Performance Analysis of Vermiculite–Potassium Carbonate Composite Materials for Efficient Thermochemical Energy Storage
Abstract
1. Introduction
2. Materials and Methods
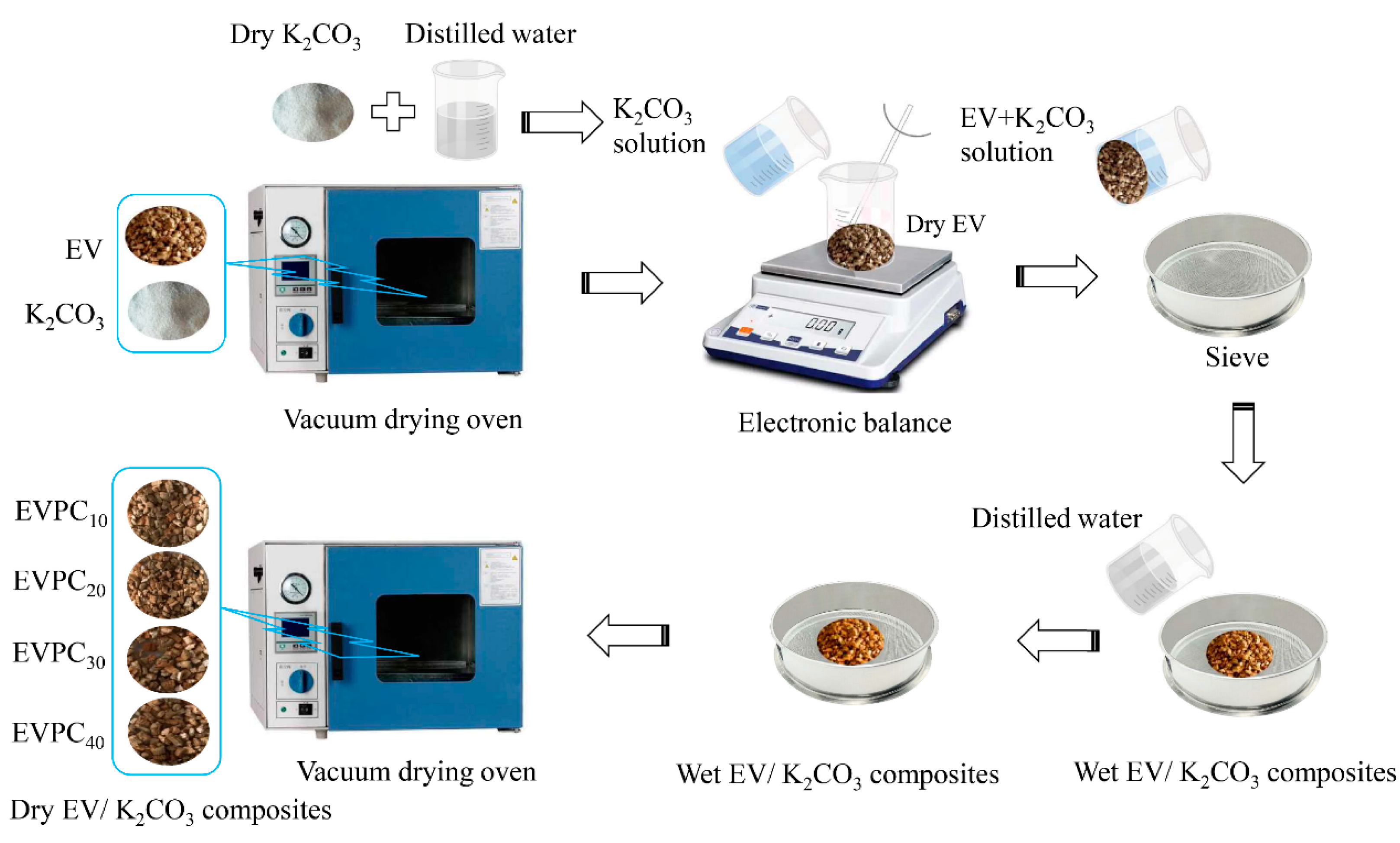
2.1. Preparation of Composite Materials
2.2. Microscopic Characterization
2.3. Sorption Experiments
2.4. Desorption Experiments
2.5. Cycle Experiments
2.6. Data Processing
3. Results and Discussion
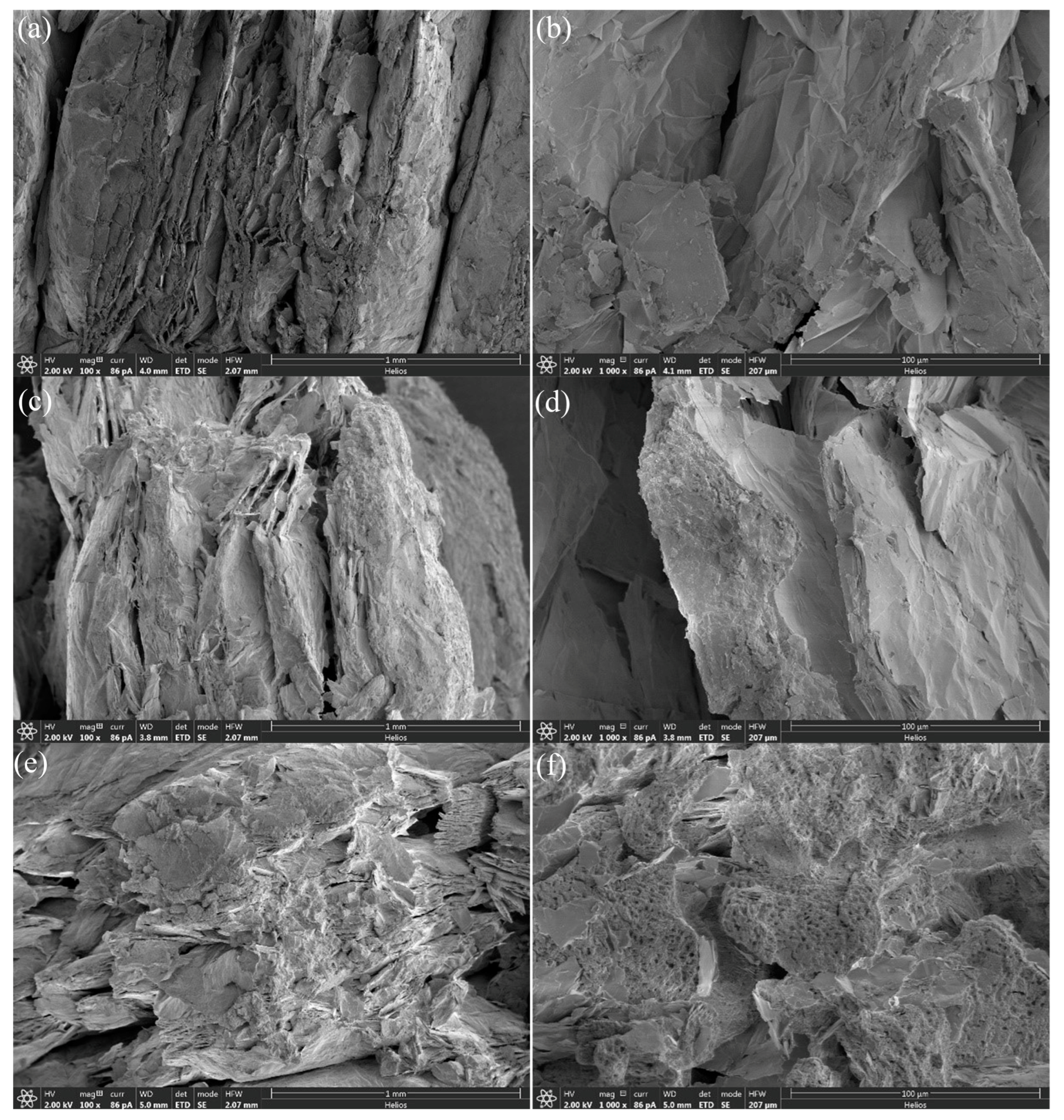
3.1. SEM Images and Salt Contents
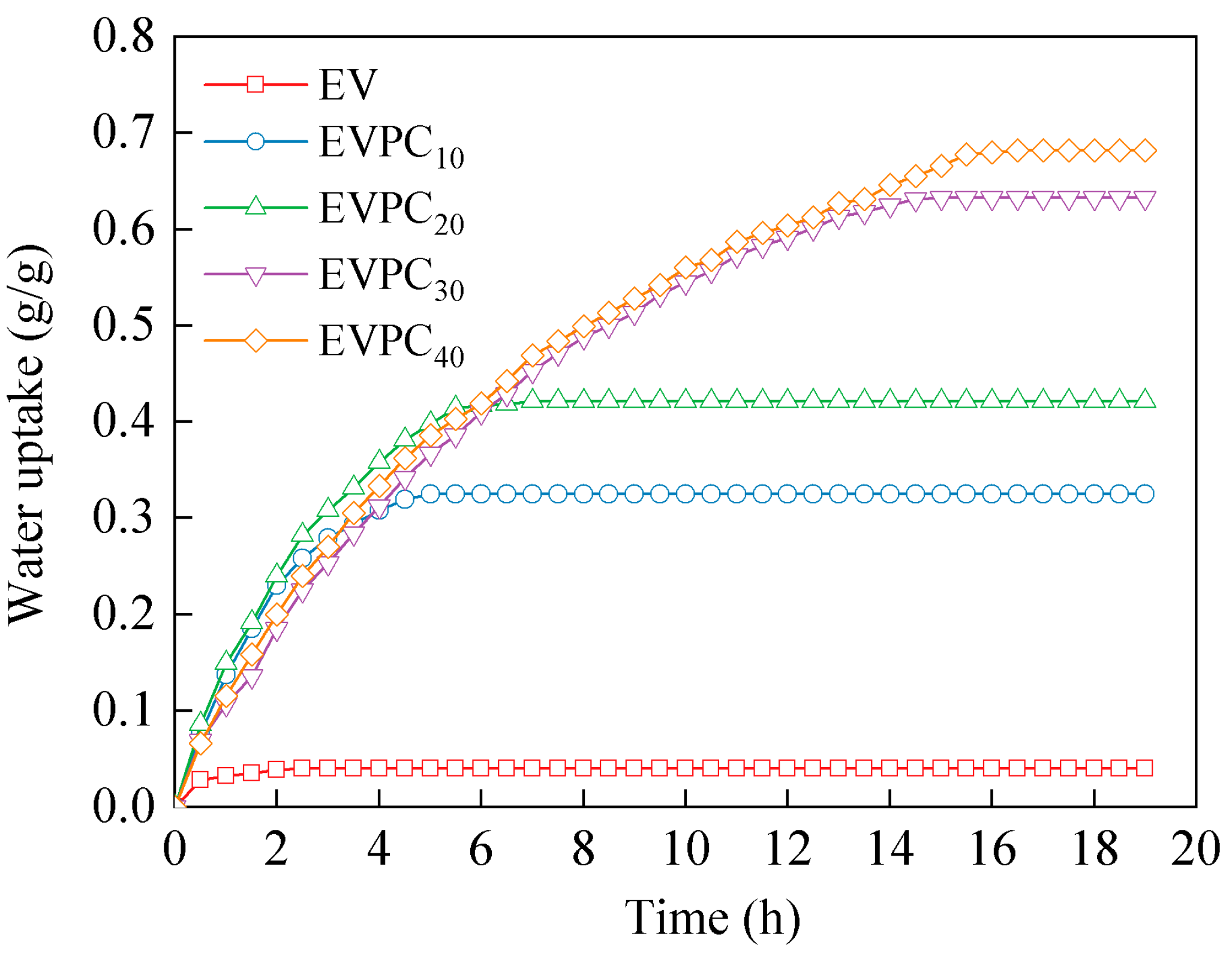
3.2. Sorption Properties
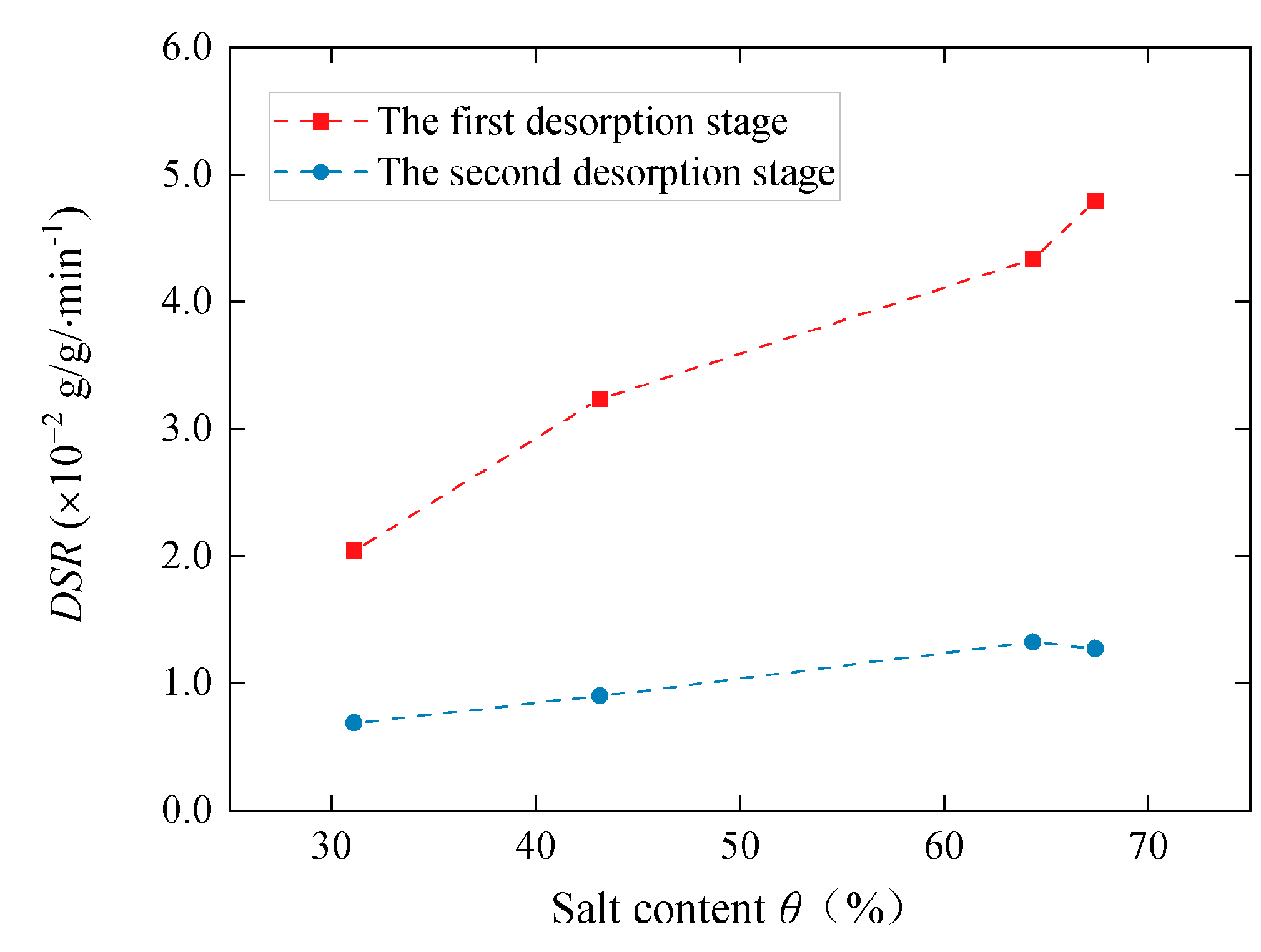
3.3. Desorption Properties
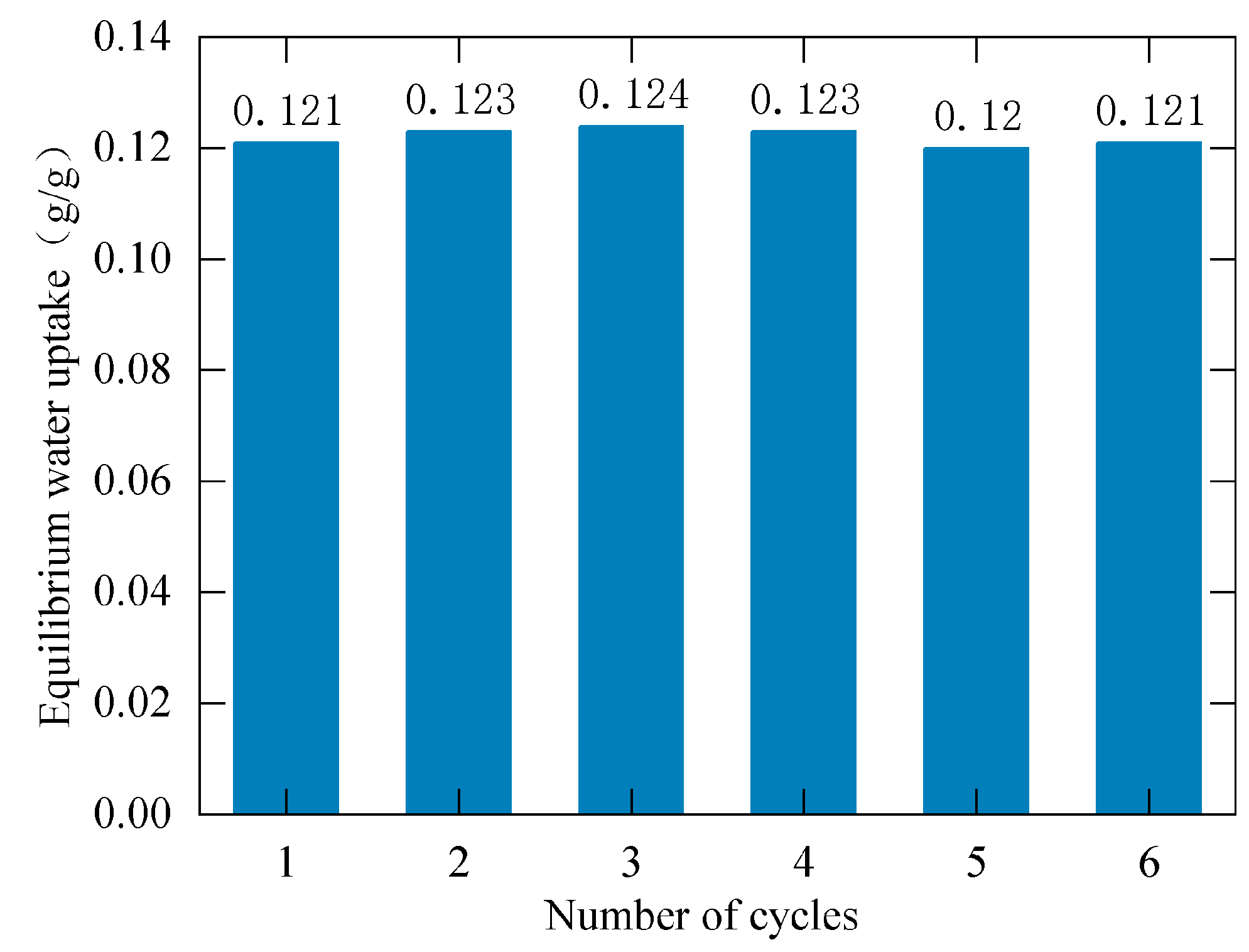
3.4. Cycle Stability
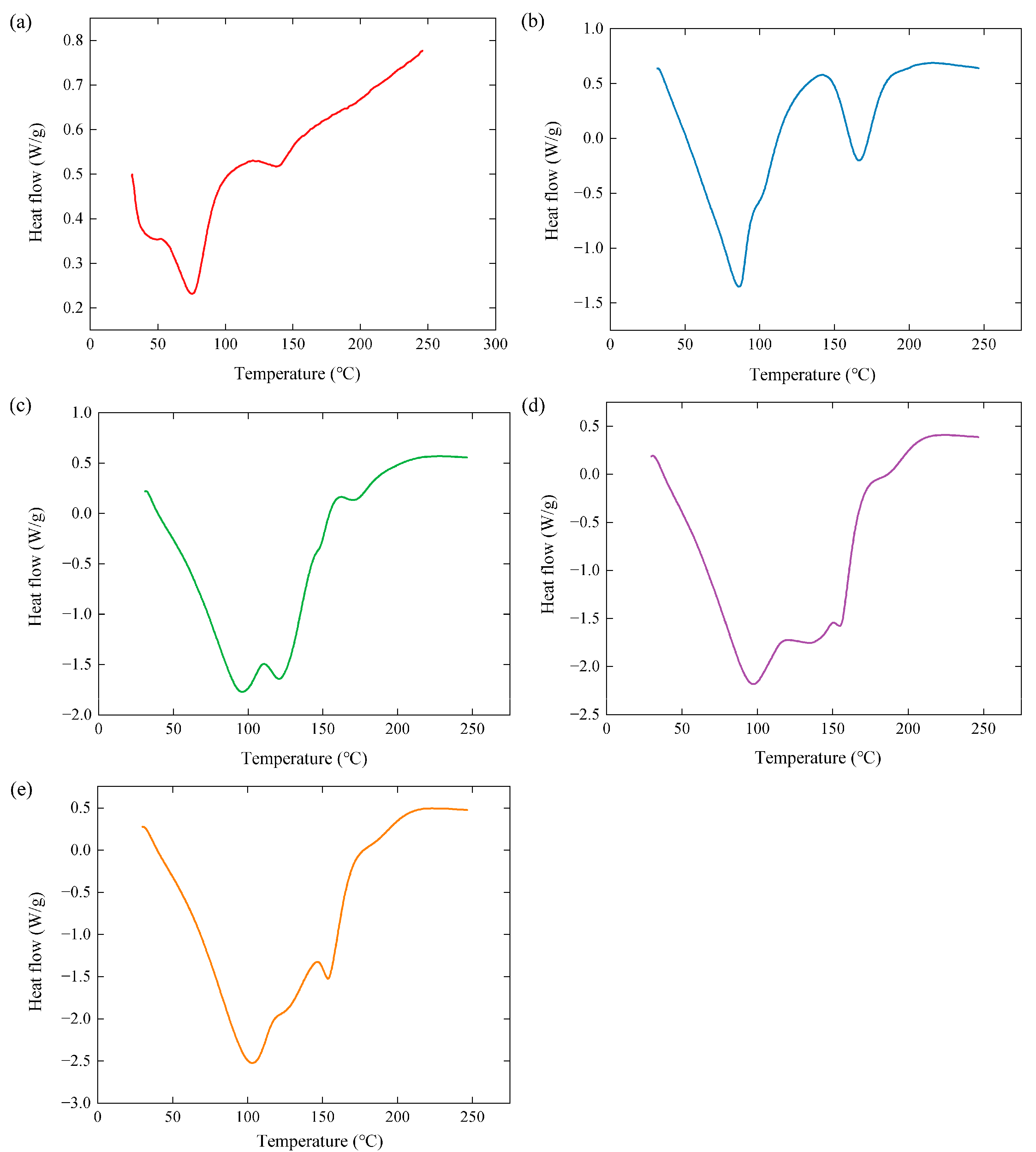
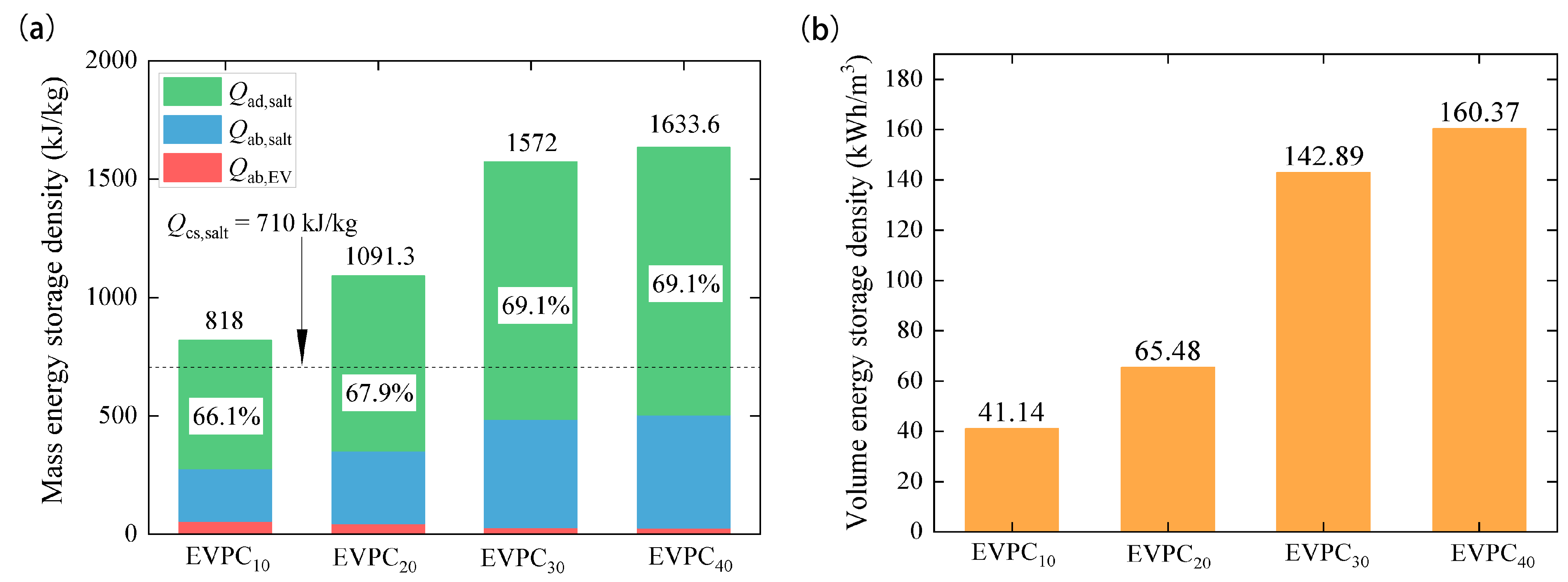
3.5. Thermal Energy Storage Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | |
| CTHC | constant temperature and humidity chamber |
| DRH | deliquescence relative humidity |
| DSC | differential scanning calorimetry |
| DSR | desorption rate |
| EV | expanded vermiculite |
| MESD | mass energy storage density |
| RH | relative humidity |
| SEM | scanning electron microscope |
| SR | sorption rate |
| STA | Simultaneous Thermal Analyzer |
| TCES | thermochemical energy storage |
