Abstract
This article presents the possibility of improving combustion using the effect of releasing hydrogen from a solution with nucleation of gas bubbles. This concept consists in dissolving hydrogen in diesel fuel until the equilibrium state of the solution is reached. At a later stage, the phenomenon is reversed, and this gas is released from the solution during its injection into the combustion chamber with a strong swirl. A characteristic feature of the solution is that when lowering the pressure (opening the atomizers), there is a decrease in the equilibrium thermodynamic potential, which results in the excess, dissolved hydrogen being released spontaneously, and this process is of a volumetric nature. This article is a continuation of the work carried out at Poznan University of Technology on the development of this concept. This article presents the results of tests for the impact of hydrogen dissolved in diesel fuel on the combustion process within a turbulent-flow environment. The tests were conducted in the combustion chamber of an engine equipped with a toroidal combustion chamber and direct injection. During the tests, the following factors were measured: the main indicators of motor operation, emission of hydrocarbons, carbon monoxide, nitrogen oxides, and particulate matters.
Keywords:
hydrogen; gas desorption; fuel atomization; combustion process; emission; ecology; CI engine 1. Introduction: A New Concept of Hydrogen Combustion
For many years, research on internal combustion (IC) engines has been aimed at reducing their harmfulness to the natural environment [1]. In many countries, scientists are conducting research to check the emission of conventional fuels in typical or less typical applications of self-ignition (SI) and compression ignition (CI) engines [2,3]. However, it seems that the greatest interest in recent years has been the research on modern alternative fuels such as, e.g., alcohols [4,5,6]. Within the group of alternative fuels, hydrogen is probably the most popular and forward-looking fuel. Considering the use of hydrogen in conventional internal combustion engines, it can be noticed that most of the work focuses on hydrogen use in spark-ignition engines. Much less work is devoted to compression-ignition engines, especially considering that hydrogen may not be the main volumetric share in the fuel but only an addition to conventional fuel. The addition of hydrogen to diesel fuel, often referred to as “mixing hydrogen with diesel” or “hydrogen enrichment”, has become of interest due to its potential to improve combustion properties and reduce emissions [7,8]. Hydrogen is considered to be a clean fuel and, when mixed with diesel fuel, it can affect various engine performance parameters. Here is the potential impact on engine parameters (Figure 1).
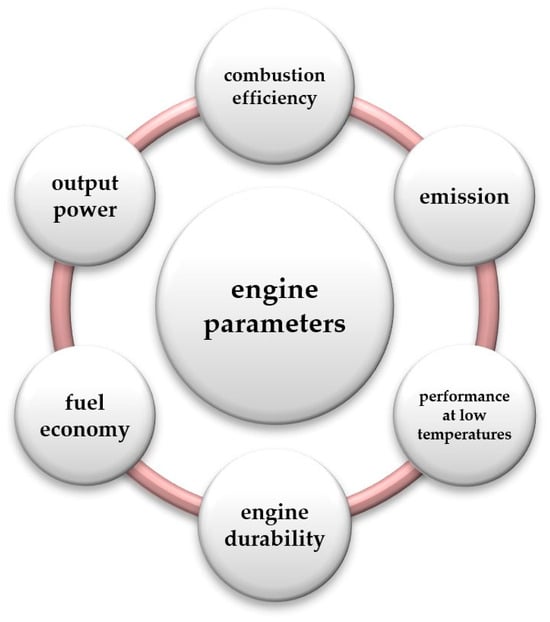
Figure 1.
Parameters affecting the operation of the engine.
Research on the addition of hydrogen to liquid hydrocarbon fuels has been ongoing since the 1990s, especially in the case of supersonic combustion. The work of Gruenig et al. [9] shows that the high temperature of the burner entrance allows the stabilization of the supersonic flame through self-ignition of hydrogen. The effectiveness of this process depends on establishing a flammable hydrogen–air mixture and ensuring thermal self-ignition conditions. Diagonal waves play an important role in raising the temperature of the fuel–air mixture, which can accelerate the self-ignition process by reactions on the surfaces of the whirling fuel. There is a close link between gas dynamics and chemical kinetics in responsive supersonic flow. Changes in temperature and pressure caused by the diagonal wave structure affect the local starting reactions on the surfaces of the whirling fuel, which also affects the reaction rates in the main combustion zone. Additionally, the emitted combustion heat may affect the flow structure, forming a diagonal wave structure extending back to the combustion zones.
The paper [10] presents experimental and computational studies of supersonic flow and hydrogen combustion in the LAPCAT-II chamber, using ramjet/scramjet. The chamber was divided into four sections with increasing cross-sectional area. The research included experiments and simulations using the large vortices (LES) method for various values of total temperature, total pressure, and equivalence coefficient. Changing the chamber temperature led to different combustion behaviors. For lower temperatures, combustion took place only in a separated area, for medium temperatures—between the first and second sections and for higher temperatures—earlier. The results of LES and the experiments showed consistent pressure increases on the chamber walls. Both LES and the experiments showed the sensitivity of the chamber to changes in the total temperature, which resembled turbulent supersonic combustion of hydrogen and air.
In work [11], the effect of hydrogen admixture on self-ignition of homogeneous and hybrid mixtures of heavy hydrocarbons in air was carried out with a detailed mechanism of n-decane oxidation reaction. It turned out that the reactivity of mixtures containing hydrogen is not always higher than the reactivity of pure hydrocarbon–air mixtures. At temperatures below about 1050 K, the addition of hydrogen to such mixtures increases the self-ignition delay, so it acts as an inhibitor. As the hydrogen content increases, the duration of the blue flame reaction becomes shorter and may even degrade. This is due to the reaction of hydrogen with intermediate products of hydrocarbon oxidation, which leads to the formation of less active forms, hindering the chain branching processes. However, at temperatures exceeding 1050 K, the addition of hydrogen reduces the overall self-ignition delay, indicating its role as a promoter.
