1. Introduction
In the pursuit of sustainable and renewable energy sources, biomass has emerged as a promising resource. However, efficient biomass conversion into proper energy forms, as a by-product for pellets as a fuel to produce electric power [
1], requires a profound understanding of its behavior across multiple scales, from particles to reactor systems. Biomass, derived from organic matter such as agricultural residues, forest residues, and dedicated energy crops, has gained increasing attention as a sustainable and renewable energy source. Its conversion into valuable bioenergy and biofuels offers a promising solution to meet the growing energy demands while reducing greenhouse gas emissions. To unlock the full potential of biomass as a renewable resource, a comprehensive understanding of its conversion behavior is crucial. This requires a detailed investigation that encompasses the entire spectrum, from particle-level phenomena to reactor-scale operations [
2].
To understand transport phenomena at a particle level, thermogravimetric analysis (TGA) is the most useful method. This is a thermo-analytical method widely implemented to investigate and compare thermal degradation events and kinetics during the conversion of solid materials, such as coal and biomass [
3]. The mass reduction is measured, under controlled conditions, while the thermal process is taking place, as the temperature increases. It is known that the composition, structure, and properties of biomass particles significantly influence the conversion process. Consequently, a comprehensive experimental exploration of particle behavior elucidates critical insights into the kinetics of the reaction. This knowledge is indispensable for designing effective particle heating strategies, optimizing reactions, and enhancing overall conversion efficiency. A good example of the TGA potential in the study of biomass conversion was presented by Lelis et al. [
4]. Their study explored the impact of various conditions (heating rate, particle size, flow rate, and atmosphere) on the thermal behavior and kinetics of pine wood particles. The authors verified a more noticeable effect of the heating rate when compared to the effect of the air flow rate on biomass conversion and its kinetics. Furthermore, the atmosphere effect on the thermal decomposition behavior was evident, and the presence of O
2 changes the mass-loss curve mainly at high temperatures.
While understanding particle phenomena is fundamental, the true impact of biomass conversion is observed at the reactor scale. An inclusive experimental study spanning this scope allows us to evaluate how particle-level dynamics translate into macroscopic reactor performance. This type of evaluation is possible using macro-TGA since it is similar to TGA but uses larger particles and weights. Within this subject, Samuelsson et al. [
5] investigated mass-loss rates for wood chips under isothermal pyrolysis conditions, comparing their findings with low-heating-rate powder data, highlighting valuable insights into the pyrolysis behavior of biomass. Gauthier et al. [
6] presented novel experimental developments for studying the pyrolysis of centimeter-scale wood particles, offering new insights into the pyrolysis process at a larger scale and contributing to a deeper understanding of wood biomass conversion. Bruch et al. [
7] developed a comprehensive model for wood combustion under fixed bed conditions, providing a valuable tool for predicting and optimizing combustion processes and offering practical implications for biomass energy utilization. The numerical approach used macro-kinetic data to provide deeper insights into the processes within a packed bed during the combustion of solid fuel. The results showed a good fit to experimental and theoretical results. Becidan et al. [
8] conducted an experimental study on the pyrolysis of thermally thick biomass residue samples, analyzing intra-sample temperature distribution and the scaling effect of sample weight, which highlighted important factors influencing biomass pyrolysis behavior.
These studies were developed considering an inert atmosphere and by performing experiments in an oxidative atmosphere. The research was essential in providing additional insights into biomass conversion processes, which is especially relevant for industrial applications where combustion and gasification occur in the presence of oxygen. In this field, in the literature, there are some relevant studies. For instance, Hu et al. [
9] investigated gaseous production kinetics and solid structure analysis during the isothermal conversion of biomass pellets under different atmospheres. The study provided insights into biomass conversion processes and their dependence on environmental conditions. The results revealed that CO
2 formation was largely promoted when using the O
2 atmosphere. Baumgarten et al. [
10] studied the kinetics of wood devolatilization during start-up, offering valuable insights into the thermal decomposition of wood and its implications for combustion processes. The author also highlighted the importance of the availability and valid kinetic data for combustion models, and this was the motivation of the work. Nikku et al. [
11] characterized the reactivity of municipal and industrial solid wastes and biomass using a vertical tube reactor and by also developing a TGA. This work aimed to obtain information to contribute to the understanding of combustion behavior and its potential applications in energy conversion. One relevant observation was the reduction in the char reactivity when the oxygen concentration was reduced, while the volatile reactivity was reduced for some materials and increased for others. Weissinger [
12] conducted in situ Fourier-transform infrared spectroscopic investigations of species from biomass fuels in a laboratory-scale combustor, elucidating the release of nitrogenous species and the major carbon species (CO, CO
2, and CH
4) during biomass combustion. The author observed that the propagation velocity of the reaction front was the main parameter and the most suitable to describe the overall conversion of nitrogen. Furthermore, NH
3 was the major nitrogenous species released, while significantly lower levels of NO and almost negligible amounts of HCN were measured. Orang and Tran [
13] explored the effect of feedstock moisture content on biomass boiler operation, providing insights into the influence of moisture levels on combustion efficiency and emissions. Brunner et al. [
14] conducted advanced biomass fuel characterization based on tests with a specially designed lab-scale reactor, offering valuable data for optimizing biomass utilization and energy conversion processes.
