Upgrading/Deacidification of Bio-Oils by Liquid–Liquid Extraction Using Aqueous Methanol as a Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preliminary Tests
2.3. Experimental Procedure
2.3.1. Deacidification of BOs by LLE (Group of Experiments I)
2.3.2. Deacidification of BOs by LLE (Group of Experiments II)
2.4. Analytical Methods
2.4.1. Physical–Chemical Analysis
2.4.2. FTIR Analysis
2.4.3. Chemical Derivatization
2.4.4. GC-MS Analysis
2.5. Determination of LLE Process Parameters
3. Results and Discussion
3.1. Deacidification of BOs by LLE (Experiment Group I)
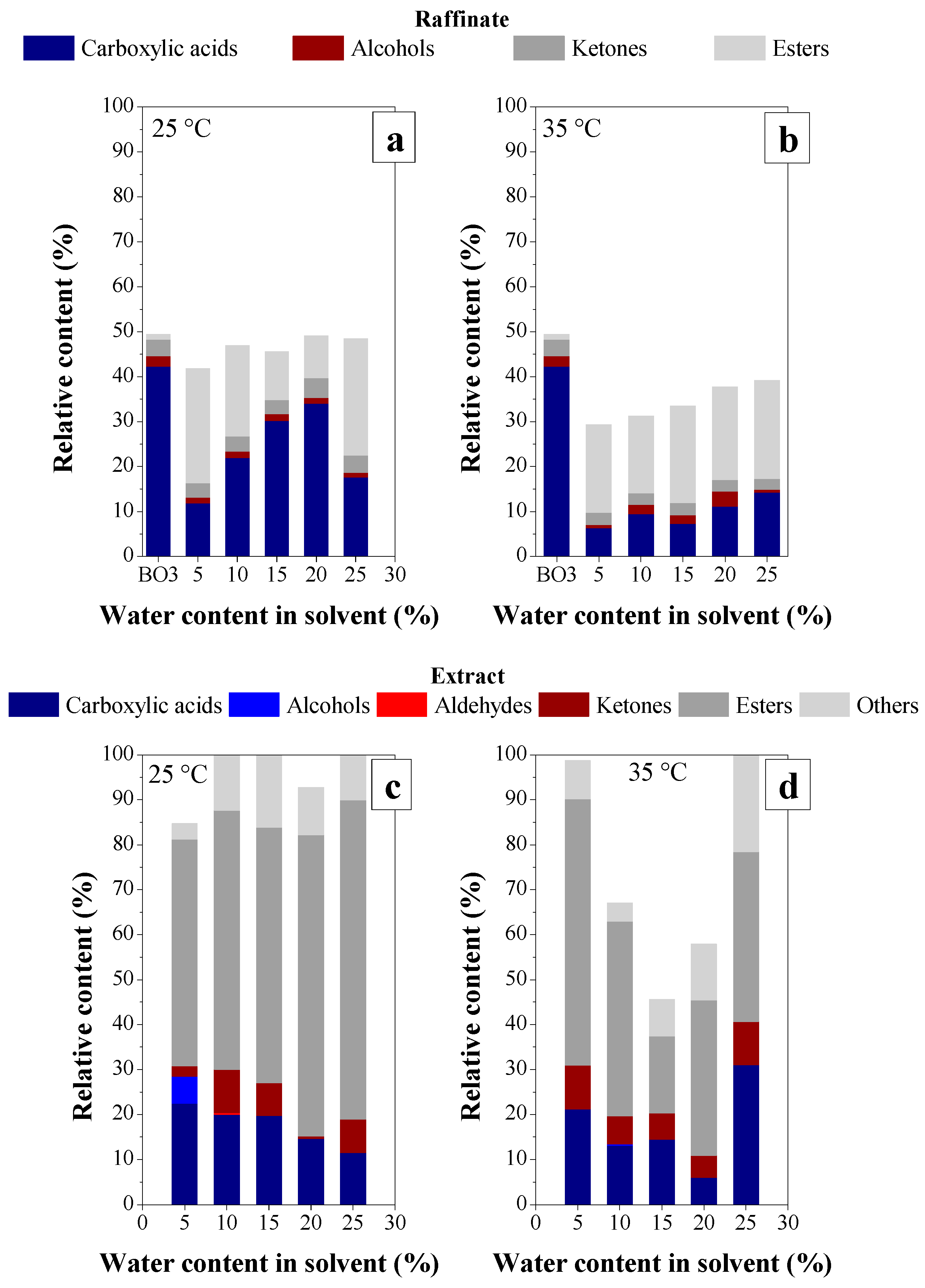
3.1.1. Effect of BO Deacidification by LLE on the Quality of Raffinates
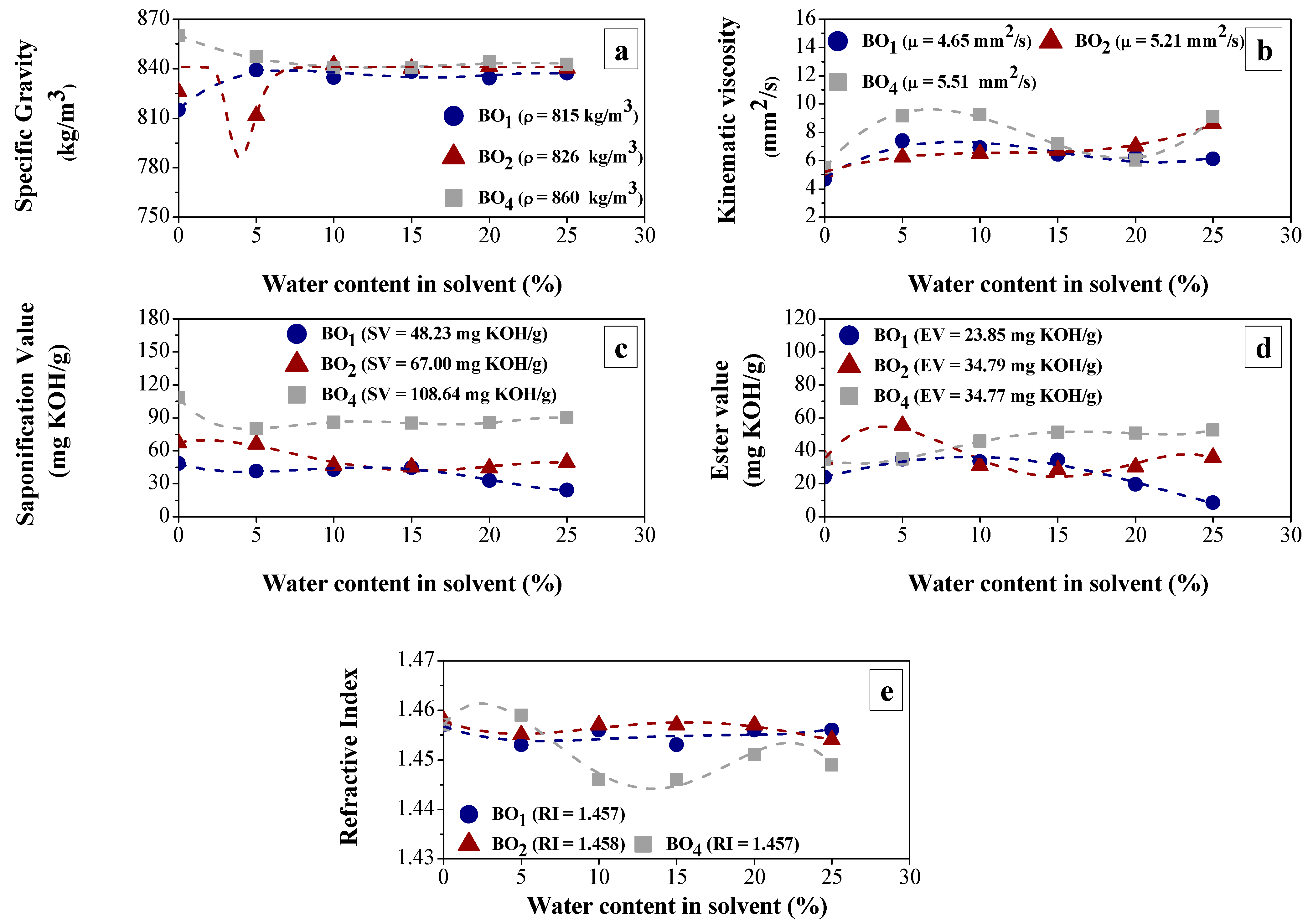
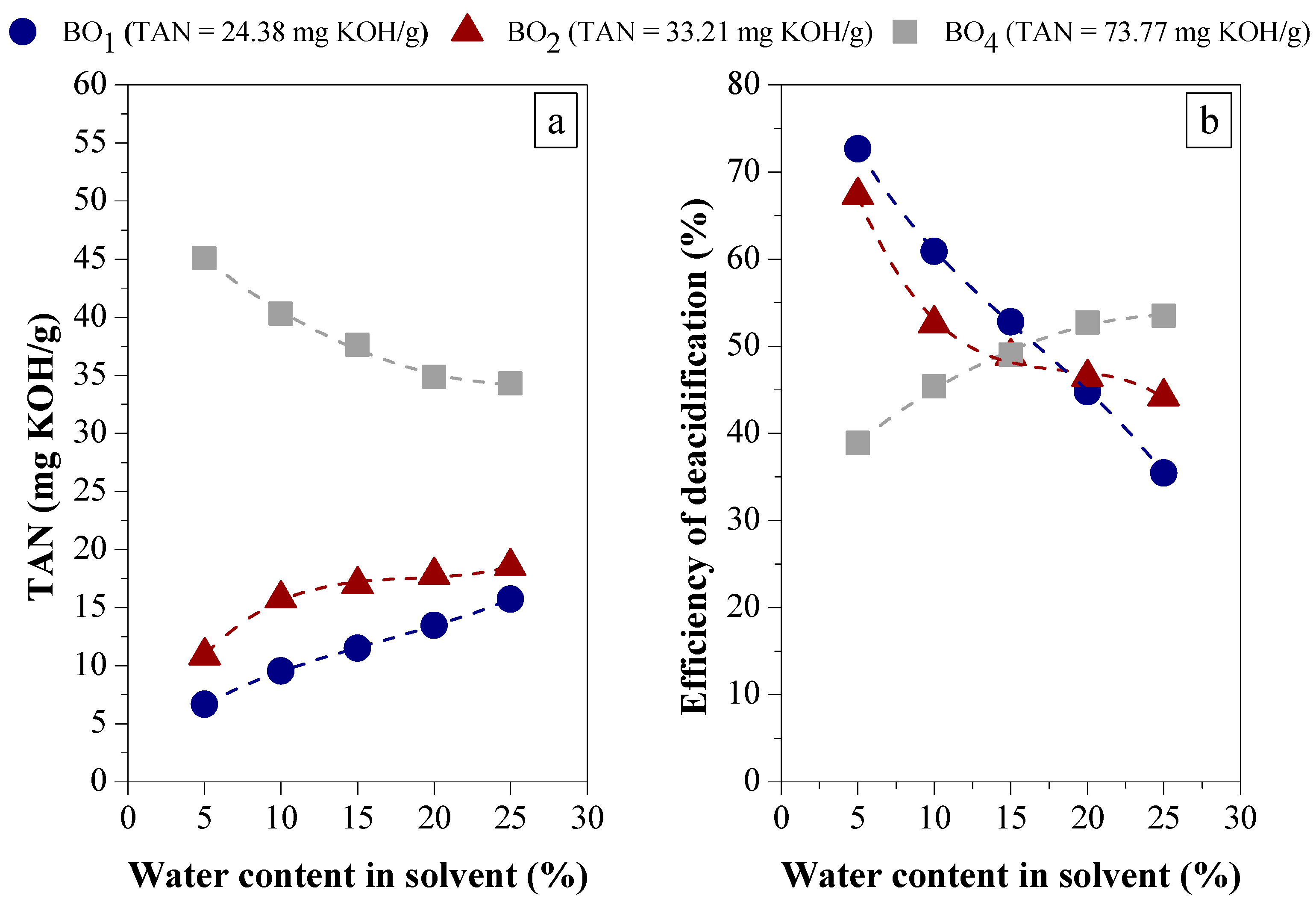
3.1.2. Efficiency of LLE Deacidification
Effect of Water Content on Deacidification
Effect of Acid Content in the Feedstock on Deacidification
3.1.3. FTIR Analysis of Original Bio-Oils and Raffinate Streams

3.2. Deacidification of BOs by LLE (Experiment Group II)
3.2.1. Effect of BO Deacidification on the Quality of Raffinates
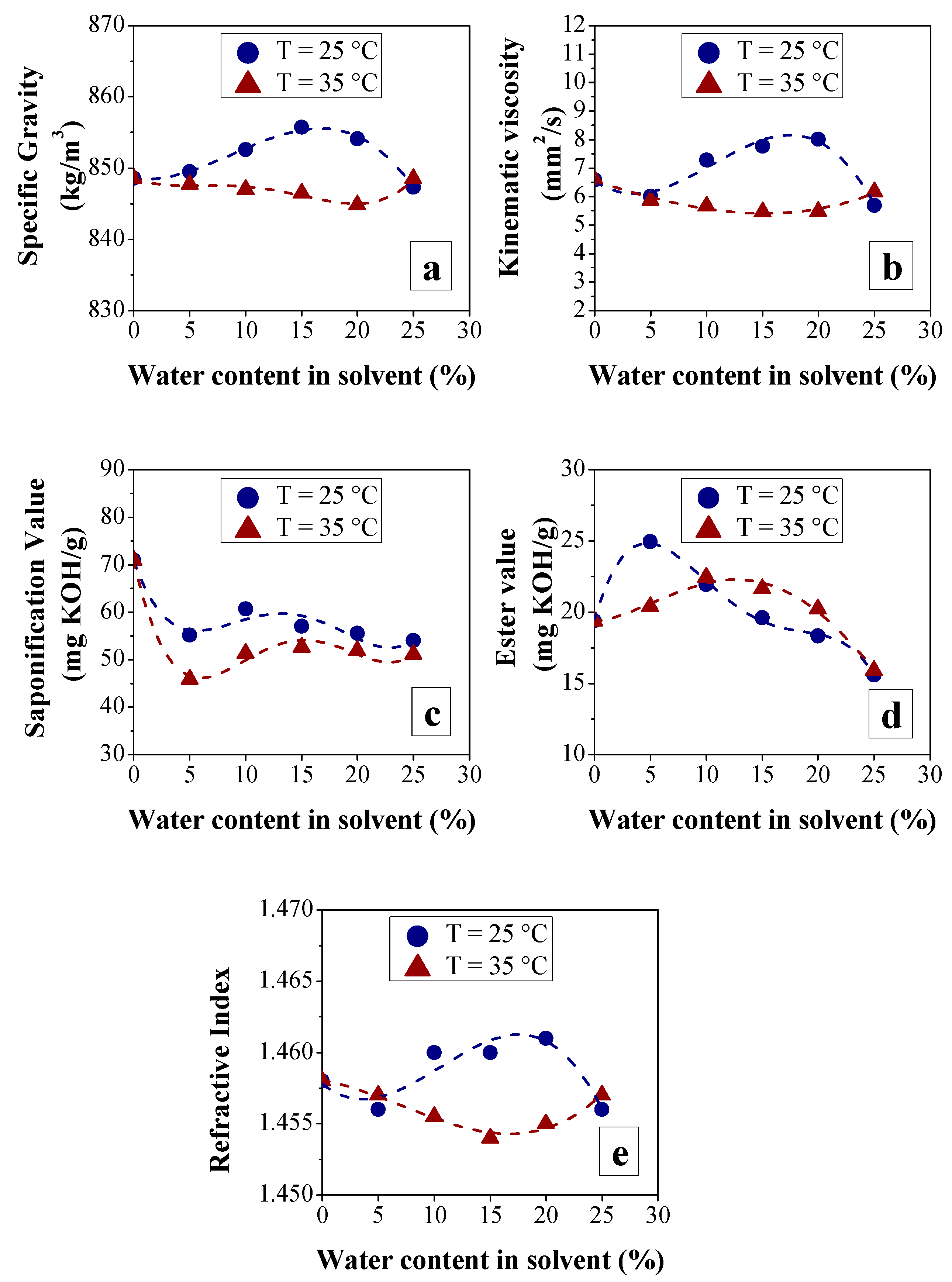
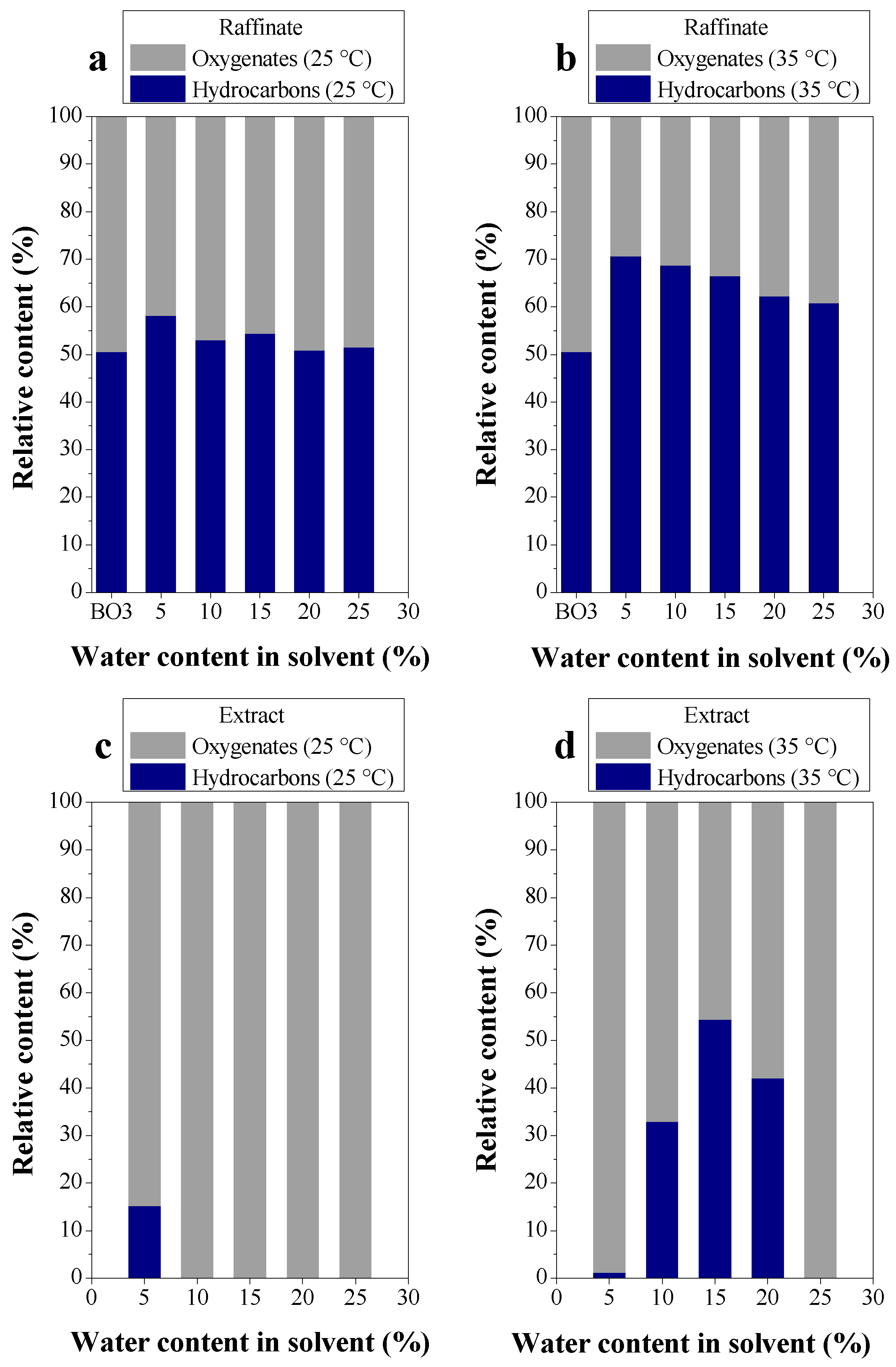
3.2.2. Efficiency of LLE Deacidification
Effect of Temperature on Deacidification

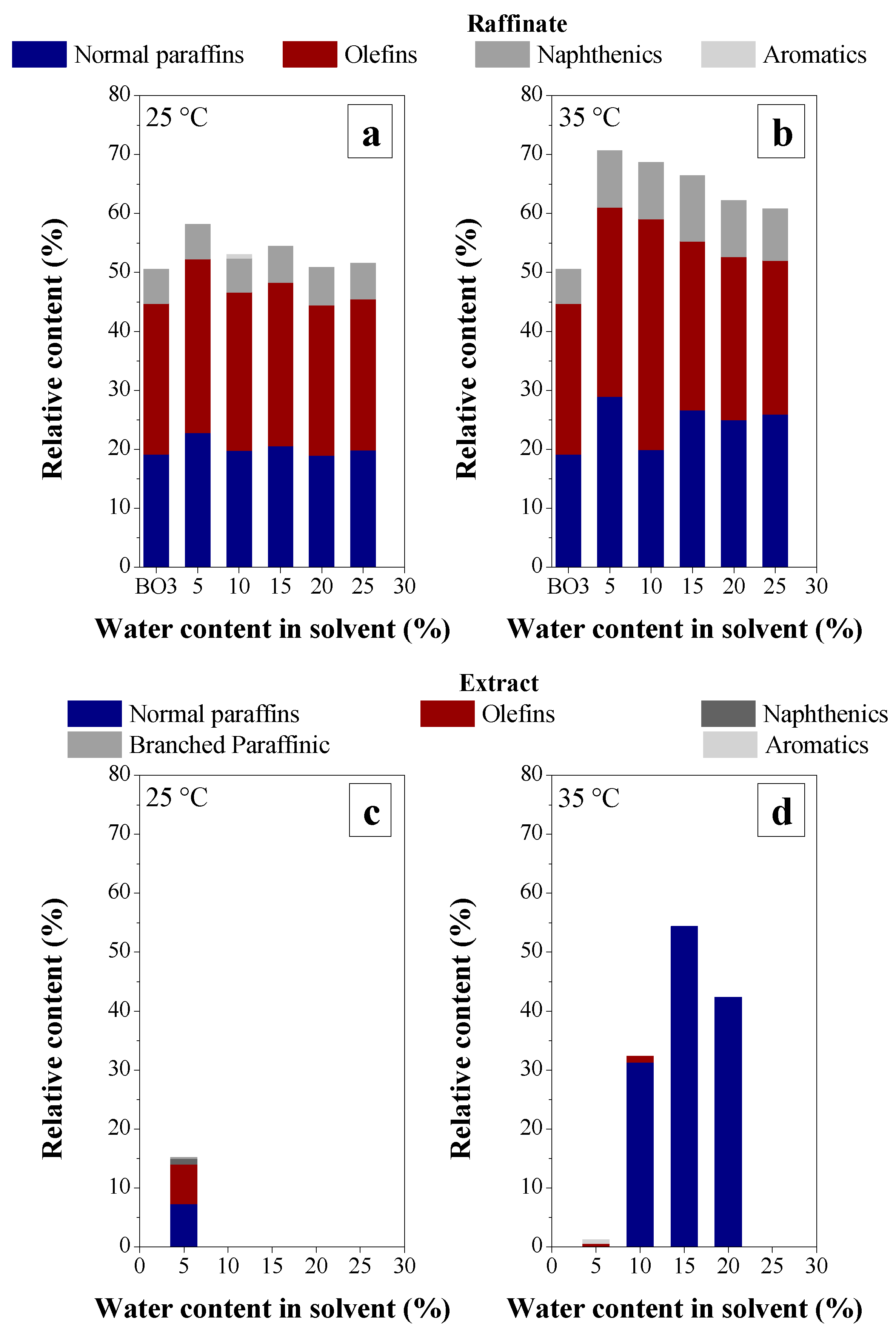
3.2.3. Chemical Composition

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chan, Y.H.; Loh, S.K.; Chin, B.L.F.; Yiin, C.L.; How, B.S.; Cheah, K.W.; Wong, M.K.; Loy, A.C.M.; Gwee, Y.L.; Lo, S.L.Y.; et al. Fractionation and Extraction of Bio-Oil for Production of Greener Fuel and Value-Added Chemicals: Recent Advances and Future Prospects. Chem. Eng. J. 2020, 397, 125406. [Google Scholar] [CrossRef]
