Abstract
This review analyzes the main methods for cleaning up oil pollution in natural ecosystems, with a particular focus on the synergy between chemical and microbiological techniques for environmental remediation. While biological methods are a green and inexpensive soil remediation technique, they have a major limitation in their inability to clean up high concentrations of toxic contaminants. The poor performance of chemical methods stems from the high cost of chemicals and concerns over their negative and toxic effects on the environment. Physical methods also have high costs due to energy consumption and the need for additional treatment of gases generated during decontamination, making them ineffective for soil remediation. The main principle of bioremediation is based on microorganisms’ ability to degrade complex organic compounds, such as petroleum. This process is described in this review. This combination of methods allows for a higher level of decontamination of soil and water ecosystems, even against pollutants that are usually resistant to degradation, such as oil derivatives. While existing methods for cleaning oil-contaminated ecosystems are highly effective, they require significant material costs to implement. Additionally, the review discusses how the joint use of current and future biotechnology techniques can lead to the development of an effective set of strategies to protect soil and water systems from oil pollution. The reviewed studies show that a hybrid biotechnological approach is the most effective remediation method. When biological decontamination methods are adopted, the optimized combination of different remediation strategies can overcome the limitations of each technique, allowing efficiencies of even more than 70% to be achieved, given that the choice still depends on the type of contaminant, its concentration, and the properties of the receiving substrate.
1. Introduction
In terms of scale and toxicity, oil pollution poses a significant global environmental threat, as petroleum substances have a widespread impact on all forms of life [1,2,3]. The characteristics of surface and groundwater can be significantly affected both during oil field exploitation and transportation through oil pipelines. The most significant scale of oil pollution occurs due to accidents related to oil pipelines and spills caused by violations of well operational regulations [4]. During the drilling process and well running, natural aquifer equilibriums may be disturbed, leading to a deterioration of their hydrodynamic and geochemical conditions. Additionally, increased groundwater flow can increase the risk of spreading pollution to larger areas. Possible migration paths of oil in a soil environment include migration of oil metabolites to deeper layers of the soil, deposition in the capillary fringe zone, and deposition in the area where groundwater rises seasonally. These phenomena are well known in geochemical literature [5]. For these reasons, estimating soil diffusion parameters is essential [6], often requiring specific mathematical tools [7]. During drilling and standard well operations, hazardous wastes are generated, including waste drilling fluids, drill cuttings—a mixture of rock fragments and drilling fluid—and drilling wastewater [8,9].
Drilling fluids may contain cements, petroleum products, salts, acids, alkalis, dispersed clay, polymers, polyacrylamides, surfactants, methanol, phenols, acetone, graphite, heavy metals, and much more. An additional issue of drilling fluids of complex composition is related to the fact that their components, which have a combined effect on the body, can lead to an increased toxic effect compared to that of individual substances [10]. Figure 1 shows the distribution of the most significant oil spills volumes from the following sources: ships (25%), tankers (20%), and tank barges (15%), as well as from oil refinery terminals (25%) [11].
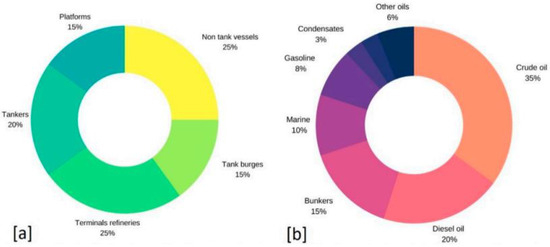
Figure 1.
(a) Percentage (%) of origin of oil spills in the water; (b) percentage (%) of oil types, according to data collected from Ref. [11].
Oil spills have numerous socio-economic consequences, as reported in Figure 2. The fundamental areas of the socio-economic life of population suffering from oil pollution are fishing, tourism, the transport sector, as well as a wide range of areas, whose disruption leads to the vulnerability and resilience of the population [12,13]. According to forecasts concerning the field of commercial and recreational fishing [12], the highest losses will be about USD 4.9 billion and USD 3.5 billion, respectively. Namely, in commercial fishing, the shrimp industry was the most vulnerable to oil pollution, accounting for almost 85% of the study’s projected impact on this sector.

Figure 2.
A summary of research findings about human effects related to nine large oil spills: Exxon Valdez (USA), Braer (UK), Sea Empress (UK), Prestige (Spain), Tasman Spirit (Pakistan), Erika (France), Nadhodka (Japan), Hebei Spirit (Republic of Korea), and Deepwater Horizon (USA) [13].
To model the expected damage, biophysical forecasts can be used, so researchers, using the Atlantis ecosystem model with input–output analysis, modeled the impact on the economic part of the fishing sector for 10 years after the spill. Their calculations showed that both the commercial and amateur sectors could involve losses totaling more than USD 2.3 billion. Assessing damage to tourism and to the transport sector is more difficult; for example, the tourism sector is greatly influenced by the geopolitical situation and by quarantine measures introduced in some countries (the situation with SARS-CoV-2) [14].
Crude oil contains a complex mixture of aliphatic, aromatic, and heterocyclic compounds. Oil contains trace amounts of sulfur and nitrogen compounds, which are themselves dangerous and can react with the environment to form toxic secondary chemicals. One of the most noticeable sources of oil pollution is catastrophic oil spills in both soil and aquatic environments [15].
Oil spills can be defined as accidental releases of liquid petroleum components into the environment, with a destructive effect on its equilibria. In soil environments, oil spills are usually limited, and their impact can be easily remedied compared to oil spills in aquatic areas. The main cause of marine oil spills is related to the transportation of oil by tankers and pipelines, which accounts for about 70% of all oil spills. The remaining percentage comes from offshore drilling and wells. A map of several oil spill accidents during the years 2018–2023 can be seen in Figure 3.
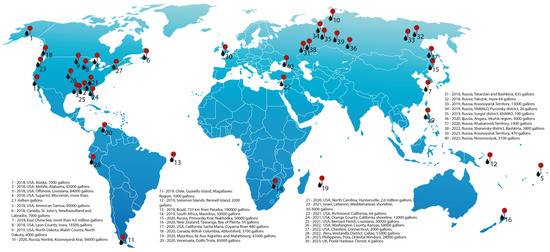
Figure 3.
The world’s largest oil and petroleum product spills in the years 2018–2023.
There are several factors that lead to oil spills, ranging from accidents and negligence, to intentional dumping. Tanker accidents are one of the most common causes of oil spills, and are associated with problems encountered during the loading or unloading of petroleum products [16]. Another cause of oil pollution is a release of petroleum substances deriving from car engines using conventional fuels as well as petroleum-based lubricants. They slowly release petroleum substances, leading to their large-scale accumulation in the soil environment. This amounts to saying that even minor daily oil spills and leaks from cars may significantly contribute to soil pollution.
Additionally, natural leakage caused by the movement of the Earth’s tectonic plates releases oil from natural reservoirs located under the seabed. Natural oil leakage accounts for about 6% of all oil spills, but human activities such as drilling tend to increase this amount. Oil installations are another cause of oil spills in the environment. For oil production, a complex array of production facilities and unit operations is required: wells (for drilling, operating, injection and observation), compressor and pumping stations, collection points, oil storage facilities, primary oil treatment points, pipelines, settling tanks, sites for burning gas and condensate, electrical substations, etc. Many of the aforementioned structures represent a potential source of oil spills [17,18]. First, the main source of oil flows in the field is wells, including those that are being drilled and those that are already in operation. At the stage of drilling a well and preparing it for operation, the main components of oil flows are drilling fluids and various chemical reagents (acids, surfactants, salts, and cement solutions). They are the dominant pollutants at the drilling stage. At the operational stage, wells may act as a source of temporary oil flows arising during emergencies, repair work, and for other reasons, interrupting the well’s operational continuity.
The main substance that makes up oil flows from production wells is formation fluid, which is oil containing dissolved gas and a certain amount of formation water, usually of high mineralization. Oil flows from wells pollute soil, surface, groundwater and disrupt soil and aquatic biocenoses. The main mechanism of their distribution is gravitational. The movement of these flows occurs along the surface towards the slope of the area with infiltration into soil horizons and loose sediments. Once in moving watercourses, petroleum flows are dispersed and mixed with flows from other sources, polluting large areas. Petroleum flows are similar in composition to leakages occurring during breakthroughs (accidents) of field pipelines, by which a reservoir fluid flows from wells to collection points and primary oil treatment installations.
Oil spills cause long-term and enormous damage to the environment. The oily mass that forms the oil slick covers the coastline with black tar, making it extremely dangerous for all marine life. Oil and petroleum products cause poisoning, death of organisms, and soil degradation. Natural self-purification of natural objects from oil pollution is a protracted process, especially in conditions where low temperatures persist for a long time [19]. Therefore, the problem of reclamation of oil-contaminated soils is of exceptional relevance. When it comes to solving the problem of cleaning soil from oil contamination, developing new and improving existing technologies for restoring oil-contaminated lands are among the priority tasks. The modern pace of development of oil production and oil refining requires effective techniques that make it possible to quickly neutralize the effects of oil and oil products on soil and water bodies. There are a significant number of studies on standard techniques for cleaning soil [20,21,22] and water [23,24,25] contaminated with crude oil and petroleum products. These works generally describe advances and minor improvements to traditional cleaning methods. This work provides a review of modern methods for cleaning oil-contaminated ecosystems using standard technologies and the currently promising method of bioremediation.
2. Characteristics of Petroleum Hydrocarbons
Petroleum is a complex mixture of organic compounds with chain sizes ranging from C6 to C60, including aliphatic hydrocarbons (n-alkanes, cycloalkanes), polycyclic aromatic hydrocarbons (PAHs), and their alkylated derivatives, as well as polar compounds and resins, mainly consisting of heterocyclic compounds such as aromatic molecules containing S, N, and O (for example, thiophene, carbazole, and their derivatives) [26]. These components are inherited by oil from the original bioorganic material source or introduced during its contact with host rocks and formation waters [27]. The main elements that make up oil are presented in Figure 4.
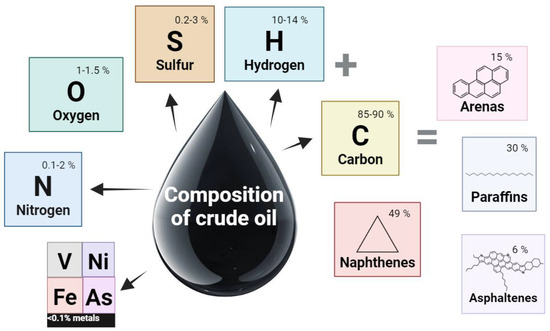
Figure 4.
The main elements that make up oil.
The quality of crude oil and the resulting petroleum products depends on its composition, including impurities such as naphthenic acids, resins, phenols, and amines, which determine the compliance of these products with modern environmental standards. For crude oil, the main quality indicators include density, sulfur content, and fractional composition. The density of oil is influenced by the content of paraffinic hydrocarbons and resins, with a lower density indicating an easier refining process and higher product quality.
The fractional composition of crude oil, which is determined by the boiling point of its components, is an important indicator of its chemical characteristics. An oil fraction is a subset (group) of hydrocarbons whose boiling point is within a certain temperature range. Start and end boiling temperatures are called boiling limits of fractions or boiling limits. Fractions boiling at temperatures up to 350 °C are called light distillates. The fraction that boils away after the selection of light distillates is called fuel oil. Fuel oil and fractions obtained from it are dark. Crude oil contains fuel oil—boiling point above 430 °C; gas oil—230–430 °С; kerosene—160–230 °C; naphtha—105–160 °C; gasoline—32–105 °C; hydrocarbon gases—below 32 °C. Diverse types of oil vary greatly in composition. Light oil usually contains more gasoline, naphtha and kerosene, while heavy oil contains more gas oil and fuel oil. The most common oil contains 20–30% gasoline.
The quality of oil is also influenced by its water content and mechanical impurities. Water not only complicates oil refining processes, but also degrades the quality of oil products by reducing thermal conductivity and causing corrosion to metal parts of equipment. Mechanical impurities such as sand, clay, and rock particles reduce the efficiency of oil equipment. Importantly, the viscosity of oil plays a significant role in its quality. A lower viscosity makes it easier to transport oil through pipelines and process it. Due to differences in physical (density and viscosity) and chemical properties (sulfur content, resin, paraffin composition) between oils from different geographical origins, it is only possible to speak about an average composition conditionally. The properties of oil determine the principles of its processing and significantly influence the quality of the final petroleum products. This makes it essential to find environmentally friendly schemes and methods for processing each type of oil.
