Abstract
Integrity of wellbores and near wellbore processes are crucial issues in geological carbon storage (GCS) projects as they both define the confinement and injectivity of CO2. For the proper confinement of CO2, any flow of CO2 along the wellbore trajectory must be prevented using engineered barriers. The effect of cyclic stimuli on wellbore integrity, especially in the context of GCS projects, has been given less attention. In this study, the effect of pressure- and temperature-cycling on two types of wellbore composites (i.e., casing-cement and cement-caprock) have been investigated experimentally in small- and large-scale laboratory setups. The experiments have been carried out by measuring the effective permeability of the composites under pressure and thermal cyclic conditions. Furthermore, the permeability of individual samples (API class G and HMR+ cement and caprock) was measured and compared to the permeability of the composites. The results indicate that the permeability of API class G cement when exposed to CO2 is in the order of 10−20 m2 (10−5 mD) as a result of the chemical reaction between the cement and CO2. In addition, the tightness of the composite cement–rock has been confirmed, while the permeability of the composite casing–cement falls within the acceptable range for tight cement and the CO2 flow was identified to occur through or close to the interface casing–cement. Results from thermal cycling within the range −9 to 14 °C revealed no significant effect on the integrity of the bond casing–cement. In contrast, pressure cycling experiments showed that the effective pressure has a larger influence on the permeability. The potential creation of micro-cracks under pressure variations may require some time for complete closing. In conclusion, the pressure and temperature cycling from this study did not violate the integrity of the casing–cement composite sample as the permeability remained low and within the acceptable range for wellbore cement.
1. Introduction
The Paris Agreement (2015) aims to keep global warming well below 2 °C (preferably 1.5 °C), compared to preindustrial levels, and requires immediate response owing to the ever-globally increasing crude oil and other fossil fuel demand and consumption. CO2 is the main greenhouse gas that causes heat entrapment in the atmosphere and, eventually, climate change. Many options have been introduced and evaluated to decrease CO2 emissions into the atmosphere [1,2]. However, geological CO2 storage is currently the most feasible option that enables rapid and large-scale storage simultaneously in underground formations like aquifers, depleted hydrocarbon reservoirs, coalbed seams, and basalt rocks [3,4,5,6,7,8,9]. Furthermore, the IPCC [10], in their last report, pointed out that without carbon capture and storage (CCS), climate targets will not be met on time.
Wellbore integrity is a critical certification requirement of geological carbon storage (GCS) projects, ensuring the safe and efficient injection operations and confinement of CO2 in geological formations. If the CO2 is not continuously injected—a likely challenge to occur during the start-up phase of many GCS field projects due to disrupted CO2 availability, planned maintenance, and/or unexpected responses and accidents, as well as unscheduled events in the near wellbore region, such as salt precipitation or hydrate formation—it may mean that the operations need to stop and restart more often. Most studies and projects today provide general knowledge on the separate challenges of wellbore integrity and near-wellbore effects in cases where CO2 is injected continuously. Much less investigated and understood are the challenges when CO2 is injected in a cyclic (intermitted/fluctuating/pulse) mode. Cyclic pressure conditions (due to multiple starts and stops) and/or cyclic temperature conditions (due to cold CO2 injection or Joule–Thomson cooling (J-T)) can induce significant stresses and impact on well integrity, leading to various damage issues such as casing failures, cement sheath degradation, and leaks. Understanding the effects of cyclic pressure and temperature on well integrity is crucial for designing and maintaining reliable well systems. Figure 1 illustrates the various potential pathways for leakage within an injection or a plugged well, extending from the storage formation to the wellhead. The figure identifies the following leakage pathways:
- ○
- leakage along the interface between the casing and cement, involving gas channels or de-bonding at the casing wall;
- ○
- leakage along the interface between cement and formation (caprock), specifically de-bonding at the formation;
- ○
- leakage through fractures in the cement sheath induced by mechanical or thermal stresses;
- ○
- leakage through channels within the cement sheath or cement bridge;
- ○
- leakage through the damaged caprock near the wellbore region;
- ○
- leakage through the casing itself, encompassing pipe corrosion and pipe/connection failures.
The first three leakage pathways can be induced during cyclic CO2 injection. Reinicke and Fichter [11] have listed the failure mechanisms responsible for the defects mentioned earlier in the system, including the wellbore, casing, cement, and formation. They have categorized the failure mechanisms into chemical loading, mechanical–thermal factors, and construction deficiencies [11]. However, cement, casing–cement, and cement–rock integrity are the most essential to avoid damage mechanism in general, this issue becomes more emphasized during cycling CO2 injection, where the failure due to cement damage or deboning from the casing or formation is most likely to occur.
The integrity of wellbores and their individual components is still a major topic for research in various geo-energy systems where the wellbore is the only path to the target formation. Its integrity guarantees a safe and long-term operation. Any failure of wellbore integrity will have serious consequences on the operations and, most likely, on the environment as well [12,13].
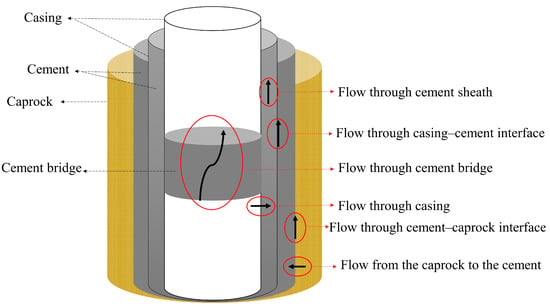
Figure 1.
Possible leakage pathways of CO2 during injection or well abandonment.
The reactivity of CO2 in the presence of water with cement is one of the main concerns in GCS operations. Many studies on typical wellbore cement (like class G and H cement) reported cement degradation due to interaction with CO2 [14,15,16,17,18,19,20]. Other studies focused on the reactivity of CO2 with casing or rocks [12,21,22,23]. Moreover, the studies have been extended to study composites or wellbore sections (two components, e.g., casing–cement or cement–rock) under various CO2 operation conditions [24,25,26,27,28]. In recent years, the whole wellbore section was investigated under reservoir conditions [29,30,31]. However, just a few studies have reported on real-time permeability measurements for composites (casing–cement or rock–cement) on a large scale while exposed to CO2 [29]. Therefore, insight details through wellbore size section experiments are essential to understand the hazards caused by intermittent CO2 injection operations. Studies like Wu et al. [32] investigated experimental integration of casing–cement composite at high pressure and temperature for oil and gas industry applications and identified that the integrity of the cement sheath is progressively damaged by cyclic loading. Cyclic CO2 injection intensifies pressure and thermal effects on wellbores and near wellbore components. This will be more pronounced when injecting CO2 in depleted hydrocarbon reservoirs owing to the low reservoir pressure at the onset of injection. Most recent studies of the effect of pressure cycling on wellbore elements have concentrated on the effect of internal casing pressure on the integrity of cement [33,34,35,36,37,38]. Shadravan et al. [39] applied high pressure and high temperature (HPHT) conditions on a down-scaled casing–cement model, and their results showed cement failure after a certain number of cycles according to the applied pressure. However, their results may not apply to CO2 injection wells, especially for low-pressure reservoirs (depleted reservoirs). Several studies have been implemented on down-scaled composites to investigate the thermal cycling effect [40,41]. Nevertheless, these studies were limited to temperatures above 0 °C. The pioneering work of Todorovic et al. [42] applied deep cooling on dry and wet casing–cement–rock composites. However, they used only computed tomography scans to evaluate cement integrity. Most recently, Udebhulu et al. [43] reviewed cement sheath integrity evaluation techniques for GCS and reported that the permeability of cement is the most critical parameter for cement integrity.
Depleted oil and gas reservoirs are very attractive candidates for GCS owing to the fact that they are well characterized. Their storage capacity and reservoir and well integrity are proven, while surface facilities already exist. However, at the onset of operations, such depleted reservoirs will be at low pressure, and when they are filled up to their maximum capacity, they will retain almost the original pressure. This means that the effective pressure on the cement sheath at the onset of CO2 injection will be very high, and when the reservoir is full, the effective pressure will reach the minimum value. During the life span of a well in such an operation scenario, we are interested in ensuring that the cement will not lose its integration/bond with the casing and the surrounding rock. Two factors play a role here, namely, effective pressure change and temperature drop due to J-T cooling especially at the starting point of injection. The impact of these two parameters on different wellbore components (single components and composites) is the main purpose of this experimental study. Figure 2 shows how confining and reservoir pressure act on wellbore cement. Effective pressure is the difference between them.
