Abstract
Asphaltene-resin-paraffin deposition (ARPD) is a complicated and prevalent issue in the oil and gas industry, impacting the efficiency and integrity of petroleum extraction, production, transportation and processing systems. Considering all witnessed ARPD problems in Azerbaijan oil fields, this paper proposed a chemical method and optimized the type and concentration of chemical inhibitors. Then, the effect of selected chemical reagents on inhibiting the ARPD amount and thus enhancing oil recovery was detected by reservoir simulation during both waterflooding and CO2 flooding production. Three new chemical compounds (namely, Chemical-A, Chemical-B and Chemical-C) were examined in laboratory conditions, and their impact on rheological properties of high-paraffin oilfield samples of Azerbaijan (X, Y and Z) were investigated. Experimental results show that Chemical-C with a concentration of 600 g/t has the best efficiency for alleviating the problems. After adding Chemical-C to the crude oil, the freezing point of oil was decreased from 12 °C to (−4) °C, the ARPD amount declined from 0.185 to 0.016 g, and oil effective viscosity was reduced from 16.2 mPa·s to 3.1 mPa·s. It was determined that for water and CO2 flooding, higher injection pressure resulted in reduced asphaltene precipitation. Adding the selected ARPD inhibitor, the oil recovery for waterflooding can increase from 52% to 62%, while it can rise from 55% to 68% for CO2 flooding.
1. Introduction
Asphaltene, resin, and paraffin deposits present significant challenges in the petroleum industry, causing blockages and reducing the efficiency of oil production and transportation. In bottomhole sections of wells, on the surfaces of well equipment, on the walls of tubing strings, and in places where oil has settled, asphaltene-resin-paraffin deposits (ARPD) can often be found. Almost all oil fields face this kind of severe problem. It is reported that this problem is encountered very often in Baku (Azerbaijan) and Grozny (Russia) oilfields. The main problems in Baku oil fields were caused by salt and in separate oil fields by paraffin deposits. In the Grozny oil fields, due to the high temperature, paraffin precipitation was not observed in the wells; nevertheless, the well flowlines were often subjected to paraffinization [,,].
At present, there are various theories that explain the formation of ARPD based on modern concepts. Among them, a relatively widespread theory explains the formation process of oil deposits from the view of crystallization temperature of solid paraffin-naphthenic hydrocarbons. This theory, however, does not account for critical factors such as adhesion, adsorption, and the influence of resin-asphaltene components on oil-dispersed systems [,,,]. In contrast, another advanced theory highlights the significant influence of ARPD on the paraffinization process in oil field equipment. The authors of this theory explain the formation of paraffin deposits through the effects of coagulation, aggregation, and micelle-forming properties of naphthenic hydrocarbons and asphaltenes in oil-dispersed systems []. Additionally, this theory considers the dynamic interactions between these components and the equipment surfaces, emphasizing the complexity of the deposition process. The theory suggests that understanding these interactions is essential for developing more effective strategies to mitigate ARPD formation and improve the efficiency of oil production operations. By incorporating these factors, the conditions under which ARPD forms can be better predicted and controlled, leading to more targeted and effective prevention methods [,,].
Currently, the application of chemical methods, particularly chemical inhibitors, is considered to be the prevailing approach for removing ARPD. ARPD inhibitors are used to effectively dissolve and disperse asphaltene, resin and paraffin deposits that can accumulate in production wells and pipelines. By inhibiting the agglomeration of these compounds, ARPD inhibitors prevent the formation of blockages and flow restrictions, ensuring sustainable production and transportation of crude oil. This technology helps maintain operational efficiency, reduces downtime for maintenance, and minimizes the need for costly interventions such as mechanical cleaning or chemical treatments [].
To address the challenge of asphaltene, resin, paraffin deposits in the petroleum industry, this study integrates laboratory experiments and numerical simulations to optimize a chemical method. By integrating laboratory experiments with reservoir simulations, the research offers a practical and reliable methodology for selecting the most effective reagent and predicting its performance in real reservoir conditions. The findings of this study not only contribute to improving the efficiency of ARPD removal processes but also enhance overall petroleum production operations, leading to cost savings, increased productivity and prolonged reservoir condition.
2. Materials and Methods
2.1. Materials
In this study, new oil-based ARPD inhibitors were proposed and developed using depressor additive of Difron-3970 and reagents of BAF-1, D1, and Gossypol, respectively. They were labeled as Chemical-A, Chemical-B, and Chemical-C, respectively. The efficient ratios of chosen chemical inhibitors were studied, and it was determined that for Chemical-A, the best ratio was BAF-1 + Difron-3970 = 1:1; for Chemical-B, D1 + Difron-3970 = 3:1; and for Chemical-C, Gossypol + Difron-3970 = 4:1. Therefore, the effects of these inhibitors at specified ratios were thoroughly studied for this investigation.
The oil sample analyzed in this paper was collected from Azerbaijan oilfield, referenced as X. The crude oil used in this study was sampled from downhole conditions to ensure it was representative of the actual reservoir fluid. By sampling directly from downhole, the risk of prior asphaltene or paraffin deposition, which can occur during surface handling and transportation, was minimized. The reservoir temperature was 55 °C, and the reservoir pressure was 16.8 MPa. The physicochemical properties of the high-paraffin oilfield sample are listed in the following Table 1.

Table 1.
Physicochemical characteristics of “X” oilfield sample.
As can be seen from the table, the contents of asphaltene, paraffin and resin components in the oilfield sample were quite high. It was characterized by a high freezing point. Therefore, asphaltene-resin-paraffin deposits are formed as soon as the temperature drops below the freezing point during production, storage and transportation of these oil samples.
