Electrode Materials with High Performance of Nickel Sulfide/Titanium Nitride@Co-Based Metal–Organic Frameworks/Nickel Foam for Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Substances and Chemical Agents
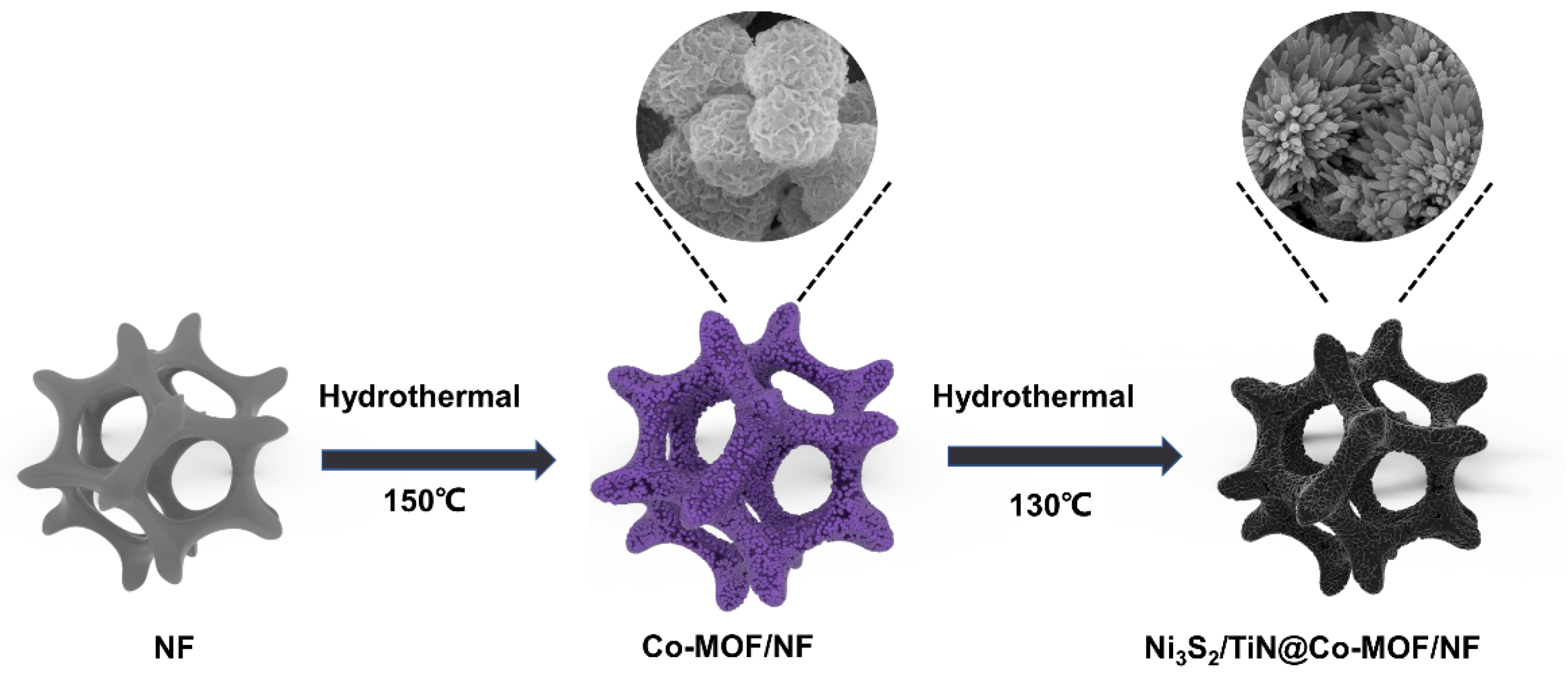
2.2. Electrode Material Preparation
2.3. Preparation of ASC
2.4. Structural Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
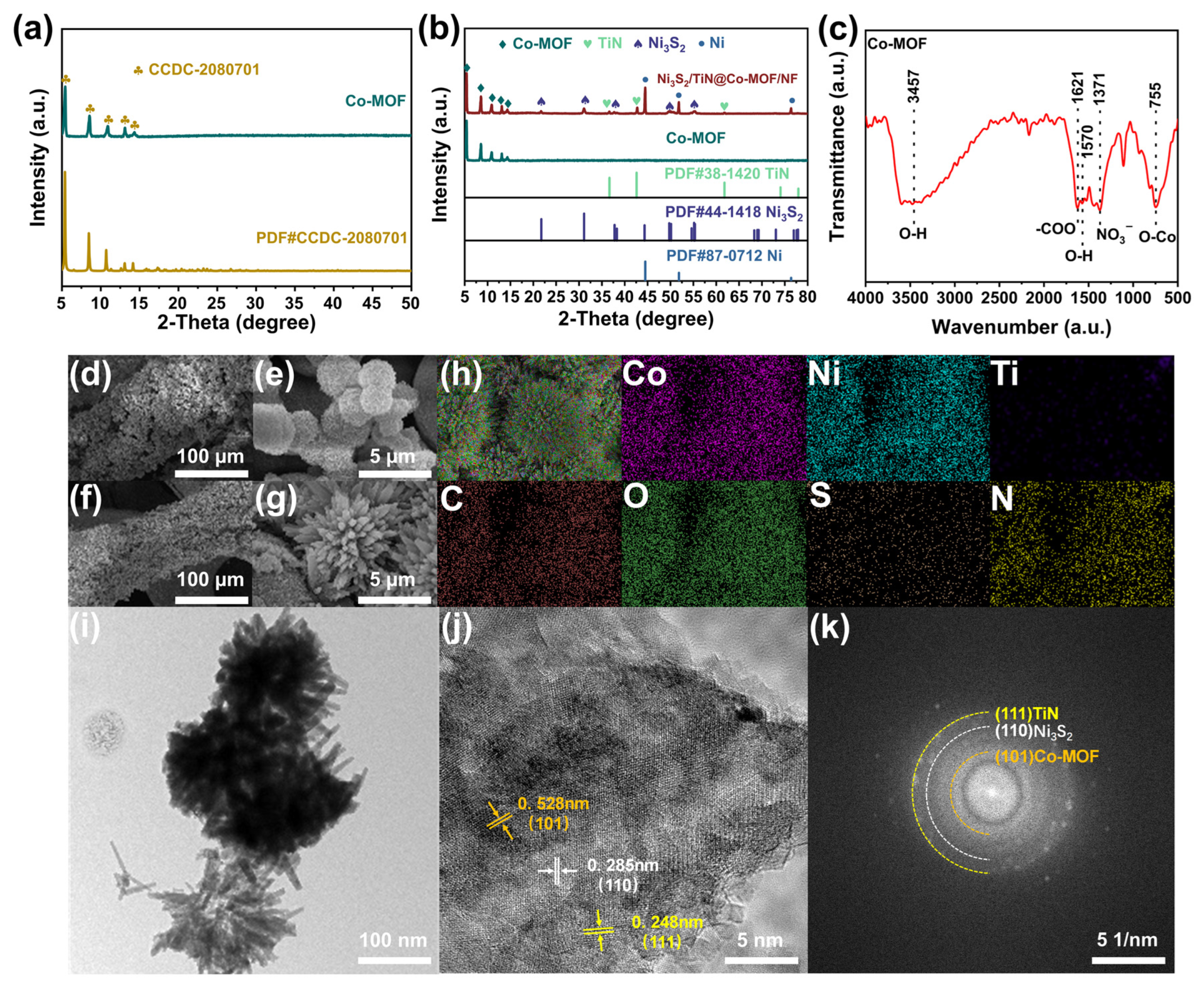
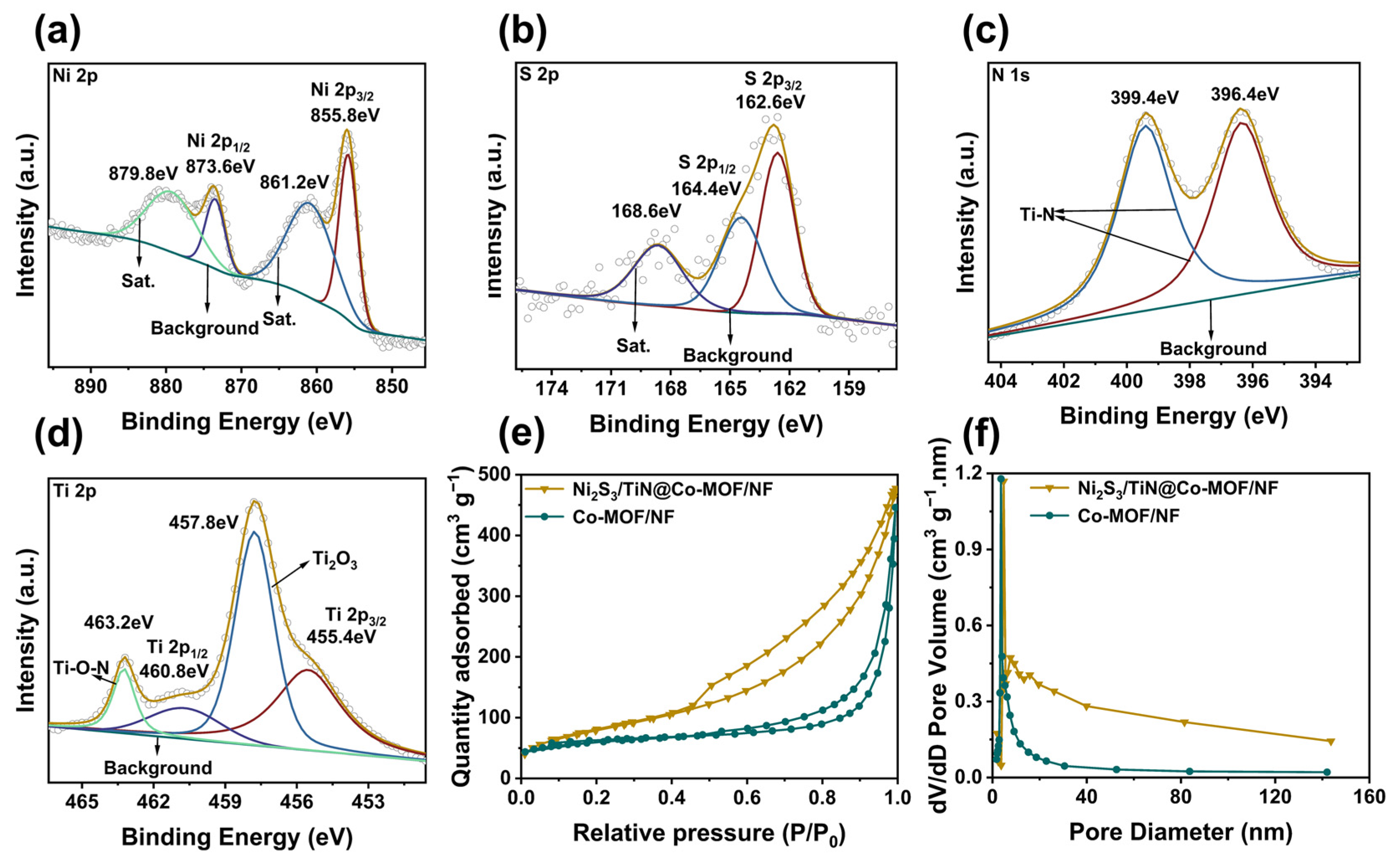
3.1. Characterizing Structure
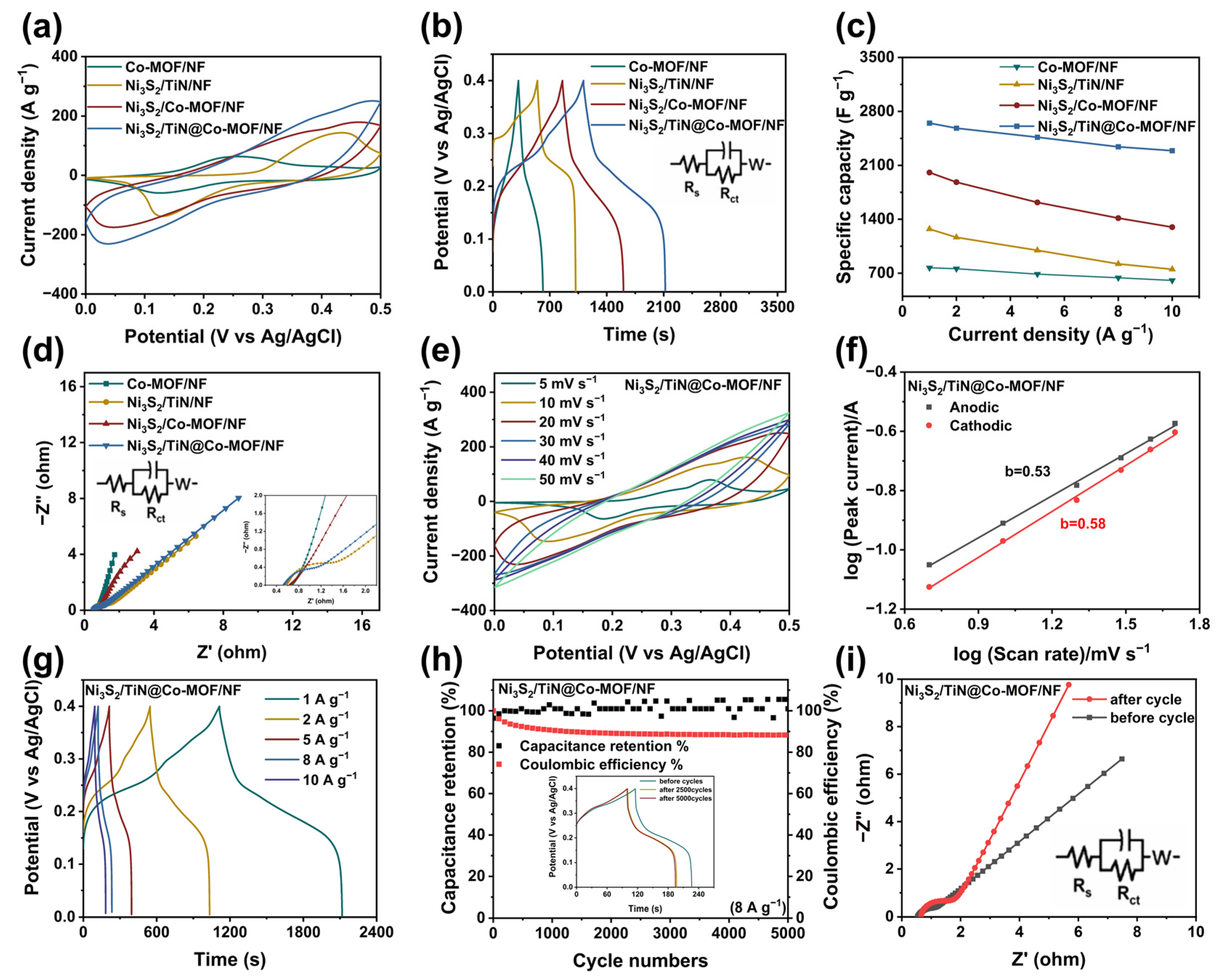
3.2. Assessment of Electrochemical Properties
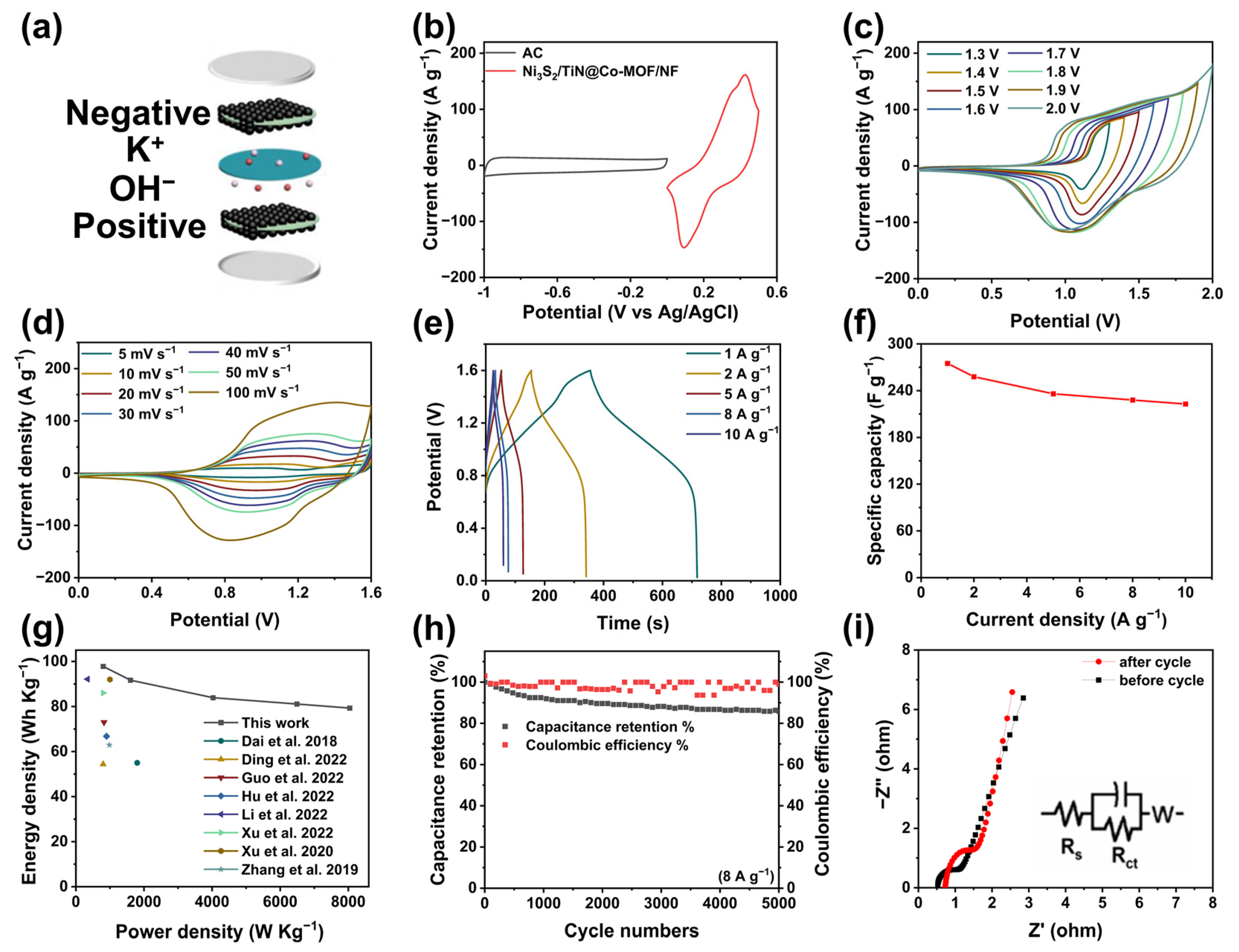
3.3. Electrochemical Measurements of Non-Symmetrical Supercapacitor (ASC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Libich, J.; Sedlaříková, M.; Máca, J.; Čudek, P.; Kazda, T.; Fafilek, G.; Rodríguez; Batteries, J.J.S. Supercapacitors vs. Lithium-ion Batteries: Properties and Applications. Chem. Ing. Tech. 2023, 29, 279–285. [Google Scholar] [CrossRef]
- Pameté, E.; Köps, L.; Kreth, F.A.; Pohlmann, S.; Varzi, A.; Brousse, T.; Balducci, A.; Presser, V. The Many Deaths of Supercapacitors: Degradation, Aging, and Performance Fading. Adv. Energy Mater. 2023, 13, 2301008. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, Q. Chemical Strategies for Making Strong Graphene Materials. Angew. Chem. Int. Ed. 2021, 60, 18397–18410. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, T.; Sana, S.S.; Kumar, K.D.; Kumar, Y.A.; Hegazy, H.H.; Kim, S.C. Asymmetric supercapacitors: Unlocking the energy storage revolution. J. Energy Storage 2023, 70, 109096. [Google Scholar] [CrossRef]
- Shah, S.S.; Niaz, F.; Ehsan, M.A.; Das, H.T.; Younas, M.; Khan, A.S.; Rahman, H.U.; Nayem, S.M.A.; Oyama, M.; Aziz, M.A. Advanced strategies in electrode engineering and nanomaterial modifications for supercapacitor performance enhancement: A comprehensive review. J. Energy Storage 2024, 79, 110152. [Google Scholar] [CrossRef]
- Pandey, D.; Kumar, K.S.; Thomas, J. Supercapacitor electrode energetics and mechanism of operation: Uncovering the voltage window. Prog. Mater. Sci. 2024, 141, 101219. [Google Scholar] [CrossRef]
- Benjamin, M.; Manoj, D.; Karnan, M.; Saravanakumar, D.; Thenmozhi, K.; Ariga, K.; Sathish, M.; Senthilkumar, S. Switching the solubility of electroactive ionic liquids for designing high energy supercapacitor and low potential biosensor. J. Colloid Interface Sci. 2021, 588, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xie, M.; Zhao, S.; Qin, Q.; Huang, F. Materials design and preparation for high energy density and high power density electrochemical supercapacitors. Mater. Sci. Eng. R Rep. 2023, 152, 100713. [Google Scholar] [CrossRef]
- Meena, D.; Kumar, R.; Gupta, S.; Khan, O.; Gupta, D.; Singh, M. Energy storage in the 21st century: A comprehensive review on factors enhancing the next-generation supercapacitor mechanisms. J. Energy Storage 2023, 72, 109323. [Google Scholar] [CrossRef]