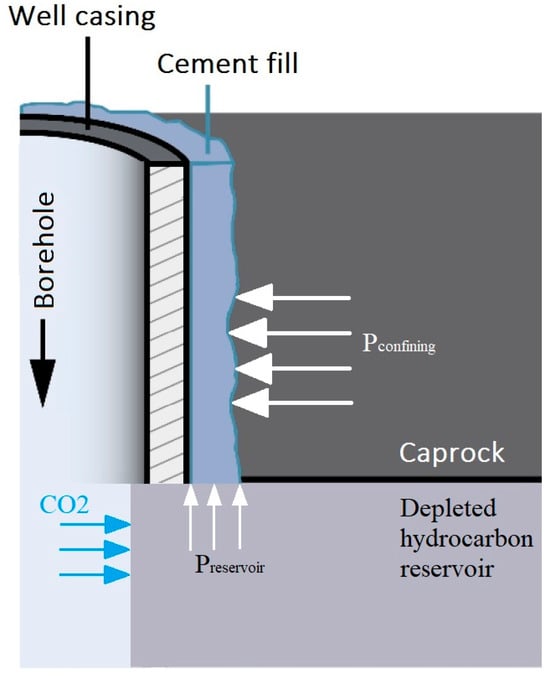
Figure 2.
The schematic shows how the reservoir and confining pressures act on wellbore cement (dimensions not to scale). Effective pressure is the difference between both pressures (peffective = pconfining − preservoir).
In this study, two types of cement were used to examine well integrity, namely, API class G and HMR+. Previously, we have investigated experimentally the resistance of both cements to CO2-carbonated water under static conditions in an autoclave [20]. In the current study, we evaluate the effective permeability of both cements and caprock (anhydrite) with CO2. Furthermore, the permeability of cement–anhydrite and cement–coiled tubing (CT) was investigated under temperature cycling, while the permeability of casing–cement composite was evaluated under thermal and pressure cyclic conditions. Our performed thermal cycling experiments did not initiate any J-T cooling, but rather cooling was reached via heat conduction of pre-cooled fluid to imitate the J-T effect.
2. Methodology
2.1. Principle of Effective Permeability Measurement of Tight Materials
There are two well-known methods to measure absolute permeability in the laboratory, namely, steady-state and transient methods [44,45]. The steady-state method requires the injection pressure and flow rate of the fluid through the sample to be constant over a period of time and the permeability is calculated simply by applying Darcy’s law. Therefore, the steady-state method is not appropriate to measure the permeability of tight materials where a very little amount of fluid can flow through the sample over a long period of time, and it is hard to attain steady-state conditions within an acceptable measurement time. Conversely, the transient method is very effective and accurate for tight materials of less than 1 mD. At our institute, we developed a setup based on this method to measure the permeability of rocks and any tight material down to 1 × 10−24 m2. Figure 3 illustrates the measurement principle, which is described as a two-chamber permeability measurement system. The measurement setup consists of an inlet and outlet chambers and a core holder for cylindrical samples. Before starting the experiment, the inlet chamber is pressurized to a high pressure (e.g., 2 MPa) while the outlet chamber is set to a lower pressure (near or atmospheric pressure). When the connection between both chambers across the sample is open, the measurement begins by recording the pressure profiles at the inlet and outlet of the sample (normally inlet and outlet chambers). Based on the recorded pressure development and the known geometry of the sample, the permeability of the sample is calculated through the following partial differential equation that is developed for the system,
where p is the gas pressure, k is the effective permeability, μ is gas viscosity, zg is the real gas factor, φ is the ample porosity, and c is the total isothermal compressibility (gas and rock).
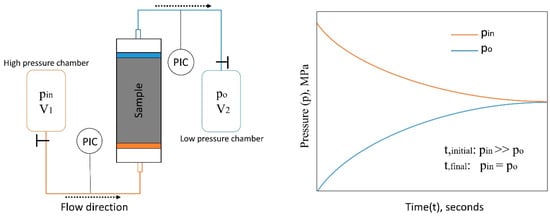
Figure 3.
The schematic of the transient two-chamber permeability measurement setup (left) and the typical recorded pressure curves (right).
The equation is solved using the finite volume approach and it is coupled with an automatic parameter identification method, and coded in Python to calculate the permeability. The calculated pressure is compared with the measured pressure at each time step, and the permeability is obtained when the best match between both pressures is reached. The measurement principle and permeability calculation method can be reviewed in detail in [46,47,48,49].
2.2. Experimental Setup
The test facility allows a sample gas to flow through a cylindrical specimen to measure its permeability. All gas-carrying parts of the system are designed to have a high resistance to hydrogen (H2) and carbon dioxide (CO2).
We developed two different transient setup systems to measure permeability using the principle described in the previous section, according to sample size, and they are termed as small-scale and large-scale setups. The large-scale setup is particularly special since it can handle samples up to 80 cm in length and 30 cm in diameter.
Figure 4 shows the small-scale setup. The system consists of two pressure chambers and an autoclave where the sample to be measured is located. The autoclave is filled with hydraulic fluid, which allows jacket pressure to be applied to the sample. To protect the sample from hydraulic fluid, it is covered with a rubber sleeve. There are stainless steel head plates on both sides of the cylindrical sample, which connect to the gas line. The pressure chambers, lines, and the pore space of the sample form a closed flow loop. The volumes of all the gas-carrying add-on parts are known and the pressure on the inlet and outlet side is recorded by pressure sensors. All attachments (pressure sensors, diaphragm valves, and piping) are attached to an aluminum panel placed above the autoclave.
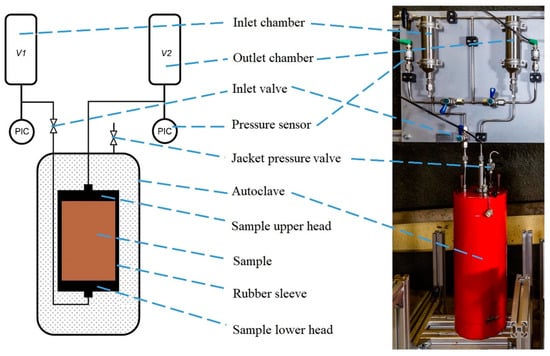
Figure 4.
Small-scale two-chamber setup system.
Each chamber (inlet and outlet) has a volume of approx. 150 mL and a maximum working pressure of 34 MPa. Both pressure chambers are connected to pressure sensors, which have a measuring range of 0–20 MPa (inlet side) and 0–6 MPa (outlet side). For measurements in the lower pressure range, identical sensors with measuring ranges of 0 to 1 MPa and 0 to 2.5 MPa are available. The system can be filled with the sample gas via a gas-filling line. Commercially available gas cylinders are used to fill the inlet chamber, and different gases are used through the experiments, e.g., CO2, H2, and N2. Two diaphragm valves, which establish the connection to the gas filling line, are used to fill and relieve the pressure chambers with gas. The input chamber is connected to the autoclave via a third diaphragm valve, which opens to start the measurement. The gas lines lead from the inlet side to the lower head plate so that the sample flows through from the bottom to the top.
The autoclave has a volume of 4 L, a height of 46 cm, and a diameter of 18 cm. It is filled with hydraulic oil and can be loaded up to a pressure of 25 MPa. This confining pressure is used to simulate the overburden pressure that acts on the samples in situ and prevents edge currents during the measurement. The pressure is applied by a pressure booster, which compresses air with a transmission ratio of 1:40. There are four through-holes in the lid of the autoclave, two for the gas lines and two for applying the confining pressure. One connection is used for the task of confining pressure; the other allows the air bubble introduced by the sample installation to circulate out.
To create a closed flow space, the sample is covered with a rubber sleeve before installation and is attached to two head plates. The head plates are stainless steel cylinders with a diameter of 10 cm, a height of 1 cm, and a central hole through which the gas lines are connected. Recesses are milled into the surface of the head plates facing the sample, creating an injection space between the steel and the sample’s flat surface. The rubber sleeve encases the sample, including the head plate, and it is locked to it with hose clamps. These prevent the hydraulic oil from flowing between the rubber and steel before the confining pressure creates the sealing effect. The gas pressure must not exceed the confining pressure otherwise, the sealing effect and the cohesion of the head plates will be lost. During the tests with H2 and CO2, it was found that these gases diffused through the rubber sleeve. To reduce the leakage rate, the sample can also be wrapped with aluminum or lead foil.
The large-scale setup works according to the same measuring principle as the small-scale facility. It also enables the measurement of hollow cylinder specimens on a field scale and under variable pressure conditions. Differences between the systems result from the sample geometry, which necessitates the use of a larger autoclave. Figure 5 shows a picture and a schematic of the large-scale setup that is equipped with a cooling/heating unit.
In addition to the external confining pressure, the sample may be subjected to an internal casing pressure to simulate the pressure of wellbore fluid during production and injection intervals of the well. This is made possible by adapted head plates and two separately controllable hydraulic lines.
