Nb2CTx-Based MXenes Most Recent Developments: From Principles to New Applications
Abstract
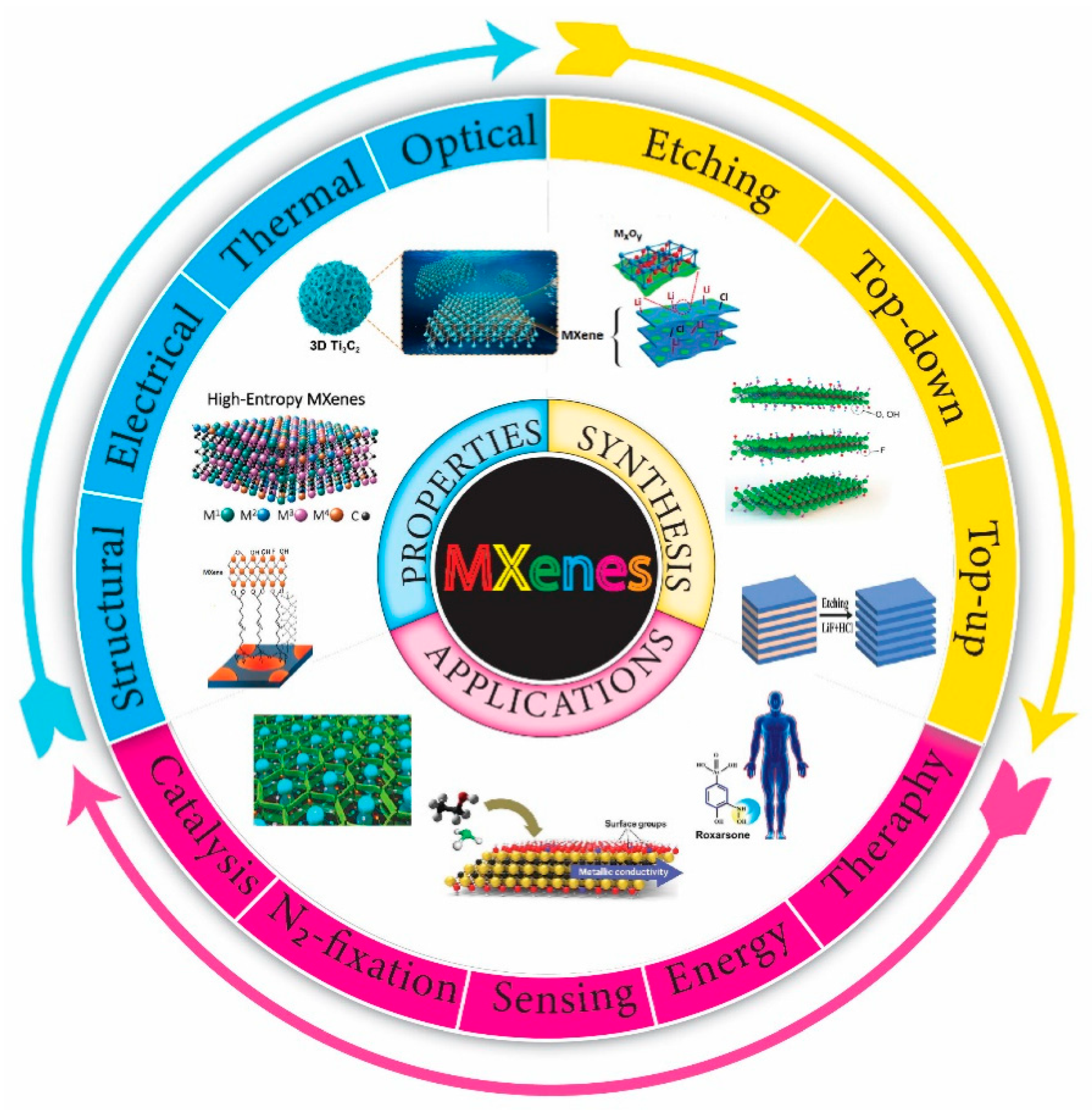
1. Introduction
2. Synthetic Approach for Nb2CTx
2.1. By HF Etching
2.2. Molten Salt Method
2.3. Other Methods
2.4. Synthesis Summary
3. Properties of MXenes
3.1. Mechanical Properties
3.2. Magnetic Property
3.3. Conductivity
3.4. Oxidizable Fraction Properties
3.5. Electrochemical Behavior