However, it is generally accepted that hydrogen requires shorter ignition delays due to low ignition energy and high flame velocity. Wide range of flammability of hydrogen means that it can ignite and burn in a wide range of concentrations in the air (from about 4% to 75% by volume). In order to achieve a high concentration of hydrogen in diesel fuel, mix proportions must be carefully managed and controlled to optimize combustion without exceeding safety limits. Hydrogen and diesel fuel differ significantly in terms of combustion properties due to different chemical properties and combustion behavior. Hydrogen is highly flammable and has a low demand for ignition energy. It may ignite at low energy levels and wide concentration ranges. Diesel fuel, on the other hand, is less flammable and requires higher temperatures or a constant heat source to ignite in comparison to hydrogen [12].
Hydrogen tends to reduce emissions of carbon monoxide (CO), particulate matters (PM), and unburnt hydrocarbons due to its cleaner combustion properties. Additionally, it can potentially reduce nitrogen oxide (NOx) emissions by changing combustion conditions. Improving combustion efficiency through the addition of hydrogen has the potential to increase fuel economy [13] by obtaining more energy from the fuel and achieving a better output with a given amount of diesel fuel. The use of hydrogen in a CI engine can positively affect its power due to a more efficient combustion process. As a result, this can increase torque and power, providing additional efficiency benefits. The presence of hydrogen in diesel fuel may affect engine components and materials. The lubricating properties change, which can affect the wear characteristics and the durability of the engine. Therefore, modifications may be necessary to ensure compatibility and durability. The use of hydrogen admixture can positively affect the performance of the engine when starting cold due to its properties, by facilitating ignition especially in colder climate conditions.
As already mentioned, the combustion of diesel fuel typically involves a longer ignition delay and burning time compared to hydrogen. However, it should be remembered that hydrogen, due to its unique properties, can under certain conditions potentially extinguish the flame in a CI engine; it has a high flame speed and a wide range of flammability, but it also has a high demand for ignition energy. Hydrogen, if added to a CI engine, can change the combustion characteristics and affect the spread of flame [14,15].
During combustion, hydrogen releases a significant amount of heat per unit mass. The high energy content results in efficient combustion, contributing to higher thermal efficiency in properly optimized systems. Diesel fuel has a lower energy content per unit mass compared to hydrogen. CI engines operate with lower thermal efficiency compared to hydrogen-powered engines due to factors such as incomplete combustion and heat loss.
The comparison of both fuels presented in the above summary shows how big the differences between them are. Modifications to the engine’s fuel system may be required to ensure proper mixing, injection, and combustion in order to effectively utilize hydrogen–diesel mixtures. Therefore, several factors seem to be crucial, including the percentage of hydrogen content, engine design, operating conditions, and control systems used. Research and development in this area is ongoing, with the aim of optimizing the proportions of mixtures and engine modifications to achieve the best performance and emission reductions. As with any modification to the fuel composition, thorough testing, adherence to engine manufacturer guidelines, and consideration of safety and performance implications are essential before hydrogen–diesel blends can be used in engines.
All this prompted the authors of this article to propose a new concept of improving combustion by dissolving hydrogen in diesel fuel. This approach focuses on the fuel injection and atomization process, which is one of the most important factors affecting the combustion efficiency of CI engines. It largely determines the efficiency of the engine and the emission of toxic exhaust components.
In currently used injection systems, the dispersion of the fuel stream into droplets occurs under the influence of only one physical stimulus. This is the velocity of the fuel flow in the atomizer holes, caused by the difference in pressure between the atomizer and the combustion chamber. The dispersion of the fuel stream initiated by this stimulus is facilitated by a secondary factor, which is the inertial and discrete medium into which the fuel is injected. It is believed that due to the low compressibility of the fuel, the expansion process does not play an important role in the atomization mechanism. In order to achieve an environmentally friendly spraying mechanism, not only is it necessary to apply a very high pressure but also to control this pressure according to the operating conditions of the engine. There are some physical implications that allow us to believe that the observed tendency in the development of injection systems is not necessarily the only possibility. The spray mechanism can be improved by increasing the injection pressure or modifying the fuel stream dispersion process.
In order to improve the combustion process, it is proposed to enrich the already existing spray mechanism with an additional physical stimulus, which is the spontaneous release of hydrogen from the liquid solution in the non-equilibrium phase. This process is volumetric. The concept of improving combustion consists in obtaining a fuel–gas solution by dissolving an appropriate amount of hydrogen in the diesel fuel. The equilibrium phase of the solution can be achieved at the assumed injection pressure. During the injection, a rapid pressure drop can be observed, which consequently reduces the thermodynamic potential of the solution in the equilibrium phase. As a result, the equilibrium phase is significantly disturbed, and the dissolved hydrogen is released from the entire volume of the solution. It can be expected that the kinetics of hydrogen release, if combined with the kinetics of the stimulus inducing the imbalance phase, may be significant.
The gas atoms in the solution are uniformly dispersed throughout the volume of the liquid. By releasing themselves simultaneously from the entire volume, they form diffuse microbubbles that expand and tend to aggregate. If the pressure drop is carried out dynamically, the microbubbles will not be able to accumulate in one volume and the expansion of hydrogen will take place in micro areas dispersed in the liquid. The energy generated during the gas release, and thus the work performed during hydrogen decompression, is absorbed by the solution. Thus, the release of hydrogen from the liquid induces additional internal forces that exceed the binding forces of the liquid particles and consequently ruptures the microbubble environment.
In the case of supplying the gas–fuel solution to the injector, there are physical premises that hydrogen can spontaneously release from the solution due to a sudden decrease in thermodynamic potential during fuel injection into the combustion chamber and thus due to a strong disturbance of the equilibrium state. As a result, the described mechanism should rupture the fuel droplets from the inside. Therefore, it will be an additional factor facilitating the fuel atomization mechanism and, as a result, improving the combustion process. This concept is illustrated in Figure 2.
