A New Straightforward Darcy-Scale Compositional Solver in OpenFOAM for CO2/Water Mutual Solubility in CO2 Storage Processes in Aquifers
Abstract
1. Introduction
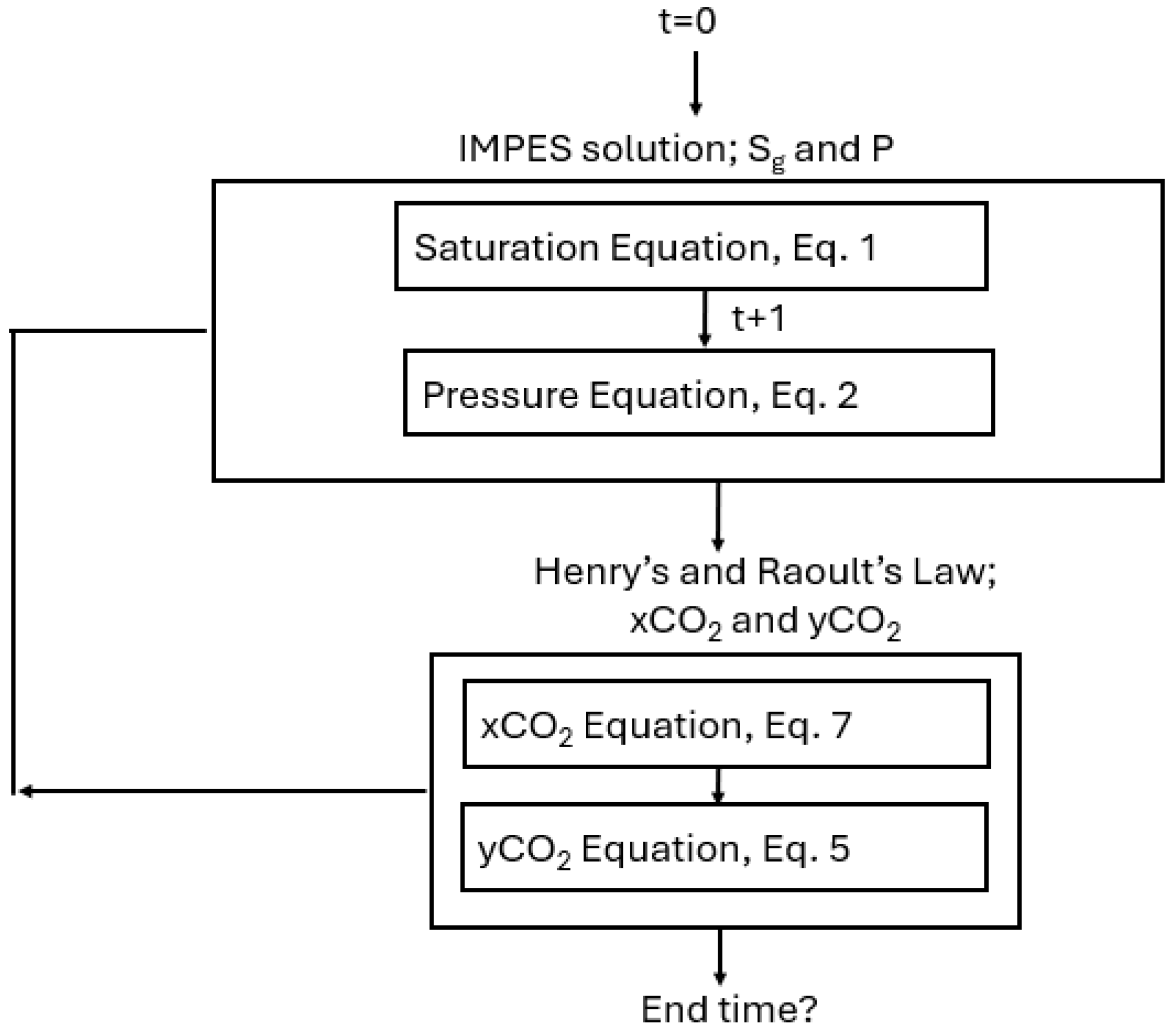
2. Numerical Method
3. Results
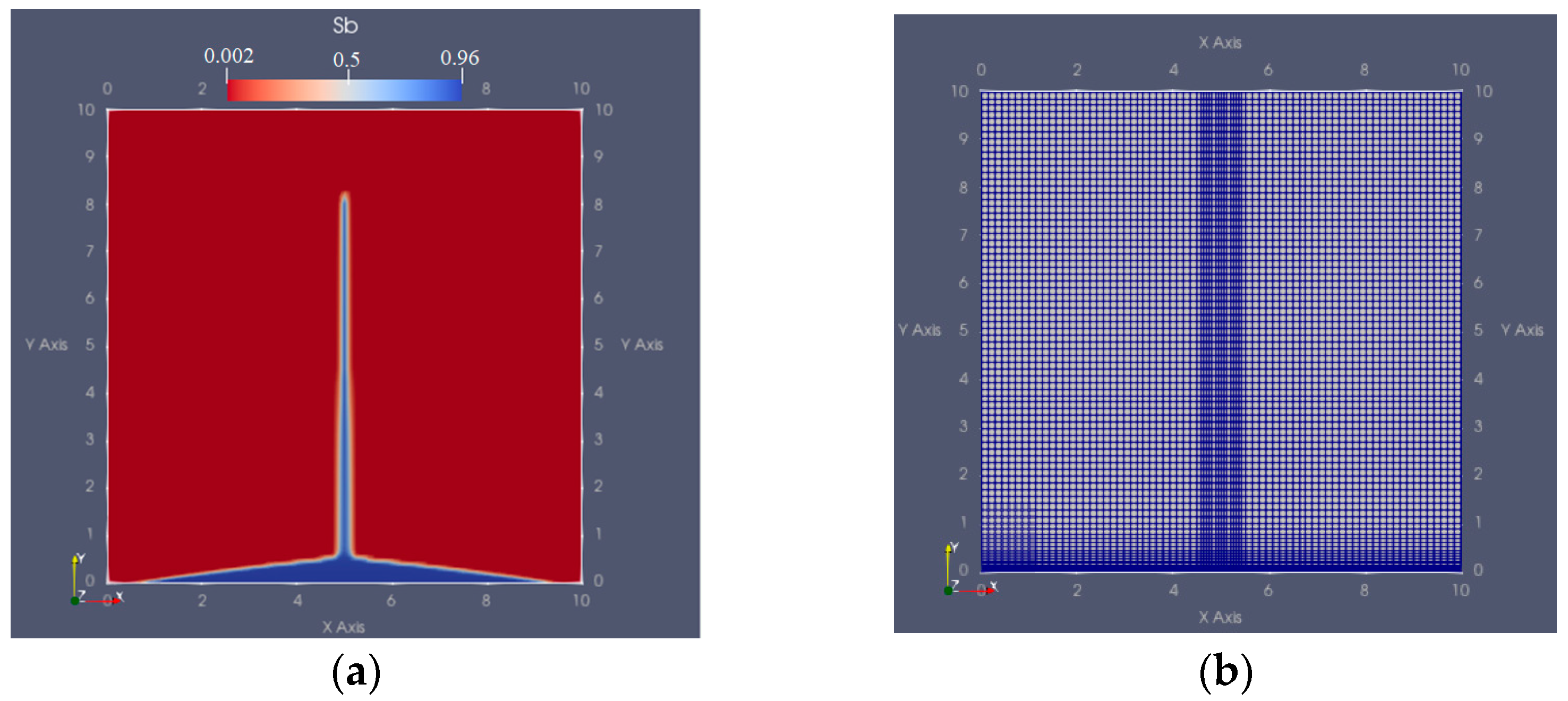
Models: Water Injection and Gas Injection
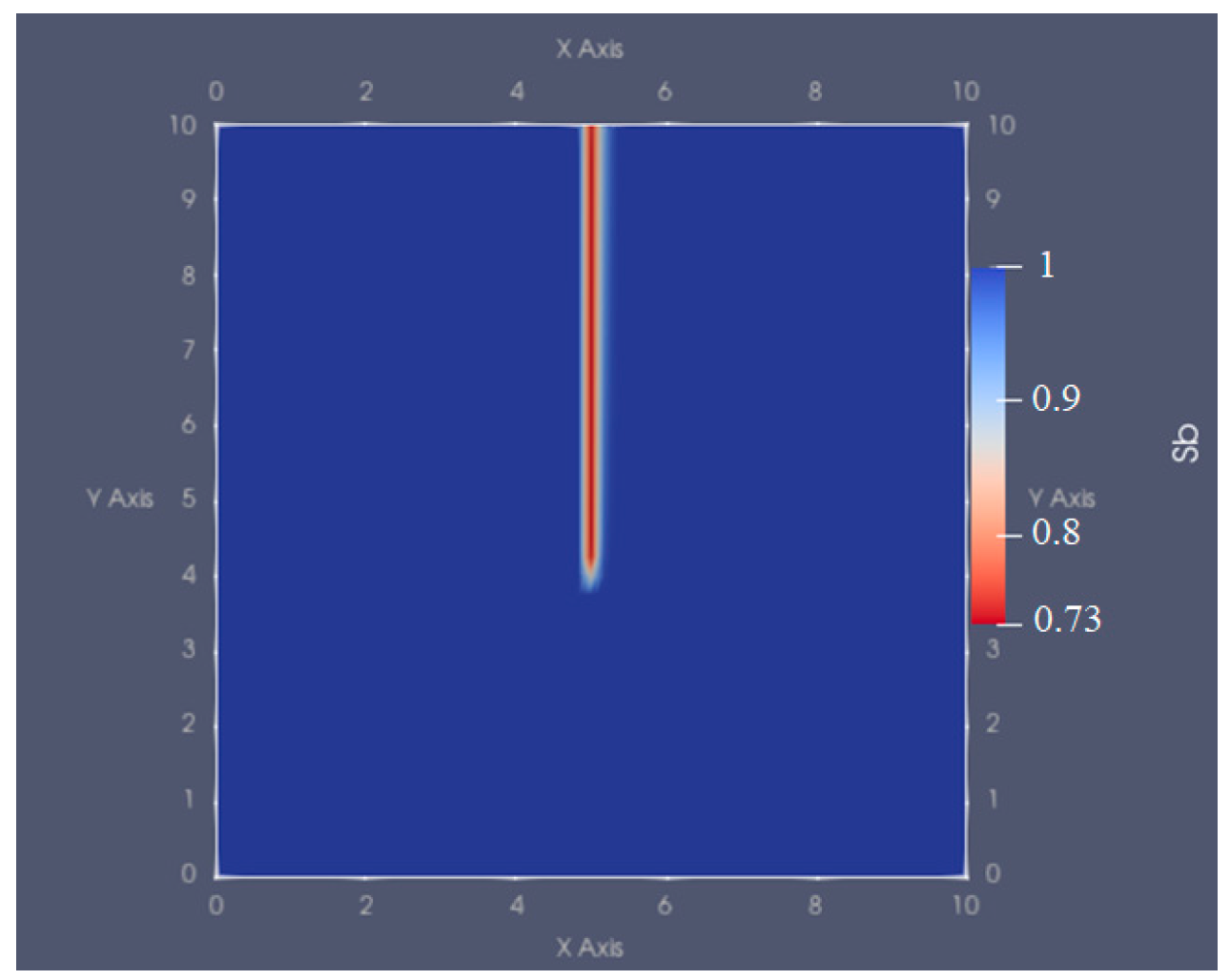