3.6. Superior Hydrophilicity
3.7. Thermal Property
3.8. Optical Property
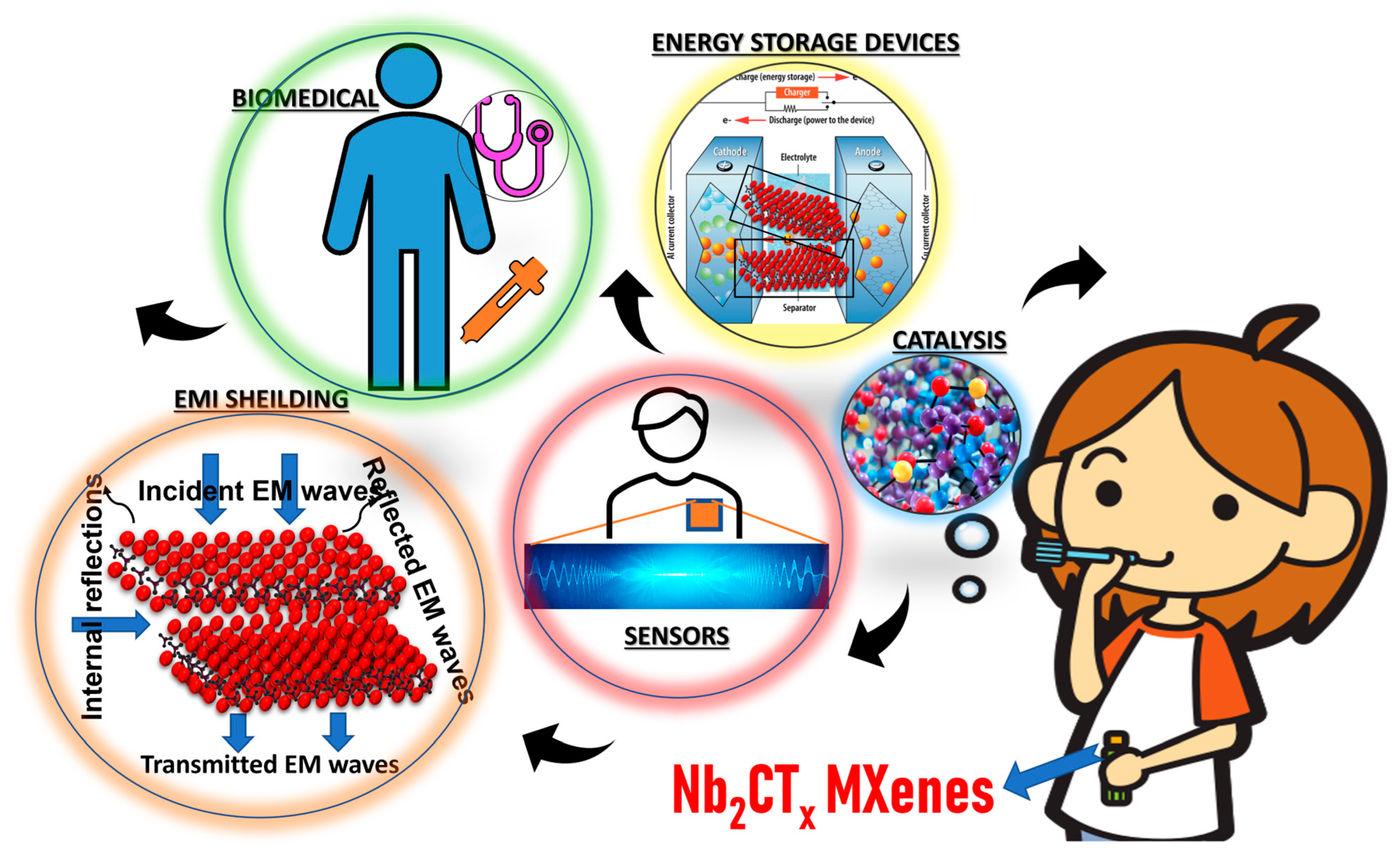
4. Applications of Nb2CTx MXene
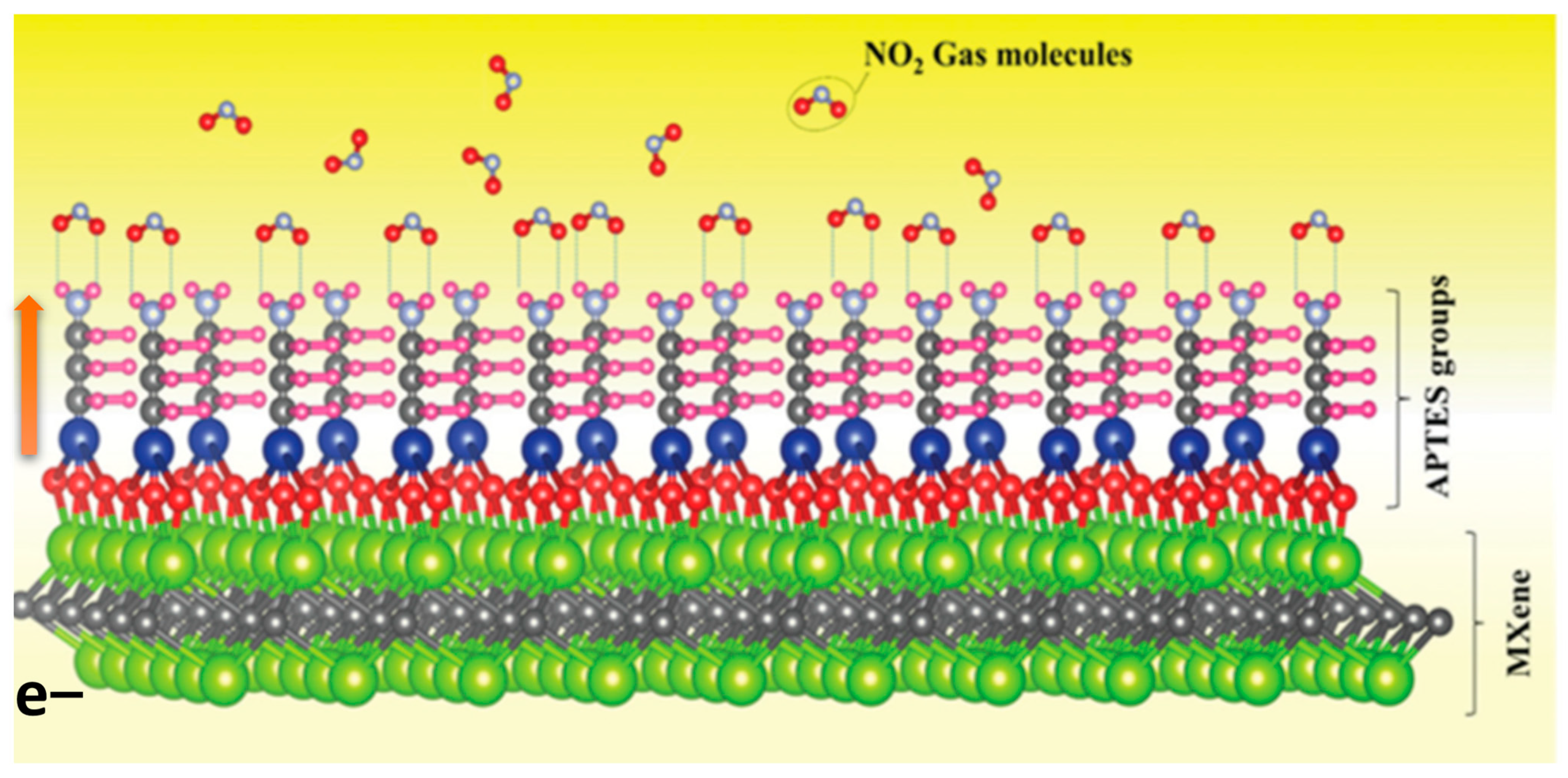
4.1. In Gas Sensors
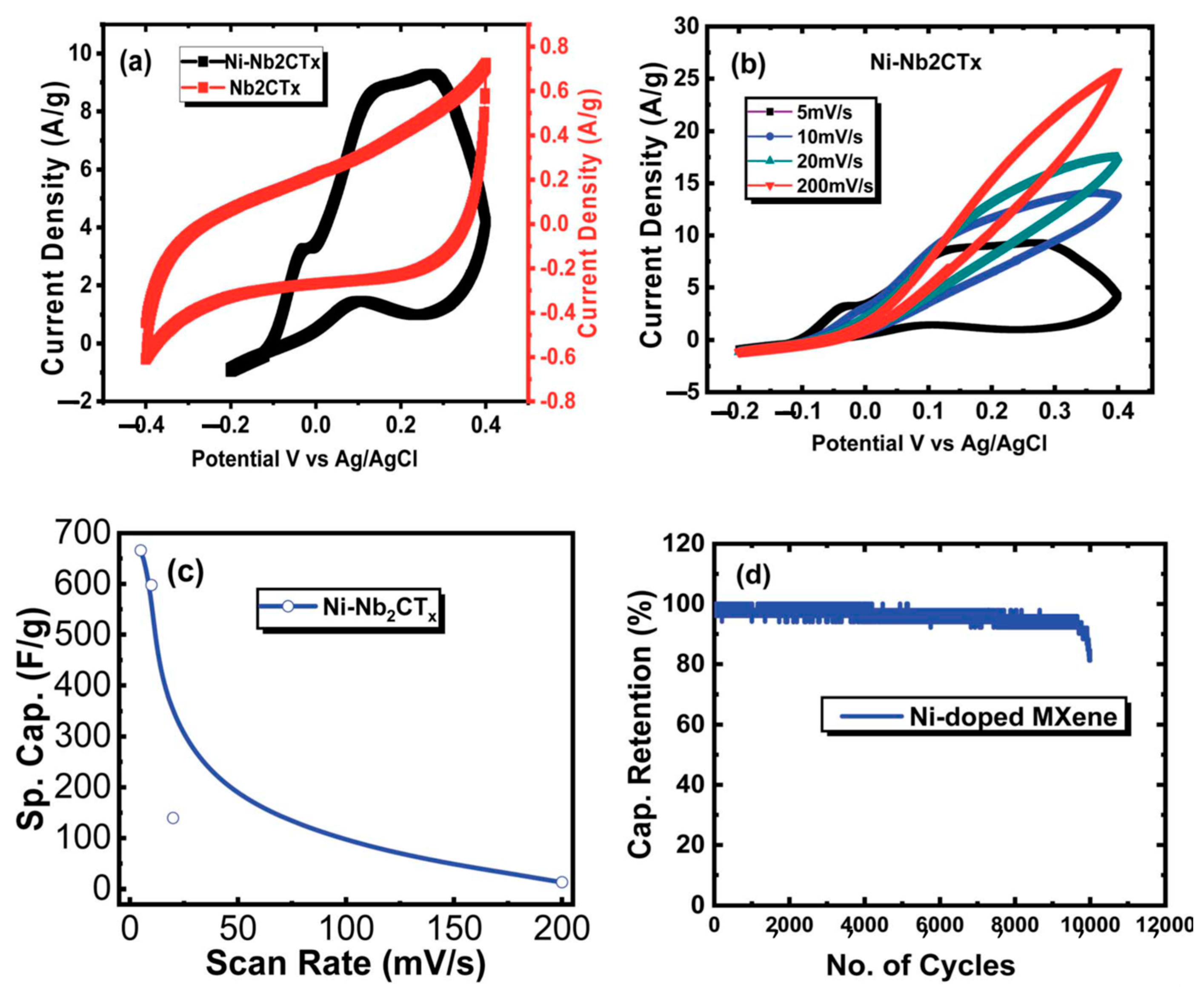
4.2. Supercapacitor Applications
4.3. Battery Applications
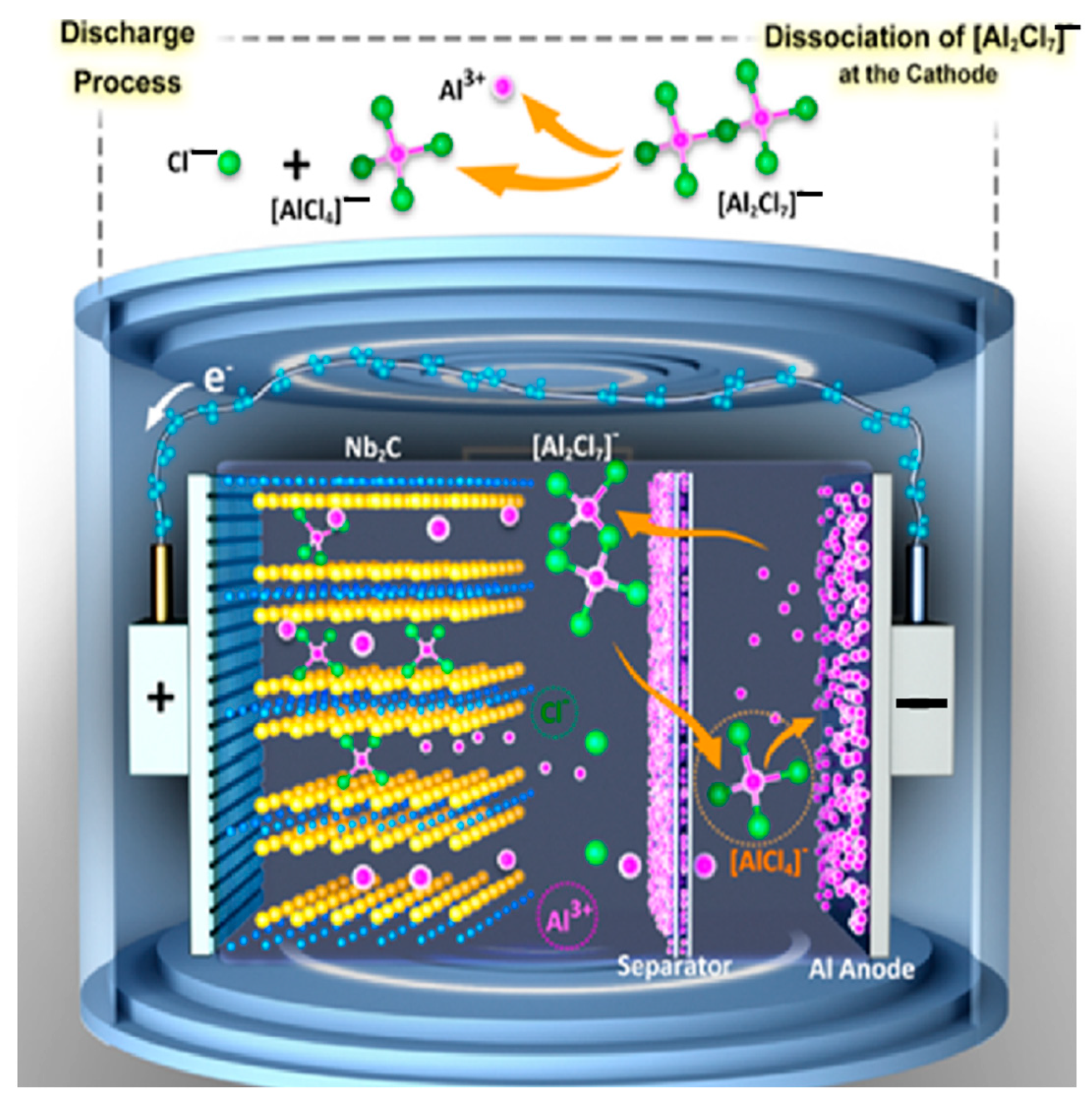
4.3.1. Aluminum Batteries
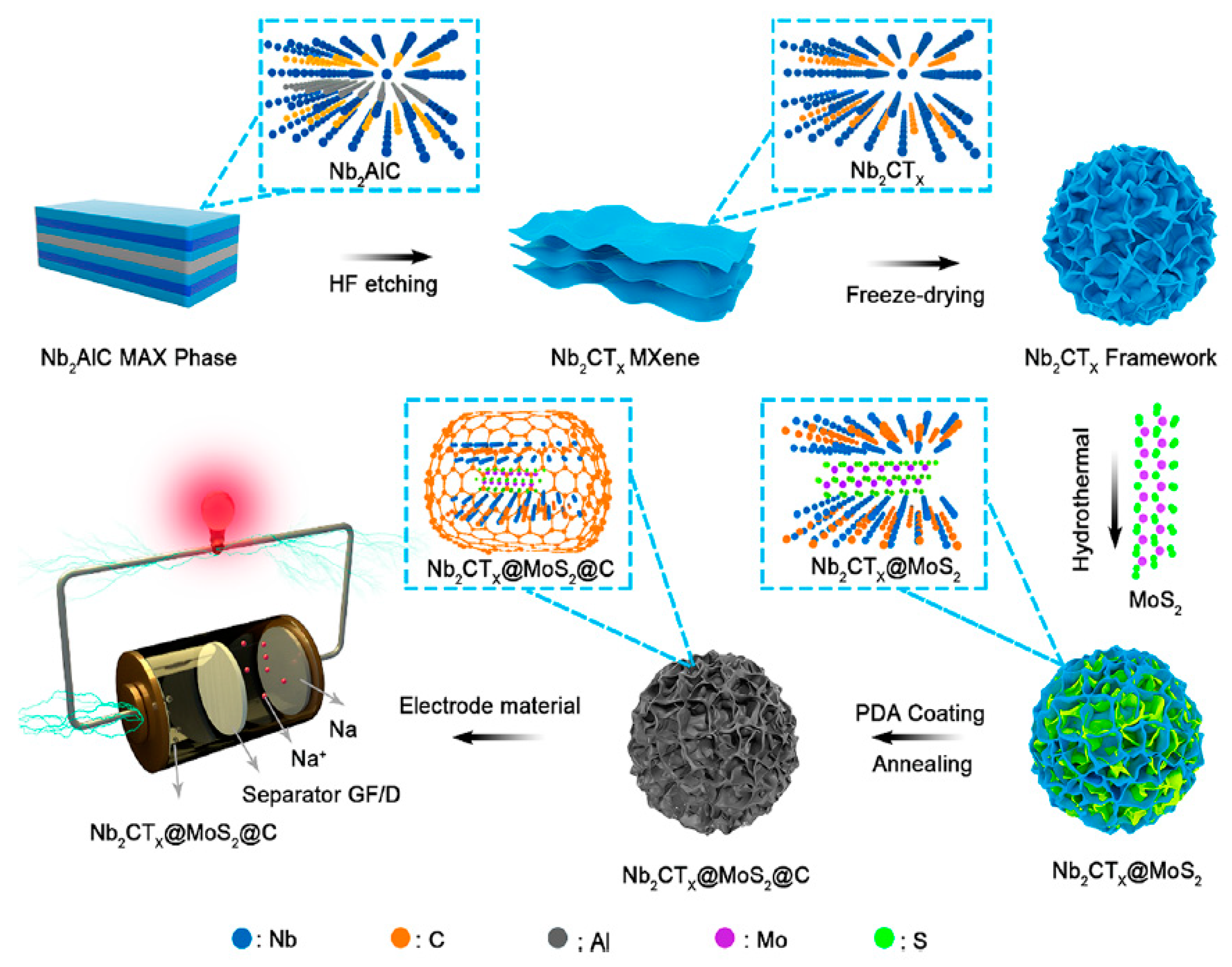
