Abstract
This review presents a recent study on improving energy crops (ECs) in the EU and discusses the potential use of biostimulants to enhance CO2 sequestration processes in these plants. The novelty of this study lies in demonstrating alternative directions for improving productivity and increasing plant yield without relying on introducing new hybrids (GM) or using advanced agrotechnology. There is a great deal of discussion about using energy crops for direct combustion or biogas production. However, there is a paucity of information regarding the use of biostimulants and their role in increasing the yield of energy crops, particularly in terms of CO2 compensation. In CO2 sequestration, increasing the intensity of the photosynthetic process is considered crucial for the more efficient growth of energy crops. Traditionally, fertilization aimed at improving photosynthesis results in a large amount of alkaline elements, which can cause negative effects in boilers. This paper describes the use of amino acid biostimulants extracted from industrial waste, either chemically or biotechnologically, and their different forms of application. It addresses the current challenges and benefits of using biostimulants in energy crops to increase photosynthesis without the use of genetic engineering tools in plants.
1. Energy Crops and Their Use in Biomass Energy
In order to combat climate change, it is necessary to reduce the concentration of greenhouse gases in the atmosphere, with a particular focus on CO2. The European Parliament has enacted a European climate law that sets a new target of reducing greenhouse gas emissions to at least 55% by 2030 (up from the previously planned 40%) and establishes the achievement of climate neutrality by 2050 as a legally binding target [1]. One of the most effective strategies for achieving this goal is the replacement of traditional transport fuels with biofuels and coal with biomass [2]. In 2021, renewable energy sources accounted for 21.8% of the EU’s gross final energy consumption. In 2023, the co-legislators increased the EU’s 2030 renewable energy target to 42.5%, with an additional 2.5% target to reach the 45% target [3]. One of the primary objectives of the European Union’s energy production and environmental policy is to increase the proportion of renewable fuels in the Community’s energy balance. In accordance with developments in the energy market, biomass production has the potential to become a significant contributor to the achievement of these goals. The straightforward combustion technology of biomass, which is readily adaptable to a multitude of traditional energy systems, and the availability of inexpensive raw materials make biomass a competitive alternative to traditional fuels for energy purposes [4].
One of the key aspects of biomass production is the cultivation of fast-growing annual and perennial energy crops through plantations. Energy crops are a group of plants from which the biomass extracted can be used to produce thermal energy, liquid, or gaseous fuel. It is, therefore, rational to establish energy crops, which have a high yield potential per unit area of land. Consequently, by utilizing a smaller area, it is possible to produce significantly higher quantities of biomass in comparison to other crops [5]. The yields of energy crops exhibit considerable variation across species. The data on these crops’ yields that is available in the literature is typically presented on a country- or geographical-area basis [6,7]. Among the most popular plants used for energy purposes are perennial plants (perennial grasses, woody species, and perennial herbaceous crops) and annual plants (oilseeds and annual herbaceous crops) [8]. The most commonly cultivated perennial lignocellulosic plants for energy purposes are willow (Salix viminalis L.), poplar (Populus sp.), and false acacia (Robinia pseudoacacia L.). Perennial grass species are gaining increasing popularity, mainly miscanthus (Miscanthus sp.) as well as prairie cordgrass (Spartina pectinata Bosc ex Link), reed canary grass (Phalaris arundinacea L.), big bluestem (Andropogon gerardii Vitman), and switchgrass (Panicum virgatum L.). Also, perennial dicotyledons such as virginia mallow (Sida hermaphrodita L. Rusby) and jerusalem artichoke (Helianthus tuberosus L.) are classified as plants used for energy purposes [9,10]. The way and intensity of management of energy crop plantations have a significant impact on biodiversity, as well as on water and soil quality and CO2 emissions [11].
In recent years, different varieties of Miscanthus sp. have become a very popular energy crop. Its popularity is primarily due to its high biomass yield and very low habitat requirements. In addition to its widespread use for biofuel production, research indicates that miscanthus has greater potential for mitigating CO2 emissions than Zea mays (maize). It has been shown that the photosynthetic rate for Miscanthus, a member of the C4 plant family, is about 20 mmol CO2 m2s−1. This photosynthetic efficiency provides a much higher plant growth rate for C4 plants (50 g m2 per day) compared to C3 plants (30–40 g m2 per day) [12].
The majority of energy crops cultivated for the bioenergy sector are produced through conventional agricultural techniques. The selection of biomass (i.e., woody or herbaceous species) for energy production is dependent on the intended end use of the bioconversion process, such as combustion, gasification, pyrolysis, fermentation, or mechanical oil extraction. Any plant species can be used as a raw material for the production of bioenergy, but in practice, only plants that meet the requirements for a good energy raw material are grown. Among the parameters for these requirements are the following: high yield, efficient solar capture, water use efficiency, nutrient use efficiency, pest resistance, perennial growth habit, nutrient cycling, amenability to existing farm equipment, non-invasiveness, and feedstock quality [13]. Due to the nature of plant cultivation, annual crops are linked to biofuels, while perennial crops are linked to the area of electricity and heat [14]. It is of significant importance that these plants are typically designed to maximize the energy yield per hectare at the lowest possible cost [15]. It is of great significance that how crops are cultivated can augment not only their costs but also their greenhouse gas emissions. In general, the management of energy crops should prioritize maintaining a proper energy balance. This is particularly relevant to the drought tolerance of plants, the efficient use of water, increasing resistance to pests and diseases, minimizing changes in the use of agricultural machinery, and improving the quality of the raw material, which will be described in detail in the following sections.
The selected properties of the material become particularly important depending on the planned energy conversion process and how the resulting plant material is processed. In the context of subsequent processing, the main material properties of importance are related to moisture content (intrinsic and extrinsic), calorific (heating) value, proportions of fixed carbon and volatiles, ash/residue content, alkali metal content, and cellulose/lignin ratio [16]. A comparison of selected physicochemical parameters for several common energy crops is presented in Table 1. If phytomass is to be utilized for energy purposes, it is essential to determine its calorific value. The calorific value of selected energy plants, given in Table 1, is approximately 19 MJ kg−1. For comparison, the value of brown coal is estimated at 17.00 to 24.00 MJ kg−1 [17,18]. In comparison to other biomass groups, energy crops exhibit a higher heat of combustion. When utilized in methane fermentation technologies, they result in enhanced biogas production efficiency and a greater proportion of methane in biogas.

Table 1.
Several parameters characterizing selected energy plants, based on our own study [19,20,21,22,23,24,25,26].
It is important if the cultivation of certain energy crops shows a certain versatility, as the resulting plants can be used to produce several energy products. An advantageous feature of many energy crops is that they can be used to produce several types of energy products. Examples include hemp, which can be used to produce both oil and solid biomass, and cereals, which can be used to produce ethanol in addition to straw biomass [8].
The literature provides data on the application of the LCA method to estimate the environmental impact of biomass grown for energy purposes. The focus of the research is on analyzing different plantation management options to quantify the differences in carbon emissions and thus determine the environmental impact. To illustrate, the Stand of Poplar Trees study analyzed the effects of fertilization (inorganic or organic), agricultural activities, machinery, and field emissions. Calculations were conducted using specialized software for data collected over several decades of experiments. The results show that fertilizing the soil with compost reduces CO2 emissions and that the application of organic manure has a very significant effect on reducing emissions of this gas [26]. In contrast, research conducted on miscanthus proved that the energy balance of its cultivation was more favorable in the variant without mineral fertilizers. In the case of the control trial (without fertilizer), the highest energy inputs were generated by diesel fuel consumption and the use of herbicides. In the case of crops cultivated with mineral fertilizers, it was found that the energy balance of the crop is strongly influenced by the production of fertilizers and the fuel consumption associated with their application. The use of mineral fertilizers in Miscanthus cultivation is not justified by the yield of biomass obtained, but it was found to hurt the energy balance of the crop [27].
When analyzing the factors influencing greenhouse gas emissions during the cultivation of energy crops, the most significant factor is the management of fertilizer use [28]. For typical crops, the use of mineral fertilizers is characteristic. However, the production of these fertilizers requires a considerable amount of energy, and they also have adverse effects on the environment. For example, they can run off into groundwater or volatilize into the atmosphere. As an alternative to mineral fertilizers, fertilizers that are a natural source of carbon, including biostimulants, can also be used for energy crops.
2. The Potential for Carbon Dioxide (CO2) Sequestration and the Utilization of Energy Crops
2.1. Current CO2 Sequestration Strategies
The phenomenon of population growth, particularly in Africa and Asia, the industrialization and globalization that have occurred over the past 150 years, and the resulting significantly increased demand for energy have caused carbon dioxide emissions into the atmosphere to increase to a large extent [29]. Although carbon dioxide is a major component of photosynthesis, its increased emissions have a negative impact on accelerating climate change due to the release of greenhouse gases into the atmosphere. In fact, CO2 accounts for as much as 68% of their total emissions [30]. Moreover, the increase in CO2 release from the latter part of the 20th century and into the 21st century was as much as 50% [31]. Consequently, it is imperative to pursue the development of alternative energy sources that diminish reliance on fossil fuels and CO2 emissions. Additionally, it is crucial to devise technologies that reduce the concentration of CO2 in the atmosphere, including methods such as sequestration and conversion into forms that do not exacerbate global warming. The number of strategies, also referred to as methods, is vast (Figure 1). A classification that is both simultaneous and consistent is a challenging process due to the complexity of the subject matter. For the purposes of this article, the authors have identified three main categories: engineered sequestration, biological sequestration, and non-biological sequestration [32,33].
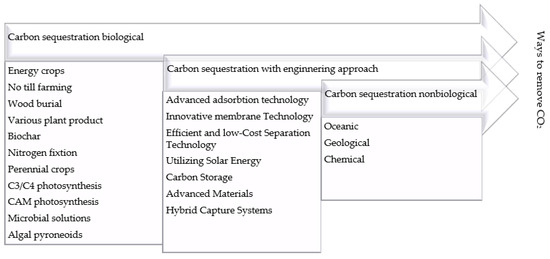
Figure 1.
Methods of carbon sequestration—own study based on [34,35,36,37,38].
These major methods can be further subdivided into smaller strategies, as shown in Figure 1, which include the following:
- Biological sequestration occurs through capture in plants that use CO2 in photosynthesis.