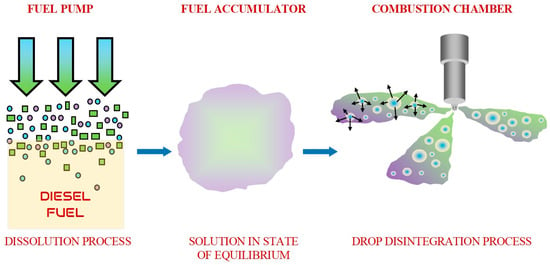
Figure 2.
The concept of fuel-spraying improved by H2 dissolved in diesel fuel [16].
2. Materials and Methods
Hydrogen is a gaseous fuel, so it was necessary to prepare an appropriate system for storing and supplying fuel to the high-pressure pump. Preparing an injection system that allows gases to dissolve is a big challenge, due to the differences in the compressibility of both the components and the presence of different phases of concentration. For this purpose, a high-pressure pump equipped with a special pumping section (Figure 3) was used, which allows gas to be supplied and mixed with diesel fuel during the compression phase. A high-pressure pump for dissolving gases in diesel fuel was developed and manufactured at Poznan University of Technology Institute of Combustion Engines and Powertrains.
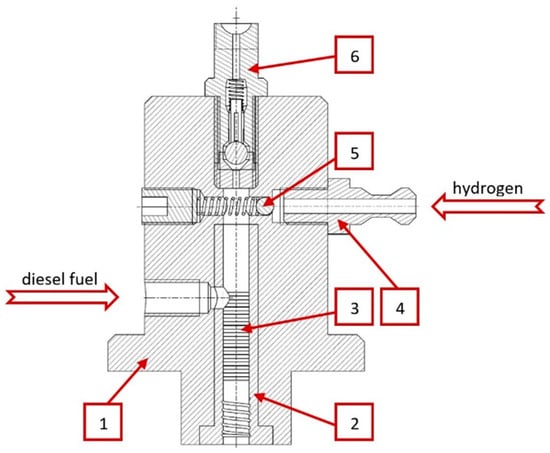
Figure 3.
Delivery section of the pump: 1—body of the delivery section, 2—cylinder, 3—section labyrinth seal, 4—gas stub pipe, 5—one-way gas valve, 6—one-way outlet valve [16].
The fuel pump is driven by an independent system consisting of an electric motor controlled by an inverter. The rotational speed is adjusted in such a way so as to ensure adequate pressure and output of the pumped fuel, while it is overheating, since it may affect the measurement results.
Diesel fuel is supplied to the pump at a pressure of 5 bar generated by the initial fuel pump, while gaseous fuel, due to the fact that it is stored in a pressurized cylinder, is supplied to the system directly using a conditioning system equipped with a filter unit and a pressure regulator to control the amount of dissolved gas (Figure 4).
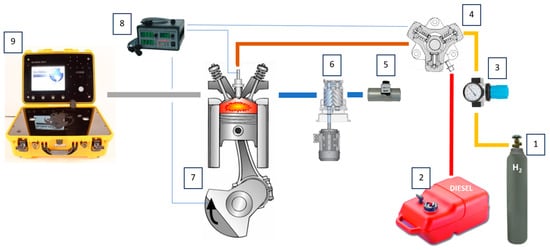
Figure 4.
Test bench consisting of 1—H2 tank, 2—diesel fuel tank, 3—filter block with pressure regulator, 4—fuel pump/dissolving pump, 5—air flow meter, 6—mechanical compressor with independent drive, 7—test engine, 8—injection controller, 9—exhaust gas analyzer.
It is difficult to precisely determine the concentration of dissolved gas in the fuel obtained in this way; however, under fixed operating conditions of the engine (i.e., constant rotational speed and constant load), it remains at an even level, depending on the pressure at which it is supplied to the high-pressure pump. The tests were carried out on an engine dynamometer equipped with a single-cylinder SB3.1 test engine coupled to an electric swirl brake. It is a proprietary single-cylinder research engine that is based on Leyland’s SW680 engine. The basic parameters of the test engine are presented in Table 1.

Table 1.
Selected parameters of the SB3.1 test engine.
The measurement of concentrations of harmful exhaust components was carried out with the Micro PEMS Axion R/S+ mobile analyzer manufactured by Global MRV (Munich, Germany). This analyzer allows the measurement of gaseous toxic compounds using a non-dispersive infrared (NDIR) analyzer and an electrochemical analyzer. The exhaust gas stream was calculated on the basis of the data obtained from sensors of crankshaft rotational speed, pressure, and temperature in the intake manifold. The equipment also allows the concentration of PM to be tested using the method based on Laser Scatter, in which the speed of particle movement is measured (taking into account the values assigned to PM10). The gas data sampling rate was 1Hertz, and the sample flow was 10 L/min. The device is characterized by high measurement accuracy. The technical characteristics of the analyzer are presented in Table 2.

Table 2.
Selected parameters of harmful exhaust components analyzer.
The measuring devices and test equipment mentioned in the previous section were selected to carry out the tests because to the scope of work was adequate to achieve the expected results.
Engine tests were performed at a constant rotational speed (load characteristic) of 900 rpm and a load in the range from 0 to 40 N∙m with a step of 10 N∙m.
The control panel of the dedicated injection controller allows one to adjust the injection angle setting by exactly 2.8125 degrees (720°/256 bit). The limitation is caused by the resolution of the absolute encoder and is related to the place of its installation, which is the camshaft. The adjustment of the injection time is carried out with an accuracy of 0.1 ms.
3. Results
During the tests, three measurement series were performed. The first for a conventional injection system equipped with a standard Bosch CP3 pump, which is a reference for further measurements. The two following measurements were carried out on a dedicated high-pressure pump enabling the dissolution of hydrogen in diesel fuel.
The fuel pressure during the measurements was regulated by an external controller based on the reading from the pressure sensor in the Common Rail tank and by regulating the operation of the electronic fuel dose valve on the pump and the pressure regulator on the CR tank. In order to demonstrate the favorable features of gas dissolution in the fuel, the injection pressure in each measurement series was 40 MPa.
