Abstract
Systematic studies on the mechanism underlying Fe2O3-catalyzed NO reduction in a reducing atmosphere during sludge combustion remain limited. In this study, density functional theory was employed to investigate the adsorption properties of NH3, CO, and NO on the α-Fe2O3(001) surface, and the mechanisms underlying the NH3 and CO reduction of NO during the adsorption process. The results demonstrated that NH3, CO, and NO chemically adsorbed on the surface Fe top site, thereby generating distinctly high adsorption energies. NO exhibited the highest adsorption energy. With regard to the catalytic mechanisms of NH3 and CO during NO reduction, the α-Fe2O3(001) surface exhibited different characteristics. NH3 reduction of NO tended to follow the Eley–Rideal (E-R) mechanism. The dissociation of -NH2NO is the rate-determining step for the NH3 reduction of NO. The presence of α-Fe2O3(001) reduced the dissociation energy barriers of NH3 and NH2NO, thereby catalyzing the reduction reaction. In contrast, NO dissociation was more challenging during the CO reduction of NO. The α-Fe2O3(001) surface reduced the dissociation barrier of the NO-NO dimer from 2.04 to 1.53 eV. Two adsorbed NO molecules first formed NO-NO dimers; these then dissociated into N2O and atomic oxygen, thereby catalyzing the reduction reaction.
1. Introduction
The production of sludge, a by-product of the sewage treatment process, has increased significantly with rapid urbanization. Sludge contains hazardous substances such as organic pollutants, pathogens, and heavy metals. These can pose severe hazards to the ecological environment and public health if not treated effectively [1]. However, after sludge is dried, its calorific value becomes equivalent to that of lignite. This makes it suitable for use as a fuel, an approach which would promote sludge reduction, stabilization, unhazardous treatment, and resource utilization. Furthermore, the utilization of a relatively complete flue gas purification system in coal-fired power plants can mitigate the environmental impacts of gaseous pollutants generated during combustion, making it a potential approach for sludge resource utilization [2]. However, sludge contains 3–9% nitrogen [3]. Thus, the use of sludge may result in higher concentrations of nitrogen oxides (NOx) than that of coal during combustion. Additionally, because of the significant differences in fuel characteristics between sludge and coal, the generated and emitted NOx exhibit distinct properties.
It is generally considered that when sludge is heated, organic nitrogen enters the volatile fraction in the form of -HCN and -NH4 and transforms to NH3 or NO through intermediates such as -NCO, -NH2, and -NH. Further nitrogen transformation follows two main pathways. First, in an oxidizing atmosphere, NH3 or NO is oxidized further to form NOx. Second, in a reducing atmosphere, NO is reduced to N2 by reducing agents such as coke, NH3, and CO [4]. It is noteworthy that the abundant minerals in sludge can catalyze reduction reactions [5].
In contrast, Fe-containing coagulants are added during wastewater treatment. These eventually get mixed with sludge and transform to Fe2O3. Relevant studies have shown that Fe2O3 can effectively catalyze the reduction of NO, thereby achieving denitrification [6,7]. Zhao et al. observed that minerals containing Fe could catalyze the reduction of NO by coke during coal combustion [7]. Sun et al. determined that iron-based additives can suppress the emission of NO during algal biomass combustion because of the catalytic effect of such additives on HCN and NO [8]. Hu et al. added high-Fe2O3-content sludge ash to the process of bamboo biomass combustion and observed a significant reduction in NO emissions [9]. Zhao et al. observed that the crystalline phase of Fe2O3 is a critical factor affecting the efficiency of NO reduction, with α-Fe2O3 demonstrating higher NO reduction catalytic performance than γ-Fe2O3 [10]. With regard to the mechanism through which Fe2O3 catalyzes NO reduction, Larrubia proposed that NH3 is adsorbed on the surface of Fe2O3 and dehydrogenates to form NH2, which functions as an intermediate during NO reduction. However, Yang and Liu indicated a strong adsorption interaction between NO and the Fe2O3 surface [11,12,13].
Quantum chemistry methods have been widely used in various fields, including adsorption and catalysis [14,15]. Fan [16] studied the mechanism underlying NO reduction by NH3 on the γ-Fe2O3(110) surface. He observed that NO tends to adsorb at the top O site and that oxygen vacancies promote the progress of the reduction reaction. Kai [17] used density functional theory (DFT) to study the reduction pathway of NO by CO on the Rh(111) surface.
Therefore, although the catalytic reduction effect of Fe2O3 on NO has been verified, systematic research on the mechanisms underlying the adsorption, catalysis, and reduction of NO on the Fe2O3 surface at the molecular level is insufficient. In this study, based on DFT, the interactions of NH3, CO, and NO with the Fe2O3(001) surface were investigated computationally to reveal the pathways for the generation of reduction products. Additionally, the mechanisms underlying the reaction of NH3 and CO-reducing NO on the Fe2O3(001) surface were studied and compared with those underlying homogeneous reactions. The effect of the Fe2O3(001) surface on the NO reduction process was revealed. The research has significant implications for industrial applications, whether in reducing NOx concentrations in flue gas by incorporating iron-containing reactants, or by placing iron-based catalysts in the flue to promote NOx reduction during production processes.