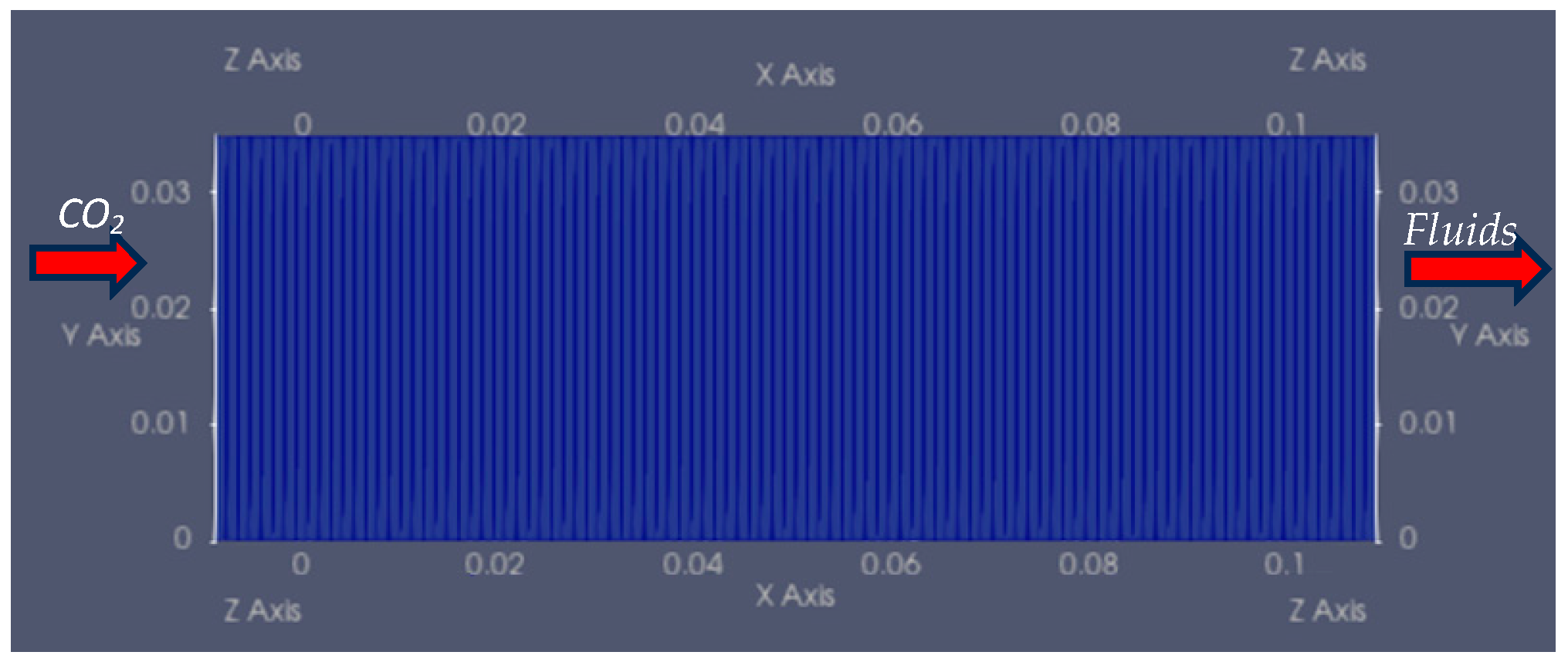
4. Validation
The Water-Saturated Coreflood Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papi, A.; Jahanbakhsh, A.; Maroto-Valer, M.M. compositionalIGFoam, a new Darcy-scale compositional solver in OpenFOAM for CO2/water interactions in CO2 storage processes in aquifers. In Proceedings of the InterPore 2024—16th Annual Meeting & Conference Courses, Qingdao, China, 13–16 May 2024; pp. 695–697. [Google Scholar]
- Amrollahinasab, O.; Jammernegg, B.; Azizmohammadi, S.; Ott, H. Simulation of CO2-Brine Primary Displacement in Heterogeneous Carbonate Rocks. In Proceedings of the 85th EAGE Annual Conference & Exhibition; European Association of Geoscientists & Engineers, Edinburgh, UK, 22–25 May 2023. [Google Scholar]
- Parvin, S.; Masoudi, M.; Sundal, A.; Miri, R. Continuum scale modelling of salt precipitation in the context of CO2 storage in saline aquifers with MRST compositional. Int. J. Greenh. Gas Control 2020, 99, 103075. [Google Scholar] [CrossRef]
- Available online: https://www.openfoam.com/documentation/user-guide (accessed on 1 June 2023).