Oil degradation by natural transformations, owing to abiotic physical and chemical processes, often begins very quickly after petroleum products enter the environment. Gasoline and kerosene can be partially removed from the environment through weathering. During the first 15 days, about 30% of petroleum products can evaporate from the top layer in the summer due to light fractions removal [28]. Components of oil and petroleum products on the soil surface may be partially subject to photo-oxidation. Abiotic factors of oil degradation significantly depend on the climatic characteristics of different geographical zones. Diesel fuel and light oils do not weather much, but they can easily penetrate the lower layers of soil, sedimentary rocks, and groundwater. Heavy oil products and asphaltenes do not weather and slowly penetrate the soil. Spilled oil and petroleum products are adsorbed by the soil and are mostly localized in its upper horizon. Only a small portion of hydrocarbons can penetrate the subsoil layers and groundwater. This applies to the low-molecular-weight paraffins and naphthenic and aromatic hydrocarbons of oil with the simplest structures. Most of the light fraction consists of alkanes with 5–10 carbon atoms in their structure. This fraction of oil exhibits the highest ecotoxicity, but it is easily removed due to leaching or evaporation [29]. The latter is perhaps one of the most significant processes of oil spill transformation, together with leaching and photo-oxidation. During the first day in summer, up to 80% of technical gasoline, 22% of kerosene, 2–15% of crude oil, and only 0.3% of volatile components of fuel oil evaporate from an oil slick on the soil’s surface. Volatility and fluidity of an oil polluting the environment are its most important physical characteristics. Further degradation of oil hydrocarbons is associated with the process of their biochemical oxidation, which only occurs through the action of oil-oxidizing microorganisms [30].
3. Inactivation Methods of Petroleum Hydrocarbons in Environment
Remediation of natural ecosystems contaminated with hydrocarbons can be achieved by four main widely used methods: mechanical, chemical, physicochemical and biological treatments.
Petroleum impurities can be divided into the following categories: easily separable, difficult to remove, and soluble. Generally, impurities that are difficult to remove are coarsely dispersed in a droplet state. Depending on their quantity, they can form either a floating film or a solid surface layer on the surface of water and they represent the main contributors to oil pollution. Easily separable impurities make up a much smaller portion, appearing in the form of emulsion when they are dispersed in water. Such dispersion is often stable for long periods of time, unless proper measures are taken, aiming at transforming it into an easily removable phase [31]. Finally, soluble compounds constitute the fraction present in the smallest quantity, since the organic components that make up the structure of oil and petroleum products are poorly soluble in water. However, the concentration of petroleum products, or more precisely, their water-soluble compounds, gradually increases with prolonged contact of contaminants with water.
3.1. Mechanical Purification from Oil and Petroleum Products
Mechanical wastewater treatment is used as an independent method in cases where the water purified by this method is suitable either to be used for the needs of the technological production process or can be discharged into a natural reservoir without causing any damage to the ecosystem. In all other cases, this method only represents a primary purification of water from oil impurities. The mechanical cleaning method makes it possible to remove from 60 to 65% of suspended particles of various substances.
The most common methods of mechanical wastewater treatment from oil pollution are sedimentation, centrifugal removal, and filtration of water pollutants [32,33,34,35,36]. Mechanical methods include oil booms, namely stationary floating devices aimed at preventing the movement of an oil slick. The use of these elevated temperature-resistant barriers allows oil to be burned on site [37]. In addition to booms, skimmers are used as mechanical devices designed to physically remove oil from the surface of water [38]. The method of mechanical wastewater treatment from oil products is most effective in the first hours after a spill, owing to a sufficient thickness of the oil layer. Over time, the layer becomes thinner, and the area of contamination increases. In addition, the use of this method is more problematic when cleaning waters of shipyards and ports. In fact, such water areas are polluted with various debris: boards, wood chips and other objects, which prevents the purification of water from oil products. All over the world, various modifications of oil skimmers are used to eliminate oil spills in the aquatic environment. This technology does not solve the problem completely, as about 30% of oil products remain on the water’s surface after collection. Another negative aspect of this method is that oil skimmers absorb a significant amount of water, about 40–80%, when collecting oil using these suction devices. The collected water contains both floating and emulsified petroleum products in various states of aggregation. Thus, additional purification of these waters is necessary before returning them to the reservoir, leading to additional costs which may even double in this situation. As a consequence, the use of this technology with an oil film thickness of 1–3 mm is not rational.
Mechanical methods are also used to decontaminate petroleum hydrocarbons in highly polluted soil ecosystems when the concentration of hydrocarbons exceeds 50 g/kg or when the penetration depth of pollution in soils and grounds attains a thickness of 0.3–1 m. With mechanical methods, contaminated soils are removed manually or using special equipment and moved to reclamation sites. Collected oil, oil products, and oil-containing sludge are transported to ponds or sludge storage tanks.
A well-known method of cleaning up oil pollution is land reclamation by loosening soils to increase the penetration of oxygen and the development of redox reactions [39]. Currently, all over the world, in addition to mechanical soil cleaning, a physical cleaning method is widely used, based on the following technologies for local cleaning of heavily contaminated soils [40].
The use of ultrasound is an effective method for cleaning soil ecosystems from oil pollution [41,42]. During the collapse of cavitation bubbles that arise with a critical value of sound pressure of acoustic waves, the resulting microjets with linear velocities of 300–800 m/s tear off oil contaminants from the surface of solid particles. The cleaning efficiency can reach 99.5–99.8%. During cavitation, ruptures of liquid layers, ionization and activation of molecules may occur, stimulating the oxidation and polymerization of hydrocarbon molecules [43,44]. However, a significant disadvantage of this method is the prohibitive cost of hardware design.
Waste disposal in landfills is a traditional method of cleaning contaminated soil by removing and burying it at specially designated sites. However, additional soil treatment may be necessary to ensure complete removal of oil contamination [45]. This method is relatively economical, but it is not completely environmentally friendly, as oil-contaminated soil can persist for hundreds of years, remaining a potential source of toxicity for ecosystems. When creating landfills for oil waste, careful consideration should be given to ensuring their complete and permanent isolation from the surrounding natural environment [46].
Incineration. One method for in situ removal of oil contaminants from soil is their destruction by burning [47]. Excess petroleum products are pre-collected in any suitable way, for example, by booms. Shrimp and fishing vessels (VoOs) can be deployed in the event of large-scale offshore oil spills. The key components of a single-stage combustion system are an oleophilic skimmer and a floating burner [48].
This method has many negative sides. When it is implemented, secondary pollution of the environment occurs due to the formation of products of incomplete combustion of hydrocarbons [49]. There is also burning of plants, seeds, organic components of the soil, and disruption of the biocenosis as a whole; therefore, this method is applicable only in the event of a critical emergency situation, in case of large oil spills, when there is a threat to sources of drinking water supply and nearby groundwater [50].
3.2. Chemical Treatment of Wastewater and Lands Contaminated by Oil and Petroleum Products
One of the most adopted chemical methods of cleaning water areas from oil consists of using various chemical agents that react with oil hydrocarbons and precipitating them in the form of insoluble precipitates to the polluted water environment. By the aforementioned method, the reduction of insoluble impurities by up to 95 percent and soluble impurities by up to 25 percent is achieved. The principle of this technique is based on the creation of an oil slick on the surface of water with the help of surfactants and oil–water emulsifiers and on the absorption of oil of different types by adsorbents. Among them, graphene is considered to be highly effective [51,52,53]. The degradation time of oil when using a six-cycle purification method spans 6–7 days [54]. The determining parameters in the selection of sorbents are buoyancy, oil absorption, water absorption, oil capacity, degree of hydrophobicity, possibility of oil removal from the sorbent, possibility of sorbent regeneration, and recyclability [55]. The use of activated carbon as a sorbent for crude oil and petroleum products is expensive and it needs to be treated with a solvent prior to reuse. However, solvent is also costly, and alternative treatments such as thermal and advanced oxidation processes (UV/H2O2 and H2O2/O3) have been explored [26]. However, thermal treatment has been found to be ineffective, and homogeneous processes have also been deemed impractical due to cost. Regeneration was faster for smaller particles in the H2O2/O3 process, and in some cases, 100% of the original capacity was recovered. However, these processes consumed more oxidants than theoretically required. Also, another main disadvantage of activated carbon is its ability to become fouled by natural organic matter (NOM). This can prevent other organic pollutants from entering the micropores of the carbon, as it competes with them for adsorption sites. Hopman et al. investigated the use of activated carbon fiber (ACF) as a potential alternative to granular activated carbon, claiming that it is less affected by NOM, but the question of cost remains unanswered. [56]. Although activated carbon has high adsorption capacity, its disadvantages make synthetic resins a practical alternative. For example, syndiotactic PS monoliths are capable of emulsifying oil and crystallizing the resulting emulsion with a high internal phase within 12 h, which can be applied in aquatic ecosystems [57]. But its use is limited by a high production cost, so it is preferable to use other substances like zeolites, clays, and agricultural and industrial wastes. However, the main disadvantage associated with the use of these adsorbents for oil pollution removal is their availability, which varies from country to country and region to region [58]. At the same time, many scientists are investigating the possibility of using nanoparticles for oil pollution removal. In this context, some authors [59] proposed the use of functionalized magnetic iron oxide nanoparticles as a substrate that has a high adsorption capacity for oil pollutants. A simplified scheme of the relevant sorption mechanism is illustrated in Figure 5.
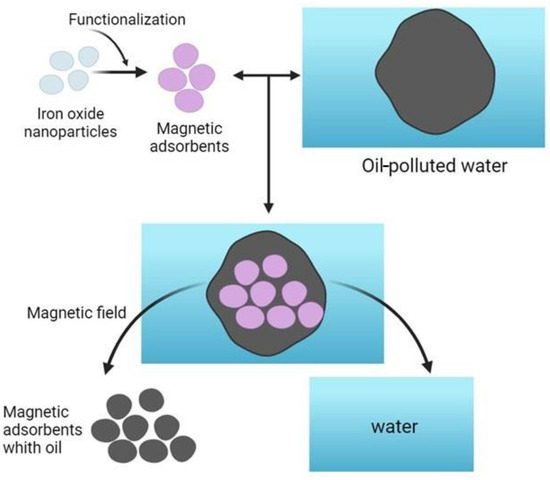
Figure 5.
Oil adsorption using iron oxide nanoparticles.
Most dispersants consist of surfactants that can emulsify oil droplets into smaller droplets and transfer them into water, where they undergo rapid decomposition [60]. Chemical dispersants reduce the likelihood of oil droplets adhering to the surface and accelerate the natural biodegradation of noxious compounds by increasing the surface area of the droplets [61]. The disadvantage of this method is the possibility of accumulation of petroleum products at the bottom of the reservoir, which leads to secondary pollution of the aquatic environment.
As for treatments of oil-contaminated lands, electrochemical methods are appealing as they do not require excavation of polluted soils. When using this method, electrodes are immersed in contaminated soil, to which a direct electric current is supplied. The principle of electrochemical treatment is based on the fact that most soils contain a certain amount of aqueous salt solutions in the pores between particles, and therefore have electrical conductivity. Most pollutants dissolve in soil moisture and, under the influence of an electric field, move to the electrodes, are deposited on them, and are further decomposed. Depending on the properties of the soil, the movement of pollutants can occur by migration or electroosmosis, or by both mechanisms simultaneously [62]. The main advantage of the electrochemical purification method is its use for low-permeability (clay) soils, and it proved to be highly efficient in the abatement of a wide variety of pollutants, including metals and organic compounds.
Electrochemical treatment of contaminated lands. One of the methods for cleaning soil that does not require excavation is electrochemical treatment of oil-contaminated soils. When using this method, electrodes are immersed in contaminated soil, to which a direct electric current is supplied. The principle of electrochemical treatment is based on the fact that most soils contain a certain amount of aqueous salt solutions in the pores between particles, and therefore have electrical conductivity. Most pollutants dissolve in moist soil and, under the influence of an electric field, move to the electrodes, are deposited on them, and are then removed. Depending on the properties of the soil, the movement of pollutants can occur due to migration or electroosmosis, or both mechanisms simultaneously [63]. Electrochemical cleaning technologies depend on the electric generator used [64,65] and the material of the electrodes [65]. The use of metal (SK-ORTA) electrodes shows that they are more energy-efficient than graphite ones and also have the longest service life [65]. In this case, the pH balance is maintained by the addition of electrolytes. The initial pH does not play a significant role. The main advantage of the electrochemical purification method is its use for low-permeability (clay) soils and the ability to extract a wide variety of pollutants, including metals and organic compounds. However, the cost of the hardware is quite high, even though the cleaning process is effective.
3.3. Physicochemical Treatment of Ecosystems Contaminated with Oil and Petroleum Products
The main unit operations for physicochemical wastewater treatment are coagulation, flotation, and sorption.
The essence of coagulation is the accelerated transformation of finely dispersed particles (with sizes from 1 to 100 μm) and emulsified types of contaminants into larger formations, which then fall out in the form of sediment. This process is promoted by coagulants, namely by special chemical reagents [66], whose action leads to the formation of flakes on the water’s surface, favoring an interaction with oil impurities present in a colloidal state and having a weak positive electrostatic charge. The coagulant is capable of electrostatically attracting these impurities, which, under the influence of gravity, fall out in the form of a sediment of a loose structure to the bottom of treatment tanks [63]. Petroleum compounds, represented by two types of oil emulsions with different initial concentrations, were prepared from diesel oil and a mix of engine oil. They were then removed from water using a volumetric coagulation method. During this process, several reagents were used, including a powdery diatomite with a particle size of less than 0.5 microns, as well as coagulants such as aluminum sulphate and iron chloride, and a cationic flocculant. Depending on the coagulant dose and treatment time, the efficiency of this technique reached 80% [67].
