Abstract
The contents of alkali and alkaline earth metals are higher in Zhundong coal, and there are serious problems of slagging and fouling during the combustion process. Therefore, it is of great significance to reveal the mechanism of slagging and fouling in the boiler of Zhundong coal. In this paper, first-principle calculations based on density functional theory are used to study the competition mechanism of alkaline metal oxides during the combustion process in Zhundong coal by establishing the Na2O(110)/CaO(100)-SiO2(100) double-layer interface model. The results show that the bond lengths of the surface of Na2O(110) and CaO(100) with SiO2(100) after adsorption were generally lengthened and the value of bond population became smaller, which formed a stable binding energy during the reaction. The electron loss of Na is 0.05 e, the electron loss of Ca is 0.03 e, and the electron loss of Na2O is greater than that of CaO. The charge transfer on the surface of Na2O with SiO2 is obviously higher than that of CaO and the orbital hybridization on the surface of CaO with SiO2 is weaker than that on the surfaces of Na2O with SiO2. Na2O is easier to react with SiO2 than CaO. The adsorption energies on the surface of Na2O and CaO with SiO2 are −5.56 eV and −0.72 eV, respectively. The adsorption energy of Na2O is higher than that of CaO, indicating that Na2O is more prone to adsorption reactions and formation of Na-containing minerals and other minerals, resulting in more serious slagging. In addition, the XRD analyses at different temperatures showed that Na-containing compounds appeared before Ca-containing ones, and the reaction activity of Na2O is stronger than that of CaO in the reaction process. The experimental results have good agreement with the calculation results. This provides strong evidence to reveal the slagging and fouling of Zhundong coal.
1. Introduction
As a large country of coal resources, it is a great significance to make clean, efficient and rational use of the existing coal resources in China, which has abundant coal reserves, which are good for power, and low mining costs [1,2]. However, Zhundong coal has a special quality, with a high content of alkali and alkaline earth metals in the ash. The Na, Ca, and K salts are precipitated during the combustion process and easily react with other minerals in the coal ash, which further melts the compounds. The molten inorganic compounds condense on the lower temperature pipe wall to form a low-temperature eutectic, which makes melting and slagging in the high-temperature zone easy. Further, it results in slagging/fouling of the boiler and reduces its operation efficiency [3,4,5].
As an important indicator to measure whether the boiler can operate normally, the melting characteristics are related to the composition of coal ash [6]. Researchers have shown that Na reacts with SiO2 during combustion to form a thin layer of silicate. Silicate further reacts with sodium to form portil, which condenses on the pipe wall at low temperatures to form a low-temperature eutectic [7,8,9,10,11]. Some researchers have studied the deposits on the heating surface of the boiler and found that sulfates containing Na and Ca deposited on the heating surface are a prominent cause of slagging and fouling problems in Zhundong coal [12,13]. Previous studies have shown that wollastonite and sodium silicate generated by the reaction of Na2O and CaO with SiO2 were the main factors causing the slagging of Zhundong coal. However, the study on slagging characteristics is limited to the macroscopic viewing analysis of slag samples. Therefore, it is crucial to explore the influence of its micro-formation mechanism on the slagging and fouling of Zhundong coal. By calculating the electronic structure and adsorption energy of minerals, slagging, a trend caused by the reaction of Na2O and CaO with SiO2, is predicted, which provides data support for the discussion of slagging characteristics.
In recent years, with the development of quantum chemistry theory, the first-principle calculation has been widely used to determine the structure and ground state properties of materials [14,15]. It explains the reaction activity of minerals at the molecular level and provides a theoretical basis for analyzing the reaction mechanism of substances during the phase transition process [16]. One way to study the relationship between a minerals’ structure and property based on quantum chemistry theory is to model the actual problem, and selection of model is key to solving the problem. The bilayer model can calculate the situation of individual molecules that are adsorbed on the surface and the interaction of molecules between layers, and it can explain the properties of minerals from the electronic structure and surface reaction. The researchers of [17] applied quantum chemistry theory to reveal the macroscopic phenomenon of substances from the perspective of the microscopic characteristics of the molecular structure, which provided a theoretical basis for analyzing the physical and chemical properties in the reaction process. Researchers have studied the heterogeneous adsorption of HCN on the CaO(100) surfaces by using the first-principle calculation, indicating that HCN was more easily adsorbed on the Ca vacancy surface of the defective CaO(100) surface and the adsorption capacity was decreased with increasing temperature. Lu [18] studied the action mechanism of Cd on the SiO2(001) surface from the microscopic point of view and found that the O site is the active site on the SiO2(001) surface. The adsorption mode of CdO on the SiO2(001) surface was chemical adsorption, which made the reaction with the SiO2(001) surface easier. The results have provided theoretical support for the removal of Cd pollutants. Lin [19] used the density functional theory (DFT) to study the adsorption reaction of CO on different crystal surfaces of Fe2O3 in the chemical ring combustion process. The results showed that a CO adsorption reaction could occur on both (001) and (100) crystal surfaces of Fe2O3 and that the addition of Fe2O3 could promote the adsorption of CO.