In Figure 5, a significant reduction in NOx emissions when supplied with a solution of diesel fuel with hydrogen indicates that the temperature of the medium has decreased during combustion, in particular the maximum values. However, the decrease in temperature due to the increasing combustion rate means that an additional factor must have appeared, which consequently accelerated combustion. The decrease in temperature could not have been caused by a change in the amount of fuel fed, because in both cases the same injection time and the same pressure in the tank were set. The acceleration of combustion must therefore have resulted from changes in the structure of the H2 injection dissolved in diesel fuel.
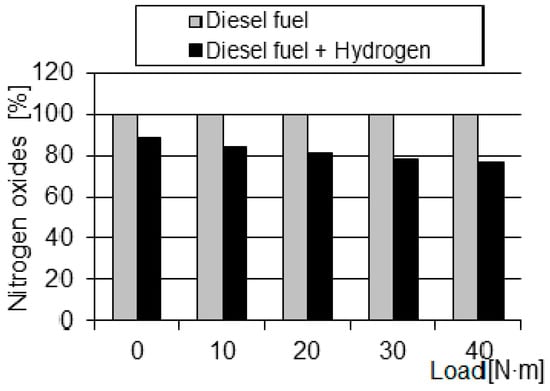
Figure 5.
Comparison of the relative changes in the NOx concentrations in exhaust gas depending on the load.
A reduction in NOx emissions of up to 21% for a diesel blend containing 0.3% hydrogen suggests that changes in combustion dynamics due to the presence of hydrogen have led to fuller and more controlled combustion, thereby limiting the formation of nitrogen oxides. This result highlights the potential of hydrogen enrichment to reduce NOx emissions in internal combustion engines. The reduction of NOx emissions from hydrogen enrichment in a diesel engine is associated with complex combustion reactions.
The following are simplified reactions illustrating the potential impact of hydrogen on the reduction of NOx emissions. In a typical combustion process without hydrogen enrichment, nitrogen oxides (NOx) are formed as a result of high temperatures:
N2 + O2 → NO (Nitric oxide formation)
NO + O2 → NO2 (Conversion of NO to nitrogen dioxide)
High temperatures during combustion cycles in the engine promote the formation of nitrogen oxides. The addition of hydrogen to the fuel mixture affects the combustion process, leading to a reduction in NOx emissions due to the changed combustion dynamics:
H2 + O2 → H2O (Combustion of hydrogen with steam generation)
H2 + O2 → H2O2 (Formation of hydrogen peroxide)
The hydrogen–air mixture exhibits combustion in a wide range from 4 to 75% by volume of air, which represents a particularly wide combustion spectrum. Such a wide range ensures fuel savings because combustion is possible even with a lean mixture. The reduction of NOx content is facilitated by a slight increase in temperature at the end of combustion when using a lean mixture [17,18]. Taking into account that the self-ignition temperature of hydrogen (584.4 °C) exceeds the temperature of gasoline and natural gas, it becomes necessary to use an internal source [19,20] when using hydrogen in internal combustion engines. It is worth noting that a key aspect of internal combustion engines is to increase efficiency by increasing the compression ratio. The increased self-ignition temperature of hydrogen allows for the improvement in engine efficiency by achieving a higher compression ratio. Nevertheless, the shorter flame extinguishing distance for hydrogen, compared to gasoline and natural gas, increases the likelihood of flame reflection. In addition, the combustion rate of hydrogen under stoichiometric conditions and at atmospheric pressure is 2.83 m/s, while for gasoline, it is 0.3 m/s. Given the faster hydrogen combustion, adjustments to the existing system should take into account more rapid ignition time of hydrogen.
The presence of hydrogen can alter the formation pathways of nitrogen oxides during combustion. In the conventional combustion process, based on hydrocarbon fuels such as diesel fuel, the formation of NOx is intermediated with various nitrogen-containing radicals (e.g., NH, NH2, and N). However, in the case of hydrogen enrichment, reactions involving these intermediates may be disrupted or altered.
The chemical kinetics of the combustion reaction plays a key role in determining the formation of nitrogen oxides (NOx) in combustion engines [21]. Hydrogen introduced into the combustion process significantly affects chemical kinetics, affecting the pathways and rate of formation of NOx intermediates.
The dynamics of rich combustion includes the combustion of fuel with a fuel–air equivalence ratio greater than 1. Compared to lean combustion conditions, total combustion requires more oxygen or oxygen atoms. As a result, the concentration of the OH radical decreases. At the same time, the share of hydrogen in OH radicals increases, which leads to hydrogen enrichment and an increase in the tendency of NHx radicals (x = 1, 2, 3) to combine with hydrogen and undergo oxidation.
One common example of a chain-termination reaction is the reaction between a radical and a stable molecule to form non-radical products. For instance, in the context of hydrocarbon combustion, the reaction of a hydroxyl radical (OH•) with a hydrocarbon molecule can be a chain-termination step:
OH• + Hydrocarbon → Products
In this reaction, the hydroxyl radical (OH•) reacts with a hydrocarbon, leading to the formation of stable products. The removal of the radical terminates the chain reaction because the radical is no longer available to initiate further propagation steps.
Chain-termination reactions play a crucial role in controlling the overall kinetics and dynamics of various chemical processes, including combustion reactions and polymerization reactions.
Within a certain temperature and oxygen concentration range, the reduction of NOx in the flames can be improved, in a process known as thermal DeNOx [22,23]. The main route of NO reduction, especially through reactions R1 and R2 (listed in Table 3) [24,25], which are key elements of thermal DeNOx. The self-sufficient nature of the DeNOx mechanism results from the direct or indirect production of O and OH from R1 at a rate controlled by the branching ratio α = k1/(k1 + k2). In addition, R1 exhibits high sensitivity to flame velocity and NO generation. When mixed with hydrogen, the increase in O/H radicals and the importance of the H2/O2 reaction make R1 with the NH2 radical even more important [26,27]. R1 is considered a chain-branching reaction, whereas R2 is a chain-termination reaction. When the reaction reaches R2, N2 + H2O is terminated and formed. A low branching coefficient indicates that R1 is slower than R2, which makes the formation of O/H radicals difficult. R2 in a larger proportion allows for the continuous production of NO in the reaction. The large branching coefficient suggests that R1 produces a significant amount of NNH, promoting the production of OH and O in the subsequent steps:

Table 3.