The flotation process leads to the formation of a stable foam on the water’s surface, through which harmful impurities of petroleum products are captured and retained for a long time. Such a foamy layer is easily separated from treated wastewater. The basis of the foam described above is a stable connection of air or gas bubbles with oil particles. Based on the principle of formation of flotation bubbles, this process is divided into mechanical flotation, vacuum flotation, and electroflotation [68].
As a general trend, a highest degree of purification of oil-contaminated wastewater is achieved only by means of a sorption method, which is based on a capture of toxic impurities, including petroleum products, by a suitable solid substrate [69]. Various materials with a porous structure are used as sorbents: peat, coke, ash, silicate gel, and several types of active clays. Typically, aluminosilicate microspheres and aluminum oxide are one of the most popular choices, thanks to the possibility of their reuse by subsequent burning of oil from the pores of the sorbent by combustion. The adsorption capacity of aluminosilicates is 800 mg/g (470 mg/cm3). The level of purification of water from oil by this technique can even exceed 98%, but this method is limited by the area of the source of oil contamination, i.e., it is applicable to purify localized quantities of water from oil. Several types of activated carbon are ranked first among sorbents with the highest efficiency [70,71,72], which is due to their high porosity and large specific surface area. The porosity of coal varies from 60% to 70%, while its specific surface area (depending on the technology used to manufacture the coal) is in the range of 500 to 1500 m2/g [71].
Physicochemical methods for cleaning soil ecosystems include treating contaminated soils in special devices with heated aqueous solutions in the presence of surfactants or other chemical agents (sorbents); extraction of petroleum products from soils with various solvents, including vacuum extraction, etc. Liming of oil-contaminated soils—that is, treating the soil with quicklime in an amount of 0.5–5% of the mass of the spilled petroleum substance—leads to the formation of a solid product that firmly holds petroleum-derived products in the form of complex compounds [73]. Table 1 contains a short list of green and conventional sorbents for oil-derived products.

Table 1.
A list of natural and artificial materials used as sorbents for oil-derived pollutants.
Currently, the methods of physicochemical and biological purification of water and soil ecosystems are the most effective and safe techniques for environmental remediation [88,89]. The choice of a sorbent depends on the scale of pollution, the stage of purification, the required quality of purification, and the state of petroleum pollutants. In this direction, there is an active search for ways to improve the quality of existing sorbents and the development of new ones. Natural sorbents, with particular reference to plant residues like humic acid and peat moss, are among the most economical and accessible biosorbents [90,91]. The high porosity and large surface area of peat make it an excellent natural sorbent for oil spills [92]. Figure 6 shows some of the most recent sorbents made from various materials, including polymers, biomass, and waste.
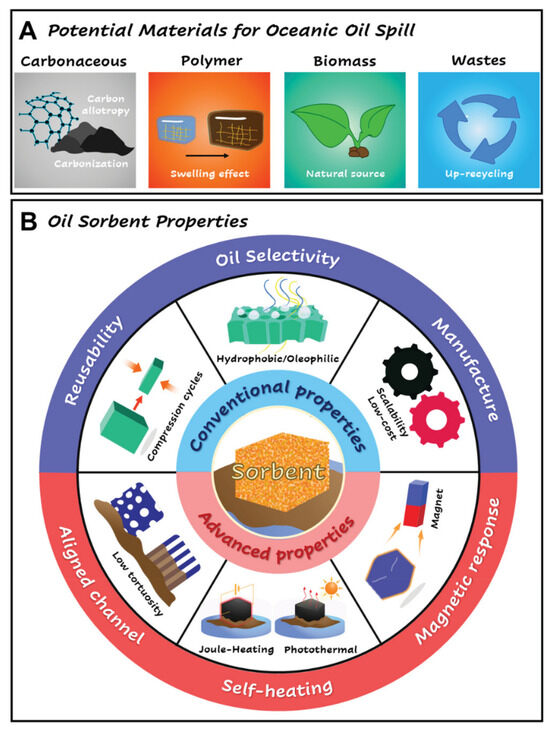
Figure 6.
(A) Schematic illustration of potential materials for oceanic oil spill decontamination procedures. (B) Schematic diagram of conventional and advanced properties of sorbents [72]. Copyright 2016.
As is well known, humic acids (HAs) can accelerate the processes of abiotic and biotic decomposition of various ecotoxicants: they increase the solubility of highly hydrophobic organochlorine pesticides in water and accelerate the photolysis of polyaromatic hydrocarbons, catalyzing the hydrolysis of s-triazines [93].
Due to their redox mediator properties, humic acids act as terminal electron acceptors, accelerating the processes of anaerobic decomposition of organic pollutants [94]. Studying the role of humic acids in decontaminating the environment from oil and petroleum products is of great practical interest for the search for new detoxifying agents of plant origin, the use of which is not associated with the danger of secondary pollution of various ecosystems. In Figure 7, some steps of the purification process carried out by humic acids are visually summarized.
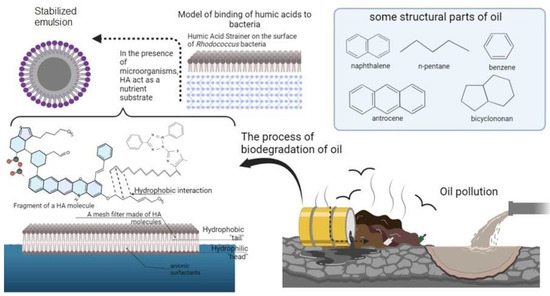
Figure 7.
Detoxification of oil and petroleum products with humic acids.
3.4. Biological and Biotechnological Methods for Cleaning Soil and Water Environments from Oil Pollution
The biotechnological approach is the most promising technique for cleaning up oil-contaminated soils and water areas in economic and environmental terms. It is based on the use of various groups of microorganisms characterized by an increased ability to biodegrade components of oil and petroleum products. The possibility of degrading substances of anthropogenic origin (xenobiotics) that are difficult to decompose has been found in many organisms [88,89,90,91,92,93,94,95,96]. This property is ensured by the presence of specific enzyme systems in microorganisms that catabolize such compounds. Microorganisms have a relatively high potential for the destruction of xenobiotics; they exhibit the ability to undergo rapid metabolic restructuring and exchange of genetic material; they are progressively gaining great importance in the development of strategies for bioremediation of contaminated objects [97].
Bioremediation consists of the use of technologies and devices designed for biological purification of soils, i.e., for removing pollutants that are already present in a contaminated environment.
Phytoremediation relies upon using plant organisms to degrade, contain, or immobilize toxic substances from soil, sludge, groundwater, surface water, and wastewater [98]. The phytoremediation method is based on the ability of plants to absorb, accumulate, and decompose components present in a polluted environment [99,100,101].
Plants can be used for phytoremediation of various classes of pollutants [100,101]; Table 2 presents a list of the main plants used in this method. Additionally, several new species used in phytoremediation have been proposed in recent studies, namely Cannabis sativa L. [102], Phragmites australis [103], Broussonetia papyrifera [104], Eichhornia crassipes, Pistia stratiotes, Lemna minor [105], and Salvinia molesta [106].

Table 2.
Typical plants used in phytoremediation.
Phytoremediation is a long-term process for restoring oil-contaminated soil. Despite its high efficiency, it can take years for almost complete removal of oil and petroleum products [125]. Recently, the phytoremediation potential of the plant species Alhagi camelorum was explored. The research found that after six months of treatment, the average percentage of total petroleum hydrocarbon removal was 53.6 ± 2.8% [126].
The processes of degradation of oil and petroleum products during phytoremediation are usually divided into rhizodegradation and phytodegradation (phytotransformation). During the decomposition of petroleum products, their structure is subject to biochemical change or destruction [127,128]. Rhizodegradation, which is a natural biodegradation of oil pollutants in an ecosystem under the action of plant roots, leads to a nearly complete destruction or detoxification of most organic pollutants by means of an enhanced microbial activity carried out in the root zone or rhizosphere [101]. It is believed that bacteria carry out rhizodegradation, and their number increases in the rhizosphere. Growth in the population and activity of microbes in the rhizosphere leads to increased biodegradation of petroleum products [129]. The potential of plants used for the remediation of oil-contaminated soil was assessed by the prevalence of genes encoding enzymes involved in the decomposition of the main components of oil pollution, like alkanes (alkB gene) and aromatic hydrocarbons (nahH gene, nahAc gene) [120].
Lin and Mendelssohn [130] indicate that Spartina alterniflora potentially enhances aerobic biodegradation of oil underground by transporting oxygen to its roots. The advantages of rhizodegradation include in situ destruction of the contaminant, possible complete mineralization of organic contaminants, and minimal likelihood of toxic compounds being transferred to plants or to the atmosphere compared to other phytoremediation technologies, as degradation occurs directly at the source of the contaminant.
Phytodegradation (phytotransformation) is the absorption of organic and nutrient substances from soil and groundwater and their subsequent transformation by plants. The phytodegradation method is used to detoxify the soil and aquatic environment owing to the degradation of petroleum products as a result of plant metabolism [128].
The process of phytodegradation does not depend on microorganisms associated with the rhizosphere [89] (microorganisms metabolize organic pollutants into carbon dioxide and water), but on plant enzymes that completely metabolize or mineralize toxicants into carbon dioxide and water [131].
The method of rhizofiltration and phytoextraction leads to the accumulation of pollutants in plants. With rhizofiltration, accumulation occurs in the roots or in the above-ground part of the plant; with phytoextraction, it occurs underground, but not in the roots. Rhizofiltration differs from phytoextraction in that the pollutant is initially located in the aquatic environment and not in the soil [101]. To acclimatize plants after large root systems have developed, contaminated water is collected from the waste site and delivered to the plants, where it replaces the moisture source. The plants are then planted in the contaminated area, where the roots absorb water and oil products along with it. When they become saturated with pollutants, they are collected [127]. Rhizofiltration uses both aquatic and terrestrial plants. Given their platform support for growth on water, terrestrial plants have the advantage of greater biomass and longer, faster-growing root systems than aquatic plants [128]. Rhizofiltration can be carried out in situ to remediate contaminated surface water bodies or ex situ, in which a specially designed reservoir system can be used to retain the introduced contaminated water and plants. Any of these systems will require an understanding of speciation and the interactions of all pollutants with each other.
Microorganisms are used to degrade oil pollution by bioremediation according to the processes of biostimulation and bioaugmentation [74]. The former consists of the activation of the degrading ability of native microflora by introducing nutrients, oxygen, and various substrates, while bioaugmentation is the introduction of natural and genetically engineered strains that destruct foreign compounds. At the same time, the preferential and selective growth of those microorganisms that are able to most effectively utilize a pollutant is ensured. “Activated” microflora is introduced into the contaminated object simultaneously with the necessary additives that increase the efficiency of pollutant disposal.
Bioaugmentation may involve the use of genetically engineered microorganisms suitable for the biodegradation of hydrocarbon pollutants in toxic water [132,133,134]. In addition, bioaugmentation is not as effective for use in oil spill responses [133,134].
As stated before, biostimulation is the addition of nutrients required by indigenous hydrocarbon-degrading microorganisms to achieve maximum degradation of the toxic compounds present [102]. During an oil spill, the increase in carbon stimulates the growth of pre-existing oil-degrading microorganisms. Since microorganisms are limited in growth and recovery due to available nitrogen and phosphorus, adding additional nutrients in proper concentrations promotes hydrocarbon degradation by microorganisms. This is due to the fact that microorganisms are able to achieve maximum growth rates and therefore maximum absorption rates of pollutants. To achieve maximum biostimulation, an important factor is to obtain the ideal concentration of nutrients required for maximum microbial growth and to maintain an optimal concentration of microorganisms for as long as possible [135].
As is well known, natural microorganisms like fungi, yeast, and bacteria are largely used for the breakdown of petroleum products in the process of bioremediation [136]. In addition, bioremediation is a process in which microorganisms degrade and metabolize chemicals and restore the quality of the environment [137]. The main microorganisms used for hydrocarbon degradation are shown in Table 3 [138].

Table 3.
Oil-degrading microorganisms.
Although many studies [158,159,160] report the ability of bacteria to degrade hydrocarbons of various chain lengths, most bacteria consume only a narrow range of hydrocarbons. For example, Geobacillus jurassicus grows only at C6–C16, and Bacillus thermoleovorans degrades n-alkanes to C23. Indeed, there are a small number of strains capable of degrading the wide range of hydrocarbons currently identified [151]. An exception is the strain Acinetobacter sp., which decomposes long-chain alkanes (C13–C44).
The goal of bioremediation is to accelerate the natural degradation process of hydrocarbons by which microorganisms assimilate organic molecules throughout the biomass and produce by-products such as carbon dioxide, water, and heat [161]. The bioremediation method uses the natural biological activity of microorganisms or enzymes to convert toxic components of oil into less toxic or harmless metabolites [162]. It is known that bioremediation is a preferable alternative for long-term restoration of ecosystems contaminated with petroleum products, the advantages of which are economic efficiency and environmental friendliness [163].
