1. Introduction
Reducing CO
2 emissions is a significant way to solve the problem of global warming, as CO
2 is the main component of greenhouse gases [
1]. The establishment of low-carbon cities is promoted in China to keep CO
2 emissions under control [
2]. In the past 30 years, nearly 40% of CO
2 in China has been released by fossil fuel power plants [
3]. Therefore, it is necessary to lower China’s fossil fuel power plants’ CO
2 emissions. Gas turbines (GT) are employed more frequently than conventional coal-fired power plants because of their dependability, flexibility and comparatively low CO
2 emissions [
4]. Because the gas turbine uses around 2.5 times as much air as is required for full combustion, the CO
2 content in the exhaust stream is quite low (about 3–4 percent points), necessitating a further expensive CO
2 extraction process [
5].
Due to its unique properties, the molten carbonate fuel cell (MCFC) may be utilized to remove CO
2 from the gas turbine exhaust. Gas turbine exhaust gas’s CO
2 and O
2 combine to form carbonate ions when it is injected into the MCFC’s cathode. The MCFC’s molten electrolyte is then used to carry the carbonate ions from the cathode to the anode. At the anode of MCFC, the carbonate ions react with H
2, then H
2O and CO
2 are generated. Once the anode exhaust gas is burned in the afterburner with pure O
2 acting as the oxidizer, all that is left are CO
2 and H
2O. Pure CO
2 gas can be achieved after being dehydrated. Stefano Campanari et al. [
6] investigated the performance of MCFC as a CO
2 separator integrated into natural gas combined cycles. According to the findings, with a CO
2 recovery rate of around 71%, the specific energy consumption for CO
2 avoidance was less than 0.5 MJ/kgCO
2. A gas–steam combined cycle (GSCC) system that incorporates CO
2 capture was investigated by Duan et al. [
7]. The system efficiency was around 54.96% when the CO
2 avoidance rate was 85%. Nonetheless, it has been established that the MCFC efficiency is sensitive to the gas turbine exhaust gas’s
content [
8]. Wang et al. [
9] introduced the comparison among different fuel cells for carbon capture. The results showed that MCFC-based configurations could gain better performance than the solid oxide fuel cell (SOFC) based configurations.
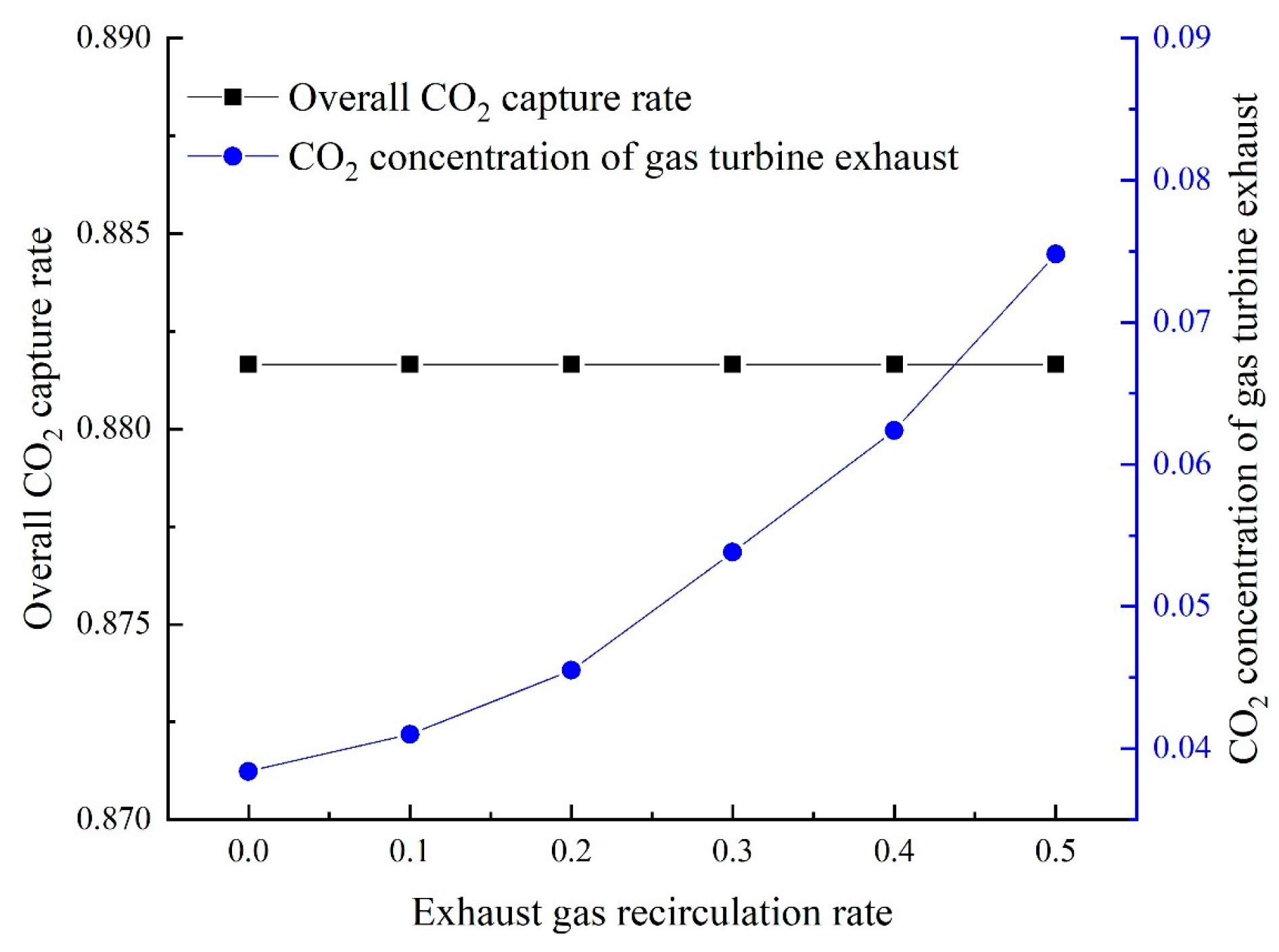
Gas turbine exhaust gas recirculation (EGR) is a popular technique for increasing the cycle’s
while lowering the energy required for the carbon capture process [
10]. The EGR process is as follows: a part of the exhaust gas is returned to the air compressor (AC) after the heat recovery steam generator releases it (HRSG). Air mass flow into the AC is reduced to maintain a constant total mass flow of the working medium, which raises the CO
2 concentration in the cycle. The application of EGR also reduces the emissions of nitrogen oxide because both the oxygen and nitrogen concentrations are decreased in the cycle [
11]. The effects of EGR on full load and partial load operation performances of GTs were studied by Joe Hachem et al. [
4]. By using four distinct techniques, Hailong Li et al. [
10] assessed the impact of raising the CO
2 content in the exhaust gas of GT-based power plants. According to the research, the combined cycle with EGR had the highest electrical efficiency and could change the CO
2 molar fraction over the widest range, from 3.8 mol% to at least 10 mol%. In order to explore strategies for increasing efficiency, Pan et al. [
12] assessed the effectiveness and effects of a post-combustion CO
2 capture (PCC) on the natural gas combined cycle (NGCC). The findings demonstrated that when the EGR ratio grew, the stripper’s energy consumption fell because EGR reduced the mass flow rate of exhaust gas and increased
, which reduced the stripper’s need for steam in a PCC. The upper bound of EGR ratio was 33% due to the restriction of the minimum oxygen concentration. The impact of a carbon capture procedure on the NGCC was investigated by Woo-Sung Lee et al. [
13]. As a consequence, 533 MW of net electricity was produced using an NGCC with a 90% CO
2 recovery rate. The total cost of CO
2 collection was around 46.5 USD/ton. Carapellucci et al. [
14] investigated the functioning of NGCC integrated with MCFC using EGR without CO
2 capture and with CO
2 capture. The results showed that the addition of an MCFC fed by GT exhaust gases markedly increases the rated plant capacity.