2.2. Methodology
2.2.1. Oil Sample’s Freezing Point Determination
Within the study, a temperature range of 0–60 °C was chosen for all experiments to align closely with the actual reservoir conditions of taken oilfield samples. Throughout the analysis, oil reservoirs had a temperature of overall 50 °C. By water injection, this temperature could be lowered, promoting asphaltene deposition. As oil is produced and transported through the wellbore, the temperature changes further, increasing the possibility of asphaltene precipitation due to reduced solubility at lower temperatures.
In laboratory conditions, the freezing point of oil examples without and with reagents was determined according to RD 39-3-812-82 methodology []. A 100 mL volume of oil sample was poured into spherical test tubes with a diameter of 20 mm and a height of 160 mm, then heated to a temperature of 55–60 °C. Chemical compounds of various concentrations were added to it, and the mixture was gradually cooled to a temperature of 30–40 °C (for conducting comparison, one test tube had no added chemical compound). Then, the test tubes were placed in the thermostat, and the cooling process was continued. As the temperature gradually dropped, precise measurements were taken at intervals of every three degrees Celsius. At each interval, the test tubes were tilted to a 90° angle to ensure uniform distribution of the oil within the tube. In successive inspections, the temperature at which the level of oil in the test bottle became stationary was noted, and at this time, the test bottle was kept in a horizontal position for 5 s, and the complete solidification of the liquid was determined due to the immobility of the upper liquid layer [] (Figure 1).
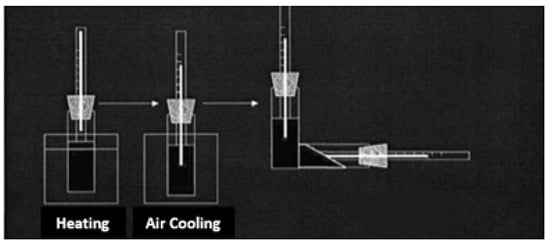
Figure 1.
Schematic description of determination of oil samples’ freezing point.
2.2.2. Determination of Oil Deposit Quantity
The aim of the “cold finger test” method is to determine the quantity of asphaltene-resin-paraffin substances deposited from oil on a cooled metal surface. In the “cold finger test” method, all stages such as the formation and accumulation of ARPD, including processes such as sediment dispersion due to oil flow effect, the precipitation, growth of heavy components of oil, paraffin crystals and the crystallization center formation are realized.
The studied oil samples with a volume of 100 mL were s poured into chemical metal cups with the same proportion (d = 36 mm, ℓ = 130 mm) equipped with a magnetic stirrer intended for the experiment (Figure 2). Then, the oil in the chemical cups was heated to 50–55 °C with stirring at 350 rpm using a magnetic stirrer to ensure a good reagent distribution throughout the oil volume, and a pre-calculated amount of the chemical compound was added to each sample (chemical compound was not added to one of the cups intentionally for comparison purposes). The chemical cups were placed in an external thermostat, and a stainless-steel U-shaped tube (d = 15 mm, ℓ = 110 mm) was inserted into each. The “cold finger” surface provides a temperature gradient with the liquid, which is perfect for the precipitation and crystallization of high-molecular oil components. The asphaltene-resin-paraffin precipitations were liquefied by heating the “cold finger” to 70 °C using a cryostat, and the precipitation mass was measured using the gravimetric technique []. The experiment duration was 40 min.

Figure 2.
Metal cups and parallel U-shaped fingers.
2.2.3. Determination Method for Effective Viscosity, Limiting Shear Stress and Non-Newtonian Index
The method was implemented by using a rotational viscometer, brand of the “Rheotest 2.1”, or its subsequent modifications with measuring devices—a special cylindrical cone-plate (Figure 3).

Figure 3.
Rotary Viscosimeter (Rheotest 2.1).
Prior to experimentation, the sample fluid was prepared while maintaining its temperature and purity. The viscometer was calibrated meticulously according to manufacturer guidelines, and precise temperature control mechanisms were employed. Initial torque and rotational speed readings were recorded before gradually increasing the rotational speed, recording torque at each increment to cover a range of shear rates. Rigorous data analysis techniques were applied to calculate the shear rate and subsequently determine the effective viscosity. In laboratory conditions, the research process was carried out in a wide temperature range (0, 10, 20, 30, 40 and 50 °C) in the “Rheotest-2.1” viscometer with 100 mL of oil samples. The experiment duration was 2 h. According to device results, the non-Newtonian index was determined and processed. Within the framework of the Gersel–Balkley model, the value of the effective viscosity depending on the temperature was calculated by the following expression []:
μe—effective viscosity, Pa·s, τ—shear stress, Pa, τ0—limit shear stress, Pa, γ—velocity gradient, s−1, K—consistency, (Pa·s), (the higher liquid viscosity means the greater K value), n—non-Newtonian index, (the more n is different from 1, the more non-Newtonian properties increase).
2.2.4. Corrosion Rate Determination by Gravimetric Method
Assessing reagents’ effectiveness in inhibiting corrosion is crucial for understanding their potential to reduce metal corrosion rates in various aggressive environments, helping optimize the reagents for industrial applications, where material durability and reliability are key factors. The essence of the gravimetric test method is based on determining the mass loss of metal samples during their stay in the tested corrosion environment. In laboratory conditions, gravimetric tests are carried out in accordance with the requirements of relevant methodology [,].
During the experiment, the samples used are prepared in the form of plates from different steel brands with 100 mL volume of reagents. The duration of the experiment is 10–12 h. The average static relative error of the measured steel sample corrosion rate is not higher than 0.5%. Ct20 steel samples, with dimensions of 30 × 20 × 1 mm, were used during the research. Table 2 shows the chemical composition of Ct20 brand steel.

Table 2.
Chemical composition of Ct20 brand steel (%).
