Elucidating Synergetic Effects of Anaerobic Co-Digestion of Slaughterhouse Waste with Livestock Manures
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrates
2.2. Experimental Design
2.3. Analytical Methods
2.4. Biochemical Methane Potential Test
2.5. Calculation
2.5.1. Theoretical Methane Yield
2.5.2. Cumulative Methane Yield
2.5.3. Biodegradability
2.5.4. Synergy Index
2.5.5. Kinetic Model
3. Results and Discussion
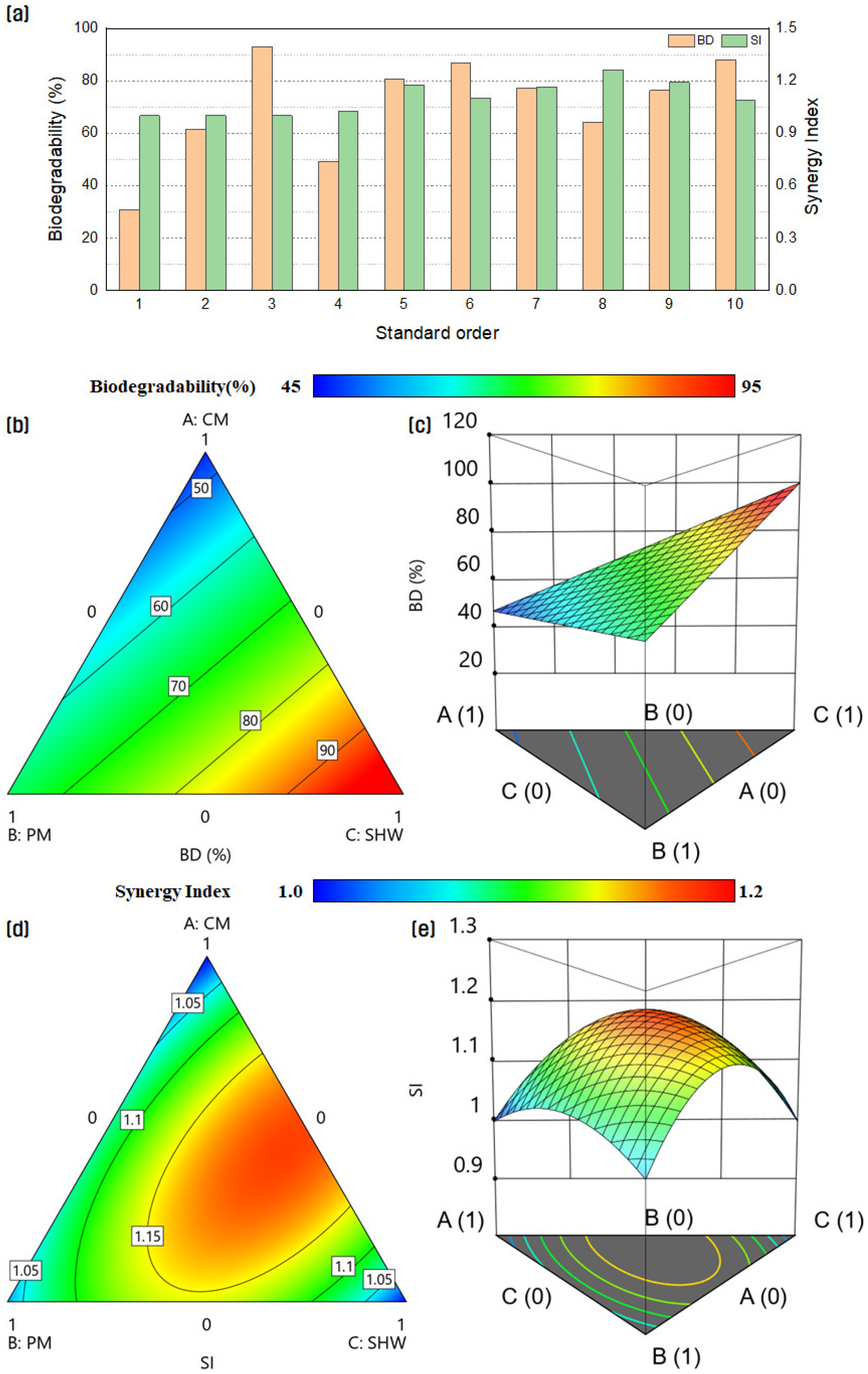
3.1. Biochemical Methane Potential Test Results
3.2. Results of Response Surface Methodology Model
3.3. Optimal Substrate Mixture Ratio
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rhee, C.; Kim, D.W.; Yu, S.I.; Lee, M.E.; Shin, J.; Kim, H.-W.; Chung, J.W.; Shin, S.G. Biogas potential assessment and characterization of Korean slaughterhouse waste for anaerobic digestion. Environ. Technol. Innov. 2021, 24, 101858. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef]
- Wang, S.; Jena, U.; Das, K.C. Biomethane production potential of slaughterhouse waste in the United States. Energy Convers. Manag. 2018, 173, 143–157. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.; Kim, D.; Ryu, J.; Lee, K.; Chung, H.; Park, K.Y. Conversion of slaughterhouse wastes to solid fuel using hydrothermal carbonization. Energies 2021, 14, 1768. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic digestion of slaughterhouse by-products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Ware, A.; Power, N. Biogas from cattle slaughterhouse waste: Energy recovery towards an energy self-sufficient industry in Ireland. Renew. Energy 2016, 97, 541–549. [Google Scholar] [CrossRef]
- Broughton, M.J.; Thiele, J.H.; Birch, E.J.; Cohen, A. Anaerobic batch digestion of sheep tallow. Water Res. 1998, 32, 1423–1428. [Google Scholar] [CrossRef]
- Salminen, E.; Rintala, J.; Lokshina, L.Y.; Vavilin, V. Anaerobic batch degradation of solid poultry slaughterhouse waste. Water Sci. Technol. 2000, 41, 33–41. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Otero, M.; Morán, A. Anaerobic digestion of solid slaughterhouse waste (SHW) at laboratory scale: Influence of co-digestion with the organic fraction of municipal solid waste (OFMSW). Biochem. Eng. J. 2008, 40, 99–106. [Google Scholar]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Hidalgo, D.; Martín-Marroquín, J.; Corona, F. The effect of feed composition on anaerobic co-digestion of animal-processing by-products. J. Environ. Manag. 2018, 216, 105–110. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Z.; Wang, L. Improving slaughterhouse byproducts utilization via anaerobic digestion, composting, and rendering. Renew. Sustain. Energy Rev. 2024, 189, 113881. [Google Scholar] [CrossRef]
