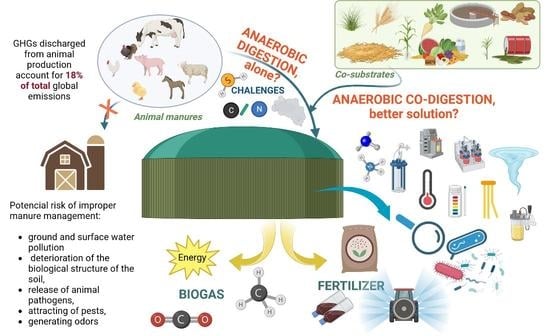
Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review
Abstract
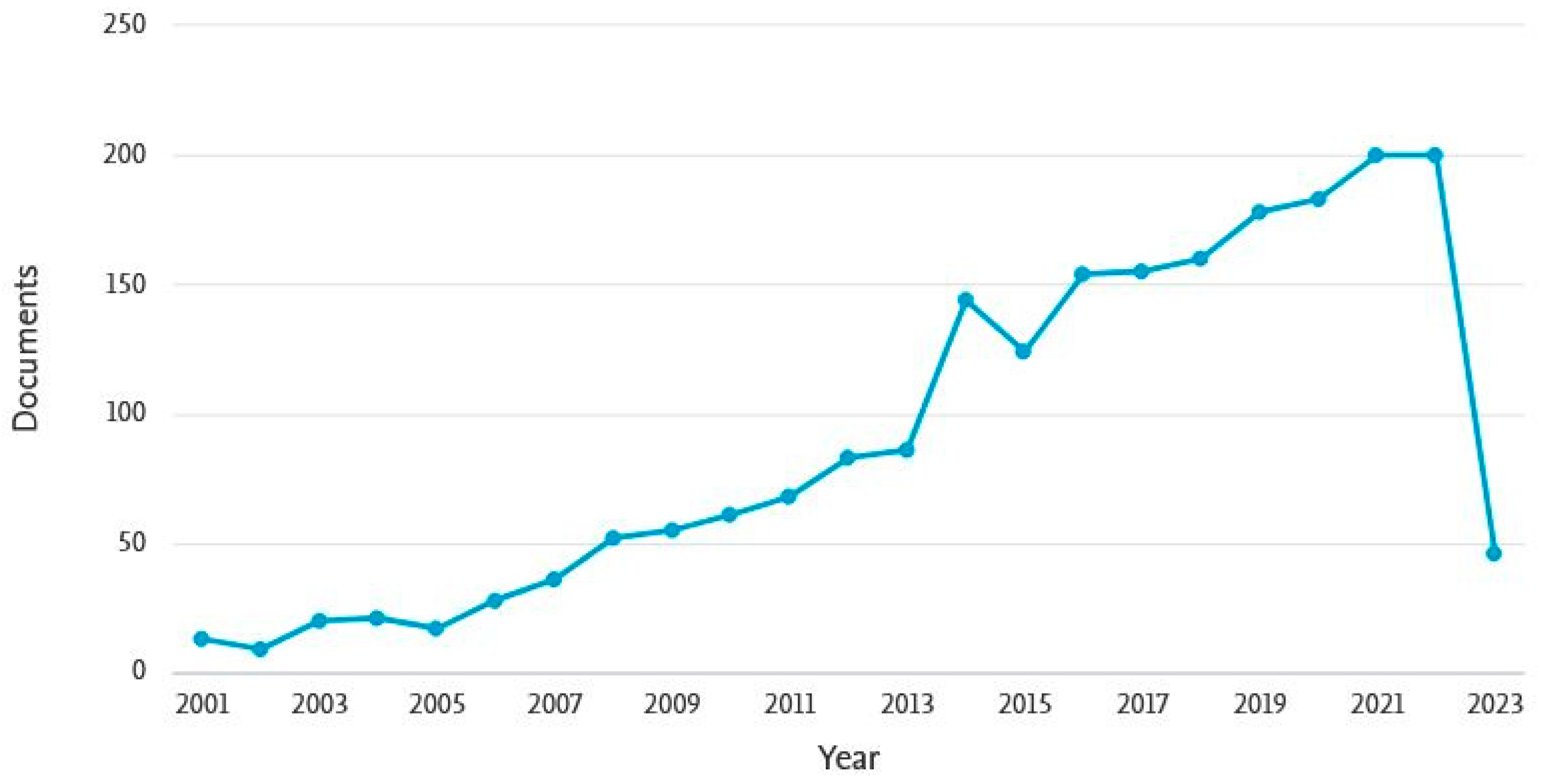
1. Introduction
2. Anaerobic Digestion in the Face of Rational Animal Manure Management
3. The Most Important Factors Affecting the Anaerobic Digestion of Animal Manure
3.1. pH
3.2. Volatile Fatty Acids, Alkalinity
3.3. Ammonia
3.4. C/N Ratio
3.5. Temperature
3.6. Mixing
3.7. Reactor Type
3.8. Hydraulic Retention Time
3.9. Water Content
3.10. Pre-Treatment
3.11. Other Process Inhibitors
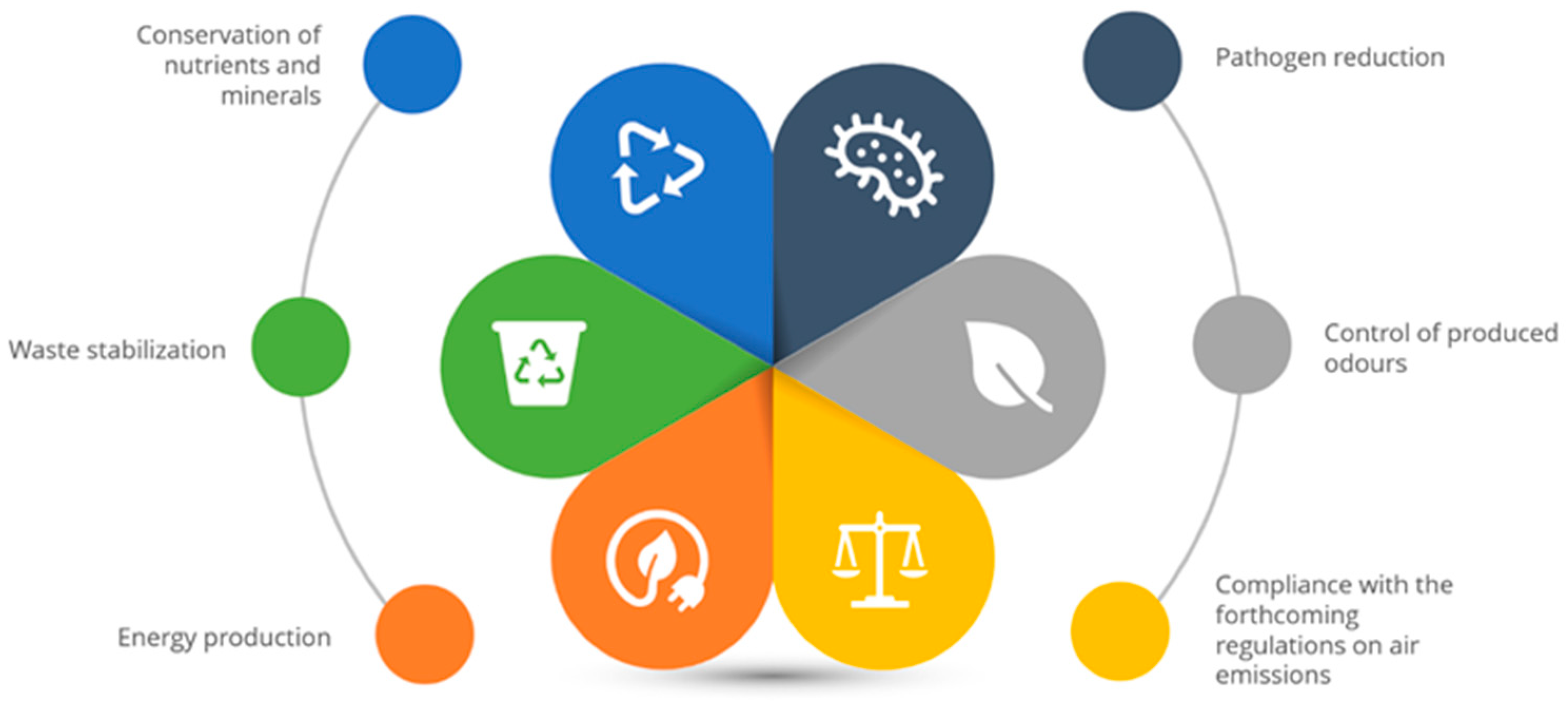
4. Importance of Anaerobic Co-Digestion in the Treatment of Animal Manure
| Substrate | Type of Reactor (Total Volume, L/ Working Volume, L) | Description of Process | VS Removal (%) | Biogas or Methane Production (Increase *) | Methane (%) | Ref. |
|---|---|---|---|---|---|---|
| COW MANURE | ||||||
| FW + CM | CSTR (140/86) | 55 °C; 16 rpm; Recirculation rate: 11.40 m3/h OLR: 1, 2, 3, 4 kg VS/(m3d) | 63.01–82.81 | 0.60–0.8 1 (up to 88.6%) 2 | 61.34–65.89 (up to +4.7%) 2 | [91] |
| CM + barley | Batch (1/0.75) | 55 °C; 100 rpm; CM to barley mixing ratio equal 1:1, VS basis; trials inoculated with sewage sludge (SS) last trial co-inoculation of CRF with inoculum | NA | 0.278 1 (+18%) 3 | 53–66 | [92] |
| CM + a trace metals solution | Batch (0.120/-) | 53 d; 35 °C | NA | 0.148 1 (+24%) 4 | NA | [93] |
| CM | Batch (0.5/0.2) | 36.5 °C; I/S 0.5; manure loading a factor was 3.5 g VS/L | 58.6 | 0.204 2 | 69.1 | [94] |
| CM + steel slag | Batch (0.5/0.4) | 36 ± 1 °C, 35 d concentrations of steel slag: 0.5, 1.0, 1.5, and 2.0 wt% | 58.62 5 (+15.5%) 6 | 0.275 1 (+153%) 6 | 51.12 | [95] |
| CM + APW | Batch (0.5/0.375) | 36 ± 1 °C, APW/DM wet weight ratios: 1:0, 3:1, 1:1, 1:3, and 0:1. | 55.9–59.91 (up to +7.4%) 7 | 0.195 1 (up to 23.6%) 7 | 61.4–67.1 (up to +12.6%) 7 | [96] |
| CM + BS 8 | CSTR (20/15) | 49 ± 1 °C, HRT = 20 d; 5% of shredded straw and 95% of CM of fresh matter | NA | 0.213 1 (+28.9%) 7 | NA | [97] |
| CSTR (20/15) | 49 ± 1 °C, HRT = 20 d; 5% of briquette straw (BS) and 95% of CM of fresh matter | 0.217 1 (+30.9%) 7 | ||||
| CSTR (30 m3/-) | 50 °C, BS concentration—9% of fresh matter | 0.351 1 (+33.1%) 7 | ||||
| CM + ESBS-DP | Batch (-/2) | 35 ± 0.5 °C ESBC-DP:CM mixture ratios were tested: 0:100, 25:75, 50:50, 75:25, and 100:0 | 65.3–77.5 (up to +33.2%) 7 | 0.323–0.557 1 (up to+24.6) 7 | NA | [98] |
| the lactating CM + FeW | Batch (0.05/-) | 37 °C added to feed at 30% of the total sample VS weight, 88 d | 45.45 (+22.9%) 7 | 0.374 1 (−9.4%) 7 | NA | [99] |
| CM from young cow + FeW | 42.98 (+23.2%) 7 | 0.349 1 (+5.1%) 7 | ||||
| Dry CM + FeW | 41.60 (+26.9%) 7 | 0.257 1 (−5.7%) 7 | ||||
| the lactating CM + WM | 37 °C, manure with waste milk was tested at two mixing ratios, 70:30 and 30:70; 88 d | 45.44–47.3 (up to +27.9%) 7 | 0.413–429 1 (up to +3.9%) 7 | |||
| CM from young cow + WM | 40.03–43.08 (up to +20.9%) 7 | 0.408–0.470 (up to 41.6%) 7 | ||||
| Dry CM + WM | 40.22–42.17 (up to +28.7%) 7 | 0.301–0.335 1 (up to 22.3%) 7 | ||||
| FR + CM | Pilot scale (-/850) | 35 °C, 27% radish and 73% dairy manure (ww); 13% radish and 87% dairy manure (ww), | NA | 0.208–0.210 1 (up to 38.7%) 7 | NA | [100] |
| CM + MS | Batch (1/0.8) | 35 ± 1 °C, mixing ratio of 3:1, 2:1, 1:1, 1:2 for CM/MS | NA | 0.534–0.614 1 (up to +39.8%%) 7 | 51.21–58.66 (up to +39.5%) 7 | [101] |
| Ss + CM | 35 ± 1 °C, mixing ratio of 3:1, 2:1, 1:1, 1:2 for Ss/CM | 0.352–0.470 1 (up to +7.1%) 7 | 48.4–58.7 (up to +39.6%) 7 | |||