- Marine sequestration by injecting CO2 in gaseous form into the oceans.
- Mineral sequestration by binding CO2 in natural mineral resources.
- Geological sequestration, by capturing CO2 that is a by-product of industrial production and storing it in underground geological formations.
In addition, we can divide the different types of sequestration into direct, indirect, and advanced sequestration as follows:
- Direct sequestration is the process of capturing CO2 before it is released into the atmosphere, followed by its storage.
- Indirect sequestration is the process of removing CO2 by using plants to convert it to oxygen through photosynthesis.
- Advanced methods include mineral sequestration [34].
In the context of direct sequestration, we can describe the various CCS technologies currently in use, the most important of which are listed as follows [35]:
- Absorption—absorption of CO2 using chemical compounds such as amines, ammonia, ionic liquids, or physical absorption using gas removal processes such as Selexol, Rectisol, Purisoll.
- Adsorption—absorption of CO2 using adsorbent beds such as carbon, silica, zeolites, organometallic structures, polymers, or regenerative cycles, such as PSA, TSA, steam, moisture.
- Membrane—involving the use of permeable membranes to separate CO2 from gas mixtures such as flue gas from power plants or industrial processes.
- Cryogenic—where CO2 is frozen and stored as a liquid product.
- Microalgae—involving the use of certain types of algae that naturally contribute to carbon sequestration through photosynthesis.
- Chemical loops—involving the burning of fossil fuels in a process known as chemical loop combustion (CLC), so that the only products of the process are water vapor and CO2, which can be stored or disposed of after drying.
In recent years, there has been a lot of interest in biosequestration, or in ways to intensify it. It seems important to describe this type of sequestration in terms of energy crops. They can provide a method of reducing CO2 emissions by storing carbon in the soil, and the use of these plants in the fight against climate warming [36,37,38].
2.2. Utilization of Energy Crops in Sequestration Processes
In recent years, an increasing number of research teams have been investigating the use of biological methods for CO2 removal. The principal advantage of this process over other sequestration methods is its low cost and low environmental impact [39].
One method of biological sequestration is the use of energy crops for this purpose. Discussions on the necessity of using biomass resources (forest and agricultural) to reduce greenhouse gases have been ongoing since the 1990s. One concept that has received considerable attention is the use of bioenergy plants in CO2 sequestration processes. This is due to their energy potential, ability to produce large amounts of biomass, and low soil requirements. As early as 2005, analyses were conducted, which demonstrated that 60 million hectares of land in the United States and 757 million hectares worldwide were available for the cultivation of energy crops. This would result in a potential CO2 sequestration of 318 million tons in the United States and 1631 million tons worldwide. Poplars, willows, and mesquite have been identified as potential species for this purpose [40]. In recent years, the majority of studies have indicated that the potential for carbon dioxide (CO2) sequestration in both cropland and grassland is approximately 4–5 gigatonnes of CO2 per year. The development of new technologies could potentially double this figure to 8 GtCO2/year. The challenge, however, is to implement such technologies within the next 20–30 years, as only then will the effects of the soil sequestration process be maximized [41]. Energy crops also have the advantage that, as an energy source, they are almost neutral with regard to CO2 emissions. The available data indicate that for every 0.6 kg of CO2 produced as a result of the cultivation of such plants, there is 1 kg of CO2 produced in the form of biomass [42]. The issue is not the cultivation of energy crops per se but rather the introduction of changes to reduce CO2 emissions, which may be caused by, for example, the addition of mineral fertilizers.
The utilization of energy crops is contingent upon the geographical region and prevailing climatic conditions. In Europe, for instance, giant miscanthus is a particularly popular species, largely due to its low input requirements and high yields [43]. Furthermore, miscanthus exhibits the advantage of sequestration potential due to its capacity for cultivation in regions with colder climates [44].
Another species of energy plant with a high sequestration potential is the willow wattle. It displays high shoot regeneration capabilities and adaptability to climatic and soil conditions in a given region. For this reason, for example, in the United Kingdom, it is considered the energy crop with the highest potential for sequestration over its lifetime of 15 to 30 years [45].
In contrast, research conducted by scientists in China has demonstrated that, in addition to miscanthus or willow, other perennial crops, such as switchgrass, also possess the potential to sequester CO2. The planting of miscanthus on marginal agricultural soils can result in a 50% increase in soil carbon content [46].
In contrast, research conducted by a team from the University of Illinois has demonstrated that the combination of different energy crops in a single area can also result in significant CO2 sequestration for perennial crops. Cultivated miscanthus, switchgrass, and plants specific to the Illinois prairie resulted in increased carbon allocation (by 400%), biomass (from 400 to 750%), and root respiration (up to 2500%) [47].
Additionally, poplar is a widely cultivated energy crop in South Asian regions. In a study conducted by Chavan et al., 79 trees from five different plantings were felled in order to estimate the biomass and carbon sequestration values. The results demonstrated that both dry biomass production and sequestration exhibited considerable variation between the different plantings, with differences of up to two and three times. These findings demonstrate that regional conditions and planting methods for energy crops can influence the outcomes observed [48].
Additionally, Chavan and colleagues conducted an analysis of the utilization of various species of energy crops in agroforestry, including, among others, eucalyptus and poplar, which are highly prevalent in the Indo-Gangetic plains. The findings of their study indicated that both species exhibited potential for carbon sequestration, with eucalyptus demonstrating a sequestration rate of 237.2 Mg C ha−1 and poplar exhibiting a sequestration rate of 212.7 Mg C ha−1. Consequently, the net carbon sequestration rate was found to be 12.7 Mg C ha−1 yr−1 for eucalyptus and 10.3 Mg C ha−1 yr−1 for poplar [49].
It is also important to consider the role that properly carried out decommissioning of an energy crop plantation will play. The intensive use of energy plantations and the export of aboveground biomass can result in the withdrawal of a portion of the biogenic pool from the biological cycle. In order to facilitate the aforementioned process, it is necessary to remove (uproot) the root system from the soil or shred the roots into fragments that will not regrow. The preferable solution is to shred the roots and leave them in the soil, as this allows for the possibility of compensating for the depleted nutrient pool by biomass extraction. Furthermore, it is advisable to consider the cultivation of multiple species of plants, which has the beneficial effect of increasing the rate of sequestration [50].
The potential for the use of energy crops in the intensification of biosequestration is considerable. However, the manner in which they are cultivated and the increase in the amount of plant biomass, which may be achieved without an increase in CO2 emissions, are also of significance.
3. The Various Methods Used to Increase the Productivity of Established Energy Crops
The utilization of biomass as a source of energy derived from waste materials and plant and animal remains has long been a known possibility. As global demand continues to increase year on year, this type of renewable energy is likely to be further developed in the future [51]. However, the efficiency of energy obtained from biomass is contingent upon the conversion of the biomass into heat, fuel, and electricity via thermochemical and biochemical methods (Figure 2) [52]. The potential for converting biomass into energy is contingent upon several physicochemical parameters intrinsic to the biomass, collectively delineating its quality. These include factors such as moisture content, calorific value, plant age, size, and the presence of contaminants.
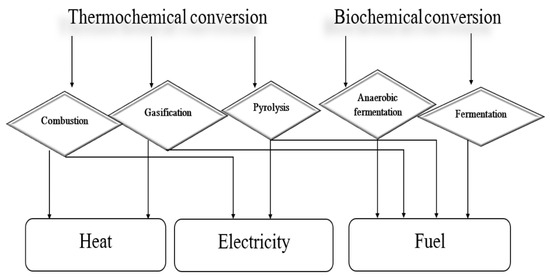
Figure 2.
The methods of biomass conversion, based on our own study based on [51].
In the field of plant production for energy crops, it is essential to assess the quality parameters of biomass. To achieve an optimal energy profit, it is first necessary to ascertain an appropriate biomass productivity (Table 2). This is largely determined by the distribution of biomass to the organs that constitute the average yield of the selected energy plants [52].

Table 2.
Energy profit and the quantity of biomass, own study based on [45,53,54,55,56,57].
The process of biomass formation is the result of the plant’s ability to capture the energy of solar radiation, subsequently converting it into chemical energy stored within organic compounds. It can be reasonably asserted that biomass, in its various forms, is an energy material utilized by the vast majority of living organisms on Earth, with humans being no exception. The biomass of crop plants (productivity of plants) is of particular importance for those engaged in the production of energy crops. It cannot be forgotten that the efficiency of biomass production depends on the photosynthesis process. It can be observed that biomass exhibits a relatively lower energy efficiency compared to photovoltaics or wind energy, which is attributed to its low density. Accordingly, the key to increasing the use of biomass as an energy source appears to lie in the development of methods to enhance the net efficiency of photosynthesis. It is also crucial to consider its resistance to pests, its capacity to grow on low-quality soil, and its potential for increasing the calorific value through changes in the physicochemical properties of biomass [58,59,60].