- Kadam, R.; Jo, S.; Lee, J.; Khanthong, K.; Jang, H.; Park, J. A Review on the Anaerobic Co-Digestion of Livestock Manures in the Context of Sustainable Waste Management. Energies 2024, 17, 546. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Zheng, G.; Yang, H.; Li, L. Optimization of cattle manure and food waste co-digestion for biohydrogen production in a mesophilic semi-continuous process. Energies 2020, 13, 3848. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 2006, 53, 7–22. [Google Scholar] [CrossRef]
- Rao, P.V.; Baral, S.S. Experimental design of mixture for the anaerobic co-digestion of sewage sludge. Chem. Eng. J. 2011, 172, 977–986. [Google Scholar] [CrossRef]
- Reza, A.; Chen, L.; Kruger, K. Microwave irradiated ammonia nitrogen removal from anaerobically digested liquid dairy manure: A response surface methodology and artificial neural network-based optimization and modeling. J. Environ. Chem. Eng. 2022, 10, 108279. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.; Guwy, A.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Suruagy, M.V.T.; Ross, A.; Camargo-Valero, M.A. Particle size, inoculum-to-substrate ratio and nutrient media effects on biomethane yield from food waste. Renew. Energy 2020, 151, 311–321. [Google Scholar] [CrossRef]
- Shelton, D.R.; Tiedje, J.M. General method for determining anaerobic biodegradation potential. Appl. Environ. Microbiol. 1984, 47, 850–857. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.; Lee, H.; Park, K.; Kwon, H.; Na, Y.; Lee, S. Modeling methane potential yield and chemical composition of bedded pack barn cattle manure: Influence of cattle, season, growth stage, its retention time and particle distribution. J. Mater. Cycles Waste Manag. 2020, 22, 1006–1018. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Krooneman, J.; Euverink, G.J.W. Strategies to boost anaerobic digestion performance of cow manure: Laboratory achievements and their full-scale application potential. Sci. Total Environ. 2021, 755 Pt 1, 142940. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, H.; Li, W.; Zhang, R.; Liu, G.; Chen, C. Anaerobic digestion of lipid-rich swine slaughterhouse waste: Methane production performance, long-chain fatty acids profile and predominant microorganisms. Bioresour. Technol. 2018, 269, 426–433. [Google Scholar] [CrossRef]
- Tapparo, D.C.; Viancelli, A.; Amaral, A.C.D.; Fongaro, G.; Steinmetz, R.L.R.; Magri, M.E.; Barardi, C.R.M.; Kunz, A. Sanitary effectiveness and biogas yield by anaerobic co-digestion of swine carcasses and manure. Environ. Technol. 2020, 41, 682–690. [Google Scholar] [CrossRef]
- Moukazis, I.; Pellera, F.M.; Gidarakos, E. Slaughterhouse by-products treatment using anaerobic digestion. Waste Manag. 2018, 71, 652–662. [Google Scholar] [CrossRef]
