Abstract
The aim of the study was to determine the feasibility of using maize biomass for the phyto-stabilisation of iron-contaminated soils under conditions involving the application of humic acids (HAs). The biomass yield content of maize trace elements was analysed. In the absence of HAs, the first dose of Fe-stimulated plant biomass growth was compared to the absence of Fe contamination. The highest soil Fe contamination resulted in a very large reduction in maize biomass yield, with a maximum of 93%. The addition of HAs had a positive effect on plant biomass, with a maximum of 53%, and reduced the negative effect of Fe. There was an almost linear increase in maize biomass yield with increasing doses of HAs. Analogous changes were observed in dry matter content in maize. Soil treatment with Fe caused a significant increase in its content in maize biomass, with a maximum increase of three times in the series without HAs. There was also a decrease in Co, Cr and Cd content (by 17%, 21% and 44%, respectively) and an increase in Cu, Ni, Pb, Zn and Mn accumulation (by 32%, 63%, 75%, 97% and 203%, respectively). The application of HAs to the soil reduced the content of this trace element and its growth in the biomass of this plant under the influence of Fe contamination. They had a similar effect on other trace elements contained in the maize biomass. HAs contributed to a decrease in the level of most of the tested trace elements (except Ni and Pb) in the maize biomass. The reduction ranged from 11% (Cr and Mn) to 72% (Cd). The accumulation of Ni and Pb in the maize biomass was higher in the objects with HAs application than in the series without their addition. Humic acid application is a promising method for the reduction of the effects of soil Fe contamination on plants.
1. Introduction
Plant biomass is a renewable, abundant and CO2-neutral energy source [1]. It can be a raw material for the production of solid (wood chips, pellets and briquettes), liquid (ethanol and methanol) and gaseous (wood gas and methane) energy carriers [2]. Its versatility makes it an alternative to coal and other exhaustible energy sources such as natural gas and oil [3]. In addition, biomass is an important component of sustainable development, climate change mitigation [4] and enhancing the country’s energy security.
As a processed fuel, biomass can meet a wide range of final energy needs. Unlike wind or hydropower, from which we currently receive only electricity, biomass can be used to produce electricity, heat, or as biofuel for vehicles [5]. Importantly, wind and hydropower production is significantly affected by climate change, which can reduce it by up to 40% in some regions [6], and hydropower plants themselves cause disruptions to the aquatic environment. Burning biomass is the simplest and most widespread method of converting it into energy [7]. Biomass energy is even more important for the development of unconventional energy, as it is one of the more stable renewable energy sources compared to solar or wind [6]. Increased cultivation of energy crops can also contribute to the creation of new local and independent energy markets [8], as well as stimulate agricultural development and reduce unemployment.
Raw materials from the processing of various types of plant biomass can also be used to produce polymeric fibres and composites and nanomaterials [9,10]. Waste plant biomass can be used as a feedstock for the production of graphene, graphene oxide (GO) [11] and reduced graphene oxide (rGO) [12] via hydrothermal carbonisation or two-step pyrolysis. These carbon nanomaterials are characterised by very good thermal and electrical conductivity, mechanical strength, transparency and high specific surface area [10]. They are used in water purification, CO2 sequestration, batteries and capacitors for energy storage; in medicine as drug carriers; and for the bio-imaging of cell structures in vitro [9], as well as a substitute for conventional antibiotics in medical implants [12]. In a study by Supriyanto et al. [11], GO was obtained from graphite produced from three waste materials (sugarcane bagasse, rice husk and coconut shell) using a modified Hummers method. The authors obtained the most promising results for GO prepared from graphite obtained from rice husk. On the other hand, Thangaraj et al. [12] used dry sugar cane leaves subjected to double pyrolysis to prepare rGO. The resulting rGO nanoparticles exhibited antibacterial properties against Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae.
One of the crops that provides a suitable feedstock for green energy production is maize. The high yield potential of this crop, which reaches 12 to 15 tonnes of dry matter (DM) of whole plants per hectare [13], promotes a growing interest in its use not only for biogas or bioethanol production but also for direct combustion. High competition in the market for agricultural products, as well as the possibility of growing maize in monocultures and reducing production costs, are additional factors in favour of using maize for energy purposes. Maize straw has a calorific value of 17.65–18.60 MJ kg−1 DM [14], depending on the different fractions, moisture content, plant age and variety. Due to its low soil requirements and high adaptability to different agroclimatic conditions [15], maize can be grown on marginal land, low-productivity land and soils contaminated with trace elements, including iron (Fe) [16]. Iron is an essential element for normal plant growth and development, as it is involved in many important metabolic pathways, including chlorophyll synthesis, photosynthesis and mitochondrial respiration [17]. However, its excess in plant tissues leads to increased production of free radicals, which irreversibly damage structural elements of the cell, as well as protein, lipid and DNA molecules [18]. They also affect the accumulation of oxidised polyphenols, leading to leaf browning [19], a decrease in the rate of photosynthesis and oxidation of chlorophyll [20]. In addition, reduced plant growth, root dwarfism (especially of adventitious roots), leaf necrosis [18], delayed flowering and maturity by up to 20–25 days [19], and consequently reduced crop yields are observed under conditions of soil iron excess [21]. The phytotoxic effect of iron can also be manifested by reduced uptake of other nutrients, including phosphorus, potassium, calcium, manganese and copper [22], due to the deposition of its oxide precipitate on the root surface (so-called indirect toxicity) [23].