- Khan, A.J.; Gao, L.; Sajjad, M.; Khan, S.; Mateen, A.; Ghaffar, A.; Malik, I.A.; Liao, X.; Zhao, G. Synthesis of heterostructured ZnO-CeO2 nanocomposite for supercapacitor applications. Inorg. Chem. Commun. 2024, 159, 111794. [Google Scholar] [CrossRef]
- Khan, A.J.; Sajjad, M.; Khan, S.; Khan, M.; Mateen, A.; Shah, S.S.; Arshid; Gao, Z. Telluride-Based Materials: A Promising Route for High Performance Supercapacitors. Chem. Rec. 2024, 24, e202300302. [Google Scholar] [CrossRef] [PubMed]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Molahalli, V.; Chaithrashree, K.; Singh, M.K.; Agrawal, M.; Krishnan, S.G.; Hegde, G. Past decade of supercapacitor research—Lessons learned for future innovations. J. Energy Storage 2023, 70, 108062. [Google Scholar] [CrossRef]
- Shaheen, I.; Hussain, I.; Zahra, T.; Javed, M.S.; Shah, S.S.A.; Khan, K.; Hanif, M.B.; Assiri, M.A.; Said, Z.; Arifeen, W.U.; et al. Recent advancements in metal oxides for energy storage materials: Design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 2023, 72, 108719. [Google Scholar] [CrossRef]
- Du, W.; Bai, Y.-L.; Xu, J.; Zhao, H.; Zhang, L.; Li, X.; Zhang, J. Advanced metal-organic frameworks (MOFs) and their derived electrode materials for supercapacitors. J. Power Sources 2018, 402, 281–295. [Google Scholar] [CrossRef]
- Senthil, R.A.; Osman, S.; Pan, J.; Liu, X.; Wu, Y. Recent progress on porous carbon derived from Zn and Al based metal-organic frameworks as advanced materials for supercapacitor applications. J. Energy Storage 2021, 44, 103263. [Google Scholar]
- Akkinepally, B.; Kumar, G.D.; Reddy, I.; Rao, H.; Nagajyothi, P.C.; Alothman, A.A.; Alqahtani, K.N.; Hassan, A.M.; Javed, M.S.; Shim, J. Investigation of Supercapacitor Electrodes Based on MIL-101(Fe) Metal-Organic Framework: Evaluating Electrochemical Performance through Hydrothermal and Microwave-Assisted Synthesis. Crystals 2023, 13, 1547. [Google Scholar] [CrossRef]
- Yue, L.; Chen, L.; Wang, X.; Lu, D.; Zhou, W.; Shen, D.; Yang, Q.; Xiao, S.; Li, Y. Ni/Co-MOF@aminated MXene hierarchical electrodes for high-stability supercapacitors. Chem. Eng. J. 2023, 451, 138687. [Google Scholar]
- Wang, G.; Yan, Z.; Wang, N.; Xiang, M.; Xu, Z. NiO/Ni Metal–Organic Framework Nanostructures for Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2021, 4, 9034–9043. [Google Scholar] [CrossRef]
- Ajdari, F.A.; Kowsari, E.; Shahrak, M.N.; Ehsani, A.; Kiaei, Z.; Torkzaban, H.; Ershadi, M.; Eshkalak, S.K.; Haddadi-Asl, V.; Chinnappan, A.; et al. A review on the field patents and recent developments over the application of metal organic frameworks (MOFs) in supercapacitors. Coord. Chem. Rev. 2020, 422, 213441. [Google Scholar] [CrossRef]
