Fast Pyrolysis of Municipal Green Waste in an Auger Reactor: Effects of Residence Time and Particle Size on the Yield and Characteristics of Produced Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Sample Preparation
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Characterization of MGW and Produced Bio-Oil
3. Results and Discussion
3.1. Characterization of MGW
3.2. Yield of Pyrolysis Products
3.2.1. Effect of Residence Time
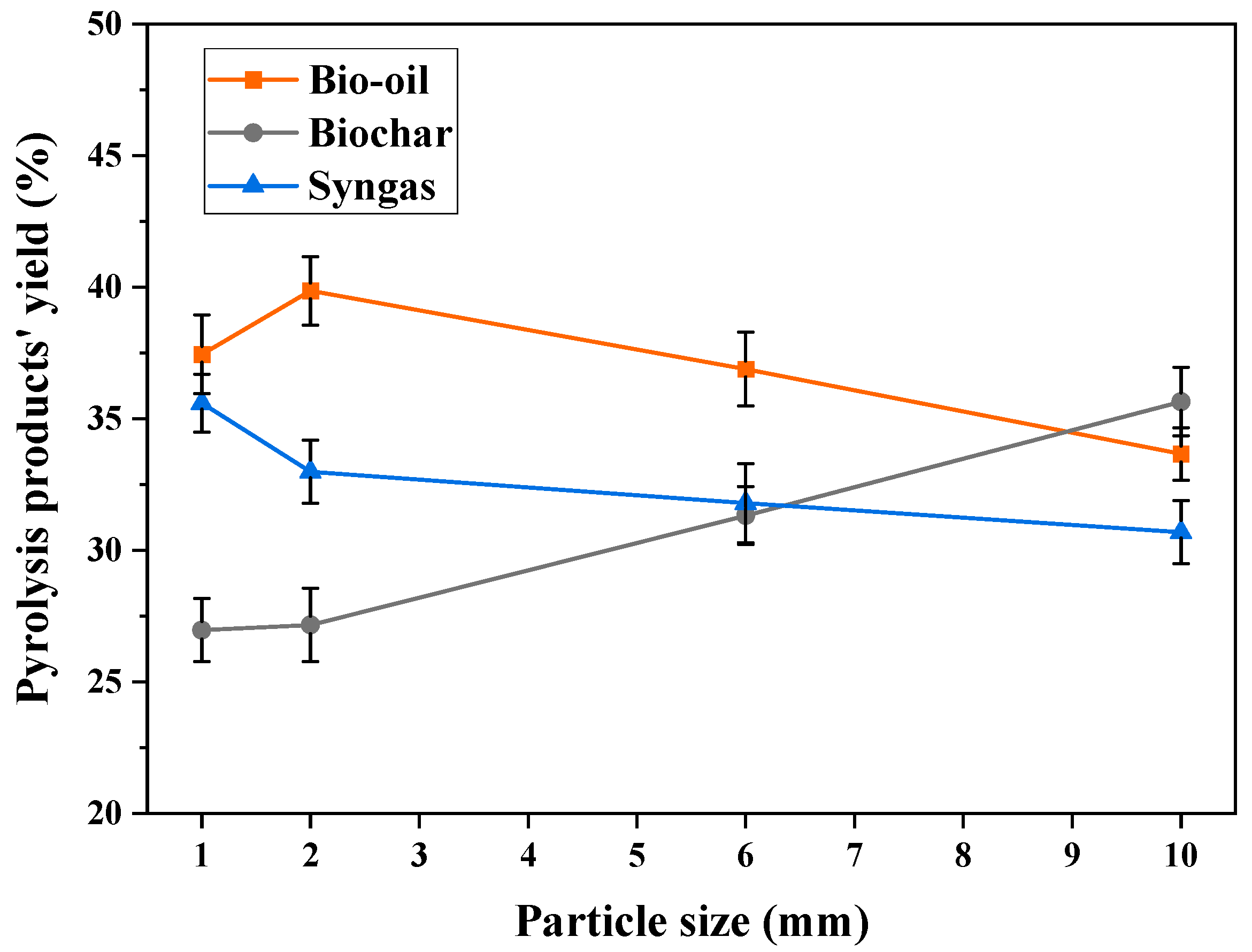
3.2.2. Effect of Particle Size
3.3. Characterization of Bio-Oil
3.3.1. FTIR Analysis
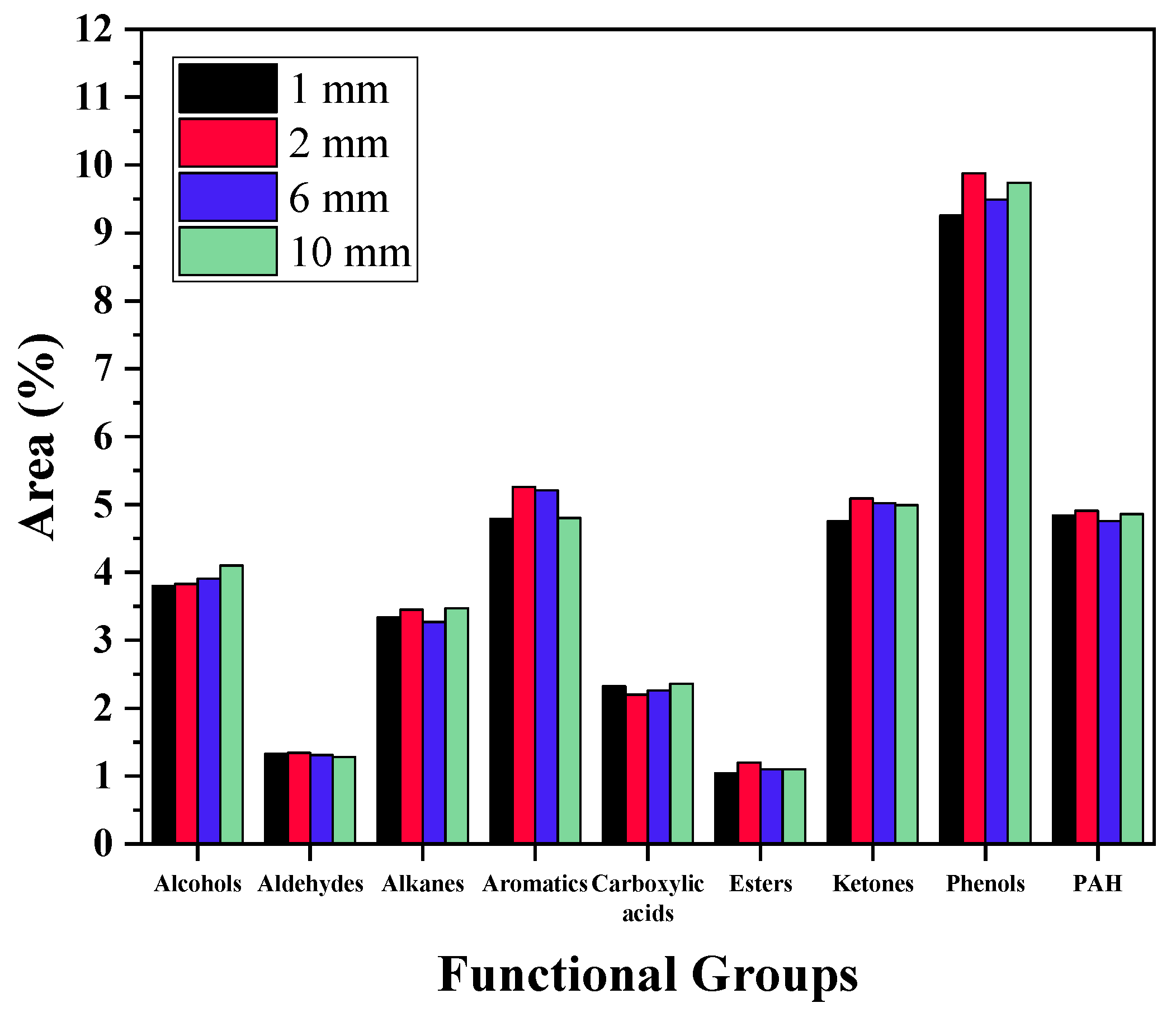
3.3.2. GC–MS Analysis
3.3.3. Physicochemical Properties Analysis
| Properties | Residence Times | Particle Sizes | ASTM Grade G [81] | ASTM Grade D [82] | Heavy Fuel Oil [83] | Light Fuel Oil [75] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 min | 2 min | 3 min | 4 min | 1 mm | 2 mm | 6 mm | 10 mm | |||||
| Kinematic viscosity @40 °C (cSt) | 13.21 | 12.78 | 12.17 | 12.51 | 12.68 | 12.17 | 12.99 | 13.54 | Maximum 125 | Maximum 125 | 180–420 | 2–4.5 |
| Density @30 °C (g/cc) | 1.17 | 1.14 | 1.13 | 1.14 | 1.17 | 1.13 | 1.19 | 1.21 | 1.1–1.3 | 1.1–1.3 | 0.99–0.995 | Maximum 0.845 |
| pH | 3.7 | 3.5 | 3.6 | 3.7 | 3.5 | 3.6 | 3.6 | 3.7 | – | – | – | – |