The main advantage of bioremediation over other remediation processes is its low cost and savings in the time spent by workers cleaning up the contaminated site. The environmental friendliness of bioremediation is due to the fact that no foreign or toxic chemicals are added to the site, and therefore no disturbance of the natural habitat is allowed [135,137]. Namely, natural organisms convert toxic hydrocarbons to simple compounds that do not pose a threat to the environment. Despite all the advantages, the disadvantages of bioremediation include the length of the process depending on the location of the pollution, which causes difficulties in providing adequate concentrations of nutrients for oil-degrading microorganisms [137,164].
There are two existing ways to intensify the biodegradation of xenobiotics in the environment: stimulation of natural microflora and introduction of active strains. They not only do not contradict, but also complement each other. The restoration of vital processes depends on the ability of soil and water to process organic toxicants into environmentally friendly, easily digestible metabolic products.
3.5. Biodegradation of Petroleum Hydrocarbons by Microbial Consortia
A study of bacteria responsible for bioremediation showed that more than one type of microorganism is required for complete biodegradation of oil and petroleum products [165]. Individual microorganisms can only metabolize a partial range of hydrocarbon substrates, so mixing populations with a common enzymatic capacity requires a further increase in the rate and extent of oil biodegradation [166]. Another study proved that microbial strains belonging to various genera were found in oil-contaminated soil or water [167]. It is assumed that each strain or genus played a role in hydrocarbon conversion processes. More studies have been reported on mixed-culture symbiosis in biodegradation processes [90,167] with sequential changes in the composition of oil-degrading bacteria over a period in oil-contaminated sand samples. Relevant observations have been reported for successive enrichments in environments containing residual crude oil.
The study of oil-degrading microorganisms has shown that in nature, there are many bacterial communities capable of decomposing oil hydrocarbons, which are found at relatively high densities in oil-contaminated areas [168]. The most common sites are around estuaries, oceans and aquatic sediments, fresh water, deep sea, and thermal plates. For this reason, these areas are being studied to isolate hydrocarbon-degrading microorganisms. Among oil-degrading microorganisms isolated from aquatic habitats, Pseudomonas, Vibrio, Achromobacter, Rhodococcus, Arthrobacter, Micrococcus, Corynebacter, Acinetobacter, Nocardia, etc., are the predominant utilizers of oil hydrocarbons [169].
Bioremediation of petroleum hydrocarbons using oil destructor microorganisms is another aspect deserving of attention, in line with a cost-effective and environmentally friendly solution for the removal of petroleum contaminants. Bacteria capable of using alkanes as a source of carbon and energy have received considerable interest over the past few decades. Many bacterial species have been identified as potential hydrocarbon degraders [170,171,172,173,174,175]. By the way, the most promising microorganisms that degrade petroleum hydrocarbons include representatives of the genus Rhodococcus. They produce various surfactants, including low-molecular-weight trehalolipids [176,177] or high-molecular-weight exopolysaccharides [178], thus reducing surface tension and increasing the bioavailability of water-insoluble substrates. Rhodococcus cell walls contain mycolic acids, which play a basic role in redox reactions. Moreover, Rhodococcus is also capable of changing its membrane composition and cell surface properties in response to organic substrates [179].
The process of bioremediation of petroleum carbohydrates is a complex biological system where different strains of bacteria are responsible for the destruction of aliphatic and aromatic fragments of petroleum products. Bioremediation occurs through oxidation reactions; however, the pathways vary greatly due to the specific oxygenases found in different bacterial species. For example, some bacteria can metabolize certain alkanes, while others break down the aromatic or tar fractions of hydrocarbons. This phenomenon is associated with the chemical structure of petroleum hydrocarbon components [180], as described in Figure 8, Figure 9 and Figure 10.
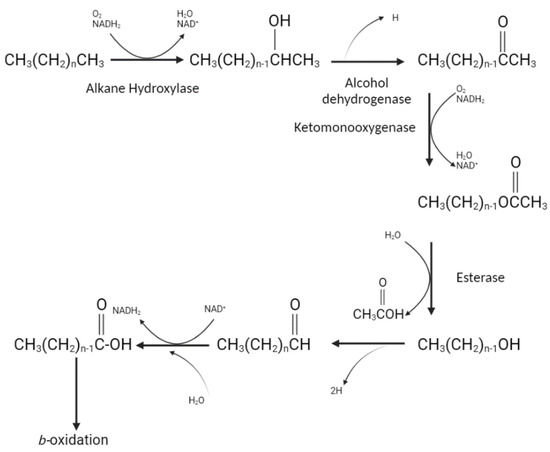
Figure 8.
Degradation of straight chain alkanes.
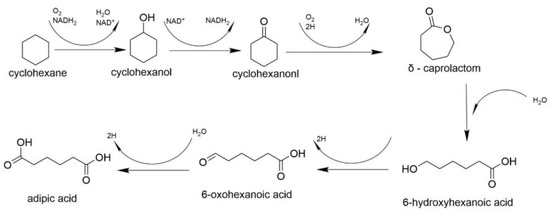
Figure 9.
Degradation of cycloalkanes.
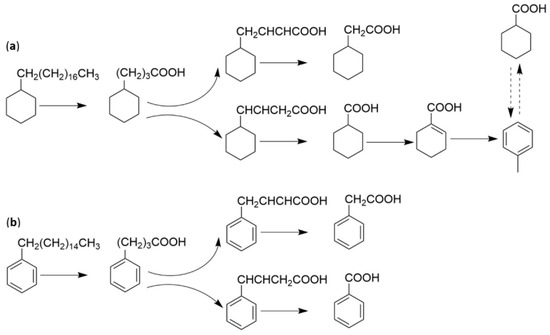
Figure 10.
Metabolic pathway of biodegradation of n-alkylcyclohexane (a) and n-alkylbenzene (b) under the influence of Alcanivorax sp. MBIC4326.
The degradation of C6H12 occurs as follows:
The basic principle of aerobic degradation of hydrocarbons by oil-degrading microorganisms is presented in Figure 11:
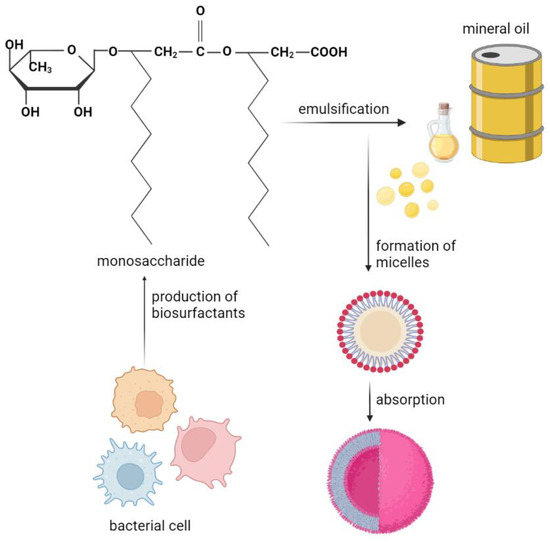
Figure 11.
Participation of biosurfactant (rhamnolipid—monosaccharide) produced by microorganisms Pseudomonas sp. in the absorption of petroleum hydrocarbons.
In addition to traditional methods for detoxifying organic toxicants, a biosorption method for cleaning oil-contaminated ecosystems is currently being developed to effectively combat oil pollution. Biosorption is a modern biotechnological approach aimed at sustainable development [181], as it allows a deep purification of wastewater from petroleum products contained in it. An example of a one-step biosorption process is illustrated in Figure 12. A number of authors have reported that the use of various agricultural lignocellulosic wastes is an effective way to purify water contaminated with hydrocarbons [182,183,184,185,186,187]; in this case, these wastes are cheap and readily available biosorbents. The principle of this method is based on the combined use of sorbents and microorganisms. Biomodification of a resistant contaminants in the microporous structure of the sorbent into a biodegradable form, followed by its oxidation by a biofilm on the surface of the sorbent [188,189], are the basis for many biotechnological applications in environmental remediation. In [185], the possibility of using a biosorbent to remove hydrocarbons from water was investigated. In their work, they used the Langmuir isotherm model to design a one-step batch biosorption process (Figure 12) to treat a specified volume of oil-contaminated wastewater.
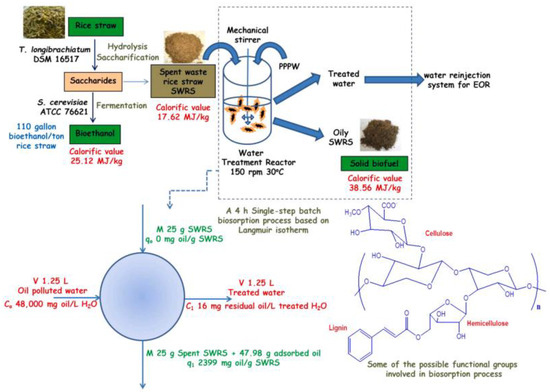
Figure 12.
Schematic diagram of a one-step batch biosorption process [185]. Copyright 2022, Journal of Hazardous Materials.
The basic principle of biosorption is defined as a metabolism-independent process (passive absorption) based on the use of biomass waste to remove pollutants of different classes. It is thanks to the recycling of biomass that numerous advantages are achieved, including lower cost compared to synthetic sorbents [190]. This method is the most effective for purifying aqueous media from oil and petroleum products. This kind of result cannot be achieved through the separate use of microbiological and sorption methods. In addition, these biosorbents are preferable to synthetic adsorbents due to their higher sorption capacity and biodegradability [191], which is a key point from an environmental point of view.
3.6. Hybrid Biotechnological Methods for Detoxification of Oil-Polluted Ecosystems
The bioavailability of oil and petroleum products in the environment is the main limitation of a complete bioremediation process. Organic toxicants are considered bioavailable if they are dissolved in an aqueous phase, and non-bioavailable if they are bound to a sorbent [135]. However, kinetic analysis of mineralization of a sorbed substrate (i.e., the release of substrate carbon in the form of CO2) by organisms in soil or bioreactors has shown that in some cases the rates of mineralization exceed the rates of desorption [135,136]. These results indicate that some organisms interact with environment sorbent in such a way that a path is created for the absorption of the substrate, which prevents desorption into the main aqueous solution or increases the rate of mass transfer from the sorbent to the bacterial cell. Known technologies for oil-recovery hybrid preparations are presented in Table 4.

Table 4.
Technologies for cleaning oil-contaminated ecosystems with biohybrid sorbents.
Humic acids are the most abundant form of organic matter in terrestrial ecosystems [202], and microbial interactions with these materials can be fundamental in many ways. In anaerobic environments, humic acids are used as electron acceptors or electron shuttles by various bacteria [203]. For aerobic microorganisms, exposure to humic acids has different effects on metabolic activity in general and on the biodegradation of organic pollutants in particular. For example, the growth of heterotrophic and nitrifying bacteria has been reported to be stimulated by the addition of humic acids to culture media, and in both cases this effect has been attributed to changes in membrane characteristics resulting in increased nutrient uptake [137]. Exposure to humic acids contributed to an increase in the lifespan of Arthrobacter crystallopoietes microorganisms [138]. The decomposition of homo- and heterocyclic aromatic compounds was stimulated by humic substances, which is explained by an increase in the rate of substrate absorption [140]. However, Shimp and Pfaender [140] observed a decrease in the ability of mixed bacterial cultures to degrade phenolic compounds after exposure to humic acids and suggested that the latter caused changes on the cell surface that prevented the uptake of phenol. Exposure to humic acids has also been shown to stimulate PAH degradation, and this postulated effect reflects improved solubilization of PAHs by humic acids or the concentration of PAHs on humic acid surfaces [141].
Several studies on the bioavailability of organic pollutants, including PAHs [142], using humic acids showed several mechanisms concerning the influence of HA on bioremediation. Among them, an important role is played by increased solubility of toxicants, increased microbial activity, a growth in the population of microorganisms, and their ability to produce useful enzymes for biodegradation. A study of the effect of extractable humic acids on bacterial density during anthracene bioremediation in liquid systems showed that, in tests with humic acids, an 8-fold increase in bacterial density was observed even after 12–15 days [143]. In tests where anthracene was the only substrate, no increase in bacterial population was detected. Thus, the increase in the bacterial population indicates that humic acids can be used as a pre-prepared substrate for the degradation of organic toxicants. The presence of humic acids can stimulate the bacterial community and its activity, causing an increase in the rate of bioremediation of toxicants. Some examples describing the action of humic acids are reported in Figure 13. In the study, associations of oil-degrading bacteria, including Rhodococcus X5, Rhodococcus S67, Pseudomonas 142 NF, and humic acids were used. The degradation of oil product films occurred within 7 days when a monobacterial mixture was used, and within 2 days when a polybacterial mixture was used [204].
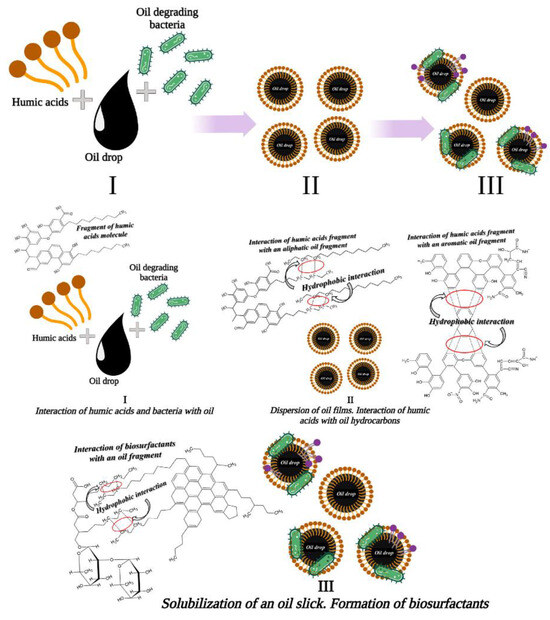
Figure 13.