| TCM | thermochemical material |
| TG | thermogravimetry |
| VESD | volumetric energy storage density |
| Nomenclature | |
| m | masses of samples |
| Q | heat flux or sorption heat |
| x | water uptake of samples |
| csalt | mass concentration of the K2CO3 salt solution |
| Greek Symbols | |
| α | material conversion |
| θ | salt content |
| ρ | mass density |
| τ | reaction time |
| Subscripts | |
| ab,salt | absorption of K2CO3 solution for EV/K2CO3 composites |
| ad,EV | physical adsorption of EV for EV/K2CO3 composites |
| ad,salt | chemical adsorption of K2CO3 for EV/K2CO3 composites |
| cs,salt | theoretical saturation chemical adsorption capacity of pure K2CO3 |
| m,EVPC | sorption of EV/K2CO3 composites |
| w,EV | sorption of pure EV |
Appendix A
References
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A Review of Promising Candidate Reactions for Chemical Heat Storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Lefebvre, D.; Tezel, F.H. A Review of Energy Storage Technologies with a Focus on Adsorption Thermal Energy Storage Processes for Heating Applications. Renew. Sustain. Energy Rev. 2017, 67, 116–125. [Google Scholar] [CrossRef]
- Almendros-Ibáñez, J.A.; Fernández-Torrijos, M.; Díaz-Heras, M.; Belmonte, J.F.; Sobrino, C. A Review of Solar Thermal Energy Storage in Beds of Particles: Packed and Fluidized Beds. Sol. Energy 2019, 192, 193–237. [Google Scholar] [CrossRef]
- Sullivan, G.; Griffiths, C.; Jewell, E.; Searle, J.; Elvins, J. Cycling Stability of Calcium-Impregnated Vermiculite in Open Reactor Used as a Thermochemical Storage Material. Energies 2023, 16, 7225. [Google Scholar] [CrossRef]
- Shchegolkov, A.; Shchegolkov, A.; Demidova, A. The Use of Nanomodified Heat Storage Materials for Thermal Stabilization in the Engineering and Aerospace Industry as a Solution for Economy. MATEC Web Conf. 2018, 224, 03012. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Galunin, E.V.; Popova, A.A.; Krivosheev, R.M.; Memetov, N.R.; Tkachev, A.G. Graphene-Modified Heat-Accumulating Materials and Aspects of Their Application in Thermotherapy and Biotechnologies. Nano Hybrids Compos. 2017, 13, 21–25. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, H. A Critical Review of Salt Hydrates as Thermochemical Sorption Heat Storage Materials: Thermophysical Properties and Reaction Kinetics. Sol. Energy 2022, 242, 157–183. [Google Scholar] [CrossRef]
- Hua, W.; Yan, H.; Zhang, X.; Xu, X.; Zhang, L.; Shi, Y. Review of Salt Hydrates-Based Thermochemical Adsorption Thermal Storage Technologies. J. Energy Storage 2022, 56, 106158. [Google Scholar] [CrossRef]