The effects of trace element contamination of soils are rarely immediately apparent. Instead, they can lead to dangerous ecological changes with a time lag. Importantly, unlike other types of pollution, trace elements do not biodegrade, but only biotransform as a result of various physical, chemical and biological processes in the soil [24]. This, in turn, can lead to the accumulation of trace elements downstream in the trophic chain, which is threatening to human health [25]. Soil processes influence trace element mobility and bioavailability in plant–soil interactions [26]. Among the factors that determine the amount of soil trace elements in phyto-available form, the most important are pH, presence of organic matter [27], granulometric composition and sorption capacity [28], moisture content, presence of iron, aluminium and manganese hydroxides and clay fractions [29]. Most trace elements, with the exception of molybdenum, are more readily taken up by plants when soil pH is acidic. Under alkaline conditions, insoluble anion complexes of trace elements are formed, inhibiting their uptake by plants [30]. Increasing the organic matter content of soils decreases the mobility and availability of trace elements for plants, mainly due to precipitation of insoluble compounds, formation of complexes and adsorption by humic acids [31,32]. As shown by some authors, humic acids (HAs) influence the content of trace elements in plants [33] and soil [34,35]. Humic acids are used as organic amendments to improve the efficiency of remediation of contaminated soils, especially those with low sorption capacity [36]. These acids affect the speciation, solubility and bioavailability of trace elements by altering soil pH and reducing soil conditions [37]. In addition, they are a source of nitrogen and phosphorus needed for plant growth [38,39], affect soil physicochemical properties by increasing cation exchange capacity and shaping water–air relations [40], and promote the growth of beneficial microorganisms [41,42]. High-value elements are particularly susceptible to irreversible binding by organic matter. The ions of these elements are capable of forming very strong covalent bonds with carboxyl and phenolic groups in HAs, which significantly reduces their desorption into soil, migration and availability to plants, even under very low soil pH conditions [43].
There is a paucity of publications in the literature on the potential of phyto-stabilisation of iron-contaminated soils. The use of humic acids as a support seems to be a promising method for the phyto-stabilisation of iron-contaminated soils (with the possibility of using the plants for energy purposes) and represents a new element in research. Humic acids have a beneficial effect on the growth of plant biomass in soils contaminated with iron [44].
Any action to support climate–energy policies is necessary because of the threats posed by global warming, pollution and dependence on fossil fuels [45]. For this reason, a study was carried out in which maize biomass was used to phyto-stabilise iron-polluted soils with humic acids (HAs) application. The effects on both biomass yield and the trace element contents in the aerial parts of this plant were determined.
2. Materials and Methods
2.1. Pot Vegetatiwe Experiment
The vegetation pot experiment was carried out in a vegetation hall on light soil taken from an arable layer. It had the granulometric composition of loamy sand [46]. The characteristics of the soil were as follows: pH1M KCl—6.51; cation exchange capacity (CEC)—82.83 mmol(+) kg−1; total organic carbon (TOC)—3.183 g kg−1 dry matter (DM); total N—0.313 g kg−1 DM; with available forms of P—128.80 mg kg−1 DM, K—112.0 mg kg−1 DM, and Mg—49.55 mg kg−1 DM; total Fe—10.46 g kg−1 DM. The experiment investigated the effect of soil contamination with iron at 0, 250, 500 and 750 mg kg−1 of soil on maize biomass yield and trace element content. Iron was applied as FeCl3. To reduce the potential negative effects of iron on maize, humic acids were added to the soil as follows: 0, 0.3, 0.6 and 0.9 g kg−1 of soil. They were applied when the experiment was set up and at the 5-leaf maize stage (BBCH 15). In addition, nitrogen, phosphorus and potassium were applied in equal amounts throughout the experiment as follows: 160 mg of N, 60 mg of P and 170 mg of K kg−1 of soil, which prevented additional stress to the plants due to a lack of basic nutrients. All the above additives were thoroughly mixed with 9 kg of soil (according to the experimental design) and transferred into polyethylene pots. A test crop—maize (Zea mays L.)—was then sown, which has good phytoremediation properties and is often used as an energy crop. The experiment was replicated 3 times with 6 plants planted per pot. During plant vegetation, the maximum soil water capacity was 60%. The plants were watered with distilled water. The photoperiod ranged from 15 h 24 min to 16 h 55 min, and the insolation ranged from 218.1 h to 335.6 h. The maize was harvested at the tasseling stage (BBCH 59). At the same time, plant samples were taken from each pot.
2.2. Analytical Methods
After harvesting, plant samples were cut, dried at 60 °C and ground to flour. Plant samples were microwave wet-digested in 65% nitric acid. Digestion was performed according to the standard method US-EPA3051 [47]. Trace elements were determined by atomic absorption spectrometry [48] using standard (Cd 51994, Cr 02733, Cu 38996, Co 119785.0100, Fe 16596, Mn 63534, Ni 42242, Pb 16595 and Zn 188227—Fluka Company, Charlotte, NC, USA) and certified reference materials (NCS ZC 73030—Chinese National Analysis Center for Iron and Steel, Beijing, China) to ensure adequate precision of the analyses performed. The limits of quantification of the method were as follows: Cd: 0.02–3 mg L−1 for 228.8 nm; Co: 0.05–15 mg L−1 for 240.7 nm; Cr: 0.06–15 mg L−1 for 357.9 nm; Cu: 0.03–10 mg L−1 for 324.7 nm; Fe: 0.06–15 mg L−1 for 248.3 nm; Mn: 0.02–5 mg L−1 for 279.5 nm; Ni: 0.1–20 mg L−1 for 232.0 nm; Pb: 0.1–30 mg L−1 for 217.0 nm; Zn: 0.01–2 mg L−1 for 213.9 nm. The maximal limits of quantification were as follows: Cd—1000 mg L−1, Co—30,000 mg L−1, Cr—6000 mg L−1, Cu—2000 mg L−1, Fe—3200 mg L−1, Mn—14,000 mg L−1, Ni—8000 mg L−1, Pb—8000 mg L−1 and Zn—14,000 mg L−1.