To determine the corrosion rate by the gravimetric method, firstly steel samples are prepared by cutting, shaping and cleaning them with sandpaper and acetone, then drying and accurately weighing each sample to record the initial mass (m0). The samples were submerged in a prepared corrosive solution for a specified duration, ensuring they were fully immersed and maintained under consistent environmental conditions. After the exposure period, the samples were removed, rinsed with distilled water, cleaned to remove corrosion products without affecting the base metal, dried thoroughly, and reweighed to obtain the final mass (m1). The area of the samples taken for testing was calculated according to the following formula:
Sn—area of steel sample, mm2, a—length of sample, mm, b—width of sample, mm, h—height of sample, mm.
Since a = 30 mm, b = 20 mm, and h = 1 mm, the area of the steel sample for testing was determined as SN = 2 × 30 × 1 + 2 × 30 × 20 + 2 × 20 × 1 = 1300 mm2 = 0.0013 m2.
Metal loss was calculated for three steel plates, and the average mass was found. During gravimetric tests, the corrosion rate mass indicator in conditions both without reagent and with reagent was characterized by Km and calculated by the following mathematical equation:
Km—corrosion rate mass index, g/m2·h, m0—mass of the sample before the tests, g; m1—mass of the sample after the tests, S—average surface area calculated for three samples, m2; τ—duration of the test, h.
The protection effect of reagent was calculated as
Z—protection effect, %, K0—corrosion rate without reagent, g/m2·h, Kinh—corrosion rate with reagent, g/m2·h.
3. Experimental Results
3.1. Effect of Chemical Compounds on Oil Sample Freezing Point
It is obviously seen from Figure 4 that as the concentrations of Chemicals A, B and C increased, their effect on the oil freezing point increased first, then decreased. In the chemical compound concentration range of 200–1000 g/t, the freezing point varied between 12–0, 12–2, and 12–(−1) °C for the three types of chemicals, respectively. Additionally, it should be noted that for Chemical-A after 800 g/t, Chemical-B after 500 g/t, and Chemical-C after 600 g/t, the freezing point of the mentioned oil samples increased; as a result, it was determined that the best effect occurred at the mentioned concentration rates, which means they are optimal concentrations for these compound chemicals. Among the three chemical compounds, Chemical-C had a great impact on the freezing point of the oil sample, reducing it by 13 °C. The other experiments were then carried out on samples with the determined optimal concentration rates, which were, respectively, 800, 500 and 600 g/t for Chemicals A, B and C.
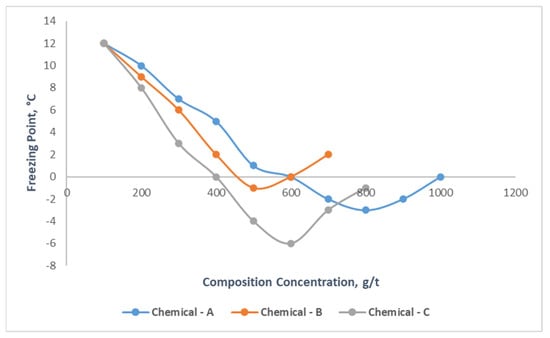
Figure 4.
Effect of chemical compounds on freezing point of high-paraffin oil sample.
3.2. Effect of Chemical Compounds on Oil Sample Deposition
The effect of the chemical compounds on asphaltene, paraffin, and resin deposits (ARPD) in the oil samples was studied by the “cold finger test” method. Experiments were carried out in the temperature range of 5–50 °C on oil samples without and with the chemical compounds (Figure 5). It can be noticed from the graph that, in the case of samples without the use of any chemical compound, the amount of ARPD decreased as temperature increased, and the ARPD amount over increased temperatures changed from 0.338 to 0.031 g. This reduction is attributed to the temperature effect, which likely influences the solubility and deposition tendencies of ARPD components, resulting in lower deposit formation at higher temperatures.
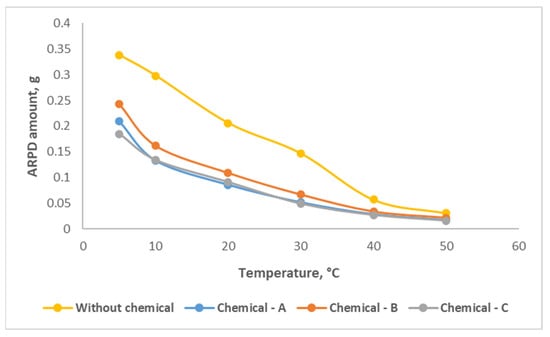
Figure 5.
Graph of amount of oil deposits formed with change in temperature, without and with compound chemicals.
After the application of 800 g/t concentration of Chemical-A, the ARPD amount at 5 °C was 0.209 g. At the final temperature of 50 °C, this value decreased to 0.018 g, resulting in a reduction of 42%. The significant reductions in ARPD deposition are explained by the effects of temperature increase and the effective chemical compositions of the applied chemical compounds. Similarly, when Chemical-B was applied at 500 g/t, the depositions declined from 0.243 g to 0.022 g, which was a decrease of nearly 31%. For Chemical-C, with a concentration of 600 g/t, the depositions reduced from 0.185 g to 0.016 g, indicating a reduction of approximately 48%. These significant reductions are attributed to the effective action of the chemical compounds in mitigating ARPD deposition. It is clearly observed that the efficiency of Chemical-C efficiency was better than that of the other two chemicals, reducing the ARPD amount by almost 2 times for the final condition.
3.3. Effect of Chemical Compounds on Oil Samples’ Effective Viscosity
Figure 6 illustrates that before application of the chemicals, the effective viscosity for the oil is varied from 29.2 to 5.9 mPa·s as temperature changed from 5 °C of 60 °C. This substantial reduction in viscosity was a direct result of the temperature effect. As temperature rises, the increased thermal energy enhances molecular movement within the oil, weakening intermolecular forces and consequently lowering its resistance to flow.