- Qin, P.; Huang, C.; Gao, B.; Pi, C.; Fu, J.; Zhang, X.; Huo, K.; Chu, P.K. Ultrathin carbon layer-encapsulated TiN nanotubes array with enhanced capacitance and electrochemical stability for supercapacitors. Appl. Surf. Sci. 2020, 503, 144293. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, Q.; Traynor, B.; Zhang, D.; Li, J.; Ye, H.; Che, F.; Han, N.; Wang, Y.; Sun, X.; et al. Stabilizing nickel sulfide nanoparticles with an ultrathin carbon layer for improved cycling performance in sodium ion batteries. Nano Res. 2016, 9, 3162–3170. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, H.; Ryu, H.; Kim, D.; Cho, G.; Kim, K.; Nam, T.; Ahn, J.H. The discharge properties of Na/Ni3S2 cell at ambient temperature. J. Power Sources 2008, 178, 852–856. [Google Scholar] [CrossRef]
- Li, J.; Deng, Z.; Liu, C.; Rong, H.; Zeng, Z. TiN nano arrays on nickel foam prepared by multi-arc ion plating for fast-charging supercapacitors. Appl. Surf. Sci. 2022, 593, 153360. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, L.; Xie, F.; Hu, J.; Tan, H.; Qian, J.; Shi, X.; Zhang, Y. Tuning the crystal structure of NiS/carbon by Mo doping for asymmetric supercapacitor application. Mater. Today Chem. 2022, 26, 101188. [Google Scholar] [CrossRef]
- Peng, X.; Huo, K.; Fu, J.; Gao, B.; Wang, L.; Hu, L. Porous Dual-Layered MoOx Nanotube Arrays with Highly Conductive TiN Cores for Supercapacitors. ChemElectroChem 2015, 2, 512–517. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Han, M.; Xia, Q.; Chen, Q.; Chen, M. Constructing ultra-thin Ni-MOF@NiS2 nanosheets arrays derived from metal organic frameworks for advanced all-solid-state asymmetric supercapacitor. Mater. Res. Bull. 2021, 137, 111186. [Google Scholar] [CrossRef]
- Bhagwan, J.; Han, J.I. Formation of MWCNT/LiCo2O4 nanoplates and their application for hybrid supercapacitor. Ceram. Int. 2024, 50, 10676–10687. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Huang, F.; Lu, Y.; Wang, L.; Chen, H.; Zhang, H. Hydrothermally formed three-dimensional hexagon-like P doped Ni(OH)2 rod arrays for high performance all-solid-state asymmetric supercapacitors. Appl. Surf. Sci. 2018, 428, 250–257. [Google Scholar] [CrossRef]
- Arbizzani, C.; Yu, Y.; Li, J.; Xiao, J.; Xiao, Y.; Yang, Y.; Santato, C.; Raccichini, R.; Passerini, S. Good practice guide for papers on supercapacitors and related hybrid capacitors for the Journal of Power Sources. J. Power Sources 2020, 450, 227636. [Google Scholar] [CrossRef]
- Hu, C.; Miao, L.; Yang, Q.; Yu, X.; Song, L.; Zheng, Y.; Wang, C.; Li, L.; Zhu, L.; Cao, X.; et al. Self-assembly of CNTs on Ni foam for enhanced performance of NiCoO2@CNT@NF supercapacitor electrode. Chem. Eng. J. 2021, 410, 128317. [Google Scholar] [CrossRef]
- Huang, C.; Lv, S.; Gao, A.; Ling, J.; Yi, F.; Hao, J.; Wang, M.; Luo, Z.; Shu, D. Boosting the energy density of supercapacitors by designing both hollow NiO nanoparticles/nitrogen-doped carbon cathode and nitrogen-doped carbon anode from the same precursor. Chem. Eng. J. 2022, 431, 134083. [Google Scholar] [CrossRef]