3.1. The Interrelationship between Genetic Engineering and Photosynthesis Efficiency
In the early 21st century, the production of energy crops saw an appreciable increase; however, it is thought that the yield currently achieved represents the upper limit for varieties cultivated through traditional methods. It can be anticipated that demand for plant-based products for energy purposes will increase further. Consequently, it is necessary to identify alternative strategies to enhance plant productivity. The most advanced area of research is the utilization of genetic engineering techniques. This article presents an overview of research initiatives aimed at enhancing the efficacy of biomass production at energy facilities [61]. Genetic variability is a pervasive aspect of biological systems. Genetically modified organisms, and therefore plants with new genotypes, are and have always been part of the natural world. These organisms emerge due to the occurrence of mutations, which are natural occurrences within the context of the natural world. The expansion of scientific knowledge has led to an increased number of activities. Their objective is to intentionally integrate new characteristics through the meticulously planned hybridization of well-characterized energy crops [62]. The advent of novel techniques for enhancing the perennial energy grasses of the Miscanthus genus commenced in the 1990s and continues to the present day. Energy grasses belonging to the genus Miscanthus employ C4 photosynthesis, a process that is more efficient than C3. However, this method of converting light into chemical energy is not without its limitations. They exhibit a correlation with the amount of pyruvate-orthophosphate dikinase (PPDK) and the RuBisCO enzyme, which impairs the regeneration of phosphoenolpyruvate (PEP). It has been reported that overexpression of PPDK may lead to an improvement in the rate of C4 photosynthesis, and that increasing the expression of genes related to NPQ non-photochemical quenching may lead to an increase in the efficiency of C4 photosynthesis [63]. Another study aimed at optimizing the photosynthetic process in plants with a C3 photosynthetic system involved an attempt to introduce genes from C4 plants. Upgrading plants with additional enzymes, such as phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphodikinase (PPDK), did not result in improved light assimilation and accumulation [64]. This phenomenon was likely the result of the accumulation of products from a pathway other than C4 photosynthesis [65]. Another study has focused on concepts for research into the improvement of RuBisCo, the introduction of CO2 compaction mechanisms into crop plants, the reduction of losses due to photorespiration, and the improvement of the dark-phase regeneration step. The RuBisCO enzyme in plant cells accounts for approximately 50% of all proteins present in chloroplasts. RuBisCO is involved in the dark phase of photosynthesis, where it catalyzes the reaction of carbon dioxide attachment to ribulose-1,5-bisphosphate. The main focus of research has been on Rubisco ‘activase’, which regulates the efficient function of the above enzyme. It is found in the stroma of chloroplasts and is strongly activated by light, allowing the carboxylase to function optimally under conditions of atmospheric CO2 concentration. The mechanism of regulation of Rubisco activity represents one of the most complex issues in plant biochemistry. Studies have been conducted to induce overexpression of this factor in Miscanthus at elevated temperatures, resulting in improved photosynthetic efficiency [66]. It has been demonstrated that study at elevated temperatures is associated with a reduction in the efficiency of RuBisCO at lower temperatures. The objective of the research is to create more stable versions of RuBisCO activase enzymes from these plants using targeted mutagenesis to make changes to the protein sequence, followed by protein thermal shift assays to measure improvements in thermal tolerance [67]. Similar methodologies are employed in the production of energy crops such as poplars to obtain their respective clones. Seedlings of Populus maximowiczii × Populus nigra (M × N) and Populus maximowiczii × Populus balsamifera (M × B) clones were grown at two day/night temperatures of 23 °C/18 °C and 33 °C/27 °C in soil with low and high nitrogen levels. The effect on thermal acclimation of the net photosynthetic rate (An) was studied. The results of their study indicated that the contributions of RARCA (RuBisCO activase), apparent activation energy Vc max, and stomatal conductance to the thermal acclimation of An were significant [68]. Further study on a range of willow clones (hybrids (R13, S3, and ‘Ulbrichtweide’) being the parents of the mapping population family, with R13 and S3 being full siblings) has identified significant differences in carboxylation and oxidation rates and CO2 affinities that have been rationalized in terms of LSU sequence polymorphism. Carboxylase rate constants were up to 29% higher for LSU sequences with isoleucine instead of methionine at position 309. In this way, genetic traits have also been identified that are specifically related to RuBisCO and, consequently, to photosynthetic efficiency [69]. Additionally, research has been conducted on the issue of restricted electron flux in cytochrome C6 (CYTOC6). Overexpression of this factor affects electron transport in photosynthesis. This process can be improved by overexpressing aquaporin, which improves CO2 conductivity in the mesophyll and influences the properties of stomata. This process can be enhanced by overexpression of aquaporin, which improves CO2 conductance in the mesophyll and affects stomatal properties. It has also been shown that a phosphotriose/phosphate transporter (TNT) reduces photosynthesis in C4 plants under conditions of elevated CO2. The solution to this problem seemed to be to overexpress the sucrose symporter. This is a system that studies particularly well in the presence of high atmospheric CO2 concentrations in both Miscanthus and Willow (S. suchowensis and S. integra hybrids) [70,71]. At the same time, other technologies are being developed, such as: nuclear or genomic transformation of chloroplasts to simplify the process of photosynthesis [72].
3.2. Gene Technology to Increase Tolerance to Biotic and Abiotic Stresses in Energy Plants
The resistance of plants cultivated under abiotic or biotic stresses may be augmented through several methodologies. One traditional approach involves gradual habituation. Another entails reducing the impact of stressors through the utilization of plant growth and development (PGA) promoters. A further option is to obtain cloned stressed plants through the use of artificially produced autotetraploid varieties. Alternatively, genetic tools may be employed to target more than just the photosynthetic system, although conventional breeding, as well as molecular techniques and genetic engineering, have significantly contributed to the production of plant varieties resistant to biotic stresses [73]. Despite the use of new genetic tools, including genome editing, to develop resistant crop plants, the complex genetics involved in resistance mechanisms have resulted in limited success in combating abiotic stresses. The use of genome-edited plants has been hindered by stringent regulations on their cultivation, which remain a significant obstacle to their wider adoption [74]. One illustrative example is the research on cell wall differentiation in energy plants, most notably Miscanthus. The researchers classified 1200 genes related to cell wall synthesis and assembly, as well as Miscanthus cell wall destruction, into six categories or stages of cell wall biogenesis. These included genes responsible for substrate production, polysaccharide synthesis, membrane transport, renewal, secondary cell wall formation, and signal transduction [75]. In recent years, the CRISPR (clustered regularly interspaced short palindromic repeats) method has become popular, allowing for the precise editing of the genome of the target cell. This method is relatively inexpensive, efficient, and simple to perform. All that is required to perform the genetic transformation is the appropriate gRNA and to ensure the availability of the Cas enzyme (usually Cas9) in the target cell [76]. The CRIPS method was initially described in a 2022 paper by Trieu et al. and has since been employed to develop gene-editing procedures for Miscanthus utilizing the CRISPR/Cas9 system. This approach has enabled the targeted mutation of an endogenous gene, thereby eliminating its function. Utilizing sequence information from Miscanthus and Sorghum, orthologues of maize lw1 (lemon withe 1) were identified. A multistep screening approach was employed to select three gRNAs that could target lw1 homologues. Embryogenic calli of M. sacchariflorus, M. sinensis, and M. × giganteus were transformed by particle bombardment (biolistics) or Agrobacterium tumefaciens insertion of the Cas9 gene and three gRNAs to edit lw1. In the future, this may result in the appropriate identification of genes and the introduction of changes that will be necessary to achieve greater resistance to, for example, salt stress, drought, limited light, or oxidative stress [77]. In 2023, comparable research was conducted on shrub willows (Salix section Vetrix). Transient gene editing was successfully achieved. In 2023, a similar program was initiated for the poplar. To achieve the integration of nptII and 2XCamV 35S into the MKK2 promoter zone, CRISPR-Cas9 was recruited, and three variables—Agrobacteria inoculator concentration, pDDT/pgRNA ratio, and homologous arm length—were designed. MKK2 is a transcriptional regulatory gene stimulated by environmental stresses, such as salinity and cold, to promote plant resistance. Poplars (Populus trichocarpa) that were recovered on kanamycin-treated media demonstrated an increase in MKK2 expression, which was influenced by the precise integration of 2XcamV 35S and nptII. This lead to an improvement in biochemical and phenotypic properties [78].
3.3. Other Methods to Enhance Tolerance to Abiotic and Biotic Stresses of Established Energy Crops
It was previously assumed that plants grown for energy were relatively simple to cultivate and resilient to environmental challenges. It is preferable to cultivate perennial plants with a useful life of at least 15–20 years for energy purposes, as this reduces the cost of cultivation. Nevertheless, the specific species selected is contingent upon soil and climatic conditions, the technical equipment of the farm, and the requirements of consumers (energy utilities) in terms of biomass quality [79]. It is unlikely that clones genetically adapted to Asian conditions will thrive under European conditions. Similarly, fluctuations in the environment, such as droughts, heavy precipitation, and contamination in the vicinity of cultivated farms, are beyond the control of farmers and can occur at the individual plantation level [80]. Furthermore, the intensification of agriculture has resulted in a decline in biodiversity and is also influenced by competition between farmers for good soil resources for food and plants for energy purposes. In this context, extensive research has been conducted for several years on the cultivation of plants for energy purposes on degraded or contaminated soils, including hydrocarbons and salt. One of the strategies for intensifying energy crops in line with the European Green Deal concept is to introduce more natural compounds into the fields, such as plant biostimulants and substances, to improve soil conditions for both food and energy crops [81]. This is discussed in greater detail in Table 3. To achieve this goal, a significant amount of research has been conducted on the innovative utilization of biostimulants in energy crops. This research has focused on the accumulation of amino acids and soluble sugars in plant cells, the maintenance of adequate levels of antioxidants and enzymes, and the promotion of the saturation of fatty acids in cell membranes [82].

Table 3.
Biostimulants for energy crops, own study based on [80,83,84,85,86,87].
The majority of the study is carried out on Miscanthus grasses [80,83,84,85,86]. It has been demonstrated that biostimulants can alleviate salt stress in these plants, including that associated with disruption of photosynthesis, which can result in growth limitations and reduced plant productivity. In the study, the yields of Miscanthus × giganteus J.M. Greef & Deuter and Cannabis sativa L. grown on agricultural soil contaminated with metals (11 mg Cd, 536 mg Pb, and 955 mg Zn kg−1) enriched with biostimulants and/or arbuscular mycorrhizal fungi and the uptake of Cd, Pb, and Zn by the shoots were evaluated. After 90 days, it was demonstrated that fulvic and humic acids were more effective than mycorrhizal fungi or protein hydrolysates in stimulating the biomass growth of energy crops on metal-contaminated soils [86]. The potential for exploiting increasingly well-documented plant–microorganism interactions has prompted efforts to introduce endophytic inoculants from the genera Clostridium, Enterobacter, or Bacillus sp. into the soil with energy crops. The presence of endophytes has been demonstrated to enhance the growth of energy crops cultivated on salt-contaminated soils [86]. Additionally, there is a substantial body of research investigating the potential applications of algal extracts, which are rich in nitrogen, auxins, cytokinins, alginates, brassinosteroids, gibberellins, phlorotannins, and polyamines. In 2018, Digruber et al. demonstrated the positive effects of algal extracts on plant stem weight, increased shoot stimulation, and more efficient electron transport rates (ETRs) of photosystems [87]. The biomass yield of cultivated energy crops can be enhanced through genetic modifications or the application of innovative cultivation technologies. These studies demonstrate the potential for enhancing biomass yield and, under unfavorable environmental conditions, partially substituting traditional mineral fertilizers. This is of significant importance as there is a potential risk of alkaline pollutants entering biomass and negatively affecting biomass-fired boilers [88,89].