Basic reactions contributing to the reduction of NO.
NNH = N2 + H,
- (1)
- H + O2 = O + OH
- (2)
- O + H2O = OH + OH.
The lifespan of NNH radicals ranges from 10−11 to 10−8 s. During this period, NH2 radicals react with O/H radicals to form NO, replacing the reduction reaction. This gives
NH2 + OH = NH +H2O,
NH2 + O = HNO + H
and finally
HNO + OH = NO +H2O [28].
An interesting observation concerns the effect of pressure on NOx emission in the mixture of NH3/H2/air. Below 1 atm, the increase in pressure reduces NO emission [29,30,31]. Similarly, NOx emissions at a high pressure show a significant decrease compared to emissions at 1 atm, at levels below 5 and 1 ppm, and when the pressure exceeds 10 and 20 atm, respectively. Moreover, the addition of steam to the NH3/H2 mixture showed increased energy generation potential while mitigating Zeldovich’s NOx emissions and introducing O/H radicals into the system. The benefits include reductions in both NOx and unburnt NH3, particularly with lower equivalence factors, φ [32].
Another harmful compound analyzed during this research was carbon monoxide. Under these conditions, the CO concentration was higher by approx. 100% (Figure 6). The presence of CO in the exhaust gas signals a local shortage of oxygen necessary for combustion. The main cause of oxygen deficiencies is the improper matching of vortexes occurring in the combustion chamber with the jet of sprayed fuel. The shortage also depends on the atomization structure of the fuel but to a lesser extent. Therefore, it is treated as a secondary indicator when assessing the preparation of fuel for combustion.
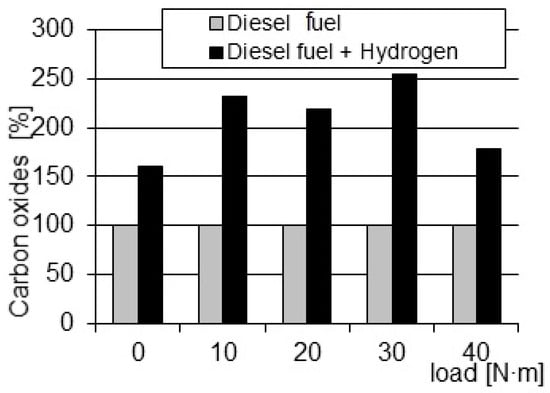
Figure 6.
Comparison of the relative changes in the CO concentrations in exhaust gas depending on the load.
The occurrence of increased CO emissions suggests a disturbance of the ideal balance of fuel and oxygen in the combustion chamber. In a properly functioning combustion process, the fuel is expected to mix evenly with oxygen, facilitating full and efficient combustion, resulting in lower CO emissions. However, the observed increase in CO levels indicates that, under certain conditions, this balance can be disturbed. The basic factor contributing to these deficiencies is the improper matching of vortexes in the combustion chamber to the sprayed dose of fuel. The vortexes play a key role in creating a well-mixed air–fuel mixture, ensuring optimal combustion efficiency. When the vortexes are not properly aligned in the fuel stream, this leads to uneven mixing, resulting in local insufficiency of oxygen. This oxygen deficiency hinders the combustion process, resulting in increased carbon monoxide production. Although the fuel atomization structure is considered a contributing factor, its influence is considered secondary to the key role played by the vortexes. The complicated relationship between the vortexes and the setting of the fuel stream is necessary to obtain an optimal air–fuel mixture and, consequently, to minimize CO emissions. The noticeable increase in CO emissions observed under these conditions highlights the importance of fine adjustments in the combustion chamber to ensure efficient mixing of fuel and air. Solving the problem of vortex–fuel misalignment is essential to alleviate local oxygen deficiencies, thereby increasing combustion efficiency and reducing carbon monoxide emissions.
Compared to the diesel fuel supply system (Figure 7 and Figure 8), in all measurements carried out on the solution supply system, the concentration of PM in the exhaust gas decreased significantly (in some cases up to 20%). PM emission indicates, to a large extent, the soot content in the exhaust gas. If the mechanism of soot formation is simplified to cracking, it can be concluded that the liquid fuel phase causes soot formation. The amount of soot produced depends on the physical conditions under which the airless liquid hydrocarbon phase is maintained.
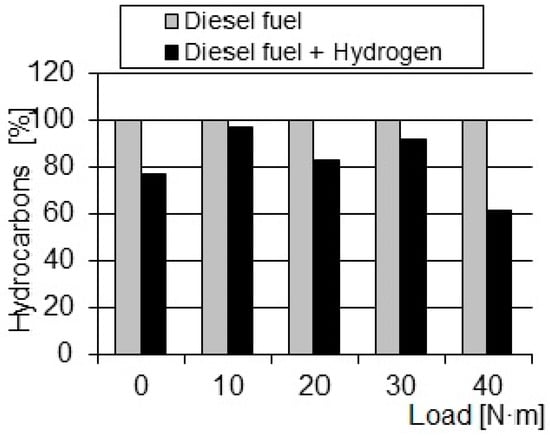
Figure 7.
Comparison of the relative changes in the HC concentrations in exhaust gas depending on the load.
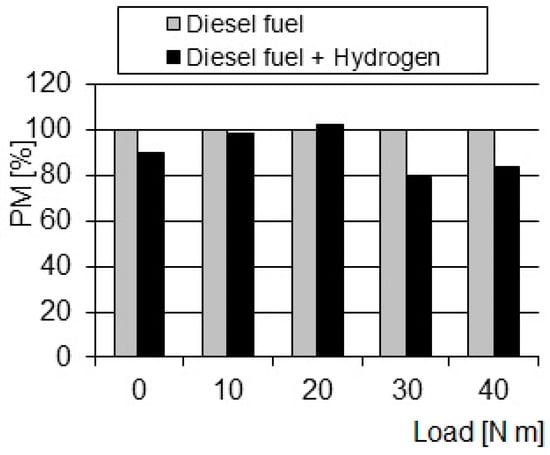
Figure 8.
