Rational Design of Electrolyte Additives for Improved Solid Electrolyte Interphase Formation on Graphite Anodes: A Study of 1,3,6-Hexanetrinitrile
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Agents
2.2. Preparation of the Electrolyte
2.3. Preparation of the Graphite Based Electrode
2.4. Material Characterization
2.5. Electrochemical Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Guo, X.; Du, X.; Liang, J.; Wu, J.; Zhao, G.; Li, X.; Gui, S.; Zheng, F.; Zhao, J.; et al. Revealing the complex lithiation pathways and kinetics of core-shell NiO@CuO electrode. Energy Storage Mater. 2022, 51, 11–18. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Yang, J.; He, Z.; Zheng, J.; Li, Y. Electrochemical methods contribute to the recycling and regeneration path of lithium-ion batteries. Energy Storage Mater. 2023, 55, 606–630. [Google Scholar] [CrossRef]
- Yang, T.; Luo, D.; Zhang, X.; Gao, S.; Gao, R.; Ma, Q.; Park, H.W.; Or, T.; Zhang, Y.; Chen, Z. Sustainable regeneration of spent cathodes for lithium-ion and post-lithium-ion batteries. Nat. Sustain. 2024, 7, 776–785. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Deng, R.; He, T. Flexible Solid-State Lithium-Ion Batteries: Materials and Structures. Energies 2023, 16, 4549. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Chen, Z.; Chao, Y.; Li, W.; Wallace, G.G.; Bussell, T.; Ding, J.; Wang, C. Abuse-Tolerant Electrolytes for Lithium-Ion Batteries. Adv. Sci. 2021, 8, 2003694. [Google Scholar] [CrossRef]
- Logan, E.R.; Dahn, J.R. Electrolyte Design for Fast-Charging Li-Ion Batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, G.; Xie, T.; Dong, D.; Ma, C.; Su, L.; Gong, L.; Lou, X.; Guo, X.; Wang, J.; et al. Carbon/Polymer Bilayer-Coated Si-SiOx Electrodes with Enhanced Electrical Conductivity and Structural Stability. ACS Appl. Mater. Interfaces 2020, 12, 19023–19032. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Q.; Chen, H.; Li, M.; Zhou, S.; Wen, S.; Pan, J.; Zheng, Q.; Jiang, B.; Liu, H.; et al. Building better solid-state batteries with silicon-based anodes. Interdiscip. Mater. 2023, 2, 635–663. [Google Scholar] [CrossRef]