2.3. Statistical Methods
Statistical analysis of the obtained experimental results was performed using multiple statistical methods: ANOVA two-factor analysis of variance, Tukey’s HSD post hoc test, simple correlation coefficients (r) and percentage of the observed variability. Tukey’s HSD test was calculated for a p ≤ 0.01 and simple correlation coefficients for ** p ≤ 0.01 and * p ≤ 0.05. Statistica software version 13.3 [49] was used to perform these calculations.
3. Results
3.1. Biomass Yield and Dry Mater Content
In the series without HAs, the first Fe dose (250 mg kg−1 soil) stimulated a 13% increase in plant biomass compared to the control without Fe (Figure 1 and Figure 2). The highest soil Fe dose resulted in a very large reduction in maize biomass yield, with a maximum of 93%. The HAs application had a positive effect on plant biomass by reducing the negative effect of Fe. The reduction in biomass yield was 84% (at the first dose of HAs) and 64% (at the highest dose of HAs) in relation to the control site (without Fe). There was an almost linear increase in maize biomass yield with increasing doses of HAs. Average maize aerial fresh matter yields from all objects in the series were 13, 34 and 53% higher at the lowest, medium and highest doses of HAs, respectively, compared to the series without HAs application.
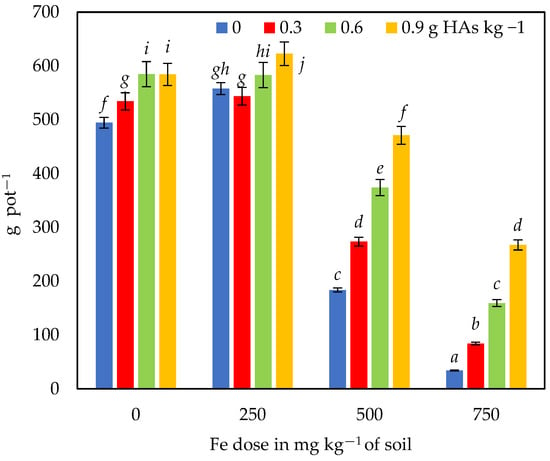
Figure 1.
Biomass yield of maize—Zea mays L. Different letters (a–j) for interactions between HAs and Fe dose are significant at p ≤ 0.01.
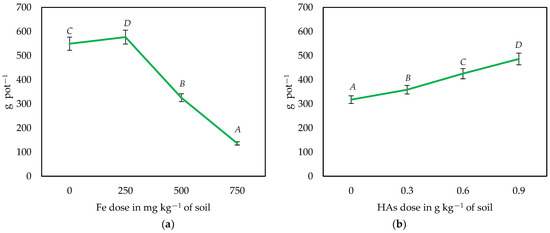
Figure 2.
Influence of the dose of Fe (a) and the application of HAs (b) on the biomass yield of maize—Zea mays L. (averaged over all series). Different letters (A—D) for HAs and Fe doses are significant at p ≤ 0.01.
Analogous changes were observed in the DM content of maize following soil iron contamination and HAs application (Figure 3 and Figure 4). The DM content of the aerial parts of maize decreased with increasing soil iron contamination (especially in the series without HAs) and increased as a result of the application of HAs (especially in most Fe-contaminated objects).
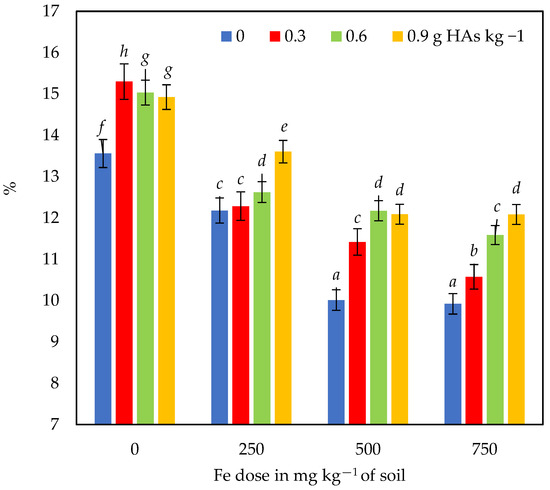
Figure 3.
Dry matter content in maize biomass. Different letters (a–h) for interactions between HAs and Fe dose are significant at p ≤ 0.01.
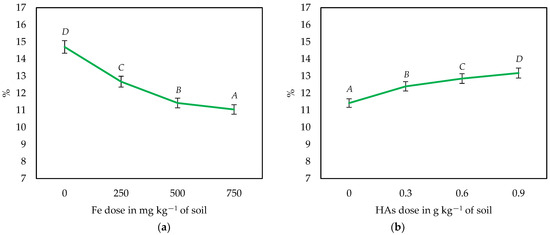
Figure 4.
Influence of the dose of Fe (a) and the application of HAs (b) on the dry matter content in maize biomass (averaged over all series). Different letters (A–D) for HAs and Fe doses are significant at p ≤ 0.01.
3.2. Iron
Iron contamination of the soil significantly increased its content in the maize biomass (Table 1). In the series without HAs, Fe contamination increased its content in the maize biomass almost 3-fold compared to the control object without Fe addition. HAs application reduced the Fe content and its growth in the biomass of this plant under the influence of Fe contamination. In the objects with the first dose of HAs, the increase in iron content in maize was more than double that of the control. The average iron content in this series was 39% lower than in the series without HAs.