The above research is the application of quantum chemistry theory to the adsorption and surface properties of a single molecule on the crystal surface. Compared with the single-molecule model, crystal-surface models with periodic boundary conditions are better to describe the solid surface. However, there are few studies on the molecular interactions between layers on crystal surfaces. Han [20] et al. studied the interaction of surface modified colloidal silica with polyvinyl alcohol by molecular simulation, which showed that the interaction of modified silica with polyvinyl alcohol molecules was significantly enhanced. By using quantum chemistry theory, Yang [21] studied the interaction between the NaCl surface and Fe2O3, Al2O3, and NiO surface in high-alkali coal and found that the adsorption energy was positively correlated with the slagging condition in the boiler. The greater the adsorption energy, the more serious the slagging.
In order to more accurately describe the real situation, the adsorption models of oxides are established in this paper. Density functional theory plays a key role in the calculation of micro parameters such as adsorption energy, charge transfer, and the bond length of gases and solids on the coal surface. The adsorption capacity of different substances on the coal surface is obtained by calculation. The theory provides an important theoretical basis for the study of spontaneous coal combustion, gasification, and the combustion process. Through the analysis of adsorption characteristics, we can deeply understand the interaction mechanism between substances, that is, a higher adsorption capacity means a stronger intermolecular interaction.
In this paper, the competition mechanism of alkaline metal oxides during the reaction between compounds in Zhundong coal is studied by means of simulations and experiments. Na2O(110)-SiO2(100) and CaO(100)-SiO2(100) models were established and the adsorptions were calculated using DFT. Orbital density and Mulliken population analysis were used to reveal the competitive relationship between Na2O and CaO, which reacted with SiO2 on the surface and predicted the trend of slagging caused by the reaction of Na2O and CaO with SiO2. In addition, the competition between Na2O and CaO in the mixture was studied by XRD results of the complex under different temperature conditions. Combined with the simulation results, it is shown that the formation of Na-containing minerals and other compounds in the high-temperature zone is more serious, which provides an effective way to solve the slagging and fouling problem of Zhundong coal.
2. Simulation Calculation and Experimental Research
2.1. Selection of Crystal Cells
The initial layer of coal ash slag mainly consists of volatile substances such as Na2O and CaO reacting with SiO2 to generate wollastonite and sodium silicate. Therefore, Na2O, CaO, and SiO2 are selected as the research object to analyze the reaction mechanism of alkali metals reacting with high-melting point mineral SiO2 in the coal ash. Na2O, CaO, and SiO2 belong to the cubic crystal system, Na2O and CaO are part of the Fm3m space group, and SiO2 is part of the Fd3m space group. The cell parameters are shown in Table 1, and Figure 1 shows the initial crystal cell structure of three compounds.

Table 1.
Lattice constants of Na2O, CaO, and SiO2.
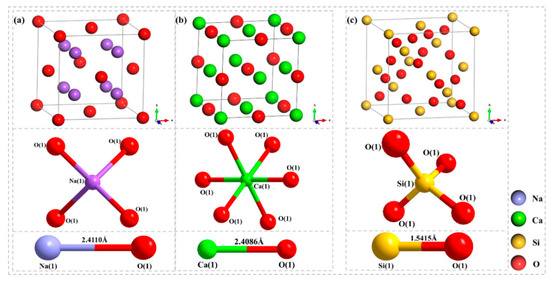
Figure 1.
Initial cell structure diagrams of (a) Na2O, (b) CaO, and (c) SiO2.
2.2. Selection of Stable Crystal Planes
One method to study the adsorption between two compounds is generally conducted through the interaction forces between surfaces. Therefore, an optimized 3D structural model was sectioned using Build in the toolbar of the Materials Studio software before crystal modeling. It is calculated that Na2O(110), CaO(100), and SiO2(100) crystal faces are relatively stable and easy for interface modeling, so the optimized crystal cell structure is faceted and the thickness of the three faceted cuts is 5 layers. Clean Na2O(110), CaO(100), and SiO2(100) surfaces were constructed on this basis, respectively.
2.3. Establishment of a Double-Layer Model
The Na2O(110) and CaO(100) crystal planes were modeled with SiO2(100) crystals by using the Build Layer tool, and the constructed model is shown in Figure 2. The top two layers of the crystal cell play a major role in surface adsorption and the deep solid molecules have little effect on it. The optimization was carried out using the bottom three layers of atoms fixed for the relaxation of the surface and the vacuum layer thickness was set at 15 Å to ensure that the bottom liner atoms were not affected by other nearby atoms in the calculation. Table 2 shows the geometrical parameters after the establishment of the adsorption model. Compared with Table 1, the lattice parameters changed after the establishment of the interface model. During the modeling process, it was necessary to match the adaptability of the upper and lower models by establishing the supercell structure. To facilitate data acquisition, approximate numerical values were used for both the upper and lower layers of materials, which were within the error tolerance.
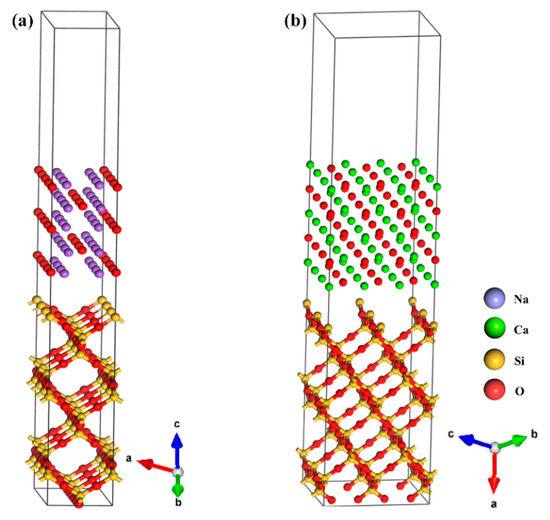
Figure 2.