Stages of the mechanism of bioorganic compositions action in relation to oil hydrocarbons [204]. Copyright 2023, Energies.
Microorganisms capable of gaining access to sorbed substrates occupy an important niche in the process of biodegradation of petroleum substances. Moreover, most of them have wide and effective applications [205,206,207,208,209]. Systems of microorganisms belonging to different species, but capable of effective destruction of organic toxicants individually, are known to be used [144]. These data support the hypothesis that a unique microbial population can adapt to and mediate the degradation of sorbed toxic substances. However, it is unknown whether microbes can adapt to interact with heterogeneous natural materials (e.g., organic matter in soil) that serve as primary environmental sorbents for oil and petroleum products in ways that facilitate the uptake of petroleum products sorbed by these materials. Both mineral and organic constituents of soil and sediment can act as sorbents, but the latter most often play a major role. The organic phase is a complex system and may consist of contaminants (e.g., non-aqueous liquid phases) as well as a variety of natural materials. Among the latter, humic acids predominate. The chemistry of humic acids and the sorption of hydrophobic compounds such as oil resemble the partition process between an aqueous solution and the organic phase [146,210]. Desorption of compounds sorbed by humic acids is usually considered necessary for entry into the bioavailable phase and subsequent biodegradation of toxicants.
4. Conclusions
Pollution from oil and petroleum products is one of the most significant forms of human impact on the environment. During the extraction, transportation, storage, and processing of oil, spills and leaks of petroleum hydrocarbons frequently occur, leading to environmental accidents. These substances, traveling hundreds and thousands of kilometers from their point of release, can enter aquatic and terrestrial ecosystems, infiltrating deep into the sea and accumulating in sediments, contaminating soils, and affecting all forms of life. There are various methods available for mitigating the effects of oil pollution, including standard cleanup techniques such as mechanical, chemical, physicochemical, and biological methods. The advantages and disadvantages of different remediation methods were considered and are presented in Figure 14.
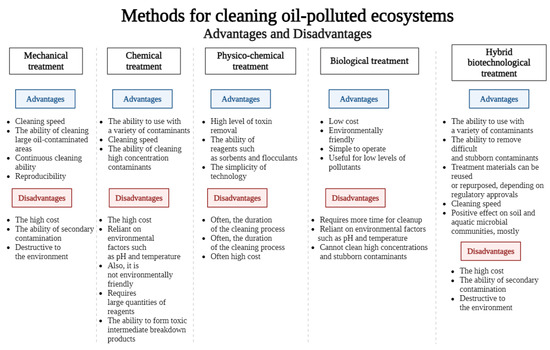
Figure 14.
Advantages and disadvantages of remediation methods.
Mechanical removal is often the first step in cleaning up oil spills, and it can remove up to 65% of the visible oil. The most promising technology for mechanical cleaning is the use of ultrasound. This method can remove up to 99% of oil, but its effectiveness is limited by the size of the area of contamination and requires expensive equipment. In the case of chemical cleaning, up to 95% of insoluble impurities and up to 25% of soluble impurities are removed. However, using synthetic petroleum-based oxidizers can lead to a high risk of secondary pollution, and the cost of this cleaning method is also very high. Physicochemical methods for cleaning soil ecosystems involve treating contaminated soils in specialized devices using heated aqueous solutions with surfactants or other chemical agents (sorbents). In the case of bioremediation, low remediation costs and less environmental impact represent the major strengths. However, the inability of bioremediation to detoxify high concentrations of toxic substances in a short time is a significant limitation of this method. But a key role in the overall process of cleaning natural ecosystems seems to be played by biological factors, particularly microorganisms that oxidize hydrocarbons, with increasing frequency. The process of bioremediation of ecosystems contaminated with oil and petroleum products relies on the ability of these microorganisms to break down the complex organic compounds found in oil. Most petroleum hydrocarbons eventually degrade or metabolize in the environment, thanks to local microorganisms that use them as a source of energy and carbon for growth and reproduction. Advances in microbial biotechnology and high-throughput sequencing techniques, such as microfluidics, may be useful in identifying and screening for functional microorganisms in oil-contaminated areas. Indeed, many studies have shown that bacteria capable of breaking down hydrocarbons are present in oil-rich environments, such as oil spill zones and oil reservoirs. Their abundance is closely linked to the types of hydrocarbons present and environmental factors. However, no single microorganism is able to completely decompose all types of hydrocarbons. Instead, most bacteria can only break down or utilize certain components of oil, leaving other components inaccessible. This is because different microorganisms have different enzymes that allow them to perform different tasks. In order to effectively clean up oil-contaminated areas, it is important to combine the efforts of multiple bacteria with different capabilities. Additional “green” chemicals may also be used to enhance the process. Our research has shown that a clean-up efficiency of 70% or more can be achieved by optimizing the strengths of two or more techniques in order to overcome the limitations of each individual method.
Author Contributions
Conceptualization, A.P.R. and Y.M.A.; methodology, V.A.A. and L.V.P.; validation, M.M.G., A.P.R. and A.N.G.; formal analysis, M.M.G.; investigation, M.M.G.; resources, A.N.G. and A.M.C.; writing—original draft preparation, M.M.G., V.A.A. and A.S.K.; writing—review and editing, M.M.G. and V.A.A.; visualization, M.M.G., A.N.G. and A.S.K.; project administration, V.A.A. and A.P.R.; funding acquisition, V.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Russian Science Foundation grant No. 23-73-01220 (https://rscf.ru/project/23-73-01220/, accessed on 10 June 2024) and was carried out by a grant from the Government of the Tula Region in the field of science and technology, contract DS/101/BASiB /23/TO (ДC/101/БACиБ/23/TO) dated 27 September 2023.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oberschelp, C.; Pfister, S.; Hellweg, S. Global site-specific health impacts of fossil energy, steel mills, oil refineries and cement plants. Sci. Rep. 2023, 13, 13708. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, P.; Mafigholami, R. Optimisation of soil washing method for removal of petroleum hydrocarbons from contaminated soil around oil storage tanks using response surface methodology. Sci. Rep. 2023, 13, 15457. [Google Scholar] [CrossRef]
- Sadrara, M.; Khorrami, M.K. Designing an efficient organic–inorganic hybrid nanocomposite for simultaneous oxidative/adsorptive desulfurization of model and real fuel oils. Sci. Rep. 2023, 13, 15134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xie, Z.; Xu, H.; Wang, N.; Zhang, L.; Mao, N.; Cheng, J. Oil spill environmental risk assessment and mapping in coastal China using automatic identification system (AIS) data. Sustainability 2022, 14, 5837. [Google Scholar] [CrossRef]
- Neukirchen, F. The Formation of Mountains; Springer Nature: London, UK, 2022. [Google Scholar]
- Vocciante, M.; Reverberi, A.P.; Pietrelli, L.; Dovì, V.G. Improved remediation processes through cost-effective estimation of soil properties from surface measurements. J. Clean. Prod. 2017, 167, 680–686. [Google Scholar] [CrossRef]
- Vocciante, M.; Reverberi, A.P.; Dovì, V.G. Approximate solution of the inverse Richards’ problem. Appl. Math. Model. 2016, 40, 5364–5376. [Google Scholar] [CrossRef]
- Gaurina-Međimurec, N.; Pašić, B.; Mijić, P.; Medved, I. Deep underground injection of waste from drilling activities—An overview. Minerals 2020, 10, 303. [Google Scholar] [CrossRef]
- Michel, J.; Fingas, M. Oil spills: Causes, consequences, prevention, and countermeasures. In Fossil Fuels: Current Status and Future Directions; Crawley, G.M., Ed.; World Scientific: Singapore, 2016; Volume 7, pp. 159–201. [Google Scholar]
- Kazzaz, A.E. Interaction of Lignin Derivatives with Polymers, Ions and Soft Surfaces in Aqueous Systems. Ph.D. Thesis, Faculty of Graduate Studies of Lakehead University, Thunder Bay, ON, Canada, 2021. [Google Scholar]
- Zamparas, M.; Tzivras, D.; Dracopoulos, V.; Ioannides, T. Application of sorbents for oil spill cleanup focusing on natural-based modified materials: A review. Molecules 2020, 25, 4522. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, L.; Yang, C.; Kang, W.; Yan, Z.; Zhu, Y.; Wang, J.; Zhang, H. Removal kinetics of petroleum hydrocarbons from low-permeable soil by sand mixing and thermal enhancement of soil vapor extraction. Chemosphere 2019, 236, 124319. [Google Scholar] [CrossRef]
- Sandifer, P.A.; Ferguson, A.; Finucane, M.L.; Partyka, M.; Solo-Gabriele, H.M.; Walker, A.H.; Wowk, K.; Caffey, R.; Yoskowitz, D. Human health and socioeconomic effects of the Deepwater Horizon oil spill in the Gulf of Mexico. Oceanography 2021, 34, 174–191. [Google Scholar] [CrossRef]
- Yan, K.; Zhao, F.; Pan, L.; Jiang, Y.; Shi, Y.; Yu, G. High-throughput clean-up of viscous oil spills enabled by a gel-coated mesh filter. Nat. Sustain. 2023, 6, 1654–1662. [Google Scholar] [CrossRef]
- Brown, I.; Tari, E. An evaluation of the effects of petroleum exploration and production activities on the social environment in Ogoni Land Nigeria. Int. J. Sci. Technol. Res. 2015, 4, 273–282. [Google Scholar]
- Helle, I.; Mäkinen, J.; Nevalainen, M.; Afenyo, M.; Vanhatalo, J. Impacts of oil spills on arctic marine ecosystems: A quantitative and probabilistic risk assessment perspective. Environ. Sci. Technol. 2020, 54, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Little, D.I.; Sheppard, S.R.J.; Hulme, D. A perspective on oil spills: What we should have learned about global warming. Ocean. Coast. Manag. 2021, 202, 105509. [Google Scholar] [CrossRef]
- Kumar, M.; Bolan, N.S.; Hoang, S.A.; Sawarkar, A.D.; Jasemizad, T.; Gao, B.; Keerthanan, S.; Padhye, L.P.; Singh, L.; Kumar, S.; et al. Remediation of soils and sediments polluted with polycyclic aromatic hydrocarbons: To immobilize, mobilize, or degrade? J. Hazard. Mater. 2021, 420, 126534. [Google Scholar] [CrossRef] [PubMed]
- Igalavithana, A.D.; Ok, Y.S.; Usman, A.R.A.; Al-Wabel, M.I.; Oleszczuk, P.; Lee, S.S. The effects of biochar amendment on soil fertility. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; SSSA Special Publications: Fitchburg, WI, USA, 2015; pp. 123–144. [Google Scholar]
- Ahmad, A.A.; Muhammad, I.; Shah, T.; Kalwar, Q.; Zhang, J.; Liang, Z.; Mei, D.; Juanshan, Z.; Yan, P.; Zhi, D.X.; et al. Remediation Methods of Crude Oil Contaminated Soil. World J. Agric. Soil. Sci. 2020, 4, 595. [Google Scholar]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O. A systematic review on the effectiveness of remediation methods for oil contaminated soils. Environ. Adv. 2022, 9, 100319. [Google Scholar] [CrossRef]
- Sultanova, G.; Abdulaeva, M. Treatment method of the soil, polluted by oil and oil products in climatic conditions of Azerbaijan. Bull. Sci. Pract. 2021, 7, 31–38. (In Russian) [Google Scholar] [CrossRef]
- Azizian, S.; Khosravi, M. Chapter 12—Advanced oil spill decontamination techniques. Interface Sci. Technol. 2019, 30, 283–332. [Google Scholar]
- Loyeh, E.N.; Mohsenpour, R. Investigation of oil pollution on aquatic animals and methods of its prevention. J. Aquac. Mar. Biol. 2020, 9, 160–165. [Google Scholar]
- Ndimele, P.E.; Saba, A.O.; Ojo, D.O.; Ndimele, C.C.; Anetekhai, M.A.; Erondu, E.S. Chapter 24—Remediation of Crude Oil Spillage. In The Political Ecology of Oil and Gas Activities in the Nigerian Aquatic Ecosystem; Ndimele, P.E., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 369–384. [Google Scholar]
- Arjoon, K.K.; Speight, J.G. Petroleum Biodegradation and Oil Spill Bioremediation; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Rajbongshi, A.; Gogoi, S.B. A review on anaerobic microorganisms isolated from oil reservoirs. World J. Microbiol. Biotechnol. 2021, 37, 1–19. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Bartha, R. Biotechnology of petroleum pollutant biodegradation. Microb. Ecol. 1986, 12, 155–172. [Google Scholar] [CrossRef]