- Stedile, T.; Ender, L.; Meier, H.F.; Simionatto, E.L.; Wiggers, V.R. Comparison between Physical Properties and Chemical Composition of Bio-Oils Derived from Lignocellulose and Triglyceride Sources. Renew. Sustain. Energy Rev. 2015, 50, 92–108. [Google Scholar] [CrossRef]
- Wei, Y.; Lei, H.; Wang, L.; Zhu, L.; Zhang, X.; Liu, Y.; Chen, S.; Ahring, B. Liquid–Liquid Extraction of Biomass Pyrolysis Bio-Oil. Energy Fuels 2014, 28, 1207–1212. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T.; Abd, A.A. State of the Art of Vegetable Oil Transformation into Biofuels Using Catalytic Cracking Technology: Recent Trends and Future Perspectives. Process Biochem. 2021, 109, 148–168. [Google Scholar] [CrossRef]
- Maher, K.D.; Bressler, D.C. Pyrolysis of Triglyceride Materials for the Production of Renewable Fuels and Chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.G.; Soares, V.C.D.; Ribeiro, E.B.; Carvalho, D.A.; Cardoso, É.C.V.; Rassi, F.C.; Mundim, K.C.; Rubim, J.C.; Suarez, P.A.Z. Diesel-like Fuel Obtained by Pyrolysis of Vegetable Oils. J. Anal. Appl. Pyrolysis 2004, 71, 987–996. [Google Scholar] [CrossRef]
- Bridgwater, A. V Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Wang, S. High-Efficiency Separation of Bio-Oil. In Biomass Now—Sustainable Growth and Use; Matovic, M.D., Ed.; InTech: Houston, TX, USA, 2013; pp. 401–418. [Google Scholar]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.H.; Yin, R.Z.; Mei, Y.F. Upgrading of Bio-Oil from Biomass Fast Pyrolysis in China: A Review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Xiu, S.N.; Shahbazi, A. Bio-Oil Production and Upgrading Research: A Review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A. Conversion of Bio-Oil to Bio Gasoline via Pyrolysis and Hydrothermal: A Review. Renew. Sustain. Energy Rev. 2017, 80, 538–549. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Jatoi, A.S.; Dumbre, D.K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Recent Advances in Production and Upgrading of Bio-Oil from Biomass: A Critical Overview. J. Environ. Chem. Eng. 2018, 6, 5101–5118. [Google Scholar] [CrossRef]
- Wisniewski, A.; Wosniak, L.; Scharf, D.R.; Wiggers, V.R.; Meier, H.F.; Simionatto, E.L. Upgrade of Biofuels Obtained from Waste Fish Oil Pyrolysis by Reactive Distillation. J. Braz. Chem. Soc. 2015, 26, 224–232. [Google Scholar] [CrossRef]
- Drugkar, K.; Rathod, W.; Sharma, T.; Sharma, A.; Joshi, J.; Pareek, V.K.; Ledwani, L.; Diwekar, U. Advanced Separation Strategies for Up-Gradation of Bio-Oil into Value-Added Chemicals: A Comprehensive Review. Sep. Purif. Technol. 2022, 283, 120149. [Google Scholar] [CrossRef]
- Jacobson, K.; Maheria, K.C.; Dalai, A.K. Bio-Oil Valorization: A Review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Hu, X.; Gunawan, R.; Mourant, D.; Hasan, M.D.M.; Wu, L.; Song, Y.; Lievens, C.; Li, C.-Z. Upgrading of Bio-Oil via Acid-Catalyzed Reactions in Alcohols—A Mini Review. Fuel Process. Technol. 2017, 155, 2–19. [Google Scholar] [CrossRef]
- Leng, L.; Li, H.; Yuan, X.; Zhou, W.; Huang, H. Bio-Oil Upgrading by Emulsification/Microemulsification: A Review. Energy 2018, 161, 214–232. [Google Scholar] [CrossRef]
- Gharib, J.; Pang, S.; Holland, D. Synthesis and Characterisation of Polyurethane Made from Pyrolysis Bio-Oil of Pine Wood. Eur. Polym. J. 2020, 133, 109725. [Google Scholar] [CrossRef]