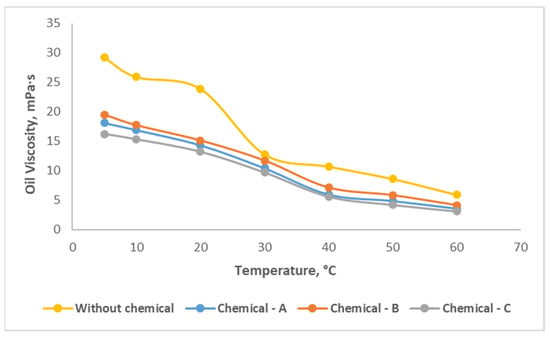
Figure 6.
Graph of effective viscosity with change in temperature, without and with chemical compounds.
After application of each chemical compound with its optimal concentration rates, an obvious oil viscosity reduction was observed. From Figure 6, it is apparent that at the initial condition (5 °C), the effective viscosity declines slightly for all the chemical compounds. At the final condition of 60 °C, it reaches values of 3.5, 4.1 and 3.1 mPa·s, which corresponds to reductions of approximately 41%, 31%, and 48% for Chemical-A, Chemical-B, and Chemical-C, respectively. As can be seen from the comparison, Chemical-C notably reduced the effective viscosity, performing better than the other two chemicals. Furthermore, it is important to note that the measurement error for determining the effective viscosity using the Rheotest 2.1 viscometer (Rheotec Messtechnik GmbH, Ottendorf-Okrilla, Germany) was ±2%.
3.4. Effect of Chemical Compounds on Oil Sample Corrosion Rate
Determination of the corrosion rate was conducted by the gravimetric method, and the results are provided in Figure 7. It was observed that the simultaneously protective effect increased with Chemical-A concentration increases in the range of 200–1000 g/t. The highest protective effect, about 95.4%, was obtained at a concentration of 800 g/t of the chemical compound. Above the 800 g/t concentration rate of the chemical compound, its effect on the corrosion rate declined. For Chemical-B and Chemical-C, the highest protective rates against corrosion were 91.8 and 98.1% at their optimal concentration rates of 500 and 600 g/t, respectively. So, Chemical-C has the best protective effect among the three chemicals.
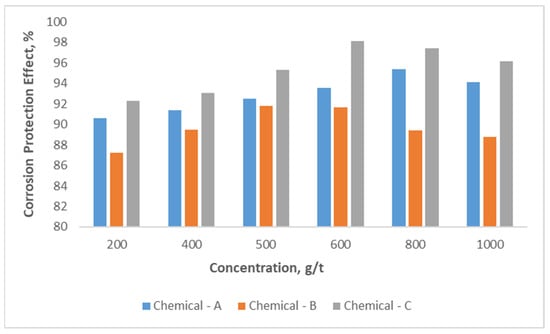
Figure 7.
Corrosion protection effect with change in chemical compound concentration.
3.5. Effect of Chemical Compounds on Limiting Shear Stress and Non-Newtonian Index
The experiments were conducted at the oil temperatures of 5,10, 20, 30, 40 and 50 °C. Experiments were carried out on samples with the determined optimal concentration rates, which were respectively 800, 500 and 600 g/t for Chemicals A, B and C. For all chemical compounds, when the oil temperature was 40 and 50 °C, experimental values for limit shear stress were very close to 0, and experimental values for the non-Newtonian index were almost 1, which are signs of a Newtonian liquid that easily flows. Below 40 °C, the non-Newtonian index became notable, which is sign of flowing liquid. Consequently, according to the results for the non-Newtonian index and limiting shear stress, it is apparent that oil began to flow from low temperatures, which means viscosity decreased (Table 3). The results correlate with comparable information for other chemical compounds, while with the application of Chemical-C, the oil began to flow at a temperature much lower than the freezing temperature of high-paraffin oil, and in that situation, the liquid properties were moderately better, allowing easier flow.

Table 3.
Effect of chemical reagents on limiting shear stress and non-Newtonian index of oilfield samples.
3.6. Effect of Chemical Compounds on Y and Z Oilfield Samples’ Rheological Properties
Next, to analyze the effect of the chemical compounds on oil samples from the other two high-paraffin oilfields of Azerbaijan (Y and Z oilfields), laboratory experiments were conducted according to the previously mentioned methodology. The obtained high-paraffin oilfield samples’ physicochemical properties are described as follows:
Table 4 demonstrates that the samples obtained from the other two oilfields also are high-paraffin oils with notable physicochemical properties. The reservoir temperatures for Y and Z were 48 and 44 °C, and the reservoir pressures were 16.1 and 15.3 MPa, respectively. Following the same procedure for the primary oilfield sample, the mentioned samples were also obtained directly from downhole conditions. Their freezing points are quite high, which is very convenient condition for ARP deposition.

Table 4.
Physicochemical characteristics of “Y” and “Z” oilfield samples.
Table 5 indicates that the studied chemicals were also very efficient for these oil samples, reducing their freezing points considerably and avoiding paraffin deposition noticeably. The ARPD amount for the Y and Z oilfields was reduced overall by 35% and 37%, respectively after application of the chemicals, and again, Chemical-C performed the best. Additionally, it is essential to emphasize that starting from 30–40 °C, the obtained oil samples displayed Newtonian characteristics, maintaining a constant viscosity across varying shear rates with Chemical-A and Chemical-B applications. However, after application of Chemical-C, it was witnessed that the transition temperature was already 20 °C, which is a quite successful result to achieve Newtonian liquid characteristics for lower temperature conditions.

Table 5.
Experimental results for chemical compounds for Y and Z oilfield samples.