| Cetane number | 38 | 38 | 40 | 39 | 39 | 40 | 38 | 39 | – | – | 35–55 | 38–40 |
| Water content (wt%) | 26.43 | 27.87 | 28.72 | 29.51 | 28.97 | 28.72 | 26.34 | 25.93 | Maximum 30 | Maximum 30 | ~0 | ~0 |
| Flash point (°C) | 86 | 87 | 90 | 91 | 91 | 90 | 85 | 83 | – | – | 90–180 | 52–82 |
| Calorific value (MJ/kg) | 25.81 | 25.45 | 25.13 | 24.99 | 24.51 | 25.13 | 25.71 | 25.89 | Minimum 15 | Minimum 15 | 40.6 | 42.6 |
4. Conclusions
- MGW contained high carbon (47.32%) and lignin (24.92%) content, highlighting its suitability for pyrolysis.
- A maximum bio-oil yield of 39.86% was achieved with a residence time of 3 min and a particle size of 2 mm, optimizing the pyrolysis reaction and heat transfer.
- Phenolic compounds increased with longer residence times and larger particle sizes, while acidic compounds decreased.
- The presence of hydrocarbons like benzene (5.67% peak area) and toluene (2.45% peak area) indicates potential for fuel production.
- High acetic acid content (15.44% peak area) suggests challenges related to acidity that must be addressed for fuel applications.
- Bio-oils exhibited high viscosity and water content, necessitating upgrading for engine use but suitable for heating applications in boilers and furnaces.