- Kim, J.H.; Kim, W.T. Numerical investigation of gas-liquid two-phase flow inside PEMFC gas channels with rectangular and trapezoidal cross sections. Energies 2018, 11, 1403. [Google Scholar] [CrossRef]
- Keser, R.; Ceschin, A.; Battistoni, M.; Im, H.G.; Jasak, H. Development of a Eulerian multi-fluid solver for dense spray applications in OpenFOAM. Energies 2020, 13, 4740. [Google Scholar] [CrossRef]
- Carrillo, F.J.; Bourg, I.C.; Soulaine, C. Multiphase flow modeling in multiscale porous media: An open-source micro-continuum approach. J. Comput. Phys. X 2020, 8, 100073. [Google Scholar] [CrossRef]
- Maes, J.; Menke, H.P. GeoChemFoam: Direct modelling of multiphase reactive transport in real pore geometries with equilibrium reactions. Transp. Porous Media 2021, 139, 271–299. [Google Scholar] [CrossRef]
- Horgue, P.; Renard, F.; Gerlero, G.S.; Guibert, R.; Debenest, G. porousMultiphaseFoam v2107: An open-source tool for modeling saturated/unsaturated water flows and solute transfers at watershed scale. Comput. Phys. Commun. 2022, 273, 108278. [Google Scholar] [CrossRef]
- Hafsteinsson, H.E. Porous Media in OpenFOAM; Chalmers University of Technology: Gothenburg, Sweden, 2009; p. 14. [Google Scholar]
- Gooya, R.; Petit, O.; Chen, C.B. Description of porousSimpleFoam and Adding the Brinkmann Model to the Porous Models. 2014. Online Source. Available online: https://www.tfd.chalmers.se/~hani/kurser/OS_CFD_2013/RezaGooya/Final%20OF.pdf (accessed on 1 October 2023).
- Missios, K.; Jacobsen, N.; Moeller, K.; Roenby, J. Extending the isoAdvector Geometric VOF Method to Flows in Porous Media. OpenFOAM® J. 2023, 3, 66–74. [Google Scholar] [CrossRef]
- Soulaine, C.; Pavuluri, S.; Claret, F.; Tournassat, C. porousMedia4Foam: Multi-scale open-source platform for hydro-geochemical simulations with OpenFOAM®. Environ. Model. Softw. 2021, 145, 105199. [Google Scholar] [CrossRef]
- Pavuluri, S.; Tournassat, C.; Claret, F.; Soulaine, C. Reactive transport modeling with a coupled openfoam®-phreeqc platform. Transp. Porous Media 2022, 145, 475–504. [Google Scholar] [CrossRef]
- Horgue, P.; Soulaine, C.; Franc, J.; Guibert, R.; Debenest, G. An open-source toolbox for multiphase flow in porous media. Comput. Phys. Commun. 2015, 187, 217–226. [Google Scholar] [CrossRef]
- Chen, Z.; Huan, G.; Li, B. An improved IMPES method for two-phase flow in porous media. Transp. Porous Media 2004, 54, 361–376. [Google Scholar] [CrossRef]
- Lange, R.; Magalhães, G.M.; Rocha, F.F.; Coimbra, P.V.; Favero, J.L.; Dias, R.A.; Moraes, A.O.; Schwalbert, M.P. upstreamFoam: An OpenFOAM-based solver for heterogeneous porous media at different scales. arXiv 2023, arXiv:230403390. [Google Scholar]
- Maes, J.; Soulaine, C. A new compressive scheme to simulate species transfer across fluid interfaces using the Volume-Of-Fluid method. Chem. Eng. Sci. 2018, 190, 405–418. [Google Scholar] [CrossRef]
- Maes, J.; Soulaine, C. A unified single-field Volume-of-Fluid-based formulation for multi-component interfacial transfer with local volume changes. J. Comput. Phys. 2020, 402, 109024. [Google Scholar] [CrossRef]
- Ahmed, T. Equations of State and PVT Analysis; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon: Exposition et Application des Principes à Suivre et des Formules à Employer Dans Les Questions de Distribution D’eau; Victor dalmont: Paris, France, 1856. [Google Scholar]
- Brown, G. Henry Darcy and the making of a law. Water Resour. Res. 2002, 38, 11-1–11-12. [Google Scholar] [CrossRef]
- Ahmed, T. Reservoir Engineering Handbook; Gulf Professional Publishing: Houston, TX, USA, 2018. [Google Scholar]
- Henry, W. III. Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. In Abstract of the Papers Printed in the Philosophical Transactions of the Royal Society of London; Royal Society: London, UK, 1803; pp. 29–74. [Google Scholar]
- Raoult, F. General law of the vapor pressure of solvents. Comptes Rendus 1887, 104, 1430–1433. [Google Scholar]
- Available online: https://github.com/phorgue/porousMultiphaseFoam/tree/openfoam-v2206/tutorials/impesFoam-tutorials/injectionExtraction/injection (accessed on 1 April 2024).