- Wang, S.R.; Wang, Y.R.; Cai, Q.J.; Wang, X.Y.; Jin, H.; Luo, Z.Y. Multi-Step Separation of Monophenols and Pyrolytic Lignins from the Water-Insoluble Phase of Bio-Oil. Sep. Purif. Technol. 2014, 122, 248–255. [Google Scholar] [CrossRef]
- Park, L.K.E.; Ren, S.; Yiacoumi, S.; Ye, X.P.; Borole, A.P.; Tsouris, C. Separation of Switchgrass Bio-Oil by Water/Organic Solvent Addition and PH Adjustment. Energy Fuels 2016, 30, 2164–2173. [Google Scholar] [CrossRef]
- Valdebenito, F.; Ramírez-Álvarez, R.; Alexandra Muñoz, M.; Pecchi, G.; Canales, R.; Ormazabal, S.; Muñoz, R.; Alejandro-Martín, S.; Quero, F.; Adam, R.; et al. Biomass Characterization and Solvent Extraction as Tools to Promote Phenol Production from Urban Pruning. Fuel 2024, 362, 130830. [Google Scholar] [CrossRef]
- Osmanbegovic, N.; Bhatnagar, A.; Konttinen, J.; Louhi-Kultanen, M. Freeze Concentration of Aqueous Pyrolysis Oil Extract and Levoglucosan Recovery by Cooling Crystallization. Powder Technol. 2023, 427, 118700. [Google Scholar] [CrossRef]
- Arroyo-Avirama, A.F.; Ormazábal-Latorre, S.; Jogi, R.; Gajardo-Parra, N.F.; Pazo-Carballo, C.; Ascani, M.; Virtanen, P.; Garrido, J.M.; Held, C.; Mäki-Arvela, P.; et al. Improving the Separation of Guaiacol from N-Hexane by Adding Choline Chloride to Glycol Extracting Agents. J. Mol. Liq. 2022, 355, 118936. [Google Scholar] [CrossRef]
- Fardhyanti, D.S.; Kadarwati, S.; Fatriasari, W.; Prasetiawan, H. Mass Transfer Study of Phenolic Compound Liquid-Liquid Extraction Process from Bio-Oil of Coffee Shell Pyrolysis Product. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook, Eighth Edition, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2008; ISBN 9780071422949. [Google Scholar]