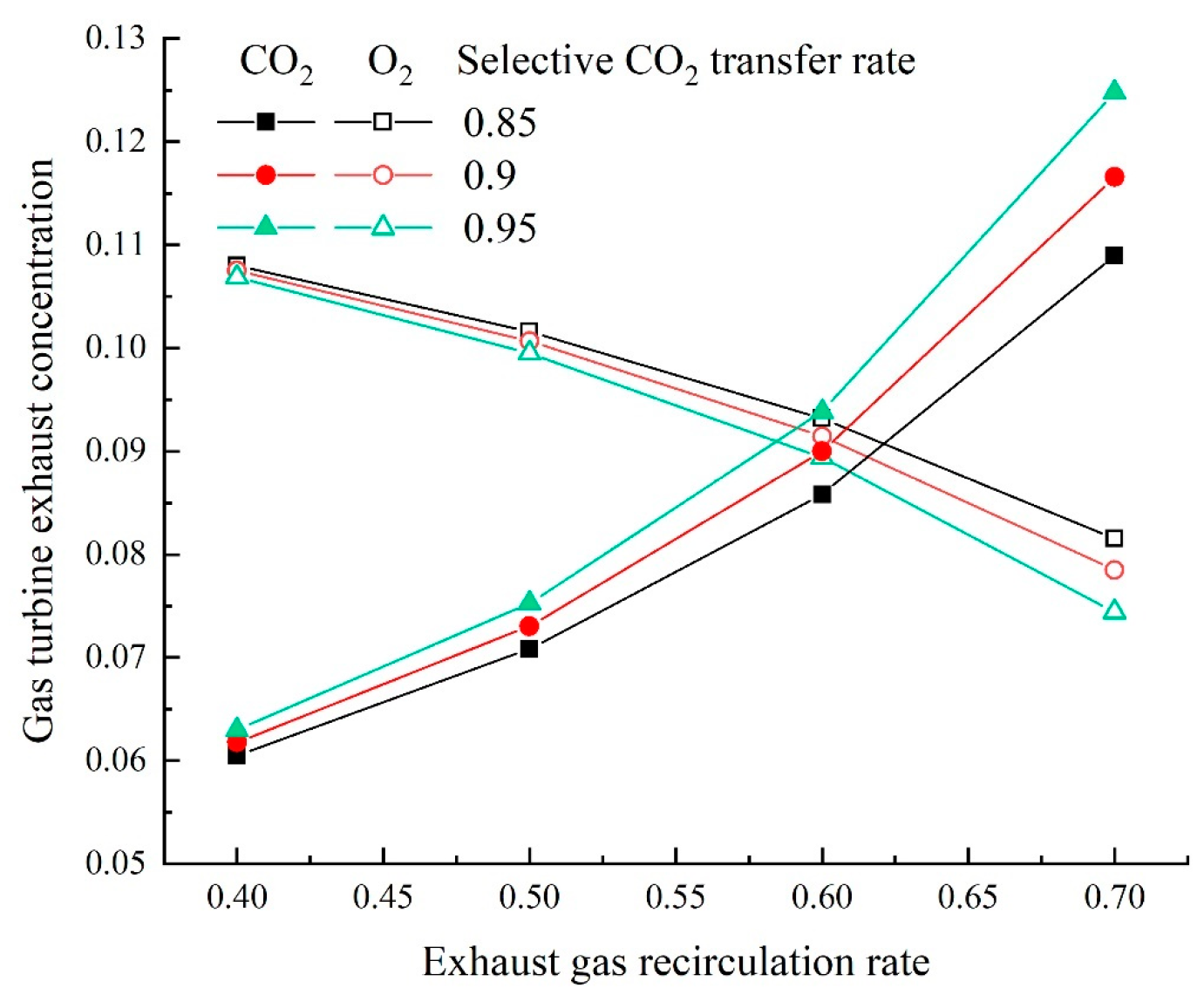
The term “selective exhaust gas recirculation” (SEGR) refers to a different method of recycling CO
2 from gas turbine exhaust gas with membranes to increase the
in the cycle. Zhao et al. [
15] used multi-stage membrane systems to capture CO
2 from coal-fired power plants. The results showed that CO
2 purity of 95 mol% and 90% CO
2 capture rate could be achieved with the SEGR method. Merkel et al. [
16] used membranes to capture CO
2 in the post-combustion power plant. The results showed that processes using a vacuum at the permeate side required less energy than processes using the compression of the feed gas. The CO
2 generated by the integrated gasification combined cycle (IGCC) power plants was captured by Merkel et al. [
17] using the H
2-selective and CO
2-selective membranes. The results showed that, compared to cold absorption, 60% of the capital cost and 50% of the energy consumption could be reduced. The impact of the sweep gas on the membrane area and the degree of CO
2 separation was studied by Franz J et al. [
18]. The findings demonstrated that for a 600 MW reference power plant, the technological approach utilizing sweep gas allowed for an efficiency reduction of 3.8 percentage points with a 70% CO
2 capture. The membrane techniques were employed by Richard et al. [
5] to remove CO
2 from the gas turbine combined cycle power plants. The results revealed that the SEGR method made CO
2 capture easier, and the lowest capture cost occurred with CO
2 capture rates of 60–70%. Herraiz L et al. [
19] used SEGR and amine-based chemical absorption technology to capture CO
2 of the natural gas-fired combined cycle plants. The results showed that the SEGR increased
in gas turbine flue gas to 13–14 vol%. Gatti M et al. [
20] presented the preliminary technical and economic assessment of four alternative technologies (including MCFC and CO
2 permeable membranes) suitable for post-combustion CO
2 capture from NGCC exhaust gases. The results showed that MCFC seemed to outperform the baseline from both performance and cost; CO
2 permeable membranes are affected by the large area and high capital costs.
According to the previous description, both the EGR and SEGR can enrich CO
2 with almost no energy input. The SEGR is driven by the difference in CO
2 partial pressure between air and flue gas while employing the air stream as the sweep gas. Compressors or vacuum pumps are not needed. To move the gas streams through the membrane unit, fans or blowers are the sole energy consumption sources. However, the enrichment of
of flue gas by EGR or SEGR is limited, only 15–20 vol% [
5]. With the combination of the EGR or SEGR with MCFC, the
of flue gas will further increase, which encourages CO
2 collection while using less energy. There is no research on the combination of SEGR with MCFC currently.