- Xie, T.; Liu, H.; Wang, L.; Wang, J.; Ma, C.; Xuan, C.; Su, L.; Gong, L. Rational Regulation of Cu-Co Thiospinel Hierarchical Microsphere to Enhance the Supercapacitive Properties. Batter. Supercaps 2023, 6, e202300317. [Google Scholar] [CrossRef]
- Sammawipawekul, N.; Kaeosamut, N.; Autthawong, T.; Watwiangkham, A.; Suthirakun, S.; Wannapaiboon, S.; Mahamai, N.; Sarakonsri, T.; Chimupala, Y.; Yimklan, S. Isostructural dual-ligand-based MOFs with different metal centers in response to diverse capacity lithium-ion battery anode. Chem. Eng. J. 2024, 482, 148904. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; He, A.; Li, J.; Ma, W.; Record, M.-C.; Boulet, P.; Wang, J.; Albina, J.-M. Core–Double-Shell TiO2@Fe3O4@C Microspheres with Enhanced Cycling Performance as Anode Materials for Lithium-Ion Batteries. Materials 2024, 17, 2543. [Google Scholar] [CrossRef]
- Sung, J.H.; Kim, T.; Kim, S.; Hasan, F.; Mohanty, S.K.; Srinivasa, M.K.; Reddy, S.C.; Yoo, H.D. Li3PO4-Coated Graphite Anode for Thermo-Electrochemically Stable Lithium-Ion Batteries. Energies 2023, 16, 6141. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, H.; Jiang, X. Preparation of graphite anode slurry by one-pot method. J. Energy Storage 2023, 73, 108973. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Z.; Fang, J.; Li, K.; Zhang, M.; Li, Z.; Yang, L.; Pan, F. Electrolyte Optimization for Graphite Anodes toward Fast Charging. J. Phys. Chem. C 2023, 127, 2755–2765. [Google Scholar] [CrossRef]
- Min, W.; Chen, X.; Huang, S.; Liao, Y.; Liang, Z.; Lei, Y.; Xu, J. High performance lithium ion battery cathode based reduced holey graphene oxides from spent lithium ion batteries. Carbon 2023, 210, 118038. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Hu, B.; Ding, J.; Zhang, Z.; Ge, S. Oriented-Etched Graphite for Low-Temperature Lithium-Ion Batteries. Batter. Supercaps 2023, 6, e202200499. [Google Scholar] [CrossRef]
- Lochala, J.A.; Zhang, H.; Wang, Y.; Okolo, O.; Li, X.; Xiao, J. Practical Challenges in Employing Graphene for Lithium-Ion Batteries and Beyond. Small Methods 2017, 1, 1700099. [Google Scholar] [CrossRef]