| POME + CM | SABr (5/3.5) | 35 °C, 25:75, 50:50, 75:25, and 100:0 mixing ratios of POME and CM | 41–63 (up to +90.9%) 7 | 357–1005 9 (up to +292%) 7 | NA | [102] |
| CM + ShM | CSTR (-/2.4) | HRT: 25 d; 37 ± 1 °C, 120 rpm Ratio 1:1 | NA | 0.179 1 (+22.6%) 7 | 61 (+8.9%) | [103] |
| CM | Batch (2/0.25) | Mechanical Pre-treatments: shredded (SP), then mixed (MP), and finally blended (BP). | NA | 0.216–0.235 1 (up to +11.9%) 10 | NA | [104] |
| CM + WS | Reactor (23.6/20.9 m3) | 35 ± 1 °C, daily flow of feedstock on the level of 0.39 m3/d ratio of 1:1 w/w; Ultrasonic pretreatment | 0.460 1 (+24.6%) 10 | 53 (+1.3%) 10 | [105] | |
| 35 ± 1 °C, daily flow of feedstock on the level of 0.39 m3/d ratio of 1:1 w/w; hydrodynamic cavitation | 0.430 1 (+16.5%) 10 | 54.1 (+3.4%) 10 | ||||
| CM + CRS + SBP | Batch (0.5/-) | Mixing ratio: 2:1:1; 39 ± 2 °C Thermal pre-treatments: at 100, 120, 150 and 180 °C with 10, 20, 30, 60, and 120 min | NA | AcD: 0.180 11 (+11.4%) 7 (+100.6%) 10 | NA | [106] |
| CM + CST | Batch (2/1) | Mixing ratio 1:1 w/w 35 ± 2 °C, 60 rpm Pre-treatment: 1.5% Ca(OH)2 and 120 °C | NA | 0.290 1 (+31.82%) 10 | NA | [107] |
| EGSB (3.4/2) | HRT: 1–16 d, 35 ± 2 °C, OLR: 2.18–35.21 kg SCOD/(m3d) Mixing ratio 1:1 w/w 35 ± 1 °C, Pre-treatment: 1.5% Ca(OH)2 and 120 °C | 85.12–96.41 12 | 0.23–0.31 13 | 48.21–69.32 | ||
| CM + tea waste | Batch (0.6/-) | Mixing ratio 1:1 w/w; 40 d, 25–35 °C; Pre-treatment: 4% NaOH g/g TS | NA | 43.85 14 (+55.9%) 10 | NA | [108] |
| Mixing ratio 1:1 w/w; 40 d, 25–35 °C; Pre-treatment: microbial consortium | 52.55 14 (+86.8%) 10 | |||||
| CM:RS | Batch (0.25/-) | Mixing ratio of 1:1, based on TS mass, 35 °C Pre-treatment: limonite concentrations of 1%, 5%, and 10% | NA | 1351–1462 15 (+18.5–30.3%) 10 | NA | [109] |
| CM | Batch (0.5/0.4) | 37 ± 1 °C, 0.18 wt% microwave pyrolytic carbon material | NA | 0.380–0.502 14 (up to +70.7%) 7 | NA | [110] |
| CM + acorn slag waste | Batch (0.5/0.4) | 36 ± 1 °C; 3:1wet weight ratio Additive: biochar dose: 0.72, 1.08, 1.44, 1.80, and 2.16 g/L | 57.4–67.75 (up to +27%) 7 | 0.431–0.581 16 (up to +42%) 7 | 62.3–66.4 (up to +11%)7 | [111] |
| CM | Batch (1/-) | 38 °C, 30 d Additives: microscale waste iron powder or iron oxide nanoparticles | 46.39–55.06 (up to +77.8%) 7 | 0.67–0.222 1 (up to +39.6%) 10 | 54.33–58.94 (up to +11.6%) 7 | [112] |
| CM | Batch (-/0.4) | 36 ± 1 °C; Additives: nano-scale tungsten (WC, W2N, and W18O49) | 50.08–71.11 5 (up to +73.9%) 7 | 0.426–0.580 16 (up to +58.5%) 7 | NA | [113] |
| CM + APW | Batch (0.5/0.4) | 36 ± 1 °C; 35 d, ratio CM:APW 1:3 w/w Additive: Ti-sphere core-shell structured (0.03 g/L); the magnetic field | 53.03–78.25 5 (up to +73.9%) 7 | 0.366–0.510 1 (up to +65.53%) 10 | NA | [114] |
| CM + Cereal crops | Batch (1/0.75) | 37 ± 1 °C, 100 rpm Pre-treatment: 10% v/v of Orpinomyces sp. (anaerobic fungus) and spent medium | NA | 0.115–0.430 1 (up to +33%) 10 | NA | [115] |
| CM | CSTR (3.0–3.5/-) | 37 ± 1 °C, 120 rpm; HRT = 30–40 d, Pre-treatment: bioaugmentation culture containing Bathyarchaeota | NA | 0.179 1 (+20.1%) 10 | NA | [116] |
| CM | Batch | A meta-analysis AD, 160 of case studies. | NA | Mean:0.204 1 (+38.5%) 7 | NA | [90] |
| CM | Continuous mixed | A meta-analysis AD, 72 of case studies. | NA | Mean:0.299 1 (+70.9%) 7 | NA | |
| POULTRY MANURE | ||||||
| PM + a trace metals solution | Batch (0.120/-) | 53 d; 35 °C | NA | 0.407 1 (+12%) 3 | NA | [93] |
| PM + B | Batch (0.5/0.2) | 36.5 °C; I/S 0.5; manure loading the factor was 3.5 g VS/L | 81.4 | 0.259 1 | 61.1 | [94] |
