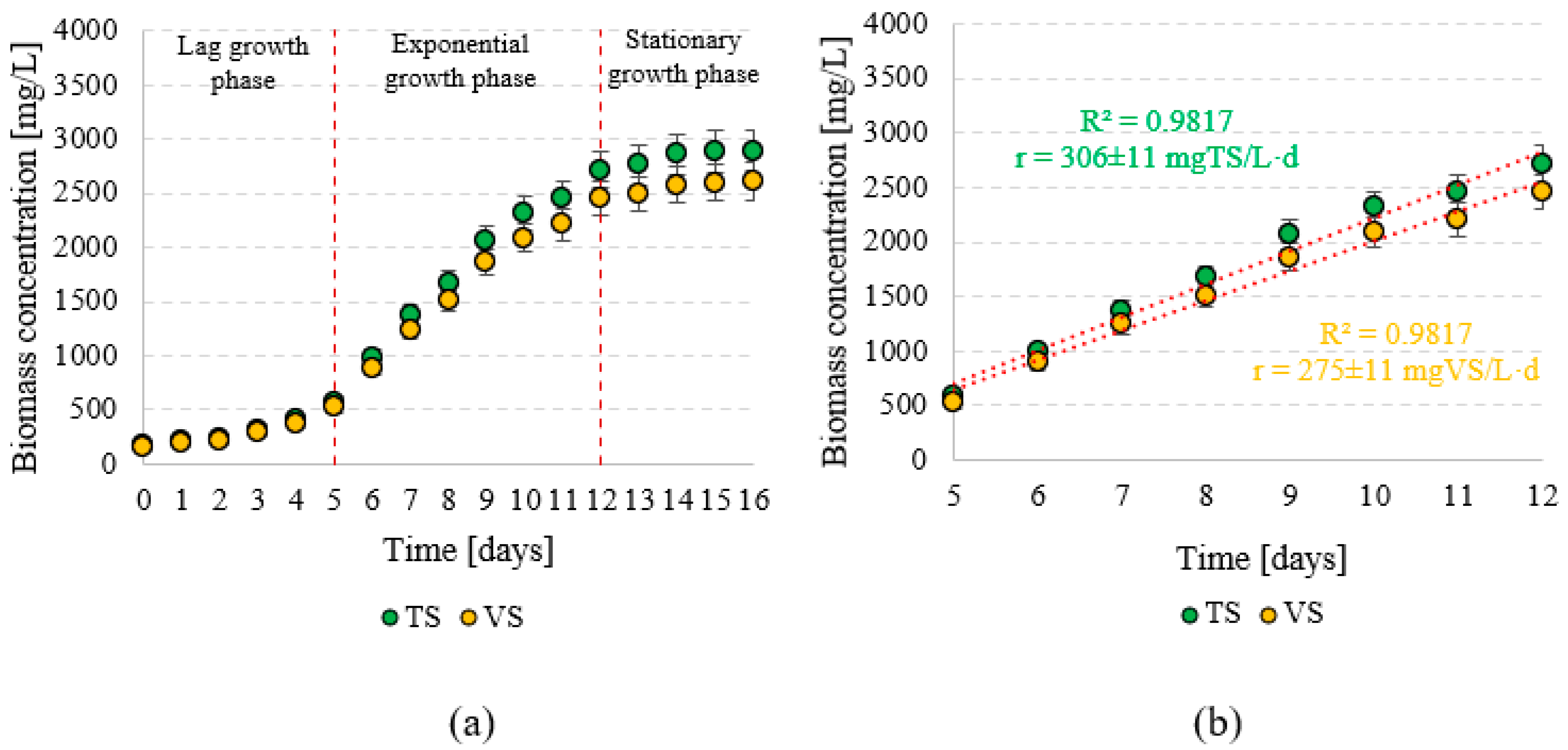
3.1. Stage 1 (Biomass Production)
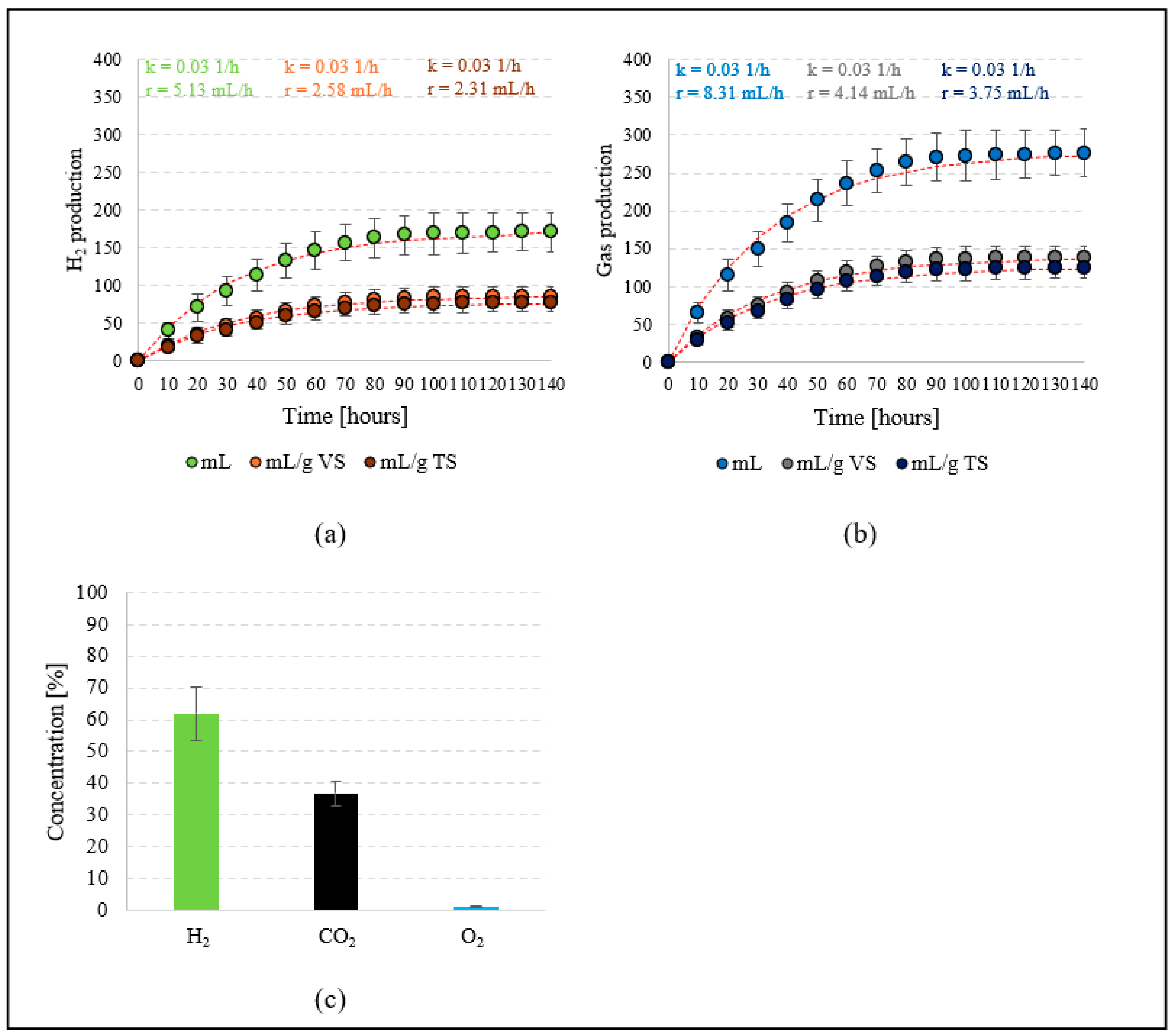
3.2. Stage 2 (Hydrogen Production)
3.3. Stage 3 (Bio-Oil Production)
3.4. Stages 4–6 (Methane Production)
3.5. Gross Energy Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choudhary, S.; Tripathi, S.; Poluri, K.M. Microalgal-Based Bioenergy: Strategies, Prospects, and Sustainability. Energy Fuels 2022, 36, 14584–14612. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Chew, K.W.; Selvarajoo, A.; Chen, W.H.; Chang, J.S.; Show, P.L. Microalgae: The Future Supply House of Biohydrogen and Biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Javed, M.A.; Zafar, A.M.; Aly Hassan, A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The Role of Oxygen Regulation and Algal Growth Parameters in Hydrogen Production via Biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003. [Google Scholar] [CrossRef]
- Condor, B.E.; de Luna, M.D.G.; Chang, Y.H.; Chen, J.H.; Leong, Y.K.; Chen, P.T.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Bioethanol Production from Microalgae Biomass at High-Solids Loadings. Bioresour. Technol. 2022, 363, 128002. [Google Scholar] [CrossRef] [PubMed]
- Morais, K.C.C.; Conceição, D.; Vargas, J.V.C.; Mitchell, D.A.; Mariano, A.B.; Ordonez, J.C.; Galli-Terasawa, L.V.; Kava, V.M. Enhanced Microalgae Biomass and Lipid Output for Increased Biodiesel Productivity. Renew. Energy 2021, 163, 138–145. [Google Scholar] [CrossRef]
- Martins, M.F.; Soares, R.B.; Gonçalves, R.F. Microalgae: The Challenges from Harvest to the Thermal Gasification. In Algal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–258. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Li, D.; Yang, Z.; Wang, X.; Lin, R. Research on the Thermochemical Conversion Utilization of Nitrogen-Rich Microalgae: Two-Step Catalytic Pyrolysis of Nannochloropsis Sp over ZSM-5. Energy Convers. Manag. 2022, 258, 115475. [Google Scholar] [CrossRef]
- Kuo, P.C.; Illathukandy, B.; Wu, W.; Chang, J.S. Energy, Exergy, and Environmental Analyses of Renewable Hydrogen Production through Plasma Gasification of Microalgal Biomass. Energy 2021, 223, 120025. [Google Scholar] [CrossRef]