- Adebiyi, F.M. Air quality and management in petroleum refining industry: A review. Environ. Chem. Ecotoxicol. 2022, 4, 89–96. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed analysis of the composition of selected plastic packaging waste products and its implications for mechanical and thermochemical recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M.; Lee, J.; Risser, M.; Ullrich, P.; Gleckler, P.; Collins, W.D. Evaluation of extreme sub-daily precipitation in high-resolution global climate model simulations. Philos. Trans. R. Soc. A 2021, 379, 20190545. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Galdino, C.J.S., Jr.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar]
- Lehtonen, J.; Chen, X.; Beaumont, M.; Hassinen, J.; Orelma, H.; Dumée, L.F.; Tardy, B.L.; Rojas, O.J. Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration. Carbohydr. Polym. 2021, 251, 117073. [Google Scholar] [CrossRef]
- Ye-Eun, L.; I-Tae, K.; Yeong-Seok, Y. Stabilization of High-Organic-Content Water Treatment Sludge by Pyrolysis. Energies 2018, 11, 3292. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Sarbatly, R.; Krishnaiah, D.; Kamin, Z. A review of polymer nanofibres by electrospinning and their application in oil–water separation for cleaning up marine oil spills. Mar. Pollut. Bull. 2016, 106, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Chen, F.; Yang, B.; Ma, J.; Qu, J.; Liu, G. Decontamination of electronic waste-polluted soil by ultrasound-assisted soil washing. Environ. Sci. Pollut. Res. 2016, 23, 20331–20340. [Google Scholar] [CrossRef]
- Mat-Shayuti, M.S.; Tuan Ya, T.M.Y.S.; Abdullah, M.Z.; Megat Khamaruddin, P.N.F.; Othman, N.H. Progress in ultrasonic oil-contaminated sand cleaning: A fundamental review. Environ. Sci. Pollut. Res. 2019, 26, 26419–26438. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okoye, C.O.; Chidike Ezeorba, T.P.; Mao, G.; Chen, Y.; Xu, H.; Song, C.; Feng, W.; Wu, X. Emerging bio-dispersant and bioremediation technologies as environmentally friendly management responses toward marine oil spill: A comprehensive review. J. Environ. Manag. 2022, 322, 116123. [Google Scholar] [CrossRef]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef]
- Flathman, P.E.; Lanza, G.R. Phytoremediation: Current views on an emerging green technology. J. Soil Contam. 1998, 7, 415–432. [Google Scholar] [CrossRef]
- Sayed, K.; Baloo, L.; Sharma, N.K. Bioremediation of total petroleum hydrocarbons (Tph) by bioaugmentation and biostimulation in water with floating oil spill containment booms as bioreactor basin. Int. J. Environ. Res. Public Health 2021, 18, 2226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nedwed, T.; Tidwell, A.; Urbanski, N.; Cooper, D.; Buist, I.; Belore, R. One-step offshore oil skim and burn system for use with vessels of Opportunity. In International Oil Spill Conference Proceedings; American Petroleum Institute: Washington, DC, USA, 2014; Volume 2014, pp. 1834–1845. [Google Scholar]
- Clayson, I.G.; Hewitt, D.; Hutereau, M.; Pope, T.; Slater, B. High throughput methods in the synthesis, characterization, and optimization of porous materials. Adv. Mater. 2020, 32, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, K.G.; Von Gleich, A.; Große Ophoff, M.; Körner, W. The necessity of a global binding framework for sustainable management of chemicals and materials—Interactions with Climate and Biodiversity. Sustain. Chem. 2022, 3, 205–237. [Google Scholar] [CrossRef]
- Riser-Roberts, E. Remediation of Petroleum Contaminated Soils: Biological, Physical, and Chemical Processes; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, H.; Jing, Y.; Liu, M.; Wang, D.; Li, Y.; Bao, M. An efficient and environmental-friendly dispersant based on the synergy of amphiphilic surfactants for oil spill remediation. Chemosphere 2019, 215, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Abdala, A.A. Oil spill cleanup using graphene. Environ. Sci. Pollut. Res. 2013, 20, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.K.; Mckay, G.; Manawi, Y.; Malaibari, Z.; Hussien, M.A. Outstanding adsorption performance of high aspect ratio and super-hydrophobic carbon nanotubes for oil removal. Chemosphere 2016, 164, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Hopman, R.; Siegers, W.G.; Kruithof, J.C. Organic micropollutant removal by activated carbon fiber filtration. Water Supply Int. Water Supply Assoc. 1995, 13, 257–262. [Google Scholar]
- Gui, H.; Zhang, T.; Guo, Q. Nanofibrous, emulsion-templated syndiotactic polystyrenes with superhydrophobicity for oil spill cleanup. ACS Appl. Mater. Interfaces 2019, 11, 36063–36072. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; Almomani, F.; Adham, S.; Khraisheh, M. Polymeric adsorbents for oil removal from water. Chemosphere 2019, 233, 809–817. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Wang, L.; Zhao, J.; Du, Z.; Gong, X. Application of magnetic adsorbents based on iron oxide nanoparticles for oil spill remediation: A review. J. Taiwan Inst. Chem. Eng. 2019, 97, 227–236. [Google Scholar] [CrossRef]
- Adofo, Y.K.; Nyankson, E.; Agyei-Tuffuor, B. Dispersants as an oil spill clean-up technique in the marine environment: A review. Heliyon 2022, 8, e10153. [Google Scholar] [CrossRef] [PubMed]
- Keramea, P.; Spanoudaki, K.; Zodiatis, G.; Gikas, G.; Sylaios, G. Oil spill modeling: A critical review on current trends, perspectives, and challenges. J. Mar. Sci. Eng. 2021, 9, 181. [Google Scholar] [CrossRef]
- Meshalkin, V.P.; Shulaev, N.S.; Kadyrov, R.R.; Pryanichnikova, V.V.; Kulov, N.N.; Garabadzhiu, A.V. Electrochemical remediation of oil-contaminated soils factoring in terrain: Theoretical and experimental studies. Russ. J. Gen. Chem. 2022, 92, 2965–2971. [Google Scholar] [CrossRef]
- Mikryukova, E.M.; Suvorova, E.V. Overcoming problems with waste water treatment from dense emulsions in the oil refining industry. In International Conference on Construction and Development: Life Cycle; Springer: Berlin/Heidelberg, Germany, 2020; pp. 311–318. [Google Scholar] [CrossRef]
- Shulaev, N.; Meshalkin, V.; Pryanichnikova, V.; Kady’rov, R.; By’kovskij, N. E’lektroximicheskaya ochistka neftezagryaznenny’x gruntov s uchetom rel’efa mestnosti. E’kologiya i promy’shlennost’ Rossii. 2022, 26, 9–13. (In Russian) [Google Scholar]
- Shulaev, N.S.; Pryanichnikova, V.V.; Kadyrov, R.R. Regularities of electrochemical cleaning of oil-contaminated soils. J. Min. Inst. 2021, 252, 937–946. [Google Scholar] [CrossRef]
- Santo, C.E.; Vilar, V.J.P.; Botelho, C.M.S.; Bhatnagar, A.; Kumar, E.; Boaventura, R.A.R. Optimization of coagulation–flocculation and flotation parameters for the treatment of a petroleum refinery effluent from a Portuguese plant. Chem. Eng. J. 2012, 183, 117–123. [Google Scholar] [CrossRef]
- Puszkarewicz, A. Removal of petroleum compounds from water in coagulation process. Environ. Prot. Eng. 2008, 34, 5–14. [Google Scholar]
- Li, H. The Roles of Non-Polar Oil in Froth Flotation of Fine Particles. Ph.D. Thesis, University of Alberta, ERA Education and Research Archive, Edmonton, AB, Canada, 2019. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Sadegh, H.; Mazloumbilandi, M.; Chahardouri, M. Low-cost materials with adsorption performance. In Handbook of Ecomaterials; Torres Martínez, L.M., Kharissova, O.V., Kharisov, B.I., Eds.; Springer Nature: Cham, Switzerland, 2017. [Google Scholar]
- Zaitsev, A.S.; Egorov, R.I.; Strizhak, P.A. Light-induced gasification of the coal-processing waste: Possible products and regimes. Fuel 2018, 212, 347–352. [Google Scholar] [CrossRef]
- Kim, H.; Zhang, G.; Chung, T.C.M.; Nam, C. A role for newly developed sorbents in remediating large-scale oil spills: Reviewing recent advances and beyond. Adv. Sustain. Syst. 2022, 6, 2100211. [Google Scholar] [CrossRef]
- Han, F.; Wu, L. Industrial Solid Waste Recycling in Western China; Springer: Singapore, 2019. [Google Scholar]
- El-Din, G.A.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jinka, S.; Hake, K.; Parameswaran, S.; Kendall, R.J.; Ramkumar, S. Novel natural sorbent for oil spill cleanup. Ind. Eng. Chem. Res. 2014, 53, 11954–11961. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; El Saqa, F.A.; Amer, A.A.E.R.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Li, D.; Zhu, F.Z.; Li, J.Y.; Na, P.; Wang, N. Preparation and characterization of cellulose fibers from corn straw as natural oil sorbents. Ind. Eng. Chem. Res. 2013, 52, 516–524. [Google Scholar] [CrossRef]
- Lim, T.T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic–oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef]
- Kudaybergenov, K.K.; Ongarbayev, E.K.; Mansurov, Z.A. Petroleum sorption by thermally treated rice husks derived from agricultural byproducts. Eurasian Chem. J. 2013, 15, 57–66. [Google Scholar] [CrossRef]
- Asadpour, R.; Sapari, N.B.; Isa, M.H.; Kakooei, S. Acetylation of oil palm empty fruit bunch fiber as an adsorbent for removal of crude oil. Environ. Sci. Pollut. Res. 2016, 23, 11740–11750. [Google Scholar] [CrossRef]
- Kumagai, S.; Noguchi, Y.; Kurimoto, Y.; Takeda, K. Oil adsorbent produced by the carbonization of rice husks. Waste Manag. 2007, 27, 554–561. [Google Scholar] [CrossRef]
- Ratcha, A.; Yoosuk, B.; Kongparakul, S. Grafted methyl methacrylate and butyl methacrylate onto natural rubber foam for oil sorbent. Adv. Mater. Res. 2014, 844, 385–390. [Google Scholar] [CrossRef]
- Abdelwahab, O. Assessment of raw luffa as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. Alex. Eng. J. 2014, 53, 213–218. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Ding, B.; Yang, J.; Yu, J.; Al-Deyab, S.S. Nanoporous polystyrene fibers for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, W.; Wang, T.; Gu, J.; Zhong, S.; Zhou, S.; Xie, T.; Fu, J. Facile preparation of magnetic poly (styrene-divinylbenzene) foam and its application as an oil absorbent. Ind. Eng. Chem. Res. 2015, 54, 11033–11039. [Google Scholar] [CrossRef]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F.; Yang, R.D. Evaluation of nonwoven polypropylene oil sorbents in marine oil-spill recovery. Mar. Pollut. Bull. 2003, 46, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Borthakur, A.; Singh, R.; Bhadouria, R.; Singh, V.K.; Devi, P. A critical review on the research trends and emerging technologies for arsenic decontamination from water. Groundw. Sustain. Dev. 2021, 14, 100607. [Google Scholar] [CrossRef]
- Perelomov, L.; Gertsen, M.; Burachevskaya, M.; Hemalatha, S.; Vijayalakshmi, A.; Perelomova, I.; Atroshchenko, Y. Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants. Sustainability 2024, 16, 4804. [Google Scholar] [CrossRef]
- Gertsen, M.M.; Golysheva, A.N.; Perelomov, L.V. Stabilizaciya neftyany’x i maslyany’x e’mul’sij bioorganicheskimi kompoziciyami na osnove guminovy’x kislot. Vestn. Ross. Univ. Druz. Nar. Seriya E’Kologiya I Bezop. Zhiznedeyatel’Nosti. 2023, 31, 476–493. (In Russian) [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef]
- Ramachandran, V.; Shriram, M.K.; Mathew, E.R.; Ramkumar, K.; Prakash, D.G.; Venkatachalam, C.D. Oil spill remediation and valorization of oil-soaked peat sorbent to biofuel by hydrothermal liquefaction. Biomass Convers. Biorefin. 2023, 13, 9325–9337. [Google Scholar] [CrossRef]
- Saljnikov, E.; Lavrishchev, A.; Römbke, J.; Rinklebe, J.; Scherber, C.; Wilke, B.-M.; Tóth, T.; Blum, W.E.H.; Behrendt, U.; Eulenstein, F.; et al. Understanding and monitoring chemical and biological soil degradation. In Advances in Understanding Soil Degradation; Saljnikov, E., Mueller, L., Lavrishchev, A., Eulenstein, F., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 75–124. [Google Scholar]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Chernov, T.I.; Semenov, M.V. Management of soil microbial communities: Opportunities and prospects (a review). Eurasian Soil. Sci. 2021, 54, 1888–1902. [Google Scholar] [CrossRef]
