Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables
Abstract
1. Introduction
2. Feedstock for Anaerobic Digestion
2.1. Agricultural Wastes
2.1.1. Food Waste
2.1.2. Animal (Livestock) and Poultry Manure
2.1.3. Slaughterhouse Waste
2.1.4. Bioenergy Crops/Crop Residues
2.2. Municipal Solid Waste
2.3. Industrial Waste
2.4. Aquatic Biomass
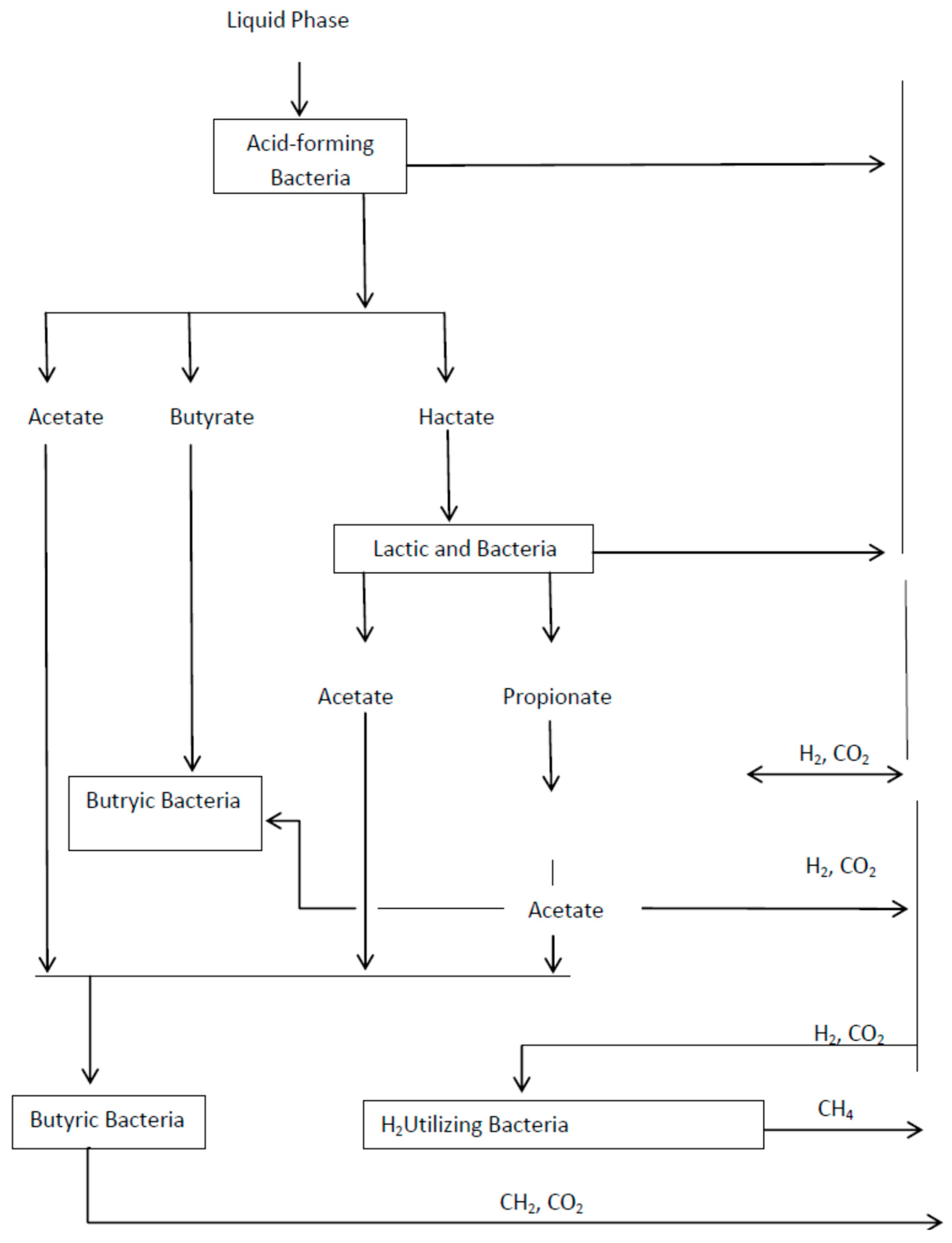
3. Process and Mechanism of AD (Biomethanation)
4. Anaerobic Digestion Technology
4.1. Classification of the AD Process Technology
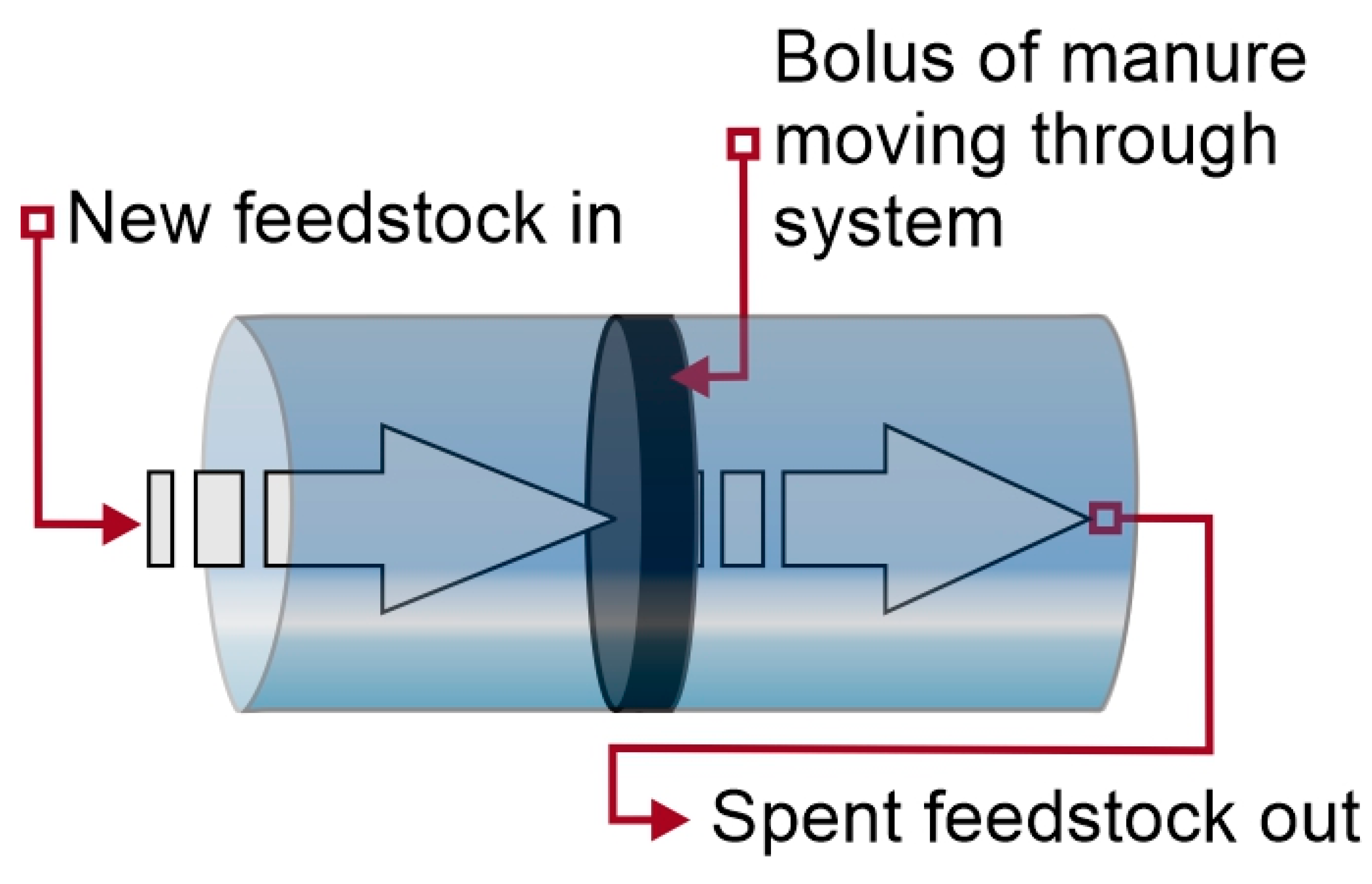
4.1.1. Dry and Wet AD Process Technology
4.1.2. Batch and Continuous AD Process Technology