| PM + RS | Batch (0.120/-) | SS-AcD, 35 °C; 180 rpm, I/S: 0.5–4.0 | 80.92–93.25 17 | 0.123–270 1 | NA | [117] |
| PM + CC | 54.55–88.89 17 | 0.131–0.291 1 | ||||
| PM + PS | 56.66–75.94 17 | 0.084–0.157 1 | ||||
| PM + SW | 49.89–87.61 17 | 0.098–0.262 1 | ||||
| PM + CH | 30.67–81.03 17 | 0.116–0.155 1 | ||||
| PM + SB | 33.82–91.7 17 | 0.140–230 1 | ||||
| PM | 32.20–89.03 17 | 0.123–0.302 1 | ||||
| PM + CST | CSTR (2.5/2) | HRT:20 d; VS ratios of CST/CM or UPCS/CM were 1:2; OLR: 2.1 g VS/(L d) Pre-treatment: Urea Pretreated CST (UPCS) Additive: 10 g/L of biochar (B) | NA | 0.449 1 (PM:CST) 0.499 1 (PM:UPCS) 0.513 1 (PM:CST+B) 0.530 1 (PM:UPCS+B) | 57.1 (PM:CST) 60 (PM:UPCS) 61.4 (PM:CST+B) 62.5 (PM:UPCS+B) | [79] |
| PM | Batch (0.5/0.4) | 35 ± 1 °C; A: Manure loading (g VS/L):31.0–58.1 Additives: Biochar dosage (%): 1.8–5.2; Cellulose loading (g VS/L): 40.0–158.1 | NA | 0.177–0.292 1 | NA | [118] |
| PM | Batch (0.5/0.4) | 37 ± 2 °C; 95 rpm, 35 d Additive: pumice | 66.83 17 | 8796 9 | 68.46 | [119] |
| PM + AWS | Batch (0.5/-) | SS-AcD (TS 20%); 35 ± 2 °C; control AD of PM | NA | 0.406 1 (+195%) 7 | NA | [120] |
| SS-AcD (TS 20%); 55 ± 2 °C, control AD of PM | 0.323 1 (+150%) 7 | |||||
| TPM + AWS | SS-AcD (TS 20%); 35 ± 2 °C, control AD of TPM Pre-treatment: stripping ammonia from PM | 0.562 1 (+63%) 7 | ||||
| SS-AcD (TS 20%); 55 ± 2 °C; control AD of TPM Pre-treatment: stripping ammonia from PM (treated PM -TPM) | 0.298 1 (+70%) 7 | |||||
| PM | Batch (1/-) |
37 ± 1 °C; enzymatic pretreatment (a mixture of
Onozuka R-10 enzyme and Macerozyme R) | NA | 0.537 18 (+35%) 7 | NA | [121] |
| PM + VW | Batch (0.25/-) | SS-AcD, 37 °C, 50 d | NA | 0.244 1 (+2.8%) 7 | NA | [122] |
| PM | CSTR (15/12) | OLR: 1.6 and 2.5 g VS/(ld) 55 °C | 42–62 | 0.094–0.220 19 | 56–67 | [123] |
| OLR: 1.6 and 2.5 g VS/(ld) 37 °C | 44.5–46.1 | 0.245–0.252 19 | 67–68 | |||
| PM | Batch (0.5/0.4) | 35 ± 1 °C Additive: biochar made up of wheat straw, discarded fruitwood, and chicken manure at temperatures of 350 °C, 450 °C, and 550 °C | NA | 0.214–0.294 1 (up to +69%) 4 | NA | [124] |
| PM + BPS | CSTR (3/2.5) | 4:1 based on VS; OLR: 0.8–3.2 gVS/(ld) | NA | 0.193 1 | NA | [125] |
| PM | Batch | A meta-analysis AD, 36 of case studies. | NA | Mean:0.260 1 (+22.4%) 7 | NA | [90] |
| PM | Continuous mixed | A meta-analysis AD, 20 of case studies. | NA | Mean:0.169 1 (+71.1%) 7 | NA | |
| SWINE MANURE | ||||||
| SM | Batch (0.25/-) | 37 °C, manually mixed once a day, manure loading factors: 8, 16, 32, and 64 g VS/L, pH adjusted to 7.0 | 54.4 (VS/L = 8) 54.2 (VS/L = 16) 52.2 (VS/L = 32) 49.4 (VS/L = 64) | 409.57 20 (VS/L = 8) 384.66 20 (VS/L = 16) 361.30 20 (VS/L = 32) 318.01 20 (VS/L = 64) | 72.8–78.8 | [126] |
| SM | Batch (0.5/0.4) | 37 °C, I/S: 1:1, manually mixed once a day Additive: zeolites (natural and sodium), at rates of 0, 10, 40, 70, and 100 g/L of SM | NA | (SM + NZ 40g/L SM) (+35% biogas, and +29% methane) | NA | [127] |
| SM | Batch (1/0.8) | Pre-treatment: use of in situ formed graphene in an electric methanogenesis system, 38 °C, 28 d, I/S: 1:5 | NA | 356.49 21 (+41.49%), 222.17 22 (+60.89%) | NA | [128] |
| SM + a trace metals solution | Batch assay (0.120/-) | 53 d; 35 °C | NA | 0.180 1 (+22%) 3 | NA | [93] |
| CM + SM + a trace metals solution | Batch assay (0.120/-) | 53 d; 35 °C | NA | 0.511 1 (+9.7%) 4 | NA | [93] |
| SM | CSTR (5.5/4) | 196 d, 60 rpm, HRT: 20 d, mesophilic conditions | 35.7–41.0 | 1.06–1.16 23 | NA | [129] |