- Chen, W.-H.; Felix, C.B. Thermo-Kinetics Study of Microalgal Biomass in Oxidative Torrefaction Followed by Machine Learning Regression and Classification Approaches. Energy 2024, 301, 131677. [Google Scholar] [CrossRef]
- Ge, S.; Brindhadevi, K.; Xia, C.; Salah Khalifa, A.; Elfasakhany, A.; Unpaprom, Y.; Whangchai, K. Performance, Combustion and Emission Characteristics of the CI Engine Fueled with Botryococcus Braunii Microalgae with Addition of TiO2 Nanoparticle. Fuel 2022, 317, 121898. [Google Scholar] [CrossRef]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A Realistic Scenario on Microalgae Based Biodiesel Production: Third Generation Biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Wang, S.; Murugesan, A.G.; Saravanakumar, M.; Selvakumar, G. Co-Cultivation of Streptomyces and Microalgal Cells as an Efficient System for Biodiesel Production and Bioflocculation Formation. Bioresour. Technol. 2021, 332, 125118. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An Overview on Microalgae as Renewable Resources for Meeting Sustainable Development Goals. J. Environ. Manag. 2022, 320, 115897. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Mendonça, H.; Assemany, P.; Abreu, M.; Couto, E.; Maciel, A.M.; Duarte, R.L.; Barbosa dos Santos, M.G.; Reis, A. Microalgae in a Global World: New Solutions for Old Problems? Renew. Energy 2021, 165, 842–862. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Cutolo, E.A.; Mandalà, G.; Dall’osto, L.; Bassi, R. Harnessing the Algal Chloroplast for Heterologous Protein Production. Microorganisms 2022, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Cyanobacteria as Renewable Sources of Bioenergy (Biohydrogen, Bioethanol, and Bio-Oil Production). In Ecophysiology and Biochemistry of Cyanobacteria; Springer: Singapore, 2021; pp. 431–454. [Google Scholar] [CrossRef]
- Dȩbowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zielínski, M. The Effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Ferreira, J.D.; Martins, C.B.; Assunção, M.F.; Santos, L.M. Microalgae Biomass as an Alternative to Fossil Carbons. In Biomass, Bioproducts and Biofuels; CRC Press: Boca Raton, FL, USA, 2022; pp. 159–172. [Google Scholar] [CrossRef]