4. New Strategies for Enhancing the Utilization of Biostimulants in Energy Crops
The primary objective of cultivating energy crops is to enhance yield quality. However, it would be advantageous if the energy efficiency of these crops could be increased and the costs reduced, with the additional benefit of influencing sequestration and the CO2 balance. To achieve more efficient growth of energy crops, it is crucial to enhance the photosynthetic rate and develop resistance to biotic and abiotic stresses. In the European Union, genetic engineering methods are still not as intensively developed and applied as in other parts of the world [90,91]. The efficient production of energy crops provides a source of energy while simultaneously reducing net CO2 emissions into the atmosphere. The fundamental objective of the strategies employed to enhance the biomass of energy crops is to optimize the utilization of their CO2 offsetting potential through the implementation of measures at various levels [92]. It is of paramount importance that any measures that do not use genetic engineering tools be energy-efficient and cost-effective, in addition to aligning with the European Green Deal, which aims to enhance soil biodiversity. The following section will present three proposals for action that are intended to improve the efficiency of biomass creation and intensify efforts towards a circular economy. In addition, the economics of production processes will be considered. Such measures may result in cost savings in cultivation, but more importantly, they may also have an impact on the CO2 balance. This is achieved by increasing CO2 sequestration while reducing the amount of CO2 produced. The three proposals will consider the breakdown of biostimulants based on their mode of application, method of preparation, and their combined use with conventional plant protection products.
4.1. Foliar Protein Biostimulants
The long-standing overuse of agrochemicals in agriculture has contributed to soil degradation, adversely affecting both the environment and human health. As a consequence of the adverse changes observed, EU legislation now seeks to reduce the use of both chemical pesticides and mineral fertilizers [93,94]. The current trend is to fine-tune fertilization to soil conditions and the requirements of crop varieties. The role of fertilization is to provide plants with readily available nutrients in the form of single elements or simple organic compounds. In this context, the use of biostimulants in agriculture is gaining popularity as a promising, safe, and environmentally sound alternative approach to more sustainable crop production [95]. Foliar application is a simple method of applying a biostimulant, the efficacy of which depends on the penetration of the active ingredients into the foliage. This parameter depends mainly on the molecular structure of the active ingredients, their particle size, and their solubility [96]. Compared to soil fertilization, foliar fertilization is more effective because it allows nutrients to be delivered to the plant in controlled amounts at specific times during its growth. It also has a lower environmental impact due to the greater precision of application [97]. The nutrients supplied to the plant can be absorbed directly through the leaves and transported to other organs. Foliar fertilization can be easily adapted to the needs of the crop, both in terms of the composition of the nutrients delivered and their concentration. It also makes it possible to combine different nutrients to take advantage of their synergistic effects [98]. However, in the context of energy crop production, which is often established on degraded land, it is important to remember that soil fertility affects the amount of biomass produced, and increased production efficiency increases carbon accumulation [99]. It is therefore essential for energy crops to choose the right, balanced way of fertilizing a plantation. For this reason, using a simple form of application and carrying out treatments in a single operation is a strategy that makes sense not only economically, but also environmentally, as it results in lower CO2 emissions.
In the cultivation of energy crops, measures are being sought that not only activate natural defense processes to stimulate plant growth and development, but also help to reduce the impact of negative environmental conditions during vegetation. One group of such measures is biostimulants. Of particular interest are biostimulants from natural sources, including those derived from waste, which have minimal or no environmental impact. Biostimulants can be produced from animal waste (animal and fish skin by-products, chicken feathers, and casein) or plant biomass (legumes and alfalfa hay) [100,101]. Of particular importance in the context of a circular economy is the development of methods for upgrading and processing animal waste to recover and reuse the polymers it contains.
As part of ongoing study on the management of waste from the tanning industry, an innovative method of obtaining collagen in the form of hydrolysates has been developed [102]. The resulting formulations can be used to develop innovative solutions that will eventually be applied to the agricultural industry, including the cultivation of energy crops. The tanning industry generates large amounts of waste. It is estimated that the processing of one ton of rawhide produces approximately 200 kg of tanned leather, 200 kg of tanned waste leather, 250 kg of untanned waste, and 50,000 kg of waste water [103]. The major protein component of rawhide is collagen, which makes up approximately 94–95% of its content. Rawhide also contains elastin (1%) and keratin (1–2%), as well as trace amounts of non-fibrous proteins [104]. The search for solutions to enable the tanning industry to move from a linear economic model to a circular economic model that ensures its sustainability and cleaner production is still ongoing. This is particularly relevant in the context of valuable raw materials that can be recovered and reused in the same or other industries. The biggest challenge for the industry is the management of all waste streams in the sector and thus the implementation of zero-waste technology.
It is believed that waste collagen and other waste-derived biopolymers (e.g., keratin) can be successfully used as biostimulants in the cultivation of energy crops. Amino acids are known biostimulants that have a positive effect on plant growth and yield [105]. They are precursors of proteins [106], which have multiple functions in plants: structural (building), metabolic (enzymatic), and transport [107]. At the same time, they play an important biological role as building blocks of enzymes, nucleic acids, antioxidants, and hormones [108]. In addition, due to their structure, amino acids act as buffers that help maintain a favorable pH in the plant cell [109]. Can mitigate the effects of environmental stress on plants [110]. The advantage of their use as biostimulants is their mobility and easy transport in plants [111].
The unique amino acid profile is the undisputed advantage of collagen preparations obtained from the hydrolysis of tannery waste [112,113]. Table 4 shows the amino acid composition of collagen hydrolysates obtained by chemical–enzymatic hydrolysis of tannery waste. Among the amino acids determined, hydroxyproline deserves particular attention, its content in the preparations studied reaching values in the range of 1658–1658 µg/mL (determination by high-performance liquid chromatography with fluorescence detector: HPLC/RF) [114]. Hydroxyproline is a marker of the plant’s stress response. This amino acid also regulates the plant’s water balance. For plants, collagen hydrolysate is primarily a source of nitrogen, and it also contains amino acids such as glycine, serine, and proline, whose increased accumulation in plants is observed in response to environmental stress [115]. Another source of nitrogen in protein preparations derived from animal waste can be keratin hydrolysate. Keratin preparations are rich in cysteine (a sulfur-containing amino acid), which distinguishes them from other biopolymers. The content of cysteine and cystine in the amino acid sequence is 7–12%. Keratin preparations are distinguished by a high content of amino acids, including glycine, alanine, serine, valine, and a comparatively low content of methionine, lysine, and tryptophan. The high potential of both proteins in improving quality and productivity in plant growth is because collagen and keratin are fibrous proteins with amphiphilic properties. These properties enable them to buffer pH fluctuations, chelate micronutrient ions, adhere to leaves and act as a reservoir of organic nitrogen and amino acids. The combination of the two proteins represents an innovative step for new amino acid-based formulations with a role in plant growth stimulation [116]. Furthermore, the specific structure of the amino acid profile of animal waste from tanning processes ensures a high proportion of L-amino acids other than hydroxyproline, which are essential for efficient photosynthesis in C4 plants, the most common of which are used for energy crops. In addition to its other functions, the enzyme RuBisCO plays a pivotal role in the efficiency of light assimilation in C4 plants. RuBisCO catalyzes the carboxylation reaction of ribulose-1,5-bisphosphate (RuBP). This involves the attachment of a CO molecule to RuBP after prior activation of the enzyme. The activation of RuBisCO involves the attachment of a CO molecule and an Mg2+ ion to lysine, the amino acid present in the enzyme’s active center. The attached carbon dioxide molecule is not a substrate, but rather an activating compound that determines the correct placement of the Mg2+ ion in the enzyme’s active center. The Mg2+ ion is the actual cofactor of RuBisCO [117].

Table 4.
Amino acid profile in selected samples of protein hydrolysates obtained from tannery waste (determined HPLC/RF) based on our own study.
It is crucial to acknowledge that the utilization of biostimulants in the form of foliar formulations expands the scope for the integrated application of agrochemicals, namely, the concurrent utilization of diverse plant protection products or the combination of plant protection products with liquid fertilizers (referred to as the tank-mix process) [118]. This approach represents an efficacious method of reducing expenditure on agricultural operations and enhancing the efficiency of the study organization, particularly when executing a multitude of agrotechnical procedures. This process is guided by the principles of good agricultural practice. Its economic benefits include enhanced study organization, material savings (water and fuel), and labor savings. Another advantage of the combined use of agrochemicals is that the treatment can be carried out rapidly, capitalizing on favorable weather conditions and the potential for simultaneous agrophage control and plant nutrition.
4.2. Biostimulants Obtained Exclusively via Biotechnological Means
Biotechnology is one of the most rapidly expanding areas of research in the European Union. The sector has been identified as one of the most innovative in recent years. Biotechnological methods are employed in the treatment of organic and mineral waste, as well as in the remediation of the environment through the use of microorganisms [90,119]. Wastewater and municipal waste management techniques [120] have been around for a long time. Unfortunately, the tanning industry is not using them. The industry always has problems, so it makes sense to develop new ways to manage waste using biotechnology [121,122,123,124]. There is no good way to treat tannery waste that contains chromium. This is because it is too expensive and because it does not meet all the environmental standards. The problem is that the processes are too expensive because they use expensive reagents, including pure enzymes. The solution was to exploit the potential for microbial biotransformation and decomposition of tannery shavings in a one-step process using the yeast strain Yarrowia lipolytica IPS21. Microorganisms, including yeasts, may act as components of biostimulants. To date, only a small number of yeast species have been evaluated for their suitability in reducing, removing, or recovering products from tannery waste or in producing biostimulants. The literature contains information on various yeasts, including those of the genera Candida, Debaryomyces hansenii, Saccharomyces cerevisiae, Torulaspora delbrueckii, and Rhodotorula mucilaginosa [125,126].