- Mohapatra, D.; Nandanavanam, J. Salt in Matrix for Thermochemical Energy Storage—A Review. Mater. Today Proc. 2023, 72, 27–33. [Google Scholar] [CrossRef]
- ElBahloul, A.A.; Zeidan, E.-S.B.; El-Sharkawy, I.I.; Hamed, A.M.; Radwan, A. Recent Advances in Multistage Sorption Thermal Energy Storage Systems. J. Energy Storage 2022, 45, 103683. [Google Scholar] [CrossRef]
- Fumey, B.; Weber, R.; Baldini, L. Sorption Based Long-Term Thermal Energy Storage—Process Classification and Analysis of Performance Limitations: A Review. Renew. Sustain. Energy Rev. 2019, 111, 57–74. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption Heat Storage for Long-Term Low-Temperature Applications: A Review on the Advancements at Material and Prototype Scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The Latest Advancements on Thermochemical Heat Storage Systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Q.; Huang, H.; Mao, H.; Liu, Y.; Xiao, Y. Applications of Low-Temperature Thermochemical Energy Storage Systems for Salt Hydrates Based on Material Classification: A Review. Sol. Energy 2021, 214, 149–178. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A Review of Salt Hydrates for Seasonal Heat Storage in Domestic Applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Stanish, M.; Perlmutter, D. Kinetics and Transport Effects in the Dehydration of Crystalline Potassium Carbonate Hydrate. AIChE J. 1983, 29, 806–812. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Ghormare, K.R.; Deshpande, N.D.; Tankhiwale, A.V. Dehydration of Crystalline K2CO3·1.5 H2O. Thermochim. Acta 1983, 66, 255–265. [Google Scholar] [CrossRef]
- Sögütoglu, L.C.; Donkers, P.A.J.; Fischer, H.R.; Huinink, H.P.; Adan, O.C.G. In-Depth Investigation of Thermochemical Performance in a Heat Battery: Cyclic Analysis of K2CO3, MgCl2 and Na2S. Appl. Energy 2018, 215, 159–173. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, J.; Huang, H.; Wu, Q.; Shen, Y.; Xiao, Y. Optimization of Thermochemical Energy Storage Systems Based on Hydrated Salts: A Review. Energy Build. 2021, 244, 111035. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Chen, X.; Riffat, S. Novel “Open-Sorption Pipe” Reactor for Solar Thermal Energy Storage. Energy Convers. Manag. 2016, 121, 321–334. [Google Scholar] [CrossRef]
