The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells
Abstract
:1. Introduction
2. Numerical Model
2.1. Model Assumptions
- The gas mixture is assumed to behave as an ideal gas due to the low-operating-pressure conditions.
- Gas flow is considered to be laminar and incompressible.
- The influence of gravity is considered to be negligible, a reasonable assumption given that the bond number—defined as the ratio of gravitational force to surface tension— is typically small for SOECs (<0.1)
- Effective porosity and permeability are utilized to characterize the porous components, namely, DL, FLs, and CCs.
- The electrochemical reactions occur uniformly in the FLs; consequently, the current density is uniformly distributed in the EL, BL.
2.2. Conservation Equations and Source Terms
| Description | Expression | ||
|---|---|---|---|
| Mass | In FLs | (4) | |
| Momentum | For porous media | (5) | |
| Energy | In FLs, BL, EL | (6) | |
| (7) | |||
| (8) | |||
| (9) | |||
| Reversible Voltage [V] | (10) | ||
2.3. Solid Mechanics Model for SOEC
2.4. Boundary/Operating Conditions and Numerical Implementation
3. Results and Discussion
4. Conclusions
- A U-type stack with a co-flow configuration exhibits a gradual temperature increase in the electrolyte, leading to a higher minimum temperature for cells at the rear end.
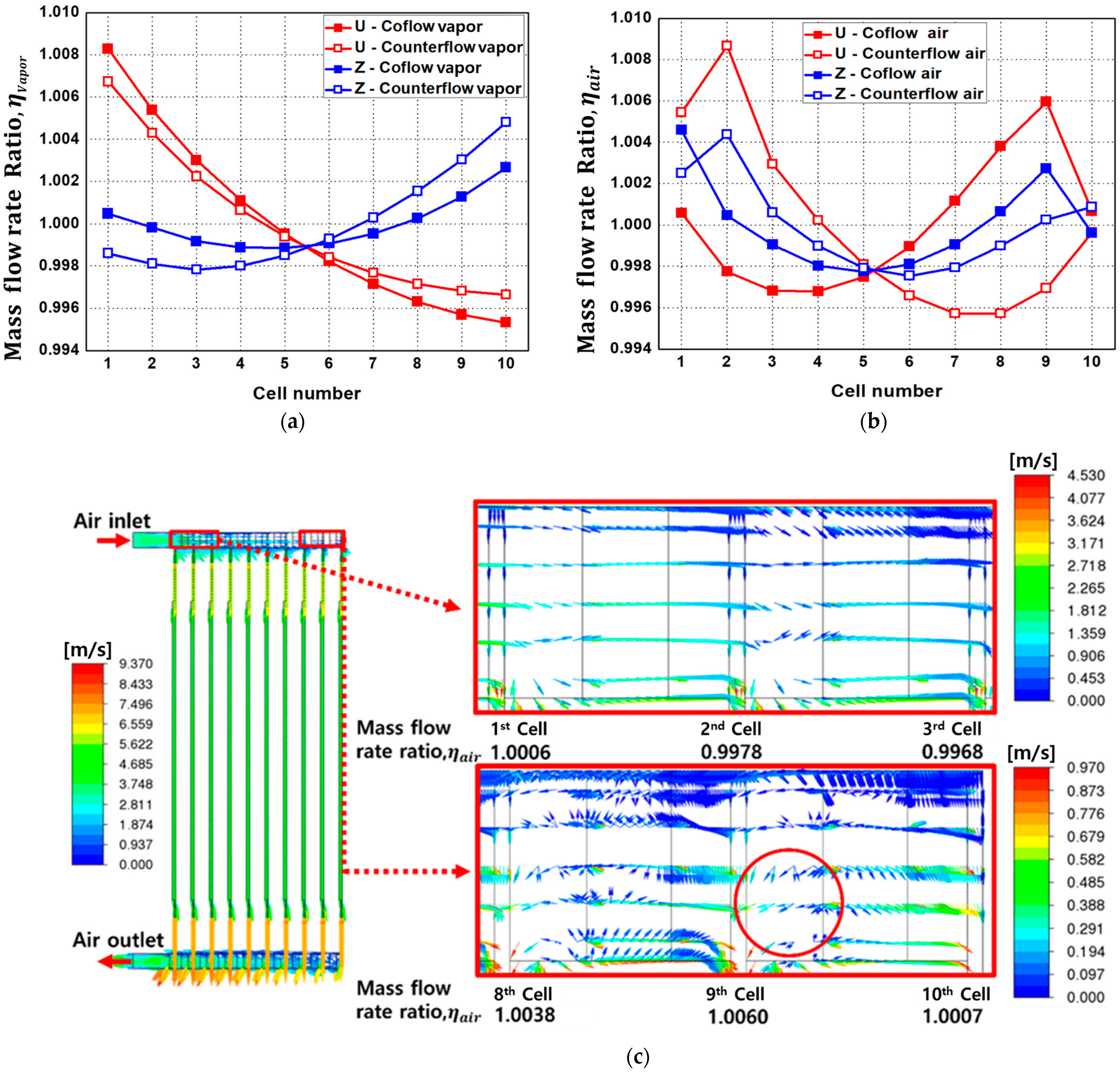
- Analysis of four different flow configurations revealed no significant differences between U-type and Z-type configurations, but the counter-flow configuration demonstrated a more uniform temperature distribution compared to the co-flow configuration. Specifically, the counter-flow exhibited an approximately 6 °C lower in the first cell compared to that of the co-flow SOEC stack.