- Dębowski, M.; Dudek, M.; Nowicka, A.; Quattrocelli, P.; Kazimierowicz, J.; Zieliński, M. Suitability of Pre-Digested Dairy Effluent for Mixotrophic Cultivation of the Hydrogen-Producing Microalgae Tetraselmis Subcordiformis. Environ. Technol. 2022, 45, 471–482. [Google Scholar] [CrossRef]
- Montoya Montoya, L.M.; Pérez, A.A.A.; Giraldo Calderón, N.D.; Garcés, L.A. Analysis of Cell Growth, Photosynthetic Behavior and the Fatty Acid Profile in Tetraselmis Subcordiformis under Different Lighting Scenarios. J. Appl. Phycol. 2024, 36, 1679–1695. [Google Scholar] [CrossRef]
- Ran, W.; Xiang, Q.; Pan, Y.; Xie, T.; Zhang, Y.; Yao, C. Enhancing Photosynthetic Starch Production by γ-Aminobutyric Acid Addition in a Marine Green Microalga Tetraselmis Subcordiformis under Nitrogen Stress. Ind. Eng. Chem. Res. 2020, 59, 17103–17112. [Google Scholar] [CrossRef]
- Xiang, Q.; Wei, X.; Yang, Z.; Xie, T.; Zhang, Y.; Li, D.; Pan, X.; Liu, X.; Zhang, X.; Yao, C. Acclimation to a Broad Range of Nitrate Strength on a Euryhaline Marine Microalga Tetraselmis Subcordiformis for Photosynthetic Nitrate Removal and High-Quality Biomass Production. Sci. Total Environ. 2021, 781, 146687. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.I.; Lv, Y.; Xiong, W.; Ong, H.C.; Xu, J. Modern Developmental Aspects in the Field of Economical Harvesting and Biodiesel Production from Microalgae Biomass. Renew. Sustain. Energy Rev. 2021, 135, 110209. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Guo, H. Characterization of H2 Photoproduction by Marine Green Alga Tetraselmis Subcordiformis Integrated with an Alkaline Fuel Cell. Biotechnol. Lett. 2016, 38, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Nowicka, A.; Zieliński, M.; Kazimierowicz, J.; Dębowski, M. The Effect of Biomass Separation Method on the Efficiency of Hydrogen Production by Platymonas Subcordiformis. Int. J. Energy Environ. Eng. 2023, 14, 167–177. [Google Scholar] [CrossRef]
- Huang, X.; Wei, L.; Huang, Z.; Yan, J. Effect of High Ferric Ion Concentrations on Total Lipids and Lipid Characteristics of Tetraselmis Subcordiformis, Nannochloropsis Oculata and Pavlova Viridis. J. Appl. Phycol. 2014, 26, 105–114. [Google Scholar] [CrossRef]
