Crystal Structure Prediction and Performance Assessment of Hydrogen Storage Materials: Insights from Computational Materials Science
Abstract
1. Introduction
2. Computational Methods for Hydrogen Storage Materials
2.1. Density Functional Theory
2.2. Molecular Dynamics Simulations
2.3. Machine Learning Methods
3. Assessment of Thermodynamic and Kinetic Properties
4. Prediction of Stable Crystal Structures
5. High-Throughput Screening and Materials Discovery
6. Multiscale Modeling and Experimental Validation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4 + Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Ding, Z.; Shaw, L. Enhancement of Hydrogen Desorption from Nanocomposite Prepared by Ball Milling MgH2 with In Situ Aerosol Spraying LiBH4. ACS Sustain. Chem. Eng. 2019, 7, 15064–15072. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Hulvey, Z.; Gennett, T.; Ahmed, A.; Autrey, T.; Camp, J.; Cho, E.S.; Furukawa, H.; Haranczyk, M.; Head-Gordon, M.; et al. An assessment of strategies for the development of solid-state adsorbents for vehicular hydrogen storage. Energy Environ. Sci. 2018, 11, 2784–2812. [Google Scholar] [CrossRef]
- Massaro, M.C.; Biga, R.; Kolisnichenko, A.; Marocco, P.; Monteverde, A.H.A.; Santarelli, M. Potential and technical challenges of on-board hydrogen storage technologies coupled with fuel cell systems for aircraft electrification. J. Power Sources 2023, 555, 232397. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New insights into the solid-state hydrogen storage of nanostructured LiBH4-MgH2 system. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Jhi, S.-H.; Kwon, Y.-K.; Bradley, K.; Gabriel, J.-C.P. Hydrogen storage by physisorption: Beyond carbon. Solid State Commun. 2004, 129, 769–773. [Google Scholar] [CrossRef]
- Kumar, A.; Muthukumar, P.; Sharma, P.; Kumar, E.A. Absorption based solid state hydrogen storage system: A review. Sustain. Energy Technol. Assess. 2022, 52, 102204. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Liu, X.; Shang, J.-X.; Yu, R.; Shui, J. Non-classical hydrogen storage mechanisms other than chemisorption and physisorption. Appl. Phys. Rev. 2022, 9, 021315. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous materials for hydrogen storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Zacharia, R.; Rather, S.U. Review of Solid State Hydrogen Storage Methods Adopting Different Kinds of Novel Materials. J. Nanomater. 2015, 2015, 914845. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.A.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X.J. Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage. Research 2021, 2021, 3750689. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Alumina based doped templated carbons: A comparative study with zeolite and silica gel templates. Microporous Mesoporous Mater. 2018, 257, 241–252. [Google Scholar] [CrossRef]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-Based Materials for Solid-State Hydrogen Storage: Strategies and Perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Samantaray, S.S.; Putnam, S.T.; Stadie, N.P. Volumetrics of Hydrogen Storage by Physical Adsorption. Inorganics 2021, 9, 45. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; Zhou, Y.; Chen, Z.; Yang, W.; Ma, W.; Shaw, L. LiBH4 for hydrogen storage—New perspectives. Nano Mater. Sci. 2020, 2, 109–119. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Effects of gaseous environments on physicochemical properties of thermally exfoliated graphene oxides for hydrogen storage: A comparative study. J. Porous Mater. 2021, 28, 875–888. [Google Scholar] [CrossRef]
- Majid, N.A.A.; Watanabe, J.; Notomi, M. Improved desorption temperature of magnesium hydride via multi-layering Mg/Fe thin film. Int. J. Hydrogen Energy 2021, 46, 4181–4187. [Google Scholar] [CrossRef]
- Baum, L.; Meyer, M.; Mendoza-Zélis, L. Hydrogen storage properties of the Mg/Fe system. Phys. B Condens. Matter 2007, 389, 189–192. [Google Scholar] [CrossRef]
- Reardon, H.; Hanlon, J.M.; Hughes, R.W.; Godula-Jopek, A.; Mandal, T.K.; Gregory, D.H. Emerging concepts in solid-state hydrogen storage: The role of nanomaterials design. Energy Environ. Sci. 2012, 5, 5951–5979. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Majzoub, E.H. Recent advances in metal hydrides for clean energy applications. MRS Bull. 2013, 38, 452–458. [Google Scholar] [CrossRef]
- Jordá-Beneyto, M.; Suárez-García, F.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen storage on chemically activated carbons and carbon nanomaterials at high pressures. Carbon 2007, 45, 293–303. [Google Scholar] [CrossRef]
- Kishor, R.; Singh, S.B.; Ghoshal, A.K. Role of metal type on mesoporous KIT-6 for hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 10376–10385. [Google Scholar] [CrossRef]
- Balaprakash, P.; Dongarra, J.; Gamblin, T.; Hall, M.; Hollingsworth, J.K.; Norris, B.; Vuduc, R. Autotuning in High-Performance Computing Applications. Proc. IEEE 2018, 106, 2068–2083. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Hippalgaonkar, K.; van Duren, J.; Jaffer, S.; Chandrasekhar, V.R.; Stevanovic, V.; Wadia, C.; Guha, S.; Buonassisi, T. Accelerating Materials Development via Automation, Machine Learning, and High-Performance Computing. Joule 2018, 2, 1410–1420. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Dubecký, M.; Mitas, L.; Jurečka, P. Noncovalent Interactions by Quantum Monte Carlo. Chem. Rev. 2016, 116, 5188–5215. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, W.; Jiang, T.; Wang, Y.; Shuai, Z. Time-dependent density matrix renormalization group method for quantum dynamics in complex systems. WIREs Comput. Mol. Sci. 2022, 12, e1614. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Wang, P.; Li, C.; Yue, Q.; Cui, W.G.; Wang, X.; Yang, Y.; Gao, F.; Zhang, M.; et al. Solid-State Hydrogen Storage Origin and Design Principles of Carbon-Based Light Metal Single-Atom Materials. Adv. Funct. Mater. 2024, 34, 2316368. [Google Scholar] [CrossRef]
- Psofogiannakis, G.M.; Froudakis, G.E. DFT Study of Hydrogen Storage by Spillover on Graphite with Oxygen Surface Groups. J. Am. Chem. Soc. 2009, 131, 15133–15135. [Google Scholar] [CrossRef]
- Marian, C.M.; Heil, A.; Kleinschmidt, M. The DFT/MRCI method. WIREs Comput. Mol. Sci. 2019, 9, e1394. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Feynman, R.P. Statistical Mechanics: A Set of Lectures; Benjamin/Cummings Publishing Company: Reading, MA, USA, 1972. [Google Scholar]
- Zhao, Y.; Zhu, Y.; Shi, R.; Jia, Z.; Zhang, J.; Liu, Y.; Cheng, H.; Tang, Q.; Ba, Z.; Hu, X.; et al. Structural inhomogeneity: A potential strategy to improve the hydrogen storage performance of metal hydrides. J. Mater. Chem. A 2023, 11, 13255–13265. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Yan, G.; Yang, W.; Gao, Z.; Ma, W.; Shaw, L. Mechanism of hydrogen storage on Fe3B. Chem. Commun. 2020, 56, 14235–14238. [Google Scholar] [CrossRef]
- Xue, W.; Zhao, B.; Liu, H.; Chen, X.; Liu, L. Ultralow Pd bimetallic catalysts boost (de)hydrogenation for reversible H2 storage. Appl. Catal. B Environ. 2024, 343, 123574. [Google Scholar] [CrossRef]
- Verma, P.; Truhlar, D.G. Status and Challenges of Density Functional Theory. Trends Chem. 2020, 2, 302–318. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Corso, A.D.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Han, Z.; Wu, Y.; Yu, H.; Zhou, S. Location-dependent effect of nickel on hydrogen dissociation and diffusion on Mg (0001) surface: Insights into hydrogen storage material design. J. Magnes. Alloys 2022, 10, 1617–1630. [Google Scholar] [CrossRef]
- Zhu, B.-C.; Liu, G.-H.; Deng, P.-J.; Liu, C.-J.; Liao, Y.-H.; Zeng, L.; Zhao, J. Study of the hydrogen absorption behaviour of a “number-sensitive” Mg atom: Ultra-high hydrogen storage in MgHn (n = 1–20) clusters. J. Mater. Chem. A 2023, 11, 13774–13782. [Google Scholar] [CrossRef]
- Prasetyo, N.; Pambudi, F.I. Toward hydrogen storage material in fluorinated zirconium metal-organic framework (MOF-801): A periodic density functional theory (DFT) study of fluorination and adsorption. Int. J. Hydrogen Energy 2021, 46, 4222–4228. [Google Scholar] [CrossRef]