- Ren, F.; Lu, Z.; Liu, X.; Wang, T.; Huang, X.; Dou, J.; Wu, D.; Yu, J.; Chen, X. Lewis acid-etched MXene self-assembled with reduced graphene oxide for symmetrical supercapacitors with liquid/ solid electrolytes. J. Alloys Compd. 2024, 978, 173480. [Google Scholar] [CrossRef]
- Chen, F.; Liu, C.; Cui, B.; Dou, S.; Xu, J.; Liu, S.; Zhang, H.; Deng, Y.; Chen, Y.; Hu, W. Regulated synthesis of Eutectic Ni3S2/NiS nanorods for quasi-solid-state hybrid supercapacitors with high energy density. J. Power Sources 2021, 482, 228910. [Google Scholar] [CrossRef]
- Phonsuksawang, P.; Khajondetchairit, P.; Ngamchuea, K.; Butburee, T.; Sattayaporn, S.; Chanlek, N.; Suthirakun, S.; Siritanon, T. Enhancing performance of NiCo2S4/Ni3S2 supercapacitor electrode by Mn doping. Electrochim. Acta 2021, 368, 137634. [Google Scholar] [CrossRef]
- Wang, J.; Chao, D.; Liu, J.; Li, L.; Lai, L.; Lin, J.; Shen, Z. Ni3S2@MoS2 core/shell nanorod arrays on Ni foam for high-performance electrochemical energy storage. Nano Energy 2014, 7, 151–160. [Google Scholar] [CrossRef]
- Yue, H.; Du, H.; Ma, X.; Zhang, X. Honeycomb-like Ni3(NO3)2(OH)4@Ni/Co-BTC composites as electrode materials for high performance supercapacitors. Mater. Sci. Eng. B 2021, 268, 115136. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Bao, Y.; Zhang, N.; Zhang, J.; Xing, Y.; Deng, W.; Liu, Z. Structures and catalytic oxidative coupling reaction of four Co-MOFs modified with R-isophthalic acid (R=H, OH and COOH) and trigonal ligands. CrystEngComm 2021, 23, 7590–7601. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Hu, N.; Liao, J.; Zong, N.; Wei, J.; Li, M.; Wang, L.; Xu, R.; Yang, L. Facile synthesis of neuronal nickel–cobalt manganese sulfide for asymmetric supercapacitors with excellent energy density. J. Electroanal. Chem. 2023, 932, 11726. [Google Scholar] [CrossRef]
- Zhao, T.; Jiang, H.; Ma, J. Surfactant-assisted electrochemical deposition of α-cobalt hydroxide for supercapacitors. J. Power Sources 2011, 196, 860–864. [Google Scholar] [CrossRef]
- Ren, C.; Jia, X.; Zhang, W.; Hou, D.; Xia, Z.; Huang, D.; Hu, J.; Chen, S.; Gao, S. Hierarchical Porous Integrated Co1−xS/CoFe2O4@rGO Nanoflowers Fabricated via Temperature-Controlled In Situ Calcining Sulfurization of Multivariate CoFe-MOF-74@rGO for High-Performance Supercapacitor. Adv. Funct. Mater. 2020, 30, 2004519. [Google Scholar] [CrossRef]
- Gurav, S.R.; Chodankar, G.R.; Sawant, S.A.; Shembade, U.V.; Moholkar, A.V.; Sonkawade, R.G. Exploring the potential of simultaneous nanoarchitectonics and utilization of Co-MOFs electrode as well as powder for aqueous supercapacitors. J. Energy Storage 2023, 73, 109254. [Google Scholar] [CrossRef]
- Qian, H.; Wu, B.; Nie, Z.; Liu, T.; Liu, P.; He, H.; Wu, J.; Chen, Z.; Chen, S. A flexible Ni3S2/Ni@CC electrode for high-performance battery-like supercapacitor and efficient oxygen evolution reaction. Chem. Eng. J. 2021, 420, 127646. [Google Scholar] [CrossRef]