- Reátegui, O.; Cardenas, H.; Pena, D.; Castro, V.; Roque, R.; Mejía, N.; Ponce, M.; Mestas, R. In Biogas production in batch in anaerobic conditions using cattle manure enriched with waste from slaughterhouse. In Proceedings of the 2017 IEEE 6th International Conference on Renewable Energy Research and Applications (ICRERA), San Diego, CA, USA, 5–7 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 819–822. [Google Scholar]
- Alvarez, R.; Riera, V.H.; Lidén, G. Batch co-digestion of manure, solid slaughterhouse waste, and fruit & vegetable waste. Rev. Boliv. Química 2006, 23, 62–70. [Google Scholar]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Sanz, J.; Lundin, M.; Taherzadeh, M.; Horváth, I. A comparison of process performance during the anaerobic mono- and co-digestion of slaughterhouse waste through different operational modes. J. Environ. Sci. 2018, 64, 149–156. [Google Scholar] [CrossRef]
- Omondi, E.A.; Ndiba, P.K.; Chepkoech, G.K.; Kegode, A.A. Modeling anaerobic co-digestion of water hyacinth with ruminal slaughterhouse waste for first order, modified gompertz and logistic kinetic models. Int. J. Renew. Energy Dev. 2023, 12, 627. [Google Scholar] [CrossRef]
- Béline, F.; Rodriguez-Mendez, R.; Girault, R.; Le Bihan, Y.; Lessard, P. Comparison of existing models to simulate anaerobic digestion of lipid-rich waste. Bioresour. Technol. 2017, 226, 99–107. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.S.; Jalalah, M.; Li, X.; Salama, E.S. Synergistic ammonia and fatty acids inhibition of microbial communities during slaughterhouse waste digestion for biogas production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef]
- Panizio, R.M.; Calado, L.F.d.C.; Lourinho, G.; de Brito, P.S.D.; Mees, J.B. Potential of biogas production in anaerobic co-digestion of Opuntia ficus-indica and slaughterhouse wastes. Waste Biomass Valorization 2020, 11, 4639–4647. [Google Scholar] [CrossRef]
- Selormey, G.K.; Barnes, B.; Kemausuor, F.; Darkwah, L. A review of anaerobic digestion of slaughterhouse waste: Effect of selected operational and environmental parameters on anaerobic biodegradability. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1073–1086. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]



| Parameter | Inoculum | CM | PM | SHW |
|---|---|---|---|---|
| pH | 8.03 | 8.21 | 7.31 | 7.22 |
| TCOD (g/kg) | 12.41 | 185.73 | 145.36 | 1162.64 |
| SCOD (g/kg) | 4.30 | 69.25 | 35.69 | 786.74 |
| SCOD/TCOD (%) | 34.65 | 37.29 | 24.55 | 67.67 |
| T-N (g/kg) | 2.74 | 4.58 | 5.23 | 8.60 |
| NH4+-N (g/kg) | 1.34 | 3.21 | 2.18 | 1.38 |
| T-P (g/kg) | 0.59 | 5.80 | 4.22 | 4.38 |
| TS (g/kg) | 17.70 | 210.02 | 69.05 | 305.01 |
| VS (g/kg) | 7.10 | 152.32 | 54.36 | 293.14 |
| VS/TS (%) | 40.11 | 72.53 | 78.73 | 96.11 |
| C (%) | 27.8 | 33.0 | 36.7 | 33.6 |
| H (%) | 6.2 | 5.6 | 7.1 | 11.6 |
| O (%) | 58.5 | 51.5 | 48.5 | 45.7 |
