Efficient Biovalorization of Oil Palm Trunk Waste as a Low-Cost Nutrient Source for Bioethanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Preparation of OPS and OPT Fiber
2.3. Ethanol Production from OPS and OPT Fiber through SSF and Repeated-Batch SSF
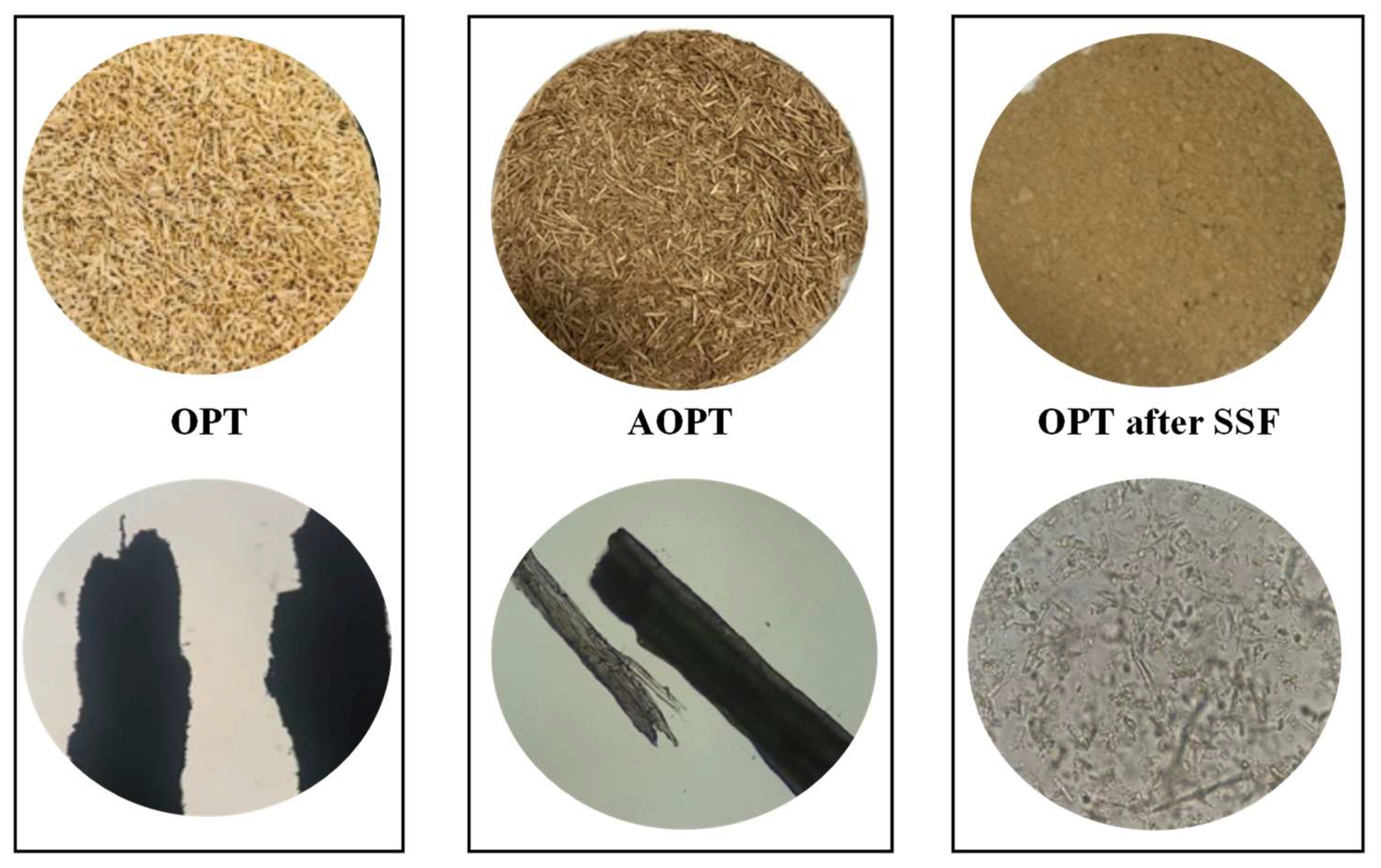
2.4. Ethanol Production from Acid-Pretreated OPT Fiber through SSF
2.5. Utilization of Residual Liquid from the SSF of AOPT for Lactic Acid Production
2.6. Analytical Methods
3. Results and Discussion
3.1. Ethanol Production from OPS and OPT Fiber through SSF
3.2. Repeated-Batch SSF for Efficient Ethanol Production from OPS and OPT Fiber
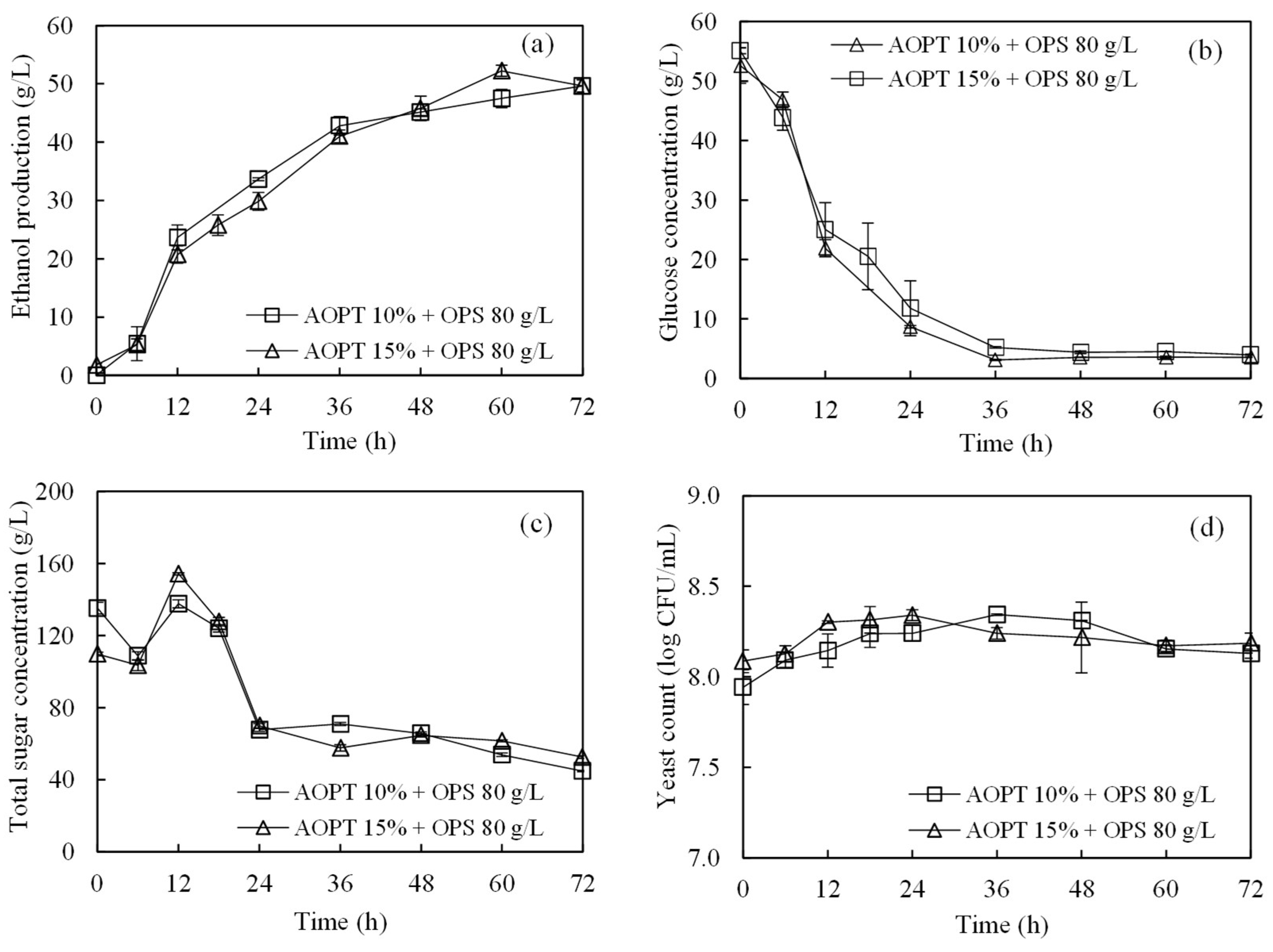
3.3. Ethanol Production from Acid-Pretreated OPT Fiber through SSF
3.4. Ethanol Production from Whole Slurry of AOPT and Concentrated OPS
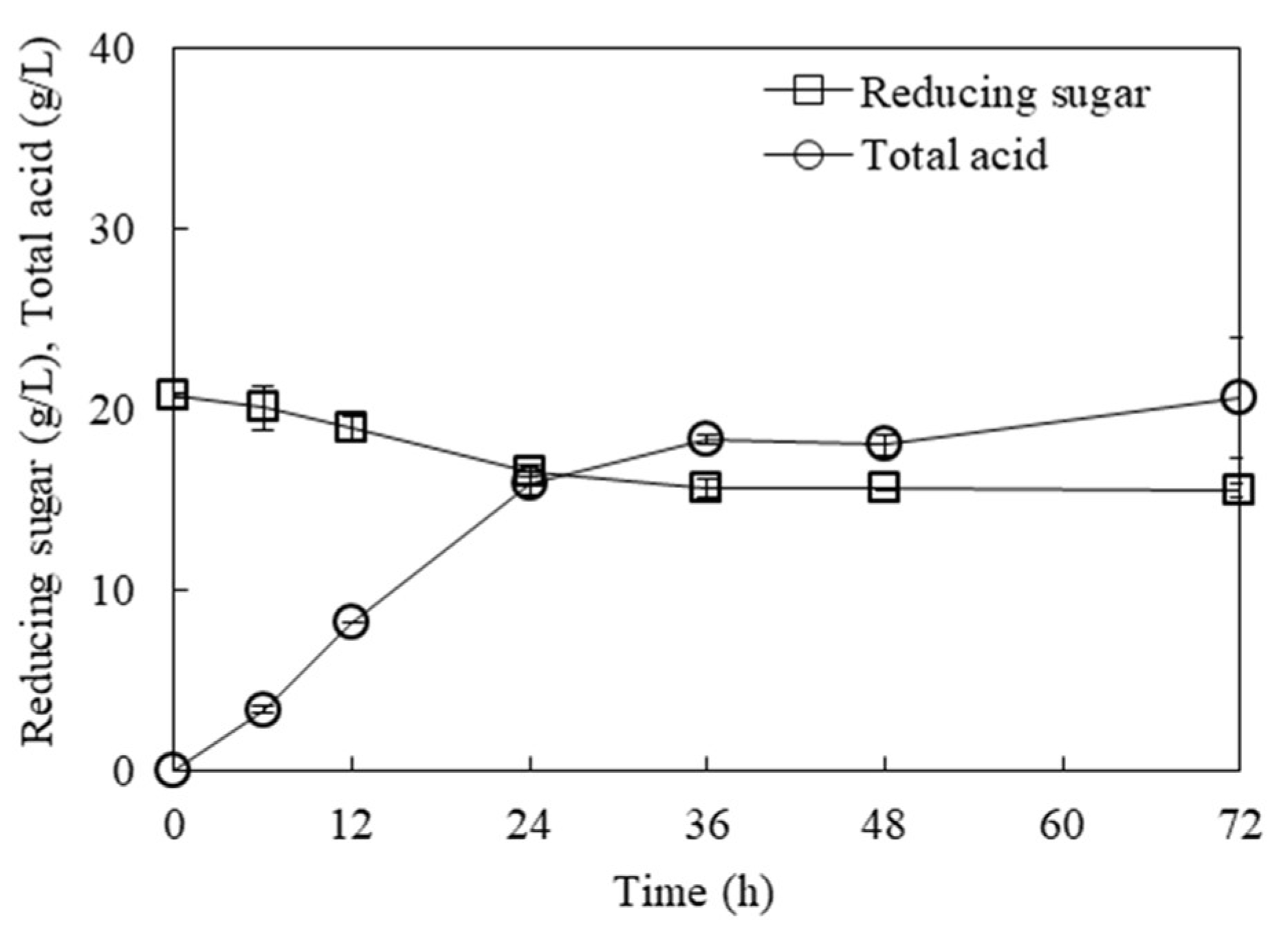
3.5. Valorization of Stillage after Ethanol Fermentation for Lactic Acid Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The production of bioethanol from lignocellulosic biomass: Pretreatment methods, fermentation, and downstream processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Patelski, A.M.; Dziekońska-Kubczak, U.; Balcerek, M.; Pielech-Przybylska, K.; Dziugan, P.; Berłowska, J. The ethanol production from sugar beet pulp supported by microbial hydrolysis with Trichoderma viride. Energies 2024, 17, 809. [Google Scholar] [CrossRef]