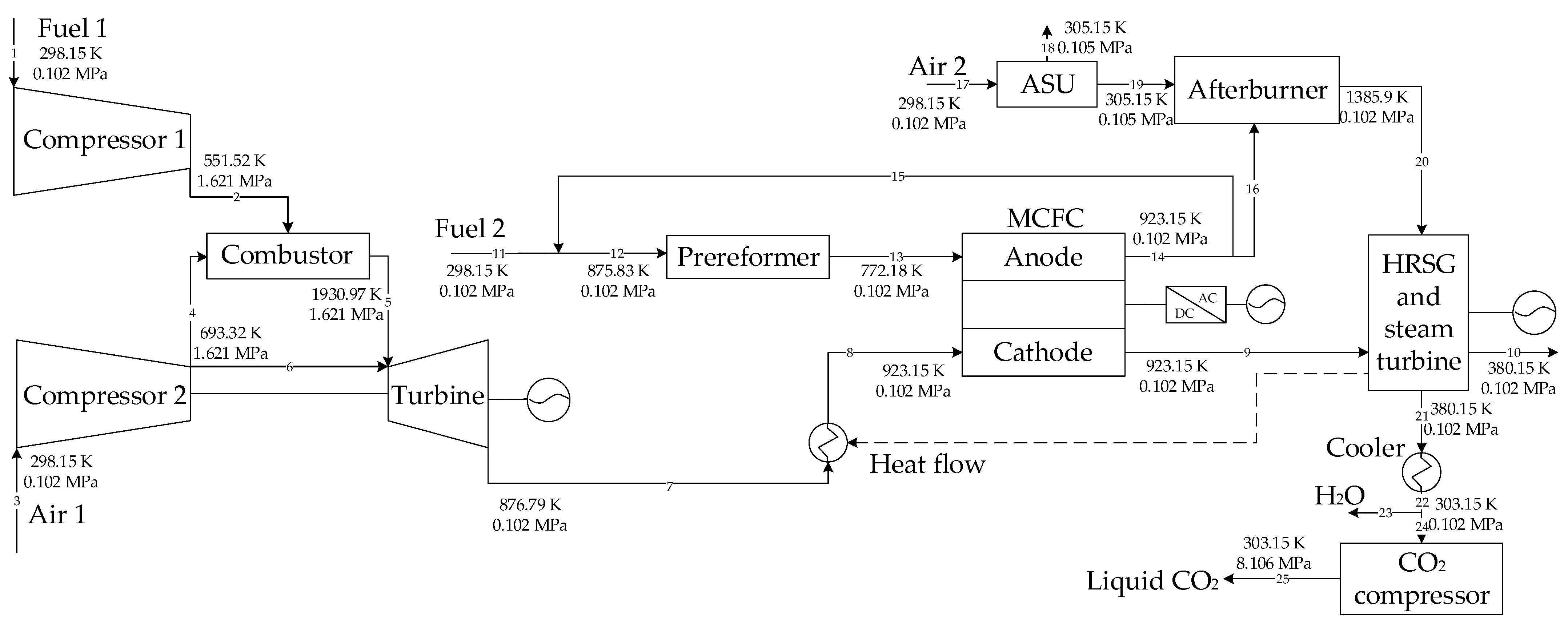
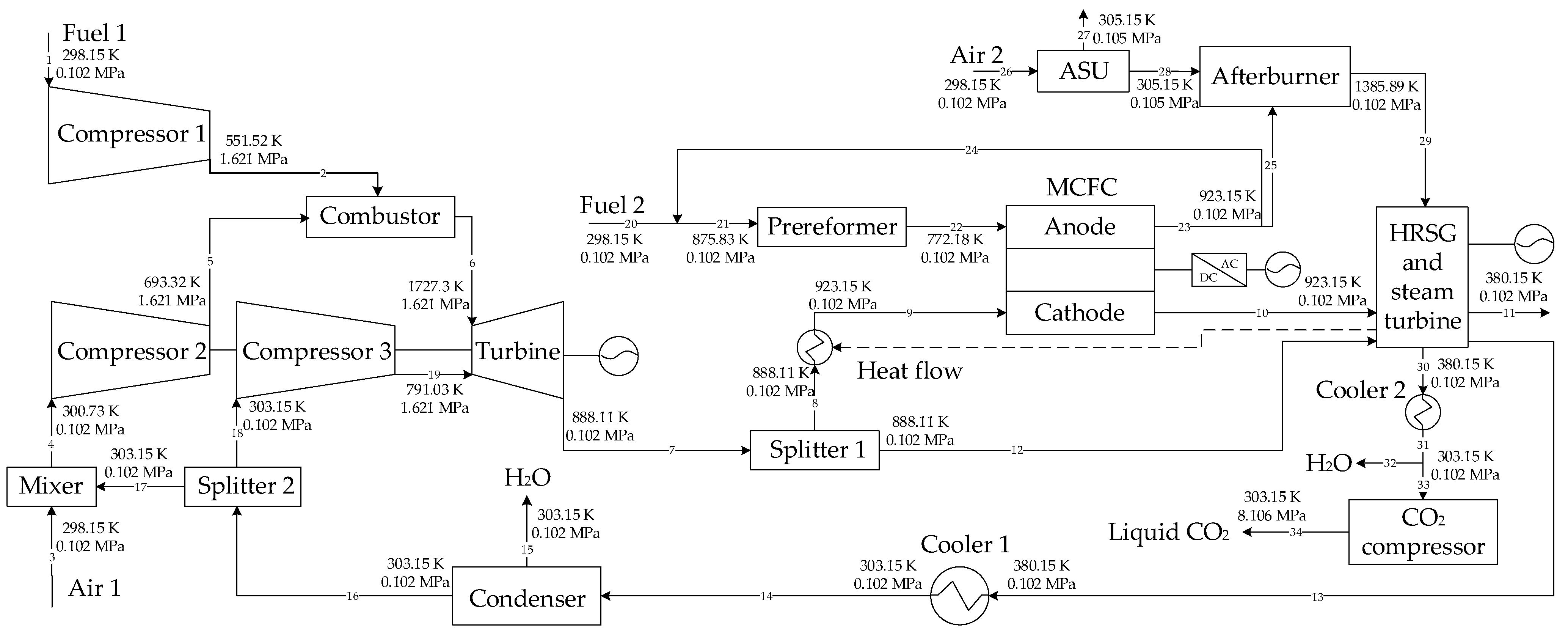
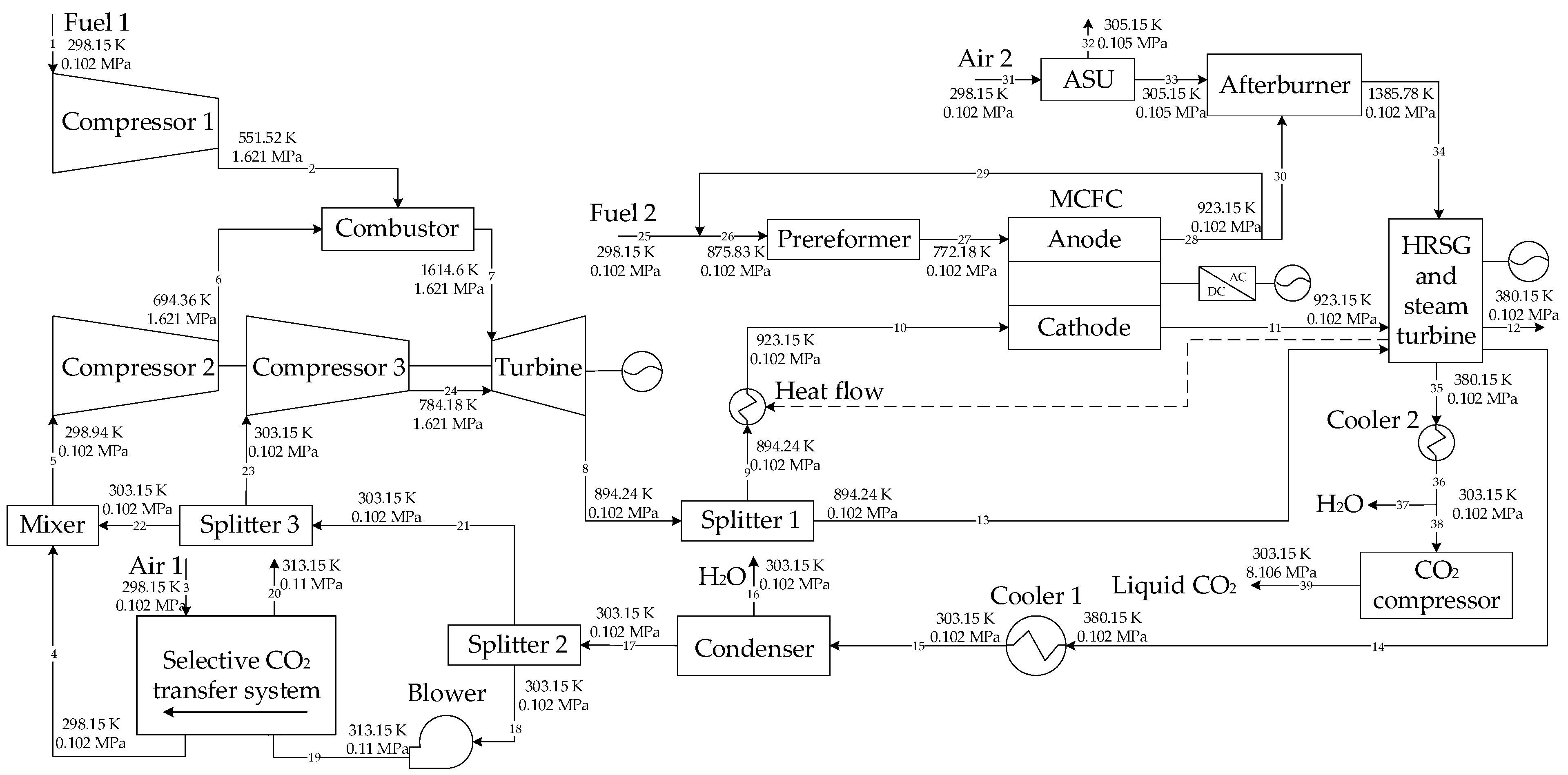
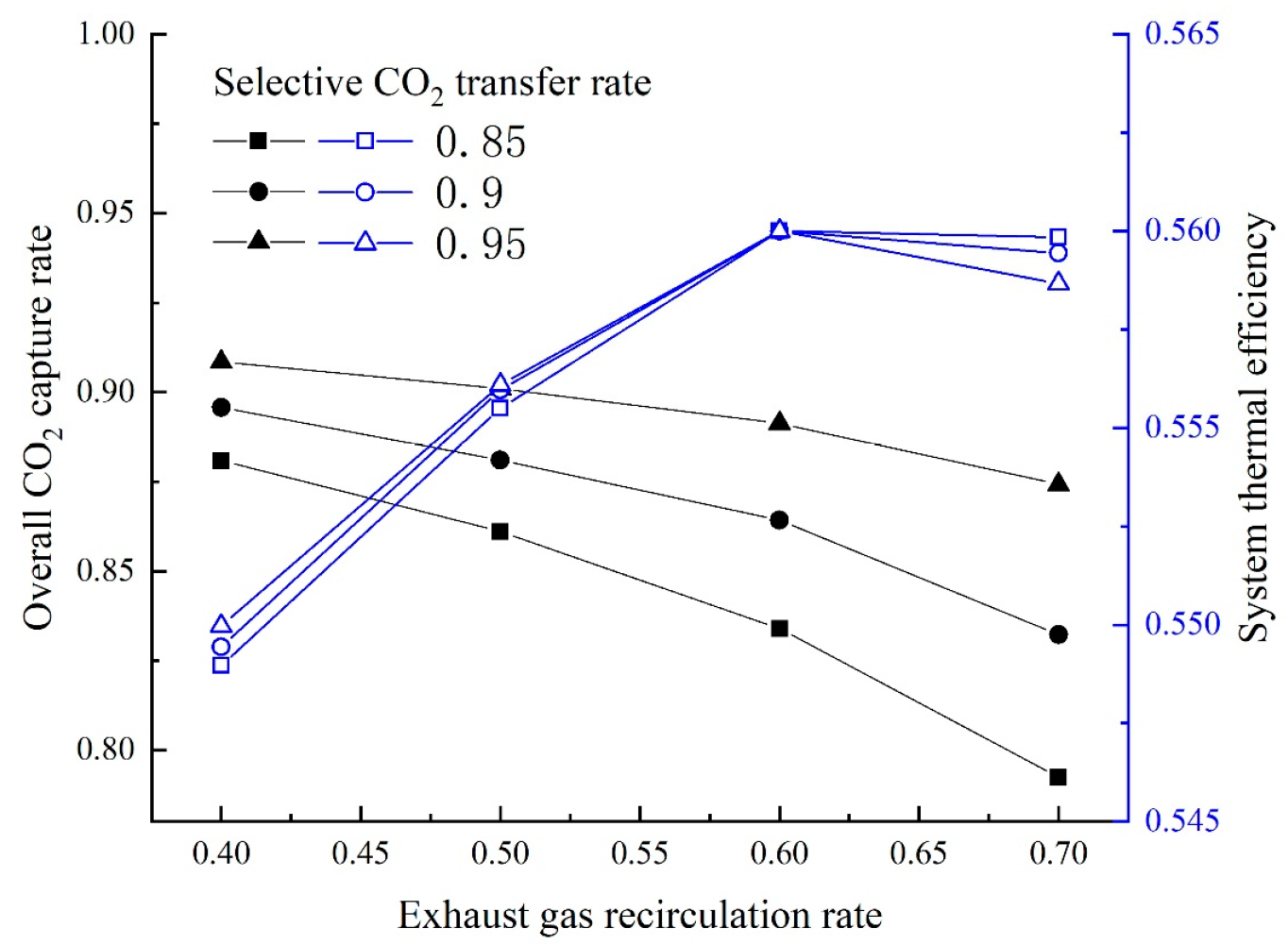
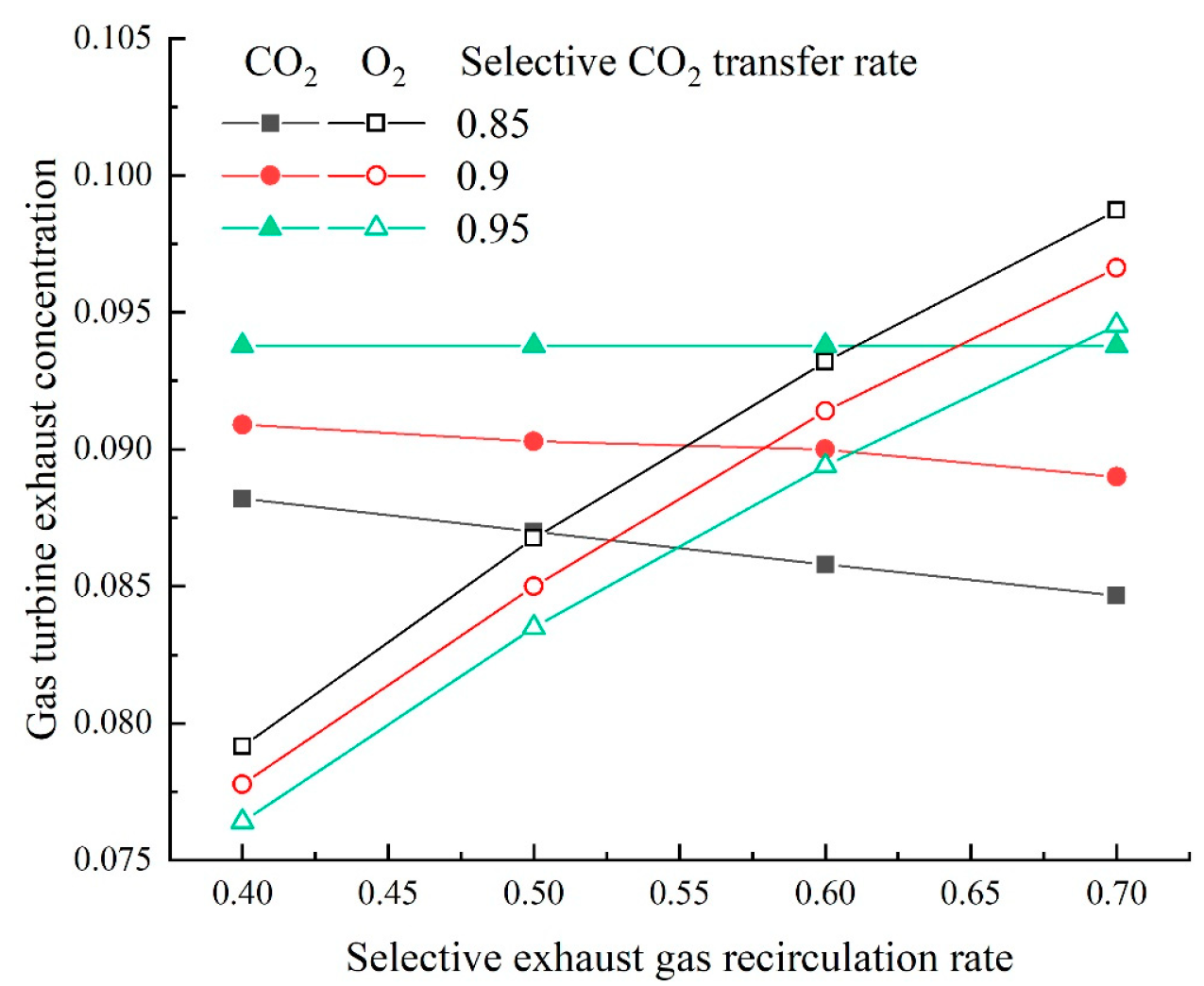
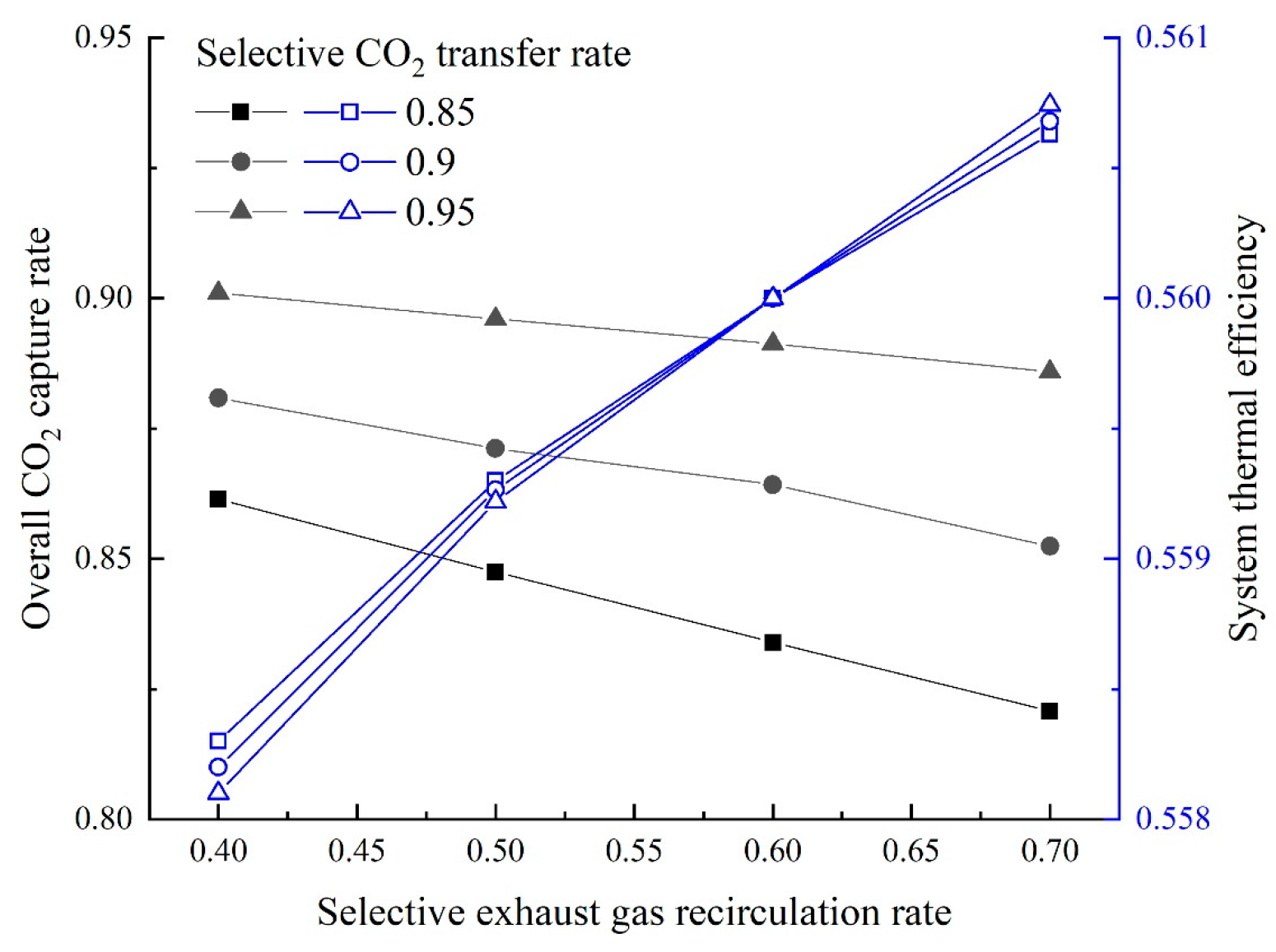
On the base of the above research, two GSCC systems integrated with MCFC, EGR or SEGR and CO2 capture are proposed in this paper. In the first system, EGR increases the of the GT exhaust gas; in the second system, SEGR and EGR increase the of the GT exhaust gas. The thermal and economic performances of different systems are analyzed and compared. On the thermal efficiency and financial performance of the new systems, the impacts of the EGR/SEGR ratio and the CO2 collection rate are investigated.