Comparison of the relative changes in PM in exhaust gas depending on the load.
The pressure and temperature values in both cases are approximate (the analysis was performed for the same torque values). Therefore, time must play a significant role. The duration of the liquid phase depends on the rate of evaporation and, thus, on the size of the droplets (the conditions of movement of the medium in the chamber are similar), resulting from the operation of the fuel-atomizing mechanism. According to the described soot formation mechanism, PM occurs due to the shortening of the liquid phase time. This means that the droplets in the solution supply systems should have had smaller diameters than the droplets in the diesel supply systems. Although the injection pressure was the same, it can be concluded on the basis of PM emissions that the atomization of diesel fuel with H2 must be preceded by significant changes in the fuel atomization mechanism to decrease droplets’ diameters, which consequently would allow for more favorable fuel atomization at the combustion site compared to a conventional diesel fuel system. The factor causing this change is the physical release effect that occurs when gas is released from the liquid in which it is dissolved and whenever an imbalance occurs, i.e., when the thermodynamic potential of the solution is higher than the thermodynamic potential of the environment into which the ON solution with H2 is injected.
Despite the identical injection pressure, the particulate matter (PM) emission test indicates that the process of hydrogen atomization of diesel fuel (H2) requires significant changes in the fuel atomization mechanism. These modifications lead to the formation of droplets with a smaller diameter, which seems to play a key role in obtaining more favorable fuel atomization at the combustion point compared to a conventional diesel fuel system. The observed differences in particulate emissions between hydrogen and conventional diesel fuel systems highlight the complex relationship between fuel atomization and combustion efficiency. Although the injection pressure remained constant, the introduction of hydrogen appears to cause changes in the atomization process, possibly affecting the droplet size distribution. The formation of smaller-diameter droplets in the case of hydrogen diesel suggests a change in microstructure and an evenly distributed stream of fuel. This finer atomization may have several combustion benefits, such as increased oxygen interaction area, improved evaporation, and a more homogeneous air–fuel mixture. These factors contribute to improving combustion efficiency and can potentially lead to a reduction in particulate emissions. The conclusions drawn from particulate emissions emphasize the importance of understanding and optimizing the fuel atomization mechanism when introducing hydrogen into diesel fuel. Tuning this process seems to play a key role in achieving more efficient combustion, highlighting the potential of cleaner and more efficient power systems. Further investigation of specific changes in the fuel atomization mechanism will provide valuable insights to improve the integration of hydrogen in CI engines, with the ultimate goal of reducing emissions and increasing overall system performance.
The general trend observed for the combustion of fuels with the addition of hydrogen indicates that the flame temperature increases with the increase in the share of hydrogen in the fuel mixture, and it can be illustrated as two curves of the flame temperature [33,34]. The rate of increase in flame temperature is particularly noticeable when the proportion of H2 exceeds 90%, indicating the dominance of H2 for the second curve. For all tested equivalence coefficients, it was observed that the normalized rate of heat release increases with the addition of H2. The maximum rate of heat release occurs at the same temperature in the fuel-rich area but shifts to a lower temperature in stoichiometric and fuel-poor areas as the proportion of hydrogen increases. In general, the rate of heat release remains higher in fuel-rich and stoichiometric areas [35].
Theoretically, the maximum power in a hydrogen engine depends on the selected air–fuel ratio and fuel injection method. Some studies indicate that the stoichiometric air-to-fuel ratio for hydrogen is 34:1. At this particular ratio, hydrogen occupies 29% of the combustion chamber, leaving only 71% for air. As a result, the energy content of this mixture is lower compared to diesel fuel. This is due to the fact that diesel fuel, being a liquid, occupies a minimum volume in the combustion chamber, allowing more air to enter and thus increasing the energy content [36,37,38].
Calculating the engine’s thermal energy when burning a mixture of hydrogen and diesel fuel involves several factors, including the energy content of each fuel and the combustion efficiency. The calculation is as follows:
Energy Content of Hydrogen: The lower heating value (LHV) of hydrogen is approximately 120 MJ/kg.
Energy Content of Diesel Fuel: The LHV of diesel fuel can vary, but it is assumed to be around 45 MJ/kg in this case.
Combustion Efficiency: The combustion efficiency varies based on engine design and operating conditions. A typical range for internal combustion engines might be from 20% to 30%.
The formula for calculating thermal energy (Q) is
where
Q = m × LHV × η
- Q is the thermal energy;
- m is the mass of the fuel burned;
- LHV is the lower heating value of the fuel;
- η is the combustion efficiency (expressed as a decimal).
The share of hydrogen energy in the fuel mixture can be calculated according to the following equation:
In the analyzed case, with the parameters set to a fuel pressure of 400 bar, fuel consumption of 1.21 kg/h, and a hydrogen pressure of 2.5 bar at the entrance to the pump, the following is obtained:
H2 energy share = 0.79 [kJ/kg]
Assuming a certain mass of hydrogen (mH2) and diesel fuel (mdiesel) burned in the engine, the thermal energy for each fuel can be calculated and then summed up.
Qhydrogen = mH2 × LHVH2 × η
Qdiesel = mdiesel × LHVdiesel × η
Qtotal = Qhydrogen + Qdiesel
It has to be kept in mind that the actual energy obtained from the combustion process can be influenced by factors such as the combustion efficiency, engine design, and the air–fuel ratio. The actual engine performance may vary, and the values provided here are for illustrative purposes.
The equilibrium constant (K) expression along with the van’t Hoff equation can be used to represent the effect of a decrease in temperature on the chemical equilibrium of the reaction involving the production of NO. Assuming a simple reaction
where a, b, c, and d are the stoichiometric coefficients.
aA + bB ⇌ cC + dD
The Equilibrium Constant Expression is given as
And the Van’t Hoff Equation is given as
Now, for the specific reaction involving the production of NO, the appropriate stoichiometric coefficients can be substituted, and the signs can be adjusted according to the direction of the reaction. The generic reaction N2 + O2 ⇌ 2NO will be used for simplicity.