- Li, Q.; Steele, P.H.; Mitchell, B.; Ingram, L.L.; Yu, F. The Addition of Water to Extract Maximum Levoglucosan from the Bio-Oil Produced via Fast Pyrolysis of Pretreated Loblolly Pinewood. Bioresources 2013, 8, 1868–1880. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Wu, S.; Jiang, W.; Wei, W.; Lei, M. Fractionation of Pyrolysis Oil Derived from Lignin through a Simple Water Extraction Method. Fuel 2019, 242, 587–595. [Google Scholar] [CrossRef]
- Bennett, N.M.; Helle, S.S.; Duff, S.J.B. Extraction and Hydrolysis of Levoglucosan from Pyrolysis Oil. Bioresour. Technol. 2009, 100, 6059–6063. [Google Scholar] [CrossRef] [PubMed]
- Vitasari, C.R.; Meindersma, G.W.; de Haan, A.B. Water Extraction of Pyrolysis Oil: The First Step for the Recovery of Renewable Chemicals. Bioresour. Technol. 2011, 102, 7204–7210. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, P.K.; Naik, D.V.; Tripathi, D.; Singh, R.; Poddar, M.K.; Konathala, L.N.S.K.; Sharma, Y.K. Pyrolysis of Jatropha Curcas Seed Cake Followed by Optimization of Liquid-Liquid Extraction Procedure for the Obtained Bio-Oil. J. Anal. Appl. Pyrolysis 2016, 118, 202–224. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.B.; Liu, Y.Q.; Lv, D.C.; Ye, Y.Y.; Zhu, S.J.; Zhang, B.B. Study of a New Complex Method for Extraction of Phenolic Compounds from Bio-Oils. Sep. Purif. Technol. 2014, 134, 132–138. [Google Scholar] [CrossRef]