Crystal interface model: (a) Na2O(110)-SiO2(100); (b) CaO(100)-SiO2(100).

Table 2.
Geometric parameters of the interface models.
2.4. Calculation Methods
Na2O, CaO, and SiO2 crystal cells were cut and interface modeling was carried out using the Build menu in the Materials Studio software. The ultra-soft pseudopotential plane wave method based on first principles was adopted to replace the ionic potential with the pseudopotential, and the electron wave function was expanded with the plane wave basis set [22]. The BFGS algorithm was used for quantum calculations in the structure optimization, [23] and the GGA-PBE algorithm in the generalized gradient approximation was used to describe the electron exchange correlation [24,25]. The CASTEP module was used for structural optimization and energy calculations. In order to ensure calculation accuracy, the cut-off energy was set to 300 eV for the optimization, and the convergence standard for geometric configuration optimization was as follows: the total energy accuracy was set to an atomic energy of 1.0 × 10−5 eV and each atom experienced a force within the crystal less than 0.03 eV/Å with a maximum displacement of 0.01 Å to make it as close as possible to the real structure under the geometric optimization. For the calculation, the periodic boundary conditions were chosen for the crystallographic protocells of three types of crystal cells, the spin polarization was neglected, and the pseudopotentials were processed using Ultrasoft. The Monkhost–Pack method was used for sampling the Brillouin zone for the k-points, taking the values of 4 × 4 × 1 and 2 × 2 × 1. Convergence of the structure was achieved by varying the number of iterations.
2.5. Experimental Verification of Simulation
The reagents Na2O, CaO, and SiO2 were purchased from Aladdin and used as reagents without any treatment.
To verify the accuracy of the simulation results, Na2O, CaO, and SiO2 with the same molar fraction as the simulated Na2O-CaO-SiO2 system were selected as the raw materials for the experiment. The three oxides were weighed and fully ground in a molar ratio of 1:1:1 to prepare synthetic coal ash. The mixture was placed in a corundum crucible and put into a muffle furnace for calcination. The experimental combustion temperature range was between 400 and 750 °C and a sample was taken at 50 °C intervals. The combustion time was set to 2 h. After calcination, the samples were measured to analyze the reaction of Na and Ca in the mixture, and three parallel experiments were conducted on each group of sample. Their chemical structures are shown in Figure 3.
Figure 3.
Chemical structure diagrams of (a) Na2O, (b) CaO, and (c) SiO2.
3. Results and Discussion
3.1. Selection of Crystal Planes
When cutting the surface of a block, the surface energy (SE) is the energy required to break chemical bonds, which directly determines the stability of the crystal surface. The stability of the crystal surface directly reflects the bonding and properties of surface atoms and is related to the bonding characteristics of atoms in the crystal cell. We calculated the surface energy of different crystal planes of SiO2, Na2O, and CaO cells to determine the relatively stable crystal planes. Table 3 shows the optimized structural parameters of different crystal planes. The calculation formula of surface energy is as shown in Equation (1): [26,27]

Table 3.
Structural parameters of SiO2, Na2O, and CaO after optimization of different crystal faces.
In Equation (1), SE is the surface energy, Esurface is the energy of the surface model, Ebulk is the energy of the system cell, N is the number of atoms contained in the surface, n is the number of individual atoms in the bulk, A is the surface area of the surface, and a, b and γ are the lattice constants of the crystal cell. The calculation formula is as follows: [28]
The most commonly used crystal planes of SiO2, Na2O, and CaO are selected for calculation [29,30]. The calculation results of surface energy for SiO2, Na2O, and CaO with different crystal planes are shown in Table 4, from which the surface energy of different crystal planes of the same cell is different. In the SiO2 cell, the (001) surface energy is 2.38 eV/Å2, the (110) surface energy is 2.49 eV/Å2, and the (100) surface energy is 2.36 eV/Å2; the relationship between the surface energy is E(100) < E(001) < E(110). The (100) surface energy of Na2O is 0.10 eV/Å2, and the (110) surface energy is 0.03 eV/Å2; the relationship between the surface energy is E(110) < E(100). The (100) surface energy of CaO is 0.03 eV/Å2, and the (110) surface energy is 0.08 eV/Å2; the relationship between the surface energy is E(100) < E(110). The lower the surface energy of the cell, the more stable the cell structure is, so subsequent calculations are performed on SiO2(100), Na2O(110), and CaO(100) surfaces.

Table 4.
Calculation process and results of surface energy of SiO2, Na2O, and CaO.
3.2. Study of the Reaction Competition between Na2O and CaO in the Combustion Process of Zhundong Coal
3.2.1. Reactive Activity Calculation
Based on the stable structure planes obtained after the geometric optimization of the interface model, the relevant calculations of its reactivity were analyzed. The adsorption model is shown in Figure 2, the upper layer of the model is Na2O(110) and CaO(100) crystal planes, and the lower layer is SiO2(100) crystal planes. The calculated number of partial atomic charges is shown in Table 5.