2. Model Construction and Computational Methods
All the modeling and calculations in this work were performed using Materials Studio 2017 software. α-Fe2O3 is reportedly the ultimate form of Fe formed during sludge combustion. It has a space group of R3c and lattice parameters of a = b = 5.035 Å, c = 13.747 Å, α = β = 90°, and γ = 120° [18]. The bulk-phase model is shown in Figure 1a. To investigate the NO reduction reaction, the stable (001) surface was selected. It terminates in -Fe-O3-Fe. A periodic slab model of 2 × 1 α-Fe2O3(001) surface consisting of nine atomic layers was constructed. The vacuum layer thickness was set to 12 Å, as shown in Figure 1b. During calculations, the bottom three layers of atoms were fixed, whereas the top six layers were permitted to relax. In this study, the effects of various adsorption sites such as Fe top sites, O top sites, O vacancies, and Fe-O bridge sites were considered; these sites are illustrated in Figure 1c.
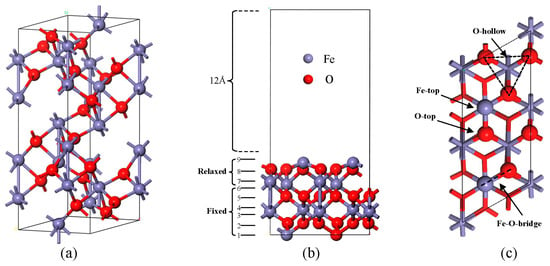
Figure 1.
Fe2O3 bulk and (001) surface. (a) bulk; (b) surface (front view); (c) surface (top view).
The adsorption energy represents the variation in system energy before and after adsorption. A negative adsorption energy indicates that adsorption is feasible. The larger the value of the adsorption energy, the stronger is the interaction between the systems. Equation (1) was used to calculate the adsorption energy. The energy barrier of the elementary reaction was determined using Equation (2). Here, a higher energy barrier indicates a more difficult reaction.
Ead = Etotal − Eslab − Emolecule
Ea = ETS − Ereactant
In these equations, the variables represent the following:
Ead: adsorption energy, in eV.
Etotal: total energy of the system after adsorption, in eV.
Eslab: surface energy of Fe2O3(001), in eV.
Emolecule: energy of the adsorbate, in eV.
Ea: activation energy of the reaction, in eV.
ETS: energy of the transition state, in eV.
Ereactant: energy of the reactants, in eV.
To investigate the reaction process, the local state transition quadratic synchronous transit (LST-QST) method was used to search for transition states, and frequency calculations were performed to ensure the accuracy of the transition states. After optimization, the lattice parameters of α-Fe2O3 were observed to be a = b = 5.051 Å and c = 13.754 Å. The optimization errors compared with the experimental values were 0.32% and 0.05 [18]. This demonstrated the reliability of the calculations.
3. Results and Discussion
3.1. Adsorption of NH3, CO, and NO on α-Fe2O3(001) Surface
Before adsorption on the α-Fe2O3(001) surface, the adsorbate molecules (NH3, CO, and NO) required geometric optimization to achieve the most stable adsorption configuration on the basis of their structures and properties. Prior to this optimization, the initial distance between the adsorbate molecules and substrate surface was set to 3.0 Å. Through optimization, we determined the most stable position and structure of the adsorbate molecules on the surface.
Table 1 presents the optimized adsorption energy (Ead), charge transfer (q), and adsorbate–substrate distance (d) for each adsorption configuration. All adsorption configurations exhibited negative adsorption energies (Ead). This indicated their stability and compliance with practical conditions.

Table 1.
Parameters for the adsorption of NH3, CO, and NO on the α-Fe2O3(001) surface.
Four configurations were observed during the adsorption of NH3 molecules on the α-Fe2O3(001) surface. Configurations 1 and 4 exhibited adsorption energies of −0.92 eV and −0.81 eV (both < 1 eV). Meanwhile, configurations 2 and 3 exhibited adsorption energies of −1.67 eV and −1.52 eV (both > 1 eV). After optimization, the NH3 molecules showed significantly reduced distances to the surface, with configuration 2 having the smallest distance of 1.918 Å. This indicated a strong interaction between NH3 and the α-Fe2O3(001) surface in configurations 2 and 3.
Mulliken population analysis of the optimized configurations revealed that the α-Fe2O3(001) surface gained partial electrons from NH3 during adsorption. Specifically, configurations 2 and 3 exhibited charge transfer values (q) of −0.34 e and −0.29 e, thereby implying a significant electron transfer. This indicated a chemical adsorption interaction between NH3 and the α-Fe2O3(001) surface in configurations 2 and 3. This result is consistent with previous research [19].
Six configurations were observed for CO molecules on the α-Fe2O3(001) surface. Configurations 2, 4, and 5 exhibited adsorption energies exceeding 1 eV, which reached the threshold for chemical adsorption. In particular, configuration 2 showed the most stable adsorption, with an adsorption energy of −1.84 eV. After optimization, the distance between CO and the nearest surface atom decreased significantly from the initial 3.0 Å to 1.801 Å. Moreover, in configuration 2, the maximum charge transfer attained −0.42 e. This implied a significant electron transfer between CO and the surface. These results indicate that the strongest interaction between the CO molecules and α-Fe2O3(001) surface occurred in configuration 2.