Table 1.
Iron, cadmium and lead content of maize biomass, mg kg−1 DM.
3.3. Other Trace Elements
Soil contamination with iron had a significant effect on the content of other trace elements in maize biomass (Table 1, Table 2 and Table 3). In the series without HAs, under the influence of the highest dose of iron, there was a maximum reduction in the content of Co by 17%, Cr by 21% and Cd by 44%, and an increase in the accumulation of Cu by 32%, Ni by 63%, Pb by 75%, Zn by 97% and Mn by 203% compared to the control (without Fe addition). The addition of HAs to the soil contributed to a reduction in the content of most of the tested trace elements (except Ni and Pb) in the maize biomass (Table 1, Table 2 and Table 3). The addition of HAs to the soil at the highest dose reduced the content of Cr and Mn by an average of 11%, Zn by 23%, Co by 30%, Cu by 32% and Cd by an average of 72% in the aerial parts of maize compared with the series without HAs. On the other hand, the accumulation of Ni in the maize biomass increased by an average of 68% and that of Pb by 167% compared with the above series of experiments.

Table 2.
Chromium, nickel and zinc content of aerial parts of maize (Zea mays L.), mg kg−1 DM.

Table 3.
Copper, manganese and cobalt content of aerial parts of maize (Zea mays L.), g kg−1 DM.
3.4. Relations beetwen Variables
The coefficient correlations confirm the existence of significant correlations between many of the study parameters (Table 4). Highly significant positive correlations were found between maize biomass yield and DM content; iron content and zinc; iron and copper; iron and manganese; manganese and nickel; manganese and zinc; manganese and copper; cadmium and chromium; cadmium and copper; copper and zinc; copper and cobalt; lead and nickel. Maize yield and DM content were negatively correlated with iron, zinc, manganese and copper. Similar (negative) relationships were found between lead and chromium; lead and copper; lead and cobalt; chromium and nickel; chromium and zinc; chromium and manganese; cadmium and lead; cadmium and nickel; and nickel and cobalt.

Table 4.
Correlation between variables in maize (Zea mays L.).
The percentage cumulative effect of first- and second-order factors (soil contamination with Fe and soil additive HAs) on maize biomass is shown in Figure 5. Overall, the effect of soil Fe contamination on maize biomass parameters was more pronounced than that of soil HAs. Soil Fe contamination had the greatest effect on manganese (95.4%), zinc (86.6%), DM (85.9%), plant biomass yield (85.5%), chromium content (47.2%) and nickel content (43.2%). The amendment of HAs had the strongest effect on cadmium (85.3%), lead (79.0%), copper (58.9%) and cobalt (56.9%) contents. In contrast, the interaction between excessive soil Fe and HAs had the greatest effect on the maize Fe content (55.3%). Excessive soil Fe also had a significant effect on iron (21.7%) and copper (23.9%) accumulation in maize biomass. HAs had a similar effect on iron content (21.3%) in maize.
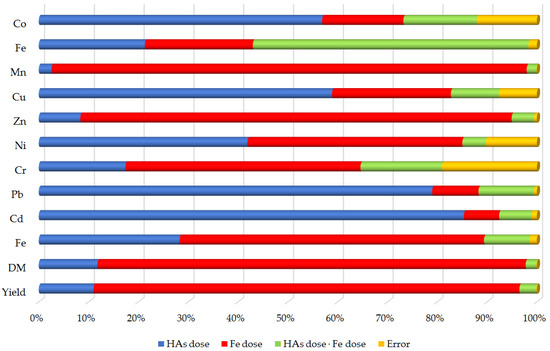
Figure 5.
Relatively effect of factors on yield, DM and trace elements of maize—Zea mays L. (in percent).
4. Discussion
Soil Fe contamination negatively affected maize biomass yield and dry matter content in our study. In the series with the highest level of contamination, biomass yield was reduced by 93% compared to the control. This is in line with observations by other researchers [50,51]. In an experiment by Olaleye et al. [52], soil iron contamination (1000–4000 µg L−1) negatively affected the yield of two rice varieties. In the study by Olaleye et al. [52], compared to the control, DM content and plant height decreased from 7% to 39% and 6% to 11%, respectively, at the highest Fe contamination. Similar findings have been reported by Ahmed et al. [51], who showed a significant reduction in rice root DM and aerial parts on Fe-contaminated soil with 300 mg L−1 Fe. The negative effect of excess iron (8 mM FeEDTA) on root growth, aerial biomass yield, and root and aerial DM content of Carica papaya was reported by Rodrigues Filho et al. [53].
Iron, as a cofactor for a number of essential enzymes required for electron transfer reactions in photosynthesis and mitochondrial respiration (including ferredoxin), affects the rate of photosynthesis, its productivity and thus plant growth and development [54]. Disruption of iron homeostasis can therefore negatively affect energy production and metabolism in the plant, resulting in stunted growth and development and reduced carbon dioxide in sugars and DM [55]. In sensitive species, over-optimal Fe accumulation can lead to total crop failure [56]. Iron affects soil nitrogen (N) cycling under both aerobic and anaerobic conditions and stimulates nitrification activity in low-pH soils [57]. Thus, its excess can interfere with N availability and transport to plants, resulting in reduced biomass yield [58]. Adamski et al. [59] also confirmed the negative effect of excess iron on the productivity of Ipomoea batatas L. The plant was grown in a hydroponic system using Fe-EDTA at four concentrations: 0.45, 0.9, 4.5 and 9.0 mmol L−1. The greatest changes were observed in the solution containing 4.5 and 9.0 mmol L−1 Fe. Under these conditions, plant height, leaf area and total biomass decreased significantly compared to the least contaminated series. There were also changes in the trace element content of the test plant leaves. In response to Fe toxicity, zinc, manganese and copper contents decreased by 22%, 79% and 21% respectively. Reduced yield, survival and stunted growth of rice grown in a hydroculture system at Fe2+ doses > 250 mg L−1 were also shown by De Dorlodot et al. [60]. The authors found that under these conditions, the levels of manganese, copper, molybdenum and zinc in the aerial parts of rice decreased compared to the control (unpolluted) object.