- Kumar Sarangi, P.; Subudhi, S.; Bhatia, L.; Saha, K.; Mudgil, D.; Prasad Shadangi, K.; Arya, R.K. Utilization of agricultural waste biomass and recycling toward circular bioeconomy. Environ. Sci. Pollut. Res. 2023, 30, 8526–8539. [Google Scholar] [CrossRef] [PubMed]
- Maluin, F.N.; Hussein, M.Z.; Idris, A.S. An overview of the oil palm industry, Challenges and some emerging opportunities for nanotechnology development. Agronomy 2020, 10, 356. [Google Scholar] [CrossRef]
- Noparat, P.; Prasertsan, P.; Sompong, O.; Pan, X. Sulfite pretreatment to overcome recalcitrance of lignocellulose for enzymatic hydrolysis of oil palm trunk. Energy Procedia 2017, 138, 1122–1127. [Google Scholar] [CrossRef]
- Komonkiat, I.; Cheirsilp, B. Felled oil palm trunk as a renewable source for biobutanol production by Clostridium spp. Bioresour. Technol. 2013, 146, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, N.A.; Loh, S.K.; Nasrin, A.B.; Luthfi, A.A.I.; Harun, S.; Abdul, P.M.; Jahim, J.M. Compatibility of utilising nitrogen-rich oil palm trunk sap for succinic acid fermentation by Actinobacillus succinogenes 130Z. Bioresour. Technol. 2019, 293, 122085. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Huang, J.; Yuan, J.; Chen, J.; Pei, Z.; Li, H.; Yang, S. Novel supramolecular deep eutectic solvent-enabled in-situ lignin protection for full valorization of all components of wheat straw. Bioresour. Technol. 2023, 388, 129722. [Google Scholar] [CrossRef] [PubMed]