| SM + G | CSTR (5.5/4) | 196 d, 60 rpm, HRT: 20 d, mesophilic conditions | 74.1–77.7 | 5.44–5.58 23 | NA | |
| SM | Batch (-/0.4) | Additive: ferrous chloride in the amount characterized by final elemental iron concentrations of 5, 10, 25 and 40 mmol/L, 37 °C, I/S: 1:3, 41 d | NA | 269.1 20 (+21.5) | NA | [130] |
| SM + CST | Batch (1/-) | Substrate combination ratios (SM/CST): 30:70, 50:50 and 70:30 (% w/w); Initial pH values adjusted to 6.0, 6.5, 7.0, 7.5, and 8.0 using 5 mol/L NaOH and 5 mol/L HCL; 35±1 °C, I/S: 1:2.5 | 7.5 (SM:CST = 30:70) 16.7 (SM:CST = 50:50) 23.8 (SM:CST = 70:30) | 11.92 20 (SM:CST = 30:70) 14.08 20 (SM:CST = 50:50) 220 20 (SM:CST = 70:30) | NA | [131] |
| Dry SM | Semi-continuous (2/1.2) | Additive: wrapped granular activated carbon: 50 g, acclimated sludge (inoculum): 1200 g, HRT: 60 d, 35 ± 1 °C | 6.6 | 1.1–1.67 24 (+10.6%) | 58.8–73.2 | [132] |
| SM | Batch | A meta-analysis AD, 73 of case studies | NA | Mean:0.287 1 (+20.6%) 7 | NA | [90] |
| SM | Continuous mixed | A meta-analysis AD, 23 of case studies | NA | Mean:0.322 1 (+52%) 7 | NA | |
| OTHER | ||||||
| HM | Batch assay (0.5/0.2) | 36.5 °C; I/S 0.5; manure loading the factor was 3.5 g VS/L | 52.9 | 0.155 1 | 70.1 | [94] |
| HM | Batch (0.5/-) | 35 °C, 35 d, HM solid ratios: 0.5, 1, 2, and 4% | 80–90 | 339 25; 203 20 (TS: 0.5%) 374 25; 239 20 (TS: 1%) 370 25; 236 20 (TS: 2%) 381 25; 247 20 (TS: 4%) | 60 (TS: 0.5%) 64 (TS: 1%) 63 (TS: 2%) 65 (TS: 4%) | [133] |
| HM + Ss | Batch (0.5/-) | AcD, 35 °C, 35 d, HM TS ratios: 2 and 4%, HM:Ss = 9:1 | 90 | 410 25; 270 20 (TS: 2%) 425 25; 280 20 (TS: 4%) | 65 (TS: 2%) 66 (TS: 4%) | |
| HM + Ss | Continuous digester (5/-) | AcD, 35 ± 2 °C, TS ratio: 4%, HM:Ss = 9:1 | >50 | NA | 66–68 | |
| GM | Batch assay (0.5/0.2) | 36.5 °C; I/S 0.5; manure loading the factor was 3.5 g VS/L | 46.4 | 0.159 | 65.8 | [94] |
| RM | Batch (0.25/-) | 37 °C, manually mixed once a day, manure loading factors: 8, 16, 32, and 64 g VS/L, pH adjusted to 7.0 | 49.5 (VS/L = 8) 48.9 (VS/L = 16) 47.5 (VS/L = 32) 46.2 (VS/L = 64) | 323.22 20 (VS/L = 8) 296.87 20 (VS/L = 16) 261.46 20 (VS/L = 32) 211.48 20 (VS/L = 64) | 68.3–76.5 | [126] |
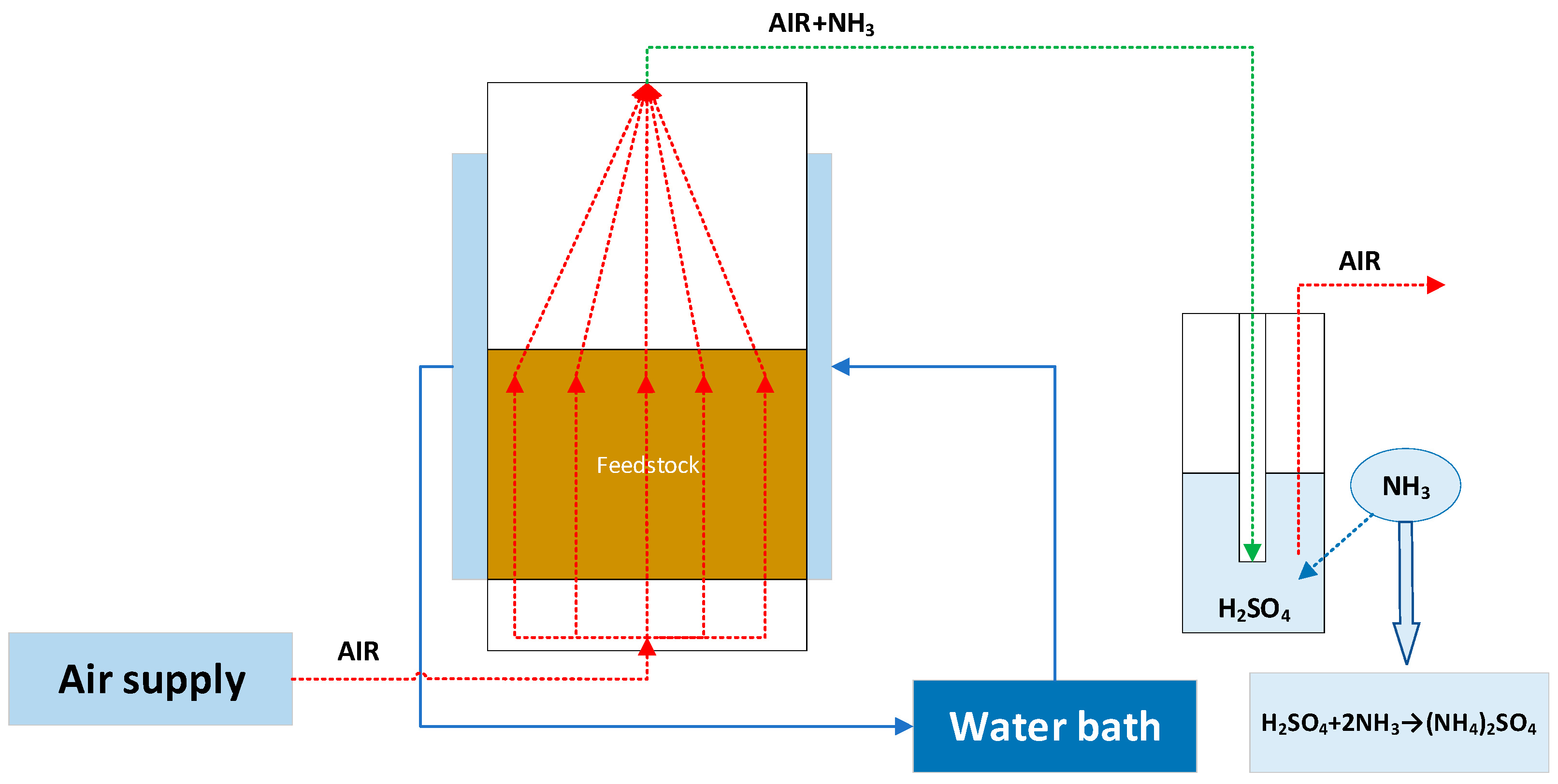
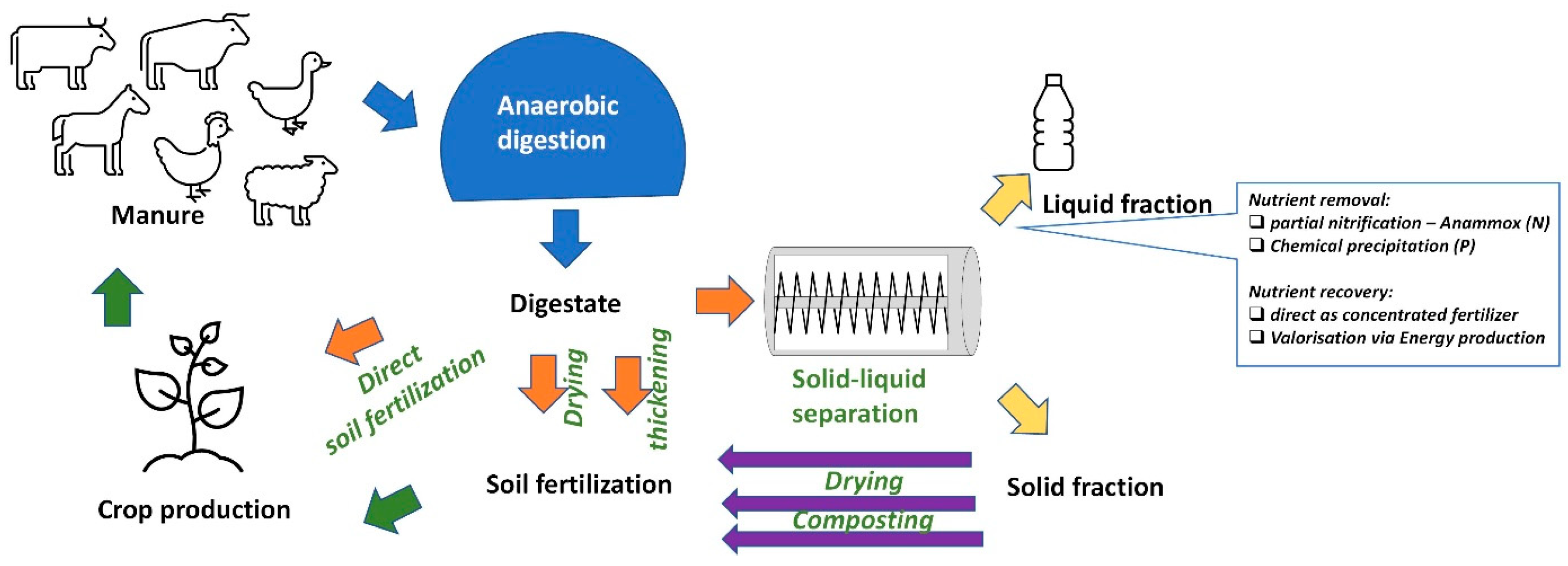
5. Ecological Potential of Digestate
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opurum, C.C.; Nwachukwu, I.N.; Christopher, E. Predicting the rate of biogas production from the anaerobic digestion of blends of cassava (Manihot esculenta) peels with poultry manure. Issues Biol. Sci. Pharm. Res. 2021, 9, 38–47. [Google Scholar]
- Urbaniec, K.; Mikulčić, H.; Duić, N.; Lozano, R. SDEWES 2014—Sustainable Development of Energy, Water and Environment Systems. J. Clean. Prod. 2016, 130, 1–11. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Osueke, C.O.; Olayanju, T.M.A.; Lawal, A.I. Co-digestion of Theobroma cacao (Cocoa) pod husk and poultry manure for energy generation: Effects of pretreatment methods. Bioresour. Technol. 2019, 283, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Fatone, F.; Bolzonella, D.; Pavan, P. Thermophilic anaerobic co-digestion of cattle manure with agro-wastes and energy crops: Comparison of pilot and full scale experiences. Bioresour. Technol. 2010, 101, 545–550. [Google Scholar] [CrossRef] [PubMed]
- European Commision Targets in Energy, Climate Change and Environment. Available online: https://ec.europa.eu/info/energy-climate-change-environment/overall-targets_en (accessed on 29 March 2020).