- Feng, S.; Yang, L.; Deng, P.; Wang, J.; Xu, R.; Liu, X.; Wang, W.; Tian, X.; Wu, Z. Hierarchical self-supported NiSe2/TiN@Ni12P5 on nickel foam for the urea oxidation reaction. Int. J. Hydrog. Energy 2022, 47, 36814–36822. [Google Scholar] [CrossRef]
- Ko, S.; Tang, X.; Gao, F.; Wang, C.; Liu, H.; Liu, Y. Selective catalytic reduction of NOx with NH3 on Mn, Co-BTC-derived catalysts: Influence of thermal treatment temperature. J. Solid State Chem. 2022, 307, 122843. [Google Scholar] [CrossRef]
- Zha, X.; Shi, L.; Yang, Y. In situ vertically growth of 2D NiCo-BTC nanosheet arrays for binder-free flexible wearable energy storage devices. J. Energy Storage 2023, 60, 106578. [Google Scholar] [CrossRef]
- Rong, H.; Chen, T.; Shi, R.; Zhang, Y.; Wang, Z. Hierarchical NiCo2O4@NiCo2S4 Nanocomposite on Ni Foam as an Electrode for Hybrid Supercapacitors. ACS Omega 2018, 3, 5634–5642. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Gong, Y.; Lin, J. Epitaxial grown self-supporting NiSe/Ni3S2/Ni12P5 vertical nanofiber arrays on Ni foam for high performance supercapacitor: Matched exposed facets and re-distribution of electron density. Nano Energy 2019, 55, 65–81. [Google Scholar] [CrossRef]
- Le, T.-L.T.; Nguyen, L.T.; Nguyen, H.-H.; Nghia, N.V.; Vuong, N.M.; Hieu, H.N.; Thang, N.V.; Le, V.T.; Nguyen, V.H.; Lin, P.-C.; et al. Titanium Nitride Nanodonuts Synthesized from Natural Ilmenite Ore as a Novel and Efficient Thermoplasmonic Material. Nanomaterials 2021, 11, 76. [Google Scholar]
- Guo, D.; Wan, Z.; Li, Y.; Xi, B.; Wang, C. TiN@Co5.47N Composite Material Constructed by Atomic Layer Deposition as Reliable Electrocatalyst for Oxygen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2008511. [Google Scholar] [CrossRef]
- Han, M.; Yang, J.; Jiang, J.; Jing, R.; Ren, S.; Yan, C. Efficient tuning the electronic structure of N-doped Ti-based MXene to enhance hydrogen evolution reaction. J. Colloid Interface Sci. 2021, 582, 1099–1106. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Chen, Z.; Wang, T.; Wang, L.; Zeng, W.; Zhang, G.; Duan, H. Synthesis of TiN nanostructures by Mg-assisted nitriding TiO2 in N2 for lithium ion storage. Chem. Eng. J. 2018, 336, 12–19. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, D.; Shi, S.; Liu, F.; Liu, W.; Chen, Q.; Zhao, P.; Li, S.; Wang, J. Superior-performance TiN films sputtered for capacitor electrodes. J. Mater. Sci. 2019, 54, 10346–10354. [Google Scholar] [CrossRef]
- Sun, J.; Gao, W.; Fei, H.; Zhao, G. Efficient and selective electrochemical reduction of nitrate to N2 by relay catalytic effects of Fe-Ni bimetallic sites on MOF-derived structure. Appl. Catal. B Environ. 2022, 301, 120829. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, H.; Yuan, C.; Song, J.; Wu, M. Electrons/ions dual transport channels design: Concurrently tuning interlayer conductivity and space within re-stacked few-layered MXenes film electrodes for high-areal-capacitance stretchable micro-supercapacitor-arrays. Nano Energy 2020, 74, 104812. [Google Scholar] [CrossRef]