- Mithra, M.G.; Jeeva, M.L.; Sajeev, M.S.; Padmaja, G. Comparison of ethanol yield from pretreated lignocellulo-starch biomass under fed-batch SHF or SSF modes. Heliyon 2018, 4, e00885. [Google Scholar] [CrossRef] [PubMed]
- Noparat, P.; Prasertsan, P.; Sompong, O.; Pan, X. Dilute acid pretreatment of oil palm trunk biomass at high temperature for enzymatic hydrolysis. Energy Procedia 2015, 79, 924–929. [Google Scholar] [CrossRef]
- Tan, J.; Yu, D.; Yuan, J.; Wu, H.; Luo, H.; Zhang, H.; Li, X.; Li, H.; Yang, S. Efficient delignification of wheat straw for microbial lipid production enabled by a novel ternary deep eutectic solvent containing ethylene glycol. Fuel 2023, 347, 128485. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, W.; Du, X.; Huang, H.; Gong, Z. Combined dilute sulfuric acid and Tween 80 pretreatment of corn stover significantly improves the enzyme digestibility, Synergistic removal of hemicellulose and lignin. Bioresour. Technol. 2023, 382, 129218. [Google Scholar] [CrossRef] [PubMed]
- Shabbirahmed, A.M.; Haldar, D.; Dey, P.; Patel, A.K.; Singhania, R.R.; Dong, C.D.; Purkait, M.K. Sugarcane bagasse into value-added products, a review. Environ. Sci. Pollut. Res. 2022, 29, 62785–62806. [Google Scholar] [CrossRef] [PubMed]