The autoclave has a height of 1.74 m and a maximum outer diameter of 0.81 m. The internal dimensions are 1.5 m in height and 0.48 m in diameter, which is in the head flange area reduced to 0.36 m. The autoclave consists of a bottom part, a top part, and a cover which are connected via flange connections. The autoclave is anchored to a concrete foundation. Its maximum operating pressure is 20 MPa, and is applied with water with the addition of an anti-corrosion medium. After installing a composite sample and connecting all lines, the cover is closed. The autoclave’s main properties and operating parameters are listed in Table 1. A schematic drawing of the autoclave with main parts and connections can be found in [50].

Table 1.
Main specifications and working parameters of the large-scale experimental setup.
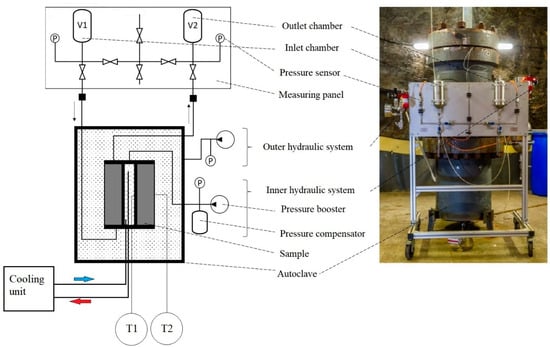
Figure 5.
Large-scale variation of the two-chamber system [51]. Dimensions in the schematic are not to scale.
Both experimental facilities (small- and large-scales) are built in an underground mine that belongs to TUBAF. The mine depth is around 150 m and the temperature is constant at 14 °C, which eliminates the need for air conditioning to maintain a constant ambient temperature. Figure 6 shows part of the laboratory with test facilities and the large-scale setup in the middle.

Figure 6.
Picture of the large-scale setup in the underground laboratory of TUBAF in the ‘Reiche Zeche’ mine at a depth around 150 m.
2.3. Samples Preparation
The samples used for the permeability measurement can be divided into the following three categories:
- -
- individual material samples (solid cylinder), cement and caprock, outer diameter (OD) = 100 mm (4 samples each);
- -
- composite samples (solid cylinder), cement-caprock, OD = 100 mm (2 samples);
- -
- composite samples (hollow cylinder), cement–coiled tubing(cement–CT), OD = 100 mm and casing–cement, OD = 290 mm (2 samples each).
Permeability measurements of the solid cylinder samples and cement CT samples were carried out in the small-scale setup, while the measurement of casing cement samples was implemented in the large-scale setup.
2.3.1. Preparing Cement Samples (API Class G and HMR+)
In the current study, six types of samples were prepared in order to evaluate well integrity under CO2 injection conditions; namely, cement (class G and HMR+), caprock (anhydrite), casing–cement composite, cement–CT composite, and cement–anhydrite composite.
Two different cements were chosen to be tested within the frame of this study. On the one hand, a blast furnace cement specially developed for cementing salt formations was under investigation, hereinafter referred to as cement HMR+ (high magnesium resistant) [52,53]. This cement was tested for resistance to CO2 attack in comparison with the other four cement types (class G cement being one of them). HMR proved to be the least attacked by CO2 [54]. HMR has been developed for wells drilled through salt formations. Further development of this cement—by changing the grain size of some of its components—has led to the new version named HMR+. The new cement has high effectiveness against gas flow due to low porosity and permeability [52]. On the other hand, a standard borehole cement, API class G cement, was utilized (“black label” type from Dyckerhoff AG) [55], hereinafter referred to as class G cement. Table 2 shows the cement recipe used for one liter of cement slurry.

Table 2.
Recipe of cement sludge for 1 L.
The cement slurries are produced using a process that differs from the standard API RP 10B-2 [56]. The API RP 10B-2 standard recommends the use of a special floor-driven blender that achieves stirring speeds of up to 12,000 rpm [20]. Corresponding devices are only available for volumes up to 600 mL or 3000 mL. Since volumes of at least 8000 mL were required to produce the composite samples, a different method was used. A laboratory stirrer with a four-bladed propeller stirrer was used to produce the cement slurry [51]. The process is identical for both cement systems, with the exception that with cement HMR+ the sodium chloride was first dissolved in the mixing water. The cement was then gradually added to the mixing water. After the addition was completed, stirring was continued for 10 min at 1000 rpm. Due to its fine particle size fraction, HMR+ cement forms a highly viscous cement slurry, which is difficult to mix. After the end of the mixing process, the homogeneous cement slurry was filled into commercially available ventilation pipes with a length of 100 cm and an internal diameter of 10 cm. In order to prevent the cement from drying out during the setting time, it was completely covered with the mixing water and left to set at ambient pressure and a temperature of 14 °C. After 28 days, the samples were cut and then stored again (until the measurement) in containers with mixing water. All cement samples were also wrapped with lead foil to avoid gas penetration through the rubber sleeve in the core holder (Figure 7a).


Figure 7.
Pictures of class G cement (a), original anhydrite core (b), and composite cement–anhydrite at different preparation stages (c–f).
2.3.2. Preparing Caprock Samples (Anhydrite)
Anhydrite samples were prepared from a core drilled from a basement layer of a salt cavern in Germany (Figure 7b). The original core (1 m length) was drilled below the cavern sump. To prepare the samples, they were cut to the desired length using a stone circular saw.
2.3.3. Preparing Cement–Rock Composites (Cement HMR+–Anhydrite)
Anhydrite samples that had already been measured were drilled through (Figure 7c) and filled with cement HMR+ (Figure 7d), while the recovered plug of 4 cm in diameter was cemented to a 10 cm diameter (Figure 7e). The drilling work was carried out on a lathe with a drill bit (4 cm outer diameter) at low speed. During the setting time of 28 days, the samples were stored in a saturated NaCl solution. All composites were also insulated with lead foil before preparation for installation in the autoclave (Figure 7f).
2.3.4. Preparing Casing–Cement Composites (Casing–Cement (Class G))
The third samples category replicates a short section of a real wellbore. They have the shape of a hollow cylinder and consist of a steel pipe surrounded by cement. The steel pipe is an X52-grade welded standard pipeline with an outside diameter of 168 mm and a wall thickness of 8 mm. The production of the cement sludge is analogous to the solid cylinder samples. The sample has an outer diameter of approx. 29 cm and a length of 16 cm. To produce the sample, the first step is to cut the steel tube to the desired length. Both ends of the pipe must then be scraped to 1.5 cm length, as these areas will later serve as sealing surfaces (Figure 8, No. 1). The pipe is then placed in a mold at both ends (Figure 8, No. 2) and covered with a rubber sleeve. The upper and lower molds determine the outside diameter and the height of the cement and thus ensure the desired sample shape. In order to prevent the casting molds from becoming detached, they were connected to one another via a spindle. After this preparation, the cement slurry was poured through the openings of the upper mold into the cavity between the steel pipe and the rubber sleeve until the level reached the desired height (Figure 8, No. 3). The two hose clamps placed at the level of the molds prevent the cement slurry from leaking out. After a setting time of 24 h, the cement was sufficiently strong to remove the rubber sleeve and molds (Figure 8, No. 4). The sample is then stored in a vessel with the respective cement mixing water for at least 28 days at ambient pressure and a temperature of 14 °C. During this time, sealing caps with O-rings were used to protect the sealing surfaces of the steel pipe from corrosion.

Figure 8.
Preparation stages of casing–cement composite; (1) sealing surfaces of the steel pipe; (2) tubular steel including casting molds; (3) composite sample immediately after cementation; (4) composite sample 24 h after the casting process.
The cement–CT composite preparation procedure follows the same casing–cement procedure and differs only in the required sealing surface of 5 mm instead of 1.5 cm from both ends.
During sample preparation, cracks formed several times in the cement sheath of the hollow cylinder samples. The timing of cracking was variable and occurred during the hardening phase (Figure 9). If the casting mold is removed no later than 24 h after casting and the composite sample is then stored in a container filled with the mixing water, cracking can be prevented. The reason for this is assumed to be the reduction in shrinkage due to sufficient water supply [57].

Figure 9.
Cracks in the cement during preparation of casing–cement composites (Red frames are used to mark the position of the cracks). The reader can refer to the online version for a better view of the cracks.
Temperature stability during transporting and installing the prepared samples prior to applying confining pressure is another important aspect to avoid cement damage. All samples were visually and carefully inspected before preparation for installation in the autoclave. Samples with visual cracks were immediately excluded from further investigations. The cement used in all experiments was ensured to be of high quality, which was proved through permeability measurements. The casing used was new, clean, and centralized.
Figure 10 shows the cement–CT sample at different preparation stages; (a–b) shows the size of the CT pipe, (c–d) shows the size of the composite after cementation, (e) shows the core holder with the upper and lower head plates, and (f) after connecting the upper and lower head plates with the rubber sleeve on the right (in red). The rubber sleeve is essential to insulate the sample from the hydraulic fluid that applies the confining pressure on the sample.