Łukasiewicz Research Network—Lodz Institute of Technology has developed an alternative method for treating tannery waste [127,128] that does not require the use of pure enzymes and chemical reagents. Consequently, the conditions were selected that had the greatest impact on proteolytic activity while having the least impact on yeast cells of the genus Yarrowia. This has enabled the following improvements: shorter substrate processing times; water savings; the resignation of chemicals typically used in chemical–enzymatic hydrolysis; reduced power costs; and reduced costs of culture substrates. This may result in a reduction in carbon dioxide emissions during the production of biostimulants. In the developed method, targeted expression of alkaline proteases is employed in a one-step process at the culture stage, as depicted in Figure 3.
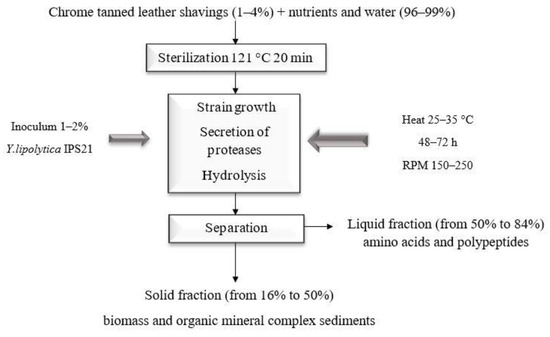
Figure 3.
This is a schematic representation of the biological transformation of solid tannery waste contaminated with hexavalent chromium, based on our own study.
Supernatants with a high nitrogen content, microbial enrichment, and a high content of protein L-amino acids, particularly those involved in the activation of the RuBisCO enzyme, were successfully obtained [129]. The resulting product, which is rich in L-lysine and other macromolecules required for RuBisCO to function properly, could guarantee the economics of energy crops and potentially increase carbon dioxide sequestration. It can be argued that enzymatic processing technology could become not only an efficient tool for the production of value-added compounds for energy crops but also an economically advantageous one. The amino acids produced biologically are distinct from those obtained by physicochemical means or in hydrochloride form. This guarantees their high bioavailability, as evidenced in the data literature [130]. The appropriate use of stimulants and the selected application method can facilitate the acceleration of net photosynthetic processes in energy crops, thereby increasing their productivity. The use of a low-cost product will enable energy crops to be grown on weak and very weak soils while simultaneously reducing mineral fertilization. Biostimulants derived from waste can be a safe, effective, and economically viable alternative to nitrogen mineral fertilization. In the context of reducing CO2 emissions and increasing biodiversity, this concept is worthy of consideration.
4.3. Biostimulators in the Form of Granules
One of the challenges currently facing the global economy is identifying alternative, environmentally friendly solutions to existing products and technologies. This chapter will present granular forms based on natural and/or waste raw materials produced by various granulation methods as potential applications in the form of biostimulants in agriculture. Granulation is widely used in a variety of industries, including agriculture, while scientists are constantly working on new applications of the process to obtain new products, manage waste, or better understand the mechanisms involved [131,132].
One of the principal products utilized in the agricultural sector is phosphorus-based fertilizers and soil additives. However, the supply of this element in nature is finite [133,134], and the excessive growth of algae resulting from phosphorus entering groundwater forces the search for new alternative solutions. One potential solution may be the use of fertilizer in the form of microgranules with a reduced phosphorus content, measuring less than 2 mm in diameter. Thielicke and colleagues conducted an experiment in which they applied fertilizer microgranules singly and in combination with biostimulants to a control corn crop. For purposes of comparison, diammonium phosphate (DAP) was also applied alone or in combination with biostimulants. The results demonstrated that the microbead fertilizer yielded comparable results to the DAP fertilizer, with a significantly lower balance-P. The experiment thus demonstrated the efficacy of utilizing fertilizers in the form of microgranules [135].
The use of granules with properties selected for their appropriateness (strength, uniform size, 1–2 mm granule size, flowability, lack of dustiness, and water solubility) and process parameters allows for the development of biostimulants using microbial strains. This is evidenced by the findings by Oancea and co-authors [136]. The specific granule form allows for the formulation of biostimulant products with optimal flowability, dust-free properties, and reduced moisture absorption, which enhances the survival rate of biostimulant microorganisms and minimizes the risk of microbial contamination. The resulting granules exhibit physicochemical properties that are comparable to those of other agrochemicals currently available on the market. Furthermore, the granular form of biostimulants offers the advantage of compatibility with commonly used application methods. Additionally, the biological activity of the strains is maintained throughout the formulation process and during storage for 12 months. This evidence supports the viability of employing the granulation process to develop competitive microbial-based biostimulants.
A team of researchers, led by J. Jimenez and his co-authors, conducted an experiment in which wastewater treatment plant waste struvite was used as a biostimulant component due to its high phosphorus content. The product, in the form of granules with a diameter of 2.4 mm, is insoluble in a humid environment. Therefore, it was combined with biostimulants in the form of bacteria and coprolites, which, in conjunction with the influence of organic acids in the soil and plant roots, facilitated its release in the test environment. The fertilizer value of struvite in oat crops was evaluated in comparison with triple superphosphate as part of the research process. The experiment was conducted on two distinct types of soil, namely, low and high phosphorus. The results demonstrated that the combination of struvite with biostimulants led to a 39% increase in the total dry weight of oats and a 33% enhancement in the plant’s phosphorus uptake. The study demonstrated that the waste product, when combined with appropriate biostimulants, is capable of increasing the release of the beneficial plant element phosphorus [137]. This suggests that it could potentially be used in agriculture.
It is also worth noting that several studies have been conducted on the use of biocarbon derived from biomass generated by agricultural waste. Previous studies have indicated that biocarbon may be a viable energy source in the form of biofuels. However, a growing body of research suggests that it could also be used in agriculture as a soil additive. In a study conducted by Sorgona and colleagues, the physical and chemical properties of biocarbon derived from vine production waste and hulls were examined in the form of a granulated pellet. The results demonstrated that the pyrolysis process had a favorable effect on the content of elements such as phosphorus and potassium. The concentration of potassium in the vine-based pellet increased by more than 2.5 times, from 8700 to 20,200 mg/kg, while in the sunflower husk-based pellet, it increased almost 3 times, from 3440 to 8590 mg/kg. A more than twofold increase in phosphorus content was also observed in both samples of the pellets tested [138]. These results indicate that the obtained pellets have the potential to be used as fertilizers or as additives to fertilizers and biostimulants. This is corroborated by the findings of other researchers, such as De la Rosa and colleagues, which demonstrate that biocarbon has a beneficial impact on soil properties, including a reduction in acidity and an enhancement in water retention capacity [139].
Moreover, more recent studies have indicated that the use of granulated biocarbon pellets has a positive effect. Kang and colleagues conducted research on the potential of biosolids, which contain biocarbon in their formulation, to improve the physical properties of soil. The additives utilized in the study were biocarbon, polyacrylamide, and horseradish (moringa) tree. The biocarbon pellets were chemically synthesized, compressed, and finally molded before being added to pots of seeded beans and corn. The results demonstrated the efficacy of the developed biostimulant in enhancing soil properties and promoting plant growth. The application of biocarbon and polyacrylamide resulted in a significant increase in water retention, with a 128.9% increase observed. Furthermore, the application of the biostimulant in the form of a pellet led to an increase in the growth parameters of both plants, including fresh and dry weight, number of leaves, diameter, and stem height. Furthermore, the stability of the soil was enhanced [140]. The results of this study indicate that soil additives/biostimulants based on biocarbon have the potential to be used on a larger scale with no adverse effects on the environment.
5. Patents
Patent Application P.442578: Method of utilization of chromium protein waste generated in leather production.
Author Contributions
Conceptualization, D.W., P.P., A.R. and K.Ł.; methodology, D.W., P.P. and A.R.; software A.R.; validation, D.W., P.P. and A.R.; formal analysis, D.W., P.P. and A.R., investigation, D.W., P.P. and A.R.; resources, D.W., P.P. and A.R.; data curation, D.W., P.P., A.R. and K.Ł.; writing—original draft preparation, D.W., P.P. and A.R.; writing—review and editing, P.P., D.W. and A.R.; visualization, D.W.; supervision, K.Ł.; project administration, K.Ł.; funding acquisition, K.Ł. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Science and Higher Education, Poland, grant number 00/BCS/08/00/1/3/0284. This study was funded by the National Center for Research and Development, Poland, contract number EUREKA/DUO_PLANT_Protect/5/2021.
Acknowledgments
This study was carried out as part of the EUREKA DUO_PLANT_Protect research project funded by the National Center for Research and Development, Poland, contract number EUREKA/DUO_PLANT_Protect/5/2021, grant title “Multifunctional foliar preparations for protect and stimulate plant growth”.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fit for 55 Package under the European Green Deal. Available online: https://www.europarl.europa.eu/legislative-train/package-fit-for-55 (accessed on 13 May 2024).
- Biomass. Green Energy for Europe. European Commission Directorate-General for Research Sustainable Energy Systems. Brussels. 2005, p. 15. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2005:0628:FIN:EN:PDF (accessed on 13 May 2024).
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 13 May 2024).
- Karekezi, S.; Kusum, L.; Suani Teixeira, C. Traditional biomass energy: Improving its use and moving to modern energy use. In Renewable Energy; Routledge: London, UK, 2012; pp. 258–289. [Google Scholar]
- Heaton, E.A.; Flavell, R.B.; Mascia, P.N.; Thomas, S.R.; Dohleman, F.G.; Long, S.P. Herbaceous energy crop development: Recent progress and future prospects. Curr. Opin. Biotechnol. 2008, 19, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, S.; Su, M.; Yang, M.; Shen, S.; Jiang, G.; Qi, D.; Chen, S.; She, L.G. Major Energy Plants and Their Potential for Bioenergy Development in China. Environ. Manag. 2010, 46, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Sims, R.E.; Hastings, A.; Schlamadinger, B.; Taylor, G.; Smith, P. Energy crops: Current status and future prospects. Glob. Chang. Biol. 2006, 12, 2054–2076. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Matyka, M.; Staniak, M. Comparison of the Effect of Perennial Energy Crops and Agricultural Crops on Weed Flora Diversity. Agronomy 2019, 9, 695. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Mola-Yudego, B.; Díaz-Yáñez, O.; Dimitriou, I. How Much Yield Should We Expect from Fast-Growing Plantations for Energy? Divergences Between Experiments and Commercial Willow Plantations. Bioenergy Res. 2015, 8, 1769–1777. [Google Scholar] [CrossRef]
- Atkinson, C.J. Establishing perennial grass energy crops in the UK: A review of current propagation options for Miscanthus. Biomass Bioenergy 2009, 33, 752–759. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Elbersen, H.W.; Cosentino, S.L.; Zatta, A.; Alexopoulou, E.; Monti, A. Agronomic aspects of future energy crops in Europe. Biofuels Bioprod. Biorefining 2010, 4, 674–691. [Google Scholar] [CrossRef]
- Biomass Action Plan, Commission of the European Communities COM. 2005. Available online: https://www.eea.europa.eu/policy-documents/com-2005-628-final.-biomass (accessed on 13 May 2024).