In our study, increasing iron contamination of the soil resulted in a decrease in the levels of cobalt, chromium and cadmium and an increase in the accumulation of iron, copper, lead, nickel, zinc and manganese. Excess iron affects the mineral composition of plant tissues as a result of so-called indirect toxicity and competition with other cations for the same physiological binding site in the transporter protein [61]. As reported by Krüeger et al. [62], the protein that binds Fe2+ and is responsible for its long-distance phloem transport in Ricinus communis can also bind Cu2+, Zn2+ and Mn2+ cations. This lack of specificity affects the intensity of uptake of other elements and their translocation to the shoots. In addition, excess iron can irreversibly inhibit the activity of the cell membrane H+-ATPase by oxidising the sulfhydryl groups of the enzyme [63], thus limiting the uptake and translocation of trace elements. Some differences in the trend of the influence of excess iron on the accumulation of selected trace elements in plant tissues in our study and that of the authors cited above may be due to the different plant species used in the experiments, as well as to the cultivation system used and the different compounds used as iron sources. The FeCl3 used in our study may have adversely affected maize growth and development as a result of the antagonistic interaction of Cl− with nitrate (NO3−) and the reduced uptake of NO3− by plants [64,65]. In addition, excess Cl− reduces stomatal conductance and transpiration rate and disrupts ion homeostasis [65], which may lead to changes in the uptake of other ions.
Our study showed that HAs application benefited maize biomass yield and DM content and reduced most of the tested trace elements (except nickel and lead) in maize biomass. Bezuglova et al. [66] found similar results. In the Bezuglova et al. [66] experiment, the addition of a humic substance preparation (HS; 4 L ha−1) significantly improved the soil mineral supply and increased the yield of winter wheat by 22.5%. These authors showed that HS application had a beneficial effect on root microflora activity and increased phosphorus availability to plants. Yuan et al. [67] also reported an increase in aerial biomass yield and DM content of the aerial parts of maize after HS application (450 mg kg−1). The positive effect of soil application of HAs (1.5 mL L−1) on the growth and yield of lettuce was reported by Raheem et al. [68]. Under these conditions, the researchers reported a 28.5% increase in fresh weight yield compared to a series without the application of HAs.
Introducing humic matter into the soil improves soil structure and fertility [69], which can lead to better crop growth and higher yields. They also increase NH4+ adsorption capacity and soil organic carbon content, promote microbial activity [70] and increase the number, diameter and length of roots [71], especially lateral and trichome roots, which are extremely important for nutrient and water uptake [72]. Nunes et al. [71] showed that the addition of HAs (5 g L−1) to maize plants, by acting on the cells at the proteomic level, resulted in an increase in fresh root weight compared to the control series. They showed that HAs increased the expression of proteins related to the energy metabolism of root cells, their cytoskeleton, cellular transport, protein conformation and degradation and DNA replication.
Due to their chelating capacity, HS has a strong effect on plant chemistry, stimulating the uptake of certain elements or reducing their toxicity through a neutralising effect [73]. In our other study [33], the application of HS at a dose of 0.15 g kg−1 significantly affected the content of maize aerial parts trace elements on two soil types and with different types of nitrogen fertilisers. The content of cadmium, cobalt, iron, zinc and manganese was reduced in plants grown on sandy loam, while the accumulation of copper, iron, manganese, zinc and cobalt was reduced in plants grown on loamy sand after the addition of HAs in the series with ammonium nitrate. Analogical results were presented by Ondrasek et al. [74], who investigated the influence of HAs (0–225 mg L−1) on the growth and trace element uptake and accumulation in roots, shoots and hypocotyls of radish (Raphanus sativus L.) grown in cadmium-contaminated soil (0.5 mg L−1). They showed that the application of HAs at the highest dose reduced the total accumulation of Cu (by 73%), Cd (by 39%), Zn (by 29%) and Mn (by 22%) in the plants. Ondrasek et al. [74] also reported that HAs (≥15 mg L−1) can be used to control the amount of soil phyto-available trace elements forms, and consequently their uptake and translocation to individual plant organs. The limiting effect of humic substances on the soil trace element mobility and accumulation in plant tissues is mainly due to the formation of metal–humate complexes with HS functional groups (-OH, -COOH, -NH3 and -OCH3) [75], as well as the increase in soil pH and organic matter content [76].
Replacing non-renewable feedstocks with biomass provides an opportunity not only for the development of green energy sources but also for increased energy security and new avenues for rural development. By enabling the use of low-productivity or polluted agricultural land, energy crops combine economic efficiency with environmental concerns. Therefore, research on renewable energy production and the factors influencing the supply of plant biomass on contaminated land is still needed, especially in view of the development of the circular economy.
5. Conclusions
In the non-HAs series, the first Fe dose (250 mg kg−1 of soil) stimulated an increase in plant biomass compared to the absence of Fe contamination. A further increase in soil Fe contamination resulted in a very large reduction in maize biomass yield, with a maximum of 93%. The application of HAs had a positive effect on plant biomass by reducing the negative effect of iron. There was an almost linear increase in maize biomass yield with increasing doses of HAs, with a maximum of 53%. Similar changes were observed in DM content.