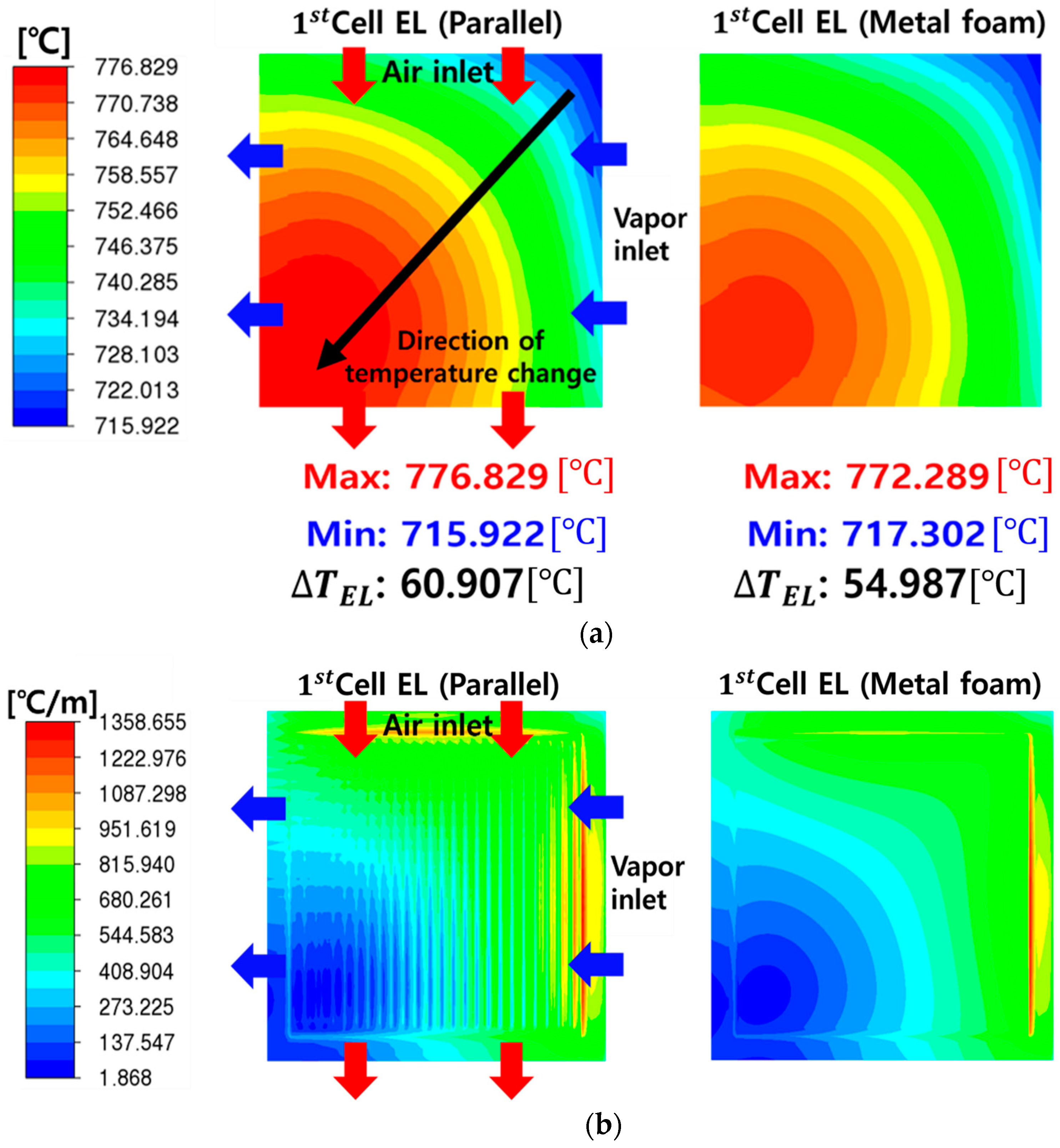
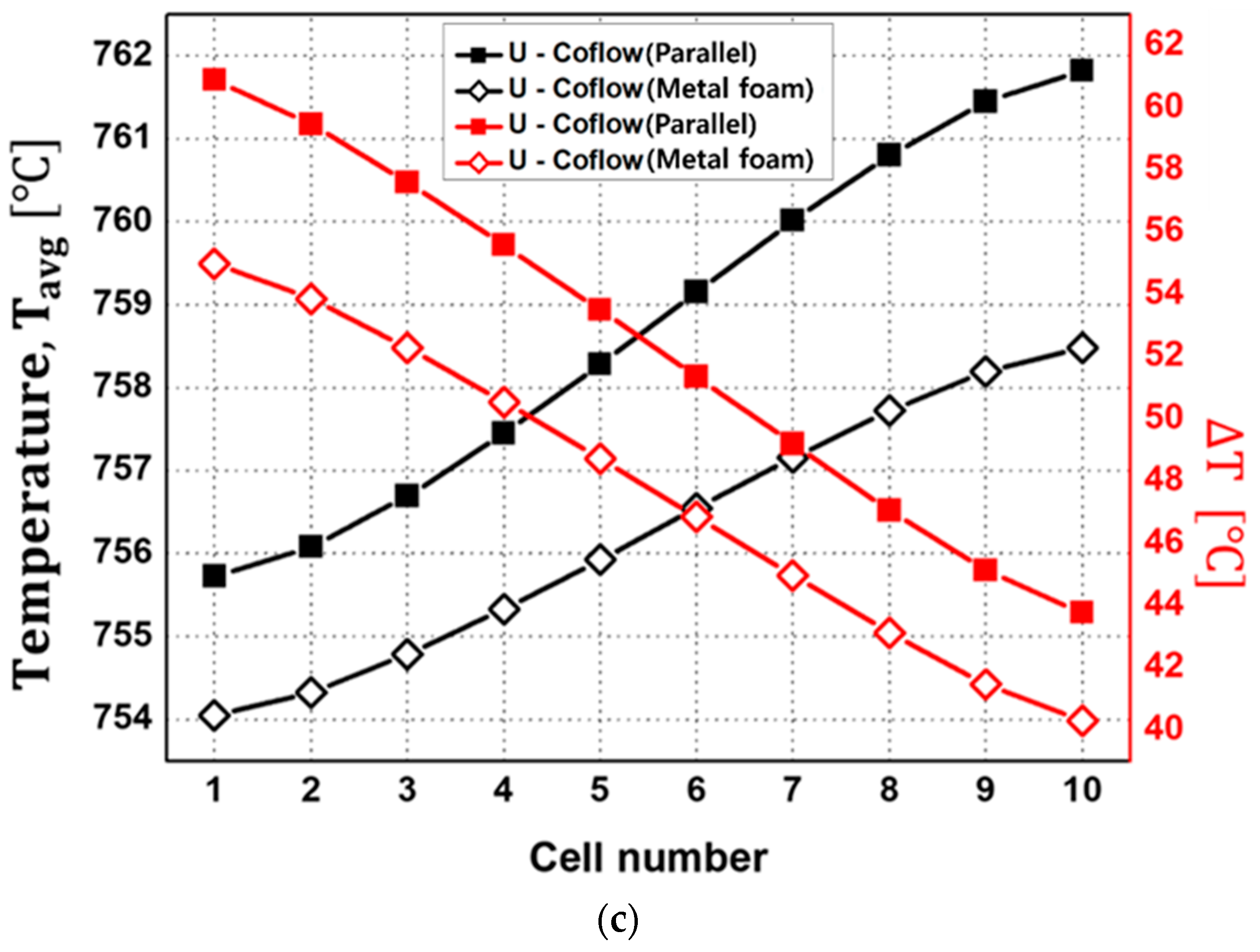
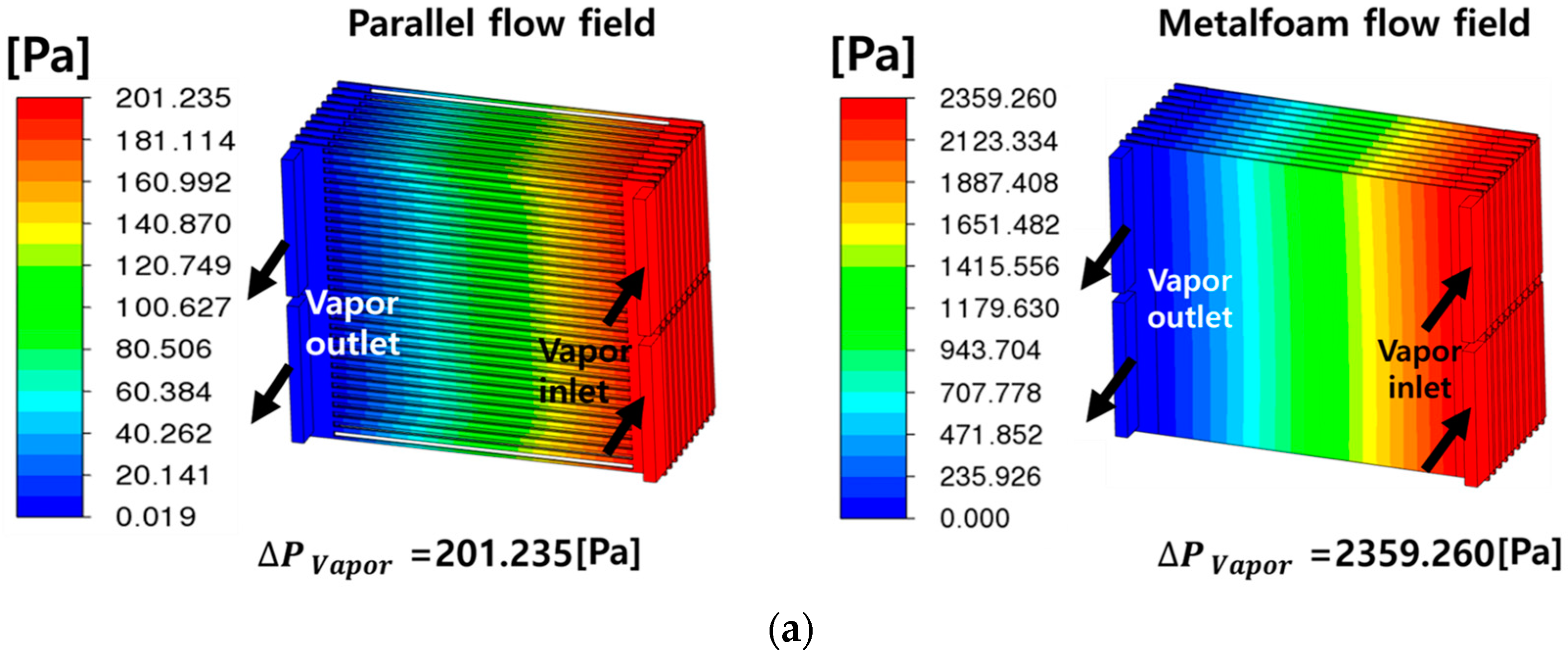
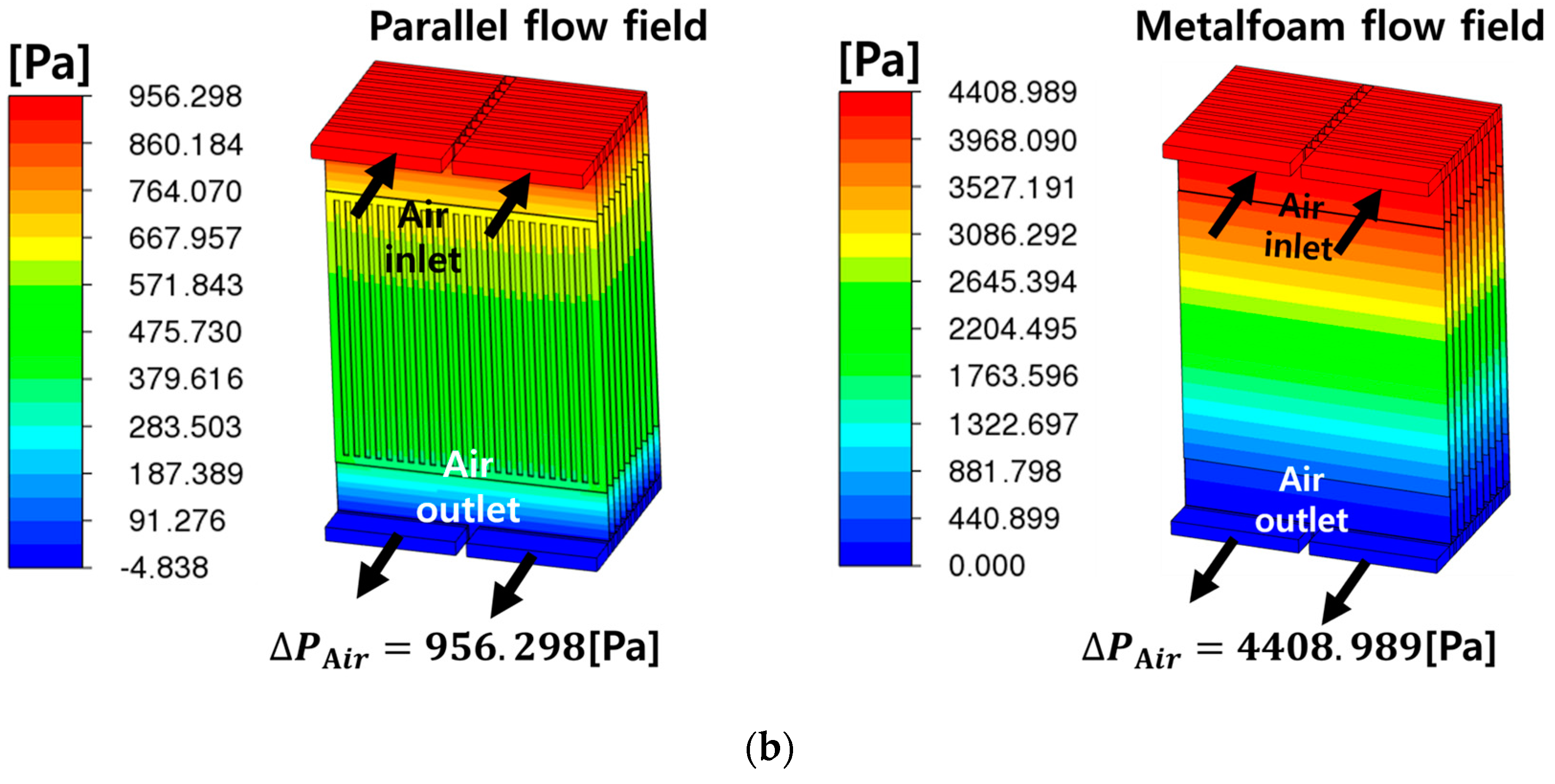
- The metal-foam-based SOEC stack outperformed the traditional parallel-flow-field design in terms of flow distribution and thermal management, with lower average temperatures and reductions of about 4–7 °C in temperature differences across cells. However, it incurred higher pressure-drops due to the increased resistance of the metal foam structure. The pressure-drop for fuel flow in the metal foam structure is particularly noteworthy, at 11 times that of the parallel flow channel structure.