Equilibrium constant expression for NO formation:
Van’t Hoff Equation for Temperature Decrease (T2 < T1):
The negative sign in the exponent indicates that a decrease in temperature (T2 < T1) leads to a decrease in the equilibrium constant (K(T2) < K(T1)), favoring the production of lower concentrations of NO. The equilibrium constant reflects the ratio of product concentrations to reactant concentrations at equilibrium, and a lower equilibrium constant suggests a shift towards lower product concentrations.
4. Conclusions
When starting this research, it was assumed that hydrogen dissolved in fuel would have a significant impact on the fuel atomization mechanism and thus on the combustion process. The presented results not only confirm the assumption, but they also indicate that this effect is beneficial for combustion. The effect of hydrogen desorption from a solution with nucleation of gas bubbles, which accompanies the release of hydrogen from diesel fuel, is clearly noticeable and should be treated as an additional physical factor facilitating combustion. This phenomenon occurs when a gas, in this case hydrogen (H2), is emitted from a liquid in which it was initially dissolved. The effect of physical release is particularly noticeable when the system is in a non-equilibrium state, which refers to the cases where the thermodynamic potential of the solution exceeds the potential of the environment into which it is introduced. In this context, the solution consists of diesel fuel with dissolved hydrogen. The effect of physical release plays a key role in changing the mechanism of fuel atomization. The release of hydrogen from liquid diesel fuel causes changes in fluid dynamics and the breakdown of the fuel stream during the injection process. The release of hydrogen may lead to the formation of droplets with smaller diameters, affecting the general characteristics of the atomization. The non-equilibrium state, characterized by greater thermodynamic potential in the solution, suggests that the system undergoes dynamic changes during the injection process. This dynamic behavior can affect the atomization of the fuel, creating conditions facilitating the production of smaller droplets. Understanding the relationship between the physical release effect and the thermodynamics of the solution is crucial to optimize the combustion process and achieve better fuel atomization. The refinement of these dynamics contributes to a more efficient use of hydrogen in the piston engine, which ultimately affects the combustion characteristics and emissions. The effect of physical release, driven by the release of hydrogen from the diesel solution, introduces dynamic changes in the atomization process. These changes, occurring in a non-equilibrium state, contribute to the formation of smaller droplets, which may positively affect the combustion efficiency. While delving into this phenomenon, it is necessary to improve the integration of hydrogen into diesel systems and use its potential for cleaner and more efficient energy generation.
The results presented in this article indicate a potentially significant improvement in spraying fuel thanks to the presence of H2 in the fuel. A decrease in the concentration of nitrogen oxides may indicate a decrease in the temperature value in the flame front zone and also causes temperature decrease in the combustion chamber. This is confirmed by the results of torque measurements, the values of which increased by 5–15% for individual engine operating points. The observed decrease in the value of hydrocarbon concentrations indicates an improvement in the course of fuel atomization, while the increase in the concentration of carbon monoxide in the exhaust gas is the result of a local oxygen shortage during combustion.
Emission changes in absolute values using the author’s concept of dissolving hydrogen in diesel oil are as follows:
- Increasing the load resulted in a gradual decrease in nitrogen oxide concentrations by approximately 21% at maximum;
- Carbon monoxide concentration increases with load, reaching a maximum of about 258% for 30 Nm, while for the maximum power load, the concentration decreases to around 180%;
- The maximum decrease in hydrocarbon concentrations occurs at a load of 40 Nm, with a reduction of approximately 38%;
- Increasing the load also led to a decrease in particulate matter emissions by up to about 18%.
Hydrogen allows for leaner fuel–air mixtures, which can reduce the formation of nitrogen oxides (NOx) during combustion. Poor mixtures tend to produce lower combustion temperatures, which in turn can reduce the formation of nitrogen oxides, the main component of NOx emissions.
Hydrogen added to the fuel mixture can act as a neutral gas that dilutes the entire charge in the combustion chamber. The high speed of the hydrogen flame ensures faster combustion.
Author Contributions
Conceptualization, M.B. and R.S.; methodology, M.B. and B.K.; validation, M.B., W.K., and M.W.; formal analysis, M.W.; investigation, M.B., W.K., and R.S.; data curation, M.B. and W.K.; writing—original draft preparation, M.B. and W.K.; writing—review and editing, W.K.; visualization, R.S.; supervision, M.W.; project administration, W.K.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish Ministry of Education and Science, grant 0911/SBAD/2402 and 0415/SBAD/0351.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Milojević, S.; Glišović, J.; Savić, S.; Bošković, G.; Bukvić, M.; Stojanović, B. Particulate Matter Emission and Air Pollution Reduction by Applying Variable Systems in Tribologically Optimized Diesel Engines for Vehicles in Road Traffic. Atmosphere 2024, 15, 184. [Google Scholar] [CrossRef]
- Rymaniak, Ł.; Merkisz, J.; Szymlet, N.; Kamińska, M.; Weymann, S. Use of emission indicators related to CO2 emissions in the ecological assessment of an agricultural tractor. Eksploat. Niezawodn. 2021, 23, 605–611. [Google Scholar] [CrossRef]