Figure 10.
Cement–CT composite at different preparation stages; (a,b) show the CT pipe dimensions and the sealing surface at both ends (5 mm each), (c,d) dimensions of the composite after cementing, and (e,f) upper and lower head plates being connected to the composite.
2.4. Experimental Procedure
The experimental procedure for all experiments on small and large-scale setups is the same for all types of samples. According to the two-chamber experiment procedure, after preparing the sample with the predefined diameter and length, the sample is placed in the core holder. Then, the gas inlet valve connected to the inlet chamber is opened while the pressure is recorded in the inlet and outlet chambers on one second time-step until the end of the experiment. The calculation of the effective permeability in real-time is applied through a code.
Preparation of all samples was straightforward, as described previously. However, for samples with casing and coiled tubing, a special preparation must be implemented, as shown in Figure 11. The procedure for such samples is reported in a previous publication [49,51]. All samples should be in wet conditions (water-saturated) when inserted in the core holder. To ensure proper sample protection, constant temperature is necessary all the time. Afterwards, a CO2 flow was applied.
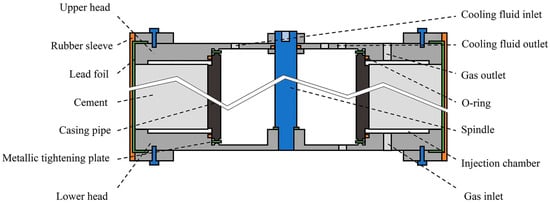
Figure 11.
Schematic of a vertical cross-sectional view of the casing–cement composite ready to be rigged up in the autoclave [51].
3. Results and Discussion
3.1. Permeability of Cement
Figure 12 and Figure 13 show the permeability measurement of class G and HMR+ cement with two different gases (CO2 and Nitrogen (N2)). It can be seen that CO2 did not flow through the cement core. The curve of the inlet chamber is decreasing while the pressure in the outlet chamber remains almost constant. The pressure drop is mainly due to CO2 solubility in water and CO2–cement interaction. This indicates that the calculated permeability (k) is within an acceptable range for wellbore cement.
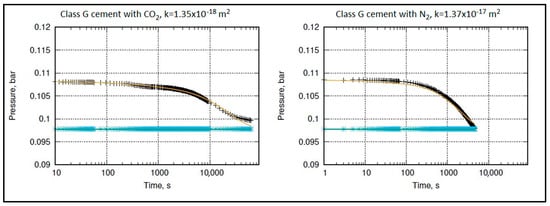
Figure 12.
Permeability measurement of class G cement with CO2 (left) and N2 (right). The black and blue marks represent the recorded pressure in the inlet and outlet chambers, repectively. The orange and blue straight lines depict the corresponding calculated pressure in the inlet and outlet chambers, repectively.
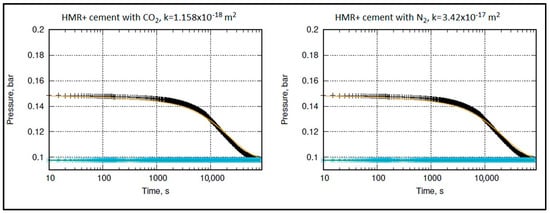
Figure 13.
Permeability measurement of HMR+ cement with CO2 (left) and N2 (right). The black and blue marks represent the recorded pressure in the inlet and outlet chambers, repectively. The orange and blue straight lines depict the corresponding calculated pressure in the inlet and outlet chambers, repectively.
The recommended API value for wellbore cement is max. 2 × 10−16 m2 (0.2 mD) [58]. However, the literature reports a sparse permeability range for class G cement. Nevertheless, it is commonly accepted that a typical value for low permeability in cement is 1 × 10−18 m2 or even less, as suggested by studies conducted by Reinicke et al. [29], Müller-Hoeppe et al. [59], Czaikowski et al. [60], Teodoriu et al. [61], and Le-Minous et al. [62]. Some researchers have reported permeabilities reaching up to 1 × 10−17 m2 for a standard class G cement, as indicated by studies conducted by Goode [63], Nelson and Guillot [64], and Lund et al. [65]. The literature also highlights various factors that can influence the measurement of cement permeability, with effective pressure being one of the significant factors, as reported by researchers.
The permeability of both types of cement was also measured at high effective pressure (4.2 MPa), and the results are shown in Figure 14 and Figure 15. The results show that both cements are tight to CO2. It can be seen from Figure 14 that there was no estimation of the permeability of HMR+ due to its tightness, while the permeability of both class G samples is on the order of 10−20 m2 or less, which confirms the high quality of the prepared cement. A second sample of HMR+ cement showed the same pressure trend in Figure 14.
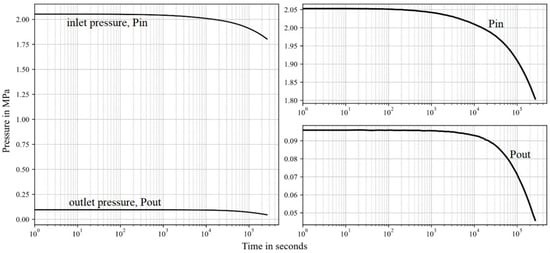
Figure 14.
Permeability measurement results of sample 1 of HMR+ cement. The left-hand side figures show the pressure on a small pressure scale to observe pressure drop accurately.
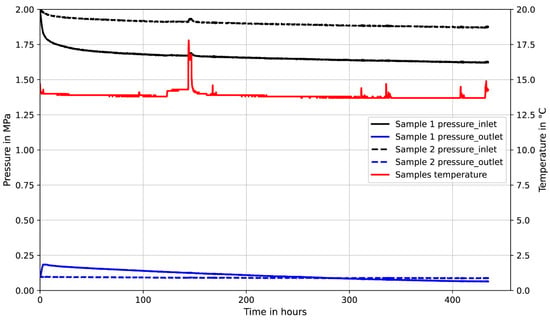
Figure 15.
Permeability measurement results of class G cement. Permeability of two samples is measured for verification. Both samples were measured at the same time. Confining pressure and permeability values are 5.2 MPa, 5.3 MPa, 8.9 × 10−20 m2, and 1.6 × 10−21 m2 for sample 1 and 2, respectively.
In order to evaluate the reactivity of class G cement with CO2, additional measurements with H2 were carried out after the samples were exposed to CO2. The results with H2 correspond to the results with CO2 for sample 2, where the permeability is very low. As for sample 1 (Figure 16), where the permeability with CO2 is relatively higher compared to sample 2, permeability with H2 is lower than with CO2 owing to reactivity with cement which is higher at higher permeability. The significant permeability reduction in sample 1 is an indication of CO2 penetration into the cement matrix having higher contact, causing more reaction and resulting in lower permeability. As for sample 2, CO2 penetration is difficult due to the very low permeability; therefore, the interaction with cement is limited, and the permeability remains almost constant. Further measurements with H2 were carried out on class G and HMR+ samples at different effective pressures to observe the effect of effective pressure on the permeability, and the results are illustrated in Figure 17. Generally, the permeability of HMR+ is lower than class G cement and for both cement types, the permeability decreases with increasing effective pressure as can be seen from Figure 17.
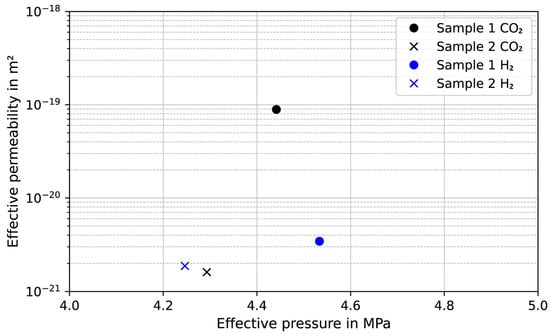
Figure 16.
Comparison of permeability measurement results of two class G cement samples with CO2 and H2.
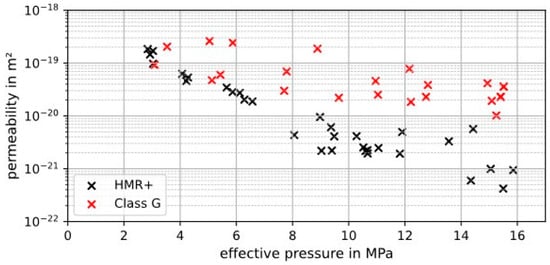
Figure 17.
Benchmarking measurements of several cement samples (class G and HMR+) with H2 as measuring gas.
3.2. Permeability of Anhydrite (Caprock)
Several measurements with different gases (H2, CH4, and N2) have been conducted on the caprock samples (anhydrite). Figure 18 shows the permeability measurement results. It is clear that the permeability is independent of the gas type. This is verified with previously published results [47]. Furthermore, permeability decreases with increasing effective pressure.