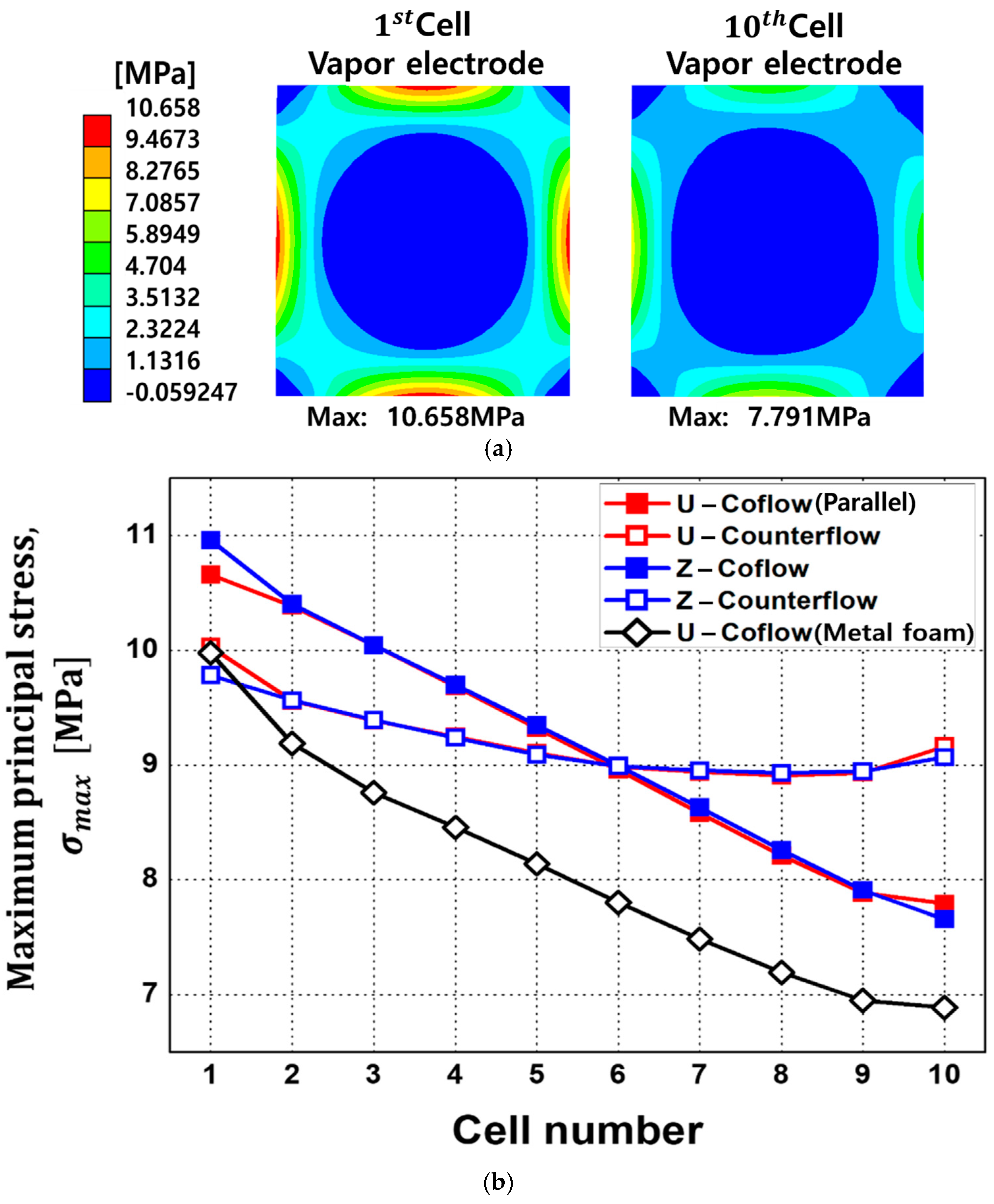
- Structural analysis showed that the co-flow configuration led to elevated stress at the front of the stack, with the maximum value of 10.658 MPa being measured at the first cell. Unlike the co-flow configuration, the counterflow configuration showed a significant mitigation in stress imbalance in each cell, and this tendency is consistent with the results of CFD temperature analysis. The metal-foam structure consistently demonstrated a reduction in thermal stress across all cells of about 1 MPa, highlighting its potential to alleviate thermal stress in SOEC stacks.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | |
| Cr | Inertial effect constant, m−1 |
| d | |
| F | |
| G | Shear modulus of elasticity, Pa |
| I | |
| K | Hydraulic permeability, m2 |
| k | Thermal conductivity, W m−1 K |
| mf | Mass fraction |
| MW | Molecular weight, kg/mol |
| n | Number of electrons transferred in the electrode reaction |
| P | Pressure, Pa |
| R | |
| r | |
| S | Source term in the transport equation or Entropy, J mol−1 K |
| T | Temperature, K |
| U | Cell potential, V |
| V | Voltage, V |
| W | |
| X | Mole fraction |
| Greek | |
| Thickness, m | |
| Volume fraction or expansion rate | |
| Overpotential, V | |
| Dynamic viscosity, | |
| Atomic diffusion volumes or Poisson’s ratio | |
| Density, | |
| Tortuosity or viscous shear stress, | |
| Superscripts | |
| air | Air electrode |
| Effective | |
| Vapor electrode | |
| T | Transposed matrix |
| th | Thermal |
| Subscripts | |
| 0 | Initial conditions or standard conditions, 298.15 K and 101.3 kPa (1 atm) |
| active | Active |
| avg | Average |
| BL | Buffer layer |
| BP | Bipolar plate |
| CC | Current collector |
| cell | Cell |
| DL | Diffusion layer |
| EL | Electrolyte layer |
| FL | Functional layer |
| Hydrogen | |
| Water | |
| i | Species or components of SOEC |
| in | Inlet |
| j | Species |
| k | Knudsen |
| m | Mass or particle diffusion |
| max | Maximum |
| metal | metal foam |
| min | Minimum |
| Nitrogen | |
| Oxygen | |
| out | Outlet |
| p | Pore |
| ref | Reference conditions |
| s | Solid |
| u | Momentum |
| vapor | Vapor |
| x | x direction |
| y | y direction |
| z | z direction |