Soil treatment with iron caused a significant increase in its content in maize biomass, with a maximum increase of three times in the series without HAs. There was also a decrease in the level of cobalt by 17%, chromium by 21% and cadmium by 44%, and an increase in the accumulation of copper, nickel, lead, zinc and manganese by 32%, 63%, 75%, 97% and 203%, respectively, compared to the control (without Fe addition).
The application of HAs to the soil reduced the content of this trace element (by 39%) and their growth in the biomass of this plant under Fe contamination. Other trace element contents in maize biomass were similarly affected. HAs contributed to a reduction in the level of most of the tested trace elements (except nickel and lead) in the maize biomass. The reduction ranged from 11% (Cr and Mn) to 72% (Cd). The accumulation of nickel and lead in the maize biomass was higher in the sites with the application of HAs than in the series without their addition.
The application of HAs is a promising method for the reduction in the effects of soil iron contamination on plants.
Author Contributions
Conceptualization, M.W.; methodology, M.W.; validation, M.W.; formal analysis, M.W. and N.K.; investigation, N.K.; resources, M.W.; data curation, M.W.; writing—original draft preparation, M.W. and N.K.; writing—review and editing, M.W. and N.K.; visualisation, M.W. and N.K.; supervision, M.W.; project administration, M.W.; funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the Department of Agricultural and Environmental Chemistry, Faculty of Agriculture and Forestry, University of Warmia and Mazury in Olsztyn (grant No. 30.610.004-110).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Okot, D.K.; Bilsborrow, P.E.; Phan, A.N.; Manning, D.A.C. Kinetics of maize cob and bean straw pyrolysis and combustion. Heliyon 2023, 9, e17236. [Google Scholar] [CrossRef]
- Kalak, T. Potential use of industrial biomass waste as a sustainable energy source in the future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Moreira, J.R. Global biomass energy potential. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 313–342. [Google Scholar] [CrossRef]
- Bilgili, F.; Koçak, E.; Bulutc, Ü.; Kuşkaya, S. Can biomass energy be an efficient policy tool for sustainable development? Renew. Sustain. Energy Rev. 2017, 71, 830–845. [Google Scholar] [CrossRef]
- Sahoo, G.; Sharma, A.; Dash, A.C. Biomass from trees for bioenergy and biofuels—A briefing paper. Mater. Today Proc. 2022, 65, 461–467. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Cost, environmental impact, and resilience of renewable energy under a changing climate: A review. Environ. Chem. Lett. 2023, 21, 741–764. [Google Scholar] [CrossRef]
- Batista, R.M.; Converti, A.; Pappalardo, J.; Benachour, M.; Sarubbo, L.A. Tools for optimization of biomass-to-energy conversion processes. Processes 2023, 11, 854. [Google Scholar] [CrossRef]
- Jasinskas, A.; Petlickaite, R.; Jotautiene, E.; Lemanas, E.; Soucek, J. Assessment of energy properties of maize and multi-croppellets and environmental impact of their combustion. In Proceedings of the 21st International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2022. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Mumtaz, F.; Abbas, Y.; Anjum, D.H.; Solomon, P.R.; Hassan, J. Synthesis of graphene oxide from sugarcane dry leaves by two-stage pyrolysis. Molecules 2023, 28, 3329. [Google Scholar] [CrossRef]
- Supriyanto, G.; Rukman, N.K.; Nisa, A.K.; Jannatin, M.; Piere, B.; Abdullah, A.; Fahmi, M.Z.; Kusuma, H.S. Graphene oxide from Indonesian biomass: Synthesis and characterization. BioResources 2018, 13, 4832–4840. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Wongyao, N.; Helal, M.I.; Abdullah, A.; Abedrabbo, S.; Hassan, J. Synthesis of reduced graphene oxide nanosheets from sugarcane dry leaves by two-stage pyrolysis for antibacterial activity. Nano Mat. Sci. 2024, 1–10. [Google Scholar] [CrossRef]
- Zbytek, Z.; Dach, J.; Pawłowski, T.; Smurzyńska, A.; Czekała, W.; Janczak, D. Energy and economic potential of maize straw used for biofuels production. MATEC Web Conf. 2016, 60, 04008. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize straw as a valuable energetic material for biogas plant feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef]
- Șimon, A.; Moraru, P.I.; Ceclan, A.; Russu, F.; Chețan, F.; Bărdaș, M.; Popa, A.; Rusu, T.; Pop, A.I.; Bogdan, I. The impact of climatic factors on the development stages of maize crop in the Transylvanian plain. Agronomy 2023, 13, 1612. [Google Scholar] [CrossRef]
- Maw, M.J.W.; Houx, J.H., III; Fritschi, F.B. Maize, sweet sorghum, and high biomass sorghum ethanol yield comparison on marginal soils in Midwest USA. Biomass Bioenerg. 2017, 107, 164–171. [Google Scholar] [CrossRef]
- Ning, X.; Lin, M.; Huang, G.; Mao, J.; Gao, Z.; Wang, X. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front. Plant Sci. 2023, 14, 1190768. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Siqueira-Silva, A.I.; Rios, C.O.; Pereira, E.G. Iron toxicity resistance strategies in tropical grasses: The role of apoplastic radicular barriers. J. Environ. Sci. 2019, 78, 257–266. [Google Scholar] [CrossRef]