Table 5.
Partial atomic charge numbers of the interface models.
The static charge of some atoms was calculated from Na2O(110)-SiO2(100) and CaO(100)-SiO2(100) models. Comparing the static charge of atoms, it is concluded that Na, Ca, and Si atoms are positively charged, while O atoms are negatively charged. Due to adsorption resulting in the transfer of electrons from the metal surface by adsorbed O atoms to make them negatively charged, and the fact that the metal surface is positively charged, the charge population changes. This phenomenon indicates that adsorption occurs between Na2O, CaO, and SiO2.
3.2.2. Mulliken Population and Bond Lengths
According to the electronic layer arrangement rule of elements in the periodic table, the valence electrons are set in a 1s22s22p63s1 configuration for Na, 1s22s22p63s23p64s2 for Ca, 1s22s22p4 for O, and 1s22s22p63s23p2 for Si. The reaction is mainly related to the outer electrons, so the outer orbitals of atoms are analyzed and studied through the charge population and the density of orbital states.
Table 6 shows the change data of Mulliken bond populations and bond lengths both before and after the adsorptions of the interface model. It can be seen from the table that after adsorption, the bond population and bond length of Na-O and Ca-O remain unchanged. Compared with before adsorption, the Mulliken bond population of Si-O decreases, the number of Si2-O6 bonds decreases from 0.51 to 0.50 e, and the number of Si4-O8 and Si6-O13 bonds decreases from 0.52 to 0.51 e after the adsorption reaction between Na2O and SiO2. After the adsorption reaction between CaO and SiO2, the number of Si-O bonds decreases from 0.51 to 0.50 e, indicating that the O in Na2O and CaO was not involved in the reaction during the adsorption reaction and that Na and Ca reacted with SiO2. The Si-O bond length of the two models is generally elongated and the structure is unstable, which makes them prone to adsorption reactions.

Table 6.
Changes in Mulliken populations and bond lengths both before and after adsorptions in interface models.
3.2.3. Orbital Density of States
Combined with the Mulliken population analysis, the orbital state densities on the surfaces of Na2O(110), CaO(100) and SiO2(100) were well analyzed in terms of the orbital hybridization of atoms on the cell surface. These results can explain the interaction between atoms and further reveal the adsorption characteristics of the interface model.
The Partial Density of States (PDOSs) before and after adsorption on the surfaces of Na2O(110) and SiO2(100) are shown in Figure 4. Compared with Figure 4a,b, it is found that the peak of O orbital energy level on Na2O(110)’s surface was almost unchanged after adsorption; the peak values of the 3s orbitals of Na decreased between −70 and −67 eV, the peak value of the 3s orbital disappeared near the Fermi level, and the peak value of the 2p orbital decreased slightly at the high energy level, indicating that Na atoms lose charge after adsorption. Figure 4c,d show the PDOS diagram of SiO2 before and after adsorption. It can be seen from the figure that the peak of 2p orbital high energy state of O(SiO2) decreases after adsorption, and the peak values of 2p orbital increase between −10 and −5 eV. The peak values of the 2s orbital are become widen at the low energy level, with the localization weakened. The peaks of the 3s and 3p orbitals low energy states of Si are reduced, and there is a significant shift to high energy states after adsorption. In addition, combined with Mulliken population, it is shown that Na exhibits charge exchange with Si and O and Na exhibits orbital hybridization with Si and O between −10~0 eV, which results in significant coupling to the substrate atoms.
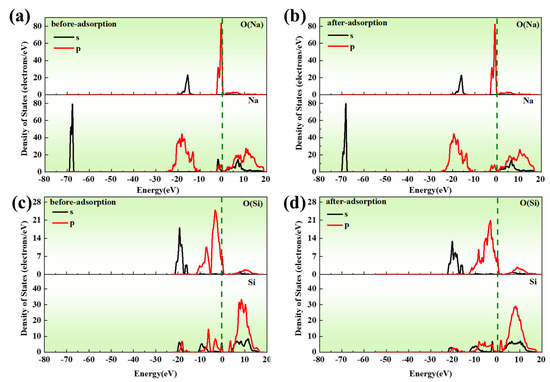
Figure 4.
PDOS diagram of Na2O(110) adsorbed on SiO2(100) surface: (a) before adsorption of Na and O; (b) after adsorption of Na and O; (c) before adsorption of Si and O; (d) after adsorption of Si and O.
Figure 5 shows the PDOS diagram on the surface of CaO(100) and SiO2(100) both before and after adsorption; comparing Figure 5a,b, it can be seen that after adsorption, the peaks of high energy states of 4s and 3p orbits of Ca decrease, while the peaks of low energy states of 4s orbits increase. The peaks of O orbitals on the surface of CaO(100) are almost unchanged. Figure 5c,d show that the peak value of the 2p orbital with low energy state of O(SiO2) is reduced after adsorption, the 2s orbitals broaden in the states at the low energy level, and the localization is weakened. The peaks of the 3s and 3p orbitals of Si increase at the high energy level of −10~0 eV, and there is a significant shift to the left of the Fermi energy level after adsorption. Combined with the Mulliken population, Ca undergoes charge exchange with Si and O. The 4s and 3p orbitals of Ca lose electrons and transfer to the SiO2 substrate, while the substrate Si returns part of the charge to the 3p orbitals of Ca. At 10−15 eV, the 4s orbitals of Ca are coupled with Si and O to varying degrees.