4.3.2. Sodium-Ion Batteries
4.3.3. Li-Sulfur Batteries
4.4. Catalysis
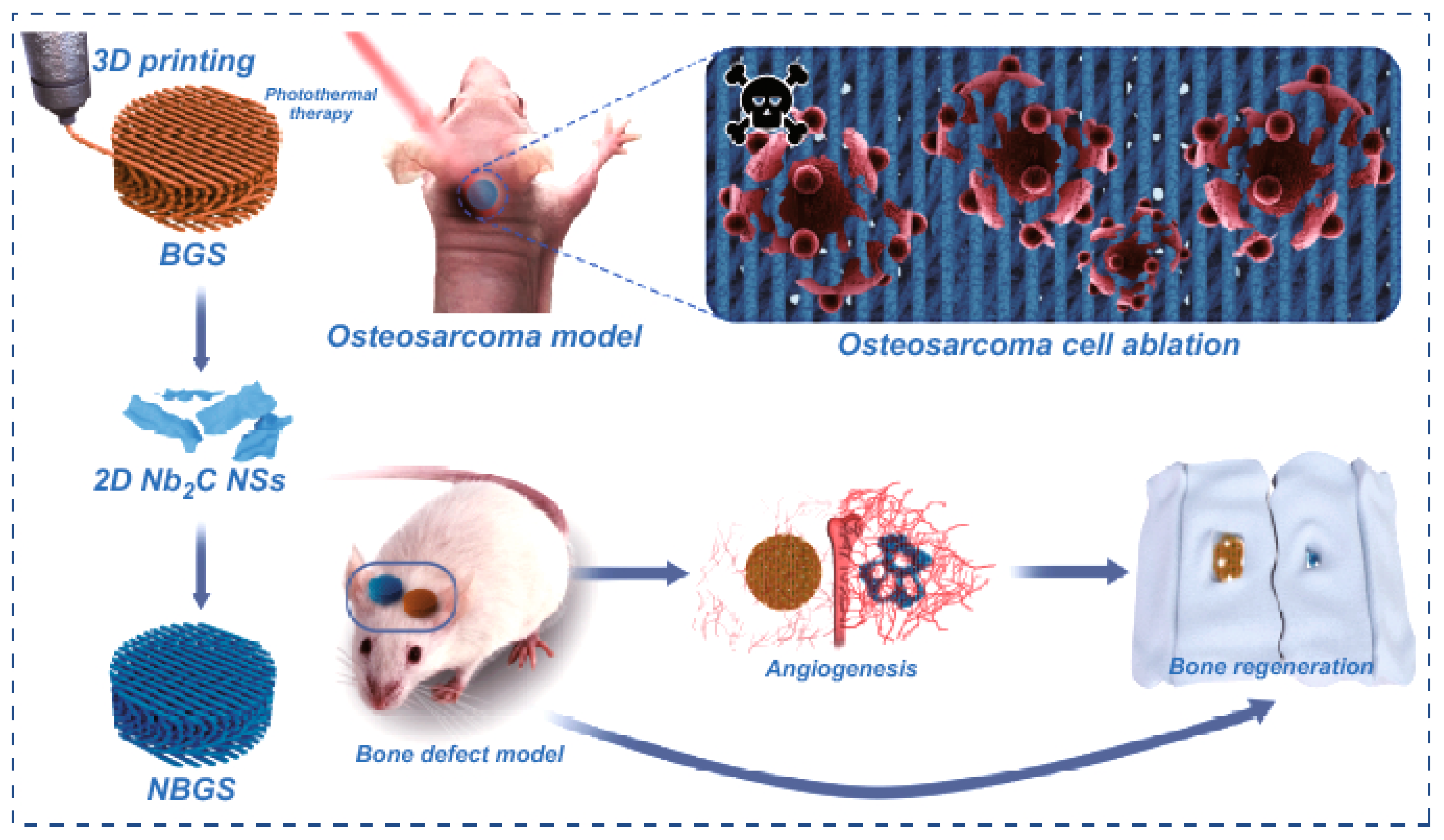
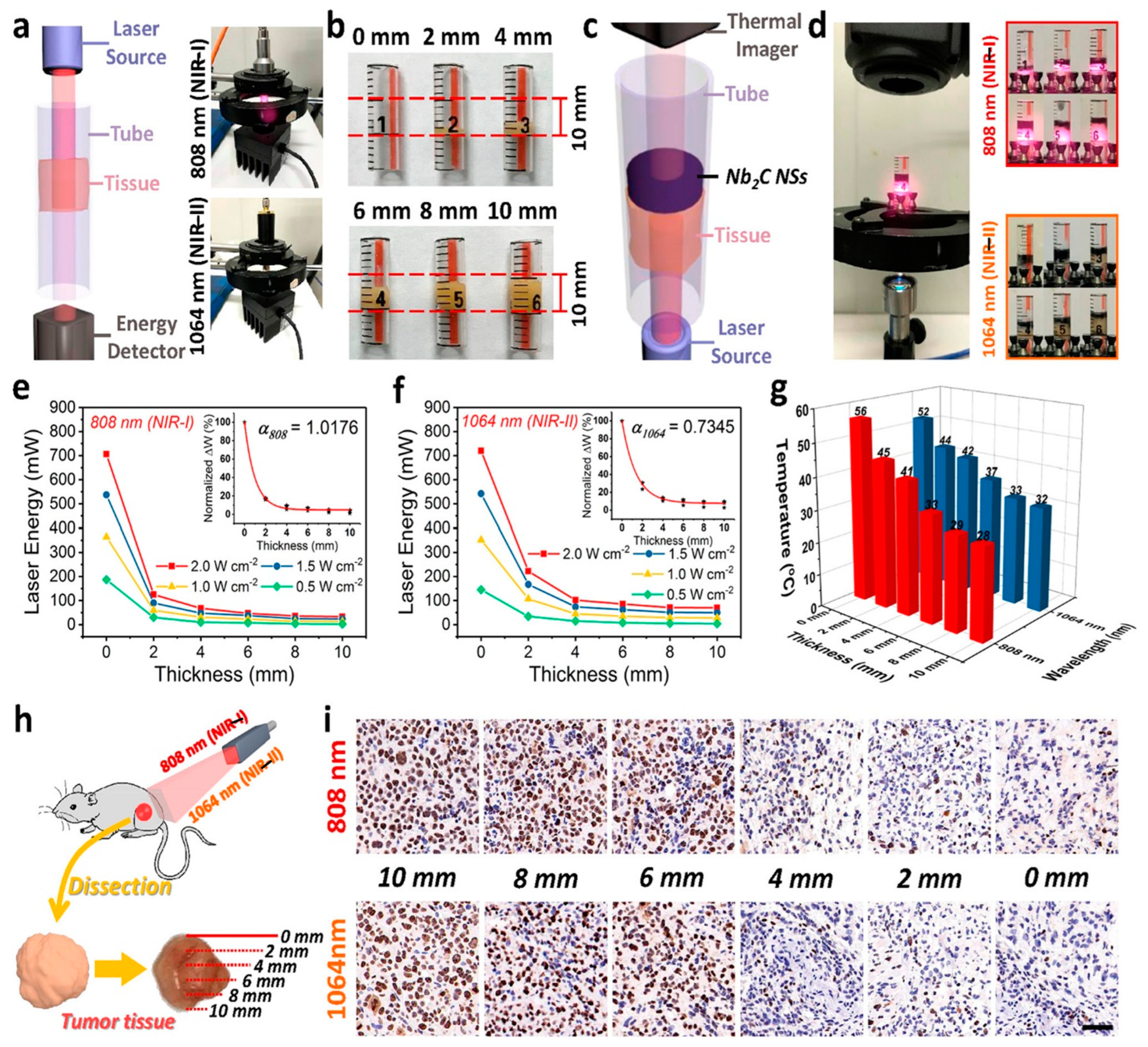
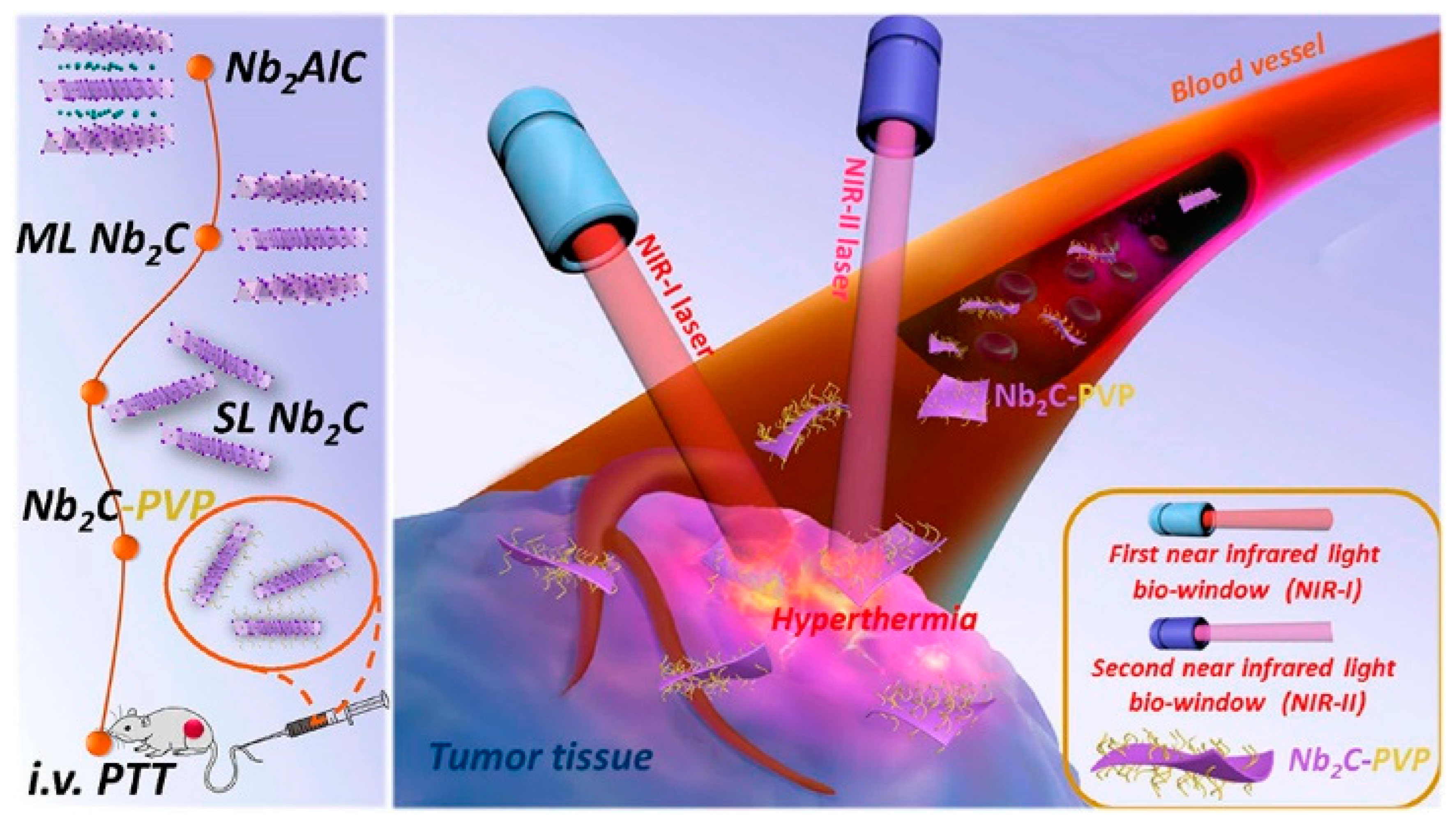
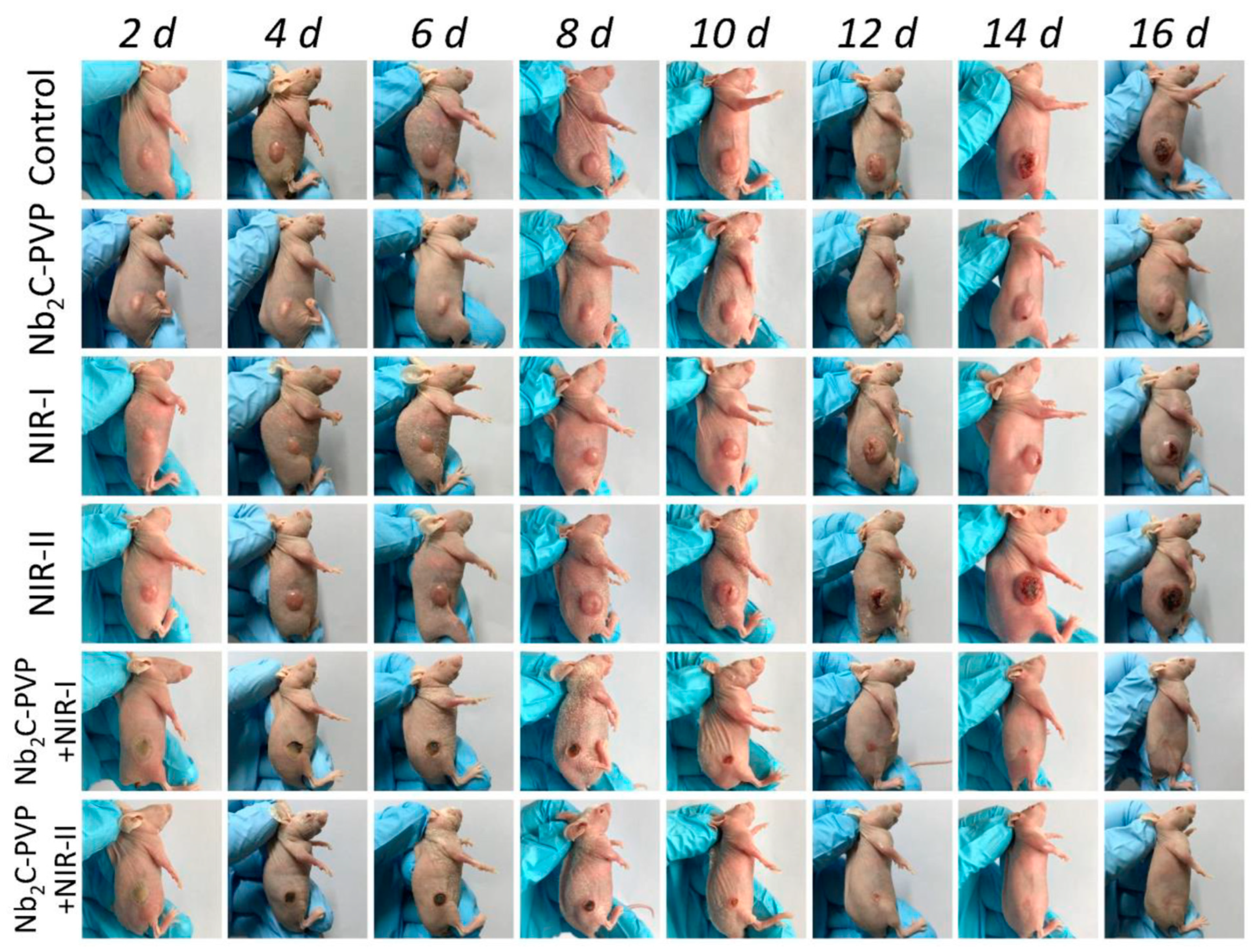
4.5. Biomedical Applications
4.6. Electromagnetic Interference (EMI) Shielding Applications
4.7. Microwave Absorption Applications
5. Possibilities for Commercial Applications
6. Conclusions and Prospects
- The traditional synthesis of Nb2CTx uses HF etching, which poses a protection and environmental risk, making large-scale production challenging. A possible area of research is the use of green materials and the replacement of HF with less toxic compounds in the manufacture of Nb2CTx. Future studies could concentrate on the production of surface terminations utilizing environmentally acceptable materials.