- Upgrading methods, such as hydrodeoxygenation, catalytic cracking, distillation, esterification, and emulsification, are suggested to enhance bio-oil quality for broader applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velghe, I.; Carleer, R.; Yperman, J.; Schreurs, S. Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrolysis 2011, 92, 366–375. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Brown, R.J.; Senadeera, W.; Ashwath, N.; Rasul, M.G.; Rahman, M.M.; Hossain, F.M.; Moghaddam, L.; Islam, M.A.; O’Hara, I.M. Physio-chemical assessment of beauty leaf (Calophyllum inophyllum) as second-generation biodiesel feedstock. Energy Rep. 2015, 1, 204–215. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Cepic, Z.; Nakomcic-Smaragdakis, B.; Miljkovic, B.; Radovanovic, L.; Djuric, S. Combustion characteristics of wheat straw in a fixed bed. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1007–1013. [Google Scholar] [CrossRef]
- Tanoh, T.S.; Ait Oumeziane, A.; Lemonon, J.; Escudero Sanz, F.J.; Salvador, S. Green Waste/Wood Pellet Pyrolysis in a Pilot-Scale Rotary Kiln: Effect of Temperature on Product Distribution and Characteristics. Energy Fuels 2020, 34, 3336–3345. [Google Scholar] [CrossRef]
- Aysu, T.; Durak, H. Catalytic pyrolysis of liquorice (Glycyrrhiza glabra L.) in a fixed-bed reactor: Effects of pyrolysis parameters on product yields and character. J. Anal. Appl. Pyrolysis 2015, 111, 156–172. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Jahirul, M.I. Energy recovery from municipal solid waste using pyrolysis technology: A review on current status and developments. Renew. Sustain. Energy Rev. 2021, 145, 111073. [Google Scholar] [CrossRef]
- Khan, S.; Kay, L.A.N.; Qureshi, K.M.; Abnisa, F.; Wan Daud, W.M.A.; Patah, M.F.A. A review on deoxygenation of triglycerides for jet fuel range hydrocarbons. J. Anal. Appl. Pyrolysis 2019, 140, 1–24. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Pyrolysis in auger reactors for biochar and bio-oil production: A review. Biosyst. Eng. 2017, 161, 80–92. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Hasan, M.D.M.; Wang, X.S.; Mourant, D.; Gunawan, R.; Yu, C.; Hu, X.; Kadarwati, S.; Gholizadeh, M.; Wu, H.; Li, B.; et al. Grinding pyrolysis of Mallee wood: Effects of pyrolysis conditions on the yields of bio-oil and biochar. Fuel Process. Technol. 2017, 167, 215–220. [Google Scholar] [CrossRef]
- Sohaib, Q.; Habib, M.; Fawad Ali Shah, S.; Habib, U.; Ullah, S. Fast pyrolysis of locally available green waste at different residence time and temperatures. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1639–1646. [Google Scholar] [CrossRef]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Cuypers, F.; Helsen, L. Pyrolysis of chromated copper arsenate (CCA) treated wood waste at elevated pressure: Influence of particle size, heating rate, residence time, temperature and pressure. J. Anal. Appl. Pyrolysis 2011, 92, 111–122. [Google Scholar] [CrossRef]
- Newalkar, G.; Iisa, K.; D’Amico, A.D.; Sievers, C.; Agrawal, P. Effect of Temperature, Pressure, and Residence Time on Pyrolysis of Pine in an Entrained Flow Reactor. Energy Fuels 2014, 28, 5144–5157. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S. Effect of particle size on pyrolysis of single-component municipal solid waste in fixed bed reactor. Int. J. Hydrogen Energy 2010, 35, 93–97. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D. Slow pyrolysis of paulownia wood: Effects of pyrolysis parameters on product yields and bio-oil characterization. J. Anal. Appl. Pyrolysis 2015, 114, 68–78. [Google Scholar] [CrossRef]
- Varma, A.K.; Thakur, L.S.; Shankar, R.; Mondal, P. Pyrolysis of wood sawdust: Effects of process parameters on products yield and characterization of products. Waste Manag. 2019, 89, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Bennadji, H.; Smith, K.; Serapiglia, M.J.; Fisher, E.M. Effect of Particle Size on Low-Temperature Pyrolysis of Woody Biomass. Energy Fuels 2014, 28, 7527–7537. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Li, K.; Zhu, X. Two-step pyrolysis of corncob for value-added chemicals and high quality bio-oil: Effects of pyrolysis temperature and residence time. Energy Convers. Manag. 2018, 166, 260–267. [Google Scholar] [CrossRef]