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Pimentel, D.; Herz, M.; Glickstein, M.; Zimmerman, M.; Allen, R.; Becker, K.; Seidel, T. Renewable energy: Current and potential issues: Renewable energy technologies could, if developed and implemented, provide nearly 50% of US energy needs; this would require about 17% of US land resources. Bioscience 2002, 52, 1111–1120. [Google Scholar] [CrossRef]
- Weijia, L.; Fushuan, W.; Yusheng, X. Power-to-gas technology in energy systems: Current status and prospects of potential operation strategies. J. Mod. Power Syst. Clean Energy 2017, 5, 439–450. [Google Scholar]
- Rashidi, S.; Karimi, N.; Sunden, B.; Kim, K.C.; Olabi, A.G.; Mahian, O. Progress and challenges on the thermal management of electrochemical energy conversion and storage technologies: Fuel cells, electrolysers, and supercapacitors. Prog. Energy Combust. Sci. 2022, 88, 100966. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High Temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef]
- Min, G.; Choi, S.; Hong, J. A review of solid oxide steam-electrolysis cell systems: Thermodynamics and thermal integration. Appl. Energy 2022, 328, 120145. [Google Scholar] [CrossRef]
- Kang, C.; Huaiwu, P.; Junfeng, Z.; Xinxin, X.; Shengchen, Z.; Jingxin, R.; Biao, L.; Yueshe, W. Analysis of performance optimization of high-temperature solid oxide electrolytic cell based on the coupling of flow, heat, and mass transfer and electrochemistry. J. Energy Sci. Eng. 2022, 10, 3918–3927. [Google Scholar] [CrossRef]
- Lang, M.; Bohn, C.; Couturier, K.; Sun, X.; McPhail, S.J.; Malkow, T.; Pilenga, A.; Fu, Q.; Liu, Q. Electrochemical quality assurance of solid oxide electrolyser (SOEC) stacks. J. Electrochem. Soc. 2019, 166, F1180–F1189. [Google Scholar] [CrossRef]
- Santhanam, S.; Heddrich, M.; Riedel, M.; Friedrich, K. Theoretical and experimental study of Reversible Solid Oxide Cell (r-SOC) systems for energy storage. Energy 2017, 141, 202–214. [Google Scholar] [CrossRef]
- Bierschenk, D.M.; Wilson, J.R.; Barnett, S.A. High efficiency electrical energy storage using a methane–oxygen solid oxide cell. Energy Environ. Sci. 2011, 4, 944–951. [Google Scholar] [CrossRef]