- Drawing-up superior Nb2CTx with the appropriate properties might be a research emphasis. This is because the surface chemistry of Nb2CTx is sensitive to synthesis conditions. Nb2CTx may have a multilayered and accordion-like structure rather than 2D sheets, limiting its utility in environmental applications. To obtain flakes of Nb2CTx with tunable morphological features, a method should be established.
- Another future challenge will be to produce Nb2CTx cheaply and with high returns. The development of inexpensive, efficient, and environmentally friendly Nb2CTx manufacturing on an industrial scale is required for the practical use of Nb2CTx. Another potential difficulty is the aggregation of Nb2CTx layers, which lowers the surface area, and thus, the storage capacity. Aggregation resolution can enhance the energy capacity of Nb2CTx-based electrodes and purifying performance for environmental purposes.
- Future research should also concentrate on the precise prediction of Nb2CTx and its composites, which is challenging owing to heterogeneous surface terminations since existing approaches call for homogeneous terminations, which are still a work in progress. There is yet no technique for evenly terminating surface groups, such as fluorine, hydroxyl, oxygen, or hydrogen. Researchers must also focus on the processes at work of structural changes brought on by functional groupings.
- Another difficulty for Nb2CTx and related materials is to create structures with controllable features for purposes such as energy storage. Many techniques including heterostructure, ion intercalation, and functional group control have been suggested to enhance the storage capability of Nb2CTx. Further research is required to better identify the energy storage process so that a combination of modifications can be scientifically preferred to obtain the best solution based on needs.
- The expense and structural instability of Nb2CTx could be a barrier to industrial use. The expense is due to the use of a costly MAX precursor during the synthesis. Advanced material synthesis with lower production costs is required. Additional composites and solvents must be investigated and assessed for varied purposes to alleviate structural instability. The creation of a packaging solution is essential for extending the service life of Nb2CTx-based devices.
- So far, only a few Nb2CTx-based MXenes have been experimentally applied to specific applications. Hence, there is a need to expand its applications to biomedical, electrodes for flexible all-solid-state supercapacitors, photothermal therapy, electronics, such as FET, for tunable band gaps, lubricant oil, biosensors, antibacterial activity, water purification, H2 generation, O2 evolution electrocatalyst, sensors such as for NH3 and CO2, nuclear waste management, nanofiltration, dye adsorption, electromagnetic interference (EMI) shielding, and more.
Author Contributions
Funding
Conflicts of Interest
References
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R.; et al. Recent Advances in Two-Dimensional Materials beyond Graphene. ACS Nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, N.V.; Barsoum, W.M.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, T.; Mourad, A.-H.I.; Raji, R.K.; Krishnapriya, R.; Cherupurakal, N.; Subhan, A.; Al-Douri, Y. KOH mediated hydrothermally synthesized hexagonal-CoMn2O4 for energy storage supercapacitor applications. Int. J. Energy Res. 2022, 46, 16823–16838. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Shao, G. Theoretical identification of layered MXene phase NaxTi4C2O4 as superb anodes for rechargeable sodium-ion batteries. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 11177–11187. [Google Scholar] [CrossRef]
- Long, Q.M.; Tang, K.K.; Xiao, J.; Li, J.Y.; Chen, J.; Gao, H.; Chen, H.W.; Liu, T.C.; Liu, H. Recent advances on MXene based materials for energy storage applications. Mater. Today Sustain. 2022, 19, 100163. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Zheng, J.; Rong, J.; Li, H.; Niu, L. MXenes: Advanced materials in potassium ion batteries. Chem. Eng. J. 2021, 404, 126565. [Google Scholar] [CrossRef]
- Nasrin, K.; Sudharshan, V.; Subramani, K.; Sathish, M. Insights into 2D/2D MXene heterostructures for improved synergy in structure toward next-generation supercapacitors: A review. Adv. Funct. Mater. 2022, 32, 2110267. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Duan, Z.; Yuan, Z.; Zha, J.; Wu, Z.; Huang, Q.; Zhou, Z.; Li, H.; He, F.; et al. A Nb2CTx/sodium alginate-based composite film with neuron-like network for self-powered humidity sensing. Chem. Eng. J. 2022, 438, 135588. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, J.; Wang, F.; Tian, F.; Zheng, W.; Bao, Y.; Zhang, K.; Zhang, Z.; Yu, J.; Xu, J.; et al. 3D Printing of Stretchable, Adhesive and Conductive Ti3C2Tx-Polyacrylic Acid Hydrogels. Polymers 2022, 14, 1992. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, L.; Zheng, W.; Zhang, H.; Wu, F.; Tian, Z.; Zhang, P.; Sun, Z.M. MXenes with applications in supercapacitors and secondary batteries: A comprehensive review. Mater. Rep. Energy 2022, 2, 100080. [Google Scholar] [CrossRef]
- Abdul Rasheed, P.; Pandey, R.P.; Banat, F.; Hasan, S.W. Recent advances in niobium MXenes: Synthesis, properties, and emerging applications. Matter 2022, 5, 546–572. [Google Scholar] [CrossRef]
- MXene Synthesis. Available online: https://nano.materials.drexel.edu/max-phases-and-mxenes-synthesis/ (accessed on 5 January 2023).
- Huang, C.; Shi, S.; Yu, H. Work Function Adjustment of Nb2CTx Nanoflakes as Hole and Electron Transport Layers in Organic Solar Cells by Controlling Surface Functional Groups. ACS Energy Lett. 2021, 6, 3464–3472. [Google Scholar] [CrossRef]
- Zhu, H.; Xue, S.; Liang, Z.; Liang, X.; Li, G.; Ren, X.; Gao, L.; Li, Q.; Ma, T.; Liu, A. Self-assembly synthesis of Ni-decorated Nb2C MXene as an efficient and stable catalyst towards electrochemical nitrogen reduction. Ceram. Int. 2022, 48, 20599–20604. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasheed, P.A.; Gomez, T.; Rasool, K.; Ponraj, J.; Prenger, K.; Naguib, M.; Mahmoud, K.A. Effect of Sheet Size and Atomic Structure on the Antibacterial Activity of Nb-MXene Nanosheets. ACS Appl. Nano Mater. 2020, 3, 11372–11382. [Google Scholar] [CrossRef]
- Li, J.; Zeng, F.; El-Demellawi, J.K.; Lin, Q.; Xi, S.; Wu, J.; Tang, J.; Zhang, X.; Liu, X.; Tu, S. Nb2CTx MXene Cathode for High-Capacity Rechargeable Aluminum Batteries with Prolonged Cycle Lifetime. ACS Appl. Mater. Interfaces 2022, 14, 45254–45262. [Google Scholar] [CrossRef]
- Gul, S.; Serna, M.I.; Zahra, S.A.; Arif, N.; Iqbal, M.; Akinwande, D.; Rizwan, S. Un-doped and Er-adsorbed layered Nb2C MXene for efficient hydrazine sensing application. Surf. Interfaces 2021, 24, 101074. [Google Scholar] [CrossRef]
- Naveen Kumar, A.; Pal, K. Amine-functionalized stable Nb2CTx MXene toward room temperature ultrasensitive NO2 gas sensor. Mater. Adv. 2022, 3, 5151–5162. [Google Scholar] [CrossRef]
- Zong, H.; Hu, L.; Gong, S.; Yu, K.; Zhu, Z. Flower-petal-like Nb2C MXene combined with MoS2 as bifunctional catalysts towards enhanced lithium-sulfur batteries and hydrogen evolution. Electrochim. Acta 2022, 404, 139781. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Chen, G.; Zhang, J. Sandwich-like N-doped carbon nanotube@Nb2C MXene composite for high performance alkali ion batteries. Ceram. Int. 2021, 47, 20610–20616. [Google Scholar] [CrossRef]
- Byeon, A.; Glushenkov, A.M.; Anasori, B.; Urbankowski, P.; Li, J.; Byles, B.W.; Blake, B.; Van Aken, K.L.; Kota, S.; Pomerantseva, E.; et al. Lithium-ion capacitors with 2D Nb2CTx (MXene)—Carbon nanotube electrodes. J. Power Sources 2016, 326, 686–694. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, B.; Zhu, J.; Li, K.; Tian, S. Hierarchical ZnO/MXene (Nb2C and V2C) heterostructure with efficient electron transfer for enhanced photocatalytic activity. Appl. Surf. Sci. 2022, 590, 153095. [Google Scholar] [CrossRef]
- Yan, Y.; Han, H.; Dai, Y.; Zhu, H.; Liu, W.; Tang, X.; Gan, W.; Li, H. Nb2CTx MXene Nanosheets for Dye Adsorption. ACS Appl. Nano Mater. 2021, 4, 11763–11769. [Google Scholar] [CrossRef]