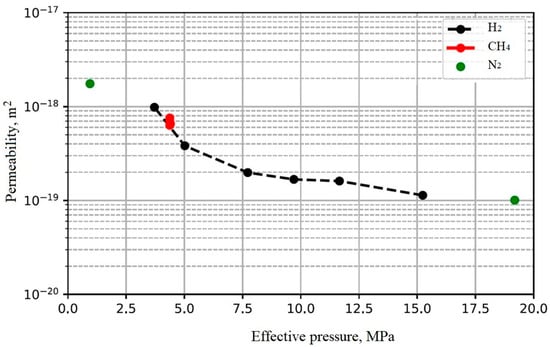
Figure 18.
Permeability measurement of caprock (anhydrite) at different effective pressures with H2, CH4, and N2.
3.3. Permeability of Cement–CT and Cement-Caprock Composites
In the following the results of cement (HMR+)–anhydrite and cement (class G)–CT are presented and discussed under pressure and thermal cyclic conditions. These composites are grouped under the same category since they are both tested in the small-scale setup.
3.3.1. Pressure Cycling
Permeability of the Composite Cement–Anhydrite
Cement–anhydrite composite was investigated under cyclic pressure variations (graphically in Figure 19). Three effective pressures were tested (4, 6, and 8 MPa). The results in Table 3 show the permeability behavior as a function of effective pressure. It is noticeable that effective pressure and permeability values are inversely proportional. The permeability reduction in the cyclic stages (effective pressure at 6 and 4 MPa) is due to long exposure time to CO2 under confining pressure. Furthermore, the values of permeability correspond to the results of the measurement for pure HMR+ and anhydrite, which confirm the tightness of the composite.
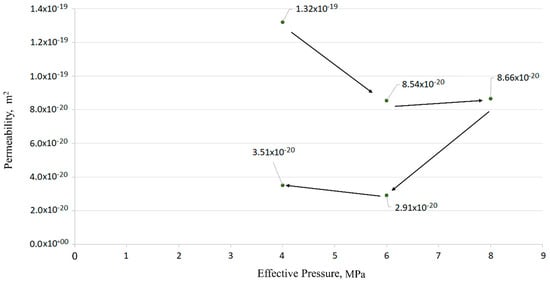
Figure 19.
Permeability of the composite sample cement-anhydrite at room temperature.

Table 3.
Permeability measurement of cement–anhydrite at different effective pressures.
Permeability of the Composite Cement–CT
Table 4 shows the results of pressure variations experiments. The gradual permeability reduction is due to composite compaction with increased effective pressure.

Table 4.
Permeability measurement of cement–CT at different effective pressures.
3.3.2. Temperature Cycling
To evaluate the effect of temperature changes on the well integrity, two different temperature cycling experiments were performed on the composite cement–CT. The first measurement started at 0 °C at an effective pressure of 5 MPa, and the test pressure (gas injection pressure) was held constant around 1 MPa. However, a minor pressure decrease was observed, most probably due to the reaction between CO2 and cement (Figure 20).
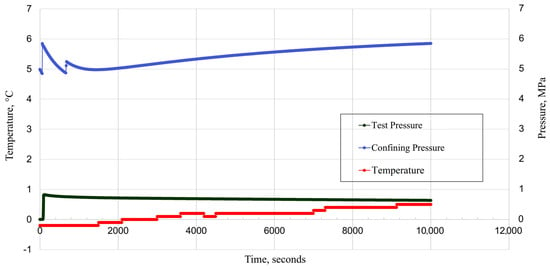
Figure 20.
Temperature variation (0 °C) effect on composite samples (cement–CT).
In the second measurement, −21 °C was the starting temperature while the test pressure was held constant at around 1 MPa, and the confining pressure was also constant at around 6 MPa. The temperature variation results (Figure 21) have shown no significant integrity effect. The outlet valve also showed no fluid discharge, meaning the cement–CT bond remained tight.
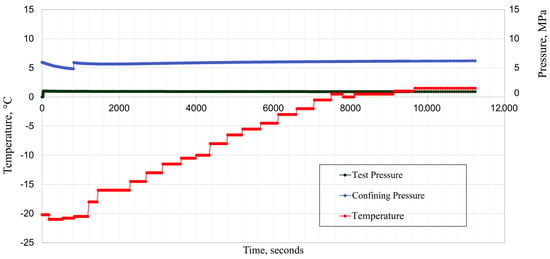
Figure 21.
Temperature variation (−21 °C) effect on composite samples (cement–CT).
To ensure the reproducibility of the results, new samples have been prepared and tested and similar results were obtained.
3.4. Permeability of Casing–Cement (Class G) Composite
In the next two sections, the results of casing–cement (class G) are presented and discussed under pressure -and thermal-cyclic conditions. Both experiments were conducted in the large-scale setup.
3.4.1. Permeability under Thermal Cycling
A number of experiments have been conducted to evaluate the impact of temperature variations in the large-scale setup. Figure 22 shows several runs on the first sample with cyclic temperatures from experiment 1 through experiment 4 at a constant effective pressure of 4.75 MPa (confining pressure 5.5 MPa), where the red line represents the measured temperature inside the casing, while the blue line displays the outside temperature between the rubber sleeve and the lead foil. Therefore, the created temperature difference between the casing and the outer boundary is the gap between the blue line and red line at the appropriate time.
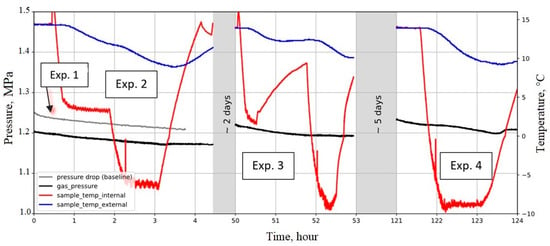
Figure 22.
Tightness test of casing–cement composite at high effective pressure (4.75 MPa) and subzero temperatures (−6 °C and −9 °C).
The following experiments series were implemented on the first sample:
- Experiment 1 (Exp.1): Reference measurement (baseline in grey color line in Figure 22) at room temperature (14 °C) and gas pressure 1.25 MPa was carried out without a cooling process. The slight pressure drop is due to CO2 solubility in water. This experiment was run for about 4 h;
- Experiment 2: The minimum temperature was set to −6 °C for a time period of 45 min. In this experiment, the temperature was held constant at the ambient temperature of around 13.7 °C during the first 30 min to confirm the start point (this reference measurement was also implemented in the subsequent runs, i.e., Exp. 3 and 4. The slight pressure drop is again referred to as CO2 solubility in water, which is present in the pores of the cement;
- Experiment 3: The temperature was decreased to 2 °C in the first stage and then increased to around 9 °C and finally decreased to subzero temperature of −9 °C;
- Experiment 4: This was running at a subzero temperature −9 °C for one hour;
The results of the four experiments show that there is no hydraulic communication between the inlet (V1) and outlet (V2) chambers at subzero temperatures in the range of −6 to −9 °C; therefore, the bond casing–cement was tight under these conditions.
The second thermal cycling experiment was implemented at a low effective pressure of about 1.6 MPa. The results are shown in Figure 23. This experiment examined the tightness of the casing–cement sample at a minimum temperature of −9 °C. During the first 30 min of the experiment, no gas flow was measured in ambient temperature conditions. At a temperature of 5 °C, the pressure of the inlet chamber starts to decrease to 1.1 MPa, while the outlet pressure increases to 0.13 MPa simultaneously due to communication between both chambers (V1 and V2). This confirms a flow in the bond zone between casing and cement. At a temperature of –7 °C, the pressure in both chambers remained at a constant level. This effect is probably due to the freezing of water in the micro-channels in the cement matrix and water in the interface casing–cement. After keeping the temperature constant at −9 °C for 45 min, the warming phase was initiated. At a temperature above 4 °C, the inlet pressure (V1) drops while the outlet pressure increases again. The regained communication between the pressure chambers is related to the thawing of the frozen pore water. It confirms that the flow occurs through and/or close to the interface casing–cement. However, the composite retains a minimum permeability of 8.3 × 10−19 m2, which is within the range for typical class G cement.
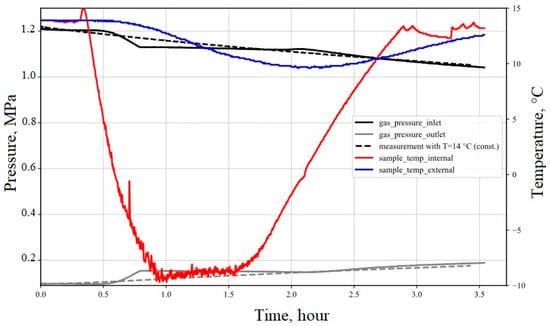
Figure 23.
The tightness test of casing–cement composite was performed at an effective pressure of 1.6 MPa and a subzero temperature of −9 °C compared with the reference test at ambient temperature.