- Kim, D.H.; Bae, Y.; Lee, S.; Son, J.-W.; Shim, J.H.; Hong, J. Thermal analysis of a 1-kW hydrogen-fueled solid oxide fuel cell stack by three-dimensional numerical simulation. Energy Convers. Manag. 2020, 222, 113213. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.H.; Lee, W.; Lee, S.; Hong, J. A novel interconnect design for thermal management of a commer-cial-scale planar solid oxide fuel cell stack. Energy Convers. Manag. 2021, 246, 114682. [Google Scholar] [CrossRef]
- Bao, J.; Karri, N.; Recknagle, K.; Wang, C.; Koeppel, B.; Marina, O.A. Modeling Framework to Analyze Performance and Structural Reliability of Solid Oxide Electrolysis Cells. J. Electrochem. Soc. 2022, 169, 054523. [Google Scholar] [CrossRef]
- Nakajo, A.; Mueller, F.; Brouwer, J.; Favrat, D. Mechanical reliability and durability of SOFC stacks. Part I: Modelling of the effect of operating conditions and design alternatives on the reliability. Int. J. Hydrogen Energy 2012, 37, 9249–9268. [Google Scholar] [CrossRef]
- Nakajo, A.; Mueller, F.; Brouwer, J.; Favrat, D. Mechanical reliability and durability of SOFC stacks. Part II: Modelling of mechanical failures during ageing and cycling. Int. J. Hydrogen Energy 2012, 37, 9269–9286. [Google Scholar] [CrossRef]
- Cui, T.; Xiao, G.; Yan, H.; Zhang, Y.; Wang, J.-Q. Numerical simulation and analysis of the thermal stresses of a planar solid oxide electrolysis cell. Int. J. Green Energy 2023, 20, 432–444. [Google Scholar] [CrossRef]
- Kim, S.-D.; Seo, D.-W.; Dorai, A.K.; Woo, S.-K. The effect of gas compositions on the performance and durability of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 6569–6576. [Google Scholar] [CrossRef]
- Choi, Y.; Byun, S.; Seo, D.W.; Hwang, H.J.; Kim, T.W.; Kim, S.-D. New design and performance evaluation of 1 kW-class reversible solid oxide electrolysis-fuel cell stack using flat-tubular cells. J. Power Sources 2022, 542, 231744. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, H.; Su, L.; Zhang, Z.; Zhang, H.; Li, K.; Fang, Y.; Ye, W. Effect analysis on thermal profile management of a cylindrical lithium-ion battery utilizing a cellular liquid cooling jacket. Energy 2021, 220, 119725. [Google Scholar] [CrossRef]