The adsorption of NO molecules on the α-Fe2O3(001) surface is similar to that of CO molecules. In configuration 2, the interaction between the systems was the strongest. This configuration exhibited a high adsorption energy of −2.34 eV, which indicated it to be the most stable adsorption configuration. Furthermore, configuration 2 showed a significant charge transfer (q) of −0.58 e. This further verified a substantial electron transfer between NO and the α-Fe2O3(001) surface.
Based on the above analysis, it is evident that when NH3, CO, and NO interact with the α-Fe2O3(001) surface, these molecules tend to adsorb near the Fe atoms, i.e., the Lewis acid sites, with weaker interactions near the surface O atoms. In terms of the adsorption energy and charge transfer, the strength of the interactions between these three molecules and the α-Fe2O3(001) surface can be ranked as follows: NO > CO > NH3. After adsorption, the three molecules retained their initial structures. This indicated that these molecules did not undergo significant activation. Figure 2 shows the most stable adsorption configurations of the three molecules on the surface.
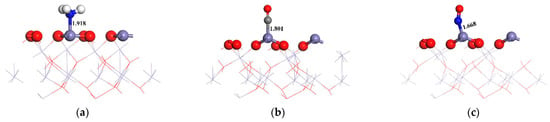
Figure 2.
Adsorption structure of the three molecules on α-Fe2O3(001). (a) NH3; (b) CO; (c) NO.
Figure 3 illustrates the density of state (DOS) distribution of the surface Fe and N atoms adsorbed at the Fe sites on the α-Fe2O3(001) surface after the strongest adsorption configurations of NH3, CO, and NO. Around −7 eV, the 2p orbitals of N in the adsorbed NH3 demonstrated a certain degree of overlap with the 3d orbitals of surface Fe. This indicated an interaction between N and Fe through the hybridization of 2p and 3d orbitals. For the adsorbed CO, the 2p orbitals of C and 3d orbitals of surface Fe displayed significant overlaps at −7, −2, the Fermi level (0 eV), and 3 eV. This indicated a stronger interaction between CO and the α-Fe2O3(001) surface. In the case of the adsorbed NO, the 2p orbitals of N exhibited an observable hybridization with the 3d orbitals of surface Fe at −8, −7, the Fermi level, and 2 eV. These results confirm the occurrence of chemical interactions among NH3, CO, and NO when adsorbed on the α-Fe2O3(001) surface.
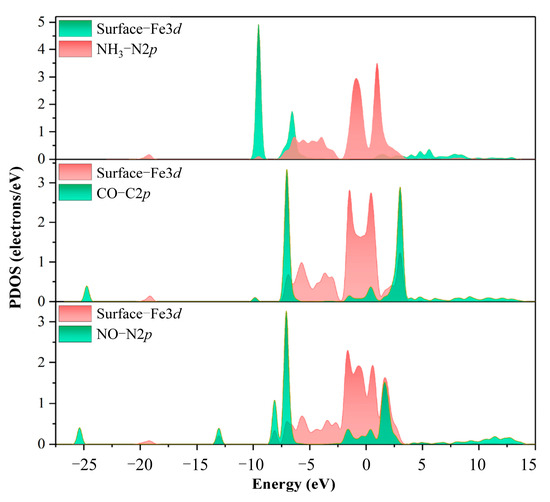
Figure 3.
DOS distribution of NH3, CO, and NO on α-Fe2O3(001) after adsorption.
3.2. Reaction Pathways of NH3 and NO on α-Fe2O3(001) Surface
The elementary reaction mechanisms on the catalyst surface can be categorized into the Eley–Rideal (E-R) and Langmuir–Hinshelwood (L-H) mechanisms [13,16]. According to the E-R mechanism [13], a component of the reactant first adsorbs on the catalyst surface and then reacts with another component in the gas phase. In contrast, the L-H mechanism indicates that all the components participating in the reaction first adsorb on the catalyst surface and then interact with each other on the surface [16].
In this study, considering both of these mechanisms, we evaluated two reaction pathways for the NH3 reduction of NO on the α-Fe2O3(001) surface:
E-R mechanism: NH3 adsorption → NH3 dissociation → NO diffusion to the surface → formation of NH2NO → dissociation of NH2NO.
L-H mechanism: co-adsorption of NH3 and NO → NH3 dissociation → formation of NH2NO → dissociation of NH2NO.
3.2.1. E-R Mechanism Pathway
The adsorption and dissociation of NH3 were the first steps of the catalytic reaction. Theoretically, NH3 can dissociate into two ammonia radicals: NH2 and NH; both radicals reduce NO. Liu [20] performed in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and detected the presence of -NH2. However, further dissociation of -NH2 into -NH requires a high energy barrier, rendering this process nearly negligible. Song [21] verified through DFT that further dissociation of -NH2 into -NH is exceptionally difficult on the MnCe1−xO2(111) surface. Zhou [22] observed that the dissociation of -NH2 into -NH on the γ-Fe2O3 surface has a high energy barrier (4.26 eV). Therefore, this study focused only on the process of NH3 dissociation into -NH2, considering -NH2 as the active site for NO reduction.