4. Investigation of Enhanced Oil Recovery Effect by Injecting Paraffin Inhibitors during Waterflooding and CO2 Flooding Based on Numerical Simulation
Modern petroleum industry developments have provided several opportunities, such as creating simulation models to anticipate asphaltene precipitation. However, in this situation, there is a challenge, as asphaltenic fluids are complex and, in turn, complex models are needed for their representation. The modeling of asphaltenic fluids has evolved comprehensive thermodynamics, beginning with simple solubility parameters and regular solutions, and currently is progressing to various modes of statistical association of fluid theory (SAFT). SAFT, a broadly used EOS for characterizing the behavior of the asphaltene phase, is based on statistical mechanics. The initial stage of SAFT EOS is the determination of a reference fluid that serves as a stand-in for the original fluid.
The GEM simulator in the commercial simulation software (CMG, https://www.cmgl.ca/, accessed date: 25 February 2024) was used in this simulation investigation. GEM and Winprop simulators were used to emulate asphaltene precipitation, which is considered during waterflooding and CO2 flooding. The asphaltene formation mechanism and its impact in the reservoir were studied. It should be mentioned that fluid modeling data are the essential and most crucial data for the research (as listed in Table 6). An “X1” oilfield sample from Azerbaijan was used and examined in our case study.

Table 6.
Reservoir fluid composition.
4.1. Asphaltene Precipitation Modeling in CMG
Winprop was used to simulate the asphaltene-related issues for reservoir oil. Nghiem proposed the asphaltene precipitation model using WinProp simulator []. Previous models presumed that asphaltene was the heaviest component of oil. The heaviest component is divided into two parts: non-precipitating components (i.e., C31+) and precipitating components (i.e., asphaltene), which share the same critical properties and acentric factors, but they interact differently with the light components. The non-precipitating components are considered, namely resins and heavy paraffins. This phase is known as the asphaltene part, and its fugacity is described as follows:
This fugacity equation is true for both liquid and solid-state asphaltene. The asphaltene stage can be classified as liquid or solid phase. As can be seen from Equation (5), fs* is referred to as the reference (asphaltene) fugacity, P* and T reference pressure and temperature, which can be determined by experimental solubility data, and Vs is the solid (asphaltene) molar volume. Asphaltene will precipitate if solid component fugacity is less than that of the other two phases.
4.1.1. Fluid Characterization
The pseudo-components of C7+ are divided into a fraction along C31+ (Figure 8). The two-stage exponent was used in order to define the molar distribution weight during the splitting process. After dividing the (C7+) fraction, the single carbon numbers were split into four pseudo-components, using the Lee–Kesler (1975) approach for determination of their critical properties and mixing rule correlation, updated after running to reflect the divided measurement results [].
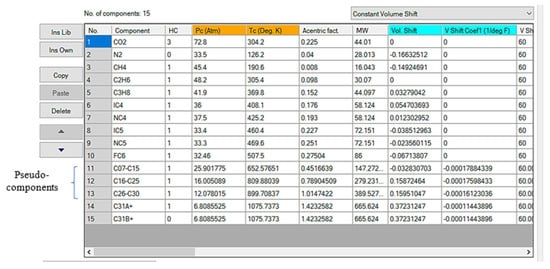
Figure 8.
Oil sample pseudo-components.
4.1.2. Prediction of Asphaltene Precipitation Behavior
Predictions related to asphaltene precipitation were made by clarifying flash calculation results with different pressure ranges. For low-pressure conditions, the precipitation content was inaccurate because it failed to forecast the offset pressure. To achieve a perfect precipitation curve, some parameters needed to be adjusted. As a result of numerous tests with various solid molar and interaction parameters, the derived curve was obtained (Figure 9). The asphaltene precipitation model predicts a pressure of 4500 psia for the onset pressure and 1500 psia for the offset, with the highest precipitation occurring around 2960 psia, near saturation pressure.
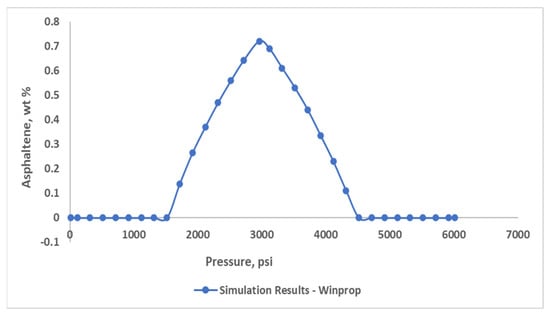
Figure 9.
The asphaltene precipitation curve at 100 °C.
In Azerbaijan oil industry experience, waterflooding is a preferred secondary oil recovery technique over others. This preference primarily originates from waterflooding being a cost-effective and readily available option, furthermore through extensive utilization and optimization over the years. Additionally, CO2 flooding can contribute to both enhanced oil recovery and greenhouse gas mitigation efforts, making it a promising approach for addressing environmental challenges. That is why, in the numerical simulation part of the paper, firstly waterflooding, then CO2 flooding was run and provided comparative outcomes.
4.2. Reservoir Modeling
A 3D model was created using Builder, which is a model in CMG, with dimensions of 250 ft × 250 ft × 6 ft and 3750 grid cells. The Z direction consisted of six equal layers. The homogeneous reservoir had a permeability of 300 mD in the X and Y directions and 50 mD in the Z direction, with all grid blocks having a porosity of 0.25. In CMG’s Builder tool, the reservoir simulator was configured as shown in Figure 10, which included the reservoir and well properties. Builder computed the rock–fluid interactions, while WinProp modeled the fluid for CO2 flooding and waterflooding simulations using GEM. The producer well was located in grid block 25, 25, 1 with a constant bottom hole pressure of 2000 psia, and the injector well was in grid block 1, 1, 1. The simulations, visualized in the figure below, included waterflooding and CO2 flooding, both with and without asphaltene precipitation.