- Profile, S.E.E. Efficacy of Biogas Production from Different Types of Livestock Manures. Int. J. Smart Grid 2021, 5, 158–166. [Google Scholar] [CrossRef]
- Han, T.; Wang, T.; Wang, Z.; Xiao, T.; Wang, M.; Zhang, Y.; Zhang, J.; Liu, D. Evaluation of gaseous and solid waste in fermentation bedding system and its impact on animal performance: A study of breeder ducks in winter. Sci. Total Environ. 2022, 836, 155672. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.; Gros, M.; Casabella, O.; Petrovic, M.; Balcazar, J.L.; Pijuan, M. Occurrence of veterinary drugs and resistance genes during anaerobic digestion of poultry and cattle manures. Sci. Total Environ. 2022, 822, 153477. [Google Scholar] [CrossRef] [PubMed]
- Lazor, M.; Hutňan, M.; Sedláček, S.; Kolesárová, N.; Špalková, V. Slovak Society of Chemical Engineering Institute of Chemical and Environmental Engineering Slovak University of Technology in Bratislava Anaerobic co-digestion of poultry manure and waste kitchen oil. In Proceedings of the 37th International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 24–28 May 2010; pp. 1399–1406. [Google Scholar]
- Magrel, L. Metodyka Oceny Efektywnosci Procesu Fermentacji Metanowejwybranych Osadów Ściekowych; Wydaw. PB: Białystok, Poland, 2002; p. 118. [Google Scholar]
- Böjti, T.; Kovács, K.L.; Kakuk, B.; Wirth, R.; Rákhely, G.; Bagi, Z. Pretreatment of poultry manure for efficient biogas production as monosubstrate or co-fermentation with maize silage and corn stover. Anaerobe 2017, 46, 138–145. [Google Scholar] [CrossRef]
- Mohammed, M.; Belkair, A.; Hamad, T.; Jirhiman, A.; Hassan, R. Improving biogas production from animal manure by batch anaerobic digestion. Alger. J. Eng. Technol. 2022, 6, 79–84. [Google Scholar]
- EUROSTAT. Poultry Meat Production in EU at New High in 2018. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20190325-1 (accessed on 25 March 2023).
- Abouelenien, F.; Namba, Y.; Kosseva, M.R.; Nishio, N.; Nakashimada, Y. Enhancement of methane production from co-digestion of chicken manure with agricultural wastes. Bioresour. Technol. 2014, 159, 80–87. [Google Scholar] [CrossRef]
- Savery, C.W.; Cruzan, D.C. Methane recovery of chicken manure digestion. Water Pollution Control Federation; Wiley: Hoboken, NJ, USA, 2013; Volume 44, pp. 2349–2354. [Google Scholar]
- Bayrakdar, A.; Molaey, R.; Sürmeli, R.Ö.; Sahinkaya, E.; Çalli, B. Biogas production from chicken manure: Co-digestion with spent poppy straw. Int. Biodeterior. Biodegrad. 2017, 119, 205–210. [Google Scholar] [CrossRef]
- Kozłowski, K.; Pietrowski, M.; Zbytek, Z.; Lewicki, A. Methane fermentation of chicken droppings. J. Res. Appl. Agric. Eng. 2016, 61, 28–30. [Google Scholar]
- EUROSTAT. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Archive:Agri-environmental_indicator_-_manure_application#Further_Eurostat_information (accessed on 23 February 2023).
- Williams, C.M. Poultry Waste Management in Developing Countries—Poultry Development Review; Food and Agriculture Organization of the United Nations (FAO): Quebec City, QC, Canada, 2008; pp. 1–2. [Google Scholar]
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of poultry manure in Poland—Current state and future perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Fahl, F.; Dallemand, J.F.; Monforti, F.; Motola, V. A spatial analysis of biogas potential from manure in Europe. Renew. Sustain. Energy Rev. 2018, 94, 915–930. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Fernández, C.; Gómez, X.; Morán, A. Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol. Bioprocess Eng. 2011, 16, 1044–1052. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.C. Anaerobic digestion of dairy manure: Design and process considerations. In Dairy Manure Management: Treatment, Handling, and Community Relations; Cornell University: Ithaca, NY, USA, 2005; pp. 301–312. [Google Scholar]
- Böske, J.; Wirth, B.; Garlipp, F.; Mumme, J.; Van den Weghe, H. Anaerobic digestion of horse dung mixed with different bedding materials in an upflow solid-state (UASS) reactor at mesophilic conditions. Bioresour. Technol. 2014, 158, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Jjagwe, J.; Mdondo, S.W.; Nagawa, C.B.; Bah, H.; Tumutegyereize, P. Synergetic effects of biochar addition on mesophilic and high total solids anaerobic digestion of chicken manure. J. Environ. Manag. 2022, 315, 115192. [Google Scholar] [CrossRef]
- Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=uriserv:OJ.L_.1991.375.01.0001.01.ENG (accessed on 23 February 2023).
- Pilarska, A.; Pilarski, K.; Dach, J.; Boniecki, P.; Dobrzański, K. Nowoczesne metody oraz perspektywy zagospodarowania nawozów naturalnych. Tech. Rol. Ogrod. Leśna 2014, 2, 9–11. [Google Scholar]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 23 February 2023).
- Fiedorowicz, G. Standardy w chowie koni w aspekcie ochrony środowiska. Probl. Inżynierii Rol. 2007, 15, 139–144. [Google Scholar]
- Liu, K.; Tang, Y.Q.; Matsui, T.; Morimura, S.; Wu, X.L.; Kida, K. Thermophilic anaerobic co-digestion of garbage, screened swine and dairy cattle manure. J. Biosci. Bioeng. 2009, 107, 54–60. [Google Scholar] [CrossRef]
- Mroczek, K.; Rudy, M.; Gil, M.; Mroczek, J.R. Możliwości zagospodarowania odpadów z produkcji drobiarskiej w zgodzie z zasadami biogospodarki. Pol. J. Sustain. Dev. 2019, 22, 93–100. [Google Scholar] [CrossRef]
- LCA of Thermal Conversion of Poultry Litter at BMC Moerdijk LCA of Thermal Conversion of Poultry Litter at BMC Moerdijk. March 2017. Available online: https://cedelft.eu/wp-content/uploads/sites/2/2021/04/CE_Delft_2H94_LCA_thermal_conversion_of_poultry_litter_BMC_Def.pdf (accessed on 23 February 2023).