- Feki, F.; Cherif, M.; Masmoudi, M.A.; Chamkha, M.; Saadaoui, I.; Das, P.; Sayadi, S. Methane Production Enhancement from Tetraselmis Biomass Co-Digestion Using Frying Oil Residue as Co-Substrate and Ultrasonication as Pretreatment. Environ. Technol. Innov. 2024, 33, 103478. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Betenbaugh, M.J.; Bouwer, E.J. The Effects of Alternative Pretreatment Strategies on Anaerobic Digestion and Methane Production from Different Algal Strains. Bioresour. Technol. 2014, 155, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Solana, M.; Riaño, B.; García-González, M.C.; Bertucco, A. Biofuels from Microalgae: Lipid Extraction and Methane Production from the Residual Biomass in a Biorefinery Approach. Bioresour. Technol. 2014, 170, 370–378. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Font-Segura, X.; Ferrer, A.S.; Barrena, R.; Rossi, S.; Valdez-Ortiz, A. Valorisation of Biodiesel Production Wastes: Anaerobic Digestion of Residual Tetraselmis Suecica Biomass and Co-Digestion with Glycerol. Waste Manag. Res. 2015, 33, 250–257. [Google Scholar] [CrossRef]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-Stage Photo-Biological Production of Hydrogen by Marine Green Alga Platymonas Subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Microwave Radiation Influence on Dairy Waste Anaerobic Digestion in a Multi-Section Hybrid Anaerobic Reactor (M-SHAR). Processes 2021, 9, 1772. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, F.; Sun, H.; Zhao, B. Effect of Culture Medium on Hydrogen Production by Sulfur-Deprived Marine Green Algae Platymonas Subcordiformis. Biotechnol. Bioprocess Eng. 2009, 14, 835–841. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Zieliński, M.; Kazimierowicz, J. The Influence of the Ultrasound Disintegration of Microalgal–Bacterial Granular Sludge on Anaerobic Digestion Efficiency. Appl. Sci. 2023, 13, 7387. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Nowicka, A.; Dudek, M.; Zieliński, M. The Use of Hydrodynamic Cavitation to Improve the Anaerobic Digestion of Waste from Dairy Cattle Farming—From Laboratory Tests to Large-Scale Agricultural Biogas Plants. Energies 2024, 17, 1409. [Google Scholar] [CrossRef]
- PN-EN 15935:2022-01; Soil, Waste, Treated Bio-Waste and Sewage Sludge—Determination of Losses on Ignition. Health, Environment and Medicine Sector. Technical Body of Soil Chemistry: Warsaw, Poland, 2022.