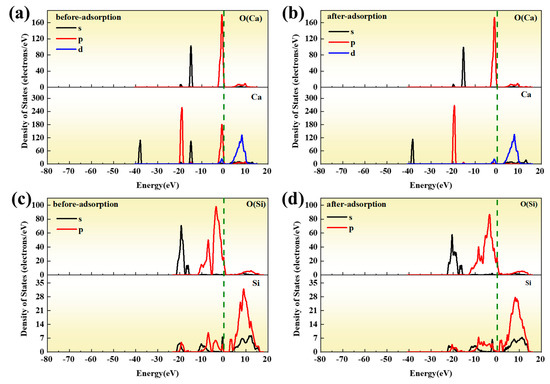
Figure 5.
PDOS diagram of CaO(100) adsorbed on SiO2(100) surface: (a) before adsorption of Ca and O; (b) after adsorption of Ca and O; (c) before adsorption of Si and O; (d) after adsorption of Si and O.
3.2.4. Adsorption Energy
As one of the important reactions occurring between solid–solid surfaces, adsorption mainly includes the adsorption of reactants to the solid surface, diffusion of intermediates on the surface (where the breaking of old bonds and the formation of new bonds occurs), and the desorption reaction of products from the surface [31,32]. Determination of the electronic and geometrical determinants of the adsorption energy on the material surface is the core goal of surface science. The calculation process of adsorption energy is that when the system energy converges, it indicates that the system reaches the equilibrium state, and the adsorption energy is calculated. Firstly, the model of structural stability is restored to the molecules fixed in the lower layer, and then Et, Es1, and Es2 are calculated respectively. The calculation formula of the adsorption energy is as shown in Equation (3): [33]
where Et is defined as the total energy of the system after adsorption; Es1 is defined as the surface energy after removal of Na2O(110) and CaO(100); and Es2 is defined as the energy to remove the SiO2(100) surface. The adsorption energy is positive, which means that the adsorption is an exothermic reaction, and the higher value indicates that the interaction is stronger, the adsorption process is more likely to occur, and the adsorption structure is more stable [34]. The calculated data are shown in Table 7.

Table 7.
Adsorption energy on the surface of the interface models.
Through simulation calculations, the adsorption energy of the Na2O(110) and SiO2(100) surface is −5.56 eV and that of the CaO(100) and SiO2(100) surface is −0.72 eV. The adsorption energy of the Na2O(110) surface is larger than that of the surface of CaO(100), which indicates that the adsorption model generated by the reaction between Na2O(110) and SiO2(100) is relatively stable. In addition, the adsorption energy is positively correlated with the amount of coal ash slagging, so the effect of Na2O and SiO2 on the slagging of Zhundong coal is stronger than that of CaO.
3.3. Experimental Verification of Basic Metals in Model Compounds
According to the calculation results of the Na2O(110)-SiO2(100) and CaO(100)-SiO2(100) models, it is concluded that the effect of Na2O on the slagging of Zhundong coal is stronger than that of CaO during the alkaline metal oxides’ reaction with high melting point minerals. In this experiment, the model compounds were calcined in the experimental combustion temperature range of 400–750 °C, and samples were taken at 50 °C intervals. The competitive relationship of alkaline metals in the mixture was studied by X-ray diffraction, and the results are shown in Figure 6.
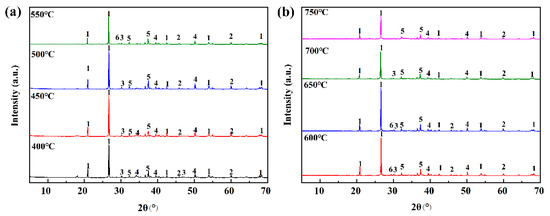
Figure 6.
XRD patterns of the complexes at different combustion temperatures: (a) 400–550 °C; (b) 600–750 °C. 1—SiO2, 2—Na2Si2O5, 3—CaSiO3, 4—Na2CaSi3O8, 5—CaO, 6—Ca6Si4O14.
It can be seen from the figure that both Na2O and CaO participate in the reaction within the mixture’s temperature increase. The peak of Ca6Si4O14 gradually diminishes at 700 °C, while the peak of CaSiO3 gradually disappears when reaching 750 °C. At 2θ of 30°, CaSiO3 belongs to the (120) crystal plane (PDF Card no.29-0372). However, the peaks of CaO and Na2Si2O5 persist throughout, indicating that CaO does not partake in the reaction at 750 °C. Na2Si2O5 is always present at 2θ of 60° (PDF Card no.19-1236). And it is a (421) crystalline surface. The reactivity of Na2O progressively emerges with higher temperatures during the reaction process. The reaction of the formation process is as follows:
Figure 7 shows the SEM images of Na2Si2O5 and CaSiO3 models. It can be seen from the figure that the mineral morphology generated after the reaction of Na2O, CaO, and SiO2 is blocky. The previous thermodynamic calculation results also show that the Gibbs free energy change (ΔG) for the generation of sodium containing minerals in coal ash is smaller at 1000 °C and occurs more easily at high temperatures. The ΔG (−442.0 KJ/mol) of the reaction of Na2Si2O5 and Na2Ca2Si3O9 generated by the reaction of Na2O with SiO2 is significantly lower than that of wollastonite (−88.57 KJ/mol). The results demonstrate that the formation of wollastonite with high melting point is effectively inhibited by Na2O present in coal ash, ref. [35], indicating its preferred reaction with SiO2 before CaO. The results are in agreement with the simulation results.