- Qin, Y.; Lyu, Y.; Chen, M.; Lu, Y.; Qi, P.; Wu, H.; Sheng, Z.; Gan, X.; Chen, Z.; Tang, Y. Nitrogen-doped Ni2P/Ni12P5/Ni3S2 three-phase heterostructure arrays with ultrahigh areal capacitance for high-performance asymmetric supercapacitor. Electrochim. Acta 2021, 393, 139059. [Google Scholar] [CrossRef]
- Yu, Q.; Gong, J.; Kong, W.; Long, Y.; Chen, J.; Pu, L.; Zhang, H.; Dai, Y. Preparation of NiAl LDH@Mn3O4@Co-MOF ternary composites using MOFs as a framework for high-performance asymmetric supercapacitors. Electrochim. Acta 2022, 428, 140913. [Google Scholar] [CrossRef]
- Khadka, A.; Samuel, E.; Pradhan, S.; Joshi, B.; Aldalbahi, A.; El-Newehy, M.; Lee, H.-S.; Yoon, S.S. Hydrothermal growth of FeMoO4 nanosheets on electrospun carbon nanofibers as freestanding supercapacitor electrodes. Ceram. Int. 2024, 50, 9398–9406. [Google Scholar] [CrossRef]
- Ramesh, S.; Bathula, C.; Ahmed, A.T.A.; Haldorai, Y.; Vijay, K.; Karthikeyan, C.; Selvaraj, M.; Shin, K.; Lee, Y.J.; Kim, H.-S.; et al. Nanostructurally fabrication of nickel oxide-interfaced carbon nanotubes for supercapacitors and exploration of electrochemical correlation via computer vision techniques and artificial intelligence. J. Energy Storage 2024, 82, 110429. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, S.; Wong, S.; Andrei, C.; Yuan, H.; Zhou, J.; Wang, J.; Zhuo, Z.; Zhong, Y.; Su, H.; et al. Revealing unprecedented cathode interface behavior in all-solid-state batteries with oxychloride solid electrolytes†. Energy Environ. Sci. 2024. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Xu, X.; Liu, Y.; Song, R.; Lu, G.; Li, Y. Cobalt Hexacyanoferrate Nanoparticles as a High-Rate and Ultra-Stable Supercapacitor Electrode Material. ACS Appl. Mater. Interfaces 2014, 6, 11007–11012. [Google Scholar] [CrossRef] [PubMed]
- Babu, C.R.; Avani, A.V.; Xavier, T.S.; Tomy, M.; Shaji, S.; Anila, E.I. Symmetric supercapacitor based on Co3O4 nanoparticles with an improved specific capacitance and energy density. J. Energy Storage 2024, 80, 110382. [Google Scholar] [CrossRef]
- Liu, H.; Yao, Z.; Liu, Y.; Diao, Y.; Hu, G.; Zhang, Q.; Li, Z. In situ synthesis of nitrogen site activated cobalt sulfide@N, S dual-doped carbon composite for a high-performance asymmetric supercapacitor. J. Colloid Interface Sci. 2021, 585, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.V.; Mohan, M.; Rakhi, R.B. High performance supercapacitors based on WS2 nanoflower electrodes with commercial-level mass-loading. Surf. Interfaces 2023, 42, 103496. [Google Scholar] [CrossRef]
- Varghese, S.M.; Mohan, V.V.; Suresh, S.; Gowd, E.B.; Rakhi, R.B. Synergistically modified Ti3C2Tx MXene conducting polymer nanocomposites as efficient electrode materials for supercapacitors. J. Alloys Compd. 2024, 973, 172923. [Google Scholar] [CrossRef]
- Dai, S.; Liu, Z.; Zhao, B.; Zeng, J.; Hu, H.; Zhang, Q.; Chen, D.; Qu, C.; Dang, D.; Liu, M. A high-performance supercapacitor electrode based on N-doped porous graphene. J. Power Sources 2018, 387, 43–48. [Google Scholar] [CrossRef]