- Krajewski, M.; Chen, C.-H.; Huang, Z.-T.; Lin, J.-Y. Li4Ti5O12 Coated by Biomass-Derived Carbon Quantum Dots as Anode Material with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Energies 2022, 15, 7715. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, W.; Yang, H.; Zhou, H. Electrolyte design principles for low-temperature lithium-ion batteries. eScience 2023, 3, 100170. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Kong, L.; Chen, S.; Peng, C.; Zheng, J.; Li, Y.; Feng, W. Designing mesostructured iron (II) fluorides with a stable in situ polymer electrolyte interface for high-energy-density lithium-ion batteries. eScience 2024, 4, 100188. [Google Scholar] [CrossRef]
- Kotronia, A.; Asfaw, H.D.; Tai, C.-W.; Hahlin, M.; Brandell, D.; Edström, K. Nature of the Cathode–Electrolyte Interface in Highly Concentrated Electrolytes Used in Graphite Dual-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 3867–3880. [Google Scholar] [CrossRef]
- Domi, Y.; Doi, T.; Tsubouchi, S.; Yamanaka, T.; Abe, T.; Ogumi, Z. Irreversible morphological changes of a graphite negative-electrode at high potentials in LiPF6-based electrolyte solution. Phys. Chem. Chem. Phys. 2016, 18, 22426–22433. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, S.; Weng, S.; Li, X.; Wang, X.; Wang, Z.; Chen, L. Anionic Effect on Enhancing the Stability of a Solid Electrolyte Interphase Film for Lithium Deposition on Graphite. Nano Lett. 2021, 21, 5316–5323. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Peng, Z.; Luo, W.; Ren, F.; Li, Z.; Zhang, Q.; He, H.; Ouyang, C.; Wang, D. Tailoring Lithium Deposition via an SEI-Functionalized Membrane Derived from LiF Decorated Layered Carbon Structure. Adv. Energy Mater. 2019, 9, 1802912. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, Q.; Wang, Y.; Battaglia, V.S.; Huang, Y.; Zheng, H. The role of carbon bond types on the formation of solid electrolyte interphase on graphite surfaces. Carbon 2019, 148, 105–114. [Google Scholar] [CrossRef]
- Parhizi, M.; Caceres-Martinez, L.E.; Modereger, B.A.; Kenttämaa, H.I.; Kilaz, G.; Ostanek, J.K. Determining the Composition of Carbonate Solvent Systems Used in Lithium-Ion Batteries without Salt Removal. Energies 2022, 15, 2805. [Google Scholar] [CrossRef]
- Pang, C.; Xu, G.; An, W.; Ding, G.; Liu, X.; Chai, J.; Ma, J.; Liu, H.; Cui, G. Three-Component Functional Additive in a LiPF6-Based Carbonate Electrolyte for a High-Voltage LiCoO2/Graphite Battery System. Energy Technol. 2017, 5, 1979–1989. [Google Scholar] [CrossRef]
- Xie, J.-D.; Patra, J.; Chandra Rath, P.; Liu, W.-J.; Su, C.-Y.; Lee, S.-W.; Tseng, C.-J.; Gandomi, Y.A.; Chang, J.-K. Highly concentrated carbonate electrolyte for Li-ion batteries with lithium metal and graphite anodes. J. Power Source 2020, 450, 227657. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, Y.; Gao, Y.; Ma, Z.; Liu, Z.; Wang, W.; Younus, H.A.; Chen, Z.; Wang, X.; Zhang, S. Less is More: Trace Amount of a Cyclic Sulfate Electrolyte Additive Enable Ultra-Stable Graphite Anode for High-Performance Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 44429–44438. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Yang, T.; Zhao, X.; Fan, W.; Wang, W.; Yu, L.; Guo, S.; Zuo, X.; Zeng, R.; Nan, J. Lithium difluorophosphate as a multi-functional electrolyte additive for 4.4V LiNi0.5Co0.2Mn0.3O2/graphite lithium ion batteries. J. Electroanal. Chem. 2019, 846, 113141. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, Z.; Shan, B.; Song, Y.; Zheng, R.; Feng, W.; Cui, Y.; Xing, W. The optimization of the electrolyte for low temperature LiFePO4-graphite battery. Mater. Lett. 2024, 356, 135594. [Google Scholar] [CrossRef]
- Uchida, S.; Ishikawa, M. Lithium bis(fluorosulfonyl)imide based low ethylene carbonate content electrolyte with unusual solvation state. J. Power Source 2017, 359, 480–486. [Google Scholar] [CrossRef]
- Guan, D.; Hu, G.; Peng, Z.; Cao, Y.; Wu, J.; Huang, M.; Zhang, S.; Dai, Y.; Gong, Y.; Du, K. A nonflammable low-concentration electrolyte for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 12575–12587. [Google Scholar] [CrossRef]
- Liu, Z.; Li, R.; Deng, T. Optimized electrolyte design for improved mechanical stability of NCM523/Gr batteries at 4.6V cycles. J. Mater. Res. 2023, 38, 4795–4804. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Li, Y.; Feng, X.; Ma, Z.; Ren, D.; Lu, L.; Xu, G.-L.; Amine, K.; Ouyang, M. Solid-state interphases design for high-safety, high-voltage and long-cyclability practical batteries via ethylene carbonate-free electrolytes. Energy Storage Mater. 2024, 65, 103165. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Lu, Y. The Mystery of Electrolyte Concentration: From Superhigh to Ultralow. ACS Energy Lett. 2020, 5, 3633–3636. [Google Scholar] [CrossRef]