Figure 4 shows the potential energy variation during the reaction based on the E-R mechanism. Using configuration 2 from Section 3.1 as the starting point for NH3 dissociation on the α-Fe2O3(001) surface, the transition state was identified using the LST/QST method to obtain the pathway for NH3 dissociation on the α-Fe2O3(001) surface. This is shown in processes I and II. The dissociation of NH3 on the α-Fe2O3(001) surface is feasible. Initially, NH3 is adsorbed with the N-end at the Fe top site. During the dissociation process, a H atom of NH3 detaches gradually and is captured by a surface O atom. This forms an O-H bond with a length of 1.018 Å at transition state TS1. Simultaneously, the distance between the remaining NH2 group and the surface Fe atom decreases from 1.918 to 1.867 Å. The entire dissociation process is endothermic, with an energy barrier of 0.68 eV.
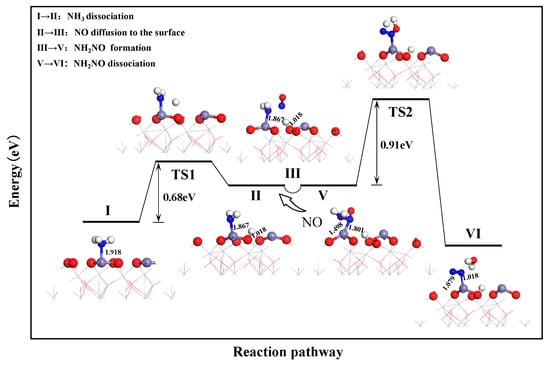
Figure 4.
Reduction process of NO by NH3 based on E-R mechanism pathway.
According to the E-R mechanism, the adsorbed NH2 species were reduced by their interaction with NO, which diffused to the catalyst surface. After NH3 dissociated on the α-Fe2O3(001) surface, forming the Fe-NH2 configuration (as shown in configuration 2), it functioned as a substrate for NO reduction. The NO molecule was placed above the Fe-NH2 configuration and subjected to structural optimization. This resulted in configuration 3. Processes II–III illustrate that under the influence of NO, -NH2 gradually shifted away from the (001) surface and the Fe-N bond was stretched, while NO approached the surface. The N atom formed bonds with both the surface Fe atom and N atom in -NH2, with lengths of 1.801 and 1.498 Å, respectively. This variation indicated a competitive adsorption relationship between -NH2 and NO at the Fe top site, with a stronger interaction between NO and the surface Fe atom. After optimization, the interaction between -NH2 and NO resulted in the formation of the NH2NO intermediate during NO reduction, which occurred spontaneously.
NH2NO is an important intermediate in NO reduction [22]. In this study, the transition state for the dissociation of NH2NO into H2O and N2 was identified. The reaction pathway obtained is shown as V → VI. During the dissociation of NH2NO, two H atoms from -NH2 detach and move toward the O atom of -NO. This results in the formation of free H2O. In addition, the N-N bond length is reduced from 1.119 to 1.079 Å. This forms a more compact N2 molecule that adsorbs on the Fe top site. Simultaneously, the Fe-N bond connecting the N2 molecule and surface Fe atoms is elongated. This indicates a weaker interaction between N2 and the surface, which favors the subsequent desorption of N2 from the surface. The dissociation of NH2NO is a strongly exothermic process. This makes the re-conversion of products to reactants unlikely. The entire process has an energy barrier of 0.91 eV. Thus, it is the rate-determining step in the NH3 reduction of NO in the E-R mechanism pathway.
3.2.2. L-H Mechanism Pathway
Figure 5 illustrates the potential energy variations during the co-adsorption and reaction of NH3 and NO on the α-Fe2O3(001) surface based on the L-H pathway. When NH3 and NO are co-adsorbed on the surface, the dissociation of NH3 needs to overcome an energy barrier of 0.64 eV. The barrier is similar to the dissociation energy barrier of NH3 when adsorbed individually. This indicates that the co-adsorbed NO had a negligible effect on the NH3 dissociation process. After co-adsorption of NH3 and NO, the NH2 and NO species co-adsorbed on the surface. These react through transition state TS2 to form the NH2NO intermediate. However, the formation of NH2NO in the L-H pathway requires a higher energy barrier of 0.71 eV than that in the E-R pathway. This indicates that the pathway based on the L-H mechanism is relatively less favorable for the α-Fe2O3(001) surface. Although NH2NO remains the rate-determining step in the reduction reaction, the reduction of NO by NH3 is more likely to follow the E-R mechanism on the α-Fe2O3(001) surface.
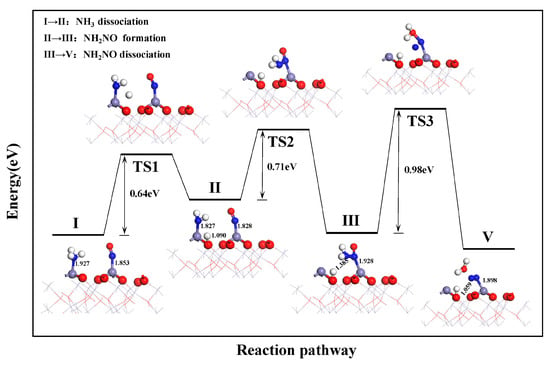
Figure 5.