The experiment has been repeated at ambient temperature (dashed lines in Figure 23) to investigate whether thermal cracks happened. The confirmation measurement shows a nearly identical pressure profile to the one initially measured prior to the start of the experiment, which indicates that the subzero temperature has no effect on the integrity of the composite sample. In other words, freeze-thaw did not initiate micro-cracks in the cement structure, at least in this experiment.
Overall, the results of temperature cycling presented here (casing–cement and cement–CT) conform with our previously published results [51,66]. Todorovic et al. [42] conducted similar experiments on wet composites of casing–cement–rock, and they observed no change in cement structure using CT scanning when they applied cooling down to −40 °C on the composite. Roy et al. [67] carried out experiments on the dry casing–cement–rock composite in the range of −50 °C–+80 °C and observed no change in cement structure using a CT scan as well. However, experimental studies by Todorovic et al. [42] and Roy et al. [67] were carried out without confining pressure, e.g., the worst-case scenario. We believe that effective permeability measurement is more reliable than a CT scan, where its results are resolution-dependent.
3.4.2. Permeability under Pressure Cycling
The second batch of experiments was to investigate the effect of pressure cycling on the composite casing–cement. This study took about six months to complete, and the results can be seen in Figure 24. By increasing the effective pressure to 9 MPa, the minimum permeability at 2 × 10−20 m2 was reached. On the other hand, when decreasing the effective pressure, the permeability increased but at a lower path than the original. At an effective pressure of about 1.7 MPa the permeability increased to 10−18 m2. It appears that micro-cracks opened at this effective pressure, which could have occurred during the period of the pressure reduction from 9.0 to 2.0 MPa (cement expansion), and these micro-cracks opened when the confining pressure was reduced. Further reduction of the confining pressure leads to an increase in permeability. After that, an increase in confining pressure caused the permeability to decrease due to the higher effective pressure. At an effective pressure of around 8.0 MPa, permeability measurement was recorded at different time steps to observe if it dropped close to the first measurement, but it retained a value at around 4 × 10−19 m2. Further increasing the effective pressure to 9.0 MPa made no difference, and the permeability value was kept constant. This is probably due to the fact that the cracks did not fully close.
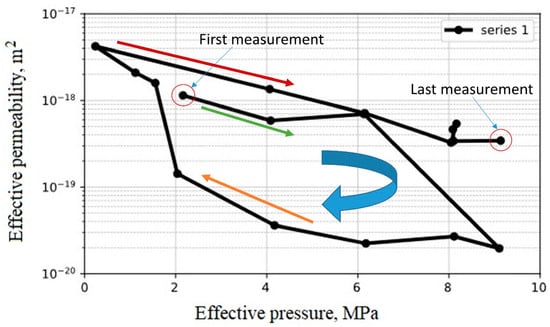
Figure 24.
Permeability of casing–cement composite as a function of effective pressure.
Overall, the permeability value during the whole experiment run (Figure 24) was within the range for class G cement, which means the bond between the casing and cement is tight.
Previously, we published the results on the composite casing–cement with an increasing confining pressure effect on the effective permeability of cement [51]. We concluded that with increasing confining pressure, the permeability decreases. Exposure time to CO2 plays the most important role; with increasing contact time, the permeability degrades. However, the previous study showed that even after decreasing the confining pressure, the permeability did not increase, which does not conform with the current results, which urges the need for more experiments in this regard to understand this behavior.
The literature on experimental work on the effect of effective pressure on the integrity of casing–cement or cement–rock is still poor. Most reported studies concentrate on the effect of the repeated internal pressure inside the casing on the bond casing–cement or cement–rock. Such studies can be found in [34,35,36,37,38].
4. Conclusions
This study represents experimental investigations to test the tightness of wellbore components in a CO2 storage operation (GCS) into depleted hydrocarbon reservoirs through real-time permeability measurement. The developed setups at our laboratory—at small and large scales—can trace the permeability down to 1 × 10−24 m2.
Different operating conditions, such as cyclic pressure and temperature (P&T) were considered in this study. Their effect on wellbore integrity issues on pure and composite samples exposed to CO2 was determined to ensure the safe operation window of GCS in depleted hydrocarbon reservoirs.
The results provide valuable insights into the behavior of cement sheath, caprock, cement-caprock, and casing–cement under different P&T conditions of a potential GCS operation.
The following conclusions are drawn:
- -
- the permeability measurements of the cement samples of class G and HMR+ showed technical impermeability to CO2;
- -
- the permeability measurement of caprock (anhydrite) showed that the higher effective pressure decreased the permeability. Furthermore, permeability measurement using different gases (H2, CH4, and N2) confirmed that it is independent of the measured gas;
- -
- temperature variations as low as subzero temperatures showed no significant integrity issues for the composite casing–cement. The results of casing–cement and cement–CT were comparable;
- -
- effective pressure and exposure time to CO2 are the main factors controlling the effective permeability of cement-anhydrite and casing–cement composites;
- -
- microcracks occurred while increasing the effective pressure on the casing–cement composite. These microcracks opened during the reduction of the effective pressure, showing a higher value of permeability. This indicates that there is a minimum effective pressure that should be maintained to ensure safe operation.
Author Contributions
Conceptualization, M.A., T.H.N., N.Z., M.G.A., O.B., J.S., and C.F.; methodology, M.A., T.H.N., and C.F.; software, D.B. and T.H.N.; validation, T.H.N.; formal analysis, T.H.N.; investigation, T.H.N.; resources, M.A.; data curation, D.B.; writing—original draft preparation, T.H.N.; writing—review and editing, T.H.N., H.A., N.Z., M.G.A., and J.S.; visualization, T.H.N.; supervision, J.S., O.B., and M.A.; project administration, J.S.; funding acquisition, M.A., J.S., and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study is implemented within the frame of “InjectWell” project (Experimental and numerical assessments of CO2 injectivity and flow assurance during storage in depleted hydrocarbon reservoirs). The project is financed by Gassnova through the CLIMIT program (grant no. 621097) and Wintershall Dea AG.
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
The authors would like to acknowledge Gassnova (Norway) and Wintershall Dea AG (Germany) for funding and giving permission to publish this paper. We also acknowledge VNG Gasspeicher GmbH (Germany) for providing anhydrite samples to accomplish this study.
Conflicts of Interest
Author Oleksandr Burachok was employed by the Wintershall Dea AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- IPCC. Special Report on Carbon Dioxide Capture and Storage, (Chapter 5): Underground Geological Storage; IPCC: Geneva, Switzerland, 2005; pp. 195–276. [Google Scholar]
- Alkan, H.; Burachok, O.; Kowollik, P. Geologic carbon storage: Key components. In Surface Process, Transportation, and Storage; Wang, Q., Ed.; Golf Professional Publishing: Houston, TX, USA, 2023; Volume IV, pp. 322–425. [Google Scholar] [CrossRef]
- Newell, P.; Ilgen, A. Overview of Geological Carbon Storage (GCS). In Science of Carbon Storage in Deep Saline Formations; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Nassan, T.; Amro, M.; Freese, C.; Wechsung, M. Modelling of supercritical CO2 (scCO2) sequestration in saline aquifers for energy harvesting. Erdöl Erdgas Kohle (EEK) 2021, 10, 35–41. [Google Scholar] [CrossRef]
- Nassan, T.; Amro, M. Carbon dioxide (CO2) as heat extraction fluid from geothermal systems. Oil Gas Eur. Mag. 2021, 47, 34–40. [Google Scholar] [CrossRef]
- Nassan, T.; Alkan, H.; Solbakken, J.; Zamani, N.; Burachok, O.; Amro, M. A Review of Reservoir Engineering Tools and Procedures to Design and Operate Geological Carbon Storage Sites. DGMK/ÖGEW Frühjahrtagung 2022—Geo-Energy-Systems and Subsurface Technologies—Key Elements towards a Low Carbon World, Celle, Germany. 2022. Ebook ISBN 978-3-947716-41-8. Available online: https://ssrn.com/abstract=4533654 (accessed on 8 May 2024).