- Nuamah, A.; Malmgren, A.; Riley, G.; Lester, E. Biomass co-firing. Compr. Renew. Energy 2012, 5, 55–73. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Chakraborty, S.; Meikap, B.C. Chemical demineralization of high ash Indian coal by using alkali and acid solutions. Fuel 2017, 196, 102–109. [Google Scholar] [CrossRef]
- Muigai, H.H.; Bordoloi, U.; Hussain, R.; Ravi, K.; Moholkar, V.S.; Kalita, P. A comparative study on synthesis and characterization of biochars derived from lignocellulosic biomass for their candidacy in agronomy and energy applications. Int. J. Energy Res. 2021, 45, 4765–4781. [Google Scholar] [CrossRef]
- Zachar, M.; Lieskovský, M.; Majlingová, A.; Mitterová, I. Comparison of thermal properties of the fast-growing tree species and energy crop species to be used as a renewable and energy-efficient resource. J. Therm. Anal. Calorim. 2018, 134, 543–548. [Google Scholar] [CrossRef]
- Butler, E.; Devlin, G.; Meier, D.; McDonnell, K. Characterisation of spruce, salix, miscanthus and wheat straw for pyrolysis applications. Bioresour. Technol. 2013, 131, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Lygin, A.V.; Upton, J.; Dohleman, F.G.; Juvik, J.; Zabotina, O.A.; Widholm, J.M.; Lozovaya, V.V. Composition of cell wall phenolics and polysaccharides of the potential bioenergy crop–Miscanthus. GCB Bioenergy 2011, 3, 333–345. [Google Scholar] [CrossRef]
- Miguez, F.E.; Villamil, M.B.; Long, S.P.; Bollero, G.A. Meta-analysis of the effects of management factors on Miscanthus× giganteus growth and biomass production. Agric. For. Meteorol. 2008, 148, 1280–1292. [Google Scholar] [CrossRef]
- Fortier, J.; Gagnon, D.; Truax, B.; Lambert, F. Biomass and volume yield after 6 years in multiclonal hybrid poplar riparian buffer strips. Biomass Bioenergy 2010, 34, 1028–1040. [Google Scholar] [CrossRef]
- Balkan Grenen Use. Energy Willow Salix viminalis—Biomass Where You Want It. 2016. Available online: https://balkangreenenergynews.com/ (accessed on 13 May 2024).
- Soriano, J.A.; García-Contreras, R.; Carpio de Los Pinos, A.J. Study of the Thermochemical Properties of Lignocellulosic Biomass from Energy Crops. Energies 2021, 14, 3780. [Google Scholar] [CrossRef]
- Cantamessa, S.; Rosso, L.; Giorcelli, A.; Chiarabaglio, P.M. The Environmental Impact of Poplar Stand Management: A Life Cycle Assessment Study of Different Scenarios. Forests 2022, 13, 464. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J.; Warmiński, K. Life cycle assessment of giant miscanthus: Production on marginal soil with various fertilisation treatments. Energies 2020, 13, 1931. [Google Scholar] [CrossRef]
- Davis, S.C.; Boddey, R.M.; Alves, B.J.; Cowie, A.L.; George, B.H.; Ogle, S.M.; Smith, P.; van Noordwijk, M.; van Wijk, M.T. Management swing potential for bioenergy crops. GCB Bioenergy 2013, 5, 623–638. [Google Scholar] [CrossRef]
- Selvasembian, R.; Rani, R.; Sinha, R.; Agrahari, R.; Joshua, I.; Santhiagu, A.; Pradhan, N.; Mal, J. Recent progress in microbial fuel cells for industrial effluent treatment and energy generation: Fundamentals to scale-up application and challenges. Bioresoure Technol. 2021, 346, 126462. [Google Scholar] [CrossRef] [PubMed]
- Bhola, V.; Swalaha, F.; Kumar, R.R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- USingh, U.B.; Ahluwalia, A.S. Change Microalgae: A promising tool for carbon sequestration. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Mason, A.R.G.; Salomon, M.J.; Lowe, A.J.; Cavagnaro, T.R. Microbial solutions to soil carbon sequestration. J. Clean. Prod. 2023, 417, 137993. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Feng, W.; Jia, R.; Liu, C.; Fu, T.; Xue, S.; Yi, Z.; Guillaume, T.; Yang, Y.; et al. Marginal land conversion to perennial energy crops with biomass removal enhances soil carbon sequestration. GCB Bioenergy 2022, 14, 1117–1127. [Google Scholar] [CrossRef]
- Lubańska, Z.; Grudniewski, T.; Chodyka, M.; Nitychoruk, J. Rodzaje metod sekwestracji CO2. JCEEA 2016, 63, 239–246. [Google Scholar] [CrossRef]
- Khan, U.; Ogbaga, C.C.; Abiodun, O.-A.O.; Adeleke, A.A.; Ikubanni, P.P.; Okoye, P.U.; Okolie, J.A. Assessing absorption-based CO2 capture: Research progress and techno-economic assessment overview. Carbon Capture Sci. Technol. 2023, 8, 100125. [Google Scholar] [CrossRef]
- Jansson, C.; Wullschleger, S.D.; Kalluri, U.C.; Tuskan, G.A. Phytosequestration: Carbon biosequestration by plants and the prospects of genetic engineering. Bioscience 2010, 60, 685–696. [Google Scholar] [CrossRef]
- Gautam, S.; Baral, N.R.; Mishra, U.; Scown, C.D. Impact of bioenergy feedstock carbon farming on sustainable aviation fuel viability in the United States. Proc. Natl. Acad. Sci. USA 2023, 120, e2312667120. [Google Scholar] [CrossRef] [PubMed]
- Bazrgar, A.B.; Ng, A.; Coleman, B.; Ashiq, M.W.; Gordon, A.; Thevathasan, N. Long-term monitoring of soil carbon sequestration in woody and herbaceous bioenergy crop production systems on marginal lands in southern Ontario, Canada. Sustainability 2020, 12, 3901. [Google Scholar] [CrossRef]
- Arora, N.K. Bioremediation: A green approach for restoration of polluted ecosystems. Environ. Sustain. 2018, 1, 305–307. [Google Scholar] [CrossRef]
- Lemus, R.; Lal, R. Bioenergy crops and carbon sequestration. Crit. Rev. Plant Sci. 2005, 24, 1–21. [Google Scholar] [CrossRef]
- Keith, P.; Eric, L.; Jeffrey, K.; Ernie, M.; Amy, S. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 482133. [Google Scholar] [CrossRef]
- Agostini, F.; Gregory, A.S.; Richter, G.M. Carbon Sequestration by Perennial Energy Crops: Is the Jury Still Out? BioEnergy Res. 2015, 8, 1057–1080. [Google Scholar] [CrossRef]
- McCalmont, J.P.; McNamara, N.P.; Donnison, I.S.; Farrar, K.; Clifton-Brown, J.C. An interyear comparison of CO2 flux and carbon budget at a commercial-scale land-use transition from semi-improved grassland to Miscanthus × giganteus. GCB Bioenergy 2017, 9, 229–245. [Google Scholar] [CrossRef]
- Nakajima, T.; Yamada, T.; Anzoua, K.G.; Kokubo, R.; Noborio, K. Carbon sequestration and yield performances of Miscanthus × giganteus and Miscanthus sinensis. Carbon Manag. 2018, 9, 415–423. [Google Scholar] [CrossRef]
- Dixit, P.N.; Richter, G.M.; Coleman, K.; Collins, A.L. Bioenergy crop production and carbon sequestration potential under changing climate and land use: A case study in the upper River Taw catchment in southwest England. Sci. Total Environ. 2023, 900, 166390. [Google Scholar] [CrossRef]
- Qin, Z.; Huang, Y.; Zhuang, Q. Soil organic carbon sequestration potential of cropland in China. Glob. Biogeochem. Cycles 2013, 27, 711–722. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Masters, M.D.; Black, C.K.; Zeri, M.; Hussain, M.Z.; Bernacchi, C.J.; DeLucia, E.H. Altered Belowground Carbon Cycling Following Land-Use Change to Perennial Bioenergy Crops. Ecosystems 2013, 16, 508–520. [Google Scholar] [CrossRef]
- Chavan, S.B.; Dhillon, R.S.; Ajit; Rizvi, R.H.; Sirohi, C.; Handa, A.K.; Bharadwaj, K.K.; Johar, V.; Kumar, T.; Singh, P.; et al. Estimating biomass production and carbon sequestration of poplar-based agroforestry systems in India. Environ. Dev. Sustain. 2022, 24, 13493–13521. [Google Scholar] [CrossRef]
- Chavan, S.B.; Dhillon, R.S.; Sirohi, C.; Uthappa, A.R.; Jinger, D.; Jatav, H.S.; Chichaghare, A.R.; Kakade, V.; Paramesh, V.; Kumari, S.; et al. Carbon Sequestration Potential of Commercial Agroforestry Systems in Indo-Gangetic Plains of India: Poplar and Eucalyptus-Based Agroforestry Systems. Forests 2023, 14, 559. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, K.; Li, J.; Shangguan, Z.; Deng, L. Mixed plantations have more soil carbon sequestration benefits than pure plantations in China. For. Ecol. Manag. 2023, 529, 120654. [Google Scholar] [CrossRef]
- Kulyk, M.; Kalynychenko, O.; Pryshliak, N.; Pryshliak, V. Efficiency of Using Biomass from Energy Crops for Sustainable Bioenergy Development. J. Environ. Manag. Tour. 2020, 11, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Sameti, M.; Fuzhan, N. Biomass-fuelled combined heat and power: Integration in district heating and thermal-energy storage. Clean Energy 2021, 5, 44–56. [Google Scholar] [CrossRef]
- Patel, N.L.; Padalia, H.; Davadas, R.; Huete, A.; Kumar, S.; Yelisetty, K.M.V.N. Estimating net primary productivity of croplands in Indo-Gangetic Plains using GOME-2 sun-induced fluorescence and MODIS NDVI. Curr. Sci. 2018, 114, 1333. [Google Scholar] [CrossRef]
- Romaneckas, K.; Švereikaitė, A.; Kimbirauskienė, R.; Sinkevičienė, A.; Balandaitė, J. The Energy and Environmental Evaluation of Maize, Hemp and Faba Bean Multi-Crops. Agronomy 2023, 13, 2316. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass production and energy balance of Miscanthus over a period of 11 years: A case study in a large-scale farm in Poland. Bioenergy 2019, 11, 1187–1201. [Google Scholar] [CrossRef]