- Van Wychen, S.; Ramirez, K.; Laurens, L.M.L. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in Situ Transesterification: Laboratory Analytical Procedure (LAP); National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016. [CrossRef]
- ISO 3104; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. International Organization for Standardization, 2023. Available online: https://www.iso.org/obp/ui/#iso:std:iso:3104:ed-4:v1:en (accessed on 10 May 2024).
- ISO 3675; Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method. International Organization for Standardization, 1998. Available online: https://www.iso.org/standard/26326.html (accessed on 10 May 2024).
- ISO 15267; Animal and Vegetable Fats and Oils—Flashpoint Limit Test Using Pensky-Martens Closed Cup Flash Tester. International Organization for Standardization, 1998. Available online: https://www.iso.org/standard/27141.html (accessed on 10 May 2024).
- EN 12662; Liquid Petroleum Products—Determination of Total Contamination. European Standard, 2024. Available online: https://standards.iteh.ai/catalog/standards/cen/8dbff4b1-811d-426a-8197-f34e172273aa/en-12662-1-2024 (accessed on 10 May 2024).
- DIN 51900; Testing of Solid and Liquid Fuels—Determination of Gross Calorific Value by the Bomb Calorimeter and Calculation of Net Calorific Value. European Standard, 2023. Available online: https://store.accuristech.com/standards/din-51900?product_id=2578000 (accessed on 10 May 2024).
- ISO 10370; Petroleum Products—Determination of Carbon Residue—Micro Method. International Organization for Standardization, 2014. Available online: https://www.iso.org/standard/57081.html (accessed on 10 May 2024).
- EN 14112; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Oxidation Stability (Accelerated Oxidation Test). European Standard, 2021. Available online: https://standards.iteh.ai/catalog/standards/cen/aa4b70be-e467-4a60-83ef-c2543b937f19/en-14112-2020 (accessed on 10 May 2024).
- ISO 12937; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. International Organization for Standardization, 2000. Available online: https://www.iso.org/standard/2730.html (accessed on 10 May 2024).
- EN 14104; Fat and Oil Derivates—Fatty Acid Methyl Ester (FAME)—Determination of Acid Value. European Standard, 2021. Available online: https://standards.iteh.ai/catalog/standards/cen/7a67b9de-1b54-4a80-bd13-c5db21ec455f/en-14104-2021 (accessed on 10 May 2024).
- ISO 10540; Animal and Vegetable Fats and Oils—Determination of Phosphorus Content. International Organization for Standardization, 2002. Available online: https://www.iso.org/standard/33121.html (accessed on 10 May 2024).
- ISO 3987; Petroleum Products—Determination of Sulfated Ash in Lubricating Oils and Additives. International Organization for Standardization, 2010. Available online: https://www.iso.org/standard/44979.html (accessed on 10 May 2024).
- EN 14111; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Iodine Value. European Standard, 2022. Available online: https://standards.iteh.ai/catalog/standards/cen/515ecaab-fec3-49e4-9d98-c2d20a515dda/en-14111-2022 (accessed on 10 May 2024).