- Ding, S.; An, J.; Ding, D.; Zou, Y.; Zhao, L. Micron-sized NiMn-glycerate solid spheres as cathode materials for all-solid-state asymmetric supercapacitor with superior energy density and cycling life. Chem. Eng. J. 2022, 431, 134100. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Zhang, Y.; Zhai, Y.; Cai, W. Functional sulfur-doped zinc-nickel-cobalt oxide nanorods materials with high energy density for asymmetric supercapacitors. J. Alloys Compd. 2022, 896, 163053. [Google Scholar] [CrossRef]
- Hu, C.; Gong, J.; Wang, J.; Zhou, T.; Xie, M.; Wang, S.; Dai, Y. Composites of NiMoO4@Ni-Co LDH@NiCo2O4 on Ni foam with a rational microscopic morphology for high-performance asymmetric supercapacitors. J. Alloys Compd. 2022, 902, 163749. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Huang, F.; Zuo, X.; Wei, X.; Li, S.; Zhang, H. Co9S8@MnO2 core–shell defective heterostructure for High-Voltage flexible supercapacitor and Zn-ion hybrid supercapacitor. Chem. Eng. J. 2022, 437, 135494. [Google Scholar] [CrossRef]
- Xu, L.; Xi, Y.; Li, W.; Hua, Z.; Peng, J.; Hu, J.; Zhou, J.-J.; Zhang, P.; Wang, J.; Wang, W.; et al. 3D frame-like architecture of N-C-incorporated mixed metal phosphide boosting ultrahigh energy density pouch-type supercapacitors. Nano Energy 2022, 91, 106630. [Google Scholar] [CrossRef]
- Xu, X.; Yang, J.; Zhou, X.; Jiang, S.; Chen, W.; Liu, Z. Highly crumpled graphene-like material as compression-resistant electrode material for high energy-power density supercapacitor. Chem. Eng. J. 2020, 397, 125525. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Yin, B.-S.; Liu, X.-X.; Gu, D.-M.; Gong, H.; Wang, Z.-B. A high energy density aqueous hybrid supercapacitor with widened potential window through multi approaches. Nano Energy 2019, 59, 41–49. [Google Scholar] [CrossRef]
- Pan, Y.; Yan, S.; Liu, Y.; Tian, Z.; Li, D.; Chen, Y.; Guo, L.; Wang, Y. Significantly enhanced electrochemical performance of 2D Ni-MOF by carbon quantum dot for high-performance supercapacitors. Electrochim. Acta 2020, 422, 140560. [Google Scholar] [CrossRef]
- Zhao, L.; Meng, F.; Zhang, W. Fabrication of 3D micro-flower structure of ternary Ni-Co-Cu hydroxide based on Co-MOF for advanced asymmetric supercapacitors. Electrochim. Acta 2023, 461, 142656. [Google Scholar] [CrossRef]
- Salunkhe, A.D.; Pawar, P.S.; Pagare, P.K.; Torane, A.P. Facile solvothermal synthesis of Ni-Co MOF/rGO nanoflakes for high-performance asymmetric supercapacitor. Electrochim. Acta 2024, 477, 143745. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Devi, S.; Reghunath, B.S.; Saravanakumar, B.; William, J.J.; Pinheiro, D. Sm-MOF/rGO/PANI composite as an electrode material for supercapacitor applications. Electrochim. Acta 2023, 467, 143031. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Tang, M.; Wang, Z.; Yang, Y.; Li, S.; Long, H. NiCo2O4 nanosheet stereostructure with N-doped carbon/Co array supports derived from Co-MOF for asymmetric supercapacitor. J. Electroanal. Chem. 2022, 923, 116818. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, W.; Wang, H.; He, L.; Wang, Y. Rational construction of MOF-derived Zn-Co-O/NiCo-LDH core/shell nanosheet arrays on nickel foam for high-performance supercapacitors. J. Alloys Compd. 2021, 884, 160931. [Google Scholar] [CrossRef]
- Darsara, S.A.; Seifi, M.; Askari, M.B.; Osquian, M. Hierarchical 3D starfish-like Ni3S4–NiS on reduced graphene oxide for high-performance supercapacitors. Ceram. Int. 2021, 47, 20992–20998. [Google Scholar] [CrossRef]
- Shwetha, K.P.; Kamath, M.S.; Athreya, Y.N.; Rastogi, C.K.; Nagaraju, K.; Khosla, A.; Manjunatha, C. Development of NiS@f-MWCNT nanocomposite-based high-performance supercapacitor coin cell prototype device. J. Energy Storage 2024, 75, 109404. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, J.; Ma, Y.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Embedding NiS nanoflakes in electrospun carbon fibers containing NiS nanoparticles for hybrid supercapacitors. Chem. Eng. J. 2022, 446, 137262. [Google Scholar] [CrossRef]
- Wang, Y.T.; He, X.F.; Chen, X.M.; Zhang, Y.; Li, F.T.; Zhou, Y.; Meng, C. Laser synthesis of cobalt-doped Ni3S4-NiS/Ni as high-efficiency supercapacitor electrode and urea oxidation electrocatalyst. Appl. Surf. Sci. 2022, 596, 153600. [Google Scholar] [CrossRef]
- Subhash, K.G.; Benoy, M.D.; Duraimurugan, J.; Prabhu, S.; Siranjeevi, R.; Ramesh, R.; Kumar, G.S.; Shkir, M. Synergistic effect of NiS/g-C3N4 nanocomposite for high-performance asymmetric supercapacitors. Inorg. Chem. Commun. 2022, 142, 109719. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, N.; Wang, J.; Liu, Z.; Wu, S.; Tong, X.; Kong, Q.; Xu, R.; Yang, L. Electrode Materials with High Performance of Nickel Sulfide/Titanium Nitride@Co-Based Metal–Organic Frameworks/Nickel Foam for Supercapacitors. Energies 2024, 17, 2788. https://doi.org/10.3390/en17112788
Zong N, Wang J, Liu Z, Wu S, Tong X, Kong Q, Xu R, Yang L. Electrode Materials with High Performance of Nickel Sulfide/Titanium Nitride@Co-Based Metal–Organic Frameworks/Nickel Foam for Supercapacitors. Energies. 2024; 17(11):2788. https://doi.org/10.3390/en17112788
Chicago/Turabian StyleZong, Naixuan, Junli Wang, Zhenwei Liu, Song Wu, Xiaoning Tong, Qingxiang Kong, Ruidong Xu, and Linjing Yang. 2024. "Electrode Materials with High Performance of Nickel Sulfide/Titanium Nitride@Co-Based Metal–Organic Frameworks/Nickel Foam for Supercapacitors" Energies 17, no. 11: 2788. https://doi.org/10.3390/en17112788
APA StyleZong, N., Wang, J., Liu, Z., Wu, S., Tong, X., Kong, Q., Xu, R., & Yang, L. (2024). Electrode Materials with High Performance of Nickel Sulfide/Titanium Nitride@Co-Based Metal–Organic Frameworks/Nickel Foam for Supercapacitors. Energies, 17(11), 2788. https://doi.org/10.3390/en17112788