3. System Modeling
The Aspen Plus software was used to build the system models.
Table 1 shows the parameters of the new systems, while
Table 2 lists the parameters of the MCFC. Some assumptions are as follows [
21]:
Steady-state conditions;
The MCFC is thermally insulated, and there is no entropy flow to the outside environment;
The permeability of the membranes is constant, and no coupling effect is taken into account;
The system’s interactions with potential or kinetic energy are disregarded;
The assumption is that all gases are perfect incompressible gases.
Pure CH4 is given to the MCFC anode as the electrochemical reaction fuel to ensure that the exhaust gas from the afterburner includes only CO2 and H2O. The MCFC is simulated with a Fortran code. The main reaction equations are as follows:
The ideal reversible voltage,
(V), can be calculated as follows [
24]:
where
represents the Gibbs free energy (kJ/kg), n indicates the number of electrons that the H
2 molecule emitted, and
is the species i’s partial pressure (MPa).
The activation loss can be calculated as follows [
24,
25]:
where
represents the activation voltage loss (V),
j represents the current density (A/m
2),
is the exchange current density (A/m
2), and
is the standard exchange current density (A/m
2).
The ohmic loss can be calculated as follows [
26]:
where
is the ohmic voltage loss (V),
is the ohmic polarization cell resistance (
),
is the thickness (mm), and
is the electrical conductivity (S/m
−1).
The gas transport model in porous media is used in Equations (17)–(21), where it is used to compute the partial pressures of gas at the three-phase boundaries (
). The concentration loss can be calculated as follows [
26]:
where
is the concentration voltage loss (V),
represents the partial pressure of the species
i at the three-phase boundary (MPa), and
is the effective diffusivity (m
2/s).
The actual MCFC voltage can be calculated as follows:
where
is the cell voltage (V).
These are the formulas for calculating the MCFC power output,
(W):
where
represents the cell active area (m
2).
The net power output can be calculated as follows:
where the efficiency of converting direct current into the alternative current is known as
.
The following formula may be used to calculate the MCFC’s thermal efficiency,