- Liu, D.; Qian, K.; He, Y.-B.; Luo, D.; Li, H.; Wu, M.; Kang, F.; Li, B. Positive film-forming effect of fluoroethylene carbonate (FEC) on high-voltage cycling with three-electrode LiCoO2/Graphite pouch cell. Electrochim. Acta 2018, 269, 378–387. [Google Scholar] [CrossRef]
- He, M.; Su, C.-C.; Feng, Z.; Zeng, L.; Wu, T.; Bedzyk, M.J.; Fenter, P.; Wang, Y.; Zhang, Z. High Voltage LiNi0.5Mn0.3Co0.2O2/Graphite Cell Cycled at 4.6 V with a FEC/HFDEC-Based Electrolyte. Adv. Energy Mater. 2017, 7, 1700109. [Google Scholar] [CrossRef]
- Kang, S.; Geng, F.; Lou, X.; Lu, G.; Liao, Y.; Shen, M.; Hu, B. Understanding the Improved Fast Charging Performance of Graphite Anodes with a Fluoroethylene Carbonate Additive by In Situ NMR and EPR. ACS Appl. Energy Mater. 2023, 6, 7596–7606. [Google Scholar] [CrossRef]
- Ehteshami, N.; Ibing, L.; Stolz, L.; Winter, M.; Paillard, E. Ethylene carbonate-free electrolytes for Li-ion battery: Study of the solid electrolyte interphases formed on graphite anodes. J. Power Source 2020, 451, 227804. [Google Scholar] [CrossRef]
- Sasanka Hewathilake, H.P.T.; Karunarathne, N.; Wijayasinghe, A.; Balasooriya, N.W.B.; Arof, A.K. Performance of developed natural vein graphite as the anode material of rechargeable lithium ion batteries. Ionics 2017, 23, 1417–1422. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Wagner, R.; Winter, M. Fluoroethylene Carbonate As an Promising Additive for γ-Butyrolactone based Electrolytes. ECS Meet. Abstr. 2014, MA2014-01, 37. [Google Scholar]
- Farhat, D.; Maibach, J.; Eriksson, H.; Edström, K.; Lemordant, D.; Ghamouss, F. Towards high-voltage Li-ion batteries: Reversible cycling of graphite anodes and Li-ion batteries in adiponitrile-based electrolytes. Electrochim. Acta 2018, 281, 299–311. [Google Scholar] [CrossRef]