- Albertz, M.; Stewart, S.A.; Goteti, R. Perspectives on geologic carbon storage. Front. Energy Res. 2023, 10, 1071735. [Google Scholar] [CrossRef]
- Bashir, A.; Ali, M.; Patil, S.; Aljawad, M.S.; Mahmoud, M.; Al-Shehri, D.; Hoteit, H.; Kamal, M.S. Comprehensive review of CO2 geological storage: Exploring principles, mechanisms, and prospects. Earth-Sci. Rev. 2024, 249, 104672. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Z.; Xie, J.; Meng, S.; Xu, J.; Ehlig-Economides, C.; Liu, H. Re-evaluation of CO2 storage capacity of depleted fractured-vuggy carbonate reservoir. Innov. Energy 2024, 1, 100019. [Google Scholar] [CrossRef]
- IPCC; Lee, H.; Romero, J. Summary for Policymakers: Climate Change 2023, Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Reinicke, K.M.; Fichter, C. Measurement strategies to evaluate the integrity of deep wells for CO2 applications. In Proceedings of the Sino-German Conference, Beijing, China, 6–7 July 2010 and the Sino-German Workshop “EOR and New Drilling Technology”, Daqing, China, 12 July 2010. [Google Scholar]
- Xiao, T.; Chen, T.; Ma, Z.; Tian, H.; Meguerdijian, S.; Chen, B.; Pawar, R.; Huang, L.; Xu, T.; Cather, M.; et al. A review of risk and uncertainty assessment for geologic carbon storage. Renew. Sustain. Energy Rev. 2024, 189, 113945. [Google Scholar] [CrossRef]
- Zhou, L.; Upchurch, E.R.; Liu, Y.; Anfinsen, B.-T.; Hashemian, Y.; Yuan, Z. Evaluating Subsea Capping Stack Usage for CO2 Blowouts. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2024. Paper number: OTC-35246-MS. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Lowry, G.V.; Dzombak, D.; Thaulow, N. Rate of CO2 attack on hydrated class H well cement under geologic sequestration conditions. Environ. Sci. Technol. 2008, 42, 6237–6242. [Google Scholar] [CrossRef]
- Abid, K.; Gholami, R.; Choate, P.; Nagaratnam, H.B. A review on cement degradation under CO2-rich environment of sequestration projects. J. Nat. Gas Sci. Eng. 2015, 27, 1149–1157. [Google Scholar] [CrossRef]
- Matteo, E.N.; Huet, B.; Jové-Colón, C.F.; Scherer, G.W. Experimental and modeling study of calcium carbonate precipitation and its effects on the degradation of oil well cement during carbonated brine exposure. Cem. Concr. Res. 2018, 113, 1–12. [Google Scholar] [CrossRef]
- Panduro, E.A.C.; Cordonnier, B.; Gawe, K.; Børve, I.; Iyer, J.; Carroll, S.; Michels, L.; Rogowska, M.; McBeck, J.A.; Sørensen, H.O.; et al. Real time 3D observations of Portland cement carbonation at CO2 storage conditions. Environ. Sci. Technol. 2020, 54, 8323–8332. [Google Scholar] [CrossRef]
- Todorovic, J.; Opedal, N.V.d.T.; Werner, B.; Clausen, J.A.; Kvassnes, A.J.S. Effect of long-term aging in carbonated brine on mechanical properties of a novel cement system with an expandable agent. In Proceedings of the SPE Norway Subsurface Conference, Bergen, Norway, 2–3 November 2020. Paper number: SPE-200753-MS. [Google Scholar] [CrossRef]
- Dalton, L.E.; Crandall, D.; Pour-Ghaz, M. Supercritical, liquid, and gas CO2 reactive transport and carbonate formation in Portland cement mortar. Int. J. Greenh. Gas Control. 2022, 116, 103632. [Google Scholar] [CrossRef]
- Nassan, T.; Muktadir, G.; Fogden, A.; Amro, M.; Solbakken, J.; Aarra, M.G.; Zamani, N.; Burachok, O. Experimental evaluations of cementing systems exposed to carbonated water for geological carbon storage (GCS) operations. Greenhouse Gases Sci. Technol. 2023; submitted. [Google Scholar]
- Carey, W.J.; Svec, R.; Grigg, R.; Zhang, J.; Crow, W. Experimental investigation of wellbore integrity and CO2-brine flow along the casing-cement microannulus. Int. J. Greenh. Gas Control. 2010, 4, 272–282. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Young, D.; Nešić, S.; Gray, L.G.S. Wellbore integrity and corrosion of carbon steel in CO2 geologic storage environments: A literature review. Int. J. Greenh. Gas Control 2013, 16, S70–S77. [Google Scholar] [CrossRef]
- Duguid, A.; Guo, B.; Nygaard, R.; Ramakrishnan, T.; Chugunov, N. Monitoring well integrity at the Cranfield field phase III CO2 storage project. Int. J. Greenh. Gas Control. 2021, 109, 103341. [Google Scholar] [CrossRef]
- Wigand, M.; Kaszuba, J.P.; Carey, J.W.; Hollis, W.K. Geochemical effects of CO2 sequestration on fractured wellbore cement at the cement/caprock interface. Chem. Geol. 2009, 265, 122–133. [Google Scholar] [CrossRef]
- Teodoriu, C.; Reinicke, K.M.; Fichter, C.; Wehling, P. Investigations on casing-cement interaction with application to gas and CO2 storage wells. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010. Paper number: SPE-131336-MS. [Google Scholar] [CrossRef]
- Panduro, E.A.C.; Torsæter, M.; Gawe, K.; Bjørge, R.; Gibaud, A.; Yang, Y.; Bruns, S.; Zheng, Y.; Sørensen, H.O.; Breiby, D.W. In-situ x-ray tomography study of cement exposed to CO2 saturated brine. Environ. Sci. Technol. 2017, 51, 9344–9351. [Google Scholar] [CrossRef]
- Azin, R.; Mehrabi, N.; Osfouri, S.; Asgari, M. Experimental study of CO2- saline aquifer-carbonate rock interaction during CO2 sequestration. Procedia Earth Planet. Sci. 2015, 15, 413–420. [Google Scholar] [CrossRef]
- Jahanbakhsh, A.; Liu, Q.; Mosleh, M.H.; Agrawal, H.; Farooqui, N.M.; Buckman, J.; Recasens, M.; Maroto-Valer, M.; Korre, A.; Durucan, S. An Investigation into CO2-brine-cement-reservoir rock interactions for wellbore integrity in CO2 geological storage. Energies 2021, 14, 5033. [Google Scholar] [CrossRef]
- Reinicke, K.M.; Teodoriu, C.; Fichter, C.; Weichmann, M.J.; Weinlich, F.H.; Krebs, R.; Zhang, C.-L. Untersuchung von Selbstheilungsmechanismen im Verbundsystem Rohr- Zement-Gebirge von CO2-Bohrungen. In Proceedings of the DGMK/ÖGEW-Frühjahrstagung, Celle, Germany, 11–12 April 2011; Fachbereich Aufsuchung und Gewinnung: Celle, Germany, 2011. (In German). [Google Scholar]
- Andrade, J.D.; Sangesland, S.; Todorovic, J.; Vrålstad, T. Cement sheath integrity during thermal cycling: A novel approach for experimental tests of cement systems. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 22 April 2015. Paper number: SPE-173871-MS. [Google Scholar] [CrossRef]
- Vrålstad, T.; Skorpa, R.; Werner, B. Experimental studies on cement sheath integrity during pressure cycling. In Proceedings of the SPE/IADC International Drilling Conference and Exhibition, The Hague, The Netherlands, 5–7 March 2019. Paper number: SPE-194171-MS. [Google Scholar] [CrossRef]
- Wu, X.; Hou, Z.; Li, Z.; Xie, Y.; Liu, J.; Song, W.; Li, J.; Sun, W. Experimental Analysis of Cyclic Loading Effect on Seal Integrity of Cement Sheath. In DGMK-Tagungsbericht 2023-1; DGMK e.V.: Hamburg, Germany, 2023. [Google Scholar]
- Goodwin, K.J.; Crook, R.J. Cement sheath stress failure. SPE Drill. Eng. 1990, 7, 291–296, Paper Number: SPE-20453-PA. [Google Scholar] [CrossRef]
- Jackson, P.B.; Murphey, C.E. Effect of casing pressure on gas flow through a sheath of set cement. In Proceedings of the SPE Drilling Conference, Amsterdam, The Netherlands, 23–25 February 1993. Paper number: SPE 25698. [Google Scholar] [CrossRef]
- Boukhelifa, L.; Moroni, N.; James, S.G.; Le Roy-Delage, S.; Thiercelin, M.J.; Lemaire, G. Evaluation of Cement Systems for Oil and Gas Well Zonal Isolation in a Full-Scale Annular Geometry. SPE Drill. Complet. 2005, 20, 44–53, Paper number: SPE-87195-PA. [Google Scholar] [CrossRef]
- Fahrman, B.P.; Huerta, N.J.; Crandall, D.; Moore, J.E. Visualizing Well System Breakdown: Experimental and Numerical Analyses. In Proceedings of the 51st US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 25–28 June 2017. Paper number: ARMA 17-867. [Google Scholar]