- Liu, Z.; El-Demellawi, K.J.; Bakr, M.O.; Ooi, S.B.; Alshareef, N.H. Plasmonic Nb2CTx MXene-MAPbI3 Heterostructure for Self-Powered Visible-NIR Photodiodes. ACS Nano. 2022, 16, 8. [Google Scholar] [CrossRef]
- Nasrin, K.; Sudharshan, V.; Arunkumar, M.; Sathish, M. 2D/2D Nanoarchitectured Nb2C/Ti3C2 MXene Heterointerface for High-Energy Supercapacitors with Sustainable Life Cycle. ACS Appl. Mater. Interfaces 2022, 14, 21038–21049. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-Ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.L.; Barsoum, M.W. Synthesis and characterization of twodimensional Nb4C3 (MXene). Chem. Commun. 2014, 50, 9517–9520. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Amine-assisted delamination of Nb2C MXene for Li-ion energy storage devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, X.; Zhu, K.; Du, F.; Chen, G.; Wei, Y.; Gogotsi, Y.; Gao, Y. Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene. Energy Storage Mater. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, J.; Bai, L.; Xiao, J.; Zheng, R.; Shan, X.; Li, L.; Gao, H.; Zhang, X. One-step synthesis of few-layer niobium carbide MXene as a promising anode material for high-rate lithium ion batteries. Dalton Trans. 2019, 48, 14433–14439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, C.; Zhao, X.; Chu, X.; Du, F.; Chen, G.; Gogotsi, Y.; Gao, Y.; Dall Agnese, Y. Flexible Nb4C3Tx film with large interlayer spacing for highperformance supercapacitors. Adv. Funct. Mater. 2020, 30, 2000815. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, K.; Qi, K.; Xue, T.; Zhang, H.; He, J.; Xiao, S. Niobium carbide MXenes with broad-band nonlinear optical response and ultrafast carrier dynamics. ACS Nano 2020, 14, 10492–10502. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, L.; Chen, H.; Zhang, F.; Yang, Q.; Ren, X.; Din, S.Z.U.; Li, C.; Leng, J.; Zhang, J.; et al. Broadband few-layer niobium carbide MXene as saturable absorber for solid-state lasers. Opt. Laser Technol. 2021, 142, 107199. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Li, Z.; Feng, K.; Cai, M.; Zhang, D.; Wang, S.; Chu, M.; Zhang, C.; Shen, J.; et al. Niobium and titanium carbides (MXenes) as superior photothermal supports for CO2 photocatalysis. ACS Nano 2021, 15, 5696–5705. [Google Scholar] [CrossRef]
- Liu, X.; Fechler, N.; Antonietti, M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem. Soc. Rev. 2013, 42, 8237. [Google Scholar] [CrossRef]
- Guan, K.; Lei, W.; Wang, H.; Liu, X.; Luo, J.; Liu, J.; Jia, Q.; Zhang, H.; Zhang, S. Efficient synthesis of Ti3AlC2 powders with high purity by microwave-assisted molten salt method. Ceram. Int. 2022, 48, 16357–16363. [Google Scholar] [CrossRef]
- Galvin, T.; Hyatt, N.C.; Rainforth, W.M.; Reaney, I.M.; Shepherd, D. Molten salt synthesis of MAX phases in the Ti-Al-C system. J. Eur. Ceram. Soc. 2018, 38, 4585–4589. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, P.; Jin, N.; Wang, B.; Liu, Y.; Lin, Z. Molten Salt Derived Nb2CTx MXene Anode for Li-Ion Batteries. Chem. Electro. Chem. 2021, 8, 957–962. [Google Scholar]
- Wang, Y.; Hu, X.; Song, H.; Cai, Y.; Li, Z.; Zu, D.; Zhang, P.; Chong, D.; Gao, N.; Shen, Y.; et al. Oxygen vacancies in actiniae-like Nb2O5/Nb2C MXene heterojunction boosting visible light photocatalytic NO removal. Appl. Catal. B 2021, 299, 120677. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Li, D.; Cao, J.; Han, W. Carbon-Reinforced Nb2CTx MXene/MoS2 Nanosheets as a Superior Rate and High-Capacity Anode for Sodium-Ion Batteries. ACS Nano 2021, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, M.; Zhang, X.; Tao, J.; Lu, L.; Qiao, G.; Liu, G. Anchoring of 2D CdS on Nb2CTx MXene nanosheets for boosting photocatalytic H2 evolution. J. Alloy. Compd. 2022, 923, 166256. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Liu, W.; Zhang, J.; Song, M.; Zhang, C.; Zhang, J.; Wang, D. Nb2CTx MXenes functionalized Co−NC enhancing electrochemical H2O2 production for organics degradation. Appl. Catal. B 2022, 317, 121737. [Google Scholar] [CrossRef]
- Fan, X.; Du, P.; Ma, X.; Wang, R.; Ma, J.; Wang, Y.; Fan, D.; Long, Y.; Deng, B.; Huang, K.; et al. Mechanochemical Synthesis of Pt/Nb2CTx MXene Composites for Enhanced Electrocatalytic Hydrogen Evolution. Materials 2021, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, Y.; Ma, Y.; Zhang, B.; Xia, B.; Zhang, P.; Qian, W.; He, D.; Zhang, X.; Li, B.W.; et al. Aqueous MXene/Xanthan Gum Hybrid Inks for Screen-Printing Electromagnetic Shielding, Joule Heater, and Piezoresistive Sensor. Small 2022, 18, 2107087. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Liu, B.; Duan, Z.; Pan, H.; Yuan, Z.; Xie, G.; Wang, J.; Fang, Z.; Tai, H. Ultrathin Nb2CTx nanosheets-supported polyaniline nanocomposite: Enabling ultrasensitive NH3 detection. Sens. Actuators B Chem. 2021, 343, 130069. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Duan, Z.; Zhao, Q.; Zhang, Y.; Xie, G.; Jiang, Y.; Li, S.; Tai, H. PANI nanofibers-supported Nb2CTx nanosheets-enabled selective NH3 detection driven by TENG at room temperature. Sens. Actuators B Chem. 2021, 327, 128923. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Yunoki, S. Topological insulators in the ordered double transition metals M2′M’’C2 MXenes (M’Mo, W.; M’’Ti, Zr, Hf). Phys. Rev. B 2016, 94, 125152. [Google Scholar] [CrossRef]
- Wyatt, C.B.; Rosenkranz, A.; Anasori, B. 2D MXenes: Tunable Mechanical and Tribological Properties. Adv. Mater. 2021, 33, 2007973. [Google Scholar] [CrossRef]
- Lipatov, A.; Loes, M.J.; Vorobeva, N.S.; Bagher, S.; Abourahma, J.; Chen, H.; Hong, X.; Gogotsi, Y.; Sinitskii, A. High breakdown current density in monolayer Nb4C3Tx MXene. ACS Mater. Lett. 2021, 3, 1088–1094. [Google Scholar] [CrossRef]
- Rahman, U.U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.M.; Khan, A. MXenes as Emerging Materials: Synthesis, Properties, and Applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Si, C.; Zhou, J.; Sun, Z. Half-Metallic Ferromagnetism and Surface Functionalization-Induced Metal-Insulator Transition in Graphene-Like Two-Dimensional Cr2C Crystals. ACS Appl. Mater. Interfaces 2015, 7, 17510–17515. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Anwar, M.S.; Mumtaz, M.; Iqbal, M.; Zheng, R.K.; Akinwande, D.; Rizwan, S. Peculiar magnetic behaviour and Meissner effect in two-dimensional layered Nb2C MXene. 2D Mater. 2020, 7, 035012. [Google Scholar] [CrossRef]
- Din Babar, Z.U.; Fatheema, J.; Arif, N.; Anwar, M.S.; Gul, S.; Iqbal, M.; Rizwan, S. Magnetic phase transition from paramagnetic in Nb2AlC-MAX to superconductivity-likediamagnetic in Nb2C-MXene: An experimental and computational analysis. RSC Adv. 2020, 10, 25669–25678. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, M.S.; Kim, H.Y.; Koo, M.C. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Liu, S.; Song, Z.; Jin, X.; Mao, R.; Zhang, T.; Hu, F. MXenes for metal-ion and metal-sulfur batteries: Synthesis, properties, and electrochemistry. Mater. Rep. Energy 2022, 2, 100077. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Ding, S.; Dai, X.; Dai, Y.; Chen, Y.; Ni, X. Self-Generated Schottky Barriers in Niobium Carbide MXene Nanocatalysts for Theory-Oriented Sonocatalytic and NIR-II Photonic Hyperthermia Tumor Therapy. Nano Today 2023, 48, 101750. [Google Scholar] [CrossRef]
- Ramachandran, T.; Thiemann, T.; Hamed, F. Phase evolution and magnetic properties of Dy3Fe5+xO12−x nanocrystalline powders: A choice of fuel approach. Mater. Chem. Phys. 2020, 240, 122138. [Google Scholar] [CrossRef]
- Hamed, F.; Ramachandran, T.; Kurapati, V. The effect of induced strains on the optical band gaps in lanthanum doped zinc ferrite nanocrystalline powders. Mod. Phys. Lett. B 2016, 30, 1650230. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Simon, P.; Xu, B. Two-dimensional MXenes for electrochemical capacitor applications: Progress, challenges and perspectives. Energy Storage Mater. 2021, 35, 630–660. [Google Scholar] [CrossRef]
- Lamiel, C.; Hussain, I.; Warner, J.H.; Zhang, K. Beyond Ti-based MXenes: A review of emerging non-Ti based metal-MXene structure, properties, and applications. Mater. Today 2023, in press. [Google Scholar] [CrossRef]
- Das, K.; Majumdar, D. Prospects of MXenes/graphene nanocomposites for advanced supercapacitor applications. J. Electroanal. Chem. 2022, 905, 115973. [Google Scholar] [CrossRef]
- Makola, L.C.; Moeno, S.; Ouma, C.N.; Sharma, A.; Vo, D.-V.N.; Dlamini, L.N. Facile Fabrication of a Metal-Free 2D-2D Nb2CTx@g-C3N4 MXene-Based Schottky-Heterojunction with the Potential Application in Photocatalytic Processes. J Alloy. Compds. 2022, 916, 165459. [Google Scholar] [CrossRef]
- Palisaitis, J.; Persson, I.; Halim, J.; Rosen, J.; Persson, P.O. On the structural stability of MXene and the role of transition metal adatoms. Nanoscale 2018, 10, 10850–10855. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Satheesh Kumar, R.; Mohamed Azarudeen, L.; Anand Kumar, G.; Vishista, K.; Hamed, F. Facile Synthesis of Cr–Doped SrS Phosphor: An Investigations on Structural, Vibrational, Morphological and Photoluminescence Properties. Mater. Focus 2016, 5, 342–346. [Google Scholar] [CrossRef]
- Echols, I.J.; Holta, D.E.; Kotasthane, V.S.; Tan, Z.; Radovic, M.; Lutkenhaus, J.L.; Green, M.J. Oxidative stability of Nbn+1CnTz MXenes. J. Phys. Chem. C 2021, 125, 13990–13996. [Google Scholar] [CrossRef]
- Zhao, X.; Vashisth, A.; Blivin, J.W.; Tan, Z.; Holta, D.E.; Kotasthane, V.; Shah, S.A.; Habib, T.; Liu, S.; Lutkenhaus, J.L. pH, nanosheet concentration, and antioxidant affect the oxidation of Ti3C2Tx and Ti2CTx MXene dispersions. Adv. Mater. Inter. 2020, 7, 2000845. [Google Scholar] [CrossRef]
- Ramachandran, T.; Natarajan, S.; Hamed, F. The role of dysprosium levels in the formation of mixed oxidation states within spinel MnCo2−xDyxO4 nanocrystalline powders. J. Electron Spectrosc. Relat. Phenom. 2020, 242, 146952. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, X.; Wang, L. Synthesis of a MoO3/Ti3C2Tx composite with enhanced capacitive performance for supercapacitors. RSC Adv. 2016, 6, 98506–98513. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, G.R.; Bachmatiuk, A.; Zhao, L.; Ta, Q.H.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wei, Y.; Zhuang, Z.; Lin, Z.; Li, X.; Hou, C.; Li, T.; Ma, Y. Fabrication of Ti3C2Tx MXene/polyaniline composite films with adjustable thickness for high-performance flexible all-solid-state symmetric supercapacitors. Electrochim. Acta 2022, 406, 139871. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, W.M. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Zaheer, A.; Zahra, S.A.; Iqbal, M.Z.; Mahmood, A.; Khand, S.A.; Rizwan, S. Nickel-adsorbed two-dimensional Nb2C MXene for enhanced energy storage applications. RSC Adv. 2022, 12, 4624. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wen, J.; Zhao, J.; Ma, X.; Gao, H.; Zhang, X. A safe etching route to synthesize highly crystalline Nb2CTx MXene for high performance asymmetric supercapacitor applications. Electrochim. Acta 2020, 337, 135803. [Google Scholar] [CrossRef]
- Raji, R.K.; Ramachandran, T.; Muralidharan, M.; Suriakarthick, R.; Dhilip, M.; Hamed, F.; Kurapati, V. Conventional synthesis of perovskite structured LaTixFe1-xO3: A comprehensive evaluation on phase formation, opto-magnetic, and dielectric properties. Int. J. Mater. Res. 2021, 112, 753–765. [Google Scholar] [CrossRef]
- Luo, J.; Lu, X.; Matios, E.; Wang, C.; Wang, H.; Zhang, Y.; Hu, X.; Li, W. Tunable MXenederived 1D/2D hybrid nanoarchitectures as a stable matrix for dendrite-free and ultrahigh capacity sodium metal anode. Nano Lett. 2020, 20, 7700–7708. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Vishista, K. Tuning the composition and magnetostructure of dysprosium iron garnets by Co-substitution: An XRD, FT-IR, XPS and VSM study. Appl. Surf. Sci. 2015, 351, 1016–1024. [Google Scholar] [CrossRef]
- Rekha, G.; Tholkappiyan, R.; Vishista, K.; Hamed, F. Systematic study on surface and magnetostructural changes in Mn-substituted dysprosium ferrite by hydrothermal method. Appl. Surf. Sci. 2016, 385, 171–181. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Zhang, J. Nb2CTx MXene as High-Performance Energy Storage Material with Na, K, and Liquid K−Na Alloy Anodes. Langmuir 2021, 37, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Huo, M.; Wang, M.; Lin, H.; Zhang, X.; Yin, J.; Chen, Y.; Chen, H. Highly catalytic niobium carbide (MXene) promotes hematopoietic recovery after radiation by free radical scavenging. ACS Nano 2019, 13, 6438–6454. [Google Scholar] [CrossRef]
- Yin, J.; Pan, S.; Guo, X.; Gao, Y.; Zhu, D.; Yang, Q.; Gao, J.; Zhang, C.; Chen, Y. Nb2C MXene-Functionalized Scaffolds Enables Osteosarcoma Phototherapy and Angiogenesis/Osteogenesis of Bone Defects. Nanomicro Lett. 2021, 13, 30. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, Z.; Zhang, X.; Zhang, J.; Zhou, Y.; Li, H.; Qin, H.; Bo, Y.; Pan, Z. Nb4C3Tx (MXene) as a new stable catalyst for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 1955–1966. [Google Scholar] [CrossRef]
- Su, T.; Peng, R.; Hood, Z.D.; Naguib, M.; Ivanov, I.N.; Keum, J.K.; Qin, Z.; Guo, Z.; Wu, Z. One-step synthesis of Nb2O5/C/Nb2C (MXene) composites and their use as photocatalysts for hydrogen evolution. Chem. Sus. Chem. 2018, 11, 688–699. [Google Scholar] [CrossRef]
- Sun, K.Y.; Wu, Y.; Xu, J.; Xiong, W.; Xu, W.; Li, J.; Sun, Z.; Lv, Z.; Wu, X.S.; Jiang, Q.; et al. Niobium carbide (MXene) reduces UHMWPE particle-induced osteolysis. Bioact. Mater. 2022, 8, 435–448. [Google Scholar] [CrossRef]
- Reding, B.; Carter, P.; Qi, Y.; Li, Z.; Wu, Y.; Wannemuehler, M.; Bratlie, K.M.; Wang, Q. Manipulate intestinal organoids with niobium carbide nanosheets. J. Biomed. Mater. Res. A 2021, 109, 479–487. [Google Scholar] [CrossRef]
- Song, S.; Liu, J.; Zhou, C.; Jia, Q.; Luo, H.; Deng, L.; Wang, X. Nb2O5/Nb2CTx composites with different morphologies through oxidation of Nb2CTx MXene for high-performance microwave absorption. J. Alloy. Compd. 2020, 843, 155713. [Google Scholar] [CrossRef]
- Jastrzebska, A.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Mitrzak, J.; Wojciechowski, T.; Chudy, M.; Moszczy_nska, D.; Wojcik, A.; Prenger, K.; Naguib, M. Juggling surface charges of 2D niobium carbide mxenes for a reactive oxygen species scavenging and effective targeting of the malignant melanoma cell cycle into programmed cell death. ACS Sustain. Chem. Eng. 2020, 8, 7942–7951. [Google Scholar] [CrossRef]
- Han, X.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Ran, H.; Li, P.; Chen, Y. Therapeutic mesopore construction on 2D Nb2C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics 2018, 8, 4491–4508. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, Y.; Lin, H.; Ge, M.; Shi, J.; Zhang, X. Niobium carbide MXene augmented medical implant elicits bacterial infection elimination and tissue regeneration. ACS Nano 2021, 15, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Z.; Fang, C.; Hou, X.; Xie, L. Recent advances in MXenes composites for electromagnetic interference shielding and microwave absorption. Compos. A Appl. Sci. Manuf. 2020, 136, 105956. [Google Scholar] [CrossRef]
- Iqbal, A.; Kwon, J.; Kim, M.K.; Koo, C.M. MXenes for electromagnetic interference shielding: Experimental and theoretical perspectives. Mater. Today Adv. 2021, 9, 100124. [Google Scholar] [CrossRef]
- Rajavel, K.; Yu, X.; Zhu, P.; Hu, Y.; Sun, R.; Wong, C. Investigation on the structural quality dependent electromagnetic interference shielding performance of fewlayer and lamellar Nb2CTx MXene nanostructures. J. Alloy. Compd. 2021, 877, 160235. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.; Ren, E.; Xiao, H.; Zhou, M.; Lai, X.; Qin, Q.; Jiang, S.; Qin, W. MXene-based rGO/Nb2CTx/Fe3O4 composite for high absorption of electromagnetic wave. Chem. Eng. J. 2021, 405, 126626. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.; Xiao, H.; Ren, E.; Song, Q.; Xiang, C.; Lai, X.; Lan, J.; Jiang, S. Bi2WO6/Nb2CTx MXene hybrid nanosheets with enhanced visible-lightdriven photocatalytic activity for organic pollutants degradation. Appl. Surf. Sci. 2020, 505, 144595. [Google Scholar] [CrossRef]
- Peng, C.; Xie, X.; Xu, W.; Zhou, T.; Wei, P.; Jia, J.; Zhang, K.; Cao, Y.; Wang, H.; Peng, F.; et al. Engineering highly active Ag/Nb2O5@Nb2CTx (MXene) photocatalysts via steering charge kinetics strategy. Chem. Eng. J. 2021, 421, 128766. [Google Scholar] [CrossRef]