4.1.3. Mesophilic and Thermophilic AD Process Technology
4.1.4. Single and Multistage AD Process Technology
4.2. Types of Anaerobic Digesters
4.2.1. Covered Anaerobic Lagoons
4.2.2. Plug-Flow Digester
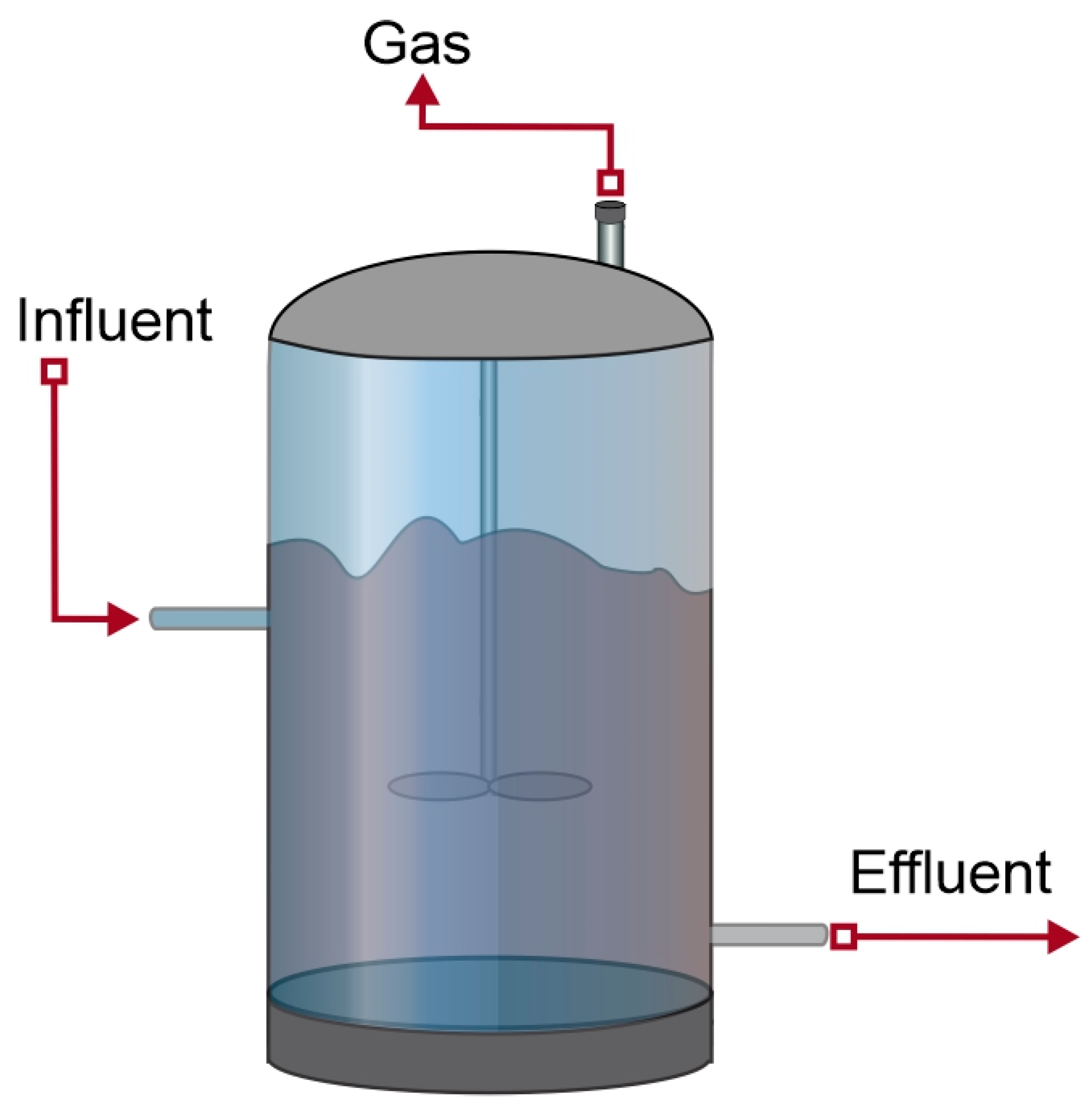
4.2.3. Total-Mixed Digester
4.2.4. Fixed-Dome Digester
4.2.5. Floating-Drum Digester
4.2.6. