A Study on the Efficient Degradation of Sulfur Hexafluoride by Pulsed Dielectric Barrier Discharge Synergistic Active Gas
Abstract
1. Introduction
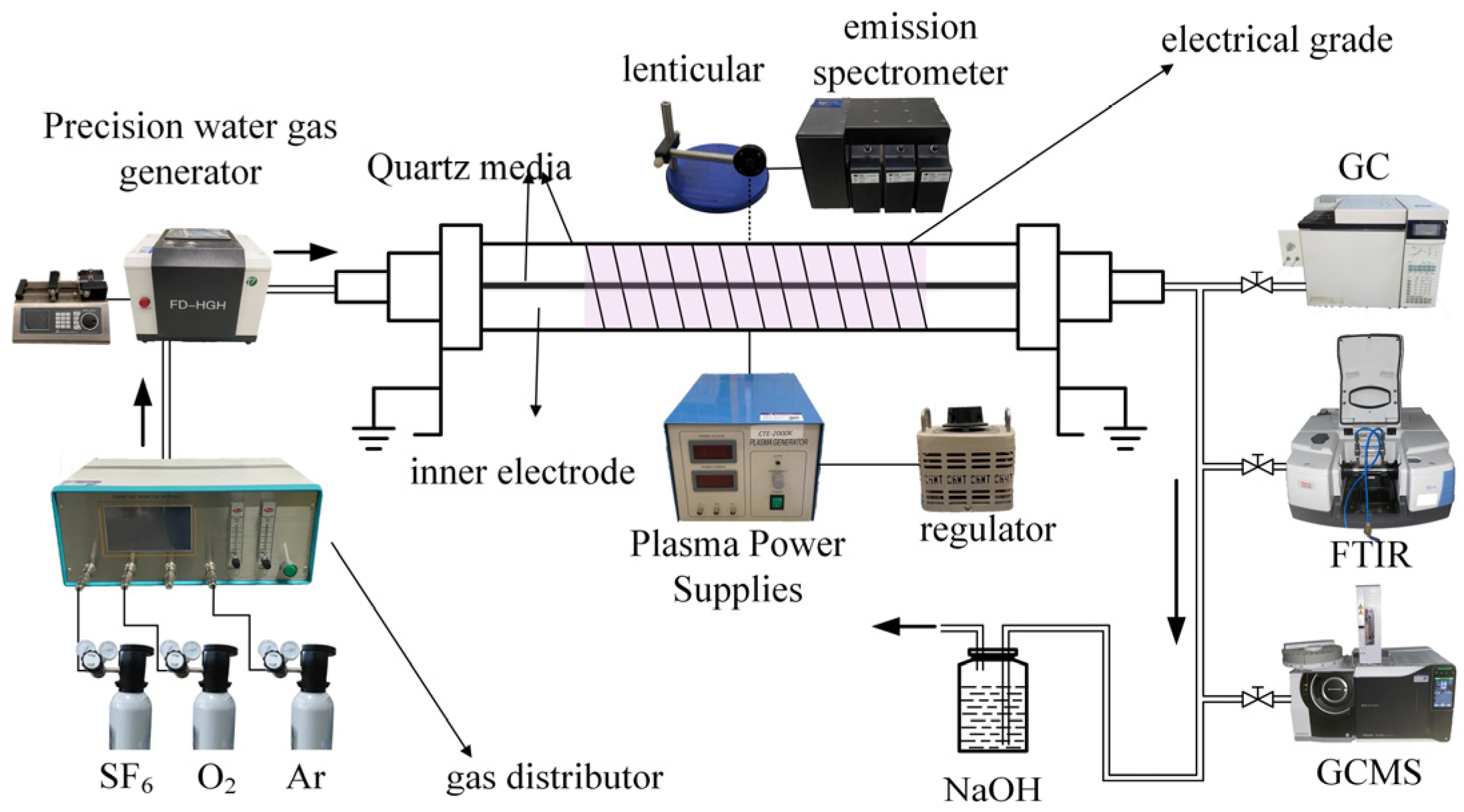
2. Experimental Platforms
3. Results
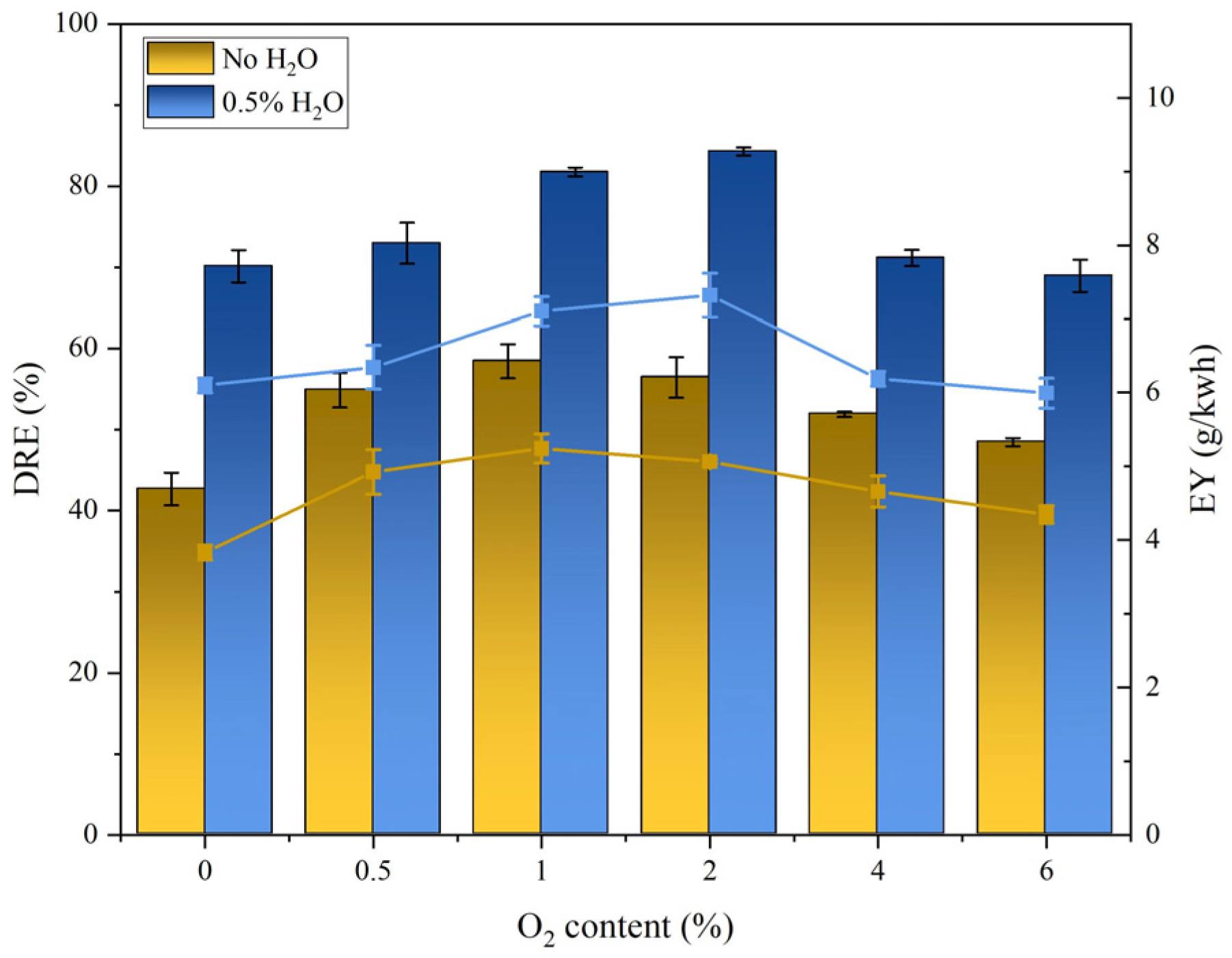
3.1. DER and EY
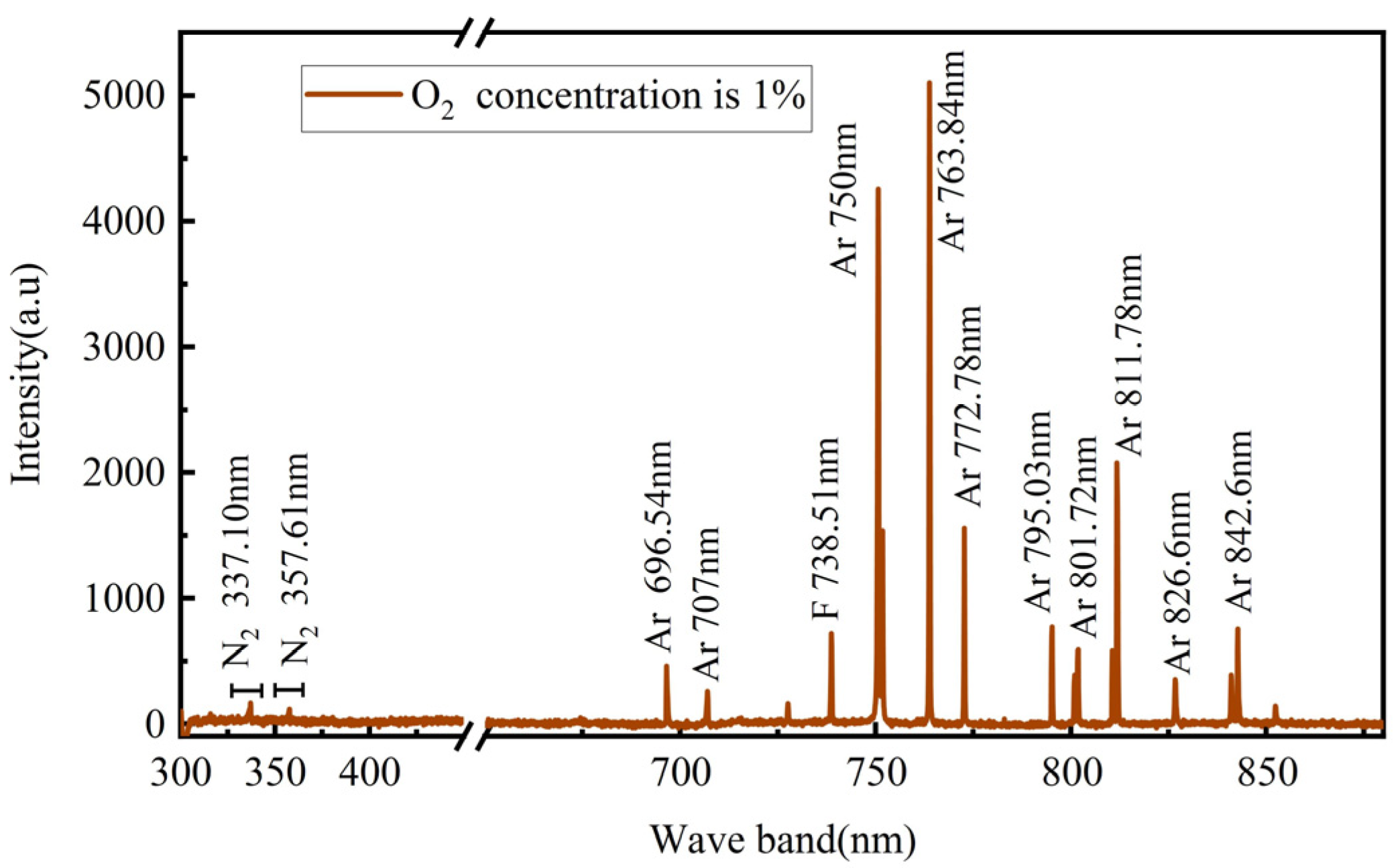
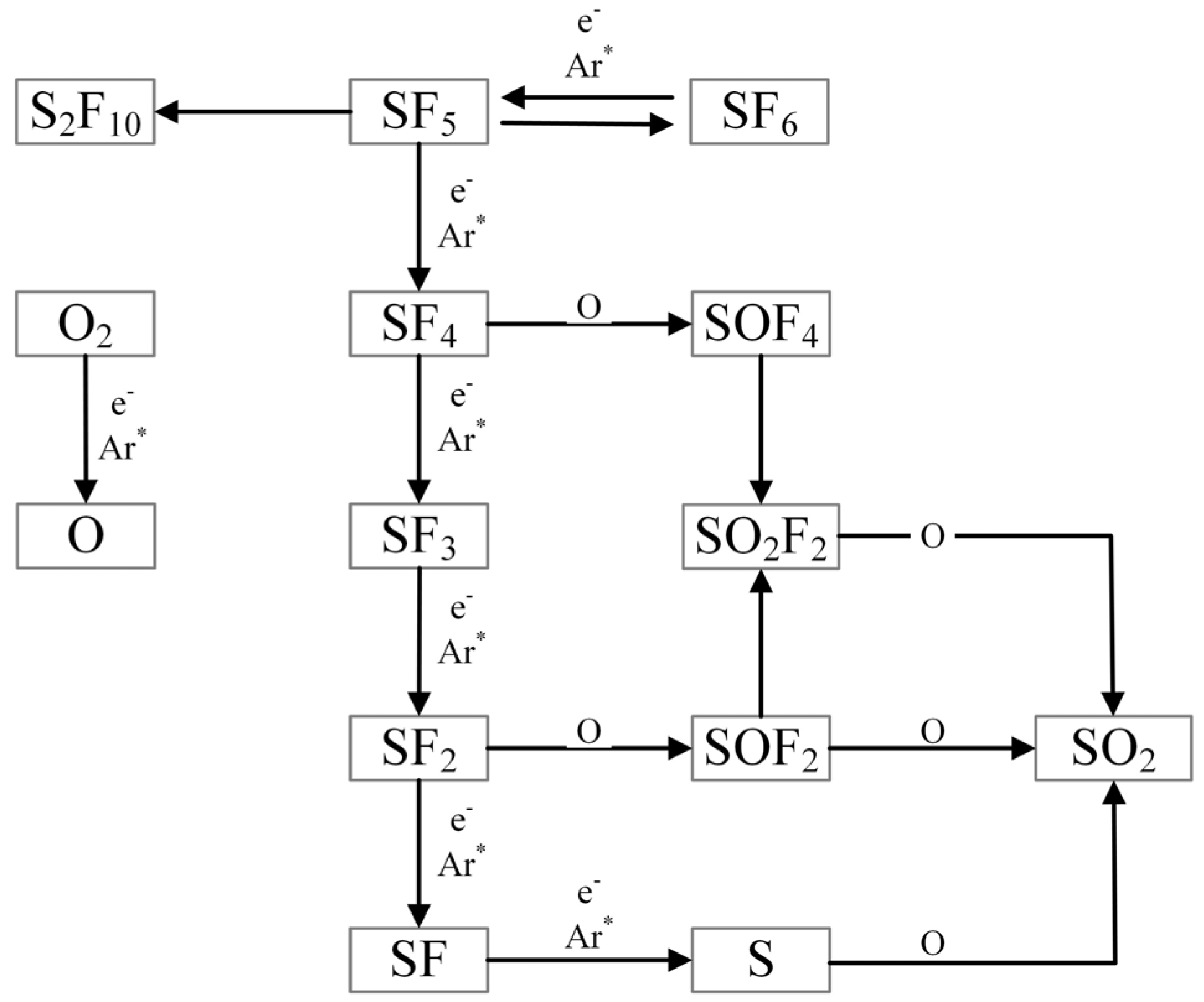
3.2. Active Particle Analysis
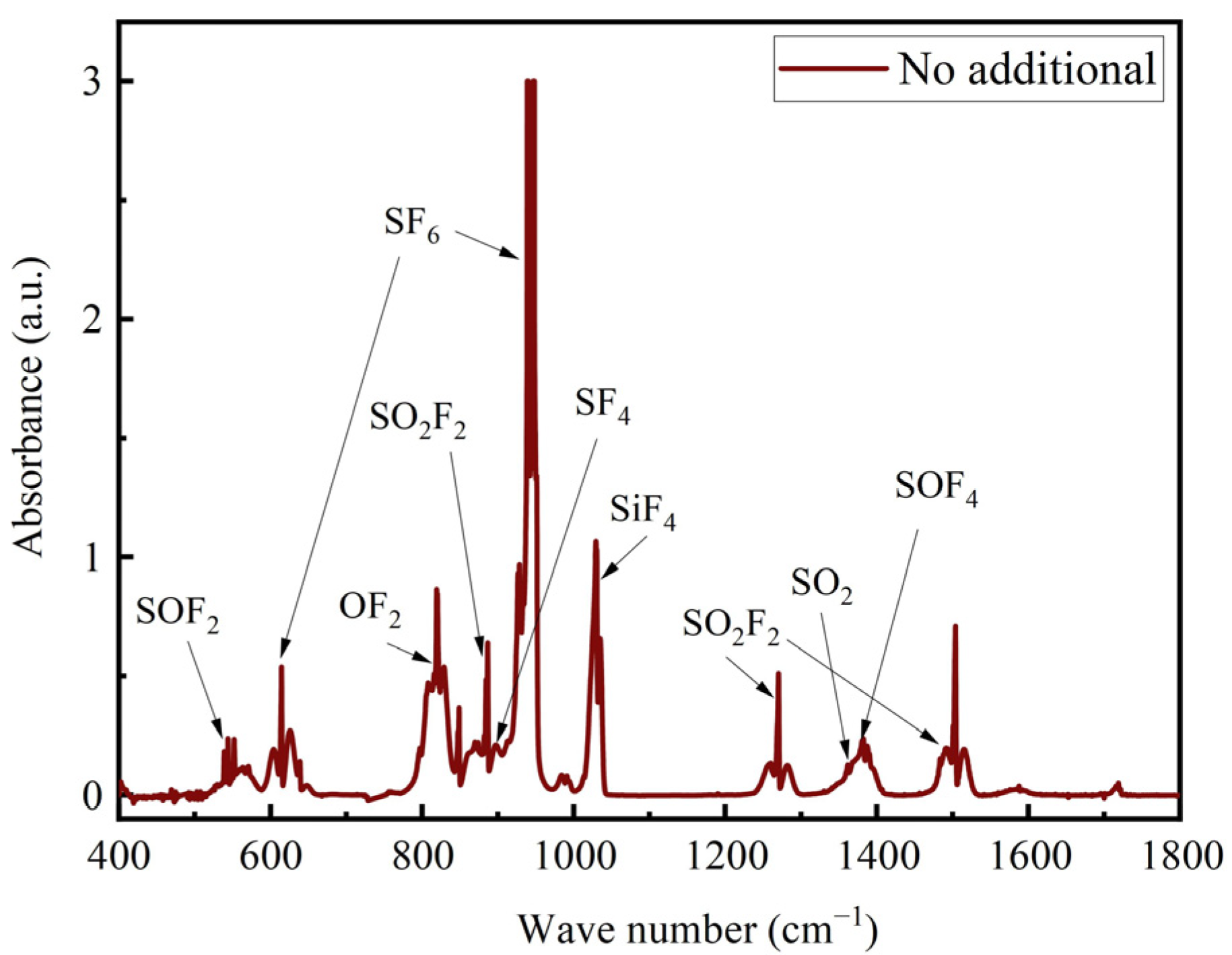
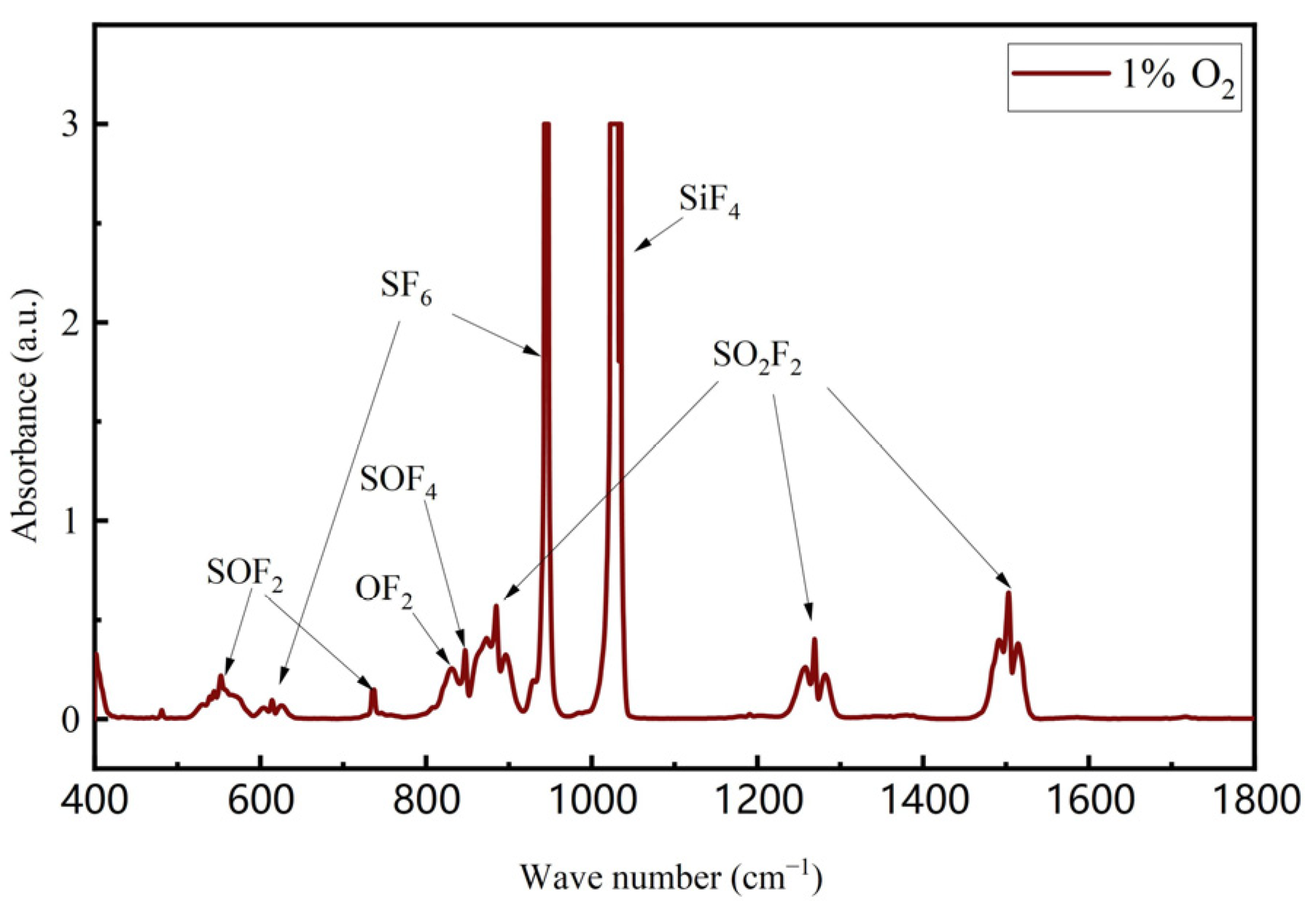
3.3. Product Analysis
3.4. Comparison of Degradation Methods and Harmless Treatment of SF6 Degradation Products
3.4.1. Comparison of Degradation Methods
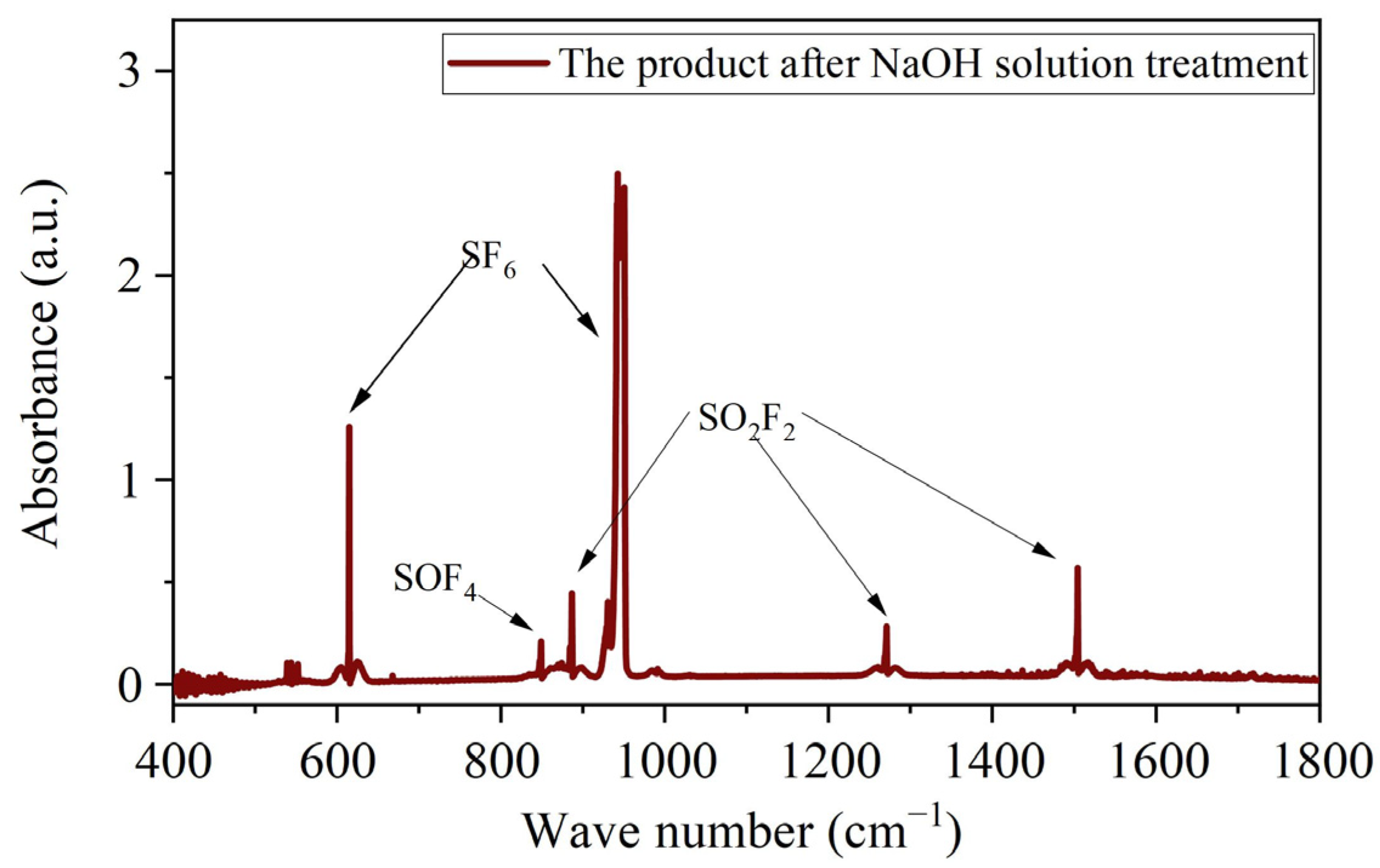
3.4.2. Harmless Treatment of SF6 Degradation Products
4. Conclusions
- The addition of O2 could effectively promote the degradation of SF6. With the increase in the addition of the O2 content, the DRE and EY of SF6 first increased and then decreased. The highest DRE and EY were obtained in the reaction system with 2% SF6 at the input power of 45 W and the gas flow rate of 50 mL/min and in the reaction system with the O2 content of 1%, which were 58.40% and 5.24 g/kWh, respectively.
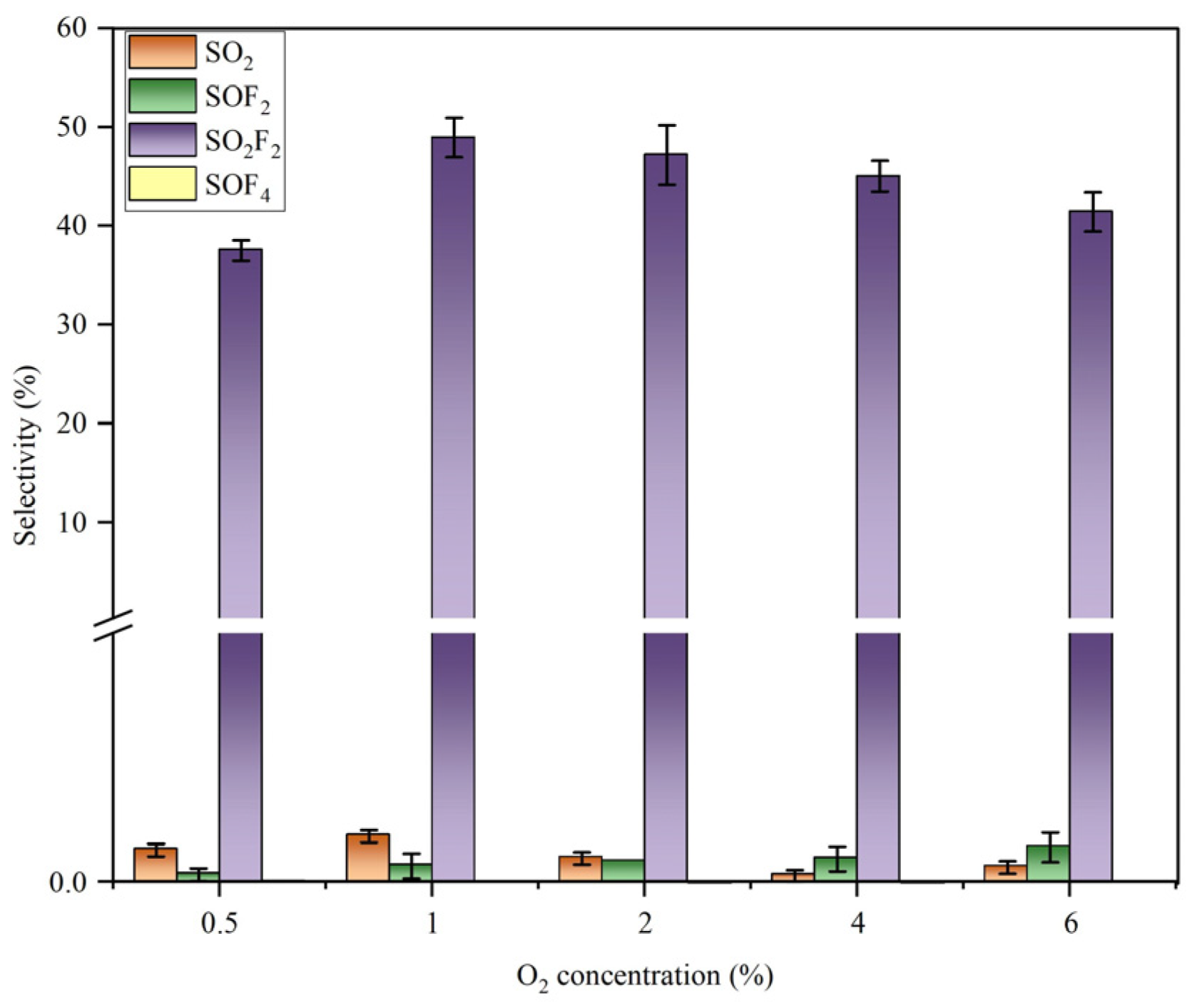
- In the SF6/Ar/O2/H2O system, the addition of H2O improved the product selectivity of SO2F2, and the highest SO2F2 selectivity of 48.96% was obtained at an O2 concentration of 1% and a concentration of 8006.76 ppm.
- The increase in the O2 content led to a decrease in the Ar content, which in turn led to a decrease in the excited-state Ar* content, resulting in a decrease in the degradation rate in this system.
- The addition of O2 inhibited the generation of SO2, and in the addition of O2, SO2F2 and SOF4 were the main components in the degradation products. In addition, there were SOF2, SO2, SiF4, SF4, and OF2.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christophorou, L.G.; Olthoff, J.K.; Van Brunt, R.J. Sulfur hexafluoride and the electric power industry. IEEE Electr. Insul. Mag. 1997, 13, 20–24. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, Y.; Bae, T. Potential of adsorbents and membranes for SF6 capture and recovery: A review. Chem. Eng. J. 2021, 404, 126577. [Google Scholar] [CrossRef]
- Ju, T.; Jianyu, P.; Qiang, Y. Decomposition Characteristic Study of SF6 With Fault Temperature Between 300~400 °C. Proc. CSEE 2013, 33, 202–210. [Google Scholar]
- Zhou, S.; Teng, F.; Tong, Q. Mitigating Sulfur Hexafluoride (SF6) Emission from Electrical Equipment in China. Sustainability 2018, 10, 2402. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, J.; Zhang, Y. Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltage insulation devices. High Volt. 2019, 4, 242–258. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Xiong, H.; Li, Y.; Tang, J.; Xiao, S.; Zhang, D. A First-Principles Study of the SF6 Decomposed Products Adsorbed Over Defective WS2 Monolayer as Promising Gas Sensing Device. IEEE Trans. Device Mater. Reliab. 2019, 19, 473–483. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Zhong, P.; Wang, W.; Liu, S. Key Issues for Negotiation of Adaptation Committee Under UNFCCC. Res. Prog. Clim. Chang. 2012, 8, 144–149. [Google Scholar]
- Li, Y.; Zhang, X.; Fu, M.; Song, X.; Ju, T.; Tian, S. Research and Application Progress of Eco-Friendly Gas Insulating Medium C4F7N, Part I: Insulation and Electrical, Thermal Decomposition Properties. Trans. Chin. Electrotech. Soc. 2021, 36, 3535–3552. [Google Scholar]
- Parthiban, A.; Gopal, A.A.R.; Siwayanan, P.; Chew, K.W. Disposal methods, health effects and emission regulations for sulfur hexafluoride and its by-products. J. Hazard. Mater. 2021, 417, 126107. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Wang, Y.; Meng, F.; Zou, Y.; Tian, S.; Cui, Z. Experimental Study on Thermal Catalytic Degradation of SF6 Waste Gas by Metal Phosphate. J. Electr. Eng. Technol. 2022, 18, 1251–1262. [Google Scholar] [CrossRef]
- Karthikeyan, N.; Nirmal, M.R.; Sathishkumar, G.; Akilan, R. Photodegradation studies of pure and cobalt doped zinc oxide nanoparticles. Mater. Res. Innov. 2023, 27, 69–74. [Google Scholar]
- Shao, T.; Yan, P. Atmospheric Pressure Gas Discharge and Its Plasma Applications; Science Press: Alexandria, Australia, 2015. [Google Scholar]
- Zhang, H.; Xu, R.; Ananthanarasimhan, J.; Zheng, J.; Wan, J.; Wang, K.; Lan, B.; Yan, J.; Lix, X. Destruction of biomass tar model compound in a rotating gliding arc plasma catalytic system: Contribution of typical transition metals in Ni-based bimetallic catalyst. Fuel 2022, 323, 124385. [Google Scholar] [CrossRef]
- Richards, C.; Jans, E.; Gulko, I.; Orr, K.; Adamovich, I.V. N2 vibrational excitation in atmospheric pressure ns pulse and RF plasma jets. Plasma Sources Sci. Technol. 2022, 31, 034001, Erratum in Plasma Sources Sci. Technol. 2022, 31, 079601. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Wang, Y.; Zhang, X.; Wan, K.; Zhang, Y. Experimental study on the Effect of H2 on the Degradation of SF6 by Pulsed Dielectric Barrier Discharge. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 448–456. [Google Scholar]
- Jung, W.; Lee, J.; Ha, K.S. A combined production technology for ethylene and hydrogen with an ethane cracking center and dielectric barrier discharge plasma reactor. Chem. Eng. J. 2023, 462, 142155. [Google Scholar] [CrossRef]
- Li, Y.; Wan, K.; Wang, Y.; Zhang, X.; Yang, Z.; Fu, M.; Zhuo, R.; Wang, D. Experimental study on the effect of H2O and O2 on the degradation of SF6 by pulsed dielectric barrier discharge. Plasma Sci. Technol. 2024, 26, 025506. [Google Scholar] [CrossRef]
- Hamed, R.; Atamalek, G. Parallel electrodes gliding plasma: Working principles and application in dry reforming of methane. Energy 2021, 230, 120753. [Google Scholar]
- Joshi, A.V. Decomposition of SF6–R134a effluents by RF plasma. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2012, 661, S245–S248. [Google Scholar] [CrossRef]
- Danhua, M.; Zhi, F.; Tao, S. Recent Progress on Characteristics and Applications of Atmospheric Pressure Low Temperature Plasmas. Proc. CSEE 2020, 40, 1339–1358. [Google Scholar]
- Lee, H.M.; Chang, M.B.; Wu, K.Y. Abatement of sulfur hexafluoride emissions from the semiconductor manufacturing process by atmospheric-pressure plasmas. J. Air Waste Manag. Assoc. 2004, 54, 960–970. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, L.; Zhang, R. Decomposition of SF6 by dielectric barriers discharge. Environ. Chem. 2007, 26, 275–279. [Google Scholar]
- Zhuang, Q.; Clements, B.; McFarlan, A.; Fasoyinu, Y. Decomposition of the most potent greenhouse gas (GHG) sulfur hexafluoride (SF6) using a dielectric barrier discharge (DBD) plasma. Can. J. Chem. Eng. 2014, 92, 32–35. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, X.; Xiao, S.; Hu, X. Experiment of Effects of Ambient Medium on Sulfur Hexafluoride Degradation for a Double Dielectric Barrier Discharge Reactor. Trans. Chin. Electrotech. Soc. 2017, 32, 20–27. [Google Scholar]
- Zhang, X.; Hu, X.; Xiao, Q.; Cui, Z. Experimental Study on the Effect of Different Dilution Gases to the Degradation of SF6 Gas by Dielectric Barrier Discharge. Proc. CSEE 2018, 38, 937–946. [Google Scholar]
- Wang, X.; Gao, Y.; Zhang, S.; Sun, H.; Li, J.; Shao, T. Nanosecond pulsed plasma assisted dry reforming of CH4: The effect of plasma operating parameters. Appl. Energy 2019, 243, 132–144. [Google Scholar] [CrossRef]
- Gao, Y.; Dou, L.; Zhang, S.; Zong, L.; Pan, J.; Hu, X.; Sun, H.; Ostrikov, K.; Shao, T. Coupling bimetallic Ni-Fe catalysts and nanosecond pulsed plasma for synergistic low-temperature CO2 methanation. Chem. Eng. J. 2020, 420, 127693. [Google Scholar] [CrossRef]
- Dong, B.; Li, Z.; Wang, P.; Luo, T.; Zou, Y.; Tu, W. 4-Chlorophenol containing wastewater joint treated by pulsed discharge. Chem. Ind. Eng. Prog. 2021, 40, 6721–6728. [Google Scholar]
- Zhang, X.; Cui, Z.; Li, Y.; Xiao, H.; Li, Y.; Tang, J.; Xiao, S. Abatement of SF6 in the Presence of NH3 by Dielectric Barrier Discharge Plasma. J. Hazard. Mater. 2018, 360, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Cheng, S.; Yao, J.; Wang, H.; Chen, S.; Xiong, L.; Zhang, G.; Zhou, C.; Zhang, X. Experimental Study on H2O Concentration on Degradation Effect of SF6 by Packed-bed Reactor. High Volt. Appar. 2021, 57, 172–179. [Google Scholar]
- Zhang, X.; Cui, Z.; Li, Y.; Xiao, H.; Li, Y.; Tang, J. Study on Degradation of SF6 in the Presence of H2O and O2 Using Dielectric Barrier Discharge. IEEE Access 2018, 6, 72748–72756. [Google Scholar]
- Zhang, Y.; Li, Y.; Cui, Z.; Xiao, H.; Zhang, X. Experiment of Effect of H2O and O2 on Degradation of High Concentration SF6 by Dielectric Barrier Discharge. High Volt. Eng. 2019, 45, 512–517. [Google Scholar]
- Cui, Z.; Hao, Y.; Yang, L.; Li, L.; Wang, Y.; Zhou, C.; Zhang, X. Review on Harmless Abatement of SF6 Waste Gas. Proc. CSEE 2023, 43, 7720–7737. [Google Scholar]
- GB 31573-2015; Emission Standards of Pollutants for Inorganic Chemical Industry. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2015.




| NO. | Reaction | Reaction Heat (kcal/mol) |
|---|---|---|
| (11) | −177.94 | |
| (12) | −529.82 | |
| (13) | −324.56 | |
| (14) | −547.64 | |
| (15) | −365.64 | |
| (16) | −246.46 | |
| (17) | −285.89 |
| Degradation Method | Degradation Rate | Energy Efficiency | Product Regulation |
|---|---|---|---|
| Thermal Degradation | High | Low | Converts to salts when reacting with corrosive materials |
| Thermal Catalysis | Moderate | Moderate | Generates toxic gases such as SO2 and SO3 |
| Photocatalysis | Low | - | Produces harmless substances |
| DBD Plasma | High | High | Produces toxic substances such as SO2F2 |
| Electrochemical Degradation | Extremely Low | - | Produces harmless substances |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, M.; Li, Y.; Yu, L.; Yang, Z.; Wan, K. A Study on the Efficient Degradation of Sulfur Hexafluoride by Pulsed Dielectric Barrier Discharge Synergistic Active Gas. Energies 2024, 17, 3648. https://doi.org/10.3390/en17153648
Zhang Y, Wang M, Li Y, Yu L, Yang Z, Wan K. A Study on the Efficient Degradation of Sulfur Hexafluoride by Pulsed Dielectric Barrier Discharge Synergistic Active Gas. Energies. 2024; 17(15):3648. https://doi.org/10.3390/en17153648
Chicago/Turabian StyleZhang, Ying, Mingwei Wang, Yalong Li, Lei Yu, Zhaodi Yang, and Kun Wan. 2024. "A Study on the Efficient Degradation of Sulfur Hexafluoride by Pulsed Dielectric Barrier Discharge Synergistic Active Gas" Energies 17, no. 15: 3648. https://doi.org/10.3390/en17153648
APA StyleZhang, Y., Wang, M., Li, Y., Yu, L., Yang, Z., & Wan, K. (2024). A Study on the Efficient Degradation of Sulfur Hexafluoride by Pulsed Dielectric Barrier Discharge Synergistic Active Gas. Energies, 17(15), 3648. https://doi.org/10.3390/en17153648