- Available online: https://www.cmgl.ca/solutions/software/gem/ (accessed on 1 October 2023).

| Package | Developer | Darcy Flow | Free Flow | Multi-Phase | Transport | Reactive Transport | Compressible |
|---|---|---|---|---|---|---|---|
| porousSimpleFoam | OpenFOAM [4] | ✓ | ✓ | × | × | × | × |
| porousInterFoam | OpenFOAM [4] | ✓ | ✓ | ✓ | × | × | ✓ |
| porousInterIsoFoam | Missios et al. (2023) [12] | ✓ | ✓ | ✓ | × | × | ✓ |
| porousMedia4Foam | Soulaine et al. (2021) [13] | ✓ | ✓ | × | ✓ | ✓ | ✓ |
| hybridPorousInterFoam | Carrillo et al. (2020) [7] | ✓ | ✓ | ✓ | × | × | ✓ |
| porousMultiphaseFoam | Horgue et al. (2022) [9] | ✓ | × | ✓ | ✓ | × | ✓ |
| impesFoam | Horgue et al. (2015) [15] | ✓ | × | ✓ | × | × | × |
| upstreamFoam | Lange et al. (2023) [17] | ✓ | × | ✓ | × | × | ✓ |
| GeoChemFoam | Maes and Menke (2021) [8] | × | ✓ | ✓ | ✓ | ✓ | × |
| Rock and Fluid Parameters | Brooks–Corey kr/Pc Model Parameters | ||
|---|---|---|---|
| T | 65 °C | krw,max | 1 |
| K | 100 md | krg,max | 1 |
| P | 107 Pa | Swr | 0.001 |
| φ | 0.5 | Sg,crit | 0.999 |
| HCO2 | 2.9 × 108 Pa | Pc0 | 5 Pa |
| Psat | 2 × 104 Pa | n | 4 |
| μw | 10−3 kg/m.s | α | 0.2 |
| μCO2 | 1.76 × 10−5 kg/m.s | ||
| ρw | 1000 kg/m3 | ||
| ρCO2 | 1 kg/m3 | ||
| Component | xH2O | xCO2 | yH2O | yCO2 | |
|---|---|---|---|---|---|
| Model | |||||
| CMG-GEM | 0.9832 | 0.0168 | 0.0062 | 0.9938 | |
| compositionalIGFoam (this work) | 0.9656 | 0.0344 | 0.0019 | 0.9981 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papi, A.; Jahanbakhsh, A.; Maroto-Valer, M.M. A New Straightforward Darcy-Scale Compositional Solver in OpenFOAM for CO2/Water Mutual Solubility in CO2 Storage Processes in Aquifers. Energies 2024, 17, 3401. https://doi.org/10.3390/en17143401
Papi A, Jahanbakhsh A, Maroto-Valer MM. A New Straightforward Darcy-Scale Compositional Solver in OpenFOAM for CO2/Water Mutual Solubility in CO2 Storage Processes in Aquifers. Energies. 2024; 17(14):3401. https://doi.org/10.3390/en17143401
Chicago/Turabian StylePapi, Ali, Amir Jahanbakhsh, and Mercedes M. Maroto-Valer. 2024. "A New Straightforward Darcy-Scale Compositional Solver in OpenFOAM for CO2/Water Mutual Solubility in CO2 Storage Processes in Aquifers" Energies 17, no. 14: 3401. https://doi.org/10.3390/en17143401
APA StylePapi, A., Jahanbakhsh, A., & Maroto-Valer, M. M. (2024). A New Straightforward Darcy-Scale Compositional Solver in OpenFOAM for CO2/Water Mutual Solubility in CO2 Storage Processes in Aquifers. Energies, 17(14), 3401. https://doi.org/10.3390/en17143401