- Skorpa, R.; Øia, T.; Taghipour, A.; Vrålstad, T. Laboratory setup for determination of cement sheath integrity during pressure cycling. In Proceedings of the ASME 2018 37th International Conference on Ocean, Offshore and Arctic Engineering OMAE2018, Madrid, Spain, 17–22 June 2018. [Google Scholar] [CrossRef]
- Kuanhai, D.; Yue, Y.; Yi, H.; Zhonghui, L.; Yuanhua, L. Experimental study on the integrity of casing-cement sheath in shale gas wells under pressure and temperature cycle loading. J. Pet. Sci. Eng. 2020, 195, 107548. [Google Scholar] [CrossRef]
- Shadravan, A.; Schubert, J.; Amani, M.; Teodoriu, C. Using fatigue-failure nvelope for cement-sheath-integrity evaluation. SPE Drill. Complet. 2015, 30, 68–75. [Google Scholar] [CrossRef]
- Albawi, A.; De Andrade, J.; Torsæter, M.; Opedal, N.; Stroisz, A.; Vrålstad, T. Experimental Set-up for Testing Cement Sheath Integrity in Arctic Wells. In Proceedings of the OTC Arctic Technology Conference, Houston, TX, USA, 10–12 February 2014. OTC 24587. [Google Scholar] [CrossRef]
- Andrade, J.D.; Torsæter, M.; Todorovic, J.; Opedal, N.; Stroisz, A.; Vrålstad, T. Influence of Casing Centralization on Cement Sheath Integrity During Thermal Cycling. In Proceedings of the IADC/SPE Drilling Conference and Exhibition, Fort Worth, TX, USA, 4–6 March 2014; Proceedings. Volume 2, pp. 870–879, IADC/SPE 168012. [Google Scholar] [CrossRef]
- Todorovic, J.; Gawel, K.; Lavrov, A.; Torsæter, M. Integrity of downscaled well models subject to cooling. In Proceedings of the SPE Bergen One Day Seminar, Grieghallen, Bergen, Norway, 20 April 2016. Paper number: SPE-180052-MS. [Google Scholar] [CrossRef]
- Udebhulu, O.D.; Aladeitan, Y.; Azevedo, R.C.; Tomi, G. A review of cement sheath integrity evaluation techniques for carbon dioxide storage. J. Petrol. Explor. Prod. Technol. 2023, 14, 1–23. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, A.M.M.; Bustin, R.M. Measurements of gas permeability and diffusivity of tight reservoir rocks: Different approaches and their applications. Geofluids 2009, 9, 208–223. [Google Scholar] [CrossRef]
- Mathur, A.; Sondergeld, C.H.; Rai, C.S. Comparison of Steady-State and Transient Methods for Measuring Shale Permeability. In Proceedings of the SPE Low Perm Symposium, Denver, CO, USA, 5–6 May 2016. Paper number: SPE-180259-MS. [Google Scholar] [CrossRef]
- Bruck, J.V.D. Zum Permeabilitätsverhalten von Kompaktiertem Salzgruz. Ph.D. Dissertation, TU Bergakademie Freiberg, Freiberg, Germany, 1999. (In German). [Google Scholar]
- Häfner, F.; Kornjaew, A.; Pohl, A.; Voigt, H.-D. Permeabilitäts- und Porositätesmessungen and Gesteinsproben mit dem instationären Zweihammerverfahren. Erdöl Erdgas Kohle 1996, 112, 401–404. (In German) [Google Scholar]
- Amro, M.; Freese, C. Novel laboratory measurement for the characterization of tight and shale formations during transient (unsteady state) flow regime. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8–11 March 2015. Paper number: SPE-172757-MS. [Google Scholar] [CrossRef]
- Kirch, M. Untersuchungen zur Bohrungsintegrität sowie dem Gasverhalten von Wasserstoff in Salzkavernen unter Berücksichtigung Variabler Randbedingungen (German). Ph.D. Dissertation, Technical University Bergakademie Freiberg (TUBAF), Freiberg, Germany, 2023. (In German). [Google Scholar]
- Nassan, T.H.; Baganz, D.; Amro, M. Well integrity evaluation and elastomer selection for hydrogen(H2) storage in salt caverns. In Proceedings of the SPE Europe Energy Conference, Turin, Italy, 26–28 June 2024. Paper number: SPE-220053-MS. [Google Scholar]
- Nassan, T.H.; Kirch, M.; Freese, C.; Alkan, H.; Baganz, D.; Amro, M. Experimental investigation of wellbore integrity during geological carbon sequestration: Thermal- and pressure-cycling experiments. Gas Sci. Eng. 2024, 124, 205253. [Google Scholar] [CrossRef]
- Maikranz, E. Historische Entwicklung des Tiefbohrzementes—Vom Portlandzement zum hoch-magnesiumresistenten HMR+ -Hochofenzement. Erdöl Erdgas Kohle (EEK) 2016, 132, 16–22. (In German) [Google Scholar]
- Lesti, M.; Tiemeye, C.; Plank, J. CO2 stability of Portland cement based well cementing systems for use on carbon capture & storage (CCS) wells. Cem. Concr. Res. 2013, 45, 45–54. [Google Scholar] [CrossRef]
- TU München. Untersuchung und Auswahl von CO2-resistenten Tiefbohrzementen; Projektabschlussbericht; TU München: Munich, Germany, 2010. (In German) [Google Scholar]
- DIN EN ISO 10426-1; Erdöl- und Erdgasindustrie—Zemente und Materialien für die Zementation von Tieflochbohrungen—Teil 1: Anforderungen. ISO: Geneva, Switzerland, 2010. [CrossRef]
- API RP 10B-2; Recommended Practice for Testing Well Cements. API: Washington, DC, USA, 2013.
- Müller, H.S.; Kvitsel, V. Kriechen und Schwinden von Beton. Beton- Stahlbetonbau 2002, 97, 8–19. [Google Scholar] [CrossRef]
- Kutchko, B.; Strazisar, B.; Huerta, N.; Lowry, G.V.; Dzombak, D.A.; Thaulow, N. CO2 reaction with hydrated class H well cement under geologic sequestration conditions: Effects of fly ash add mixtures. Environ. Sci. Technol. 2009, 43, 3947–3952. [Google Scholar] [CrossRef]
- Müller-Hoeppe, N.; Breustedt, M.; Wolf, J.; Czaikowski, O.; Wieczorek, K. Integrität Geotechnischer Barrieren—Teil 2: Vertiefte Nachweisführung, Bericht zum Arbeitspaket 9.2. Vorläufige Sicherheitsanalyse für den Standort Gorleben, GRS-288; Gesellschaft für Anlagen- und Reaktorsicherheit (GRS) mbH: Garching, Germany, 2012. [Google Scholar]
- Czaikowski, O.; Wieczorek, K.; Hertes, U. Sealing capacity of a seal system in rock salt—hydraulic impact of the EDZ long-term evolution. In Proceedings of the 49th US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 28 June–1 July 2015. Paper number: ARMA-2015-210. [Google Scholar]
- Teodoriu, C.; Asamba, P.; Ichim, A. Well integrity estimation of salt cements with application to long term underground storage systems. In Proceedings of the SPE Europec featured at 78th EAGE Conference and Exhibition, Vienna, Austria, 30 May–2 June 2016. Paper number: SPE-180073-MS. [Google Scholar] [CrossRef]
- Le-Minous, J.C.; Mutti, D.; Bouvet, A.; Unanue-Rodriguez, I.; Chang, A.; Massie, I.; Xiao, E.; Schnell, E. Permeability study of API class G and B cements considering seawater and WBM contamination. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, The Hague, The Netherlands, 14–16 March 2017. Paper number: SPE-184613-MS. [Google Scholar] [CrossRef]
- Goode, J.M. Gas and water Permeability Data for Some Common Oilwell Cements. J. Pet. Technol. 1962, 14, 851–854, Paper number: SPE-288-PA. [Google Scholar] [CrossRef]
- Nelson, E.; Guillot, D. Well Cementing, Developments in Petroleum Science; Schlumberger: Houston, TX, USA, 2006; ISBN 9780978853006. [Google Scholar]
- Lund, H.; Torsæter, M.; Munkejord, S.T. Study of thermal variations in wells during carbon dioxide injection. SPE Drill. Complet. 2016, 31, 159–165, Paper number: SPE-173864-PA. [Google Scholar] [CrossRef]
- Nassan, T.H.; Baganz, D.; Alkan, H.; Opedal, N.; Amro, M. Well integrity tests for geological CO2 storage using large-scale experimental setup. In Proceedings of the SPE Europe Energy Conference, Turin, Italy, 26–28 June 2024. Paper number: SPE-220028-MS. [Google Scholar]
- Roy, P.; Walsh, S.D.C.; Morris, J.P.; Iyer, J.; Hao, Y.; Carroll, S.; Gawel, K.; Todorovic, J.; Torsæter, M. Studying the Impact of Thermal Cycling on Wellbore Integrity during CO2 Injection. In Proceedings of the 50th U.S. Rock Mechanics/Geomechanics Symposium, Houston, TX, USA, 26–29 June 2016. Paper number: ARMA-2016-668. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).