| N (%) | 4.5 | 2.6 | 3.8 | 6.5 |
| S (%) | <0.1 | <0.1 | 1.0 | <0.1 |
| C/N ratio | 6.2 | 12.7 | 9.7 | 5.2 |
| Chemical formula | C7.26H19.6O11.4N | C15.0H30.8O17.6N | C11.4H26.5O11.3N | C6.0H25.0O6.1N |
| TMY (NmL CH4/g VS) | - | 290.5 | 358.9 | 538.8 |
| Standard Order | Mixture Ratio (Based on VS Content) | ||
|---|---|---|---|
| CM (%) | PM (%) | SHW (%) | |
| S1 | 1 | 0 | 0 |
| S2 | 0 | 1 | 0 |
| S3 | 0 | 0 | 1 |
| S4 | 0.5 | 0.5 | 0 |
| S5 | 0.5 | 0 | 0.5 |
| S6 | 0 | 0.5 | 0.5 |
| S7 | 0.33 | 0.33 | 0.33 |
| S8 | 0.67 | 0.17 | 0.17 |
| S9 | 0.17 | 0.67 | 0.17 |
| S10 | 0.17 | 0.17 | 0.67 |
| Standard Order | Mixture Ratio (Based on VS Content) | TMY (NmL CH4/g VS) | CMY (NmL CH4/g VS) | BD (%) | ||
|---|---|---|---|---|---|---|
| CM | PM | SHW | ||||
| S1 | 1 | 0 | 0 | 347.0 | 107.0 | 30.8 |
| S2 | 0 | 1 | 0 | 428.7 | 264.1 | 61.6 |
| S3 | 0 | 0 | 1 | 538.8 | 500.2 | 92.8 |
| S4 | 0.5 | 0.5 | 0 | 387.9 | 190.6 | 49.1 |
| S5 | 0.5 | 0 | 0.5 | 442.9 | 356.9 | 80.6 |
| S6 | 0 | 0.5 | 0.5 | 483.8 | 419.9 | 86.8 |
| S7 | 0.33 | 0.33 | 0.33 | 433.8 | 334.8 | 77.2 |
| S8 | 0.67 | 0.17 | 0.17 | 397.0 | 254.7 | 64.2 |
| S9 | 0.17 | 0.67 | 0.17 | 437.8 | 333.9 | 76.3 |
| S10 | 0.17 | 0.17 | 0.67 | 492.9 | 433.1 | 87.9 |
| Standard Order | Mixture Ratio (Based on VS Content) | Rmax (NmL CH4/g VS/d) | λ (d) | R2 | ||
|---|---|---|---|---|---|---|
| CM | PM | SHW | ||||
| S1 | 1 | 0 | 0 | 20.2 | 6.7 | 0.9969 |
| S2 | 0 | 1 | 0 | 45.2 | 6.6 | 0.9931 |
| S3 | 0 | 0 | 1 | 65.3 | 3.8 | 0.9926 |
| S4 | 0.5 | 0.5 | 0 | 31.4 | 6.4 | 0.9817 |
| S5 | 0.5 | 0 | 0.5 | 45.2 | 5.9 | 0.9927 |
| S6 | 0 | 0.5 | 0.5 | 58.9 | 5.2 | 0.9874 |
| S7 | 0.33 | 0.33 | 0.33 | 48.2 | 5.2 | 0.9959 |
| S8 | 0.67 | 0.17 | 0.17 | 38.5 | 5.8 | 0.9949 |
| S9 | 0.17 | 0.67 | 0.17 | 54.2 | 5.4 | 0.9924 |
| S10 | 0.17 | 0.17 | 0.67 | 61.1 | 3.9 | 0.9935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Kadam, R.; Jang, H.; Seo, D.; Park, J. Elucidating Synergetic Effects of Anaerobic Co-Digestion of Slaughterhouse Waste with Livestock Manures. Energies 2024, 17, 3027. https://doi.org/10.3390/en17123027
Jo S, Kadam R, Jang H, Seo D, Park J. Elucidating Synergetic Effects of Anaerobic Co-Digestion of Slaughterhouse Waste with Livestock Manures. Energies. 2024; 17(12):3027. https://doi.org/10.3390/en17123027
Chicago/Turabian StyleJo, Sangyeol, Rahul Kadam, Heewon Jang, Dongyun Seo, and Jungyu Park. 2024. "Elucidating Synergetic Effects of Anaerobic Co-Digestion of Slaughterhouse Waste with Livestock Manures" Energies 17, no. 12: 3027. https://doi.org/10.3390/en17123027
APA StyleJo, S., Kadam, R., Jang, H., Seo, D., & Park, J. (2024). Elucidating Synergetic Effects of Anaerobic Co-Digestion of Slaughterhouse Waste with Livestock Manures. Energies, 17(12), 3027. https://doi.org/10.3390/en17123027