Reduction process of NO by NH3 based on path 2.
To investigate the role of α-Fe2O3 in the homogeneous NH3 reduction of NO, the transition states of the key reaction steps in the E-R mechanism were investigated, and the energy barrier differences between the homogeneous and heterogeneous reactions were compared. Table 2 presents the results of this study. In the homogeneous reaction, the energy barrier for NH3 dissociation is 0.89 eV. This is marginally higher than the energy barrier for nonhomogeneous dissociation in the presence of Fe2O3. During the dissociation process, the O atoms on the α-Fe2O3(001) surface form stable O-H bonds with the H atoms dissociated from NH3. This facilitates the dissociation process.

Table 2.
Homogeneous and heterogeneous energy barriers for NO reduction by NH3.
Fe2O3 significantly reduces the energy barrier by 0.62 eV in the dissociation of NH2NO. This indicates its pronounced catalytic effect. In homogeneous reactions, NH2NO forms a stable planar structure. This renders it less vulnerable to dissociation. However, the Fe atoms on the α-Fe2O3(001) surface formed bonds with one of the N atoms in NH2NO. This disrupted the planar structure and significantly reduced the difficulty of dissociation. Therefore, α-Fe2O3(001) can catalyze the reduction of NO by NH3 during sludge combustion, with the surface Fe atoms functioning as active centers for the catalytic process.
3.3. α-Fe2O3(001) Surface: Reaction Pathway of CO with NO
3.3.1. Dissociation of NO
N2O is an important intermediate in the CO reduction of NO [23]. This study considered two pathways for the generation of intermediates (Table 3). The formation of N2O involves NO dissociation. According to the E-R pathway, NO directly dissociates and reacts with NO to form N2O. In contrast, in the L-H pathway, two NO molecules first form a dimer, (NO)2. It subsequently dissociates to generate N2O. These pathways play crucial roles in the generation of N2O.

Table 3.
Two paths for N2O formation.
The NO dissociation process on the α-Fe2O3(001) surface was determined through the E-R mechanism pathway and transition state search, as shown in Figure 6. Initially, configuration 1 was established, with two NO molecules co-adsorbed at the top Fe sites, which served as the starting point for NO dissociation. Subsequently, during the dissociation process, the N-O bond gradually stretched and broke. This resulted in the formation of a 1.562 Å-long Fe-O bond between the free O radical and neighboring Fe atom. Meanwhile, the N-Fe bond length decreased from 1.634 to 1.589 Å, as shown in configuration 1. Finally, the adjacent NO molecule at the Fe top site underwent desorption and gradually approached the dissociated N atom. This caused the formation of N2O under the influence of transition state TS2, which resulted in configuration 2. Notably, NO dissociation through the E-R mechanism is a highly endothermic process with a high energy barrier (4.25 eV). This indicates the challenging direct dissociation of NO on the α-Fe2O3(001) surface.
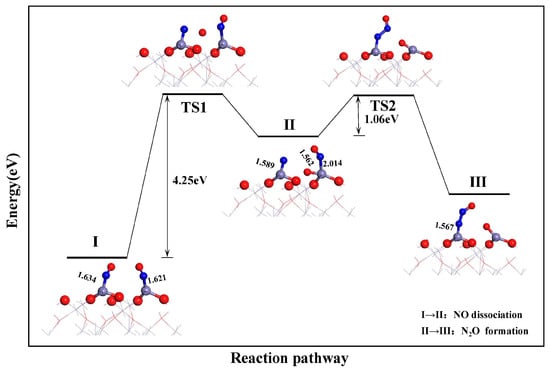
Figure 6.
N2O formation process based on path 1.
As shown in Figure 7, NO dissociation follows the L-H mechanism pathway. First, NO was adsorbed at the adjacent Fe top site on the surface, thereby forming configuration 1. Then, NO dimerized to form (NO)2 through transition state TS1. This resulted in configuration 2. Subsequently, an O atom dissociated from (NO)2 and formed bonds with two N atoms. This caused the N-Fe bond to shorten. Moreover, the free O atom formed a bond with the Fe atom, which resulted in configuration 2. Configuration 2 transformed to configuration 3 via TS2 and generated Fe-N2O and Fe-O. In the L-H mechanism, the highest energy barrier for the dissociation reaction was 1.52 eV, which was significantly lower than that in the E-R mechanism. This indicated that the direct dissociation of NO is significantly more difficult than that of (NO)2. Therefore, it can be concluded that the dissociation of NO on the α-Fe2O3(001) surface follows the L-H mechanism pathway. Here, step 2 is the rate-determining step of the dissociation reaction.
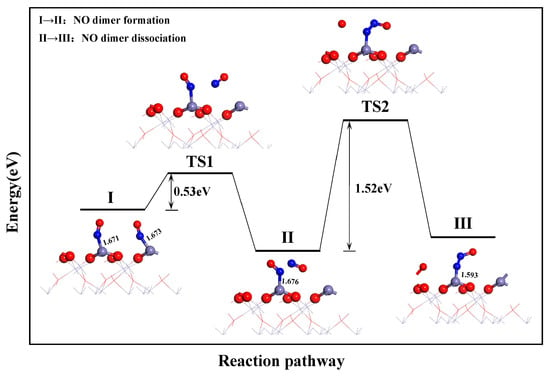
Figure 7.