- Ji, C.F.; Legrand, J.; Pruvost, J.; Chen, Z.A.; Zhang, W. Characterization of Hydrogen Production by Platymonas Subcordiformis in Torus Photobioreactor. Int. J. Hydrogen Energy 2010, 35, 7200–7205. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Li, Y.; Wang, Y. Mixotrophic Cultivation of Platymonas Subcordiformis. J. Appl. Phycol. 2001, 13, 343–347. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. Applicability of Water from the Bay of Gdańsk as a Growth Medium for Mixotrophic Culture of Platymonas Subcordiformis. Front. Biosci.-Elit. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and Characterization of Microalgae with Potential for Nutrient Removal from Municipal Wastewater and Simultaneous Lipid Production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Heo, S.W.; Ryu, B.G.; Nam, K.; Kim, W.; Yang, J.W. Simultaneous Treatment of Food-Waste Recycling Wastewater and Cultivation of Tetraselmis Suecica for Biodiesel Production. Bioprocess Biosyst. Eng. 2015, 38, 1393–1398. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban Wastewater Treatment by Tetraselmis Sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Quattrocelli, P.; Nowicka, A. The cultivation of biohydrogen-producing tetraselmis subcordiformis microalgae as the third stage of dairy wastewater aerobic treatment system. Sustainability 2022, 14, 12085. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Anderson, A.; Anbarasu, A.; Pasupuleti, R.R.; Manigandan, S.; Praveenkumar, T.R.; Aravind Kumar, J. Treatment of Heavy Metals Containing Wastewater Using Biodegradable Adsorbents: A Review of Mechanism and Future Trends. Chemosphere 2022, 295, 133724. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Verma, P. Current Perspective on Wastewater Treatment Using Photobioreactor for Tetraselmis Sp.: An Emerging and Foreseeable Sustainable Approach. Environ. Sci. Pollut. Res. 2021, 1, 61905–61937. [Google Scholar] [CrossRef] [PubMed]
- Amit; Chandra, R.; Ghosh, U.K.; Nayak, J.K. Phycoremediation Potential of Marine Microalga Tetraselmis Indica on Secondary Treated Domestic Sewage for Nutrient Removal and Biodiesel Production. Environ. Sci. Pollut. Res. 2017, 24, 20868–20875. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Guo, H.; Yan, S.; Mu, J. Microalgae Cultivation Using an Aquaculture Wastewater as Growth Medium for Biomass and Biofuel Production. J. Environ. Sci. 2013, 25 (Suppl. S1), S85–S88. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.S. Effects of Water Culture Medium, Cultivation Systems and Growth Modes for Microalgae Cultivation: A Review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-Based Livestock Wastewater Treatment (MbWT) as a Circular Bioeconomy Approach: Enhancement of Biomass Productivity, Pollutant Removal and High-Value Compound Production. J. Environ. Manag. 2022, 308, 114612. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Qiu, S.; Li, M.; Yuan, W.; Ge, S. Granular Indigenous Microalgal-Bacterial Consortium for Wastewater Treatment: Establishment Strategy, Functional Microorganism, Nutrient Removal, and Influencing Factor. Bioresour. Technol. 2022, 353, 127130. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; Li, Q.; Wang, C.; Wu, K.; Wang, C.; Zhao, X.; Yin, F.; Liang, C.; Zhang, W. A Review of Biogas Slurry Treatment Technology Based on Microalgae Cultivation. Curr. Opin. Environ. Sci. Health 2022, 25, 100315. [Google Scholar] [CrossRef]