- Králík, T.; Knápek, J.; Vávrová, K.; Outrata, D.; Romportl, D.; Horák, M.; Jandera, J. Ecosystem services and economic competitiveness of perennial energy crops in the modelling of biomass potential—A case study of the Czech Republic. Renew. Sustain. Energy Rev. 2023, 173, 113120. [Google Scholar] [CrossRef]
- Stolarski, J.M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S.; Niksa, D. Analysis of the energy efficiency of short rotation woody crops biomass as affected by different methods of soil enrichment. Energy 2016, 113, 748–761. [Google Scholar] [CrossRef]
- Tzelepi, V.; Zeneli, M.; Kourkoumpas, D.M.; Karampinis, E.; Gypakis, N.; Nikolopoulos, N.; Grammelis, P. Biomass Availability in Europe as an Alternative Fuel for Full Conversion of Lignite Power Plants: A Critical Review. Energies 2020, 13, 3390. [Google Scholar] [CrossRef]
- Madley, S.J.; Daioglou, V.; Junginer, H.M.; Van Vuuren, D.P.; Wicke, B. EU bioenergy development to 2050. Renew. Sustain. Energy Rev. 2020, 127, 109858. [Google Scholar] [CrossRef]
- Bilandžija, D.; Bilandžija, N.; Zgorelec, Ž. Sequestration potential of energy crop Miscanthus × giganteus cultivated in continental part of Croatia. J. Cent. Eur. Agric. 2021, 22, 188–200. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Wang, Y.; Evers, B.J.; Kromdijk, J. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J. Exp. Bot. 2021, 72, 5942–5960. [Google Scholar] [CrossRef] [PubMed]
- Buckley, F.; Lopez-Villalobos, N.; Heins, B.J. Crossbreeding: Implications for dairy cow fertility and survival. Animal 2014, 8, 122–133. [Google Scholar] [CrossRef]
- Waliszewska-Cerazy, J.; Jeżowski, S.; Łysakowski, P.; Białas, W.; Pniewski, T. Potential of bioethanol production from biomass of various Miscanthus genotypes cultivated in three-year plantations in west-central Poland. Ind. Crop. Prod. 2019, 141, 111790. [Google Scholar] [CrossRef]
- Zub, H.W.; Brancourt-Hulmel, M. Agronomic and physiological performances of different species of Miscanthus, a major energy crop. A review. Agron. Sustain. Dev. 2010, 30, 201–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Zahid, I.; Danial, A.; Minaret, J.; Cao, Y.; Dutta, A. Hydrothermal carbonization of Miscanthus: Processing, properties, and synergistic Co-combustion with lignite. Energy 2021, 225, 120200. [Google Scholar] [CrossRef]
- Lewandowski, A.; Lewandowska, W.; Sielski, J.; Dziku’c, M.; Wróbel, M.; Jewiarz, M.; Knapczyk, A. Sustainable Drying and Torrefaction Processes of Miscanthus for Use as a Pelletized Solid Biofuel and Biocarbon-Carrier for Fertilizers. Molecules 2021, 26, 1014. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Galle, A.; Van Rie, J.; Carmo-Silva, E.; Salvucci, M.E.; Atwell, B.J. Heat tolerance in a wild Oryza species is attributed to maintenance of Rubisco activation by a thermally stable Rubisco activase ortholog. New Phytol. 2016, 211, 889–911. [Google Scholar] [CrossRef] [PubMed]
- Benomar, L.; Moutaoufik, M.T.; Elferjani, R.; Isabel, N.; DesRochers, A.; El Guellab, A.; Khlifa, R.; Hassania, L.A.L. Thermal acclimation of photosynthetic activity and RuBisCO content in two hybrid poplar clones. PLoS ONE 2019, 14, e0206021. [Google Scholar] [CrossRef] [PubMed]
- Moll, L.; Wever, C.; Völkering, G. Pude Increase of Miscanthus Cultivation with New Roles in Materials Production—A Review. Agronomy 2020, 10, 308. [Google Scholar] [CrossRef]
- Andralojc, P.J.; Bencze, S.; Madgwick, P.J.; Philippe, H.; Powers, S.J.; Shield, J.; Karp, A.; Parry, M.A.J. Photosynthesis and growth in diverse willow genotypes. Food Energy Secur. 2014, 3, 69–85. [Google Scholar] [CrossRef]
- Guo, N.; Fan, L.; Cao, Y.; Ling, H.; Xu, G.; Zhou, J.; Chen, Q.; Tao, J. Comparison of two willow genotypes reveals potential roles of iron-regulated transporter 9 and heavy-metal ATPase 1 in cadmium accumulation and resistance in Salix suchowensis. Ecotoxicol. Environ. Saf. 2022, 244, 114065. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, E.; Babich, O.; Sukhikh, S.; Ivanova, S.; Budenkova, E.; Kalashnikova, O.; Kriger, O. Methods of Increasing Miscanthus Biomass Yield for Biofuel Production. Energies 2021, 14, 8368. [Google Scholar] [CrossRef]
- Nongpiur, R.C.; Singla-Pareek, S.L.; Pareek, A. Genomics Approaches For Improving Salinity Stress Tolerance in Crop Plants. Curr. Genom. 2016, 17, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Raman, R. The impact of Genetically Modified (GM) crops in modern agriculture: A review. GM Crop. Food 2017, 8, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Iacono, R.; Slavov, G.T.; Davey, C.L.; Clifton-Brown, J.; Allison, G.; Bosch, M. Variability of cell wall recalcitrance and composition in genotypes of Miscanthus from different genetic groups and geographical origin. Front. Plant Sci. 2023, 14, 1155188. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Trieu, A.; Belaffif, M.B.; Hirannaiah, P.; Manjunatha, S.; Wood, R.; Bathula, Y.; Billingsley, R.L.; Arpan, A.; Sacks, E.J.; Clemente, T.E.; et al. Transformation and gene editing in the bioenergy grass Miscanthus. Biotechnol. Biofuels Bioprod. 2022, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Wei, H.; Kadkhodaei, S.; Sun, W.; Zhuge, Q.; Yang, L.; Xu, C. CRISPR-mediated genome editing in poplar issued by efficient transformation. Front. Plant Sci. 2023, 14, 1159615. [Google Scholar] [CrossRef] [PubMed]
- Koçar, G.; Civa, N. An overview of biofuels from energy crops: Current status and future prospects. Renew. Sustain. Energy Rev. 2013, 28, 900–916. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Pikuła, W.; Pawlak, R.; Drygaś, B.; Szpunar-Krok, E. Physiological Response of Miscanthus sinensis (Anderss.) to Biostimulants. Agriculture 2024, 14, 33. [Google Scholar] [CrossRef]
- Dauber, J.; Miyake, S. To integrate or to segregate food crop and energy crop cultivation at the landscape scale?Perspectives on biodiversity conservation in agriculture in Europe. Energy Sustain. Soc. 2016, 6, 25. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; van der Meer, I.M.; van der Werf, A.; Karlova, R. The power of seaweeds as plant biostimulants to boost cropproduction under abiotic stress. Plant Cell Environ. 2021, 45, 2537–2553. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, F.; Waterlot, C.; Manu, J.; Laloge, R.; Cancin, R.F.; Papazoglou, E.G.; Alexopoulou, E.; Lounès-Hadj Sahraoui, A.; Tisserant, B.; Mench, M.; et al. Plant testing with hemp and miscanthus to assess phytomanagement options including biostimulants and mycorrhizae on a metal-contaminated soil to provide biomass for sustainable biofuel production. Sci. Total Environ. 2024, 912, 169527. [Google Scholar] [CrossRef] [PubMed]
- Pidlisnyuk, V.; Stefanovska, T.; Zhukov, O.; Medkow, A.; Shapoval, P.; Stadnik, V.; Sozanskyi, M. Impact of Plant Growth Regulators to Development of the Second Generation Energy Crop Miscanthus × giganteus Produced Two Years in Marginal Post-Military Soil. Appl. Sci. 2022, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Farrar, K.; Bryant, D.; Cope-Selby, N. Understanding and engineering beneficial plant–microbeinteractions: Plant growth promotion in energy crops. Plant Biotechnol. J. 2014, 12, 1193–1206. [Google Scholar] [CrossRef]
- Fei, H.; Crouse, M.; Papadopoulos, J.A.; Vessey, J.K. Improving biomass yield of giant Miscanthus by application of beneficial soil microbes and a plant biostimulant. Can. J. Plant Sci. 2020, 100, 29–39. [Google Scholar] [CrossRef]
- Digruber, T.; Sass, L.; Cseri, A.; Paul, K.; Nagy, A.V.; Remenyik, J.; Molnár, I.; Vass, I.; Toldi, O.; Gyuricza, V.; et al. Stimulation of energy willow biomass with triacontanol and seaweed extract. Ind. Crop. Prod. 2018, 120, 104–112. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Gajek, M.; Nowińska, K.; Nowak, W. Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 2020, 261, 116421. [Google Scholar] [CrossRef]
- Chai, Y.; Bai, M.; Chen, A.; Peng, L.; Shao, J.; Shang, C.; Zhou, Y. Thermochemical conversion of heavy metal contaminated biomass: Fate of the metals and their impact on products. Sci. Total Environ. 2022, 822, 153426. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Gientka, E.; Tyczewska, A.; Twardowski, T. Public opinion on biotechnology and genetic engineering in the European Union: Polish consumer study. BioTechnologia 2022, 103, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Menary, J.; Fuller, S.S. New genomic techniques, old divides: Stakeholder attitudes towards new biotechnology regulation in the EU and UK. PLoS ONE 2024, 19, e0287276. [Google Scholar] [CrossRef] [PubMed]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and Consolidated Review of Plastic Waste Management and Bio-Based Biodegradable Plastics: Challenges and Opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. In Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2020; Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 13 May 2024).