- Rymaniak, Ł.; Kamińska, M.; Szymlet, N.; Grzeszczyk, R. Analysis of harmful exhaust gas concentrations in cloud behind a vehicle with a spark ignition engine. Energies 2021, 14, 1769. [Google Scholar] [CrossRef]
- Kuszewski, H. Effect of Injection Pressure and Air–Fuel Ratio on the Self-Ignition Properties of 1-Butanol–Diesel Fuel Blends: Study Using a Constant-Volume Combustion Chamber. Energy Fuels 2019, 33, 2335–2347. [Google Scholar] [CrossRef]
- Kozak, M. Exhaust Emissions from a Diesel Passenger Car Fuelled with a Diesel Fuel-Butanol Blend. In Proceedings of the 16th Asia Pacific Automotive Engineering Conference, Chennai, India, 6–8 October 2011. SAE Technical Paper 2011-28-0017. [Google Scholar] [CrossRef]
- Kozak, M. Ethyl alcohol as a fuel for contemporary internal combustion engines. Diagnostyka 2019, 20, 27–32. [Google Scholar] [CrossRef]
- Chen, J.; Fei, Y.; Wan, Z. The relationship between the development of global maritime fleets and GHG emission from shipping. J. Environ. Manag. 2019, 242, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Geca, M.; Wendeker, M.; Sochaczewski, R.; Szlachetka, M. Mean Effective Pressure Oscillations in an IC-CI Engine with Hydrogen-Rich-Gas Addition. In Proceedings of the SAE 2013 World Congress & Exhibition, Detroit, MI, USA, 16–18 April 2013. SAE Technical Paper 2013-01-1687. [Google Scholar] [CrossRef]
- Gruenig, C.; Avrashkov, V.; Mayinger, F. Self-Ignition and Supersonic Reaction of Pylon-Injected Hydrogen Fuel. J. Propuls. Power 2000, 16, 35–40. [Google Scholar] [CrossRef]
- Vincent-Randonnier, A.; Sabelnikov, V.; Ristori, A.; Zettervall, N.; Fureby, C. An experimental and computational study of hydrogen–air combustion in the LAPCAT II supersonic combustor. Proc. Combust. Inst. 2019, 37, 3703–3711. [Google Scholar] [CrossRef]
- Frolov, S.M.; Medvedev, S.N.; Basevich, V.Y.; Frolov, F.S. Self-ignition of hydrocarbon–hydrogen–air mixtures. Int. J. Hydrogen Energy 2013, 38, 4177–4184. [Google Scholar] [CrossRef]
- Siadkowska, K.; Barański, G. Combustion stability for early and late direct hydrogen injection in a dual fuel diesel engine. Combust. Engines 2024, 196, 89–98. [Google Scholar] [CrossRef]
- Longwic, R.; Tatarynow, D.; Kuszneruk, M.; Wozniak-Borawska, G. Preliminary tests of a Diesel engine powered by diesel and hydrogen. Combust. Engines 2023, 195, 35–39. [Google Scholar] [CrossRef]
- Al-Baghdadi, M.A.R.S. Measurements and predictions of pre-ignition limited operating conditions of a four stroke spark ignition engine fueled with hydrogen. J. Sci. Eng. Res. 2009, 2, 269–277. [Google Scholar]
- Al-Baghdadi, M. An overview of hydrogen as an alternative fuel. Encyclopedia 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Bajerlein, M.; Karpiuk, W.; Smolec, R. Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design. Energies 2022, 15, 9163. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hasegawa, H.; Mikami, M.; Kojima, N.; Kabashima, H.; Urata, Y. Effect of hydrogen addition to intake gas on combustion and exhaust emission characteristics of a diesel engine. Int. J. Hydrogen Energy 2011, 36, 13138–13149. [Google Scholar] [CrossRef]
- Leon, A. Hydrogen Technology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Lılık, G.; Zhang, H.; Herreros, J.; Haworth, D. Hydrogen assisted diesel combustion. Int. J. Hydrogen Energy 2010, 35, 4382–4398. [Google Scholar] [CrossRef]
- Pant, K.K.; Gupta, R.B. Fundamentals and use of hydrogen as a fuel. In Hydrogen Fuel: Production, Transport, and Storage; Gupta, R.B., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 3–32. [Google Scholar]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and kinetic modeling investigation on the laminar flame propagation of ammonia under oxygen enrichment and elevated pressure conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Lyon, R.K. Method for the Reduction of the Concentration of NO in Combustion Effluents Using Ammonia. U.S. Patent 3,900,554, 19 August 1975. [Google Scholar]
- Lyon, R.K. The NH3-NO-O2 reaction. Int. J. Chem. Kinet. 1976, 8, 315–318. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Ramos, C.F.; Rocha, R.C.; Oliveira, P.M.R.; Costa, M.; Bai, X.-S. Experimental and kinetic modelling investigation on NO, CO and NH3 emissions from NH3/CH4/air premixed flames. Fuel 2019, 254, 115693. [Google Scholar] [CrossRef]
- Atakan, B.; Jacobs, A.; Wahl, M.; Weller, R.; Wolfrum, J. Kinetic measurements and product branching ratio for the reaction NH2+NO AT 294–1027 K. Chem. Phys. Lett. 1989, 155, 609–613. [Google Scholar] [CrossRef]
- Miller, J.A.; Klippenstein, S.J. Theoretical considerations in the NH2 + NO reaction. J. Phys. Chem. A 2000, 104, 2061–2069. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling combustion of ammonia/hydrogen fuel blends under gas turbine conditions. Energy Fuel 2017, 31, 8631–8642. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Combustion behavior of ammonia blended with diethyl ether. Proc. Combust. Inst. 2020, 38, 499–506. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Flame structure studies of premixed ammonia/hydrogen/oxygen/argon flames: Experimental and numerical investigation. Proc. Combust. Inst. 2009, 32, 1277–1284. [Google Scholar] [CrossRef]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2019, 37, 5401–5409. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Deng, L.; He, Z.; Osaka, Y.; Kobayashi, N. Effect of hydrogen addition on combustion and heat release characteristics of ammonia flame. Energy 2019, 175, 604–617. [Google Scholar] [CrossRef]
- Kumar, N. Hydrogen use in internal combustion engine. Int. J. Adv. Cult. Technol. 2015, 3, 87–99. [Google Scholar] [CrossRef]
- Faizal, M.; Chuah, L.S.; Lee, C.; Hameed, A.; Lee, J.; Shankar, M. Review of hydrogen fuel for Internal combustion engines. J. Mech. Eng. Res. Dev. 2019, 42, 35–46. [Google Scholar] [CrossRef]
- Gandhi, R.D. Use of hydrogen in internal combustion engine. Int. J. Eng. Manag. Sci. 2015, 2, 1–9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).