- Liu, H.; Naylor, A.J.; Menon, A.S.; Brant, W.R.; Edström, K.; Younesi, R. Understanding the Roles of Tris(trimethylsilyl) Phosphite (TMSPi) in LiNi0.8Mn0.1Co0.1O2 (NMC811)/Silicon–Graphite (Si–Gr) Lithium-Ion Batteries. Adv. Mater. Interfaces 2020, 7, 2000277. [Google Scholar] [CrossRef]
- Younesi, R.; Christiansen, A.S.; Scipioni, R.; Ngo, D.-T.; Simonsen, S.B.; Edström, K.; Hjelm, J.; Norby, P. Analysis of the Interphase on Carbon Black Formed in High Voltage Batteries. J. Electrochem. Soc. 2015, 162, A1289. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Zhang, Z.; Li, W.; Jian, J.; Jie, Y.; Huang, F.; Han, Y.; Li, W.; Ai, F.; et al. Stable cycling of practical high-voltage LiCoO2 pouch cell via electrolyte modification. Nano Res. 2023, 16, 3864–3871. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.-A.; Lee, D.G.; Son, J.; Bae, T.H.; Lee, T.K.; Choi, N.-S. Designing Electrolytes for Stable Operation of High-Voltage LiCoO2 in Lithium-Ion Batteries. ACS Energy Lett. 2024, 9, 262–270. [Google Scholar] [CrossRef]
- Li, X.; Han, X.; Li, G.; Du, J.; Cao, Y.; Gong, H.; Wang, H.; Zhang, Y.; Liu, S.; Zhang, B.; et al. Nonsacrificial Nitrile Additive for Armoring High-Voltage LiNi0.83Co0.07Mn0.1O2 Cathode with Reliable Electrode–Electrolyte Interface toward Durable Battery. Small 2022, 18, 2202989. [Google Scholar] [CrossRef]
- Huang, L.; Chen, S.; Zhan, J.; Liang, J.; Li, S.; Wang, H.; Xu, M.; Li, W. Insight into the role of fluoroethylene carbonate in solid electrolyte interphase construction for graphite anodes of lithium-ion batteries. J. Phys. Chem. C 2024, 128, 9586–9594. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, H.; Zhou, J.; Tian, Y.; Guo, J.; Yuan, M.; Liu, L.; Gai, L. Abnormal electrode behavior of graphite anode operating with the electrolyte containing vinylene carbonate and ethylene sulfite additives. Electrochim. Acta 2023, 462, 142768. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, D.; Gai, Y.; Cai, Y. Adiponitrile (ADN): A Stabilizer for the LiNi0.8Co0.1Mn0.1O2 (NCM811) electrode/electrolyte interface of a graphite/NCM811 Li-ion cell. ACS Appl. Mater. Interfaces 2022, 14, 11398–11407. [Google Scholar]