- Casey, S.P.; Elvins, J.; Riffat, S.; Robinson, A. Salt Impregnated Desiccant Matrices for ‘Open’ Thermochemical Energy Storage—Selection, Synthesis and Characterisation of Candidate Materials. Energy Build. 2014, 84, 412–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Li, T.; Zhao, Y. Thermochemical Characterizations of Novel Vermiculite-LiCl Composite Sorbents for Low-Temperature Heat Storage. Energies 2016, 9, 854. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Zhao, Y.J.; Li, T.X.; Riffat, S.B.; Wajid, N.M. Development and Thermochemical Characterizations of Vermiculite/SrBr2 Composite Sorbents for Low-Temperature Heat Storage. Energy 2016, 115, 120–128. [Google Scholar] [CrossRef]
- Zou, D.; Yue, X.; He, T.; Ding, J.; Ba, D. Experimental Research on the Preparation of K2CO3/Expanded Vermiculite Composite Energy Storage Material. Materials 2022, 15, 3702. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Ding, Y.; Sciacovelli, A. Hydration Kinetics of K2CO3, MgCl2 and Vermiculite-Based Composites in View of Low-Temperature Thermochemical Energy Storage. J. Energy Storage 2021, 38, 102561. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Houben, J.; Fischer, H.; Huinink, H.P. Stabilization of K2CO3 in Vermiculite for Thermochemical Energy Storage. Renew. Energy 2020, 150, 990–1000. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81 A, 89–96. [Google Scholar] [CrossRef]