- Wischral, D.; Arias, J.M.; Modesto, L.F.; de França Passos, D.; Pereira, N. Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus, integrating xylose and glucose fermentation. Biotechnol. Prog. 2019, 35, e2718. [Google Scholar] [CrossRef] [PubMed]
- Sritrakul, N.; Nitisinprasert, S.; Keawsomponga, S. Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agri. Nat. Res. 2017, 51, 512–519. [Google Scholar] [CrossRef]
- A.O.A.C. AOAC method 973.18—Fiber (acid detergent) and lignin in animal feeds, fifteenth ed. In Official Methods of Analysis of Association of Official Analytical Chemists; Helrick, K., Ed.; Association of Official Analytic Chemists: Gaithersburg, MD, USA, 1990. [Google Scholar]
- A.O.A.C. AOAC method 992.16—Total dietary fiber—Enzymatic gravimetric method, eighteenth ed. In Official Methods of Analysis of Association of Official Analytical Chemists; Association of Official Analytic Chemists: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Boonsawang, P.; Subkaree, Y.; Srinorakutara, T. Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass Bioenergy 2012, 40, 127–132. [Google Scholar] [CrossRef]
- Saini, J.K. Enhanced cellulosic ethanol production via fed-batch simultaneous saccharification and fermentation of sequential dilute acid-alkali pretreated sugarcane bagasse. Bioresour. Technol. 2023, 372, 128671. [Google Scholar]