- Van Poucke, R.; Nachenius, R.W.; Agbo, K.E.; Hensgen, F.; Bühle, L.; Wachendorf, M.; Ok, Y.S.; Tack, F.M.G.; Prins, W.; Ronsse, F.; et al. Mild hydrothermal conditioning prior to torrefaction and slow pyrolysis of low-value biomass. Bioresour. Technol. 2016, 217, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sadecka, Z.; Suchowska-Kisielewicz, M. Ko-fermentacja pomiotu kurzego. Rocz. Ochr. Środowiska 2016, 18, 609–625. [Google Scholar]
- Carlos-Pinedo, S.; Wang, Z. Assessment of a full-scale solid-state anaerobic co-digestion: A multi-component substrate analysis by using ORWARE. Waste Manag. 2022, 146, 36–43. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Investigation of the methane potential of horse manure. Agric. Eng. Int. CIGR J. 2013, 15, 161–172. [Google Scholar]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci. Total Environ. 2017, 599–600, 1885–1894. [Google Scholar] [CrossRef]
- Wang, H.; Bi, X.; Clift, R. A case study on integrating anaerobic digestion into agricultural activities in British Columbia: Environmental, economic and policy analysis. Environ. Pollut. 2021, 271, 116279. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential—Global and Irish perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Kundu, D.; Dutta, D.; Samanta, P.; Dey, S.; Sherpa, K.C.; Kumar, S.; Dubey, B.K. Valorization of wastewater: A paradigm shift towards circular bioeconomy and sustainability. Sci. Total Environ. 2022, 848, 157709. [Google Scholar] [CrossRef] [PubMed]
- Murto, M.; Björnsson, L.; Mattiasson, B. Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J. Environ. Manag. 2004, 70, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Qurrahman, A.H.; Wilopo, W.; Susanto, S.P.; Petrus, M. Energy and Exergy Analysis of Dieng Geothermal Power Plant. Int. J. Technol. 2021, 12, 175–185. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Improvement of the anaerobic digestion of sewage sludge by co-digestion with wine vinasse and poultry manure: Effect of different hydraulic retention times. Fuel 2022, 321, 124104. [Google Scholar] [CrossRef]
- Dąbrowska, L. Wpływ sposobu prowadzenia fermentacji osadów ściekowych na produkcję biogazu. Rocz. Ochr. Sr. 2015, 17, 943–957. [Google Scholar]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Braun, R.; Huber, P.; Meyrath, J. Ammonia toxicity in liquid piggery manure digestion. Biotechnol. Lett. 1981, 3, 159–164. [Google Scholar] [CrossRef]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Co-digestion of waste organic solids: Batch studies. Bioresour. Technol. 1999, 67, 117–122. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Moneti, M. Biogas production from poultry manure and cheese whey wastewater under mesophilic conditions in batch reactor. Energy Procedia 2015, 82, 811–818. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Tanner, C.C. Ammonium removal from wastewaters using natural New Zealand zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef]
- Lei, L.; Li, X.; Zhang, X. Ammonium removal from aqueous solutions using microwave-treated natural Chinese zeolite. Sep. Purif. Technol. 2008, 58, 359–366. [Google Scholar] [CrossRef]
- Kuai, L.; Verstraete, W. Ammonium removal by the oxygen-limited autotrophic nitrification- denitrification system. Appl. Environ. Microbiol. 1998, 64, 4500–4506. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhao, Q.L.; Hao, X.D. Ammonium removal from landfill leachate by chemical precipitation. Waste Manag. 1999, 19, 409–415. [Google Scholar] [CrossRef]
- Lahav, O.; Green, M. Ammonium removal using ion exchange and biological regeneration. Water Res. 1998, 32, 2019–2028. [Google Scholar] [CrossRef]
- Cabeza, A.; Urtiaga, A.; Rivero, M.J.; Ortiz, I. Ammonium removal from landfill leachate by anodic oxidation. J. Hazard. Mater. 2007, 144, 715–719. [Google Scholar] [CrossRef]
- Strous, M.; Van Gerven, E.; Zheng, P.; Kuenen, J.G.; Jetten, M.S.M. Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (anammox) process in different reactor configurations. Water Res. 1997, 31, 1955–1962. [Google Scholar] [CrossRef]
- Apellido, N.B. Revista Latinoamericana de Metalurgia y Materiales. 2018, 38, pp. 110–115. Available online: https://www.rlmm.org/ojs/index.php/rlmm/article/view/934/538 (accessed on 15 March 2023).
- Ellersdorfer, M.; Pesendorfer, S.; Stocker, K. Nitrogen recovery from swine manure using a zeolite-based process. Processes 2020, 8, 1515. [Google Scholar] [CrossRef]
- Czerwińska, E.; Kalinowska, K. Warunki prowadzenia procesu fermentacji metanowej w biogazowni. Tech. Rolinicza Ogrod. Leśna 2014, 2, 12–14. [Google Scholar]
- Rodriguez-Verde, I.; Regueiro, L.; Lema, J.M.; Carballa, M. Blending based optimisation and pretreatment strategies to enhance anaerobic digestion of poultry manure. Waste Manag. 2018, 71, 521–531. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Grosser, A.; Worwag, M.; Neczaj, E.; Grobelak, A. Półcia{ogonek}gła kofermentacja osadów ściekowych i odpadów tłuszczowych pochodzenia roślinnego. Rocz. Ochr. Sr. 2013, 15, 2108–2125. [Google Scholar]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and Carbon-Nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Huang, R.; Dykstra, C.M.; Jiang, R.; Pavlostathis, S.G.; Tang, Y. Energy and Nutrient Recovery from Sewage Sludge and Manure via Anaerobic Digestion with Hydrothermal Pretreatment. Environ. Sci. Technol. 2019, 54, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Kwon, S.J.; Woo, J.H. Mesophilic and thermophilic temperature co-phase anaerobic digestion compared with single-stage mesophilic- and thermophilic digestion of sewage sludge. Water Res. 2004, 38, 1653–1662. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Marañón, E.; Castrillón, L.; Quiroga, G.; Fernández-Nava, Y.; Gómez, L.; García, M.M. Co-digestion of cattle manure with food waste and sludge to increase biogas production. Waste Manag. 2012, 32, 1821–1825. [Google Scholar] [CrossRef]
- Smith, D.B.; Almquist, C.B. The anaerobic co-digestion of fruit and vegetable waste and horse manure mixtures in a bench-scale, two-phase anaerobic digestion system. Environ. Technol. 2014, 35, 859–867. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.C.; Lee, J.; Wang, C.H.; Dai, Y.; Wah Tong, Y. Three-stage anaerobic co-digestion of food waste and horse manure. Sci. Rep. 2017, 7, 1269. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycka, A. Przyczyny zakłóceń procesu fermentacji metanowej. Autobusy Tech. Eksploat. Syst. Transp. 2011, 12, 224–228. [Google Scholar]
- Bi, S.; Hong, X.; Yang, H.; Yu, X.; Fang, S.; Bai, Y.; Liu, J.; Gao, Y.; Yan, L.; Wang, W.; et al. Effect of hydraulic retention time on anaerobic co-digestion of cattle manure and food waste. Renew. Energy 2020, 150, 213–220. [Google Scholar] [CrossRef]
- Curkowski, A.A.; Oniszk-popławska, A. Surowce do produkcji biogazu—Uproszczona metoda obliczenia wydajności biogazowni rolniczej. Czysta Energ. 2010, 1, 25–27. [Google Scholar]
- Grosser, A. The influence of decreased hydraulic retention time on the performance and stability of co-digestion of sewage sludge with grease trap sludge and organic fraction of municipal waste. J. Environ. Manag. 2017, 203, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, C.; Liu, R.; Yellezuome, D.; Zhu, X.; Bai, R.; Liu, M.; Sun, M. Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: The effect of biochar addition and urea pretreatment. Bioresour. Technol. 2021, 319, 124197. [Google Scholar] [CrossRef]
- Castrillón, L.; Fernández-Nava, Y.; Ormaechea, P.; Marañón, E. Optimization of biogas production from cattle manure by pre-treatment with ultrasound and co-digestion with crude glycerin. Bioresour. Technol. 2011, 102, 7845–7849. [Google Scholar] [CrossRef]
- Azman, S.; Milh, H.; Somers, M.H.; Zhang, H.; Huybrechts, I.; Meers, E.; Meesschaert, B.; Dewil, R.; Appels, L. Ultrasound-assisted digestate treatment of manure digestate for increased biogas production in small pilot scale anaerobic digesters. Renew. Energy 2020, 152, 664–673. [Google Scholar] [CrossRef]
- González-Fernández, C.; León-Cofreces, C.; García-Encina, P.A. Different pretreatments for increasing the anaerobic biodegradability in swine manure. Bioresour. Technol. 2008, 99, 8710–8714. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, A. The Importance of Heavy Metal Speciation from the Standpoint of the Use of Sewage Sludge in Nature. Eng. Prot. Environ. 2018, 21, 239–250. [Google Scholar] [CrossRef]
- Scopus Database Analyze Search Results for KEY (Anaerobic AND Digestion AND of AND Animal AND Manure). Available online: https://www.scopus.com (accessed on 10 March 2023).