- European Commission. The European Green Deal. In Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2019; Available online: https://www.eea.europa.eu/policy-documents/com-2019-640-final (accessed on 13 May 2024).
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Schuman, G.E.; Janzen, H.H.; Herrick, J.E. Soil carbon dynamics and potential carbon sequestration by rangelands. Environ. Pollut. 2002, 116, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Ławińska, K.; Lasoń-Rydel, M.; Gendaszewska, D.; Grzesiak, E.; Sieczyńska, K.; Gaidau, C.; Epure, D.G.; Obraniak, A. Coating of seeds with collagen hydrolysates from leather waste. Fibres Text. East. Eur. 2019, 27, 59–64. [Google Scholar] [CrossRef]
- Black, M.; Canova, M.; Rydin, S.; Scalet, B.M.; Roudier, S.; Sancho, L.D. Best available techniques (BAT) reference document for the tanning of hides and skins. Eur. Comm. Database 2013, 46, 2013. [Google Scholar]
- Pekhtasheva, E.L.; ZAIKOV, G.E. Degradation mechanism of leather and fur. In Key Engineering Materials. Interdiscip. Concepts Res. 2014, II, 317–336. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Shukla, R.; Sharma, Y.K.; Shukla, A.K. Molecular mechanism of nutrient uptake in plants. Int. J. Curr. Res. Acad. Rev. 2014, 2, 142–154. [Google Scholar]
- Radkowski, A.; Radkowska, I.; Godyn, D. Effects of fertilization with an amino acid preparation on the dry matter yield and chemical composition of meadow plants. J. Elem. 2018, 23, 947–958. [Google Scholar] [CrossRef]
- Ali, Q.; Haider, M.Z.; Shahid, S.; Aslam, N.; Shehzad, F.; Naseem, J.; Ashraf, R.; Ali, A.; Hussain, S.M. Role of amino acids in improving abiotic stress tolerance to plants. In Plant Tolerance to Environmental Stress; Informa UK Limited: London, UK, 2019; pp. 175–204. [Google Scholar] [CrossRef]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Gendaszewska, D.; Lasoń-Rydel, M.; Ławińska, K.; Grzesiak, E.; Pipiak, P. Characteristics of collagen preparations from leather wastes by the high pressure liquid chromatography method. Fibres Text. East. Eur. 2021, 5, 149. [Google Scholar] [CrossRef]
- Niculescu, M.; Bajenaru, S.; Gaidau, C.; Simion, D.; Filipescu, L. Extraction of the protein components as amino-acids hydrolysates from chrome leather wastes through hydrolytic processes. Rev. Chim. 2009, 60, 1070–1078. [Google Scholar]
- Gendaszewska, D.; Miśkiewicz, K.; Wieczorek, D. Use of plants in Cosmetology, Medicine and Pharmacy. In Application of Selected Biostimulators in Agriculture; Janiszewska, M., Ed.; Wydawnictwo Naukowe TYGIEL: Lublin, Poland, 2023; pp. 214–231. Available online: https://bc.wydawnictwo-tygiel.pl/publikacja/C7472DDE-41C4-E694-15C3-2023B37E3B98 (accessed on 13 May 2024).
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef] [PubMed]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-based biostimulants to enhance plant growth—State-of-the-art and future direction with sugar beet as an example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Hartman, F.C.; Harpel, M.R. Structure, function, regulation, and assembly of D-ribulose-1, 5-bisphosphate carboxylase/oxygenase. Annu. Rev. Biochem. 1994, 63, 197–232. [Google Scholar] [CrossRef] [PubMed]
- Gandini, E.M.M.; Costa, E.S.P.; dos Santos, J.B.; Soares, M.A.; Barroso, G.M.; Corrêa, J.M.; Carvalho, A.G.; Zanuncio, J.C. Compatibility of pesticides and/or fertilizers in tank mixtures. J. Clean. Prod. 2020, 268, 122152. [Google Scholar] [CrossRef]
- Wrześniewska-Tosik, K.; Marchut-Mikołajczyk, O.; Mik, T.; Wieczorek, D.; Pałczyńska, M. Mats for Removing Technical Oil Contamination. Fibres Text. East. Eur. 2012, 20, 101–106. [Google Scholar]
- Gendaszewska, D.; Liwarska-Bizukojc, E. Adaptation of microbial communities in activated sludge to 1-decyl-3-methylimidazolium bromide. Water Sci. Technol. 2016, 74, 1227–1234. [Google Scholar] [CrossRef]
- Lawinska, K.; Gendaszewska, D.; Grzesiak, E.; Lason-Rydel, M.; Obraniak, A. Coating of leguminosarum seeds with collagen hydrolyzates from tanning waste. Przem. Chem. 2017, 9, 1877–1880. [Google Scholar] [CrossRef]
- Lawinska, K.; Serweta, W.; Modrzewski, R. Studies on water absorptivity and desorptivity of tannery shavings-based composites with mineral additives. Przem. Chem. 2019, 1, 106–109. [Google Scholar] [CrossRef]
- Obraniak, A.; Gluba, T.; Lawinska, K.; Derbiszewski, B. Minimisation of Environmental Effects Related with Storing Fly Ash from Combustion of Hard Coal. Environ. Prot. Eng. 2018, 4, 177–189. [Google Scholar] [CrossRef]
- Lawinska, K.; Serweta, W.; Gendaszewska, D. Applications of Bamboo Textiles in Individualised Children’s Footwear. Fibres Text. East. Eur. 2018, 26, 87–92. [Google Scholar] [CrossRef]
- Chambard, M.; Albert, B.; Cadiou, M.; Auby, S.; Profizi, C.; Boulogne, I. Living yeast-based biostimulants: Different genes for the same results? Front. Plant Sci. 2023, 14, 1171564. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.F.; Mestre, M.V.; Vergara, C.; Maturano, P.; Petrignani, D.; Pesce, V.; Vazquez, F. Residual brewer’s Saccharomyces cerevisiae yeasts as biofertilizers in horticultural seedlings: Towards a sustainable industry and agriculture. Front. Ind. Microbiol. 2024, 2, 1360263. [Google Scholar] [CrossRef]
- Wieczorek, D.; Lasoń-Rydel, M.; Gendaszewska, D. Wpływ obecności strużyn garbarskich na wzrost drożdży z rodziny Dipodascaceae. Technol. I Jakość Wyr. 2020, 66, 170–184. [Google Scholar]
- Wieczorek, D.; Miśkiewicz, K.; Gendaszewska, D.; Pipiak, P.; Lasoń-Rydel, M.; Sieczyńska, K.; Ławińska, K. Extracellular activity of proteases from IPS21 as a function of the carbon and nitrogen source. Fibres Text. East. Eur. 2023, 31, 66–74. [Google Scholar] [CrossRef]
- Wieczorek, D.; Gendaszewska, D.; Miśkiewicz, K.; Słubik, A.; Ławińska, K. Biotransformation of protein-rich waste by Yarrowia lipolytica IPS21 to high-value products—Amino acid supernatants. Microbiol. Spectr. 2023, 11, e02749-23. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef]
- Lawinska, K.; Obraniak, A.; Modrzewski, R. Granulation Process of Waste Tanning Shavings. Fibres Text. East. Eur. 2019, 27, 107–110. [Google Scholar] [CrossRef]
- Obraniak, A.; Lawinska, K. Spectrophotometric analysis of disintegration mechanisms (abrasion and crushing) of agglomerates during the disc granulation of dolomite. Granul. Matter 2018, 20, 7. [Google Scholar] [CrossRef]
- Edixhoven, J.D.; Gupta, J.; Savenije, H.H.G. Recent revisions of phosphate rock reserves and resources: A critique. Earth Syst. Dynam. 2014, 5, 491–507. [Google Scholar] [CrossRef]
- Kisinyo, P.O.; Opala, P.A. Depletion of phosphate rock reserves and world food crisis: Reality or hoax? Afr. J. Agric. Res. 2020, 16, 1223–1227. [Google Scholar] [CrossRef]
- Thielicke, M.; Ahlborn, J.; Životić, L.; Saljnikov, E.; Eulenstein, F. Microgranular fertilizer and biostimulants as alternatives to diammonium phosphate fertilizer in maize production on marshland soils in northwest Germany. Zemljište I biljka 2022, 71, 53–66. [Google Scholar] [CrossRef]
- Oancea, F.; Raut, I.; Şesan, T.E.; Cornea, P.C. Dry Flowable Formulation of Biostimulants Trichoderma Strains. Agric. Agric. Sci. Procedia 2016, 10, 494–502. [Google Scholar] [CrossRef][Green Version]
- Hernández Jiménez, J.E.; Nyiraneza, J.; Fraser, T.D.; Peach Brown, H.C.; Lopez-Sanchez, I.J.; Botero-Botero, L.R. Enhancing phosphorus release from struvite with biostimulants. Can. J. Soil Sci. 2020, 101, 22–32. [Google Scholar] [CrossRef]
- Sorgonà, A.; Longo, L.; Proto, A.R.; Cavalletti, P.; Cecchini, M.; Salvati, L.; Gallucci, F.; Colantoni, A. Characterization of Biochar and Syngas Obtained from Pellets of Grape Vine and Sun Flower Husk Using A Pyrolysis System, Procedia. Soc. Behav. Sci. 2016, 223, 871–878. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Santa-Olalla, A.; Campos, P.; López-Núñez, R.; González-Pérez, J.A.; Almendros, G.; Knicker, H.E.; Sánchez-Martín, Á.; Fernández-Boy, E. Impact of Biochar Amendment on Soil Properties and Organic Matter Composition in Trace Element-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 2140. [Google Scholar] [CrossRef]
- Kang, M.W.; Yibeltal, M.; Kim, Y.H.; Oh, S.J.; Lee, J.C.; Kwon, E.E.; Lee, S.S. Enhancement of soil physical properties and soil water retention with biochar-based soil amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).