- Zentou, H.; Zainal Abidin, Z.; Yunus, R.; Awang Biak, D.R.; Abdullah Issa, M.; Yahaya Pudza, M. A new model of alcoholic fermentation under a byproduct inhibitory effect. ACS Omega 2021, 6, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Salihu, U.Y.; Usman, U.G.; Abubakar, A.Y.; Mansir, G. Effect of pH and temperature on bioethanol production: Evidences from the fermentation of sugarcane molasses using Saccharomyces cerevisiae. Dujopas 2022, 8, 9–16. [Google Scholar] [CrossRef]
- Arif, A.R.; Natsir, H.; Rohani, H.; Karim, A. Effect of pH fermentation on production bioethanol from jackfruit seeds (Artocarpus heterophyllus) through separate fermentation hydrolysis method. J. Phys. Conf. Ser. 2018, 979, 012015. [Google Scholar] [CrossRef]
- Hsieh, C.W.C.; Cannella, D.; Jørgensen, H.; Felby, C.; Thygesen, L.G. Cellulase inhibition by high concentrations of monosaccharides. J. Agric. Food Chem. 2014, 62, 3800–3805. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.D.; Azab, M.S.; Mahin, A.A.; Gaber, M.A. High improvement in lactic acid productivity by new alkaliphilic bacterium using repeated batch fermentation integrated with increased substrate concentration. BioMed Res. Int. 2019, 1, 7212870. [Google Scholar] [CrossRef] [PubMed]