- Saaltink, R.M.; Dekker, S.C.; Eppinga, M.B.; Griffioen, J.; Wassen, M.J. Plant-specific effects of iron-toxicity in wetlands. Plant Soil 2017, 416, 83–96. [Google Scholar] [CrossRef]
- Silveira, V.C.; Oliveira, A.P.; Sperotto, R.A.; Amaral, L.; Dias, J.F.; Cunha, J.B.; Fett, J.P. Influence of iron on mineral status of two rice (Oryza sativa L.) cultivars. Braz. J. Plant Physiol. 2007, 19, 127–139. [Google Scholar] [CrossRef]
- Siqueira-Silva, A.I.; Silva, L.C.; Azevedo, A.A.; Oliva, M.A. Iron plaque formation and morphoanatomy of roots from species of restinga subjected to excess iron. Ecotox. Environ. Safe. 2012, 78, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz, M.; Niemiec, M.; Wiśniowska-Kielian, B. Post-effect of increasing bottom sediment additives to the substratum on nickel uptake by plants. Ecol. Chem. Eng. A 2012, 19, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.; Molina-Montenegro, M.; Sandoval, C.; Rivas, N.; Espinoza, J.; Basualto, S.; Fierro, P.; Vargas-Chacoff, L. Human activity in Antarctica: Effects on metallic trace elements (MTEs) in plants and soils. Plants 2021, 10, 2593. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Soils and Plants, 1st ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015; p. 468. [Google Scholar] [CrossRef]
- Qishlaqi, A.; Moore, F. Statistical Analysis of accumulation and sources of heavy metals occurrence in agricultural soils of Khoshk River Banks, Shiraz, Iran. Am.-Eurasian J. Agric. Environ. Sci. 2007, 2, 565–573. [Google Scholar]
- Ukalska-Jaruga, A.; Siebielec, G.; Siebielec, S.; Pecio, M. The effect of soil amendments on trace elements’ bioavailability and toxicity to earthworms in contaminated soils. Appl. Sci. 2022, 12, 6280. [Google Scholar] [CrossRef]
- Pikuła, D.; Stępień, W. Effect of the degree of soil contamination with heavy metals on their mobility in the soil profile in a microplot experiment. Agronomy 2021, 11, 878. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D.; Gusiatin, Z.M.; Bulkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S.; Kordala, N. Trace element contents in maize following the application of organic materials to reduce the potential adverse effects of nitrogen. Materials 2023, 16, 215. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, R.; Peng, S.; Liu, Q.; Zhu, X. Effect of humic acid on transformation of soil heavy metals. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012089. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N.; Brodowska, M.S. Trace element content in soils with nitrogen fertilisation and humic acids addition. Agriculture 2023, 13, 968. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Bassouny, M.A.; Jahin, H.S.; Abdelhafez, A.A. Stabilization of Lead and Copper in a Contaminated Typic Torripsament Soil Using Humic Substances. Clean–Soil Air Water 2019, 47, 1800309. [Google Scholar] [CrossRef]
- Alvarenga, P.; Gonçalves, A.; Fernandes, R.; De Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Organic residues as immobilizing agents in aided phytostabilization:(I) Effects on soil chemical characteristics. Chemosphere 2009, 74, 1292–1300. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Kordala, N.; Brodowska, M. Role of humic acids-based fertilisers and nitrogen fertilisers in the regulation of the macroelement content in maize biomass. J. Elem. 2023, 28, 1289–1309. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J.; Borowik, A.; Kaczyński, P. Possibilities of restoring homeostasis of soil exposed to terbuthylazine by its supplementation with HumiAgra preparation. Appl. Soil Ecol. 2022, 178, 104582. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Gao, S.; Zhang, Z.; Huang, L. Effect of humic acid on phytoremediation of heavy metal contaminated sediment. J. Hazard. Mater. Adv. 2023, 9, 100235. [Google Scholar] [CrossRef]
- Tan, L.; Yu, Z.; Tan, X.; Fang, M.; Wang, X.; Wang, J.; Xing, J.; Ai, Y.; Wang, X. Systematic studies on the binding of metal ions in aggregates of humic acid: Aggregation kinetics, spectroscopic analyses and MD simulations. Environ. Pollut. 2019, 246, 999–1007. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Effects of humic acids on calorific value and chemical composition of maize biomass in iron-contaminated soil phytostabilisation. Energies 2024, 17, 1691. [Google Scholar] [CrossRef]
- Santos, F.D.; Ferreira, P.L.; Pedersen, J.S.T. The climate change challenge: A review of the barriers and solutions to deliver a Paris solution. Climate 2022, 10, 75. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; p. 236. Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 14 April 2023).
- US-EPA Method 3051A. Microwave Assisted Acid Digestion of Sediment, Sludges, Soils, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–30. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 22 December 2022).
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plants Properties, 1st ed.; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13.3 2017. Available online: http://statistica.io (accessed on 29 February 2024).