:
where
represents the fuel mass flow after the pre-reformer (kg/s),
represents the H
2 mass fraction of the fuel mass flow after the pre-reformer, and
represents the low heat value of H
2 (kJ/kg).
The selective CO
2 transfer system is set as counter-current. The selective CO
2 transfer system is simulated with the Aspen Custom Modeler. These are the formulas for calculating a species
i’s gas permeance [
18]:
where
is the permeability of the species
i (kmol/(m
2s·MPa)),
represents the gas permeance of species i for a segment of area (kmol/s), A represents the area (m
2),
represents the partial pressure of the species
i at the feed side (MPa), and
represents the partial pressure of species
i at the permeate side (MPa).
The fuel utilization rate of MCFC can be calculated as follows:
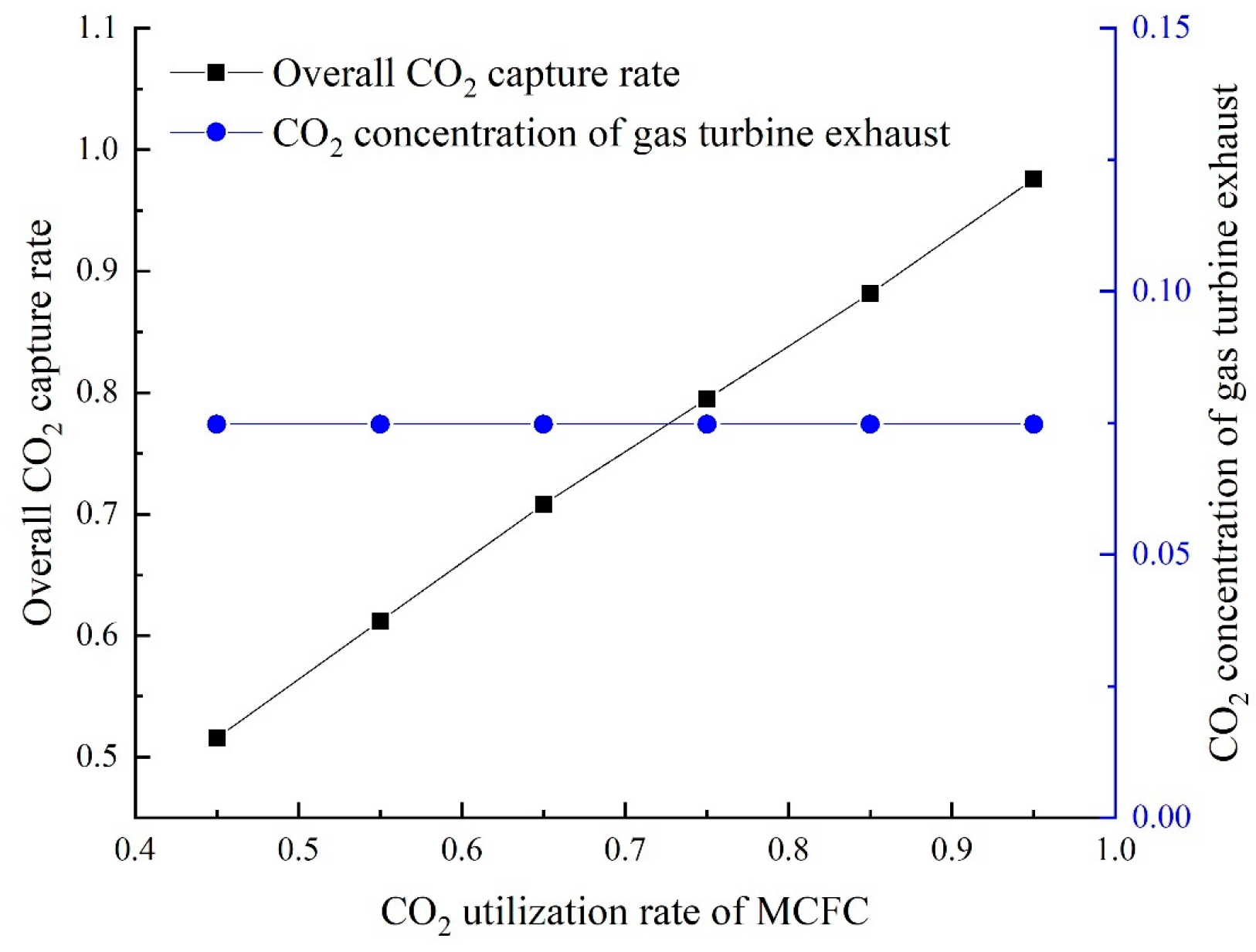
The CO
2 utilization rate of MCFC can be calculated as follows:
The overall CO
2 capture rate can be calculated as follows:
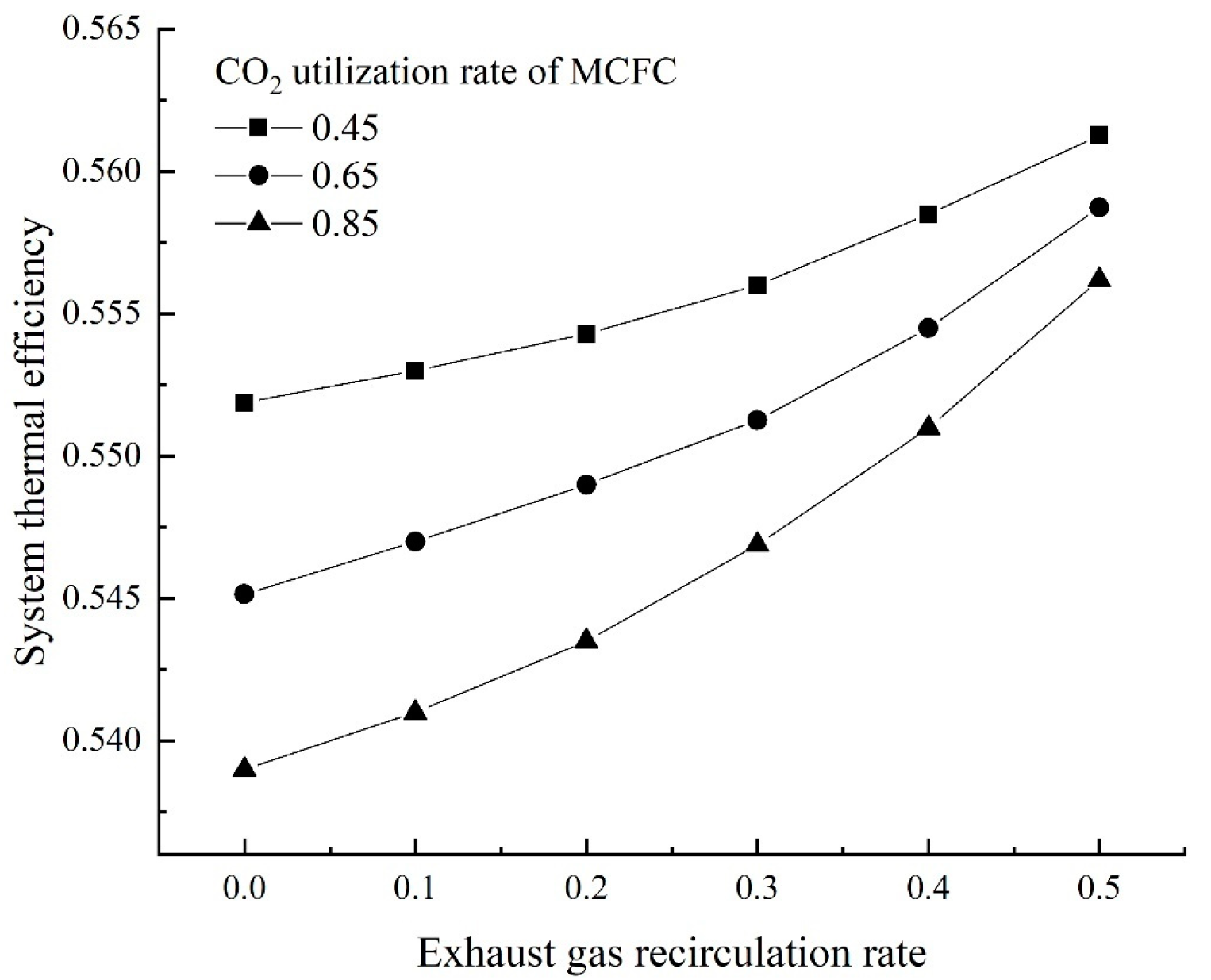
The overall system thermal efficiency can be calculated as follows:
6. Economic and Environment Performance Evaluation
This section compares the environmental and economic performances of new systems to those of the reference system. The cost of energy (COE) and the cost of CO
2 avoided (CCA) are the main economic factors considered when comparing different CO
2 collection techniques. The COE is calculated with the IEA methodology [
29,
30], which sets the net present value (NPV) of the power plant to zero. This can be achieved by varying the per kWh price until the revenues balance all the expenses over the whole lifetime of the power plant. The CCA, USD/
, is described as follows:
Table 6 displays a few significant presumptions for the COE evaluation.
Table 7 presents the comparing findings. The price per square foot for MCFC is fixed at 1891 USD/m
2 [
31].
The COE values of the new systems are substantially higher when compared to those of other systems, as shown in
Table 7. Compared with the CCA of the traditional mono-ethanol ammine (MEA) method for CO
2 capture [
34], the CCA values for the new systems in this paper are higher. Due to the high expense of MCFC, this viewpoint is short-term. The cost of MCFC will decrease as MCFC technology develops [
35].