- Sriputorn, B.; Laopaiboon, P.; Phukoetphim, N.; Polsokchuak, N.; Butkun, K.; Laopaiboon, L. Enhancement of ethanol production efficiency in repeated-batch fermentation from sweet sorghum stem juice, Effect of initial sugar; nitrogen and aeration. Electron. J. Biotechnol. 2020, 46, 55–64. [Google Scholar] [CrossRef]
- Shah, A.A.; Seehar, T.H.; Sharma, K.; Toor, S.S. Biomass pretreatment technologies. In Hydrocarbon Biorefinery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–228. [Google Scholar]
- Zhao, X.; Wen, J.; Chen, H.; Liu, D. The fate of lignin during atmospheric acetic acid pretreatment of sugarcane bagasse and the impacts on cellulose enzymatic hydrolyzability for bioethanol production. Renew. Energy 2018, 128, 200–209. [Google Scholar] [CrossRef]
- Sasmal, S.; Mohanty, K. Pretreatment of lignocellulosic biomass toward biofuel production. In Biorefining of Biomass to Biofuels. Biofuel and Biorefinery Technologies; Springer: Cham, Switzerland, 2018; pp. 203–221. [Google Scholar]
- Lee, C.; Zheng, Y.; Vander Gheynst, J.S. Effects of pretreatment conditions and post–pretreatment washing on ethanol production from dilute acid pretreated rice straw. Biosyst Eng 2015, 137, 36–42. [Google Scholar] [CrossRef]
- Sharma, S.; Swain, M.R.; Mishra, A.; Mathur, A.S.; Gupta, R.P.; Puri, S.K.; Sharma, A.K. High solid loading and multiple-fed simultaneous saccharification and co-fermentation (mf-SSCF) of rice straw for high titer ethanol production at low cost. Renew. Energy 2021, 179, 1915–1924. [Google Scholar] [CrossRef]