- Xia, L.; Bo, Z.; Liu, Q.; Zhang, X.; Pei, Y. Li-doped and functionalized metal-organic framework-519 for enhancing hydrogen storage: A computational study. Comput. Mater. Sci. 2019, 166, 179–186. [Google Scholar] [CrossRef]
- Kurth, S.; Perdew, J.P.; Blaha, P. Molecular and solid-state tests of density functional approximations: LSD, GGAs, and meta-GGAs. Int. J. Quantum Chem. 1999, 75, 889–909. [Google Scholar] [CrossRef]
- Klimeš, J.; Michaelides, A. Perspective: Advances and challenges in treating van der Waals dispersion forces in density functional theory. J. Chem. Phys. 2012, 137, 120901. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Bowler, D.R.; Miyazaki, T. methods in electronic structure calculations. Rep. Prog. Phys. 2012, 75, 036503. [Google Scholar] [CrossRef]
- Cerutti, D.S.; Case, D.A. Molecular dynamics simulations of macromolecular crystals. WIREs Comput. Mol. Sci. 2019, 9, e1402. [Google Scholar] [CrossRef]
- Shi, R.; Qian, H.-J.; Lu, Z.-Y. Coarse-grained molecular dynamics simulation of polymers: Structures and dynamics. WIREs Comput. Mol. Sci. 2023, 13, e1683. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Bai, S.; Piri, M. Hydrogen storage in nanoporous media: Molecular dynamics simulations of the confinement effects. Int. J. Hydrogen Energy 2022, 47, 24886–24896. [Google Scholar] [CrossRef]
- Akbarzadeh, F.Z.; Rajabi, M. Mechanical alloying fabrication of nickel/cerium/MgH2 nanocomposite for hydrogen storage: Molecular dynamics study and experimental verification. J. Alloys Compd. 2022, 899, 163280. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Wang, X.-D.; Zhang, W.; Ma, E.; Deringer, V.L.; Mazzarello, R. Unraveling Crystallization Mechanisms and Electronic Structure of Phase-Change Materials by Large-Scale Ab Initio Simulations. Adv. Mater. 2022, 34, 2109139. [Google Scholar] [CrossRef]
- Reilly, J.J., Jr.; Wiswall, R.H., Jr. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; von Colbe, J.M.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Azizzadenesheli, K.; Kovachki, N.; Li, Z.; Liu-Schiaffini, M.; Kossaifi, J.; Anandkumar, A. Neural operators for accelerating scientific simulations and design. Nat. Rev. Phys. 2024, 6, 320–328. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Long time scale kinetic Monte Carlo simulations without lattice approximation and predefined event table. J. Chem. Phys. 2001, 115, 9657–9666. [Google Scholar] [CrossRef]
- Biamonte, J.; Wittek, P.; Pancotti, N.; Rebentrost, P.; Wiebe, N.; Lloyd, S. Quantum machine learning. Nature 2017, 549, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Salehi, K.; Rahmani, M.; Atashrouz, S. Machine learning assisted predictions for hydrogen storage in metal-organic frameworks. Int. J. Hydrogen Energy 2023, 48, 33260–33275. [Google Scholar] [CrossRef]