- Gałwa-Widera, M. Plant-based technologies for removal of pharmaceuticals and personal care products. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 297–319. [Google Scholar]
- Mohapatra, B.; Malhotra, H.; Saha, B.K.; Dhamale, T.; Phale, P.S. Microbial metabolism of aromatic pollutants: High-throughput OMICS and metabolic engineering for efficient bioremediation. In Current Developments in Biotechnology and Bioengineering: Designer Microbial Cell Factories: Metabolic Engineering and Applications; Joshi, S., Pandey, A., Sirohi, R., Park, S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 151–199. [Google Scholar]
- Jethwa, K.; Bajpai, S.; Chaudhari, P.K.; Swankar, A. Wastewater Nutrient Removal Through Phytoremidiation: A Review. In Proceedings of the 49th Annual Convention of IWWA on “Smart Water Management”, Nagpur, India, 19–21 January 2017. [Google Scholar]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; Al-Zuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Sladkovska, T.; Wolski, K.; Bujak, H.; Radkowski, A.; Sobol, Ł. A review of research on the use of selected grass species in removal of heavy metals. Agronomy 2022, 12, 2587. [Google Scholar] [CrossRef]
- Hazaimeh, M.D.; Ahmed, E.S. Bioremediation perspectives and progress in petroleum pollution in the marine environment: A review. Environ. Sci. Pollut. Res. 2021, 28, 54238–54259. [Google Scholar] [CrossRef] [PubMed]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Y.; Xu, Z.; Zhang, W.; Jiang, K. Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavas, I.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Recent studies on applications of aquatic weed plants in phytoremediation of wastewater: A review article. Ain Shams Eng. J. 2021, 12, 355–365. [Google Scholar] [CrossRef]
- Hauwa, I.M.; Adedoyin, V. Phytoextraction potentials of Hyptis suaveolens and Euphorbia hirta weeds for Cd2+ and Co2+. Commun. Phys. Sci. 2020, 6, 869–874. [Google Scholar]
- Shakoor, A.; Abdullah, M.; Sarfraz, R.; Altaf, M.A.; Batool, S. A comprehensive review on phytoremediation of cadmium (Cd) by mustard (Brassica juncea L.) and sunflower (Helianthus annuus L.). J. Biosersity Environ. Sci. 2017, 10, 88–98. [Google Scholar]
- Angelova, V.R.; Ivanova, R.I.; Todorov, J.M.; Ivanov, K.I. Potential of rapeseed (Brassica napus L.) for phytoremediation of soils contaminated with heavy metals. J. Environ. Prot. Ecol. 2017, 18, 468–478. [Google Scholar]
- Belimov, A.A.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, M.N.; Safronova, V.I.; Litvinskiy, V.A.; Nosikov, V.V.; Zavalin, A.A.; Tichonovich, I.A. Microbial consortium of PGPR, rhizobia and arbuscular mycorrhizal fungus makes pea mutant SGECdt comparable with Indian mustard in cadmium tolerance and accumulation. Plants 2020, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Sumiahadi, A.; Acar, R. A review of phytoremediation technology: Heavy metals uptake by plants. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 142, p. 12023. [Google Scholar]
- Khan, A.G. In situ phytoremediation of uranium contaminated soils. In Phytoremediation: In-Situ Applications; Shmaefsky, B.R., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 123–151. [Google Scholar]
- Hou, Y.; Liu, X.; Zhang, X.; Hu, X.; Cao, L. Rhizosphere phytoremediation with Cyperus rotundus for diesel-contaminated wetlands. Water Air Soil Pollut. 2016, 227, 1–8. [Google Scholar] [CrossRef]
- Khandare, R.V.; Watharkar, A.D.; Pawar, P.K.; Jagtap, A.A.; Desai, N.S. Hydrophytic plants Canna indica, Epipremnum aureum, Cyperus alternifolius and Cyperus rotundus for phytoremediation of fluoride from water. Environ. Technol. Innov. 2021, 21, 101234. [Google Scholar] [CrossRef]
- Bordoloi, S.; Basumatary, B. A study on degradation of heavy metals in crude oil-contaminated soil using Cyperus rotundus. In Phytoremediation Management of Environmental Contamination, Volume 4; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 53–60. [Google Scholar]
- Jahan-Nejati, S.; Jowkar-Tangkarami, M.; Taei-Semiromi, J. Cyperus rotundus: A safe forage or hyper phytostabilizer species in copper contaminated soils. Int. J. Phytoremediat. 2021, 23, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Khan, A.A.; Aziz, T.; Ali, W.; Ahmad, S.; Rahman, S.U.; Iqbal, Z.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Phytochemical investigation, antioxidant properties and in vivo evaluation of the toxic effects of Parthenium hysterophorus. Molecules 2022, 27, 4189. [Google Scholar] [CrossRef] [PubMed]
- Boruah, T.; Chakravarty, P.; Deka, H. Phytosociology and antioxidant profile study for selecting potent herbs for phytoremediation of crude oil–contaminated soils. Environ. Monit. Assess. 2020, 192, 766. [Google Scholar] [CrossRef]
- Frantsuzova, E.; Bogun, A.; Solomentsev, V.; Vetrova, A.; Streletskii, R.; Solyanikova, I.; Delegan, Y. Whole genome analysis and assessment of the metabolic potential of Gordonia rubripertincta Strain 112, a degrader of aromatic and aliphatic compounds. Biology 2023, 12, 721. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Dynamics of natural revegetation of hydrocarbon-contaminated soil and remediation potential of indigenous plant species in the steppe zone of the southern Volga Uplands. Environ. Sci. Pollut. Res. 2018, 25, 3260–3274. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Biktasheva, L.; Galitskaya, P.; Golubev, S.; Dubrovskaya, E.; Selivanovskaya, S.; Turkovskaya, O. Study of Boraginaceae plants for phytoremediation of oil-contaminated soil. Int. J. Phytoremediat. 2022, 24, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Sasmaz, M.; Sasmaz, A. The accumulation of strontium by native plants grown on Gumuskoy mining soils. J. Geochem. Explor. 2017, 181, 236–242. [Google Scholar] [CrossRef]
- Palutoglu, M.; Akgul, B.; Suyarko, V.; Yakovenko, M.; Kryuchenko, N.; Sasmaz, A. Phytoremediation of cadmium by native plants grown on mining soil. Bull. Environ. Contam. Toxicol. 2018, 100, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Alirzayeva, E.G.; Shirvani, T.S.; Yazici, M.A.; Alverdiyeva, S.M.; Shukurov, E.S.; Ozturk, L.; Ali-Zade, V.M.; Cakmak, I. Heavy metal accumulation in Artemisia and foliaceous lichen species from the Azerbaijan flora. Forest Snow Landsc. Res. 2006, 80, 339–348. [Google Scholar]
- Germida, J.J.; Frick, C.M.; Farrell, R.E. Phytoremediation of oil-contaminated soils. Dev. Soil. Sci. 2002, 28, 169–186. [Google Scholar]
- Nemati, B.; Baneshi, M.M.; Akbari, H.; Dehghani, R.; Mostafaii, G. Phytoremediation of pollutants in oil-contaminated soils by Alhagi camelorum: Evaluation and modeling. Sci. Rep. 2024, 14, 5502. [Google Scholar] [CrossRef] [PubMed]
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Erdner, D.L.; Rosenheim, B.E.; Shetty, P.; Seitz, K.W.; Baker, B.J.; Liu, Z. Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil. ISME J. 2018, 12, 2532–2543. [Google Scholar] [CrossRef]
- Brown, D.M.; Okoro, S.; van Gils, J.; van Spanning, R.; Bonte, M.; Hutchings, T.; Linden, O.; Egbuche, U.; Bruun, K.B.; Smith, J.W.N. Comparison of landfarming amendments to improve bioremediation of petroleum hydrocarbons in Niger Delta soils. Sci. Total Environ. 2017, 596, 284–292. [Google Scholar] [CrossRef]
- Lin, Q.; Mendelssohn, I.A. Evaluation of tolerance limits for restoration and phytoremediation with Spartina alterniflora in crude oil-contaminated coastal salt marshes. IOSC Proc. 2008, 1, 869–873. [Google Scholar]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Wanapaisan, P.; Laothamteep, N.; Vejarano, F.; Chakraborty, J.; Shintani, M.; Muangchinda, C.; Morita, T.; Suzuki-Minakuchi, C.; Inoue, K.; Nojiri, H.; et al. Synergistic degradation of pyrene by five culturable bacteria in a mangrove sediment-derived bacterial consortium. J. Hazard. Mater. 2018, 342, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Singh, R.; Singh, R.; Pandey, S.; Rai, A.; Singh, V.K.; Rahul, B. Genetically engineered bacteria for the degradation of dye and other organic compounds. In Abatement of Environmental Pollutants: Trends and Strategies; Pardeep Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. [Google Scholar]
- Skinder, B.M.; Uqab, B.; Ganai, B.A. Bioremediation: A sustainable and emerging tool for restoration of polluted aquatic ecosystem. In Fresh Water Pollution Dynamics and Remediation; Qadri, H., Bhat, R.A., Mehmood, M.A., Dar, G., Eds.; Springer: Singapore, 2020; pp. 143–165. [Google Scholar]
- Adeleye, A.O.; Nkereuwem, M.E.; Omokhudu, G.I.; Amoo, A.O.; Shiaka, G.P.; Yerima, M.B. Effect of microorganisms in the bioremediation of spent engine oil and petroleum related environmental pollution. J. Appl. Sci. Environ. Manag. 2018, 22, 157–167. [Google Scholar] [CrossRef][Green Version]
- Singh, R.K.; Tripathi, R.; Ranjan, A.; Srivastava, A.K. Fungi as potential candidates for bioremediation. In Abatement of Environmental Pollutants: Trends and Strategies; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–191. [Google Scholar]
- Belovezhets, L.A.; Makarova, L.E.; Tretyakova, M.S.; Markova, Y.A.; Dudareva, L.V.; Semenova, N.V. Possible pathways for destruction of polyaromatic hydrocarbons by some oil-degrading bacteria isolated from plant endosphere and rhizosphere. Appl. Biochem. Microbiol. 2017, 53, 68–72. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Mullaeva, S.A.; Sazonova, O.I.; Petrikov, K.V.; Vetrova, A.A. Current research on simultaneous oxidation of aliphatic and aromatic hydrocarbons by bacteria of genus Pseudomonas. Folia Microbiol. 2022, 67, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Shimp, R.; Pfaender, F.K. Influence of Naturally Occurring Humic Acids on Biodegradation of Monosubstituted Phenols by Aquatic Bacteria. Appl. Environ. Microbiol. 1985, 49, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Gregson, B.H.; Metodieva, G.; Metodiev, M.V.; Golyshin, P.N.; McKew, B.A. Protein expression in the obligate hydrocarbon-degrading psychrophile Oleispira antarctica RB-8 during alkane degradation and cold tolerance. Environ. Microbiol. 2020, 22, 1870–1883. [Google Scholar] [CrossRef]
- Gorbunova, T.I.; Egorova, D.O.; Pervova, M.G.; Kir’yanova, T.D.; Plotnikova, E.G. Degradability of commercial mixtures of polychlorobiphenyls by three Rhodococcus strains. Arch. Microbiol. 2022, 204, 534. [Google Scholar] [CrossRef]
- Sun, S.; Song, X.; Bian, Y.; Wan, X.; Zhang, J.; Wang, W. Multi-parameter optimization maximizes the performance of genetically engineered Geobacillus for degradation of high-concentration nitroalkanes in wastewater. Bioresour. Technol. 2022, 347, 126690. [Google Scholar] [CrossRef]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent advances in bacterial degradation of hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Brunet, C.; van Zyl, L.J.; Trindade, M.; Golyshin, P.N.; Dell’Anno, A.; Ianora, A.; Sansone, C. Degradation of hydrocarbons and heavy metal reduction by marine bacteria in highly contaminated sediments. Microorganisms 2020, 8, 1402. [Google Scholar] [CrossRef]
- Syrova, D.S.; Shaposhnikov, A.I.; Yuzikhin, O.S.; Belimov, A.A. Destruction and transformation of phytohormones by microorganisms. Appl. Biochem. Microbiol. 2022, 58, 1–18. [Google Scholar] [CrossRef]
- Rahmati, F.; Lajayer, B.A.; Shadfar, N.; van Bodegom, P.M.; van Hullebusch, E.D. A Review on biotechnological approaches applied for marine hydrocarbon spills remediation. Microorganisms 2022, 10, 1289. [Google Scholar] [CrossRef]
- Godambe, T.; Fulekar, M. Bioremediation of petrochemical hydrocarbons (BTEX)—Review. J. Environ. Sci. Pollut. Res. 2017, 3, 189–199. [Google Scholar]
- Das, D.; Baruah, R.; Roy, A.S.; Singh, A.K.; Deka Boruah, H.P.; Kalita, J.; Bora, T.C. Complete genome sequence analysis of Pseudomonas aeruginosa N002 reveals its genetic adaptation for crude oil degradation. Genomics 2015, 105, 182–190. [Google Scholar] [CrossRef]
- Tazdaït, D.; Salah-Tazdaït, R. Polycyclic aromatic hydrocarbons: Toxicity and bioremediation approaches. In Biotechnology for Sustainable Environment; Joshi, S.J., Deshmukh, A., Sarma, H., Eds.; Springer Nature: Singapore, 2021; pp. 289–316. [Google Scholar]