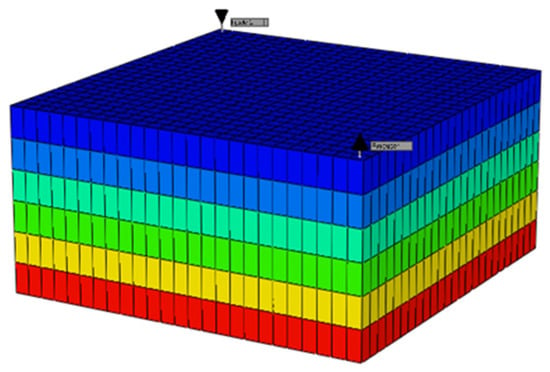
Figure 10.
Simulation model with the producer and injector locations.
4.3. Simulation of Asphaltene Deposition during Waterflooding
The simulation was run with a rate of 2000 bbl/day and a pressure of 3000 psia, for 10 years with and without asphaltene precipitation. The results of the simulation are shown below:
Figure 11 shows that the recovery factor of waterflooding considering asphaltene is almost 12% lower than that without asphaltene.
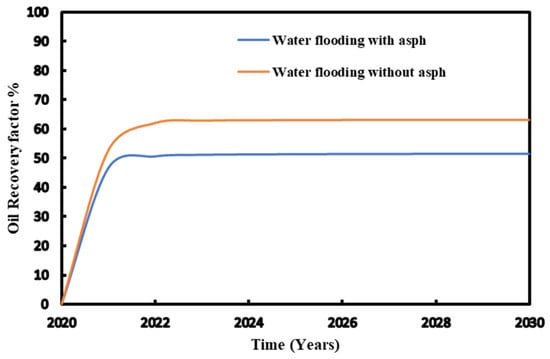
Figure 11.
Recovery factor for the injection pressure with and without asphaltene during waterflooding.
Figure 12 shows asphaltene precipitation during waterflooding with initial very low deposition (a) and occasional drops near injection and producer at the end of 10-year production (b). Water in oil reservoirs can alter asphaltene stability, affecting reservoir fluid characteristics. High asphaltene deposition occurs at block pressures of 2500 and 3000 psia, causing the liquid phases to cohabit and indicating a thermodynamic imbalance in the asphaltic oil system. The lower recovery factor observed during waterflooding with asphaltene precipitation can be explained with several mechanisms. Asphaltene particles precipitate within the reservoir, leading to permeability reduction by blocking pore spaces and impeding oil flow to production wells. This restriction in permeability limits oil movement, resulting in decreased recovery. Furthermore, asphaltene accumulation near production wells obstructs flow paths, causing productivity losses and reducing oil extraction efficiency. Other potential reasons could include formation damage caused by asphaltene deposition, altering the reservoir’s fluid flow dynamics and causing changes in fluid properties, such as viscosity, which directly affect oil displacement and recovery.
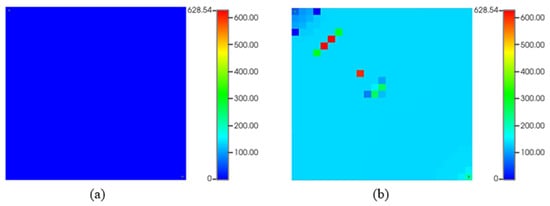
Figure 12.
Asphaltene deposited mass (lb) during waterflooding: (a) start of production; (b) at the end of production.
4.4. Simulation of Asphaltene Deposition during CO2 Flooding
The GEM model of CMG software was used to simulate CO2 flooding, adjusting the EOS with the WinProp module. Model parameters like porosity and permeability were correlated, and reservoir pressure was used for miscible flooding processes. A 3D numerical model was set up and run for 10 years with constant gas injection, both with and without asphaltene. CO2 was injected at pressures of 2500 psia, 3000 psia, and 4000 psia, but in the simulation, 3000 psia pressure was maintained, close to the bubble point pressure.
Using 3000 psia as injection pressure, the comparison of the recovery factor was conducted for the CO2 flooding with and without considering asphaltene precipitation. The results demonstrate that for both injection processes, higher pressure leads to an improved recovery factor. It shows that high pressure injection reduces asphaltene precipitation and thus enhances oil recovery (Figure 13 and Figure 14).
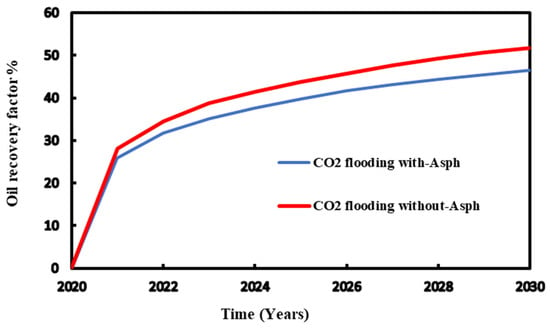
Figure 13.
Recovery factor for the injection pressure with and without asphaltene during CO2 flooding.
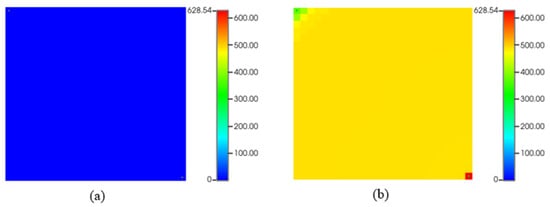
Figure 14.
Asphaltene precipitation (lb) during CO2 flooding: (a) start of production; (b) at the end of production.
Figure 14 depicts asphaltene precipitation during CO2 flooding. As we can see, (a) represents the start of the simulation, which is almost typical, with no asphaltene deposition. However, at the end of the simulation (b), there are some asphaltene drops in the reservoir near the injection and the producer due to temperature and pressure changes.