Hence, considering this background, the comprehensive experimental study of biomass conversion behavior, spanning from particle phenomena to reactor scale, encapsulates the essence of knowledge needed to unlock the full potential of biomass as a renewable energy source. Thus, the main objective of this work is to present a comprehensive experimental study focusing on biomass conversion behavior, covering both particle-level phenomena and laboratory-scale operations. Through a systematic and in-depth examination, this study aims to contribute to the fundamental understanding of biomass conversion kinetics, reaction pathways, and gaseous emissions. Although some investigations have been published in this area, we observed a need for studies in biomass combustion involving thermal conversion analysis, kinetics, and gaseous emissions. Until now, few works have succeeded in analyzing these three issues from micro to macro scales. In that sense, this work addresses the biomass conversion process in a simplified manner, but it represents the operation of industrial grate-fired boilers and properly characterizes the biomass decomposition and release of volatiles.
2. Experimental Methodology
To fairly compare the combustion behaviors of various types of solid biomass samples, experiments need to be carried out under the same conditions. For this purpose, a purpose-built macro thermogravimetric reactor and a thermogravimetric analyzer were used.
Section 2.1,
Section 2.2 and
Section 2.3 present detailed information about the samples, features, and operation procedures of the experiments.
2.1. Biomass Feedstock
The woodchips necessary for the experiments were prepared from large eucalyptus, acacia, pine, and olive trunks using a knife chipper. Pine, here referred to as
Pinus pinaster, along with eucalyptus (
Eucalyptus globulus), is the main Portuguese forest species.
Acacia—
Acacia dealbata—is an invasive species, and an interesting alternative fuel since wildfires and climatic changes have contributed to reducing the pine and eucalyptus forest area [
15]. Olive is also an interesting alternative feedstock due to the high olive production in Portugal. Consequently, olive tree pruning should be a very interesting alternative for power generation.
Thus, considering these four different biomass fuels and the use of a knife chipper machine, larger particles were obtained, with dimensions similar to the ones used in biomass power plants, ranging from a few millimeters up to hundreds of millimeters. The particles were initially spread out in a room for air-drying to reduce moisture content. Subsequently, they underwent sieving to assess particle-size distribution using horizontal screening according to standard EN 15149-1:2010 Part 2, utilizing sieves with square hole apertures of 3.15, 8, 16, and 50 mm. The most representative particle-size class fell between 8 and 16 mm, consistent with analysis from a biomass power plant [
1]. Particles within this size range were then collected and utilized for the experimental program. Following air-drying for approximately one month, the moisture content of the particles ranged between 10 and 15% (dry basis).
In addition to the particle-size determination, the woodchips were assessed in terms of their dimension and bulk density. Being of irregular shape, the equivalent diameter of a spherical particle was determined for the most representative particle-size range (8 to 16 mm), as 16.78 mm. In its turn, the bulk density ranges from 185 to 253 kg/m3.
The raw materials previously milled were also used to perform the TGA experiments. After the milling process, the samples were sieved in a vibratory sieve shaker to collect only particles with a size between 0.125 and 0.250 mm.
Regarding the elemental and proximate composition of the particles, this was also evaluated considering the standards for solid fuel characterization (CEN/TS 15414:2006 and CEN/TS 15104:2005, respectively), and the results are presented in
Table 1. The heating value of the different fuels was also determined using LECO CS-200 equipment, and the data were added to
Table 1. It is important to highlight that fuel properties are dependent on the collection sites and storage methods.
2.2. Apparatus: Macro- and Micro-Thermogravimetric Equipment
As mentioned before, two different types of equipment were considered to develop the experiments. One of them is the micro-thermogravimetric analyzer, which consists of common thermogravimetric equipment from the TA Instruments brand, model SDT 2960. The other equipment, the macro-TGA, consists of a purpose-built reactor for a scale relevant to industrial applications, developed to study the combustion of large particles and determine the mass loss and gas compounds released during the devolatilization. The reactor can replicate the behavior of a fuel portion moving along the grate from an industrial biomass boiler and, thereby, passing through the different reaction zones (drying, devolatilization, and char combustion). The reactor is 200 mm in diameter and 350 mm in height; the walls are made of refractory material to reduce heat losses and contain a 2 kW electrical heater. In the upper part of the reactor, there is a rotating lid with a rip of 10 mm to allow the support of the sample holder, which is connected to a scale. Hence, the mass loss during the experiment is measured over time. The macro-TGA reactor has been used in other studies such as in [
16]; however, in the present study, instead of a portable gas analyzer to measure the main gas compounds, a sample bag to collect the flue gas was used for subsequent analysis in a micro-gas chromatograph (GC).
Figure 1 schematically represents the experimental macro-thermogravimetric test facility used in this work.
2.3. Experimental Procedure
The TGA experiments were performed in an oxidative atmosphere with a non-isothermal heating with an in situ drying heating program defined for the thermal decomposition analysis. Non-isothermal experiments are generally adopted for the determination of kinetic parameters as they are considered more reliable and less time-consuming than isothermal analysis [
17]. After the drying stage, low heating rates were defined to avoid transport effects, ensure that the reaction was only temperature dependent, and, consequently, the experiments were performed in a pure kinetic regime.
Table 2 summarizes the operating conditions defined to perform the thermogravimetric experiments.
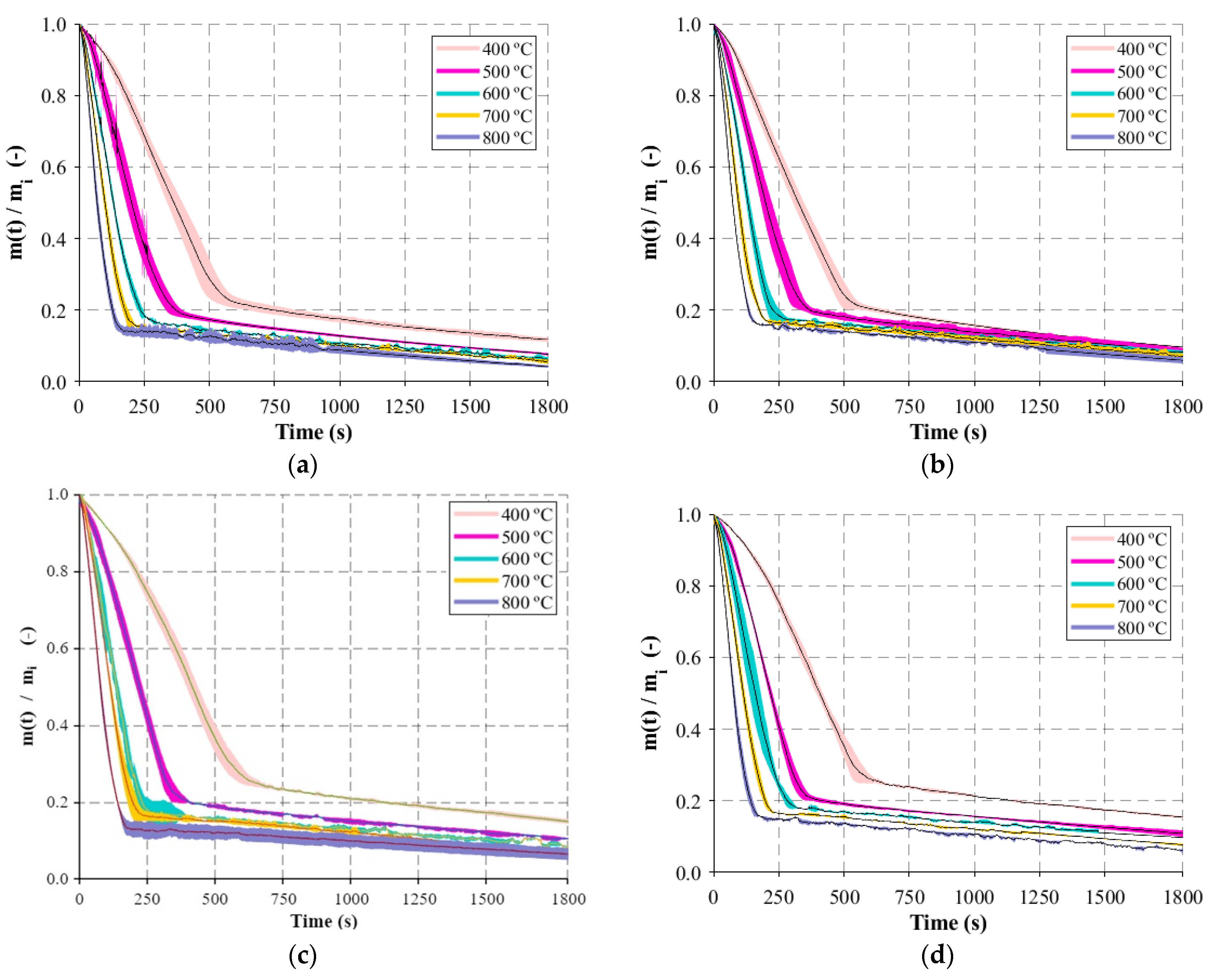
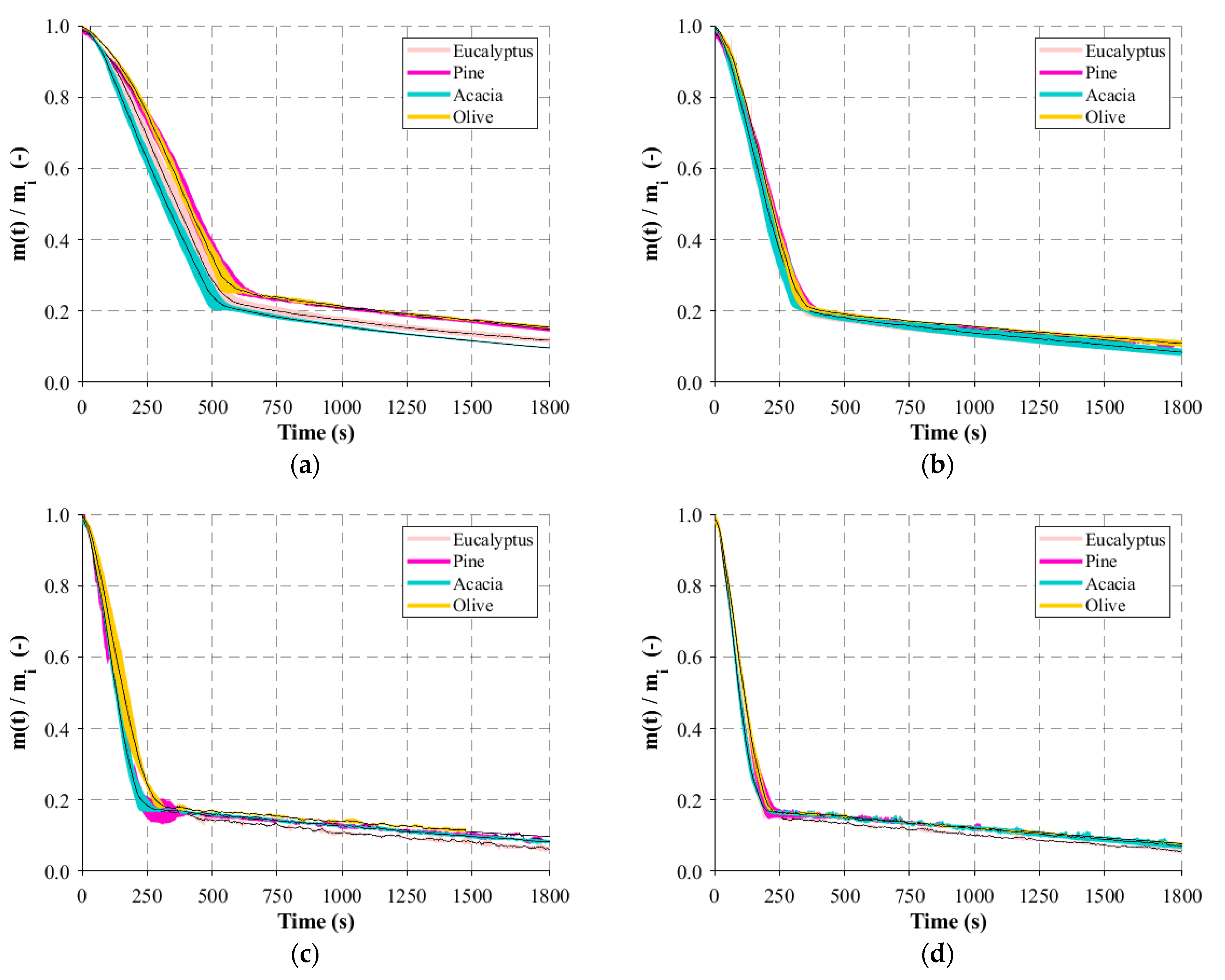
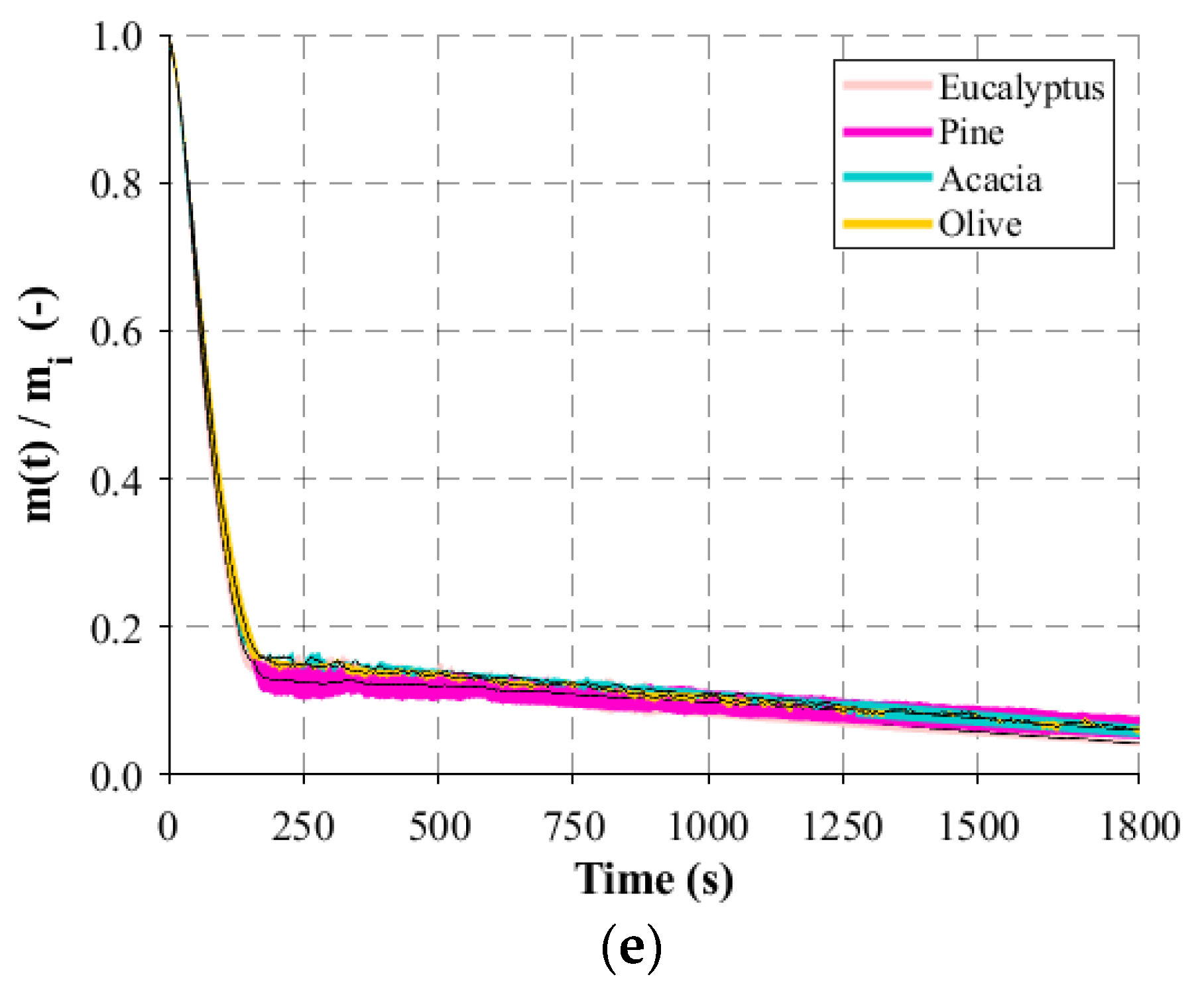
Regarding the macro-TGA, isothermal experiments were developed and, before the experimental campaign, calibration and verification procedures were carried out. First, separate blank tests were performed to evaluate the internal heat transfer and to obtain the background of the weight signal before the experiments. Three thermocouples (K type) were introduced in the reactor to record the internal temperature at different positions and to assess temperature gradients in the center of the reactor. This was important to understand which temperature should be defined in the temperature controller in order to obtain the corresponding desired value for each experiment. The runs to evaluate the weight signal were also important since there were disturbances detected due to the interference of the steel wire that connected the basket and scale. After some modifications in the lid and on both connections of the wire, basket, and scale, the gradient of the weight was minimal. Hence, the weight of the basket was appropriately tared, the basket became stable, and deviations below 8 mg were found during long experiments. Consequently, with this set of blank experiments, it was proved that the small-scale reactor had acceptable stability and reproducibility. Additionally, a set of experiments were concerned with the samples. The initial conditions are important and should be maintained in each experiment. Neglecting possible variations in the chemical composition of the same batch of particles, the moisture content and the dimension of the particles were considered parameters of particular interest, and their influence should be controlled or quantified. Experiments with random particles and particles from the 16 to 50 mm batch after the sieving process were burnt, and the mass-loss variation presented slight differences. Since the woodchips present an irregular structure, particles with fixed dimensions were produced to investigate the dimension that influences the mass-loss behavior. From the experiments performed, it was possible to observe that thickness is the key dimension. Although the woodchips present different lengths and/or widths, the thickness is approximately the same, and this is the reason for the similar profiles of mass loss. Regarding the moisture content, the strategy was to control and obtain similar values for all particles after the experiments. As the moisture content of the particles used in power plants is generally higher, the maximum value that can be replicated in the laboratory, 20% of moisture, was defined as the setpoint. In this way, the influence of moisture on the mass-loss behavior was avoided. Experiments considering the influence of the air flow rate were also considered, but, due to the limitations of the apparatus to collect the flue gases, the air flow rate needs to be as low as possible to ensure that only volatiles are captured.