- Pinheiro1, T.; Coelho1, E.; Romaní, A.; Domingues, L. Intensifying ethanol production from brewer’s spent grain waste: Use of whole slurry at high solid loadings. New Biotechnol. 2019, 53, 1–8. [Google Scholar] [CrossRef]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Lü, X. A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Combined biogas and bioethanol production: Opportunities and challenges for industrial application. Energies 2015, 8, 8121–8144. [Google Scholar] [CrossRef]
- Zhang, C.; Pang, S.; Lv, M.; Wang, X.; Su, C.; Gao, W.; Tan, T. Reduction of wastewater discharge toward second-generation acetone–butanol–ethanol production, broth recycling by the fermentation–pervaporation hybrid process. Energy Fuels 2019, 33, 1210–1218. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Y.; Chen, D.; Li, J.; Wu, H.; Meng, Y.; Huang, J.; Yuan, J.; Su, Y.; Wang, J.; et al. Relay photo/thermal catalysis enables efficient cascade upgrading of sugars to lactic acid: Mechanism study and life cycle assessment. Chem. Eng. J. 2023, 452, 139687. [Google Scholar] [CrossRef]
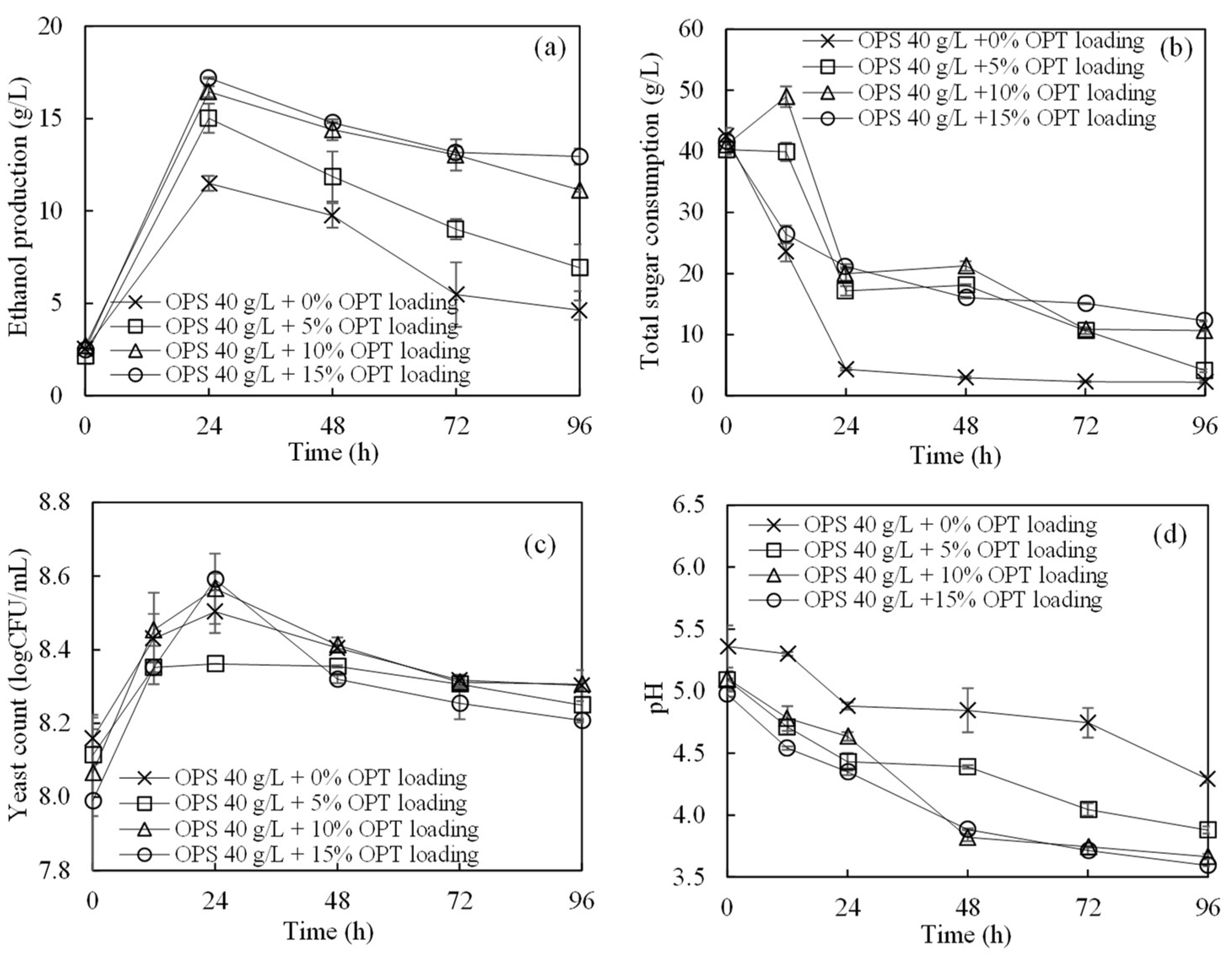

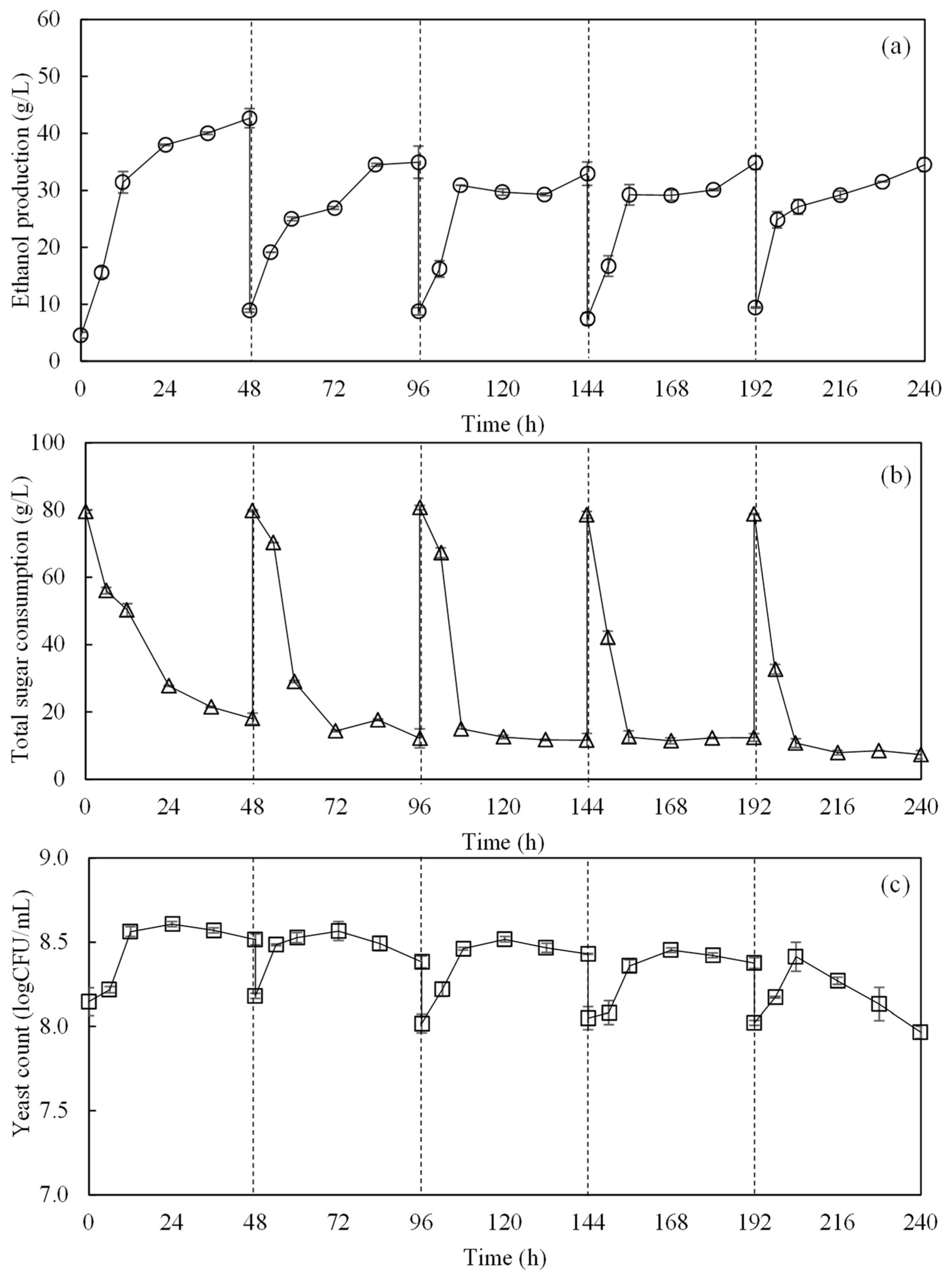
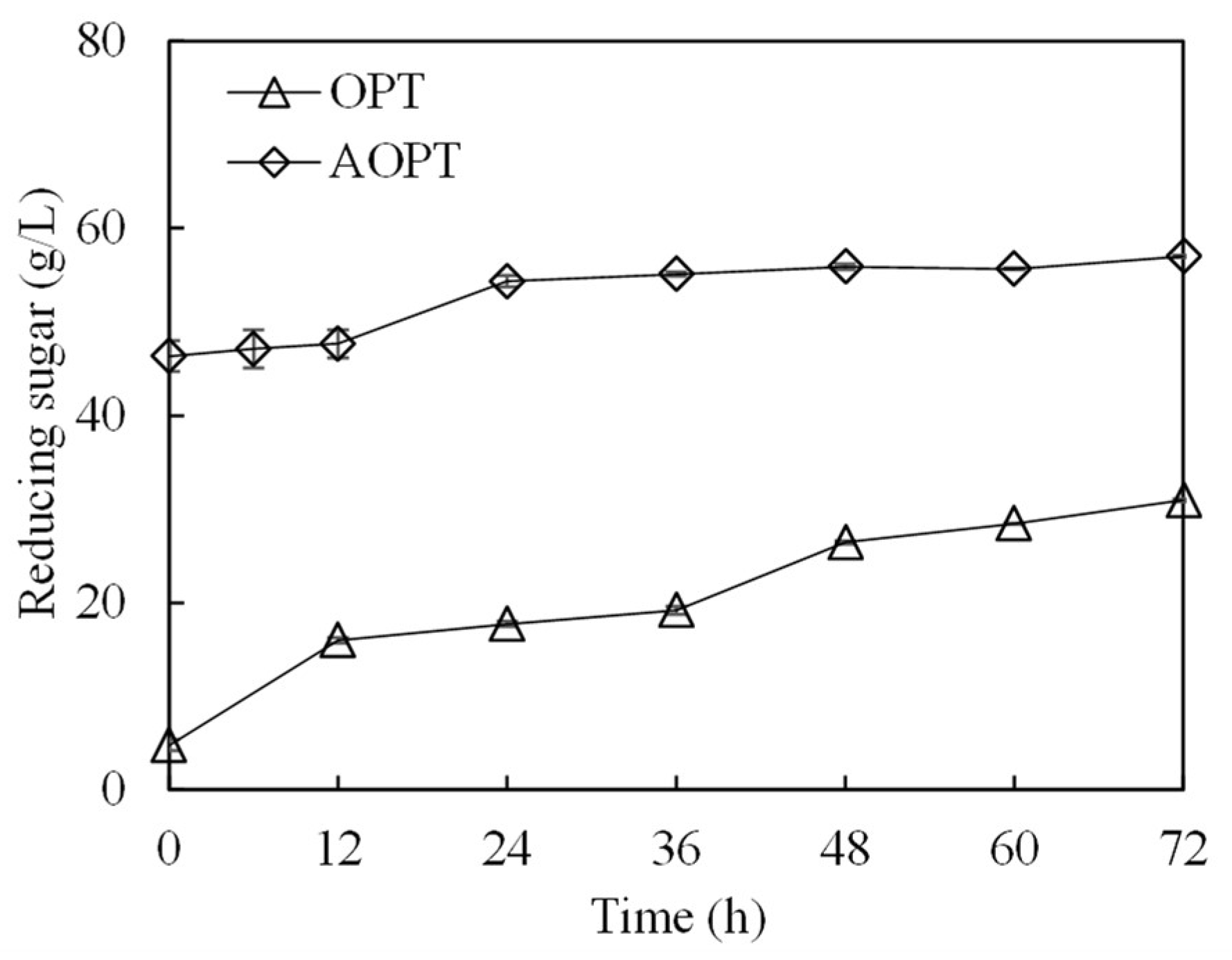
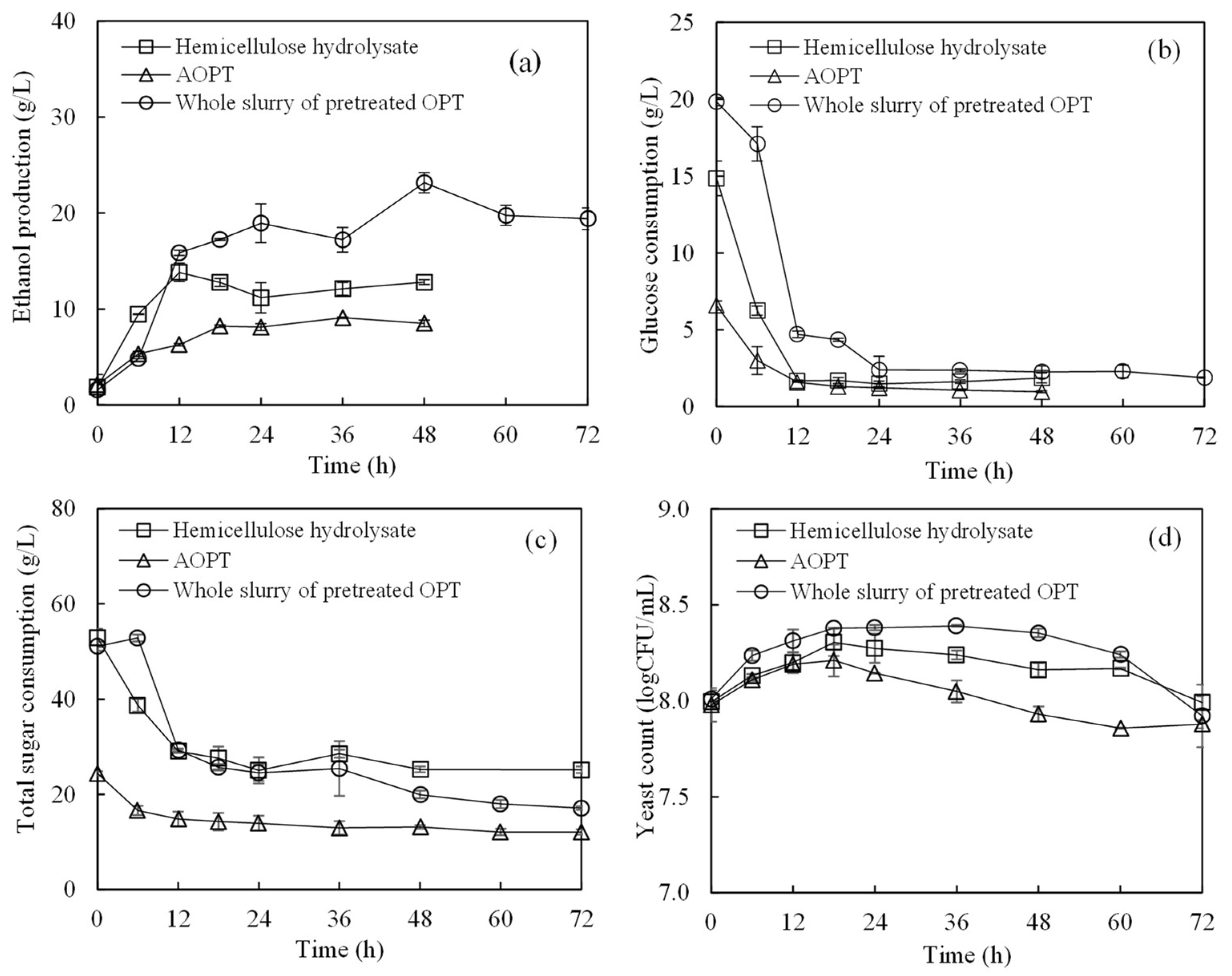
| Composition | Concentration (g/L) |
|---|---|