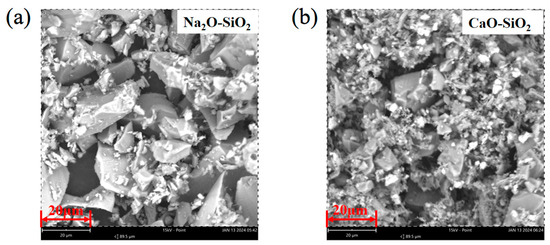
Figure 7.
SEM images of Na2Si2O5 and CaSiO3 models. (a) Na2O–SiO2; (b) CaO–SiO2.
3.4. Reaction Mechanism of Alkaline Metal Oxides in Zhundong Coal Ash
Theoretical and experimental studies have shown that compared with other coals, Zhundong coal has a higher content of alkali/alkaline earth metals, and the phase transition temperature of alkali metals is relatively lower during combustion. Gasification occurs at a certain temperature, and gaseous alkali/alkaline earth metals condense on the surface of the heat exchanger, trapping solid particles in the gas, resulting in slagging and contamination problems [36,37]. The slag sample formed at the high temperature will form stratification phenomena. The initial layer has the highest content of CaSiO3 and Na2Si2O5, mainly due to the reaction of volatile substances such as Na2O and CaO with SiO2 to form wollastonite and sodium silicate, which forms a very viscous initial layer [2,38]. The reaction equation for the formation process is shown in Section 3.3.
The reaction mechanism diagram of alkali metal with high melting point mineral SiO2 in Zhundong coal ash is shown in Figure 8. Figure 8a is the reaction of the Na2O and SiO2 surface reaction, and the given and loss of electrons between Na, Si, and O atoms are shown through the differential charge density. The cross-sectional diagram shows that Na and O atoms lose electrons and Si atoms accept electrons (blue indicates that atoms lose electrons and red indicates that atoms accept electrons). When Na2O reacts with SiO2 in reverse, the bond population and bond lengths of Na and O are basically unchanged; the population of the Si-O bond decreases and the bond lengths are generally lengthened (Table 6), indicating that the covalence of the Si-O bond is weak during the reverse adsorption reaction. Also, it is prone to fracture and react with Na2O to produce sodium-containing minerals such as Na2Si2O5.
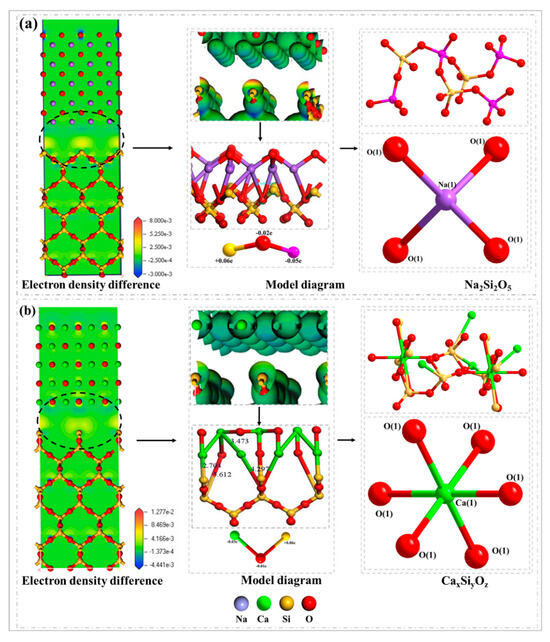
Figure 8.
Reaction mechanism diagram of alkaline metal oxides in Zhundong coal ash: (a) Na2O(110)–SiO2(100) model; (b) CaO(100)–SiO2(100) model.
The reaction between the CaO and SiO2 surface is shown in Figure 8b. During the reaction between CaO and SiO2, electron exchange occurs between Ca, Si, and O atoms. The bond population and bond length of Ca-O remain basically unchanged after the adsorption reaction; the Si-O bond populations are reduced and the bond lengths are generally elongated (Table 6). The results show that the Si-O bond has strong ionic properties and is prone to fracture during the adsorption reaction. Calcium minerals such as CaSiO3 are formed by the reaction between CaO and SiO2. According to the conclusion of the above adsorption energy, Na2O reacts with SiO2 to form sodium-containing minerals and other compounds, and the slagging performed at the high temperature is more serious. Na2O has a greater effect on slagging during the combustion of Zhundong coal. Sodium silicate further reacts with other minerals to form low-temperature eutectic, which aggravates the slagging and fouling phenomenon of Zhundong coal.