- Hu, R.; Cao, Y.; Chen, X.; Zhan, J.; Luo, G.; Hao Ngo, H.; Zhang, S. Progress on Microalgae Biomass Production from Wastewater Phycoremediation: Metabolic Mechanism, Response Behavior, Improvement Strategy and Principle. Chem. Eng. J. 2022, 137187. [Google Scholar] [CrossRef]
- Ge, S.; Qiu, S.; Tremblay, D.; Viner, K.; Champagne, P.; Jessop, P.G. Centrate Wastewater Treatment with Chlorella Vulgaris: Simultaneous Enhancement of Nutrient Removal, Biomass and Lipid Production. Chem. Eng. J. 2018, 342, 310–320. [Google Scholar] [CrossRef]
- Nagarajan, D.; Di Dong, C.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Biohydrogen Production from Microalgae—Major Bottlenecks and Future Research Perspectives. Biotechnol. J. 2021, 16, 2000124. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. The Effect of Autotrophic Cultivation of Platymonas Subcordiformis in Waters from the Natural Aquatic Reservoir on Hydrogen Yield. Resources 2022, 11, 31. [Google Scholar] [CrossRef]
- Ji, C.F.; Yu, X.J.; Chen, Z.A.; Xue, S.; Legrand, J.; Zhang, W. Effects of Nutrient Deprivation on Biochemical Compositions and Photo-Hydrogen Production of Tetraselmis Subcordiformis. Int. J. Hydrogen Energy 2011, 36, 5817–5821. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Guo, H. Effect of Light/Dark Regimens on Hydrogen Production by Tetraselmis Subcordiformis Coupled with an Alkaline Fuel Cell System. Appl. Biochem. Biotechnol. 2017, 183, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Torri, C.; Samorì, C.; Adamiano, A.; Fabbri, D.; Faraloni, C.; Torzillo, G. Preliminary Investigation on the Production of Fuels and Bio-Char from Chlamydomonas Reinhardtii Biomass Residue after Bio-Hydrogen Production. Bioresour. Technol. 2011, 102, 8707–8713. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Huang, X.; Huang, Z. Temperature Effects on Lipid Properties of Microalgae Tetraselmis Subcordiformis and Nannochloropsis Oculata as Biofuel Resources. Chinese J. Oceanol. Limnol. 2015, 33, 99–106. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Bray, S.; Mummacari, R.; Danh, L.T.; Foster, N. Assessment of Bio-Oil Extraction from Tetraselmis Chui Microalgae Comparing Supercritical CO2, Solvent Extraction, and Thermal Processing. Energy Fuels 2012, 26, 248–255. [Google Scholar] [CrossRef]



| Parameter | Unit | S1 | S2 | S3 |
|---|---|---|---|---|
| VS | [% FM] | 90.21 ± 3.4 | 88.13 ± 2.5 | 82.67 ± 3.3 |
| TS | [% FM] | 9.79 ± 3.4 | 11.87 ± 2.5 | 17.33 ± 3.3 |
| TN | [mg/gTS] | 49.22 ± 6.5 | 47.03 ± 5.9 | 43.9 ± 5.4 |
| TP | [mg/gTS] | 9.14 ± 1.3 | 9.02 ± 1.2 | 8.02 ± 1.1 |
| TC | [mg/gTS] | 512.3 ± 22.7 | 488.1 ± 23.5 | 451.6 ± 21.4 |
| TOC | [mg/gTS] | 459.8 ± 22.7 | 421.8 ± 23.5 | 372.2 ± 21.4 |
| Protein | [% TS] | 29.37 ± 3.5 | 31.2 ± 2.9 | 30.7 ± 3.3 |
| Lipids | [% TS] | 9.91 ± 1.6 | 8.41 ± 1.1 | 0.00 |
| Saccharides | [% TS] | 37.56 ± 5.2 | 23.5 ± 4.1 | 21.91 ± 3.5 |
| Fatty Acid | Content % m/m |