- Fu, D.; Farag, S.; Chaouki, J.; Jessop, P.G. Extraction of Phenols from Lignin Microwave-Pyrolysis Oil Using a Switchable Hydrophilicity Solvent. Bioresour. Technol. 2014, 154, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lange, J.-P.; Van Rossum, G.; Kersten, S.R.A. Bio-Oil Fractionation by Temperature-Swing Extraction: Principle and Application. Biomass Bioenergy 2015, 83, 96–104. [Google Scholar] [CrossRef]
- Li, H.; Xia, S.; Ma, P. Upgrading Fast Pyrolysis Oil: Solvent–Anti-Solvent Extraction and Blending with Diesel. Energy Convers. Manag. 2016, 110, 378–385. [Google Scholar] [CrossRef]
- Hu, H.-S.; Wu, Y.-L.; Yang, M.-D. Fractionation of Bio-Oil Produced from Hydrothermal Liquefaction of Microalgae by Liquid-Liquid Extraction. Biomass Bioenergy 2018, 108, 487–500. [Google Scholar] [CrossRef]
- Yang, X.; Lyu, H.; Chen, K.; Zhu, X.; Zhang, S.; Chen, J. Selective Extraction of Bio-Oil from Hydrothermal Liquefaction of Salix Psammophila by Organic Solvents with Different Polarities through Multistep Extraction Separation. Bioresources 2014, 9, 5219–5233. [Google Scholar] [CrossRef]
- Sukhbaatar, B.; Steele, P.H.; Ingram, L.L.; Kim, M.G. An Exploratory Study on the Removal of Acetic and Formic Acids from Bio-Oil. Bioresources 2009, 4, 1319–1329. [Google Scholar] [CrossRef]
- Lewis, K.R.; Daane, M.L.; Schelling, R. Processing Corrosive Crude Oils. In Proceedings of the CORROSION 99, San Antonio, TX, USA, 25–30 April 1999. [Google Scholar]
- de Bruyn, H.J. Naphthenic Acid Corrosion in Synthetic Fuels Production. In Proceedings of the CORROSION 98, San Diego, CA, USA, 22–27 March 1998. [Google Scholar]
- Qu, D.R.; Zheng, Y.G.; Jing, H.M.; Yao, Z.M.; Ke, W. High Temperature Naphthenic Acid Corrosion and Sulphidic Corrosion of Q235 and 5Cr1/2Mo Steels in Synthetic Refining Media. Corros. Sci. 2006, 48, 1960–1985. [Google Scholar] [CrossRef]