4.5. Simulation of Asphaltene Deposition during Waterflooding and CO2 Flooding with Reagent
With the addition of the chemical reagent to the numerical simulation, the approach expressing that asphaltene in solution behaves as a fluid component was followed and applied. The chemical reagent was treated as a component of the fluid composition. It involved defining the chemical reagent’s properties and interactions using the WinProp module and adjusting the EOS to reflect its impact on asphaltene solubility and stability. The reagent was then introduced into the simulation model as an additive to the injection fluid, either mixed with the injected water for waterflooding or combined with the CO2 during CO2 flooding. According to the experimental results, the most effective reagent (Chemical-C) was chosen for application in the reservoir simulation during CO2 flooding and waterflooding and compared with previous models.
Figure 15 provides the change in the recovery factor with time during waterflooding and CO2 flooding with the added reagent. The used regent reduced asphaltene deposits in the reservoir, leading more fluid to be displaced. The comparison of waterflooding and CO2 flooding with the application of chemicals is presented in Figure 16.
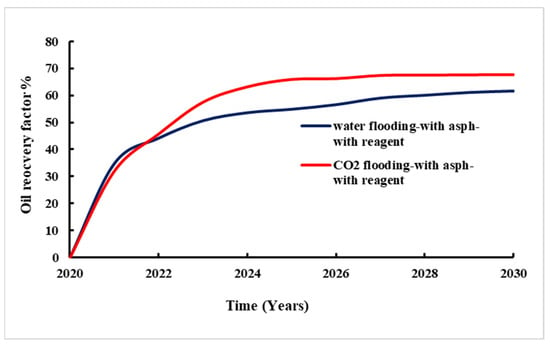
Figure 15.
Recovery factor for CO2 flooding and waterflooding with asphaltene and reagent.
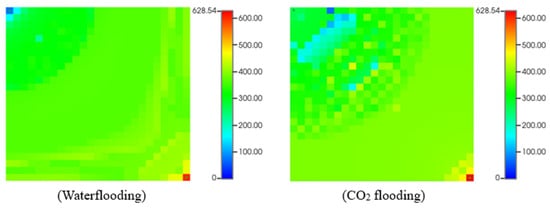
Figure 16.
Asphaltene deposited mass (lb) during waterflooding and CO2 flooding with asphaltene and reagent.
The study results show the significant impact of chemical reagent application on oil recovery in both waterflooding and CO2 flooding (Figure 17).
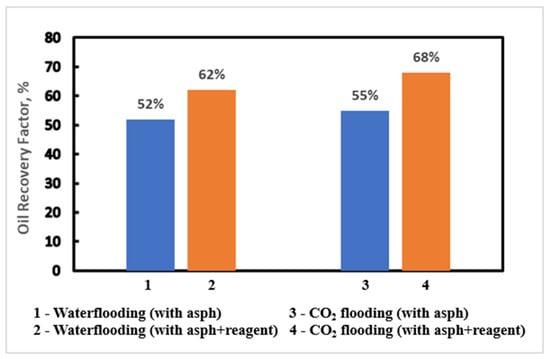
Figure 17.
Oil recovery for waterflooding and CO2 flooding with asphaltene, with and without reagent.
For waterflooding, the oil recovery rate without the chemical reagent was 52%. After application of the chemical reagent, the recovery rate increased to 60%. This improvement indicates the chemical reagent’s effectiveness in mitigating asphaltene-related issues and achieving enhanced overall oil recovery. Similarly, for CO2 flooding, the oil recovery rate was 47% without the application of chemical reagent, whereas with the chemical reagent, the recovery rate significantly increased to 67%. This suggests that the chemical reagent effectively addresses challenges, leading to more efficient oil extraction.
5. Conclusions
During the study of the effects of new, proposed chemical compounds (paraffin inhibitors) on the rheological parameters of high-paraffin oil samples chosen from Azerbaijan oilfields, it was determined that among the three applied chemical compounds, the best efficiency was possessed by Chemical-C at its optimal concentration rate of 600 g/t. With the application of Chemical-C, the following experimental and reservoir simulation results were determined:
- (1)
- The freezing point for oilfield sample X dropped from 12 °C to (−1) °C; for Y, from 17 °C to (−2) °C; and for Z, from 16 °C to 0 °C. The reason is that the chemical compounds reduced the size of the paraffin crystals and prevented them from sticking together.
- (2)
- The “cold finger test” method showed that the ARPD amount of oilfield sample X was reduced from 0.338 g to 0.031 g when the temperature changed from initial (5 °C) to final (50 °C) without adding any chemicals. However, at 50 °C, after applying Chemical-C, the ARPD amount in the sample was further reduced, from 0.031 g to 0.016 g. Chemical-C was also very effective in reducing the ARPD amount in oilfield samples Y and Z at a certain temperature.
- (3)
- On one hand, as the temperature increases, the oil effective viscosity decreases. On the other hand, at the same temperature, additives further reduce the oil effective viscosity. At 60 °C, after adding Chemical-C at 600 g/t, the oil effective viscosity for oilfield sample X decreased from 5.9 mPa·s to 3.1 mPa·s (a 48% decline); for Y, from 8.3 mPa·s to 4.8 mPa·s (42%); and for Z, from 7.8 mPa·s to 4.3 mPa·s (45%). The chemical compound has high dissolving ability and can prevent particle agglomeration.
- (4)
- Determination of the corrosion rate by the gravimetric method showed that the highest protective effect against corrosion peaked at 98.1% for Chemical-C at 600 g/t.
- (5)
- Rheological parameters determined according to the Gersel–Balkley model showed that the limit shear stress started to decrease significantly from lower temperatures. Furthermore, oil samples started to flow when the temperature was higher than 5 °C. It was observed that the non-Newtonian index for the studied oil samples gradually approached 1 from lower temperatures (20, 30 °C), which is representative of Newtonian fluid behavior, where the fluid exhibits characteristics of easy flow and predictable, stable viscosity.