- Setter, T.L.; Waters, I.; Sharma, S.K.; Singh, K.N.; Kulshreshtha, N.; Yaduvanshi, N.P.; Ram, P.C.; Singh, B.N.; Rane, J.; McDonald, G.; et al. Review of wheat improvement for waterlogging tolerance in Australia and India: The importance of anaerobiosis and element toxicities associated with different soils. Ann. Bot. 2009, 103, 221–235. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Ullah, H.; Aung, M.Z.; Tisarum, R.; Cha-Um, S. Iron toxicity tolerance of rice genotypes in relation to growth, yield and physiochemical characters. Rice Sci. 2023, 30, 321–334. [Google Scholar] [CrossRef]
- Olaleye, A.O.; Ogunkunle, A.O.; Singh, B.N.; Akinbola, G.E.; Tabi, F.O.; Fayinminu, O.O.; Iji, M.E. Ratios of nutrients in lowland rice grown on two iron toxic soils in Nigeria. J. Plant Nutr. 2009, 32, 1336–1352. [Google Scholar] [CrossRef]
- Rodrigues Filho, J.; Corte, V.B.; Perin, I.T.A.L.; dos Santos, C.R.; da Silva, R.W. Effects of iron toxicity on germination and initial growth of Carica papaya L. Sci. Plena 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Wu, L.B.; Shhadi, M.Y.; Gregorio, G.; Matthus, E.; Becker, M.; Frei, M. Genetic and physiological analysis of tolerance to acute iron toxicity in rice. Rice 2014, 7, 8. [Google Scholar] [CrossRef]
- Audebert, A.; Sahrawat, K.L. Mechanisms for iron toxicity tolerance in lowland rice. J. Plant Nutr. 2000, 7, 1877–1885. [Google Scholar] [CrossRef]
- Huang, X.; Zhu-Barker, X.; Horwath, W.R.; Faeflen, S.J.; Luo, H.; Xin, X.; Jiang, X. Effect of iron oxide on nitrification in two agricultural soils with different pH. Biogeosciences 2016, 13, 5609–5617. [Google Scholar] [CrossRef]
- Song, Y.; Wan, G.-Y.; Wang, J.-X.; Zhang, Z.-S.; Xia, J.-Q.; Sun, L.-Q.; Lu, J.; Ma, C.-X.; Yu, L.-H.; Xiang, C.-B.; et al. Balanced nitrogen–iron sufficiency boosts grain yield and nitrogen use efficiency by promoting tillering. Moleculr. Plant 2023, 16, 1661–1677. [Google Scholar] [CrossRef] [PubMed]
- Adamski, J.M.; Danieloski, R.; Deuner, S.; Braga, E.J.B.; de Castro, L.A.S.; Peters, J.A. Responses to excess iron in sweet potato: Impacts on growth, enzyme activities, mineral concentrations, and anatomy. Acta Physiol. Plant 2012, 34, 1827–1836. [Google Scholar] [CrossRef]
- De Dorlodot, S.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Lombi, E.; Tearall, K.L.; Horwath, J.R.; Zhao, F.J.; Hawkesford, M.J.; McGrath, S.P. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2002, 128, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Krüeger, C.; Berkowitz, O.; Stephan, U.W.; Hell, R. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J. Biol. Chem. 2002, 277, 25062–25069. [Google Scholar] [CrossRef] [PubMed]
- Souza-Santos, P.; Ramos, R.S.; Ferreira, S.T.; Carvalho-Alves, P.C. Iron-induced oxidative damage of corn root plasma membrane H+-ATPase. Biochem. Biophys. Acta 2001, 1512, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Abou Seeda, M.A.; Abou El-Nour, E.A.A.; Hammad, S.A.; Yassen, A.A. Chloride ions as a beneficial and essential micronutrient multifunctional, role and regulation in plant physiology: A review. Middle East J. Appl. Sci. 2021, 11, 76–125. [Google Scholar]
- Zhang, X.; Franzisky, B.L.; Eigner, L.; Geilfus, C.-M.; Zörb, C. Antagonism of chloride and nitrate inhibits nitrate reductase activity in chloride-stressed maize. Plant Growth Regul. 2021, 93, 279–289. [Google Scholar] [CrossRef]
- Bezuglova, O.S.; Gorovtsov, A.V.; Polienko, E.A.; Zinchenko, V.E.; Grinko, A.V.; Lykhman, V.A.; Dubinina, M.N.; Demidov, A. Effect of humic preparation on winter wheat productivity and rhizosphere microbial community under herbicide-induced stress. J. Soils Sediments 2019, 19, 2665–2675. [Google Scholar] [CrossRef]
- Yuan, Y.; Gai, S.; Tang, C.; Jin, Y.; Cheng, K.; Antonietti, M.; Yang, F. Artificial humic acid improves maize growth and soil phosphorus utilization efficiency. Appl. Soil Ecol. 2022, 179, 104587. [Google Scholar] [CrossRef]
- Raheem, S.M.; Al-Jaf, H.I.; Tofiq, G.K. Influence of foliar and soil application of humic acid on growth and yield of lettuce. J. Biol. Agricult. Healthc. 2018, 8, 199–204. Available online: https://core.ac.uk/download/pdf/234662566.pdf (accessed on 24 April 2024).
- Aylaj, M.; Sisouane, M.; Tahiri, S.; Mouchrif, Y.; El Krati, M. Effects of humic acid extracted from organic waste composts on turnip culture (Brassica rapa subsp. rapa) in a sandy soil. J. Ecol. Eng. 2023, 24, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Nunes, R.O.; Domiciano, G.A.; Alves, W.S.; Melo, A.C.A.; Nougeira, F.C.S.; Canellas, L.P.; Olivare, F.L.; Zingali, R.B.; Soares, M.R. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 2019, 9, 12019. [Google Scholar] [CrossRef] [PubMed]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Horuz, A. Effects of humic acids from different sources on sodium and micronutrient levels in corn plants. Sains Malays. 2020, 49, 1533–1542. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z.; Romic, D. Humic acids decrease uptake and distribution of trace metals, but not the growth of radish exposed to cadmium toxicity. Ecotoxicol. Environ. Saf. 2018, 151, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Yang, L.; Chen, Y.; Wang, M.; Xiao, L.; Yuan, G. Enhancing soil remediation of copper-contaminated soil through washing with a soluble humic substance and chemical reductant. Agronomy 2023, 13, 1754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).