- Yan, P.; Ji, L.; Liu, X.; Guan, Q.; Guo, J.; Shen, Y.; Zhang, H.; Wei, W.; Cui, X.; Xu, Q. 2D amorphous-MoO3−x@ Ti3C2-MXene non-van der Waals heterostructures as anode materials for lithium-ion batteries. Nano Energy 2021, 86, 106139. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, C.; Huang, H.; Yang, T.; Tian, G.; Xiong, D.; Chen, N.; Chu, X.; Zhong, S.; Deng, W.; et al. Microchannel-confined MXene based flexible piezoresistive multifunctional micro-force sensor. Adv. Funct. Mater. 2020, 30, 1909603. [Google Scholar] [CrossRef]
- Wei, Y.; Soomro, R.A.; Xie, X.; Xu, B. Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes. J. Energy Chem. 2021, 55, 244–255. [Google Scholar] [CrossRef]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.-W.; Yang, X.-Q.; Kolesnikov, A.I.; Kent, P.R. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Wu, Z.S. MXene for energy storage: Present status and future perspectives. J. Phys. Energy 2020, 2, 032004. [Google Scholar] [CrossRef]
- Zhang, C.J.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.-H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of flexible, coplanar micro-supercapacitors using MXene inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Yang, W.; Yang, J.; Byun, J.J.; Moissinac, F.P.; Xu, J.; Haigh, S.J.; Domingos, M.; Bissett, M.A.; Dryfe, R.A.; Barg, S. 3D Printing of Freestanding MXene Architectures for Current-Collector-Free Supercapacitors. Adv. Mater. 2019, 31, 1902725. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All pseudocapacitive MXene-RuO2 Asymmetric supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Shin, H.; Eom, W.; Lee, K.H.; Jeong, W.; Kang, D.J.; Han, T.H. Highly Electroconductive and Mechanically Strong Ti3C2Tx MXene Fibers Using a Deformable MXene Gel. ACS Nano 2021, 15, 3320–3329. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 22123–22133. [Google Scholar] [CrossRef]
- Vahid Mohammadi, A.; Mojtabavi, M.; Caffrey, M.N.; Wanunu, M.; Beidaghi, M. Assembling 2D MXenes into Highly Stable Pseudocapacitive Electrodes with High Power and Energy Densities. Adv. Mater. 2019, 31, 1806931. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, L.; Anasori, B.; Liu, Y.-T.; Zhu, Q.; Zhang, P.; Gogotsi, Y.; Xu, B. MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors. ACS Energy Lett. 2018, 3, 1597–1603. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, F.; Fang, L.; Hu, J.; Luo, H.; Guan, T.; Hu, B.; Zhou, M. Layered NiFe-LDH/MXene nanocomposite electrode for high-performance supercapacitor. Int. J. Hydrog. Energy 2020, 45, 13080–13089. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Yang, Q.; Hanif, M.; Wang, Z.; Dong, L.; Dong, M. A novel MnO2/Ti3C2Tx MXene nanocomposite as high performance electrode materials for flexible supercapacitors. Electrochim. Acta 2018, 290, 695–703. [Google Scholar] [CrossRef]
- Qian, A.; Hyeon, E.S.; Seo, Y.J.; Chung, H.C. Capacitance changes associated with cation-transport in free-standing flexible Ti3C2Tx (TO, F, OH) MXene film electrodes. Electrochim. Acta 2018, 266, 86–93. [Google Scholar] [CrossRef]
- Xia, Q.X.; Shinde, N.M.; Zhang, T.; Yun, J.M.; Zhou, A.; Mane, R.S.; Mathur, S.; Kim, K.H. Seawater electrolyte-mediated high volumetric MXene-based electrochemical symmetric supercapacitors. Dalton Trans. 2018, 47, 8676–8682. [Google Scholar] [CrossRef]
- Fu, B.; Sun, J.; Wang, C.; Shang, C.; Xu, L.; Li, J.; Zhang, H. MXenes: Synthesis, Optical Properties, and Applications in Ultrafast Photonics. Small 2021, 17, 2006054. [Google Scholar] [CrossRef]
- Shein, R.I.; Ivanovskii, L.A. Graphene-like titanium carbides and nitrides Tin+1Cn, Tin+1Nn (n = 1, 2, and 3) from de-intercalated MAX phases: First-principles probing of their structural, electronic properties and relative stability. Comput. Mater. Sci. 2012, 65, 104–114. [Google Scholar] [CrossRef]
- Tang, H.; Wang, R.; Shi, L.; Sheremet, E.; Rodriguez, D.R.; Sun, J. Post-processing strategies for improving the electrical and mechanical properties of MXenes. Chem. Eng. J. 2021, 425, 131472. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.; Goddard, A.W. Schottky-Barrier-Free Contacts with Two-Dimensional Semiconductors by Surface-Engineered MXenes. J. Am. Chem. Soc. 2016, 138, 15853–15856. [Google Scholar] [CrossRef]
- Hoque, M.M.; Hannan, A.M.; Mohamed, A.; Ayob, A. Battery charge equalization controller in electric vehicle applications: A review. Renew. Sustain. Energy Rev. 2017, 75, 1363–1385. [Google Scholar] [CrossRef]
- Ghidiu, M.; Halim, J.; Kota, S.; Bish, D.; Gogotsi, Y.; Barsoum, W.M. Ion-Exchange and Cation Solvation Reactions in Ti3C2 MXene. Chem. Mater. 2016, 28, 3507–3514. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpige, N.L.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Anil Kumar, Y.; Koyyada, G.; Ramachandran, T.; Kim, J.H.; Sajid, S.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Carbon Materials as a Conductive Skeleton for Supercapacitor Electrode Applications: A Review. Nanomaterials 2023, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, M.-Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Mater. Chem. A Mater. Energy Sustain. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z.; Hu, T.; Zhu, S.; Zhang, C.; Wang, X. High-Capacitance Mechanism for Ti3C2Tx MXene by In Situ Electrochemical Raman Spectroscopy Investigation. ACS Nano 2016, 10, 11344–11350. [Google Scholar] [CrossRef]
- Zhang, C.J.; Nicolosi, V. Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Storage Mater. 2019, 16, 102–125. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.; Soomro, R.A.; He, S.; Sun, N.; Qiao, N.; Xu, B. In situ ice template approach to fabricate 3D flexible MXene film-based electrode for high performance supercapacitors. Adv. Funct. Mater. 2020, 30, 2000922. [Google Scholar] [CrossRef]
- De, S.; Acharya, S.; Sahoo, S.; Shim, J.-J.; Nayak, G.C. From 0D to 3D MXenes: Their diverse syntheses, morphologies and applications. Mater. Chem. Front. 2022, 6, 818–842. [Google Scholar] [CrossRef]
- Wyatt, B.C.; Thakur, A.; Nykiel, K.; Hood, Z.D.; Adhikari, S.P.; Pulley, K.K.; Highland, W.J.; Strachan, A.; Anasori, B. Design of Atomic Ordering in Mo2Nb2C3Tx MXenes for Hydrogen Evolution Electrocatalysis. Nano Lett. 2023, 23, 931–938. [Google Scholar] [CrossRef] [PubMed]



| MAX | MXene | HF Acid | References |
|---|---|---|---|
| Nb2AlC | Nb2CTx films | 50% | [28] |
| Nb4AlC3 | Nb4C3Tx flake | 48–51% | [29] |
| Nb2AlC | Nb2CTx flakes | 50% | [30] |
| Nb2AlC | Nb2CTx sheet | 50% | [31] |
| Nb4AlC3 | Nb4C3Tx layer | 49% | [32] |
| Nb2AlC | Nb2CTx few-layer | 40% | [33] |
| Nb4AlC3 | Nb4C3Tx film | 49% | [34] |
| Nb2AlC | Nb2CTx nanosheets | 40% | [35] |
| Nb2AlC | Nb2CTx multi-layer | 49% | [36] |
| Sl.No. | Nb2CTx for Battery Applications | |||
|---|---|---|---|---|
| Aluminum Batteries | Sodium-Ion Batteries | Li-Sulfur Batteries | ||
| 1 | Properties | safer and less expensive | operate at room temperature | quite expensive |
| 2 | Specific capacities | Nb2CTx-108 Ah/g at 0.2 A/g | Nb2CTx@MoS2@C-403 mA h/g at 1.0 A/g | MoS2/Nb2C −919.2 mAh/g, Nb2CTx MXene 330 mAh/g at 0.05 A/g |
| 3 | References | Li et al. [80] | Yuan et al. [81] | Zong et al. [82], Dong et al. [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, T.; Mourad, A.-H.I.; ElSayed, M.S.A. Nb2CTx-Based MXenes Most Recent Developments: From Principles to New Applications. Energies 2023, 16, 3520. https://doi.org/10.3390/en16083520
Ramachandran T, Mourad A-HI, ElSayed MSA. Nb2CTx-Based MXenes Most Recent Developments: From Principles to New Applications. Energies. 2023; 16(8):3520. https://doi.org/10.3390/en16083520
Chicago/Turabian StyleRamachandran, Tholkappiyan, Abdel-Hamid Ismail Mourad, and Mostafa S. A. ElSayed. 2023. "Nb2CTx-Based MXenes Most Recent Developments: From Principles to New Applications" Energies 16, no. 8: 3520. https://doi.org/10.3390/en16083520
APA StyleRamachandran, T., Mourad, A.-H. I., & ElSayed, M. S. A. (2023). Nb2CTx-Based MXenes Most Recent Developments: From Principles to New Applications. Energies, 16(8), 3520. https://doi.org/10.3390/en16083520