- Czekała, W.; Smurzyńska, A.; Kozłowski, K.; Brzoski, M.; Chełkowski, D.; Gajewska, K. Kofermentacja osadów ściekowych sposobem na ich zagospodarowanie oraz produkcję energii. Probl. Inżynierii Rol. 2017, 2017, 5–14. [Google Scholar]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, W.; Luo, W.; Fang, H.; Lv, H.; Liu, R.; Niu, Q. Anaerobic co-digestion of chicken manure and cardboard waste: Focusing on methane production, microbial community analysis and energy evaluation. Bioresour. Technol. 2020, 321, 124429. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kuglarz, M.; Mrowiec, B. Intensyfikacja fermentacji metanowej gnojowicy świńskiej przez wprowadzenie kosubstratu w formie bioodpadów komunalnych. Nauka Przyr. Technol. 2011, 5, 53. [Google Scholar]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Ince, O.; Akyol, Ç.; Ozbayram, E.G.; Tutal, B.; Ince, B. Enhancing methane production from anaerobic co-digestion of cow manure and barley: Link between process parameters and microbial community dynamics. Environ. Prog. Sustain. Energy 2020, 39, 13292. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Liu, Y.; Dach, J.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Han, F.; Yun, S.; Zhang, C.; Xu, H.; Wang, Z. Steel slag as accelerant in anaerobic digestion for nonhazardous treatment and digestate fertilizer utilization. Bioresour. Technol. 2019, 282, 331–338. [Google Scholar] [CrossRef]
- Huang, X.; Yun, S.; Zhu, J.; Du, T.; Zhang, C.; Li, X. Mesophilic anaerobic co-digestion of aloe peel waste with dairy manure in the batch digester: Focusing on mixing ratios and digestate stability. Bioresour. Technol. 2016, 218, 62–68. [Google Scholar] [CrossRef]
- Xavier, C.A.N.; Moset, V.; Wahid, R.; Møller, H.B. The efficiency of shredded and briquetted wheat straw in anaerobic co-digestion with dairy cattle manure. Biosyst. Eng. 2015, 139, 16–24. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Evaluation of methane generation and process stability from anaerobic co-digestion of sugar beet by-product and cow manure. J. Biosci. Bioeng. 2016, 121, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Adghim, M.; Abdallah, M.; Saad, S.; Shanableh, A.; Sartaj, M. Assessment of the biochemical methane potential of mono- and co-digested dairy farm wastes. Waste Manag. Res. 2020, 38, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Belle, A.J.; Lansing, S.; Mulbry, W.; Weil, R.R. Anaerobic co-digestion of forage radish and dairy manure in complete mix digesters. Bioresour. Technol. 2015, 178, 230–237. [Google Scholar] [CrossRef]
- Wei, L.; Qin, K.; Ding, J.; Xue, M.; Yang, C.; Jiang, J.; Zhao, Q. Optimization of the co-digestion of sewage sludge, maize straw and cow manure: Microbial responses and effect of fractional organic characteristics. Sci. Rep. 2019, 9, 2374. [Google Scholar] [CrossRef]
- Khalid, Z.B.; Siddique, M.N.I.; Nasrullah, M.; Singh, L.; Wahid, Z.B.A.; Ahmad, M.F. Application of solar assisted bioreactor for biogas production from palm oil mill effluent co-digested with cattle manure. Environ. Technol. Innov. 2019, 16, 100446. [Google Scholar] [CrossRef]
- Li, Y.; Achinas, S.; Zhao, J.; Geurkink, B.; Krooneman, J.; Willem Euverink, G.J. Co-digestion of cow and sheep manure: Performance evaluation and relative microbial activity. Renew. Energy 2020, 153, 553–563. [Google Scholar] [CrossRef]
- Coarita Fernandez, H.; Teixeira Franco, R.; Bayard, R.; Buffiere, P. Mechanical Pre-treatments Evaluation of Cattle Manure Before Anaerobic Digestion. Waste Biomass Valorization 2020, 11, 5175–5184. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Cavitation-based pretreatment strategies to enhance biogas production in a small-scale agricultural biogas plant. Energy Sustain. Dev. 2019, 49, 21–26. [Google Scholar] [CrossRef]
- Şenol, H.; Açıkel, Ü.; Demir, S.; Oda, V. Anaerobic digestion of cattle manure, corn silage and sugar beet pulp mixtures after thermal pretreatment and kinetic modeling study. Fuel 2020, 263, 116651. [Google Scholar] [CrossRef]
- Yuan, Y.; Bian, A.; Zhang, L.; Chen, Z.; Zhou, F.; Ye, F.; Jin, T.; Pan, M.; Chen, T.; Yan, J.; et al. Thermal-alkali and enzymes for efficient biomethane production from co-digestion of corn straw and cattle manure. BioResources 2019, 14, 5422–5437. [Google Scholar] [CrossRef]
- Kavisa, G.H.; Sari, N.; Hawali, H.; Matin, A. The Effect of C/N Ratio and Pretreatment in Making Biogas from Tea Waste and Cow Manure in Liquid State Anaerobic Co-Digestion. Waste Technol. 2020, 8, 1–7. [Google Scholar]
- Xu, L.; Peng, S.; Dong, D.; Wang, C.; Fan, W.; Cao, Y.; Huang, F.; Wang, J.; Yue, Z. Performance and microbial community analysis of dry anaerobic co-digestion of rice straw and cow manure with added limonite. Biomass Bioenergy 2019, 126, 41–46. [Google Scholar] [CrossRef]
- Yun, S.; Fang, W.; Du, T.; Hu, X.; Huang, X.; Li, X.; Zhang, C.; Lund, P.D. Use of bio-based carbon materials for improving biogas yield and digestate stability. Energy 2018, 164, 898–909. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; Xu, H.; Wang, C.; Zhang, Y.; Chen, J.; Jia, B. Mesophilic anaerobic co-digestion of acorn slag waste with dairy manure in a batch digester: Focusing on mixing ratios and bio-based carbon accelerants. Bioresour. Technol. 2019, 286, 121394. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamamoto, Y.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Prospects for biogas production and H2S control from the anaerobic digestion of cattle manure: The influence of microscale waste iron powder and iron oxide nanoparticles. Waste Manag. 2020, 101, 141–149. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; Shi, J.; Han, F.; Liu, B.; Wang, R.; Li, X. Critical evidence for direct interspecies electron transfer with tungsten-based accelerants: An experimental and theoretical investigation. Bioresour. Technol. 2020, 311, 123519. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Yun, S.; Shi, J.; Han, F.; Wang, Z.; Chen, J.; Abbas, Y.; Xu, H.; Wang, K.; Xing, T. Enhanced anaerobic mono- and co-digestion under mesophilic condition: Focusing on the magnetic field and Ti-sphere core–shell structured additives. Bioresour. Technol. 2020, 310, 123450. [Google Scholar] [CrossRef] [PubMed]
- Akyol, Ç.; Ince, O.; Bozan, M.; Ozbayram, E.G.; Ince, B. Fungal bioaugmentation of anaerobic digesters fed with lignocellulosic biomass: What to expect from anaerobic fungus Orpinomyces sp. Bioresour. Technol. 2019, 277, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Achinas, S.; Zhang, Z.; Krooneman, J.; Euverink, G.J.W. The biomethanation of cow manure in a continuous anaerobic digester can be boosted via a bioaugmentation culture containing Bathyarchaeota. Sci. Total Environ. 2020, 745, 141042. [Google Scholar] [CrossRef]
- de Oliveira Paranhos, A.G.; Adarme, O.F.H.; Barreto, G.F.; de Queiroz Silva, S.; de Aquino, S.F. Methane production by co-digestion of poultry manure and lignocellulosic biomass: Kinetic and energy assessment. Bioresour. Technol. 2020, 300, 122588. [Google Scholar] [CrossRef]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef]
- Yılmaz, Ş.; Şahan, T. Utilization of pumice for improving biogas production from poultry manure by anaerobic digestion: A modeling and process optimization study using response surface methodology. Biomass Bioenergy 2020, 138, 105601. [Google Scholar] [CrossRef]
- Abouelenien, F.; Namba, Y.; Nishio, N.; Nakashimada, Y. Dry Co-Digestion of Poultry Manure with Agriculture Wastes. Appl. Biochem. Biotechnol. 2016, 178, 932–946. [Google Scholar] [CrossRef]
- Kucuker, M.A.; Demirel, B.; Onay, T.T. Enhanced biogas production from chicken manure via enzymatic pretreatment. J. Mater. Cycles Waste Manag. 2020, 22, 1521–1528. [Google Scholar] [CrossRef]