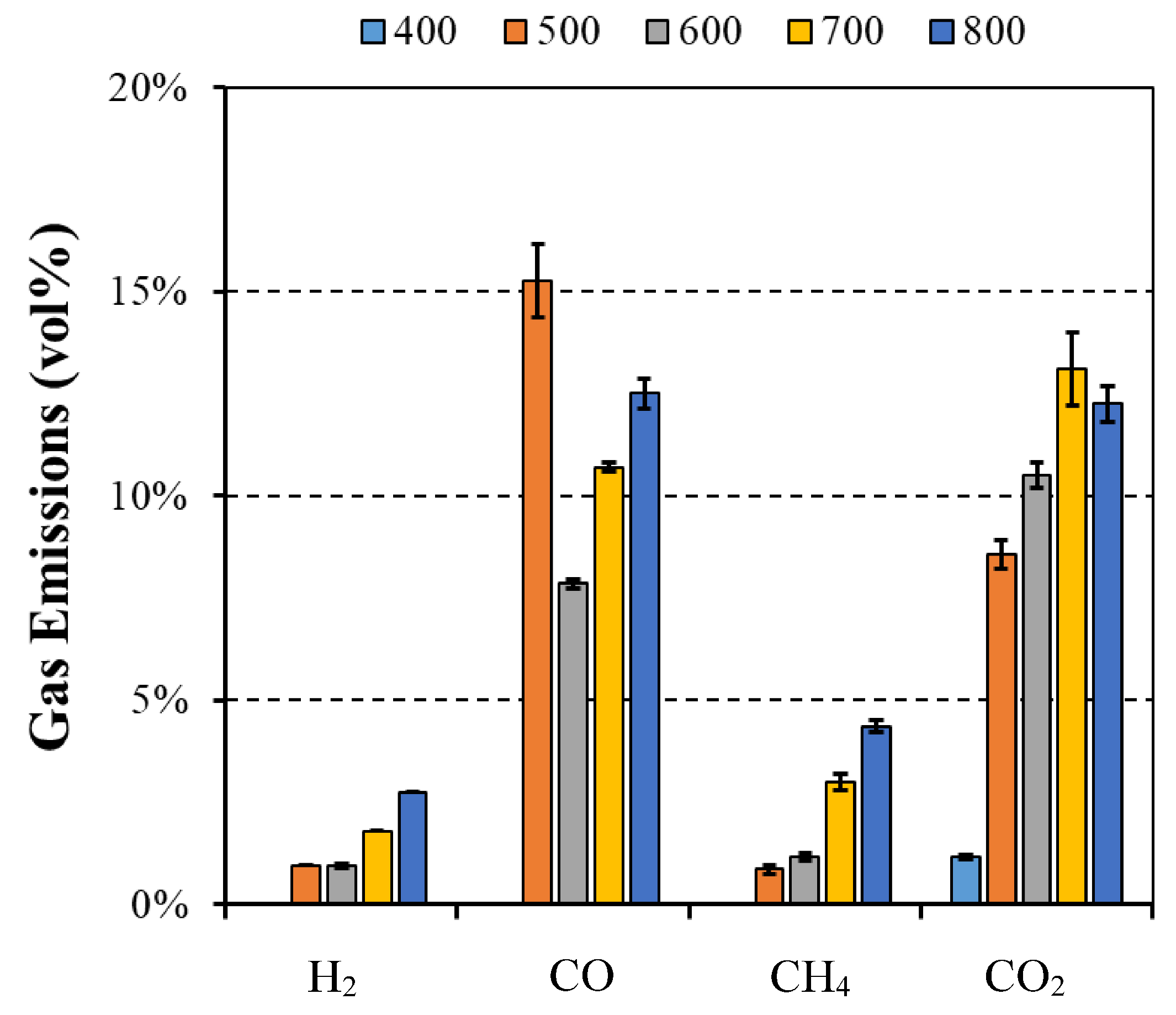
Hence, the air flow rate was set at 0.1 L/min, the initial weight at 20 g, the maximum possible weight to produce the higher flue gas amount, and the reactor temperature varied between 400 and 800 °C (the temperature range present in a typical biomass grate-fired boiler). All experiments were run in triplicate, under identical conditions, and the average values were computed and reported as well as the standard deviation. During the experiments with the eucalyptus particles, the gases, which included end-product gases such as H2, CH4, CO, and CO2, released during the devolatilization period were collected in a sample bag. In order to be able to collect the sample during the entire devolatilization period, the flow rate of the sampling pump was adjusted to fill the bag within the time frame of the experiment. Then, the composition of the samples was analyzed in a micro-GC where three samples were removed from the bag and inserted into the equipment to detect the concentration of the different compounds.