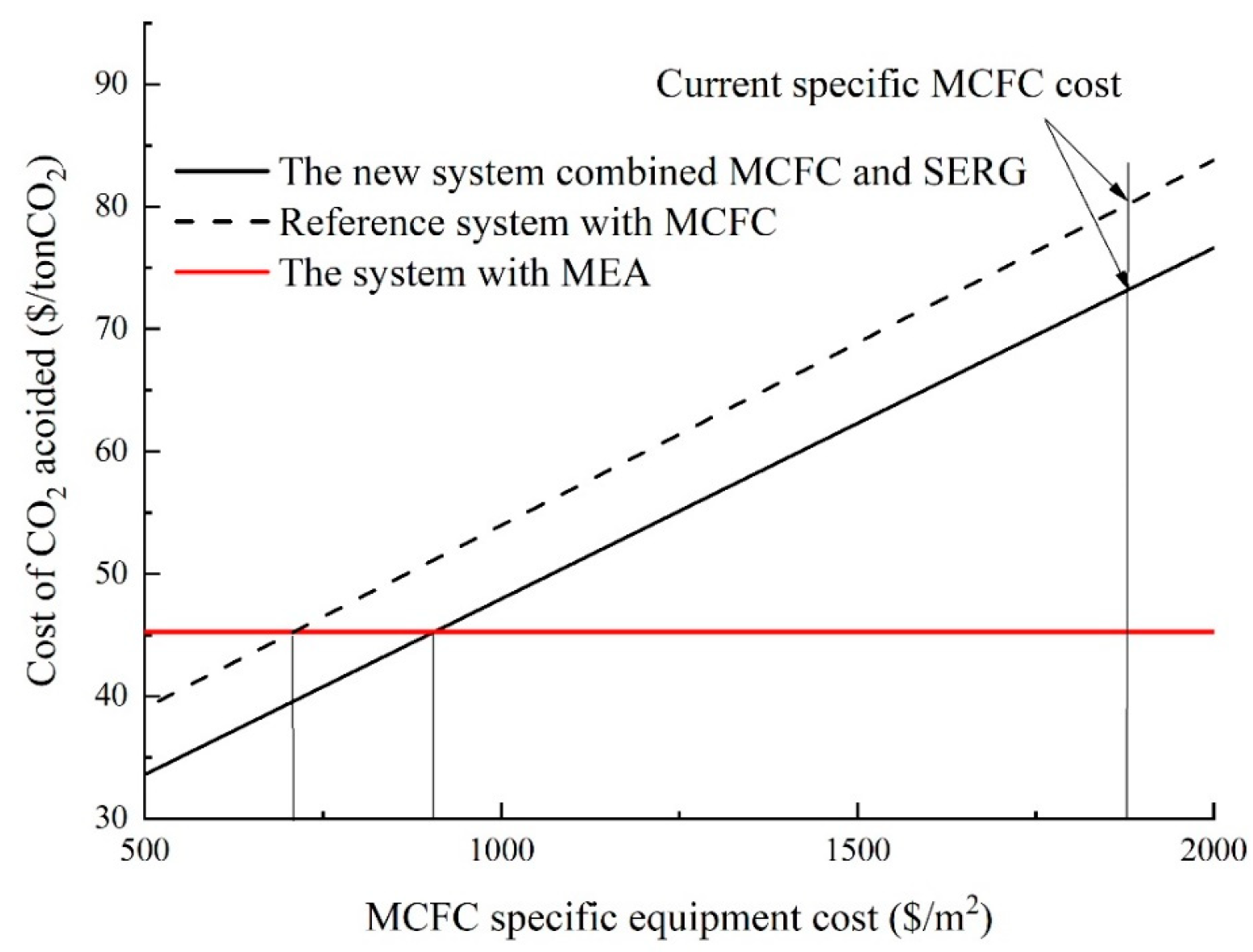
According to
Figure 13, the cost of CO
2 avoided by the system with CO
2 capture by MCFC will be equal to the CCA of the GSCC system with CO
2 capture by the MEA technique if the MCFC cost is reduced from the present 1891 USD/m
2 to 707.6 USD/m
2. The cost of CO
2 saved in Case 2 will be equal to the CCA of the system with the MEA technique for CO
2 capture if the MCFC cost is reduced from the present 1891 USD/m
2 to 904.4 USD/m
2 [
33].
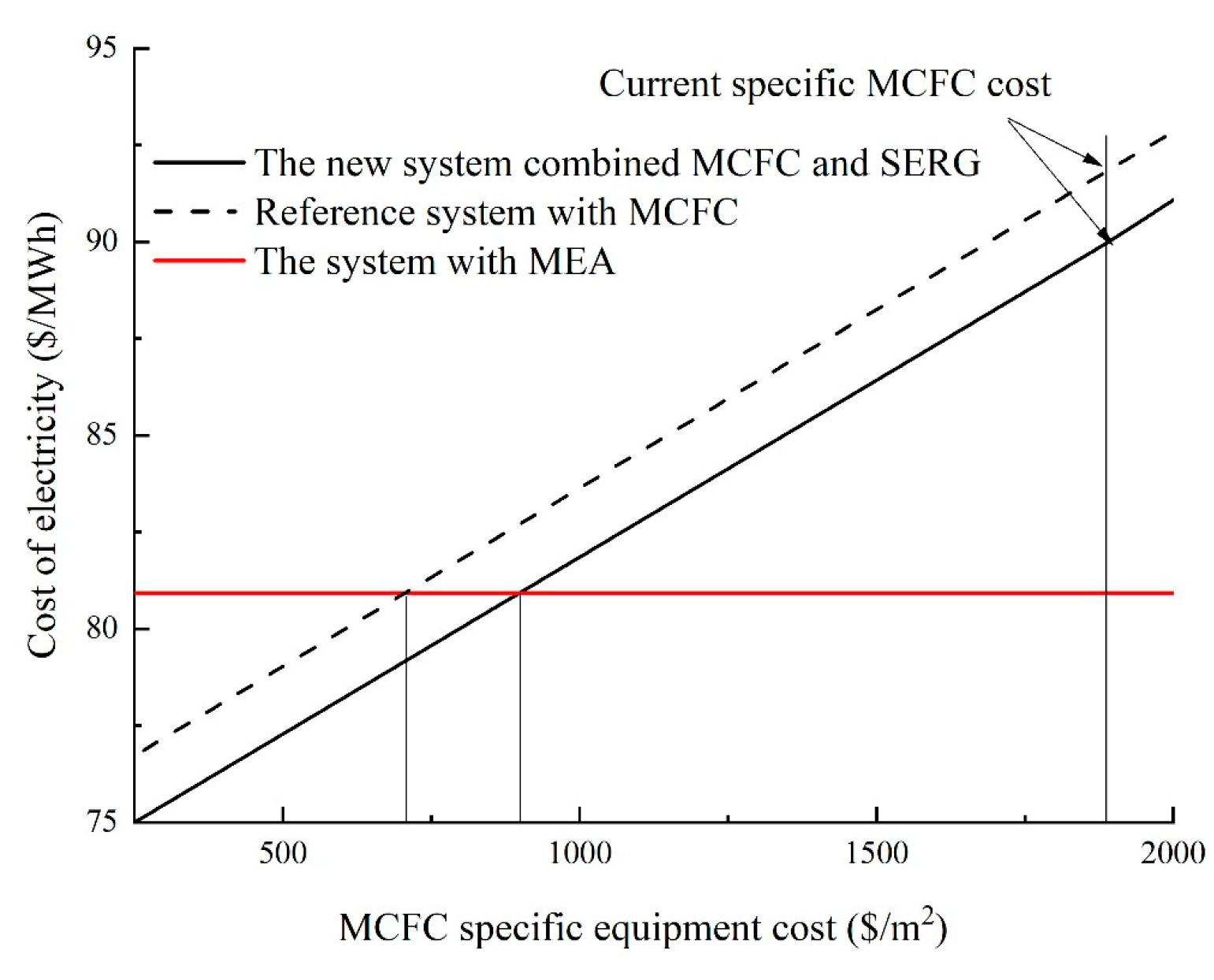
Figure 14 demonstrates that the COE for the system with MCFC-based CO
2 capture will be equal to that of the system using the MEA technique for CO
2 capture when the MCFC cost is reduced from the present 1891 USD/m
2 to 705.7 USD/m
2. When the MCFC cost is reduced from the current 1891 USD/m
2 to 897.6 USD/m
2, the COE for the system with MCFC and SEGR for CO
2 collection will be equivalent to that of the system with the MEA method for CO
2 capture [
33]. As a result, the cost of the MCFC-specific equipment strongly influences whether the new system’s COE or CCA will drop.