- Zhou, P.; Xiao, X.; Zhu, X.; Chen, Y.; Lu, W.; Piao, M.; Cao, Z.; Lu, M.; Fang, F.; Li, Z.; et al. Machine learning enabled customization of performance-oriented hydrogen storage materials for fuel cell systems. Energy Storage Mater. 2023, 63, 102964. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xiang, X.; Zu, X.; Hu, S. Hydrogen diffusion in zirconium hydrides from on-the-fly machine learning molecular dynamics. Int. J. Hydrogen Energy 2024, 56, 1057–1066. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Z.; Ma, T.; Lu, C.-T.; Ma, W.; Shaw, L. Predicting the hydrogen release ability of LiBH4-based mixtures by ensemble machine learning. Energy Storage Mater. 2020, 27, 466–477. [Google Scholar] [CrossRef]
- Krallinger, M.; Rabal, O.; Lourenco, A.; Oyarzabal, J.; Valencia, A. Information Retrieval and Text Mining Technologies for Chemistry. Chem. Rev. 2017, 117, 7673–7761. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Li, J.; Li, Y.; Wang, L.; Zhang, J. Exploration and design of Mg alloys for hydrogen storage with supervised machine learning. Int. J. Hydrogen Energy 2023, 48, 38412–38424. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Zafar, H.K.; Javed, M.S.; Din, M.A.U.; Alarfaji, S.S.; Balkourani, G.; Sohail, M.; Tsiakaras, P.; Najam, T. Mxenes for Zn-based energy storage devices: Nano-engineering and machine learning. Coord. Chem. Rev. 2024, 501, 215565. [Google Scholar] [CrossRef]
- Hu, J.; Shen, H.; Jiang, M.; Gong, H.; Xiao, H.; Liu, Z.; Sun, G.; Zu, X. A DFT Study of Hydrogen Storage in High-Entropy Alloy TiZrHfScMo. Nanomaterials 2019, 9, 461. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.-G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; Van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Assila, A.; Rkhis, M.; Alaoui-Belghiti, A.; Laasri, S.; Hlil, E.K.; Boughaleb, Y.; Hajjaji, A. Feeling the strain: Enhancing the thermodynamics characteristics of magnesium nickel hydride Mg2NiH4 for hydrogen storage applications through strain engineering. Int. J. Hydrogen Energy 2024, 67, 651–657. [Google Scholar] [CrossRef]
- Andreasen, A.; Vegge, T.; Pedersen, A.S. Dehydrogenation kinetics of as-received and ball-milled LiAlH4. J. Solid State Chem. 2005, 178, 3672–3678. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Zhang, N.; Li, Z.; Lin, X.; Zhu, W.; Lu, C.; Ding, W.; Zou, J. Nanostructuring of Mg-Based Hydrogen Storage Materials: Recent Advances for Promoting Key Applications. Nano-Micro Lett. 2023, 15, 93. [Google Scholar] [CrossRef]