4. Conclusions
This paper presents an assessment of the thermal behavior of different solid biomass fuels under an oxidizing atmosphere and in sub-stoichiometric conditions.
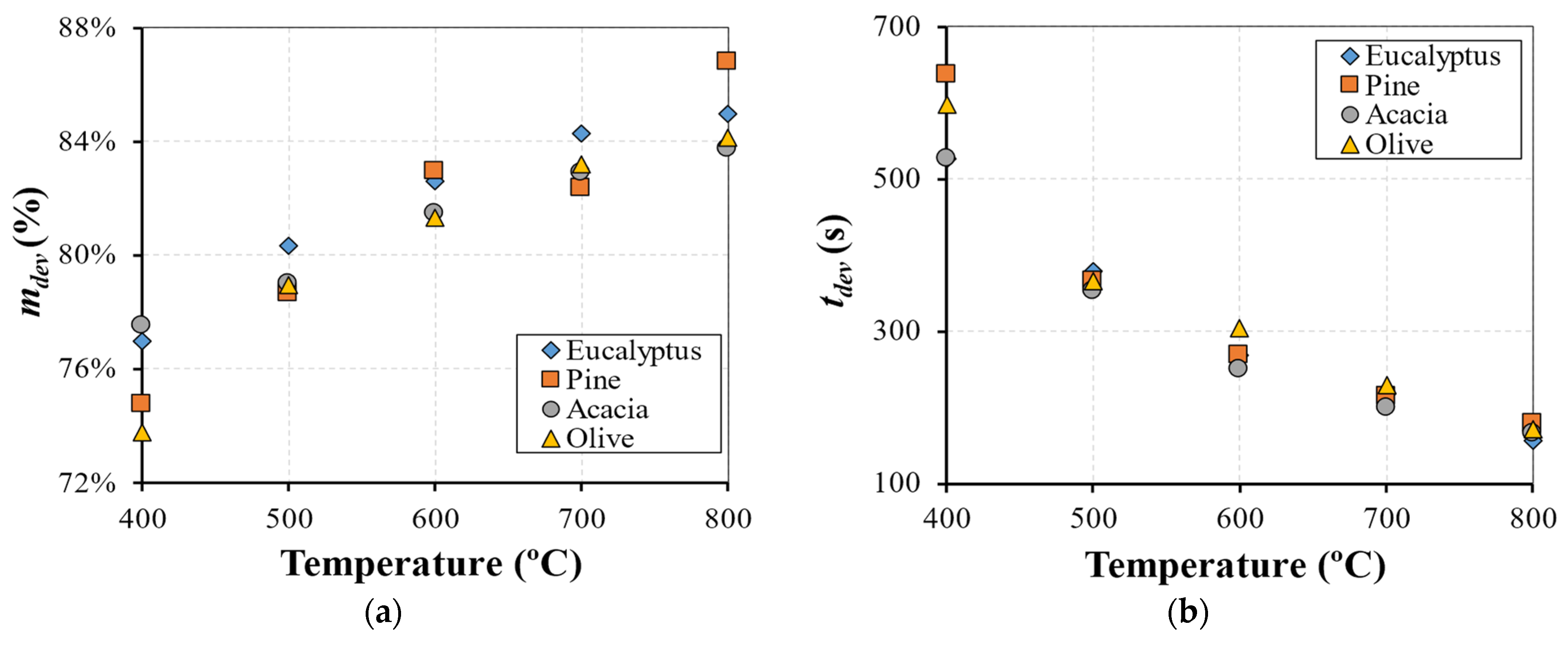
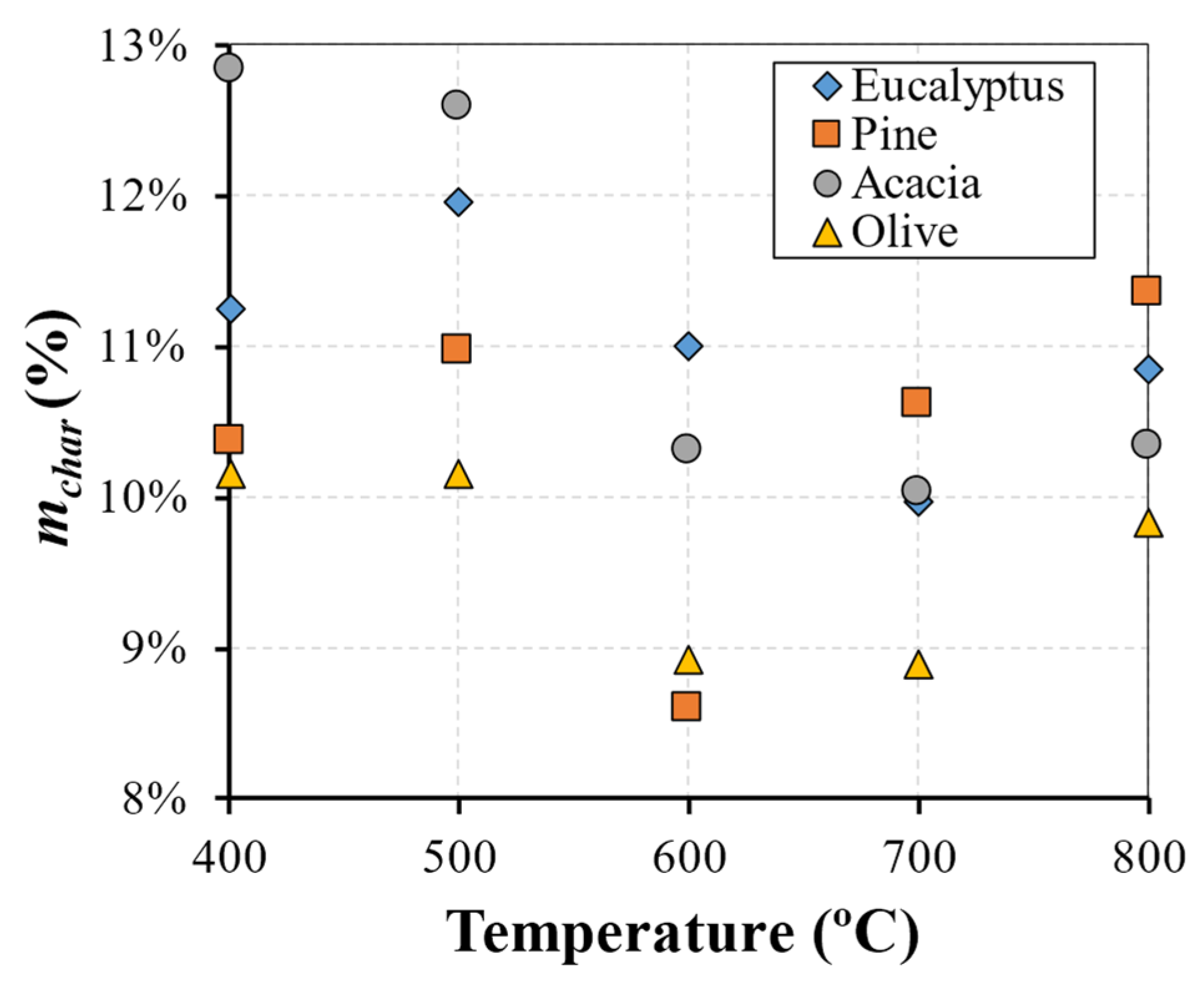
The main results and key findings have led to the conclusion that isothermal experiments showed two distinct stages that can be identified for mass loss, each one with a linear trend. This pattern of the combustion behavior is significantly different from the TGA results and revealed that, in the combustion of large particles, the presence of consecutive conversion stages cannot be clearly identified. The first stage, which includes drying and devolatilization, presented a dependency on the reactor temperature, while the last stage was not influenced by the temperature. This fact highlighted the influence of the diffusion mechanism, thereby combining the kinetic phenomenon and transport processes in the combustion of large biomass particles. The influence of fuel type on the combustion behavior was only detected at lower temperatures, while at higher temperatures, the profiles are almost coincident. This fact shows that physical, thermal, and chemical properties are only relevant at lower reactor temperatures, while at higher temperatures, the combustion behavior is only dependent on the reaction kinetics. Considering the previous findings, the gas compounds released during the combustion of eucalyptus presented a strong correlation to the reactor temperature, with CO2 and CO always being the main devolatilization products.
Despite being conducted at a laboratory scale, the experiments offered valuable insights into the transport phenomena occurring at both the particle and particle levels under various operating conditions and with different fuels. Furthermore, these experiments enabled the quantification of gas emissions, making them a crucial undertaking that yielded the reliable and significant data necessary for the development of a numerical model for biomass combustion systems. Thus, a crucial future research development would be the integration of the experimental data into a particle conversion model for coupled CFD simulations, which would allow more details to be obtained about the bed conversion in the biomass boiler and improve the gas-phase combustion inside the furnace.