| Glucose | 19.84 ± 0.27 |
| Xylose | 11.33 ± 0.23 |
| Cellobiose | 2.32 ± 0.21 |
| Arabinose | 2.50 ± 0.03 |
| Acetic acid | 5.08 ± 0.18 |
| Furfural | 0.76 ± 0.09 |
| 5-Hydroxymethylfurfural | 0.42 ± 0.00 |
| Substrate loading | Glucose (g/L) | Xylose (g/L) | Arabinose (g/L) | Cellobiose (g/L) |
|---|---|---|---|---|
| OPS 80 g/L + 10% AOPT | 3.5 ± 0.04 | 11.9 ± 0.35 | 1.70 ± 0.06 | 1.84 ± 0.06 |
| OPS 80 g/L + 15% AOPT | 4.0 ± 0.09 | 13.7 ± 0.02 | 1.90 ± 0.11 | 1.40 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billateh, A.; Cheirsilp, B. Efficient Biovalorization of Oil Palm Trunk Waste as a Low-Cost Nutrient Source for Bioethanol Production. Energies 2024, 17, 3217. https://doi.org/10.3390/en17133217
Billateh A, Cheirsilp B. Efficient Biovalorization of Oil Palm Trunk Waste as a Low-Cost Nutrient Source for Bioethanol Production. Energies. 2024; 17(13):3217. https://doi.org/10.3390/en17133217
Chicago/Turabian StyleBillateh, Asma, and Benjamas Cheirsilp. 2024. "Efficient Biovalorization of Oil Palm Trunk Waste as a Low-Cost Nutrient Source for Bioethanol Production" Energies 17, no. 13: 3217. https://doi.org/10.3390/en17133217
APA StyleBillateh, A., & Cheirsilp, B. (2024). Efficient Biovalorization of Oil Palm Trunk Waste as a Low-Cost Nutrient Source for Bioethanol Production. Energies, 17(13), 3217. https://doi.org/10.3390/en17133217