- Qu, D.R.; Zheng, Y.G.; Jiang, X.; Ke, W. Correlation between the Corrosivity of Naphthenic Acids and Their Chemical Structures. Anti-Corros. Methods Mater. 2007, 54, 211–218. [Google Scholar] [CrossRef]
- Yépez, O. On the Chemical Reaction between Carboxylic Acids and Iron, Including the Special Case of Naphthenic Acid. Fuel 2007, 86, 1162–1168. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, L.; Gan, F. High Temperature Naphthenic Acid Corrosion of Steel in High TAN Refining Media. Anti-Corros. Methods Mater. 2008, 55, 257–263. [Google Scholar] [CrossRef]
- Da Mota, S.D.P.; Mancio, A.A.; Lhamas, D.E.L.; De Abreu, D.H.; Da Silva, M.S.; Dos Santos, W.G.; De Castro, D.A.R.; De Oliveira, R.M.; Arajo, M.E.; Borges, L.E.P.; et al. Production of Green Diesel by Thermal Catalytic Cracking of Crude Palm Oil (Elaeis guineensis Jacq) in a Pilot Plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D130; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D974; Standard Test Method for Acid and Base Number by Color-Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2022.
- AOCS Cd 3-25; AOCS Official Method Cd 3-25, Saponification Value of Fats and Oils. Official Methods and Recommended Practices of the American Oil Chemists’ Society. AOCS Press: Champaign, IL, USA, 2017.
- Paquot, C. Standard Methods for the Analysis of Oils, Fats and Derivatives, 6th ed.; Pergamon: Bergama, Turkey, 1979; p. 60. ISBN 978-0-08-022379-7. [Google Scholar]
- AOCS Cc 7-25; AOCS Official Method Cc 7-25, Refractive Index of Fats and Oils. Official Methods and Recommended Practices of the American Oil Chemists’ Society. AOCS Press: Champaign, IL, USA, 2017.