- Perry, D.L. Handbook of Inorganic Compounds; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-13036-6. [Google Scholar]
- Michel, B.; Mazet, N.; Mauran, S.; Stitou, D.; Xu, J. Thermochemical Process for Seasonal Storage of Solar Energy: Characterization and Modeling of a High Density Reactive Bed. Energy 2012, 47, 553–563. [Google Scholar] [CrossRef]
- Mazur, N.; Huinink, H.; Borm, B.; Sansota, S.; Fischer, H.; Adan, O. Thermodynamic Analysis of Dehydration of K2CO3·1.5H2O. Thermochim. Acta 2022, 715, 179286. [Google Scholar] [CrossRef]
- Yan, T.S.; Li, T.X.; Xu, J.X.; Chao, J.W. Understanding the Transition Process of Phase Change and Dehydration Reaction of Salt Hydrate for Thermal Energy Storage. Appl. Therm. Eng. 2020, 166, 114655. [Google Scholar] [CrossRef]
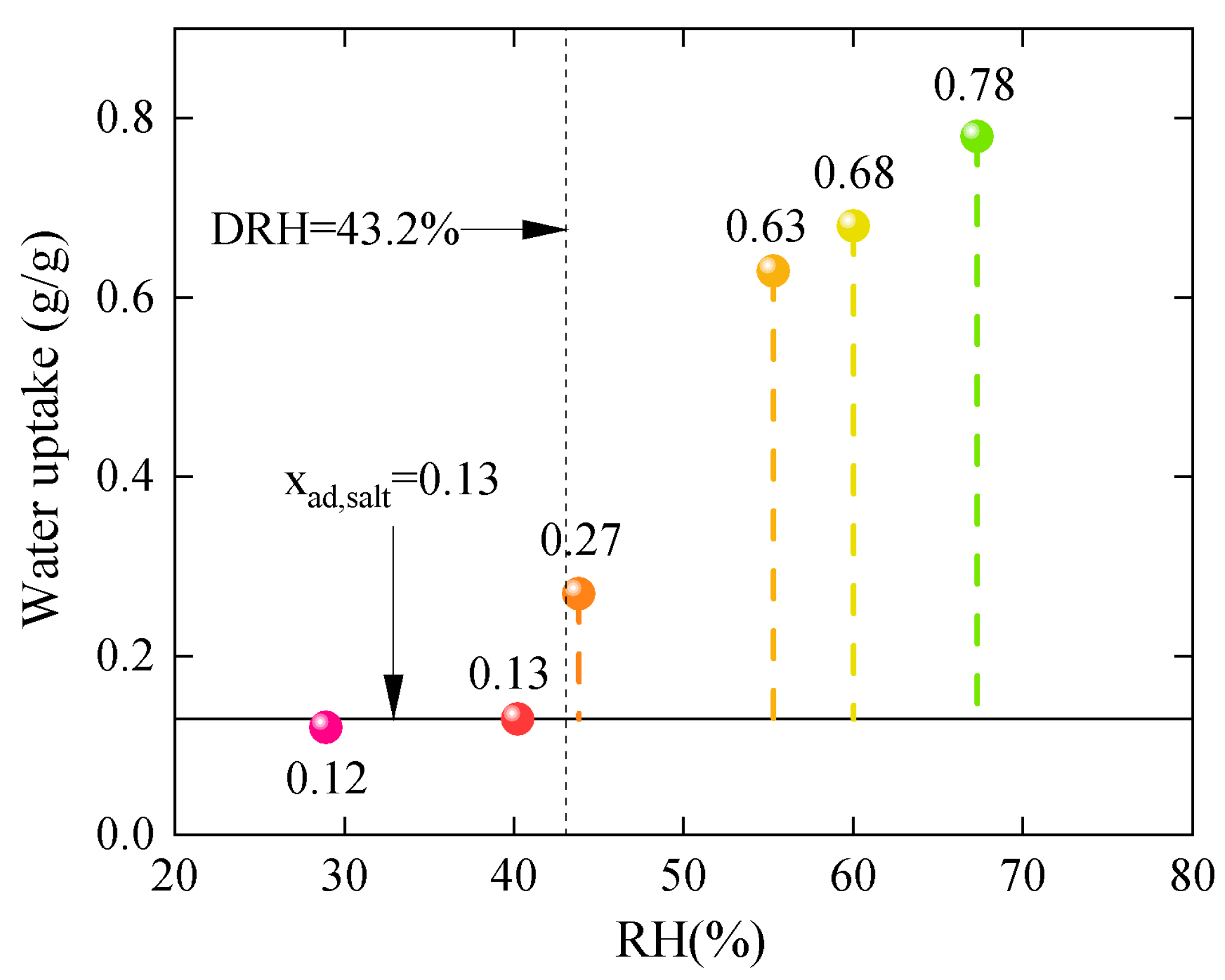
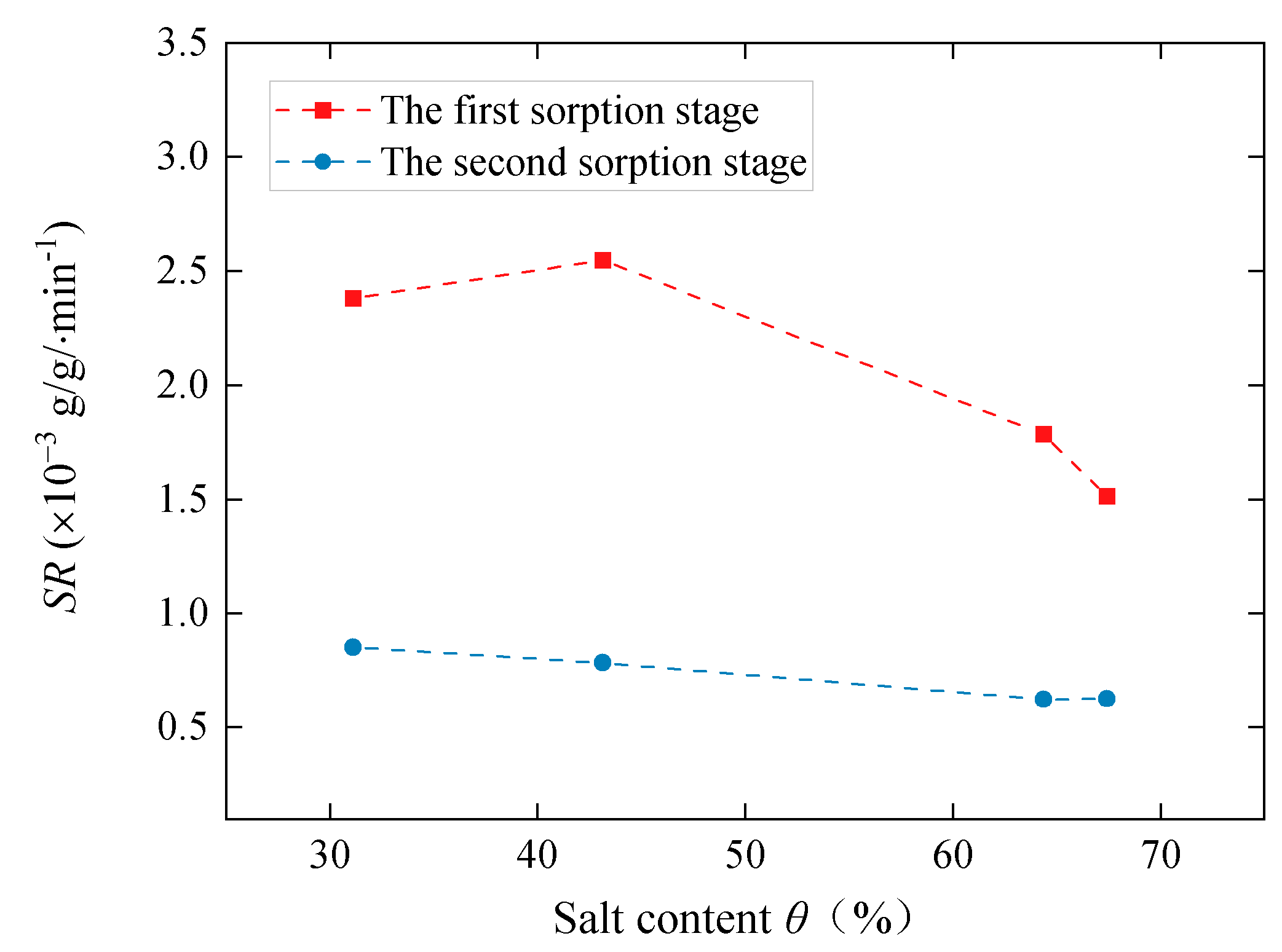
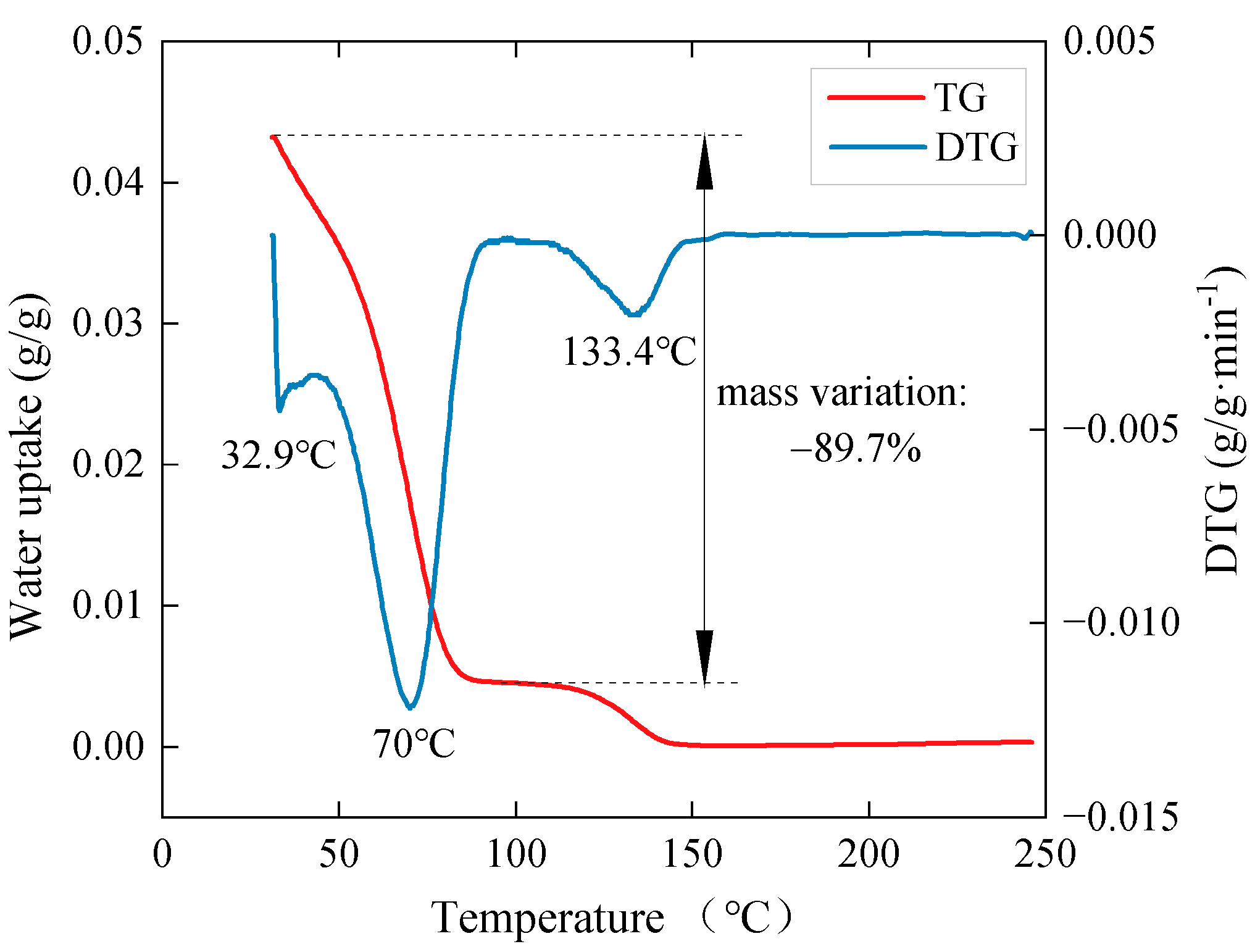
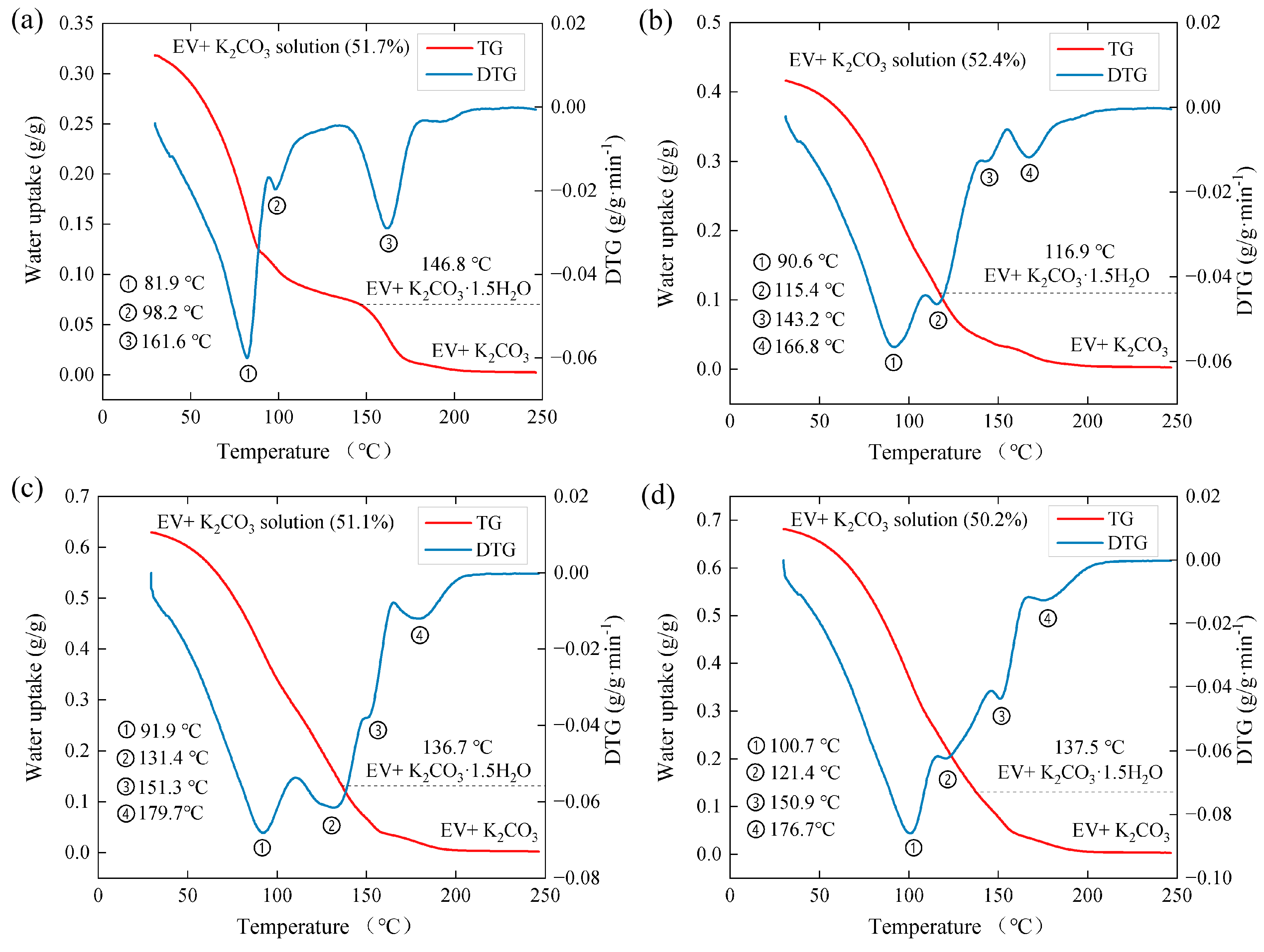

| Samples | EV | EVPC10 | EVPC20 | EVPC30 | EVPC40 | K2CO3 |
|---|---|---|---|---|---|---|
| Salt content, θ (%) | 0 | 31.1 | 43.1 | 64.4 | 67.4 | 100 |
| Bulk density, ρc (kg/m3) | 128 1 | 181 | 216 | 327 | 353 | 2428 2 |
| Samples | EVPC10 | EVPC20 | EVPC30 | EVPC40 |
|---|---|---|---|---|
| (g/g) | 0.03 | 0.02 | 0.02 | 0.02 |