4. Conclusions
DFT was used to study the adsorption between Na2O, CaO, and SiO2 surfaces. Firstly, the surface energy of different crystal surfaces of cells was calculated, and the most stable crystal surface was determined. Then, the bonding characteristics of Na2O, CaO, and SiO2 surfaces were studied, and the reaction difficulty of alkaline metals was analyzed, which provides a theoretical basis for preventing slagging of Zhundong coal. In addition, the accuracy of the simulation results was verified by experiments. The main conclusions are as follows:
- (1)
- Comparing the partial charge numbers of each atom in the two models, it is found that the O atom is negatively charged, while the Na, Ca, and Si atoms are positively charged. The charge numbers change after adsorption. The electron loss of Na is 0.05 e, and the electron loss of Ca is 0.03 e, indicating adsorption on the surfaces of both models. The electron loss of Na2O is greater than that of CaO, and it is prone to react with SiO2 to form silicates containing Na.
- (2)
- After adsorption, the adsorption energy of Na2O is −5.56 eV and that of CaO is −0.72 eV. The value of Na2O is greater than CaO, and Na2O is more likely to undergo an adsorption reaction and preferentially form silicate. Based on Mulliken population analysis, the charge transfer between Na2O(110) and SiO2(100) crystal planes is higher than that of CaO (100) during interface model adsorption. The orbital hybridization between CaO(100) and SiO2(100) crystal planes is weaker than that of Na2O(110). The adsorption effect on the surface of Na2O and SiO2 is stronger, making it easier to form slag containing Na minerals and other substances in the high-temperature zone. This also explains that the slag fouling situation of Zhundong coal is more significant than that of CaO.
- (3)
- XRD results at different temperatures show that the compounds containing Na appear earlier than those containing Ca, and the reactivity of Na2O is stronger than that of CaO. This conclusion is consistent with the simulation results.
Author Contributions
Writing—original draft preparation, M.A.; supervision, B.W.; writing—review and editing, L.H.; project administration, K.L., J.L., J.W. and Q.Z.; funding acquisition, B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region of China (No. 2022D01C377), National Natural Science Foundation of China (Nos. 22369017, 22178298), Open Foundation of State Key Laboratory of Fine Chemical Industry of Dalian University of Technology (No. KF2204), Tianshan talents (No. 2022TSYCCX0056), and Tianshan Innovation Team (No. 2023D14010).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, H.; Wu, Y.; Zhang, M.; Zhang, Y.; Lyu, J. Ash deposition of Zhundong coal in a 350 MW pulverized coal furnace: Influence of sulfation. Fuel 2020, 260, 116317.1–116317.8. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Liu, D.; Yang, H.; Kou, X. Understanding ash deposition for Zhundong coal combustion in 330 MW utility boiler: Focusing on surface temperature effects. Fuel 2018, 216, 697–706. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, J.; Liu, Z.; Kou, X.; He, X.; Shen, L. Ash formation characteristics in co-combusting coagulation sludge and Zhundong coal. Fuel 2022, 311, 122571. [Google Scholar] [CrossRef]
- Tang, S.; Fu, P.; Liu, Y. Mineral Phase Transformation and Ash Melting Mechanism of Zhundong Coal and Blended Coal. J. Combust. Sci. Technol. 2019, 25, 324–330. [Google Scholar]
- Tao, Y.; Zhang, Y.; Zhou, J.; Jing, X.; Cen, K. Mineral Conversion Regularity and Release Behavior of Na, Ca During Zhundong Coal’s Combustion. Proc. CSEE 2015, 35, 1169–1175. [Google Scholar]
- Liu, B.; He, Q.; Jiang, Z.; Xu, R.; Hu, B. Relationship between coal ash composition and ash fusion temperatures. Fuel 2013, 105, 293–300. [Google Scholar] [CrossRef]
- Eyk, P.J.V.; Ashman, P.J.; Nathan, G.J. Mechanism and kinetics of sodium release from brown coal char particles during combustion. Combust. Flame 2011, 158, 2512–2523. [Google Scholar]
- Wang, Y.; Xiang, Y.; Wang, D.; Dong, C.; Yang, Y.; Xiao, X. Effect of sodium oxides in ash composition on ash fusibility. Energy Fuels 2016, 30, 1437–1444. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Zhang, M.; Cui, L.; Dong, Y. Ash problems and prevention measures in power plants burning high alkali fuel: Brief review and future perspectives. Sci. Total Environ. 2023, 901, 165985. [Google Scholar] [CrossRef]
- Hou, G.; Yan, Z.; Sun, J.; Naguib, H.; Lu, B.; Zhang, Z. Microstructure and mechanical properties of CO2-cured steel slag brick in pilot-scale. Constr. Build. Mater. 2021, 271, 121581. [Google Scholar] [CrossRef]
- Huo, Z.; Lu, B.; Sun, J.; Jiang, R.; Hou, G.; Ji, S.; Naguib, H. Preparation of high flexural strength rankinite cement benefiting from formation of aragonite whisker during carbonation curing. J. Sustain. Cem.-Based Mater. 2024, 13, 738–753. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, L.; Zhao, Y.; Ji, J.; Bai, Y. Transformation behavior of alkali metals in high-alkali coals. Fuel Process. Technol. 2018, 169, 288–294. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Wei, B.; Zhang, L.; Tan, H.; Yang, T. The ash deposition mechanism in boilers burning Zhundong coal with high contents of sodium and calcium: A study from ash evaporating to condensing. Appl. Therm. Eng. 2015, 80, 150–159. [Google Scholar] [CrossRef]