- Li, T.; Kim, M.; Liang, Z.; Asthagiri, A.; Weaver, J.F. Dissociative Chemisorption and Oxidation of H2 on the Stoichiometric IrO2(110) Surface. Top. Catal. 2018, 61, 397–411. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. Invited paper presented at the International Symposium on Metal–Hydrogen Systems, Les Diablerets, August 25–30, 1996, Switzerland. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Pickard, C.J.; Needs, R.J. Ab initio random structure searching. J. Phys. Condens. Matter 2011, 23, 053201. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.W.; Oganov, A.R.; Hansen, N. USPEX—Evolutionary crystal structure prediction. Comput. Phys. Commun. 2006, 175, 713–720. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.; Cho, H.; Lee, H.; Lee, S.Y.; Cho, E.S.; Kim, J. Computational Screening of Trillions of Metal–Organic Frameworks for High-Performance Methane Storage. ACS Appl. Mater. Interfaces 2021, 13, 23647–23654. [Google Scholar] [CrossRef]
- Giri, S.; Chakraborty, A.; Chattaraj, P.K. Potential use of some metal clusters as hydrogen storage materials—A conceptual DFT approach. J. Mol. Model. 2011, 17, 777–784. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Yu, X.; Zhao, H.; Shao, H. Geometrical effect in Mg-based metastable nano alloys with BCC structure for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 29291–29296. [Google Scholar] [CrossRef]
- Curtarolo, S.; Hart, G.L.W.; Nardelli, M.B.; Mingo, N.; Sanvito, S.; Levy, O. The high-throughput highway to computational materials design. Nat. Mater. 2013, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Curtarolo, S.; Setyawan, W.; Hart, G.L.W.; Jahnatek, M.; Chepulskii, R.V.; Taylor, R.H.; Wang, S.; Xue, J.; Yang, K.; Levy, O.; et al. AFLOW: An automatic framework for high-throughput materials discovery. Comput. Mater. Sci. 2012, 58, 218–226. [Google Scholar] [CrossRef]
- Spotte-Smith, E.W.C.; Cohen, O.A.; Blau, S.M.; Munro, J.M.; Yang, R.; Guha, R.D.; Patel, H.D.; Vijay, S.; Huck, P.; Kingsbury, R.; et al. A database of molecular properties integrated in the Materials Project. Digit. Discov. 2023, 2, 1862–1882. [Google Scholar] [CrossRef]
- Sbailò, L.; Fekete, Á.; Ghiringhelli, L.M.; Scheffler, M. The NOMAD Artificial-Intelligence Toolkit: Turning materials-science data into knowledge and understanding. npj Comput. Mater. 2022, 8, 250. [Google Scholar] [CrossRef]
- Muy, S.; Johnston, C.; Marzari, N. AiiDA-defects: An automated and fully reproducible workflow for the complete characterization of defect chemistry in functional materials. Electron. Struct. 2023, 5, 024009. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Li, X.-B.; Zheng, H.; Chen, N.-K.; Wang, X.-P.; Zhang, X.-L.; Sun, H.-B.; Zhang, S. High-Throughput Screening for Phase-Change Memory Materials. Adv. Funct. Mater. 2021, 31, 2009803. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, X.; Pascual-San-José, E.; Campoy-Quiles, M. Accelerating organic solar cell material’s discovery: High-throughput screening and big data. Energy Environ. Sci. 2021, 14, 3301–3322. [Google Scholar] [CrossRef]