- Singh, A.; Nanda, S.; Guayaquil-Sosa, J.F.; Berruti, F. Pyrolysis of Miscanthus and characterization of value-added bio-oil and biochar products. Can. J. Chem. Eng. 2021, 99 (Suppl. S1), S55–S68. [Google Scholar] [CrossRef]
- Xiong, Z.; Fang, Z.; Jiang, L.; Han, H.; He, L.; Xu, K.; Xu, J.; Su, S.; Hu, S.; Wang, Y.; et al. Comparative study of catalytic and non-catalytic steam reforming of bio-oil: Importance of pyrolysis temperature and its parent biomass particle size during bio-oil production process. Fuel 2022, 314, 122746. [Google Scholar] [CrossRef]
- Charis, G.; Danha, G.; Muzenda, E. Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia tortilis and Pine Dust. Processes 2020, 8, 551. [Google Scholar] [CrossRef]
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- Abitbol, T.; Marway, H.; Cranston, E.D. Surface modification of cellulose nanocrystals with cetyltrimethylammonium bromide. Nord. Pulp Pap. Res. J. 2014, 29, 46–57. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Ashwath, N.; Khan, M.M.K.; Jahirul, M.I. Fast pyrolysis of Beauty Leaf Fruit Husk (BLFH) in an auger reactor: Effect of temperature on the yield and physicochemical properties of BLFH oil. Renew. Energy 2022, 194, 1098–1109. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Mohanty, K. Kinetic analysis and pyrolysis behavior of low-value waste lignocellulosic biomass for its bioenergy potential using thermogravimetric analyzer. Mater. Sci. Energy Technol. 2021, 4, 136–147. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef] [PubMed]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Domenico, M.D.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting pecan nutshell pyrolysis as a source of bioenergy and bio-based chemicals using multicomponent kinetic modeling, thermodynamic parameters estimation, and Py-GC/MS analysis. Renew. Sustain. Energy Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Ahmed, A.; Jeffery, D.M.; Hidayat, S.; Sukri, R.S.; Mahlia, T.M.I.; Jamil, F.; Khurrum, M.S.; Inayat, A.; Moogi, S.; et al. Pyrolysis of solid waste residues from Lemon Myrtle essential oils extraction for bio-oil production. Bioresour. Technol. 2020, 318, 123913. [Google Scholar] [CrossRef]
- Hla, S.S.; Roberts, D. Characterisation of chemical composition and energy content of green waste and municipal solid waste from Greater Brisbane, Australia. Waste Manag. 2015, 41, 12–19. [Google Scholar] [CrossRef]
- Taib, R.M.; Abdullah, N.; Aziz, N.S.M. Bio-oil derived from banana pseudo-stem via fast pyrolysis process. Biomass Bioenergy 2021, 148, 106034. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G.; Al-Ansari, T. Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers. Biorefinery 2022, 12, 669–681. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Mettler, M.S.; Vlachos, D.G.; Dauenhauer, P.J. Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ. Sci. 2012, 5, 7797–7809. [Google Scholar] [CrossRef]
- Mabrouki, J.; Abbassi, M.A.; Guedri, K.; Omri, A.; Jeguirim, M. Simulation of biofuel production via fast pyrolysis of palm oil residues. Fuel 2015, 159, 819–827. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast pyrolysis of rice husk: Product yields and compositions. Bioresour. Technol. 2007, 98, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.; Bhaskar, T.; Konarova, M. Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass. Renew. Sustain. Energy Rev. 2018, 90, 292–315. [Google Scholar] [CrossRef]
- Ogunsina, B.; Ojolo, S.; Ohunakin, O.; Oyedeji, O.; Matanmi, K.; Bamgboye, I. Potentials for generating alternative fuels from empty palm fruit bunches by pyrolysis. Proc. ICCEM 2012, 159, 185–190. [Google Scholar]
- Chen, Y.; Liang, S.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; Deng, H.; Yang, J. A cost-effective strategy for metal recovery from waste printed circuit boards via crushing pretreatment combined with pyrolysis: Effects of particle size and pyrolysis temperature. J. Clean. Prod. 2021, 280, 124505. [Google Scholar] [CrossRef]
- Martínez-Narro, G.; Hassan, S.; Phan, A.N. Chemical recycling of plastic waste for sustainable polymer manufacturing–A critical review. J. Environ. Chem. Eng. 2024, 12, 112323. [Google Scholar] [CrossRef]