- Kang, H.; Wang, J.; Zhang, Y. Theoretical analysis of conductive properties of La, Bi co-doped Ag/SnO2 contact materials. J. Mater. Sci. Eng. 2019, 37, 463–468. [Google Scholar]
- Zong, X.L.; Pan, H.; Xu, J. First-principles calculation on mechanical properties of Ag-Cu alloy bonding wire. J. Mater. Sci. Eng. 2022, 40, 737–741. [Google Scholar]
- Lei, Z.; Rui, S.; Xing, W.; Zhuo, W.; Wen, Z.; Jiang, W. Experimental and density functional theory investigation of the NO reduction mechanism by semichars preheated in Ar and CO2/Ar atmospheres. Fuel 2022, 326, 125080–125091. [Google Scholar]
- Hou, F.; Jin, J.; Yang, H.; Wang, Y.; Li, S. Understanding HCN heterogeneous adsorption on CaO(100) surface for the pyrolysis of sludge: A first-principles study and GCMC simulation. Appl. Surf. Sci. 2019, 475, 1033–1042. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, X.; Wu, Y. Interaction mechanism between cadmium species and SiO2 of municipal solid waste incineration fly ash: Effect of HCl. Chem. Eng. J. 2021, 425, 130604. [Google Scholar] [CrossRef]
- Lin, C.; Qin, W.; Dong, C. Reduction effect of α-Fe2O3 on carbon deposition and CO oxidation during chemical-looping combustion. Chem. Eng. J. 2016, 301, 257–265. [Google Scholar] [CrossRef]
- Lay, H.C.; Spencer, M.J.S.; Evans, E.J.; Yarovsky, I. Molecular simulation study of polymer interactions with silica particles in aqueous solution. J. Phys. Chem. B 2003, 107, 9681–9691. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z.; Wu, X. Quantum chemistry calculation for the adsorption of NaCl on waterwall in high Alkali coal. J. Univ. Shanghai Sci. Technol. 2013, 35, 409–414. [Google Scholar]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B Condens. Matter 1990, 41, 7892. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, M.I.; Lichtenstein, A.I. Electronic structure and magnetic properties of correlated metals: A local self-consistent perturbation scheme. Eur. Phys. J. B-Condens. Matter Complex Syst. 2002, 30, 9–15. [Google Scholar] [CrossRef]
- Wang, Y.; PerdewSpin, J.P. Scaling of the electron-gas correlation energy in the high-density limit. Phys. Rev. B 1991, 43, 8911–8916. [Google Scholar] [CrossRef] [PubMed]
- PerdewSpin, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar]
- Hu, Y.; Gao, Z.; Sun, W.; Liu, X. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 439–448. [Google Scholar] [CrossRef]
- Buff, P.F. The Spherical Interface. I. Thermodynamics. J. Chem. Phys. 1951, 19, 1591–1594. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, D.; Tong, J. CASTEP Calculation of Surface Energy of α-Al2O3. Key Eng. Mater. 2012, 512, 490–493. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, X.; Wu, Y.; Mi, T.; Hu, B. Migration and transformation of lead species over CaO surface in municipal solid waste incineration fly Ash: A DFT study. Waste Manag. 2021, 120, 59–67. [Google Scholar] [CrossRef]
- Lshihara, T.; Kato, K. Theoretical prediction of universal curves for carrier transport in Si/SiO2(100) interfaces. J. Appl. Phys. 2013, 114, 053713. [Google Scholar] [CrossRef]
- Bligaard, T.; Norskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.; Sehested, J. The Bronsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Theoretical Surface Science and Catalysis-Calculations and Concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar]
- Lv, D.; Wu, H.; Chen, J.; Nie, Y.; Bian, G.; Li, J.; Gong, S. Research progress of molecular dynamics simulation on adsorption mechanisms of dispersants/surfactants on the surface of coal particles. J. Fuel Chem. Technol. 2023, 52, 1–10. [Google Scholar]
- Wang, Y.; Jin, J.; Kou, X.; Hou, F. Understanding the mechanism for the formation of initial deposition burning Zhundong coal: DFT calculation and experimental study. Fuel 2020, 269, 117054. [Google Scholar]
- Chen, X.; Kong, L.; Bai, J.; Bai, Z.; Li, W. Effect of Na2O on mineral transformation of coal ash under high temperature gasification condition. J. Fuel Chem. Technol. 2016, 44, 263–272. [Google Scholar] [CrossRef]
- Ge, H.; Shen, L.; Gu, H.; Song, T.; Jiang, S. Combustion performance and sodium transformation of high-sodium Zhundong coal during chemical looping combustion with hematite as oxygen carrier. Fuel 2015, 159, 107–117. [Google Scholar] [CrossRef]
- Qiu, P.; Zhao, Y.; Chen, X.; Xu, J.; Sun, S. Effects of alkali and alkaline earth metallic species on pyrolysis characteristics and kinetics of Zhundong coal. J. Fuel Chem. Technol. 2014, 42, 1178–1189. [Google Scholar]
- Li, G.; Li, S.; Huang, Q.; Yao, Q. Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace. Fuel 2015, 143, 430–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).