N2O formation process based on path 2.
3.3.2. Catalytic Effect of Fe2O3 on NO Dissociation
In this study, we explored the transition states and calculated the energy barriers for homogeneous NO dissociation via the L-H mechanism to investigate the catalytic effect of Fe2O3 on NO dissociation. The results are summarized in Table 4. During the formation of NO dimers, the difference in the energy barriers between the homogeneous and heterogeneous cases was negligible. However, during the dissociation process, the energy barrier for heterogeneous dissociation in the presence of Fe2O3 was significantly reduced. This observation indicated that the Fe atoms on the α-Fe2O3(001) surface can capture the O radicals produced during NO dissociation, thereby facilitating the formation of N2O. Thus, the α-Fe2O3(001) catalyst induced a notable catalytic effect on NO dissociation. Moreover, the Fe surface atoms played a crucial role in capturing the dissociated O radicals. This resulted in the promotion of N2O formation.

Table 4.
Homogeneous and heterogeneous energy barriers for N2O formation.
3.3.3. CO2 and N2 Formation
The pathway for CO2 formation is illustrated in Figure 8. First, the CO molecule adsorbed on the Fe top site diffused to the vicinity of the O atom produced by the dissociation of (NO)2. Then, the CO molecule interacted with the O atom through TS1. This resulted in the formation of CO2. In the CO2 molecule, the C-O bond lengths were 1.595 and 1.543 Å, and the C-O-C bond angle was 115.6°. Simultaneously, the Fe-O bond was elongated to 2.016 Å. This indicated a weaker interaction between CO2 and the surface, which favored the subsequent desorption process. The overall energy barrier for this reaction was 0.46 eV. This indicated that CO and O are likely to react to generate CO2.
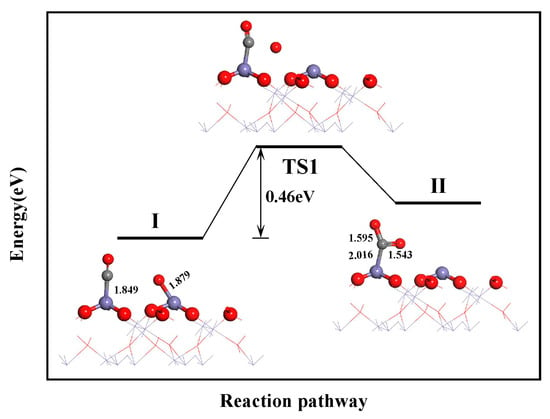
Figure 8.
CO2 formation path.
N2 was mainly formed through the further dissociation of N2O, which is a product of (NO)2 dissociation (see Figure 9). First, the top O atom of the N2O molecule adsorbed on the Fe top site dissociated and formed an Fe-O bond with the neighboring Fe atom through TS1. Simultaneously, the distance between the remaining N atoms reduced. This resulted in the formation of an N2 molecule with a bond length of 1.304 Å. In this process, the N-Fe bond was broken. This indicated that the N2 molecule can be desorbed straightforwardly from the surface. The process is exothermic with an energy barrier of 0.68 eV, which makes its occurrence feasible. From an energetic perspective, the formation of CO2 and N2 on the α-Fe2O3(001) surface during the CO reduction of NO is relatively straightforward. Furthermore, based on the configuration variations owing to the surface interactions, it can be inferred that as the final reduction products, CO2 and N2 can straightforwardly desorb from the surface, expose the active sites, and ensure the continuous progress of the reaction. Tian’s findings support this conclusion [17].
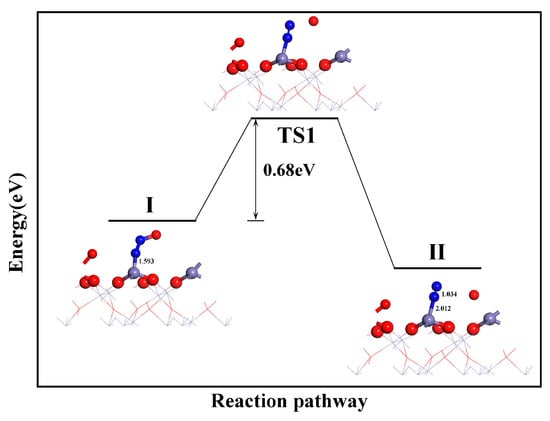
Figure 9.
N2 formation path.
3.4. Mechanism of NH3/CO Reduction of NO on α-Fe2O3(001) Surface
To conclude, during sludge combustion, the inherent α-Fe2O3 mineral played a significant role in the reduction of NO. The mechanism underlying the NH3/CO reduction of NO on the α-Fe2O3(001) surface is illustrated in Figure 10. In NH3 reduction of NO (Figure 10a), NH3 was first chemically adsorbed at the Fe top site on the α-Fe2O3 surface and dissociated to form Fe-NH2. Subsequently, NO from the flue gas diffused to the surface and reacted with -NH2 to form -NH2NO, which dissociated to produce H2O and CO2. For the CO reduction of NO (Figure 10b), NO molecules first diffused to the Fe top site on the surface and were adsorbed chemically. Then, the two NO molecules on the α-Fe2O3 surface reacted to form the NO-NO dimer, which subsequently dissociated into -N2O. CO from the flue gas then diffused to the surface and reacted with N2O to produce CO2. Meanwhile, O in N2O was removed, which resulted in the formation of N2.