| (g/g) | 0.07 | 0.11 | 0.13 | 0.13 |
| (g/g) | 0.22 | 0.29 | 0.48 | 0.53 |
| (g/g) | 0.32 | 0.42 | 0.63 | 0.68 |
| (%) | 51.7 | 52.4 | 51.1 | 50.2 |
| Samples | EV | EVPC10 | EVPC20 | EVPC30 | EVPC40 |
|---|---|---|---|---|---|
| Desorption heat (kJ/kg) | 81.6 | 818.0 | 1091.3 | 1572.0 | 1633.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Zhao, Q.; Huang, H. Performance Analysis of Vermiculite–Potassium Carbonate Composite Materials for Efficient Thermochemical Energy Storage. Energies 2024, 17, 2847. https://doi.org/10.3390/en17122847
Lin J, Zhao Q, Huang H. Performance Analysis of Vermiculite–Potassium Carbonate Composite Materials for Efficient Thermochemical Energy Storage. Energies. 2024; 17(12):2847. https://doi.org/10.3390/en17122847
Chicago/Turabian StyleLin, Jianquan, Qian Zhao, and Haotian Huang. 2024. "Performance Analysis of Vermiculite–Potassium Carbonate Composite Materials for Efficient Thermochemical Energy Storage" Energies 17, no. 12: 2847. https://doi.org/10.3390/en17122847
APA StyleLin, J., Zhao, Q., & Huang, H. (2024). Performance Analysis of Vermiculite–Potassium Carbonate Composite Materials for Efficient Thermochemical Energy Storage. Energies, 17(12), 2847. https://doi.org/10.3390/en17122847