- Sheng, L.; Su, L.; Zhang, H.; Li, K.; Fang, Y.; Ye, W.; Fang, Y. Numerical investigation on a lithium ion battery thermal management utilizing a serpentine-channel liquid cooling plate exchanger. Int. J. Heat Mass Transf. 2019, 141, 658–668. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, H.; Zhang, H.; Su, L.; Zhang, Z. Lightweight liquid cooling based thermal management to a prismatic hard-cased lithium-ion battery. Int. J. Heat Mass Transf. 2021, 170, 120998. [Google Scholar] [CrossRef]
- Sheng, L.; Su, L.; Zhang, H.; Fang, Y.; Xu, H.; Ye, W. An improved calorimetric method for characterizations of the specific heat and the heat generation rate in a prismatic lithium ion battery cell. Energy Convers. Manag. 2019, 180, 724–732. [Google Scholar] [CrossRef]
- Afshari, E.; Mosharaf-Dehkordi, M.; Rajabian, H. An investigation of the PEM fuel cells performance with partially restricted cathode flow channels and metal foam as a flow distributor. Energy 2017, 118, 705–715. [Google Scholar] [CrossRef]
- Jo, A.; Ju, H. Numerical study on applicability of metal foam as flow distributor in polymer electrolyte fuel cells (PEFCs). Int. J. Hydrogen Energy 2018, 43, 14012–14026. [Google Scholar] [CrossRef]
- Zhan, R.; Wang, Y.; Ni, M.; Zhang, G.; Du, Q.; Jiao, K. Three-dimensional simulation of solid oxide fuel cell with metal foam as cathode flow distributor. Int. J. Hydrogen Energy 2020, 45, 6897–6911. [Google Scholar] [CrossRef]
- Navasa, M.; Yuan, J.; Sundén, B. Computational fluid dynamics approach for performance evaluation of a solid oxide electrolysis cell for hydrogen production. Appl. Energy 2015, 137, 867–876. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenkij, A.; Zhou, X.Y. A review of numerical modeling of solid oxide fuel cells. Int. J. Hydrogen Energy 2007, 32, 761–786. [Google Scholar] [CrossRef]
- Green, W.; Don, H.; Robert, P. Perry’s Chemical Engineers’ Handbook; McGraw-Hill Education: New York, NY, USA, 2008. [Google Scholar]
- Li, W.; Shi, Y.; Luo, Y.; Cai, N. Theoretical modeling of air electrode operating in SOFC mode and SOEC mode: The effects of microstructure and thickness. Int. J. Hydrogen Energy 2014, 39, 13738–13750. [Google Scholar] [CrossRef]
- Nakajo, A.; Kuebler, J.; Faes, A.; Vogt, U.F.; Schindler, H.J.; Chiang, L.-K.; Modena, S.; Van Herle, J.; Hocker, T. Compilation of mechanical properties for the structural analysis of solid oxide fuel cell stacks. Constitutive materials of anode-supported cells. Ceram. Int. 2012, 38, 3907–3927. [Google Scholar] [CrossRef]