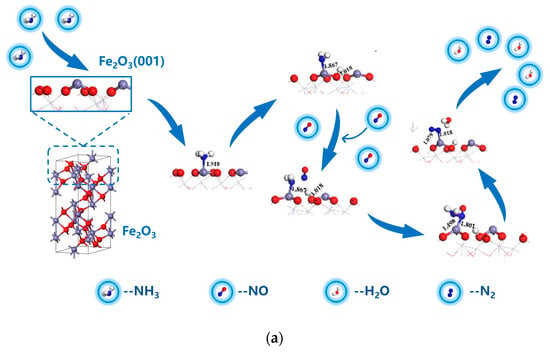
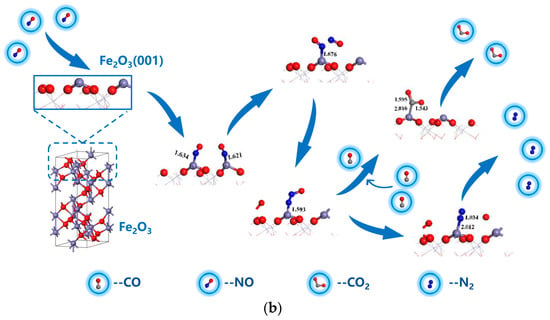
Figure 10.
Mechanism underlying the NH3/CO reduction of NO on the α-Fe2O3(001) surface. (a) Process of NH3 reduction of NO; (b) Process of CO reduction of NO.
4. Conclusions
- (1)
- The α-Fe2O3(001) surface can undergo chemical adsorption of NH3, CO, and NO preferentially on the surface Fe top sites. Among these, NO exhibits the strongest adsorption with an energy of −2.34 eV and a charge transfer of −0.58 e. The partial DOS analysis indicated significant hybridization between the surface Fe 3d orbitals and the N 2p orbitals of NO around −8, −7, the Fermi level, and 2 eV.
- (2)
- On the α-Fe2O3(001) surface, the reduction of NO by NH3 follows the E–R mechanism: NH3 adsorption → NH3 dissociation → NO diffusion to the surface → NH2NO formation → NH2NO dissociation. The dissociation of NH2NO is the rate-determining step in the reduction reaction, with an energy barrier of 0.91 eV.
- (3)
- During the reduction of NO by CO on the α-Fe2O3(001) surface, the formation of N2O follows the L-H mechanism: NO + NO → (NO)2 → (NO)2 → N2O + O. The dissociation of the NO dimer is the rate-determining step for the CO reduction of NO, with an energy barrier of 1.53 eV. The formation of CO2 and N2 requires energy barriers of 0.46 and 0.68 eV, respectively.
- (4)
- During sludge combustion, intrinsic α-Fe2O3 can catalyze the reduction of NO in a reducing atmosphere, and the surface Fe atoms function as catalytically active sites. Specifically, the catalytic effect includes a reduction in the energy barrier from 0.89 to 0.68 eV for NH3 dissociation, from 1.53 to 0.91 eV for NH2NO dissociation, and from 2.04 to 1.53 eV for NO dimer dissociation.
Author Contributions
Conceptualization, J.L. and Y.W.; methodology, Y.W.; software, X.H.; validation, J.L., Y.W. and X.H.; investigation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, Y.W.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [National Natural Science Foundation of China] grant number [No. U1910214].