- (6)
- Based on simulation results, higher injection pressures for CO2 flooding and waterflooding resulted in less asphaltene precipitation. The precipitation process happened near the saturation pressure due to the highest dissolved gas oil ratio at saturation pressure. The injection rates did not have a large impact on the precipitation of asphaltene. The use of the paraffin inhibitor could remove asphaltene deposition amount in the reservoir, leading to improved oil recovery of 62% for waterflooding and around 68% for CO2 flooding.
- (7)
- Based on the simulation results, it is obvious that CO2 flooding outperforms waterflooding in terms of oil recovery. This suggests that CO2 flooding exhibits a higher efficiency compared to traditional waterflooding techniques. Therefore, in reservoirs where both methods are applicable, CO2 flooding emerges as the superior option for enhanced oil recovery.
Author Contributions
Project administration, outline, structure, guidance, X.W.; supervision, expertise, data curation, visualization and writing—original draft preparation, X.W., H.G., M.A. and E.A.; experiment and numerical simulation, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Panahov, G.M. Development of New Methods of Combating Asphaltene-Resin-Paraffin Deposits; Panahov, G.M., Abbasov, E.M., Ismayilov, S.Z., Eds.; Azerbaijan Oil Refinery: Baku, Azerbaijan, 2019; pp. 65–70. [Google Scholar]
- Gurbanov, G.R. Research into the Influence of the Depressant Additive “Difron-4201” on the Formation of Paraffin Deposits in Laboratory Conditions; Gurbanov, G.R., Adigozalova, M.B., Akhmedov, S.F., Eds.; Azerbaijan Oil Economy: Baku, Azerbaijan, 2020; pp. 30–36. [Google Scholar]
- Gurbanov, G.R.; Adygezalova, M.B.; Pashaeva, S.M. Study of a universal combined inhibitor for the oil and gas industry. Univ. Chem. Chem. Technol. 2020, 63, 78–89. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Z.; Cao, L.; Wang, G. Study on performance of surfactant-polymer system in deep reservoir. SOCAR Proc. 2016, 1, 34–41. [Google Scholar] [CrossRef]
- Hasanvand, M.Z.; Montazeri, M.; Salehzadeh, M.; Amiri, M.; Fathinasab, M. A Literature Review of Asphaltene Entity, Precipitation, and Deposition: Introducing Recent Models of Deposition in the Well Column. J. Oil Gas Petrochem. Sci. 2018, 1, 83–89. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Louzada, H.F.; Dip, R.M.M.; Gonza, G.; Lucas, E.F. Influence of the architecture of additives on the stabilization of asphaltene and water-in-oil emulsion separation. Energy Fuels 2015, 29, 7213–7220. [Google Scholar] [CrossRef]
- Matiyev, K.I.; Aga-zade, A.D.; Keldibayeva, S.S. Removal of asphaltene-resin-paraffin deposits of various fields. SOCAR Proc. 2016, 4, 64–68. [Google Scholar] [CrossRef]
- Samadov, A.M. Research of Depressor and Inhibitory Properties of NDP-Type Reagents; Samadov, A.M., Aghazade, A.D., Alsafarova, M.E., Eds.; Azerbaijan Oil Industry: Baku, Azerbaijan, 2017; pp. 43–47. [Google Scholar]
- Gurbanov, G.R.; Adygezalova, M.B.; Pashayeva, S.M. The influence of depressant additives on the process of formation of asphalt, resin and paraffin deposits in high-paraffin oil. Transp. Storage Pet. Prod. Hydrocarb. Raw Mater. 2020, 1, 23–28. [Google Scholar]
- Kelbaliev, G.I.; Rasulov, S.R.; Tagiev, D.V.; Mustafaeva, G.R. Mechanics and Rheology of Petroleum Dispersed Systems; Maska: Moscow, Russia, 2017; 462p. [Google Scholar]
- Bakhtizin, R.N.; Karimov, R.M.; Mastobaev, B.N. The influence of high-molecular components on rheological properties depending on the structural-group and frictional composition of oil. Socar Proc. 2016, 1, 42–50. [Google Scholar] [CrossRef]
- Ivanova, L.V.; Koshelev, V.N. Removal of various kind of asphalt, resin and paraffin deposits. Electron. Sci. J. Oil Gas Bus. 2011, 2, 257–270. [Google Scholar]
- RD 39-3-812-82; Method for Determining the Freezing Point of Paraffin Oil. Rheological Properties. USSR Ministry of Oil Industry: Moscow, Russia, 2015.
- Sviridov, A.V.; Sklyuev, P.V. Assessment of the Influence of Substances of Various Classes for Asphalt Loss Using the “Cold Finger Test” Method; Samara State Technical University: Samara, Russia, 2023; pp. 250–252. [Google Scholar]
- GOST 26581-85; Method of Test for Effective Viscosity on a Rotary Viscosimeter. USSR State Committee for Standards: Moscow, Russia, 2015.
- GOST 9.506-87; Unified System of Corrosion and Ageing Protection. Corrosion Inhibitors of Metals in Water-Petroleum Environments. Methods of Protective Ability Evaluation. USSR State Committee for Standards: Moscow, Russia, 2015.
- Pashaeva, S.M. Research on the effectiveness of corrosion protection of MARZA-1 inhibitor in H2S, CO2 and H2S + CO2 environments. Sci. News Nat. Tech. Sci. 2021, 21, 42–47. [Google Scholar]
- Nghiem, L.X.; Hassam, M.S.; Nutakki, R.; George, A.E.D. Efficient Modelling of Asphaltene Precipitation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1993. [Google Scholar]
- Lee, B.I.; Kesler, M.G. A Generalized Thermodynamic Correlation Based on Three-Parameter Corresponding States. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).