- Paul, A.; Birol, T. Applications of DFT + DMFT in Materials Science. Annu. Rev. Mater. Res. 2019, 49, 31–52. [Google Scholar] [CrossRef]
- Zheng, S.; Ding, H.; Li, S.; Chen, D.; Pan, F. Application of topology-based structure features for machine learning in materials science. Chin. J. Struct. Chem. 2023, 42, 100120. [Google Scholar] [CrossRef]
- Xie, C.; He, X.K.; Liu, X.; Ye, J.H.; Chen, J.B. Phase-field modeling for anisotropic ductile damage of magnesium alloys at finite deformations. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Yu, C.; Qin, S.; Chai, B.; Huang, S.; Liu, Y. The Effect of Compressible Flow on Heat Transfer Performance of Heat Exchanger by Computational Fluid Dynamics (CFD) Simulation. Entropy 2019, 21, 829. [Google Scholar] [CrossRef]
- Chen, C.; Nguyen, D.T.; Lee, S.J.; Baker, N.A.; Karakoti, A.S.; Lauw, L.; Owen, C.; Mueller, K.T.; Bilodeau, B.A.; Murugesan, V.; et al. Accelerating Computational Materials Discovery with Machine Learning and Cloud High-Performance Computing: From Large-Scale Screening to Experimental Validation. J. Am. Chem. Soc. 2024. [Google Scholar] [CrossRef]
- Waser, R.; Ghani, F.; Maranda, S.; O’Donovan, T.S.; Schuetz, P.; Zaglio, M.; Worlitschek, J. Fast and experimentally validated model of a latent thermal energy storage device for system level simulations. Appl. Energy 2018, 231, 116–126. [Google Scholar] [CrossRef]
- Altintas, C.; Keskin, S. On the shoulders of high-throughput computational screening and machine learning: Design and discovery of MOFs for H2 storage and purification. Mater. Today Energy 2023, 38, 101426. [Google Scholar] [CrossRef]
- Basdogan, Y.; Keskin, S. Simulation and modelling of MOFs for hydrogen storage. CrystEngComm 2015, 17, 261–275. [Google Scholar] [CrossRef]

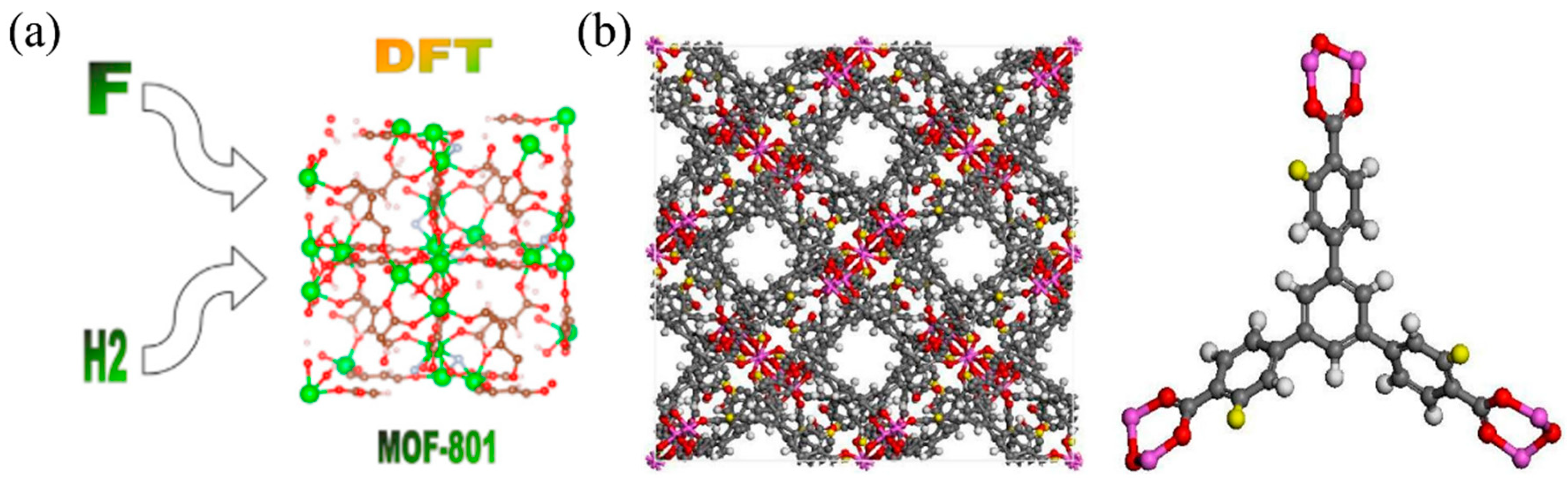


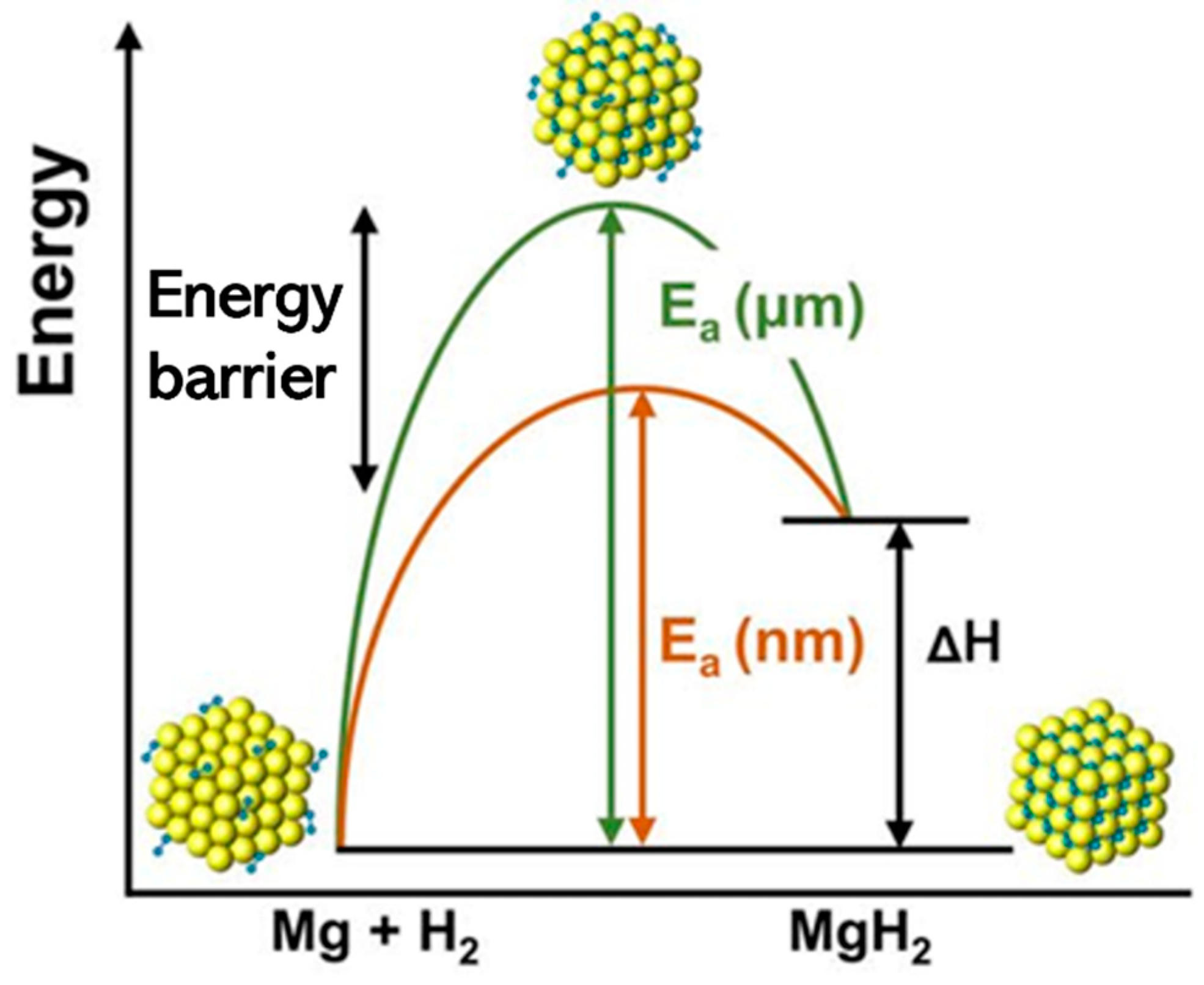
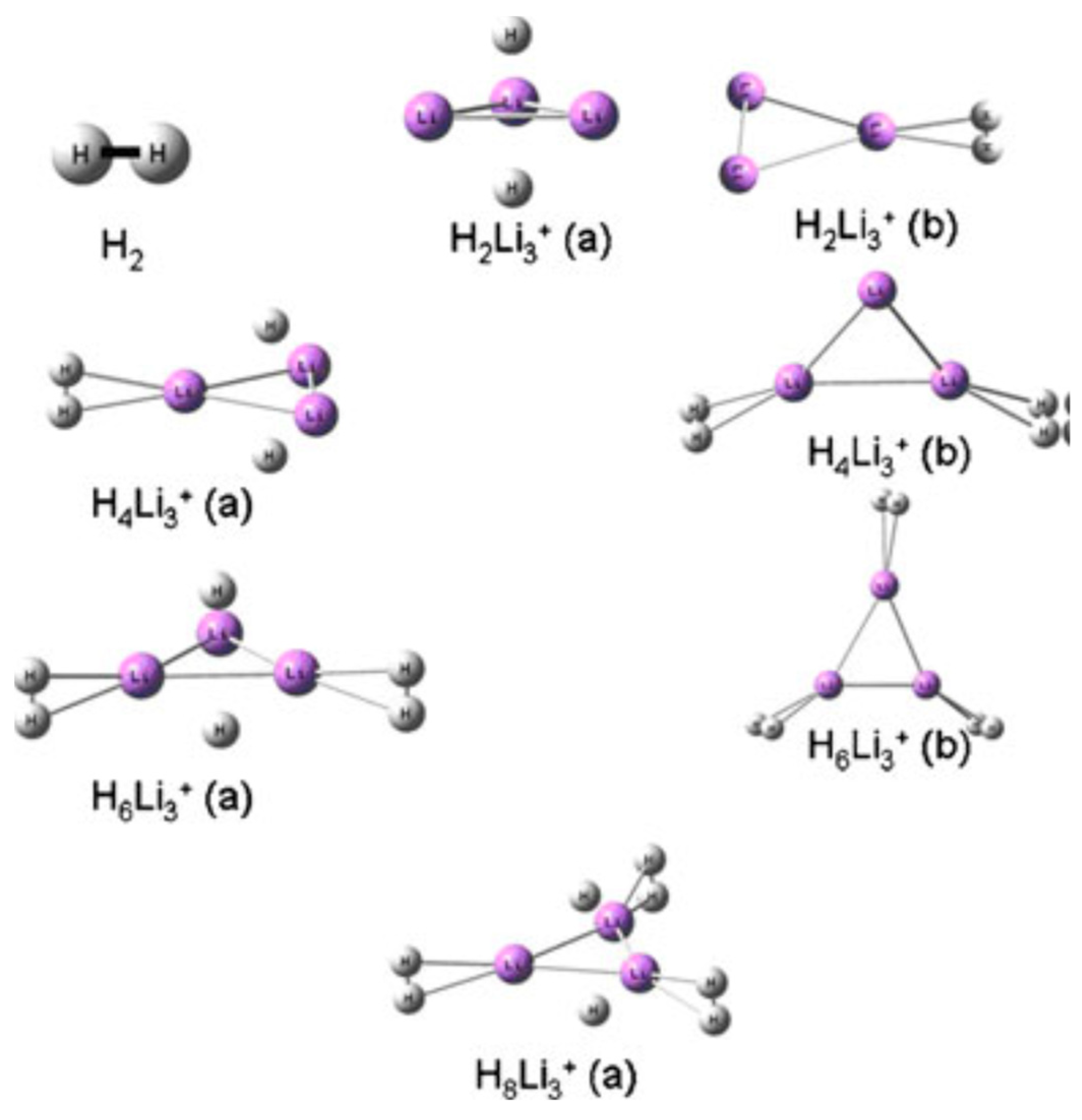
| Technology | Cost | Safety | Practicality | Major Challenges and Limitations |
|---|---|---|---|---|
| High pressure | High cost, especially for large capacity requirements | Potential for leaks and explosion risks | Suitable for small-scale applications | Heavy container weight, high safety requirements, economic feasibility for large-scale applications limited |
| Cryogenic | High cost, energy-intensive | Explosion risks during liquefaction and transportation | Suitable for long-term storage and long-distance transport | Requires complex insulation techniques, operation sensitivity to environmental conditions |