- Wen, F.; Cao, S.; Ren, X.; Cao, Y.; Ai, X.; Xu, F. Nonflammable dual-salt electrolytes for graphite/LiNi0.8Co0.1Mn0.1O2 lithium-ion batteries: Li+ solvation structure and electrode/eelectrolyte interphase. ACS Appl. Energy Mater. 2022, 5, 15491–15501. [Google Scholar] [CrossRef]
- Lu, D.; He, J.; Qiu, Y.; Zhu, J.; Zhang, M.; Cai, Y. Using triallyl phosphate as electrolyte additive to stabilize electrode–electrolyte interface of LiNi0.5Mn1.5O4/graphite high voltage lithium ion cells. ACS Appl. Energy Mater. 2022, 5, 13600–13609. [Google Scholar] [CrossRef]


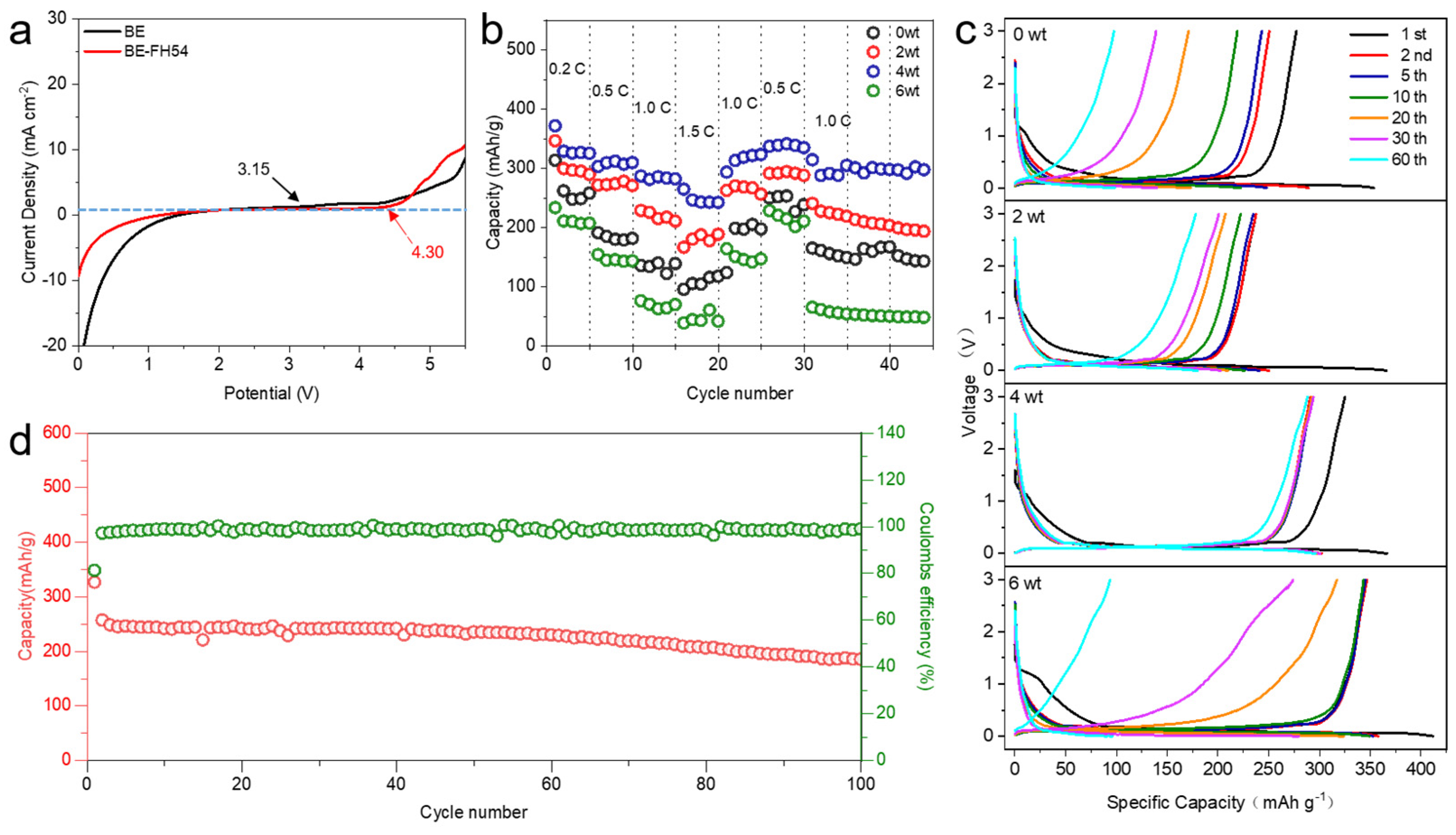
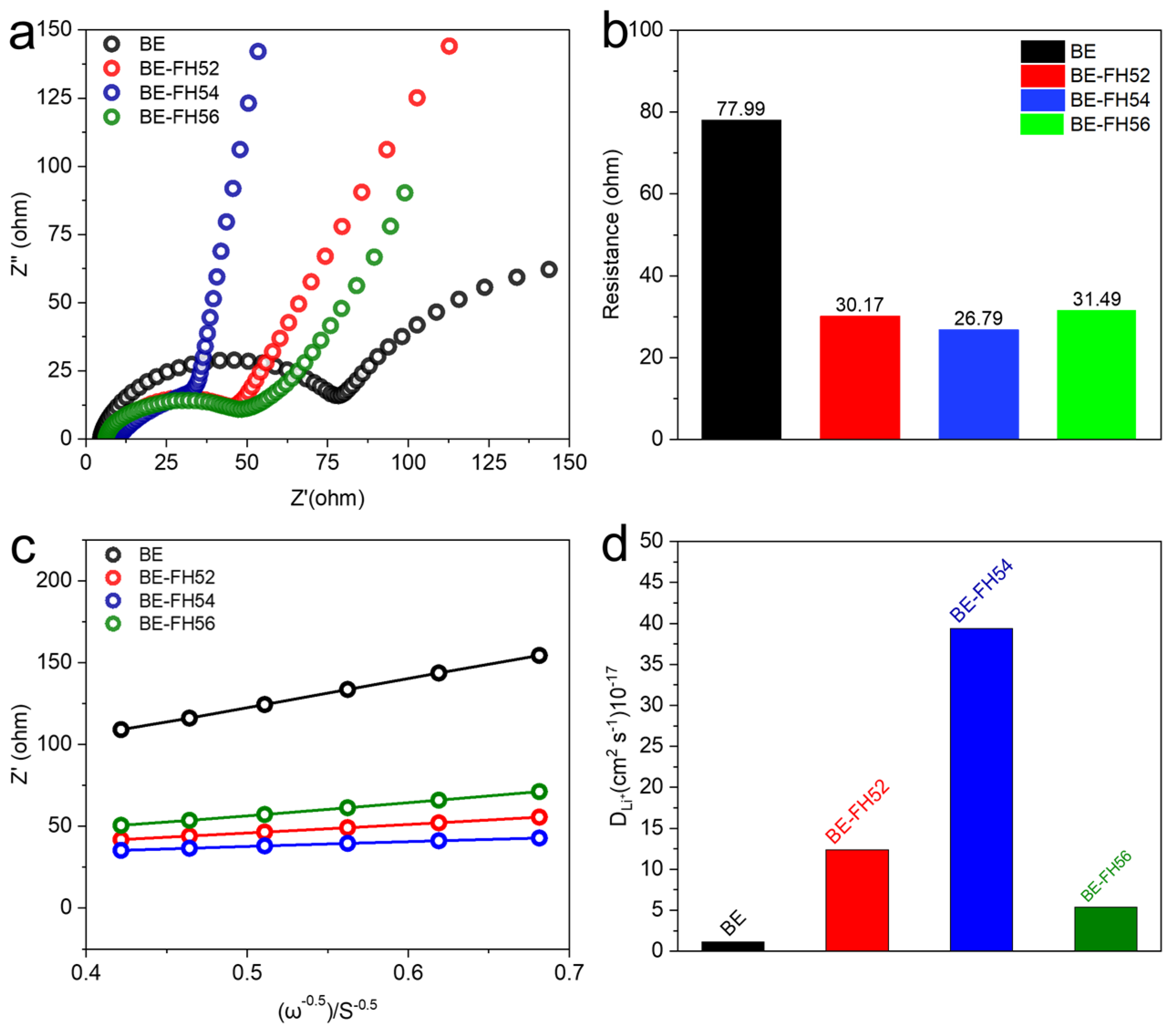

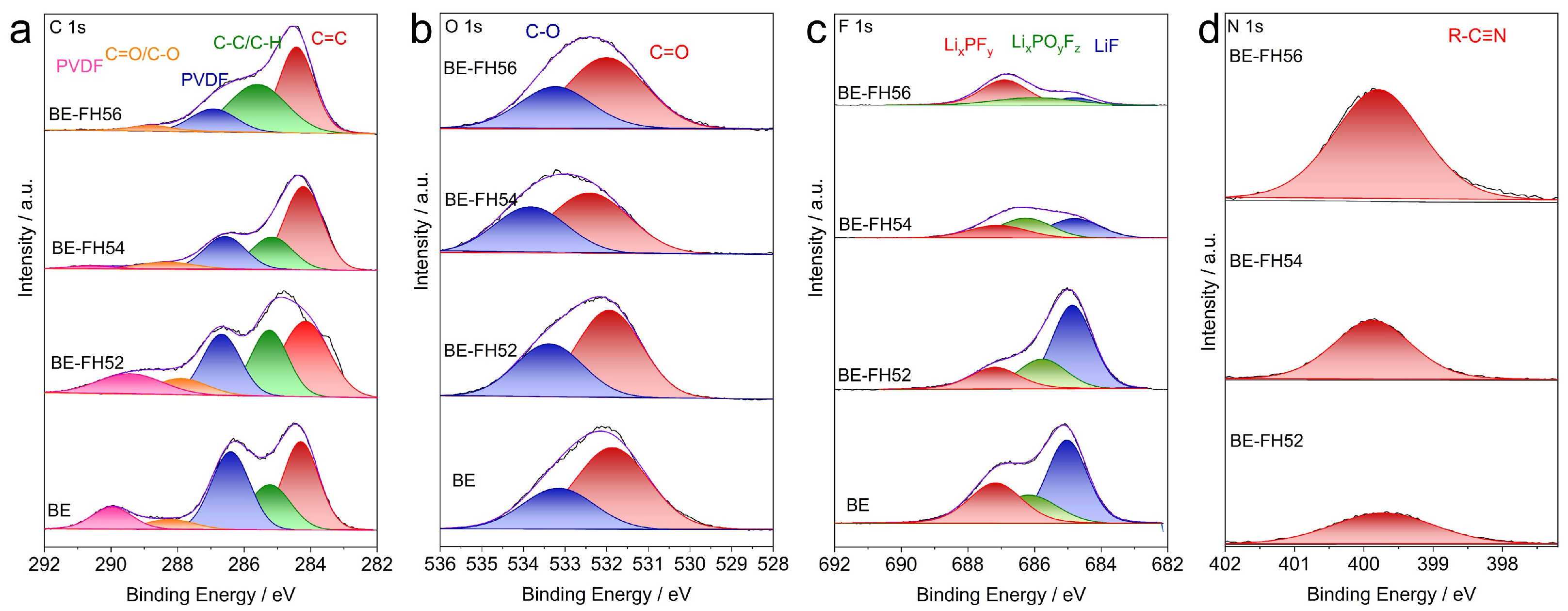
| Sample | IDC | ICC | DC after 60th Cycle | CC after 60th Cycle | ICE | CE after 60th Cycle |
|---|---|---|---|---|---|---|
| BE | 353.6 mAh/g | 277.3 mAh/g | 98.6 mAh/g | 96.3 mAh/g | 78.4% | 97.6% |
| BE-FH52 | 366.0 mAh/g | 237.6 mAh/g | 179.7 mAh/g | 177.9 mAh/g | 64.9% | 98.9% |
| BE-FH54 | 366.7 mAh/g | 326.4 mAh/g | 296.16 mAh/g | 288.1 mAh/g | 89.0% | 97.3% |
| BE-FH56 | 411.7 mAh/g | 345.4 mAh/g | 106.6 mAh/g | 104.2 mAh/g | 83.8% | 97.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, L.; Cao, Y.; Ma, Y.; Wang, S.; Wang, J.; Liu, H. Rational Design of Electrolyte Additives for Improved Solid Electrolyte Interphase Formation on Graphite Anodes: A Study of 1,3,6-Hexanetrinitrile. Energies 2024, 17, 3331. https://doi.org/10.3390/en17133331
Liu H, Wang L, Cao Y, Ma Y, Wang S, Wang J, Liu H. Rational Design of Electrolyte Additives for Improved Solid Electrolyte Interphase Formation on Graphite Anodes: A Study of 1,3,6-Hexanetrinitrile. Energies. 2024; 17(13):3331. https://doi.org/10.3390/en17133331
Chicago/Turabian StyleLiu, Hangning, Lin Wang, Yi Cao, Yingjun Ma, Shan Wang, Jie Wang, and Haidong Liu. 2024. "Rational Design of Electrolyte Additives for Improved Solid Electrolyte Interphase Formation on Graphite Anodes: A Study of 1,3,6-Hexanetrinitrile" Energies 17, no. 13: 3331. https://doi.org/10.3390/en17133331
APA StyleLiu, H., Wang, L., Cao, Y., Ma, Y., Wang, S., Wang, J., & Liu, H. (2024). Rational Design of Electrolyte Additives for Improved Solid Electrolyte Interphase Formation on Graphite Anodes: A Study of 1,3,6-Hexanetrinitrile. Energies, 17(13), 3331. https://doi.org/10.3390/en17133331