- Mani, T.; Murugan, P.; Abedi, J.; Mahinpey, N. Pyrolysis of wheat straw in a thermogravimetric analyzer: Effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chem. Eng. Res. Des. 2010, 88, 952–958. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.-S.; Garcia-Perez, M.; Mourant, D.; Rhodes, M.J.; Li, C.-Z. Effects of particle size on the fast pyrolysis of oil mallee woody biomass. Fuel 2009, 88, 1810–1817. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Berrueco, C.; Fidalgo, B.; Paterson, N.; Millan, M. Influence of temperature and particle size on structural characteristics of chars from Beechwood pyrolysis. J. Anal. Appl. Pyrolysis 2018, 130, 127–134. [Google Scholar] [CrossRef]
- Arnold, S.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Slow pyrolysis of bio-oil and studies on chemical and physical properties of the resulting new bio-carbon. J. Clean. Prod. 2018, 172, 2748–2758. [Google Scholar] [CrossRef]
- Mandal, S.; Bhattacharya, T.K.; Verma, A.K.; Haydary, J. Optimization of process parameters for bio-oil synthesis from pine needles (Pinus roxburghii) using response surface methodology. Chem. Pap. 2018, 72, 603–616. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Bio-energy generation from sagwan sawdust via pyrolysis: Product distributions, characterizations and optimization using response surface methodology. Energy 2019, 170, 423–437. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Vos, M.P.; Brilman, D.W.F. Effect of process conditions on bio-oil obtained through continuous hydrothermal liquefaction of Scenedesmus sp. microalgae. J. Anal. Appl. Pyrolysis 2018, 134, 415–426. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Pyrolysis of torrefied biomass: Optimization of process parameters using response surface methodology, characterization, and comparison of properties of pyrolysis oil from raw biomass. J. Clean. Prod. 2020, 272, 122517. [Google Scholar] [CrossRef]
- Daimary, N.; Boruah, P.; Eldiehy, K.S.H.; Pegu, T.; Bardhan, P.; Bora, U.; Mandal, M.; Deka, D. Musa acuminata peel: A bioresource for bio-oil and by-product utilization as a sustainable source of renewable green catalyst for biodiesel production. Renew. Energy 2022, 187, 450–462. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.H.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- Sowmya Dhanalakshmi, C.; Madhu, P. Utilization possibilities of Albizia amara as a source of biomass energy for bio-oil in pyrolysis process. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1908–1919. [Google Scholar] [CrossRef]
- Ren, S.; Ye, X.P. Stability of crude bio-oil and its water-extracted fractions. J. Anal. Appl. Pyrolysis 2018, 132, 151–162. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.; Scott, J. Bio-oil upgrading with catalytic pyrolysis of biomass using Copper/zeolite-Nickel/zeolite and Copper-Nickel/zeolite catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef]
- Isa, K.M.; Daud, S.; Hamidin, N.; Ismail, K.; Saad, S.A.; Kasim, F.H. Thermogravimetric analysis and the optimisation of bio-oil yield from fixed-bed pyrolysis of rice husk using response surface methodology (RSM). Ind. Crops Prod. 2011, 33, 481–487. [Google Scholar] [CrossRef]
- Hilten, R.N.; Speir, R.A.; Kastner, J.R.; Mani, S.; Das, K.C. Effect of Torrefaction on Bio-oil Upgrading over HZSM-5. Part 1: Product Yield, Product Quality, and Catalyst Effectiveness for Benzene, Toluene, Ethylbenzene, and Xylene Production. Energy Fuels 2013, 27, 830–843. [Google Scholar] [CrossRef]
- Machado, H.; Cristino, A.F.; Orišková, S.; Galhano dos Santos, R. Bio-Oil: The Next-Generation Source of Chemicals. Reactions 2022, 3, 118–137. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Ansari, F.A. Development of environmentally benign corrosion inhibitors for organic acid environments for oil-gas industry. J. Mol. Liq. 2021, 329, 115514. [Google Scholar] [CrossRef]
- Kabbour, M.; Luque, R. Chapter 10—Furfural as a Platform Chemical: From Production to Applications. In Biomass, Biofuels, Biochemicals; Saravanamurugan, S., Pandey, A., Li, H., Riisager, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–297. [Google Scholar]
- Yang, H.; de Wild, P.; Lahive, C.W.; Wang, Z.; Deuss, P.J.; Heeres, H.J. Experimental studies on a combined pyrolysis/staged condensation/hydrotreatment approach to obtain biofuels and biobased chemicals. Fuel Process. Technol. 2022, 228, 107160. [Google Scholar] [CrossRef]
- Ahmad, R.; Hamidin, N.; Ali, U.; Abidin, C. Characterization of bio-oil from palm kernel shell pyrolysis. J. Mech. Eng. Sci. 2014, 7, 1134–1140. [Google Scholar] [CrossRef]