| Solid-State | High initial investment, lower operational costs | High stability, controlled release | Applicable across various scenarios | High initial costs, need for further material design and engineering optimization to enhance hydrogen capacity |
| Material | Binding Energy (kJ/mol H2) | Storage Capacity (wt.%) | ||
|---|---|---|---|---|
| DFT | Exp. | DFT | Exp. | |
| MOF-5 | 4.8 | 4.5 | 5.2 | 4.7 |
| MgH2 | 62.3 | 65.8 | 7.6 | 7.0 |
| LaNi5 | 20.5 | 22.1 | 1.4 | 1.3 |
| TiH2 | 126.7 | 130.2 | 4.0 | 4.0 |
| Material | Diffusion Coefficient (cm2/s) | Activation Energy (kJ/mol) | ||
|---|---|---|---|---|
| MD | Exp. | MD | Exp. | |
| Pd | 2.5 × 10−5 | 3.2 × 10−5 | 22.3 | 24.1 |
| MgH2 | 1.2 × 10−8 | 2.5 × 10−8 | 116.5 | 120.7 |
| NaAlH4 | 4.1 × 10−7 | 6.8 × 10−7 | 48.2 | 51.4 |
| LiBH4 | 3.6 × 10−6 | 5.1 × 10−6 | 35.7 | 39.2 |
| MOF | Hydrogen Adsorption Capacity (wt.%) | |
|---|---|---|
| ML | Exp. | |
| HKUST-1 | 2.8 | 2.7 |
| UiO-66 | 1.6 | 1.6 |
| MOF-74 | 3.5 | 3.6 |
| MOF-177 | 1.2 | 1.3 |
| Material | Enthalpy of Desorption (kJ/mol H2) | |
|---|---|---|
| Calculated | Experimental | |
| MgH2 | 74.5 | 75.2 |
| LiBH4 | 67.3 | 66.5 |
| NaAlH4 | 37.2 | 37.8 |
| LaNi5H6 | 30.8 | 31.1 |
| Material | Space Group | Lattice Parameters (Å) | ||
|---|---|---|---|---|
| Predicted | Experimental | Predicted | Experimental | |
| MgH2 | P42/mnm | P42/mnm | a = 4.52, c = 3.01 | a = 4.51, c = 3.02 |
| LiBH4 | Pnma | Pnma | a = 7.18, b = 4.44, c = 6.80 | a = 7.17, b = 4.43, c = 6.80 |
| NaAlH4 | I41/a | I41/a | a = 5.02, c = 11.33 | a = 5.02, c = 11.32 |
| Ti-MOF-74 | R-3 | R-3 | a = 26.21, c = 6.86 | a = 26.13, c = 6.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, Y.; Liu, Y.; Li, Q.; Yang, T.; Jia, H. Crystal Structure Prediction and Performance Assessment of Hydrogen Storage Materials: Insights from Computational Materials Science. Energies 2024, 17, 3591. https://doi.org/10.3390/en17143591
Yang X, Li Y, Liu Y, Li Q, Yang T, Jia H. Crystal Structure Prediction and Performance Assessment of Hydrogen Storage Materials: Insights from Computational Materials Science. Energies. 2024; 17(14):3591. https://doi.org/10.3390/en17143591
Chicago/Turabian StyleYang, Xi, Yuting Li, Yitao Liu, Qian Li, Tingna Yang, and Hongxing Jia. 2024. "Crystal Structure Prediction and Performance Assessment of Hydrogen Storage Materials: Insights from Computational Materials Science" Energies 17, no. 14: 3591. https://doi.org/10.3390/en17143591
APA StyleYang, X., Li, Y., Liu, Y., Li, Q., Yang, T., & Jia, H. (2024). Crystal Structure Prediction and Performance Assessment of Hydrogen Storage Materials: Insights from Computational Materials Science. Energies, 17(14), 3591. https://doi.org/10.3390/en17143591