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| Fe2O3 | Iron oxide |
| NH3 | Ammonia |
| CO | Carbon monoxide |
| NO | Nitric oxide |
| DFT | Density functional theory |
| DOS | Density of state |
| PDOS | Partial density of states |
References
- Wang, Y.L.; Jia, L.; Guo, B.H.; Wang, B.R.; Zhang, L.; Zheng, X.; Xiang, J.; Jin, Y. Effects of CaO-Fe2O3-Fe3(PO4)2 in sewage sludge on combustion characteristics and kinetics of coal slime. Fuel 2022, 322, 124267. [Google Scholar] [CrossRef]
- Liu, H.M.; Wang, Y.C.; Zhao, S.L.; Hu, H.Y.; Cao, C.Y.; Li, A.J.; Yu, Y.; Yao, H. Review on the current status of the co-combustion technology of organic solid waste (OSW) and coal in china. Energy Fuels 2020, 34, 15448–15487. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.Y.; Liu, P.; Hu, X.W.; Li, A.J.; Yao, H. Catalytic role of conditioner CaO in nitrogen transformation during sewage sludge pyrolysis. P. Combust. Inst. 2015, 35, 2759–2766. [Google Scholar] [CrossRef]
- Guo, S.C.; Zhang, K.X.; Liu, S.J.; Yang, S.; Du, G.W.; Zhang, H.X.; Shang, G.J.; Zhi, G.R. Progresses of research on the formation and transformation of NOX as atmospheric pollutant during coal burning. Appl. Chem. Ind. 2020, 49, 3205–3212+3245. [Google Scholar]
- Wang, Y.L.; Jia, L.; Guo, B.H.; Shen, X.; Zheng, X.; Xiang, J.; Jin, Y. Investigation of interaction mechanisms during co-combustion of sewage sludge and coal slime: Combustion characteristics and NO/SO2 emission behavior. Sci. Total Environ. 2022, 851, 158166. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.Y.; Hu, L.L.; Song, T.X.; Zhang, Y.; Zhang, H.; Ma, S.X.; Lyu, J.F. Occurrence transformation of iron oxides and their catalytic reduction of NO under fluidized bed temperature and CO. Chem. Ind. Eng. Prog. 2020, 39, 4474–4479. [Google Scholar]
- Zhao, Z.B.; Li, B.Q. Influence of Mineral Matter in Coal on NO-Char Reaction. J. Fuel Chem. Technol. 2001, 29, 129–134. [Google Scholar]
- Sun, J.; Zhao, B.T.; Su, Y.X. Advanced control of NO emission from algal biomass combustion using loaded iron-based additives. Energy 2019, 185, 229–238. [Google Scholar] [CrossRef]
- Hu, J.W.; Yan, Q.P.; Song, Y.Y.; Liu, J.Y.; Evrendilek, F.; Buyukada, M. Catalytic combustions of two bamboo residues with sludge ash, CaO, and Fe2O3: Bioenergy, emission and ash deposition improvements. J. Clean. Prod. 2020, 270, 122418. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Li, J.W.; Chen, B.H. Effect of Fe2O3 crystal structure on the performance of Fe2O3/Cr2O3 high temperature water gas shift catalyst. Mod. Chem. Ind. 2006, S2, 143–146. [Google Scholar]
- Larrubia, M.; Ramis, G.; Busca, G. An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3-TiO2 SCR catalysts. Appl. Catal. B-Environ. 2001, 30, 101–110. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, C.Z.; Li, J.H.; Yan, N.Q.; Ma, L.; Chang, H.Z. Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B-Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Liu, F.D.; He, H.; Zhang, C.B.; Shan, W.B.; Shi, X.Y. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Zhang, X.X.; Lyu, X.X.; Ww, H.X.; Xie, M.; Lin, R.Y.; Zhou, Z.J. Microscopic mechanism for effect of sodium on NO heterogeneous reduction by char. J. Fuel Chem. Technol. 2020, 48, 663–673. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.L.; Lu, C.M.; Wang, J.X.; Peng, J.S. Molecular simulation study of NO heterogeneous reduction by biomass reburning. J. Fuel Chem. Technol. 2020, 48, 689–697. [Google Scholar]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.Y.; Hu, S.; Sun, L.S.; Zhang, A.C. NO/NH3 adsorption properties on γ-Al2O3(110) surface during SCR process. J. Chem. Ind. Eng. 2014, 65, 4056–4062. [Google Scholar]
- Tian, K.; Tu, X.Y.; Dai, S.S. Density Functional Theory Study of NO+CO on Rh(111). Chem. J. Chinese U 2008, 29, 2360–2364. [Google Scholar]
- Finger, L.W.; Hazen, R.M. Crystal structure and isothermal compression of Fe2O3, Cr2O3, and V2O3 to 50 kbars. J. Appl. Phys. 1980, 51, 5362–5367. [Google Scholar] [CrossRef]
- Fang, Q.L.; Zhu, B.Z.; Sun, Y.L.; Zhu, Z.C.; Xu, M.G.; Ge, T.T. Mechanistic insight into the selective catalytic reduction of NO by NH3 over α-Fe2O3(001): A density functional theory study. Catal. Sci. Technol. 2019, 9, 116–124. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Li, Y.P.; Guan, Q.X.; Tian, Y. Low-Temperature Selective Catalytic Reduction of NO with NH3 over Mn2O3-Doped Fe2O3 Hexagonal Microsheets. ACS Appl. Mater. Inter. 2016, 8, 5224–5233. [Google Scholar] [CrossRef]
- Song, W.Y.; Liu, J.; Zheng, H.L.; Ma, S.C.; Wei, Y.C.; Duan, A.J.; Jiang, G.Y.; Zhao, Z.; Hensen, J.M. A mechanistic DFT study of low temperature SCR of NO with NH3 on MnCe1-xO2(111). Catal. Sci. Technol. 2016, 6, 2120–2128. [Google Scholar] [CrossRef]
- Zhou, W.B.; Niu, S.L.; Wang, D.; Lu, C.M.; Han, K.H.; Li, Y.J.; Zhu, Y. Promoting e ffect of Ti in the Ti-modified γ-Fe2O3 catalyst on its performance in the selective catalytic reduction of NO with ammonia, a DFT calculation study. J. Fuel Chem. Technol. 2020, 48, 1224–1235. [Google Scholar]
- Zhou, Y.S.; Gao, F.Y.; Tang, X.L.; Yi, H.H.; Meng, J.X. Research progress on NO reduction by CO over metal oxide catalysts. Chem. Ind. Eng. Prog. 2019, 38, 4941–4948. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).