- Williams, P.T.; Horne, P.A. Analysis of aromatic hydrocarbons in pyrolytic oil derived from biomass. J. Anal. Appl. Pyrolysis 1995, 31, 15–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Thring, R.W.; Katikaneni, S.P.R.; Bakhshi, N.N. The production of gasoline range hydrocarbons from Alcell® lignin using HZSM-5 catalyst. Fuel Process. Technol. 2000, 62, 17–30. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Uzoejinwa, B.B.; Zheng, A.; Wang, Q.; Huang, J.; Abomohra, A.E.-F. A state-of-the-art review on dual purpose seaweeds utilization for wastewater treatment and crude bio-oil production. Energy Convers. Manag. 2020, 222, 113253. [Google Scholar] [CrossRef]
- Salehi, E.; Abedi, J.; Harding, T. Bio-oil from Sawdust: Effect of Operating Parameters on the Yield and Quality of Pyrolysis Products. Energy Fuels 2011, 25, 4145–4154. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Z.; Li, S.; Zhu, X. Coupling effect of condensing temperature and residence time on bio-oil component enrichment during the condensation of biomass pyrolysis vapors. Fuel 2020, 274, 117861. [Google Scholar] [CrossRef]
- Liu, s.; Zhao, H.; Fan, T.; Zhou, J.; Liu, X.; Li, Y.; Zhao, G.; Wang, Y.; Zeng, M. Investigation on chemical structure and hydrocarbon generation potential of lignite in the different pretreatment process. Fuel 2021, 291, 120205. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Kay Lup, A.N.; Khan, S.; Abnisa, F.; Wan Daud, W.M.A. Optimization of palm shell pyrolysis parameters in helical screw fluidized bed reactor: Effect of particle size, pyrolysis time and vapor residence time. Clean. Eng. Technol. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Zhou, X.; Moghaddam, T.B.; Chen, M.; Wu, S.; Zhang, Y.; Zhang, X.; Adhikari, S.; Zhang, X. Effects of pyrolysis parameters on physicochemical properties of biochar and bio-oil and application in asphalt. Sci. Total Environ. 2021, 780, 146448. [Google Scholar] [CrossRef]
- DeSisto, W.J.; Hill, N.; Beis, S.H.; Mukkamala, S.; Joseph, J.; Baker, C.; Ong, T.-H.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G.; et al. Fast Pyrolysis of Pine Sawdust in a Fluidized-Bed Reactor. Energy Fuels 2010, 24, 2642–2651. [Google Scholar] [CrossRef]
- Abdullahi, N.; Sulaiman, F.; Safana, A.A. Bio-oil and biochar derived from the pyrolysis of palm kernel shell for briquette. Sains Malays. 2017, 46, 2441–2445. [Google Scholar] [CrossRef]
- Iáñez-Rodríguez, I.; Martín-Lara, M.A.; Blázquez, G.; Calero, M. Effect of different pre-treatments and addition of plastic on the properties of bio-oil obtained by pyrolysis of greenhouse crop residue. J. Anal. Appl. Pyrolysis 2021, 153, 104977. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Onay, O. Influence of pyrolysis temperature and heating rate on the production of bio-oil and char from safflower seed by pyrolysis, using a well-swept fixed-bed reactor. Fuel Process. Technol. 2007, 88, 523–531. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Sukri, R.S.; Hussain, M.; Farooq, A.; Moogi, S.; Park, Y.-K. Sawdust pyrolysis from the furniture industry in an auger pyrolysis reactor system for biochar and bio-oil production. Energy Convers. Manag. 2020, 226, 113502. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Intermediate pyrolysis of Acacia cincinnata and Acacia holosericea species for bio-oil and biochar production. Energy Convers. Manag. 2018, 176, 393–408. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.-K.; Oh, C.-H.; Park, J.-W.; Kwon, E.E. Production of bio-oil from fast pyrolysis of biomass using a pilot-scale circulating fluidized bed reactor and its characterization. J. Environ. Manag. 2019, 234, 138–144. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Lanigan, B.A.; Shuttleworth, P.; Breeden, S.W.; Wilson, A.J.; Macquarrie, D.J.; Milkowski, K.; Jones, J.; Bridgeman, T.; et al. The preparation of high-grade bio-oils through the controlled, low temperature microwave activation of wheat straw. Bioresour. Technol. 2009, 100, 6064–6068. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Neumann, J.; Binder, S.; Apfelbacher, A.; Gasson, J.R.; Ramírez García, P.; Hornung, A. Production and characterization of a new quality pyrolysis oil, char and syngas from digestate—Introducing the thermo-catalytic reforming process. J. Anal. Appl. Pyrolysis 2015, 113, 137–142. [Google Scholar] [CrossRef]
- Kontoulis, P.; Kazangas, D.; Doss, T.P.; Kaiktsis, L. Development and CFD Validation of an Integrated Model for Marine Heavy Fuel Oil Thermophysical Properties. J. Energy Eng. 2018, 144, 04018059. [Google Scholar] [CrossRef]
- Shafaghat, H.; Kim, J.M.; Lee, I.-G.; Jae, J.; Jung, S.-C.; Park, Y.-K. Catalytic hydrodeoxygenation of crude bio-oil in supercritical methanol using supported nickel catalysts. Renew. Energy 2019, 144, 159–166. [Google Scholar] [CrossRef]
- Ibarra, Á.; Hita, I.; Arandes, J.M.; Bilbao, J. Influence of the Composition of Raw Bio-Oils on Their Valorization in Fluid Catalytic Cracking Conditions. Energy Fuels 2019, 33, 7458–7465. [Google Scholar] [CrossRef]
- de Castro, D.A.R.; da Silva Ribeiro, H.J.; Ferreira, C.C.; de Andrade Cordeiro, M.; Guerreiro, L.H.H.; Pereira, A.M.; dos Santos, W.; Santos, M.C.; de Carvalho, F.B.; Jose, O.C.S., Jr. Fractional Distillation of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea) Seeds. In Fractionation; InTechOpen: London, UK, 2019; p. 61. [Google Scholar]
- Sondakh, R.C.; Hambali, E.; Indrasti, N.S. Improving characteristic of bio-oil by esterification method. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012071. [Google Scholar] [CrossRef]
- Jiang, X.; Ellis, N. Upgrading Bio-oil through Emulsification with Biodiesel: Mixture Production. Energy Fuels 2010, 24, 1358–1364. [Google Scholar] [CrossRef]






| Analysis/Properties | ASTM Standards | Equipment |
|---|---|---|
| MGW | ||
| Proximate analysis | D3172-07a | Thermogravimetric analyzer (Mettler Toledo TGA/SDTA 851) |
| Ultimate analysis | D5373 | Vario Micro Cube CHNS analyzer |
| Higher heating value | D4809 | Oxygen-bomb calorimeter |
| Bio-oil | ||
| FTIR analysis | – | Perkin Elmer System One FTIR/ATR spectrum analyzer |
| GC-MS analysis | – | Varian CP3800 mass spectroscopy detector |
| Kinematic viscosity | D7042 | Stabinger Viscometer SVM 3000 |
| Density | D4052 | Density meter DM40 Mettler Toledo |
| pH | E70 | Omega DP24-pH meter |
| Cetane number | D613 | Ignition quality tester |
| Water content | D2709 | Centrifuge sigma |
| Flash point | D93B | Pensky Martins closed cup apparatus |
| Calorific value | D4809 | Oxygen-bomb calorimeter |
| Analysis | Property | MGW | Royal Poinciana Seed [27] | Banana Leaves [28] | Pecan Nutshell [29] |
|---|---|---|---|---|---|
| Proximate (wt%) | Moisture | 9.72 | 6.21 | 8.4 | 3.32 |
| Volatile matter | 69.57 | 73.15 | 73.05 | 67.93 | |
| Fixed carbon a | 19.78 | 17.7 | 7.26 | 29.69 | |
| Ash | 0.93 | 3.02 | 11.29 | 2.47 | |
| Ultimate (wt%) | Carbon | 47.32 | 52.12 | 43.28 | 49.22 |
| Hydrogen | 5.14 | 5.86 | 6.83 | 5.59 | |
| Nitrogen | 0.42 | 5.1 | 1.28 | 0.65 | |
| Oxygen a | 47.06 | 36.42 | 48.31 | 41.92 | |
| Sulphur | 0.06 | 0.5 | 0.3 | 0.14 | |
| Biochemical (wt%) | Cellulose | 37.53 | 27 | 43.34 | 14.99 |
| Hemicellulose a | 22.34 | 44.21 | 34.34 | 27.59 | |
| Lignin | 24.92 | 12 | 15 | 48.37 | |
| Extractives | 15.21 | 16.32 | 7.32 | 9.05 | |
| HHV (MJ/kg) | 18.24 | 20.52 | 17.8 | 19.39 |
| Chemical Compound | Molecular Formula | Retention Time (min) | Peak Area (%)—Residence Times | Peak Area (%)—Particle Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 min | 2 min | 3 min | 4 min | 1 mm | 2 mm | 6 mm | 10 mm | |||
| Alcohols | ||||||||||
| propan-1-ol | C3H8O | 7.15 | 0.91 | 0.93 | 0.99 | 0.89 | 0.97 | 0.99 | 1.01 | 1.05 |
| 1-tetradecanol | C14H30O | 34.29 | 1.07 | 1.05 | 1.23 | 1.11 | 1.29 | 1.23 | 1.19 | 1.21 |
| 1-heptadecanol | C17H36O | 40.99 | 1.63 | 1.75 | 1.61 | 1.69 | 1.54 | 1.61 | 1.71 | 1.84 |
| Aldehydes | ||||||||||
| 3-hydroxypropanal | C3H6O2 | 14.75 | 0.59 | 0.61 | 0.63 | 0.59 | 0.64 | 0.63 | 0.61 | 0.59 |
| succinaldehyde | C4H6O2 | 18.13 | 0.74 | 0.72 | 0.71 | 0.81 | 0.69 | 0.71 | 0.7 | 0.69 |
| Alkanes | ||||||||||
| cyclopropane | C3H6 | 5.44 | 0.54 | 0.56 | 0.51 | 0.57 | 0.49 | 0.51 | 0.49 | 0.56 |
| undecane | C11H24 | 16.61 | 0.74 | 0.69 | 0.75 | 0.67 | 0.71 | 0.75 | 0.77 | 0.87 |
| dodecane | C12H26 | 23.92 | 0.81 | 0.84 | 0.79 | 0.91 | 0.77 | 0.79 | 0.83 | 0.82 |
| tetradecane | C14H30 | 29.03 | 0.59 | 0.57 | 0.67 | 0.54 | 0.61 | 0.67 | 0.52 | 0.54 |
| pentadecane | C15H32 | 34.59 | 0.39 | 0.36 | 0.42 | 0.31 | 0.47 | 0.42 | 0.37 | 0.41 |
| hexadecane | C16H34 | 39.65 | 0.21 | 0.11 | 0.31 | 0.23 | 0.29 | 0.31 | 0.29 | 0.27 |
| Aromatics | ||||||||||