- Treybal, R. Liquid Extraction; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1951. [Google Scholar]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, V.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Mancio, A.A.; da Costa, K.M.B.; Ferreira, C.C.; Santos, M.C.; Lhamas, D.E.L.; da Mota, S.A.P.; Leão, R.A.C.; de Souza, R.O.M.A.; Araújo, M.E.; Borges, L.E.P.; et al. Thermal Catalytic Cracking of Crude Palm Oil at Pilot Scale: Effect of the Percentage of Na2CO3 on the Quality of Biofuels. Ind. Crops Prod. 2016, 91, 32–43. [Google Scholar] [CrossRef]
- Park, L.K.E.; Liu, J.; Yiacoumi, S.; Borole, A.P.; Tsouris, C. Contribution of Acidic Components to the Total Acid Number (TAN) of Bio-Oil. Fuel 2017, 200, 171–181. [Google Scholar] [CrossRef]
- Oasmaa, A.; Elliott, D.C.; Korhonen, J. Acidity of Biomass Fast Pyrolysis Bio-Oils. Energy Fuels 2010, 24, 6548–6554. [Google Scholar] [CrossRef]
- Santos, A.L.; Martins, D.U.; Iha, O.K.; Ribeiro, R.A.; Quirino, R.L.; Suarez, P.A. Agro-Industrial Residues as Low-Price Feedstock for Diesel-like Fuel Production by Thermal Cracking. Bioresour. Technol. 2010, 101, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Buzetzki, E.; Sidorová, K.; Cvengrošová, Z.; Kaszonyi, A.; Cvengroš, J. The Influence of Zeolite Catalysts on the Products of Rapeseed Oil Cracking. Fuel Process. Technol. 2011, 92, 1623–1631. [Google Scholar] [CrossRef]
- Haas, M.J. Animal Fats. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 267–284. [Google Scholar]
- Gunstone, F.D. The Chemistry of Oils and Fats: Sources, Composition, Properties and Uses; Blackwell Publishing Ltd: Oxford, UK, 2004. [Google Scholar]
- Canapi, E.C.; Agustin, Y.T.V.; Moro, E.A.; Pedrosa, E., Jr.; Bendaño, M.L.J. Coconut Oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Swern, D. Bailey’s Industrial Oils and Fat Products; Swern, D., Ed.; Interscience Publishers: New York, NY, USA, 1964; pp. 128–131. [Google Scholar]
- Wu, W.-L.; Tan, Z.-Q.; Wu, G.-J.; Yuan, L.; Zhu, W.-L.; Bao, Y.-L.; Pan, L.-Y.; Yang, Y.-J.; Zheng, J.-X. Deacidification of Crude Low-Calorie Cocoa Butter with Liquid–Liquid Extraction and Strong-Base Anion Exchange Resin. Sep. Purif. Technol. 2013, 102, 163–172. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Garavazo, B.R.; Rodrigues, C.E.C. Liquid-Liquid Equilibria for Systems Composed of Rice Bran Oil and Alcohol-Rich Solvents: Application to Extraction and Deacidification of Oil. J. Food Eng. 2012, 110, 418–427. [Google Scholar] [CrossRef]
- Gandhi, S.; Kadrmas, J.; Št’ávová, J.; Kubátová, A.; Muggli, D.; Seames, W.S.; Sadrameli, S.M.; Tande, B.M. Extraction of Fatty Acids from Noncatalytically Cracked Triacylglycerides with Water and Aqueous Sodium Hydroxide. Sep. Sci. Technol. 2012, 47, 66–72. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Cengage Learning: Belmont, CA, USA, 2009. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ren, S.; Ye, X.P.; Borole, A.P. Separation of Chemical Groups from Bio-Oil Water-Extract via Sequential Organic Solvent Extraction. J. Anal. Appl. Pyrolysis 2017, 123, 30–39. [Google Scholar] [CrossRef]
- Lee, Y.; Shafaghat, H.; Kim, J.K.; Jeon, J.K.; Jung, S.C.; Lee, I.G.; Park, Y.K. Upgrading of Pyrolysis Bio-Oil Using WO3/ZrO2 and Amberlyst Catalysts: Evaluation of Acid Number and Viscosity. Korean J. Chem. Eng. 2017, 34, 2180–2187. [Google Scholar] [CrossRef]



| Physical–Chemical Property | Test Method | Feedstock (Bio-Oils) | |||
|---|---|---|---|---|---|
| BO1 | BO2 | BO3 | BO4 | ||
| Specific gravity at 20 °C (kg/m3) | ASTM D4052 [46] | 815.00 | 826.00 | 848.56 | 860.00 |
| Viscosity at 40 °C cSt (mm2/s) | ASTM D445 [47] | 4.65 | 5.21 | 6.5885 | 5.51 |
| Corrosiveness to copper, 3 h at 50 °C | ASTM D130 [48] | 1A | First | 1A | 1A |
| TAN (mg KOH/g) | ASTM D974 [49] | 24.38 | 33.21 | 51.5600 | 73.77 |
| Saponification value (mg KOH/g) | AOCS Cd 3-25 [50] | 48.23 | 67.00 | 70.9480 | 108.54 |
| Ester content (mg KOH/g) | Paquot [51] | 23.85 | 34.79 | 19.388 | 34.77 |
| Refractive index | AOCS Cc 7-25 [52] | 1.457 | 1.458 | 1.4580 | 1.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.T.; Mota, S.A.P.d.; Leão, R.A.C.; Souza, R.O.M.A.d.; Duvoisin Junior, S.; Borges, L.E.P.; Mota, A.d.A.M.d. Upgrading/Deacidification of Bio-Oils by Liquid–Liquid Extraction Using Aqueous Methanol as a Solvent. Energies 2024, 17, 2713. https://doi.org/10.3390/en17112713
Machado NT, Mota SAPd, Leão RAC, Souza ROMAd, Duvoisin Junior S, Borges LEP, Mota AdAMd. Upgrading/Deacidification of Bio-Oils by Liquid–Liquid Extraction Using Aqueous Methanol as a Solvent. Energies. 2024; 17(11):2713. https://doi.org/10.3390/en17112713
Chicago/Turabian StyleMachado, Nélio Teixeira, Silvio Alex Pereira da Mota, Raquel Ana Capela Leão, Rodrigo Octavio Mendonça Alves de Souza, Sergio Duvoisin Junior, Luiz Eduardo Pizarro Borges, and Andréia de Andrade Mancio da Mota. 2024. "Upgrading/Deacidification of Bio-Oils by Liquid–Liquid Extraction Using Aqueous Methanol as a Solvent" Energies 17, no. 11: 2713. https://doi.org/10.3390/en17112713
APA StyleMachado, N. T., Mota, S. A. P. d., Leão, R. A. C., Souza, R. O. M. A. d., Duvoisin Junior, S., Borges, L. E. P., & Mota, A. d. A. M. d. (2024). Upgrading/Deacidification of Bio-Oils by Liquid–Liquid Extraction Using Aqueous Methanol as a Solvent. Energies, 17(11), 2713. https://doi.org/10.3390/en17112713