- Mlaik, N.; Sayadi, S.; Mnasri, N.; Kechaou, S.; Loukil, S.; Aloui, F.; Khoufi, S. Dry mesophilic anaerobic co-digestion of vegetable wastes with animal manures using leach bed reactor. Biomass Convers. Biorefinery 2021, 13, 697–707. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Guo, F.; Pan, S.; Lu, B.; Du, L.; Wei, Y. Synergistic digestion of banana pseudo-stems with chicken manure to improve methane production: Semi-continuous manipulation and microbial community analysis. Bioresour. Technol. 2021, 328, 124851. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef]
- Wijesinghe, D.T.N.; Dassanayake, K.B.; Scales, P.J.; Sommer, S.G.; Chen, D. Effect of Australian zeolite on methane production and ammonium removal during anaerobic digestion of swine manure. J. Environ. Chem. Eng. 2018, 6, 1233–1241. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Gan, R.; Jia, H.; Yong, X.; Yong, Y.C.; Wu, X.; Wei, P.; Zhou, J. Enhanced biogas production from swine manure anaerobic digestion via in-situ formed graphene in electromethanogenesis system. Chem. Eng. J. 2020, 389, 124510. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, J.; Li, P.; Shen, P.; Wei, Y. Enhancement of methane production and antibiotic resistance genes reduction by ferrous chloride during anaerobic digestion of swine manure. Bioresour. Technol. 2020, 298, 122519. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, T.; Wang, X.; Feng, Y.; Ren, G.; Yang, G. Process performance and methane production optimizing of anaerobic co-digestion of swine manure and corn straw. Sci. Rep. 2017, 7, 9379. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, H.; Yang, H.; Wang, H.; Zheng, D.; Liu, Y.; Pu, X.; Deng, L. Improved biogas production of dry anaerobic digestion of swine manure. Bioresour. Technol. 2019, 294, 122188. [Google Scholar] [CrossRef]
- Agayev, E.; Ugurlu, A. Biogas production from co-digestion of horse manure and waste sewage sludge. TechConnect Briefs 2011, 3, 657–660. [Google Scholar]
- Silvestre, G.; Gómez, M.P.; Pascual, A.; Ruiz, B. Anaerobic co-digestion of cattle manure with rice straw: Economic & energy feasibility. Water Sci. Technol. 2013, 67, 745–755. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R. Biogas production from co-digestion of dairy manure and food waste. Bioresour. Technol. 2010, 101, 4021–4028. [Google Scholar] [CrossRef]
- Westerholm, M.; Hansson, M.; Schnürer, A. Improved biogas production from whole stillage by co-digestion with cattle manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Misi, S.N.; Forster, C.F. Batch co-digestion of two-compqnent mixtures of agro-wastes. Process Saf. Environ. Prot. 2001, 79, 365–371. [Google Scholar] [CrossRef]
- Borowski, S.; Kucner, M.; Czyżowska, A.; Berłowska, J. Co-digestion of poultry manure and residues from enzymatic saccharification and dewatering of sugar beet pulp. Renew. Energy 2016, 99, 492–500. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Wartell, B.A.; Krumins, V.; Alt, J.; Kang, K.; Schwab, B.J.; Fennell, D.E. Methane production from horse manure and stall waste with softwood bedding. Bioresour. Technol. 2012, 112, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hadin, Å.; Eriksson, O. Horse manure as feedstock for anaerobic digestion. Waste Manag. 2016, 56, 506–518. [Google Scholar] [CrossRef]
- Borowski, S.; Weatherley, L. Co-digestion of solid poultry manure with municipal sewage sludge. Bioresour. Technol. 2013, 142, 345–352. [Google Scholar] [CrossRef]
- Borowski, S.; Domański, J.; Weatherley, L. Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Waste Manag. 2014, 34, 513–521. [Google Scholar] [CrossRef]
- Chang, H.C.; Chou, P.Y.; Cheng, M.P.; Hsiao, T.H.; Lo, K.Y.; Wang, S.L. Phosphorus conversion during anaerobic digestion of high-calcium chicken manures and phosphorus recovery as struvite. J. Environ. Chem. Eng. 2022, 10, 107615. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Van Poucke, R.; Vaneeckhaute, C.; Michels, E.; Meers, E. Manure as a resource for energy and nutrients. In Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 65–82. [Google Scholar]
- Leclerc, A.; Laurent, A. Framework for estimating toxic releases from the application of manure on agricultural soil: National release inventories for heavy metals in 2000–2014. Sci. Total Environ. 2017, 590–591, 452–460. [Google Scholar] [CrossRef]
- Czekała, W.; Lewicki, A.; Pochwatka, P.; Czekała, A.; Wojcieszak, D.; Jóźwiakowski, K.; Waliszewska, H. Digestate management in polish farms as an element of the nutrient cycle. J. Clean. Prod. 2020, 242, 118454. [Google Scholar] [CrossRef]
- Kempf, I.; Le Devendec, L.; Lucas, P.; Druilhe, C.; Pourcher, A.M. Impact of mesophilic anaerobic digestion and post-treatment of digestates on the transfer of conjugative antimicrobial resistance plasmids. Waste Manag. 2022, 152, 1–5. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.T.; Cai, Y.F.; Chen, Y.X.; Yang, Y.W.; Xing, S.C.; Liao, X. Di Occurrence of microplastic in livestock and poultry manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef] [PubMed]
- Recebli, Z.; Selimli, S.; Ozkaymak, M.; Gonc, O. Biogas production from animal manure. J. Eng. Sci. Technol. 2015, 10, 722–729. [Google Scholar] [CrossRef]
- Gao, T.; Li, X. Using thermophilic anaerobic digestate effluent to replace freshwater for bioethanol production. Bioresour. Technol. 2011, 102, 2126–2129. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kotas, P.; Kabsch-Korbutowicz, M. Charakterystyka i metody zagospodarowania masy pofermentacyjnej powstającej w biogazowniach. Ochr. Sr. 2019, 41, 39–45. [Google Scholar]
| Animal | Unit | Value |
|---|---|---|
| Laying hens | kg/(1000 birds·d) | 120–150 |
| Chicken broilers | 80 | |
| Turkeys | 200–350 | |
| Duck | 150–190 | |
| Geese | 200 | |
| Calves | kg/(head·d) | 8.0 |
| bovine | 20.0 | |
| Male bovine | 25.0 | |
| Dairy cows | 53.0 | |
| Other cows | 25.0 | |
| Piglets | 0.5 | |
| Other pigs | 4.5 | |
| Sows | 11.0 | |
| Sheep | 1.5 | |
| Goat | 1.5 | |
| Broilers | 0.10 | |
| Laying hens | 0.20 | |
| Other poultry | 0.3 |
| Type of Manure | Manure | TS (%) | C (%) | N (%) | P (%) | K (%) |
|---|---|---|---|---|---|---|
| BM | Cattle, Horse, Sheep, or pig | 20.9–69.9 | 11.9–12.0 | 0.4–2.2 | 0.2–4.0 | 0.9–4.0 |
| Poultry | 33.3–78.5 | 12.6–50.4 | 1.1–5.9 | 1.1–3.2 | 2.0–3.3 | |
| SM | Cattle, Horse | 24.4–65.0 | 10.4–48.1 | 0.6–4.6 | 0.1–2.5 | 0.1–3.2 |
| Pig | 28.0–29.0 | 35.3–41.0 | 1.3–2.7 | 1.5–3.2 | 0.7 | |
| Poultry | 33.0–79.4 | 24.9–46.2 | 1.7–7.1 | 0.7–6.7 | 1.9–5.0 | |
| SL | Cattle | 0.5–8.3 | 17.5–36.5 | 0.2–2.8 | 0.04–0.1 | 0.4–0.5 |
| Pig slurry | 0.3–8.3 | 16.3–41.4 | 0.1–3.4 | 0.01–3.1 | 0.1–2.5 | |
| Cattle, Horse | 4.9 | NA | NA | 0.05 | 0.2 | |
| Pig | <1.6 | NA | 0.1 | 1.0 | NA |
| High C/N Content Materials | Low C/N Content Materials | ||
|---|---|---|---|
| Substrate | C/N | Substrate | C/N |
| Paper | 170–800 | Kitchen waste | 12–20 |
| Scobs | 200–500 | Green waste | 10–25 |
| Wood | 700 | Fresh grass | 12–20 |
| Bark | 100–130 | Legumes | 18–20 |
| Straw | 80–100 | Non–legume plants | 11–12 |
| Leaves and weeds | 90 | Manure | 18 |
| Maize cobs | 40–80 | Poultry manure | 15 |
| Hay | 40 | Food waste | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, A.; Grosser, A.; Meers, E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies 2023, 16, 3885. https://doi.org/10.3390/en16093885
Jasińska A, Grosser A, Meers E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies. 2023; 16(9):3885. https://doi.org/10.3390/en16093885
Chicago/Turabian StyleJasińska, Anna, Anna Grosser, and Erik Meers. 2023. "Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review" Energies 16, no. 9: 3885. https://doi.org/10.3390/en16093885
APA StyleJasińska, A., Grosser, A., & Meers, E. (2023). Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies, 16(9), 3885. https://doi.org/10.3390/en16093885