- Bacosa, H.P.; Inoue, C. Polycyclic aromatic hydrocarbons (PAHs) biodegradation potential and diversity of microbial consortia enriched from tsunami sediments in Miyagi, Japan. J. Hazard. Mater. 2015, 283, 689–697. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Yang, S.; Yao, J.; Chen, K.; Yang, L.; Zheng, W.; Tian, Y. Finding novel chemoreceptors that specifically sense and trigger chemotaxis toward polycyclic aromatic hydrocarbons in Novosphingobium pentaromativorans US6-1. J. Hazard. Mater. 2021, 416, 126246. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, J.; Lai, H.; Xue, Q. Degradation of asphaltenes by two Pseudomonas aeruginosa strains and their effects on physicochemical properties of crude oil. Int. Biodeterior. Biodegrad. 2017, 122, 12–22. [Google Scholar] [CrossRef]
- Verma, S.; Bhargava, R.; Pruthi, V. Oily sludge degradation by bacteria from Ankleshwar, India. Int. Biodeterior. Biodegrad. 2006, 57, 207–213. [Google Scholar] [CrossRef]
- Jahromi, H.; Fazaelipoor, M.H.; Ayatollahi, S.; Niazi, A. Asphaltenes biodegradation under shaking and static conditions. Fuel 2014, 117, 230–235. [Google Scholar] [CrossRef]
- Shahebrahimi, Y.; Fazlali, A.; Motamedi, H.; Kord, S.; Mohammadi, A.H. Effect of various isolated microbial consortiums on the biodegradation process of precipitated asphaltenes from crude oil. ACS Omega 2020, 5, 3131–3143. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yang, X.; Lv, J.; Li, X. Study on a strain of Lysinibacillus sp. with the potential to improve the quality of oil sands. ACS Omega 2022, 7, 11654–11663. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.; Jeon, C.O. Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci. Rep. 2019, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Microbial biodegradation of paraffin wax in Malaysian crude oil mediated by degradative enzymes. Front. Microbiol. 2020, 11, 565608. [Google Scholar] [CrossRef]
- Novik, G.; Savich, V.; Meerovskaya, O. Geobacillus Bacteria: Potential commercial applications in industry, bioremediation, and bioenergy production. In Growing and Handling of Bacterial Cultures; Mishra, M., Ed.; IntechOpen: London, UK, 2018; pp. 1–36. [Google Scholar]
- Roy, A.; Dutta, A.; Pala, S.; Gupta, A.; Sarkar, J.; Chatterjee, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018, 253, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Ajona, M.; Vasanthi, P. Bioremediation of petroleum contaminated soils—A review. Mater. Today Proc. 2021, 45, 7117–7122. [Google Scholar] [CrossRef]
- Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Singh, M.; Joshi, D.; Singh, J.; Suyal, D.C.; Kumar, A.; Rajput, V.D.; et al. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: Present status and future challenges. Environ. Sci. Pollut. Res. 2021, 28, 24917–24939. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Tahir, A.; Dogar, M.; Yasar, A.; Rasheed, R.; Mahnoor, F. Role of microorganisms in the remediation of toxic metals from contaminated soil. In Phytoremediation: Management of Environmental Contaminants; Newman, L., Ansari, A.A., Gill, S.S., Naeem, M., Gill, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; Volume 7, pp. 231–259. [Google Scholar]
- Thapa, B.; Kc, A.K.; Ghimire, A. A review on bioremediation of petroleum hydrocarbon contaminants in soil. Kathmandu Univ. J. Sci. Eng. Technol. 2012, 8, 164–170. [Google Scholar] [CrossRef]
- Malik, S.; Kishore, S.; Kumar, S.A.; Dhasmana, A. Role of bacteria in biological removal of environmental pollutants. In Emerging Technologies in Applied and Environmental Microbiology; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2023; Volume 12, pp. 205–225. [Google Scholar]
- Belahmadi, M.S.O.; Charchar, N.; Abdessemed, A.; Gherib, A. Impact of petroleum refinery on aquatic ecosystem of Skikda Bay (Algeria): Diversity and abundance of viable bacterial strains. Mar. Pollut. Bull. 2023, 188, 114704. [Google Scholar] [CrossRef] [PubMed]
- Myazin, V.A.; Korneykova, M.V.; Chaporgina, A.A.; Fokina, N.V.; Vasilyeva, G.K. The effectiveness of biostimulation, bioaugmentation and sorption-biological treatment of soil contaminated with petroleum products in the Russian subarctic. Microorganisms 2021, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.M.; Ehlers, S.M.; Dick, J.T.A.; Sigwart, J.D.; Linse, K.; Dick, J.J.; Kiriakoulakis, K. High abundances of microplastic pollution in deep-sea sediments: Evidence from Antarctica and the Southern Ocean. Environ. Sci. Technol. 2020, 54, 13661–13671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, X.; Solaiman, D.K.Y.; Ashby, R.D.; Liu, Z.; Mukhopadhyay, S.; Yan, R. Inactivation of Escherichia coli O157: H7 in vitro and on the surface of spinach leaves by biobased antimicrobial surfactants. Food Control 2016, 60, 158–165. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.; Lin, J.; Wang, W.; Li, S. Biodegradation of petroleum hydrocarbons by Bacillus subtilis BL-27, a strain with weak hydrophobicity. Molecules 2019, 24, 3021. [Google Scholar] [CrossRef] [PubMed]
- Travkin, V.M.; Solyanikova, I.P. Salicylate or phthalate: The main intermediates in the bacterial degradation of naphthalene. Processes 2021, 9, 1862. [Google Scholar] [CrossRef]
- Zabolotskikh, V.V.; Vasilyev, A.V.; Tankikh, S.N. Development of bioactive composition for effective bioremediation of oil-contaminated soil. In Proceedings of the Seventh International Environmental Congress (Ninth International Scientific-Technical Conference) “Ecology and Life Protection of Industrial-Transport Complexes” ELPIT 2019, Samara, Russia, 25–28 September 2019; pp. 173–190. [Google Scholar]
- Cova, C.M.; Rincón, E.; Espinosa, E.; Serrano, L.; Zuliani, A. Paving the way for a green transition in the design of sensors and biosensors for the detection of volatile organic compounds (VOCs). Biosensors 2022, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Imam, A.; Kanaujia, P.K.; Ray, A.; Suman, S.K. Removal of petroleum contaminants through bioremediation with integrated concepts of resource recovery: A review. Indian J. Microbiol. 2021, 61, 250–261. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaabouni, S.; Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Ruengvisesh, S.; Kerth, C.R.; Taylor, T.M. Inhibition of Escherichia coli O157: H7 and Salmonella enterica isolates on spinach leaf surfaces using eugenol-loaded surfactant micelles. Foods 2019, 8, 575. [Google Scholar] [CrossRef] [PubMed]
- Kotake, T.; Matsuzawa, J.; Suzuki-Minakuchi, C.; Okada, K.; Nojiri, H.; Iwata, K. Purification and partial characterization of the extradiol dioxygenase, 2′-carboxy-2, 3-dihydroxybiphenyl 1,2-dioxygenase, in the fluorene degradation pathway from Rhodococcus sp. strain DFA3. Biosci. Biotechnol. Biochem. 2016, 80, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)–A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non. Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Luis-Zarate, V.H.; Rodriguez-Hernandez, M.C.; Alatriste-Mondragon, F.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Coconut endocarp and mesocarp as both biosorbents of dissolved hydrocarbons in fuel spills and as a power source when exhausted. J. Environ. Manag. 2018, 211, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.N.; El-Azab, W.I.M.; El-Gendy, N.S. Sustainable ecofriendly recruitment of bioethanol fermentation lignocellulosic spent waste biomass for the safe reuse and discharge of petroleum production produced water via biosorption and solid biofuel production. J. Hazard. Mater. 2022, 422, 126845. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Kaur, S.; Sodhi, A.K. A study on removal of cutting oil from wastewater by using agricultural wastes. Mater. Today Proc. 2020, 32, 719–727. [Google Scholar] [CrossRef]
- Rizaev, A. Enhancement of aqua scourings technology from petrochemical. Turk. J. Comput. Math. Educ. 2021, 12, 1987–1992. [Google Scholar]
- Jesubunmi, C.O.; Phil, E.K.; Chigbu, C.C. Isolation and optimization of hydrocarbon–degrading bacteria. Asian J. Biotechnol. Bioresour. Technol. 2022, 8, 46–54. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mukherji, S. Biosorption of diesel and lubricating oil on algal biomass. 3 Biotech 2012, 2, 301–310. [Google Scholar] [CrossRef]
- Ali, N.; Eliyas, M.; Al-Sarawi, H.; Radwan, S.S. Hydrocarbon-utilizing microorganisms naturally associated with sawdust. Chemosphere 2011, 83, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Kolotova, O.V.; Mogilevskaya, I.V.; Vladimtseva, I.V.; Arzamasova, T.D.; Kuznetsova, V.A. Biosorbents for oil-containing wastewater treatment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 421, p. 62044. [Google Scholar]
- Archegova, I.B.; Khabibullina, F.M.; Shubakov, A.A. Optimization of the purification of soil and water objects from oil using biosorbents. Contemp. Probl. Ecol. 2012, 5, 548–553. [Google Scholar] [CrossRef]
- Dashti, N.; Ali, N.; Khanafer, M.; Radwan, S.S. Oil uptake by plant-based sorbents and its biodegradation by their naturally associated microorganisms. Environ. Pollut. 2017, 227, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; El-Sheshtawy, H.S.; Khalil, N.M. Bioremediation process of oil spill using fatty-lignocellulose sawdust and its enhancement effect. Egypt. J. Pet. 2019, 28, 205–211. [Google Scholar] [CrossRef]
- Nwankwegu, A.S. Sawdust assisted bioremediation of PMS hydrocarbon impacted agricultural soil in NigerDelta Nigeria. Int. J. Pharma Biosci. 2016, 7, 47–57. [Google Scholar]
- Amajuoyi, C.A.; Wemedo, S.A. Effect of oil palm bunch ash (Elaeis guineensis) on the bioremediation of diesel oil polluted soil. Am. J. Microbiol. Biotechnol. 2015, 2, 6–14. [Google Scholar]
- Akpe, A.R.; Ekundayo, A.O.; Aigere, S.P.; Okwu, G.I. Bacterial degradation of petroleum hydrocarbons in crude oil polluted soil amended with cassava peels. Am. J. Res. Commun. 2015, 3, 99–118. [Google Scholar]
- Omoni, V.T.; Aguoru, C.U.; Edoh, E.O.; Makinde, O. Biostimulation of hydrocarbon utilizing bacteria in soil contaminated with spent engine oil using banana and plantain agro-wastes. J. Soil Sci. Environ. Manag. 2015, 6, 225–233. [Google Scholar]
- Hamzah, A.; Phan, C.-W.; Yong, P.-H.; Mohd Ridzuan, N.H. Oil palm empty fruit bunch and sugarcane bagasse enhance the bioremediation of soil artificially polluted by crude oil. Soil. Sediment. Contam. An. Int. J. 2014, 23, 751–762. [Google Scholar] [CrossRef]
- Kadim, M.K.; Risjani, Y. Biomarker for monitoring heavy metal pollution in aquatic environment: An overview toward molecular perspectives. Emerg. Contam. 2022, 8, 195–205. [Google Scholar] [CrossRef]
- Somu, P.; Narayanasamy, S.; Gomez, L.A.; Rajendran, S.; Lee, Y.R.; Balakrishnan, D. Immobilization of enzymes for bioremediation: A future remedial and mitigating strategy. Environ. Res. 2022, 212, 113411. [Google Scholar] [CrossRef]
- Gertsen, M.M.; Perelomov, L.V.; Arlyapov, V.A.; Atroshchenko, Y.M.; Meshalkin, V.P.; Chistyakova, T.B.; Reverberi, A.P. Degradation of oil and petroleum products in water by bioorganic compositions based on humic acids. Energies 2023, 16, 5320. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.S.; Medvedeva, A.S.; Kuznetsova, L.S.; Gertsen, M.M.; Kolesov, V.V.; Arlyapov, V.A.; Reshetilov, A.N. A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution. Polymers 2024, 16, 1431. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A mediator microbial biosensor for assaying general toxicity. Enzym. Microbial. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef]
- Kurbanalieva, S.K.; Arlyapov, V.A.; Kharkova, A.S.; Perchikov, R.N.; Kamanina, O.A.; Melnikov, P.A.; Popova, N.M.; Machulin, A.V.; Tarasov, S.G.; Saverina, E.A.; et al. Electroactive Biofilms of Activated Sludge Microorganisms on a Nanostructured Surface as the Basis for a Highly Sensitive Biochemical Oxygen Demand Biosensor. Sensors 2020, 22, 6049. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD using Paracoccus yeei bacteria isolated from activated sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Anielak, A.M.; Styszko, K.; Kłeczek, A.; Łomińska-Płatek, D. Humic Substances—Common Carriers of Micropollutants in Municipal Engineering. Energies 2022, 15, 8496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).