| benzene | C6H6 | 6.57 | 1.75 | 1.77 | 1.89 | 1.76 | 1.71 | 1.89 | 1.77 | 1.78 |
| pyridine | C5H5N | 9.12 | 0.73 | 0.69 | 0.76 | 0.67 | 0.72 | 0.76 | 0.73 | 0.69 |
| pyrrole | C4H5N | 11.35 | 0.43 | 0.51 | 0.39 | 0.51 | 0.33 | 0.39 | 0.37 | 0.41 |
| toluene | C7H8 | 14.21 | 1.07 | 1.01 | 1.11 | 0.99 | 1.01 | 1.11 | 1.15 | 0.91 |
| ethylbenzene | C8H10 | 18.34 | 0.32 | 0.33 | 0.41 | 0.24 | 0.42 | 0.41 | 0.39 | 0.32 |
| benzofuran | C8H6O | 23.41 | 0.21 | 0.19 | 0.23 | 0.11 | 0.11 | 0.23 | 0.29 | 0.24 |
| Carboxylic Acids | ||||||||||
| 1-allyl cyclopropane carboxylic acid | C7H10O2 | 18.72 | 0.51 | 0.52 | 0.49 | 0.53 | 0.51 | 0.49 | 0.52 | 0.5 |
| 14-pentadecynoic acid, methyl ester | C17H34O2 | 42.67 | 0.84 | 0.81 | 0.77 | 0.79 | 0.78 | 0.77 | 0.81 | 0.84 |
| hexadecenoic acid, methyl ester | C17H34O2 | 44.98 | 0.54 | 0.51 | 0.53 | 0.52 | 0.61 | 0.53 | 0.56 | 0.49 |
| octadecanoic acid, methyl ester | C19H38O2 | 48.41 | 0.45 | 0.47 | 0.41 | 0.46 | 0.42 | 0.41 | 0.37 | 0.53 |
| Esters | ||||||||||
| ethyl acetate | C4H8O2 | 9.49 | 0.54 | 0.51 | 0.59 | 0.55 | 0.47 | 0.59 | 0.57 | 0.61 |
| 2-oxopropyl acetate | C5H8O3 | 16.77 | 0.59 | 0.49 | 0.61 | 0.58 | 0.57 | 0.61 | 0.53 | 0.49 |
| Ketones | ||||||||||
| 3-Hexanone | C6H12O | 8.43 | 0.65 | 0.63 | 0.66 | 0.61 | 0.61 | 0.66 | 0.65 | 0.67 |
| ethanone,1-(2-furanyl) | C6H6O2 | 8.81 | 1.01 | 1.12 | 1.23 | 1.14 | 1.19 | 1.23 | 1.21 | 1.17 |
| cyclopentanone,2-methyl | C6H10O | 11.53 | 1.27 | 1.29 | 1.3 | 1.25 | 1.23 | 1.3 | 1.29 | 1.27 |
| 2-cyclopenten-1-one,3-methyl | C6H8O | 12.99 | 0.99 | 1.01 | 1.11 | 1.05 | 1.01 | 1.11 | 1.09 | 1.12 |
| 2-cyclopenten-1-one,2-methyl | C6H8O | 14.19 | 0.74 | 0.77 | 0.79 | 0.67 | 0.71 | 0.79 | 0.78 | 0.76 |
| Phenols | ||||||||||
| phenol | C6H6O | 17.12 | 1.45 | 1.41 | 1.47 | 1.39 | 1.42 | 1.47 | 1.39 | 1.47 |
| phenol, 2-methyl | C7H8O | 20.77 | 1.2 | 1.23 | 1.21 | 1.27 | 1.17 | 1.21 | 1.14 | 1.19 |
| phenol, 2,3-dimethyl | C8H10O | 22.79 | 1.67 | 1.69 | 1.76 | 1.75 | 1.56 | 1.76 | 1.78 | 1.79 |
| 2-methoxy-5-methyl phenol | C8H10O2 | 23.56 | 1.73 | 1.74 | 1.71 | 1.72 | 1.65 | 1.71 | 1.45 | 1.67 |
| phenol, 4-ethyl-2-methoxy | C9H12O2 | 28.91 | 0.97 | 0.94 | 0.99 | 1.01 | 1.01 | 0.99 | 0.89 | 0.92 |
| phenol,2-methoxy-4-propyl | C10H14O2 | 36.45 | 1.35 | 1.29 | 1.32 | 1.29 | 1.11 | 1.32 | 1.43 | 1.31 |
| 3-phenyl-5-t-butylpyridazine | C14H16N2 | 38.69 | 1.35 | 1.37 | 1.42 | 1.32 | 1.34 | 1.42 | 1.41 | 1.39 |
| Polycyclic Aromatic Hydrocarbons (PAH) | ||||||||||
| naphthalene | C10H8 | 27.13 | 0.97 | 0.99 | 1.05 | 0.91 | 1.01 | 1.05 | 1.02 | 0.99 |
| 2-methylnaphthalene | C11H10 | 29.42 | 0.69 | 0.67 | 0.71 | 0.84 | 0.69 | 0.71 | 0.77 | 0.73 |
| biphenyl | C12H10 | 31.63 | 0.45 | 0.49 | 0.47 | 0.48 | 0.49 | 0.47 | 0.51 | 0.45 |
| 1-methylnaphthalene | C11H10 | 33.51 | 0.48 | 0.53 | 0.45 | 0.51 | 0.51 | 0.45 | 0.43 | 0.56 |
| acenaphthene | C12H10 | 37.81 | 0.42 | 0.41 | 0.51 | 0.49 | 0.49 | 0.51 | 0.49 | 0.51 |
| fluorene | C13H10 | 39.97 | 0.97 | 1.02 | 0.97 | 0.93 | 0.99 | 0.97 | 0.91 | 0.87 |
| anthracene | C14H10 | 41.67 | 0.84 | 0.81 | 0.79 | 0.81 | 0.84 | 0.79 | 0.81 | 0.79 |
| pyrene | C16H10 | 43.78 | 0.35 | 0.41 | 0.43 | 0.39 | 0.31 | 0.43 | 0.33 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Rasul, M.G.; Jahirul, M.I.; Khan, M.M.K. Fast Pyrolysis of Municipal Green Waste in an Auger Reactor: Effects of Residence Time and Particle Size on the Yield and Characteristics of Produced Oil. Energies 2024, 17, 2914. https://doi.org/10.3390/en17122914
Hasan MM, Rasul MG, Jahirul MI, Khan MMK. Fast Pyrolysis of Municipal Green Waste in an Auger Reactor: Effects of Residence Time and Particle Size on the Yield and Characteristics of Produced Oil. Energies. 2024; 17(12):2914. https://doi.org/10.3390/en17122914
Chicago/Turabian StyleHasan, M. M., M. G. Rasul, M. I. Jahirul, and M. M. K. Khan. 2024. "Fast Pyrolysis of Municipal Green Waste in an Auger Reactor: Effects of Residence Time and Particle Size on the Yield and Characteristics of Produced Oil" Energies 17, no. 12: 2914. https://doi.org/10.3390/en17122914
APA StyleHasan, M. M., Rasul, M. G., Jahirul, M. I., & Khan, M. M. K. (2024). Fast Pyrolysis of Municipal Green Waste in an Auger Reactor: Effects of Residence Time and Particle Size on the Yield and Characteristics of Produced Oil. Energies, 17(12), 2914. https://doi.org/10.3390/en17122914