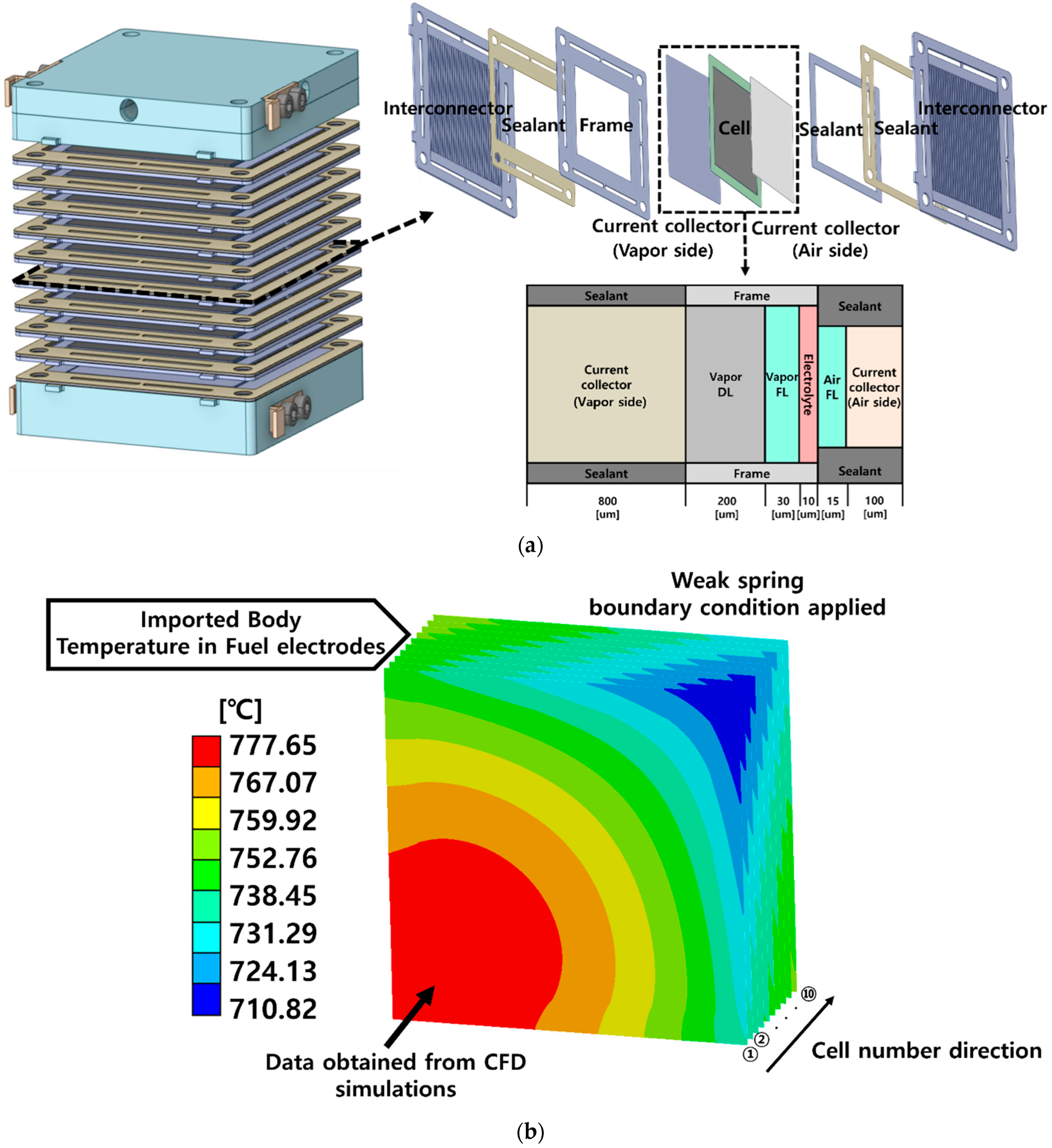

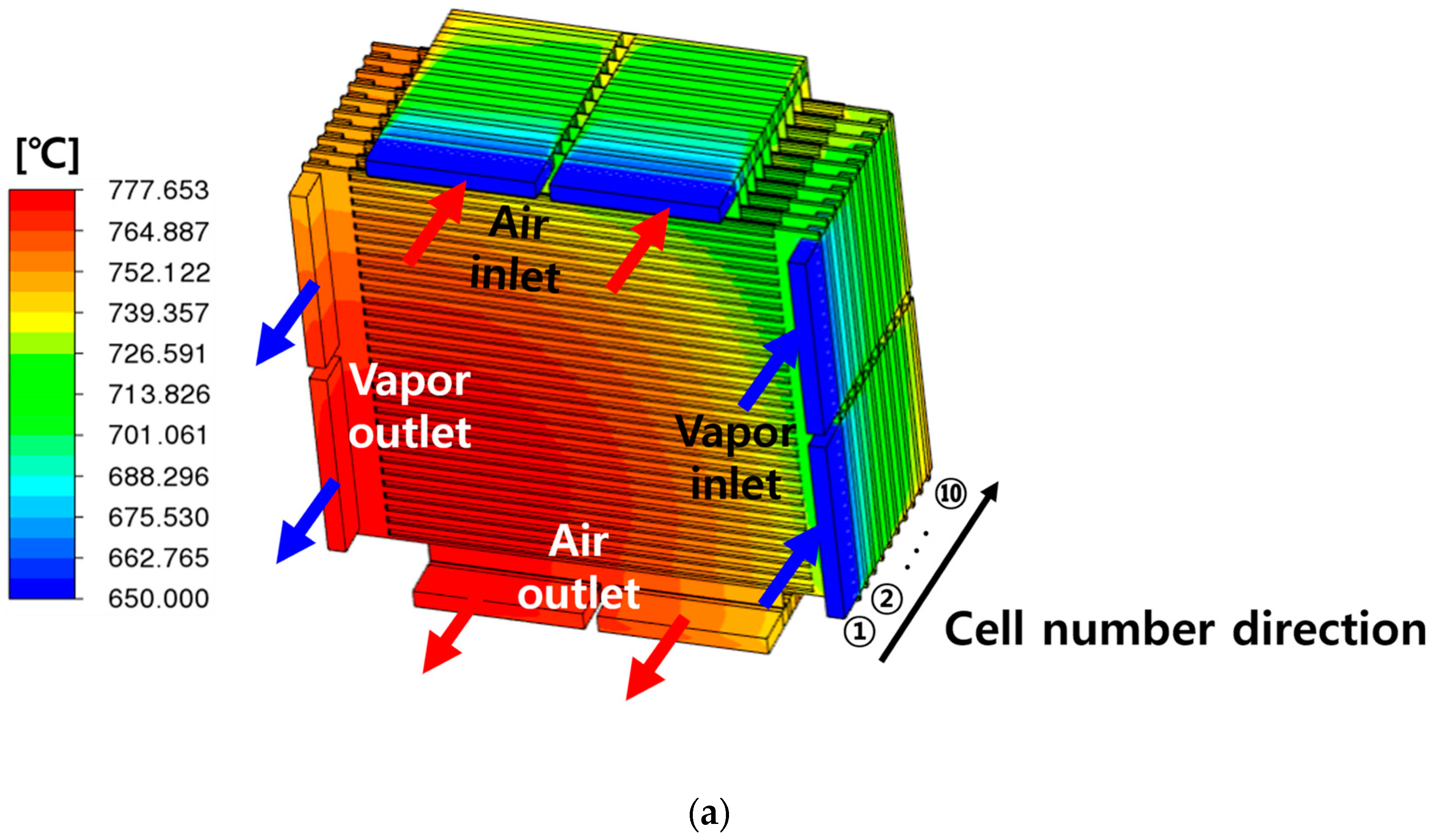
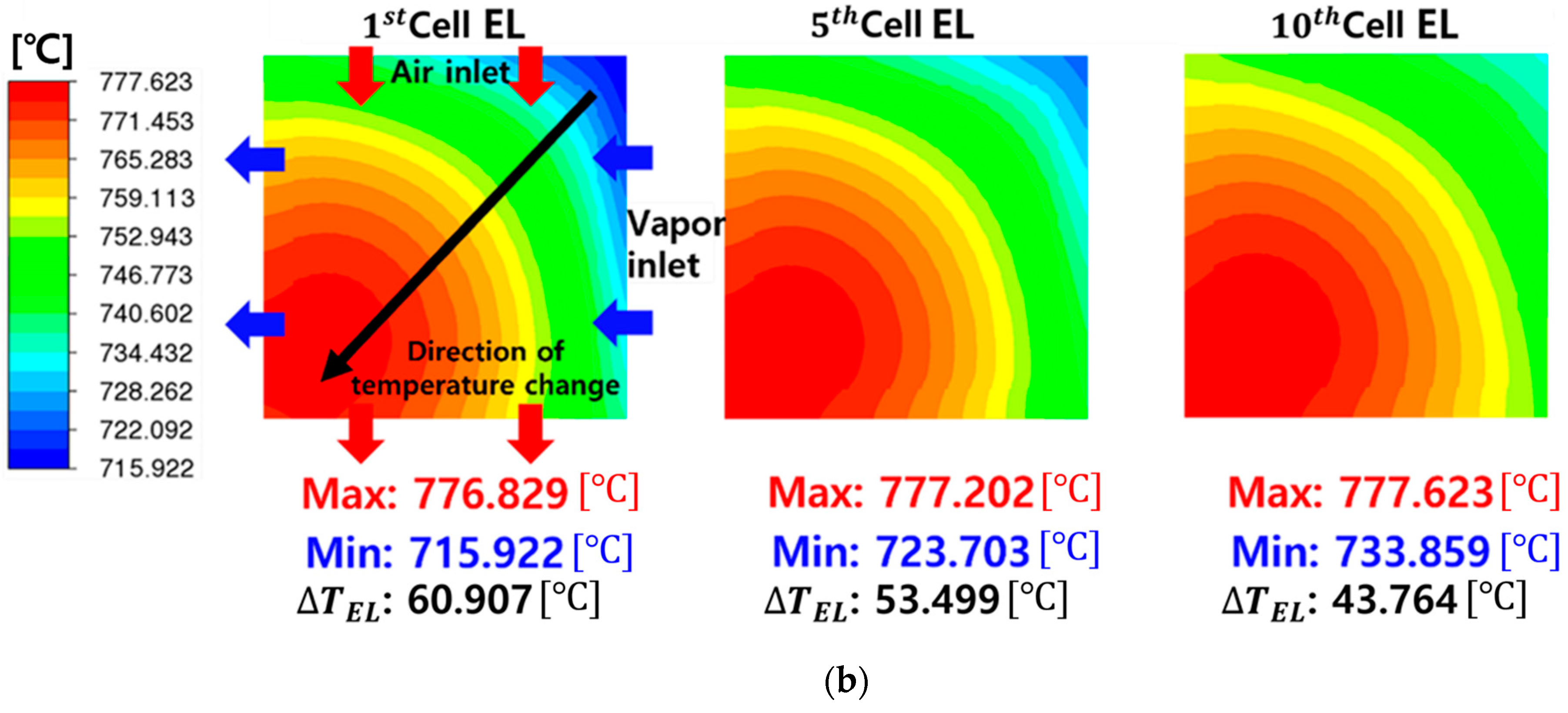
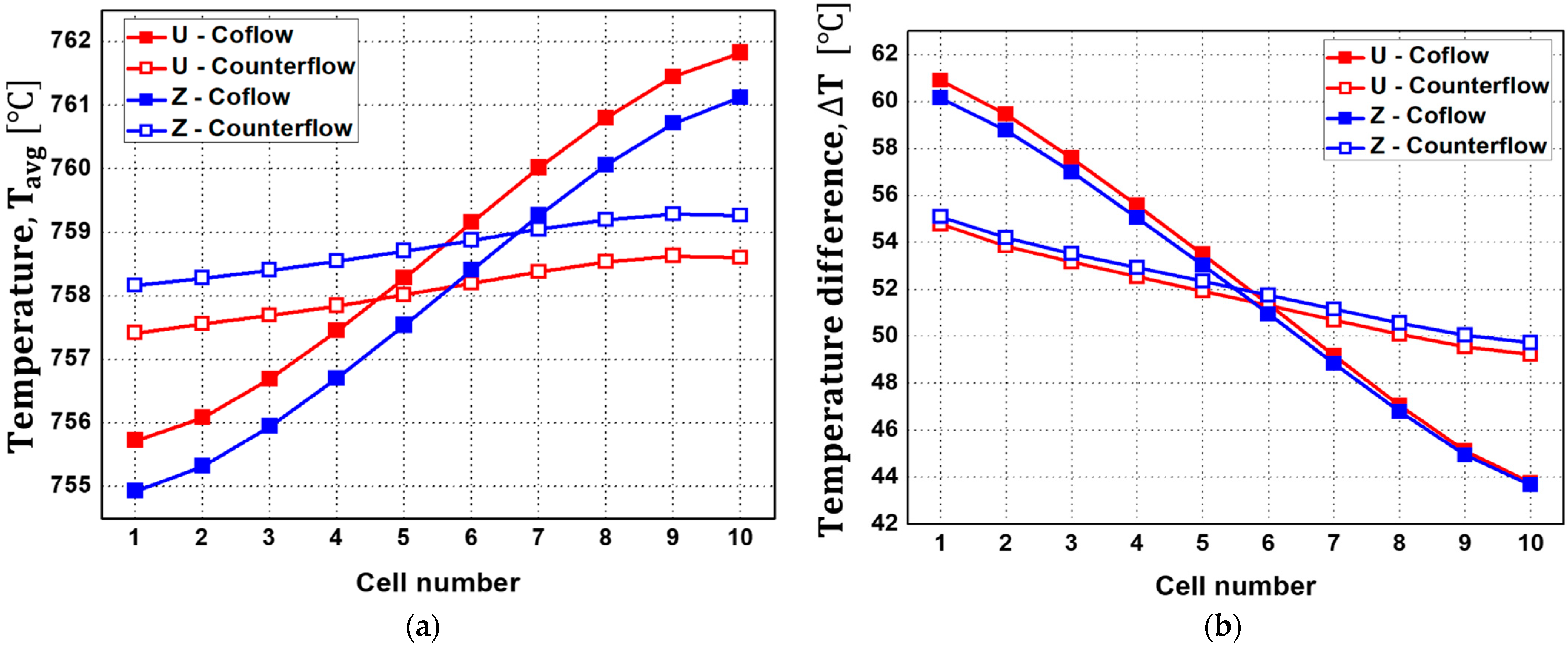


| Description | Value | Ref. |
|---|---|---|
| [28] | ||
| Exponent value in H2/H2O/N2/O2 relation of Equation (15), m | [28] | |
| Effective thermal conductivity of CC/vapor DL/vapor FL/electrolyte/BL/air FL/BP, ) | [29] | |
| Thermal conductivities of H2/H2O/N2/O2, | [30] | |
| Porosity of CC/vapor DL/vapor FL/air FL/metal foam, | /0.8 | [14,25] |
| Assumed | ||
| ) | [31] | |
| ) | [25] | |
| ) | [32] | |
| Young’s modulus, (E) | [32] | |
| Poisson’s ratio | 0.32 | [32] |
| Description | Value | |
|---|---|---|
| GC | Width, (vapor electrode/air electrode) | 2.0/2.0 |
| Depth, (vapor electrode/air electrode) | 1.0/1.0 | |
| Thickness | CC, (vapor electrode/air electrode) | 0.8/0.1 |
| Vapor DL, | 0.2 | |
| FL, (vapor electrode/air electrode) | 0.03/0.015 | |
| EL, | 0.005 | |
| BL, | 0.005 | |
| Active area, (air FL, EL) | 100/144 cm2 | |
| Inlet area, (vapor electrode/air electrode) | 5.6/4.8 | |
| Operating cell voltage | 1.4 V | |
| Operating current density, | ||
| Inlet temperature, | 650 °C | |
| Inlet pressure, (vapor electrode/air electrode) | 101.325/101.325 kPa | |
| Mole fraction, (vapor electrode (H2:H2O)/air electrode (O2:N2)) | 0.2:0.8/0.21:0.79 | |
| Gas flow rate (H2O/H2/Air) | 27.86/6.96/16.58 slpm | |
| Thermal boundary, except for the inlet and outlet regions | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lim, K.; Salihi, H.; Heo, S.; Ju, H. The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells. Energies 2024, 17, 125. https://doi.org/10.3390/en17010125
Kim Y, Lim K, Salihi H, Heo S, Ju H. The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells. Energies. 2024; 17(1):125. https://doi.org/10.3390/en17010125
Chicago/Turabian StyleKim, Youchan, Kisung Lim, Hassan Salihi, Seongku Heo, and Hyunchul Ju. 2024. "The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells" Energies 17, no. 1: 125. https://doi.org/10.3390/en17010125
APA StyleKim, Y., Lim, K., Salihi, H., Heo, S., & Ju, H. (2024). The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells. Energies, 17(1), 125. https://doi.org/10.3390/en17010125