|---|---|
| C8:0 | 0.08 ± 0.02 |
| C10:0 | 0.04 ± 0.01 |
| C11:0 | 0.02 ± 0.01 |
| C12:0 | 0.07 ± 0.01 |
| C14:0 | 0.03 ± 0.02 |
| C15:0 | 0.02 ± 0.01 |
| C16:0 | 4.77 ± 0.12 |
| C16:1 (cis-9) | 0.09 ± 0.02 |
| C17:0 | 0.07 ± 0.01 |
| C17:1 (cis-10) | 0.05 ± 0.01 |
| C18:0 | 4.33 ± 0.12 |
| C18:1 (trans-9) | 0.07 ± 0.02 |
| C18:1 (cis-9) | 27.09 ± 2.36 |
| C18:2 (all-cis-9,12) | 13.08 ± 1.17 |
| C18:3 (all-cis-6,9,12) | 0.33 ± 0.07 |
| C18:3 (all-cis-9,12,15) | 48.63 ± 3.44 |
| C20:0 | 0.17 ± 0.05 |
| C20:1 (cis-11) | 0.15 ± 0.02 |
| C20:2 (all-cis-11,14) | 0.02 ± 0.01 |
| C22:0 | 0.17 ± 0.05 |
| C22:1 (cis-13) | 0.12 ± 0.02 |
| C20:4 (all-cis-5,8,11,14) | 0.13 ± 0.03 |
| C22:2 (all-cis-13,16) | 0.13 ± 0.06 |
| C24:0 | 0.21 ± 0.07 |
| Parameter | Unit | Value |
|---|---|---|
| Density at 15 °C | kg/m3 | 823 ± 22.6 |
| Viscosity at 40 °C | Mm2/s | 3.7 ± 0.9 |
| Flash point | °C | 126 ± 6.2 |
| Carbon residue (on 10% distillation residue) | % (m/m) | 0.3 ± 0.1 |
| Total contamination | mg/kg | 2.8 ± 0.4 |
| Oxidation stability at 110 °C | Hours | 13.0 ± 1.9 |
| Calorific value | MJ/kg | 40.2 ± 4.2 |
| Acid value | mg·KOH/g | 0.2 ± 0.1 |
| Iodine value | mg·KOH/g | 53.8 ± 3.4 |
| Water content | mg/kg | 68.4 ± 4.3 |
| Sulphur content | mg/kg | 2.6 ± 0.5 |
| Phosphorus content | mg/kg | 3.1 ± 0.5 |
| Technological Protocol Used | Energy Carrier | Production Efficiency m3/MgTS | Energy Value kWh/m3 | Energy Value kWh/MgTS | Total Energy Value kWh/MgTS |
|---|---|---|---|---|---|
| S1, S2 | Hydrogen | 77.2 | 3.0 | 231.6 | 231.6 |
| S1, S2, S3 | Hydrogen | 77.2 | 3.0 | 231.6 | 232.9 |
| Bio-oil | 0.12 | 11.2 | 1.3 | ||
| S1, S2, S3, S4 | Hydrogen | 77.2 | 3.0 | 231.6 | 1415.9 |
| Bio-oil | 0.12 | 11.2 | 1.3 | ||
| Methane | 129 | 9.17 | 1182.9 | ||
| S1, S2, S6 | Hydrogen | 77.2 | 3.0 | 231.6 | 1891.4 |
| Methane | 181 | 9.17 | 1659.8 | ||
| S1, S5 | Biomethane | 193 | 9.17 | 1770 | 1769.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Dudek, M.; Kazimierowicz, J.; Quattrocelli, P.; Rusanowska, P.; Barczak, Ł.; Nowicka, A.; Zieliński, M. Analysis of Multi-Biofuel Production during Cultivation of the Green Microalga Tetraselmis subscordiformis. Energies 2024, 17, 3670. https://doi.org/10.3390/en17153670
Dębowski M, Dudek M, Kazimierowicz J, Quattrocelli P, Rusanowska P, Barczak Ł, Nowicka A, Zieliński M. Analysis of Multi-Biofuel Production during Cultivation of the Green Microalga Tetraselmis subscordiformis. Energies. 2024; 17(15):3670. https://doi.org/10.3390/en17153670
Chicago/Turabian StyleDębowski, Marcin, Magda Dudek, Joanna Kazimierowicz, Piera Quattrocelli, Paulina Rusanowska, Łukasz Barczak, Anna Nowicka, and Marcin Zieliński. 2024. "Analysis of Multi-Biofuel Production during Cultivation of the Green Microalga Tetraselmis subscordiformis" Energies 17, no. 15: 3670. https://doi.org/10.3390/en17153670
APA StyleDębowski, M., Dudek, M., Kazimierowicz, J., Quattrocelli, P., Rusanowska, P., Barczak, Ł., Nowicka, A., & Zieliński, M. (2024). Analysis of Multi-Biofuel Production during Cultivation of the Green Microalga Tetraselmis subscordiformis. Energies, 17(15), 3670. https://doi.org/10.3390/en17153670










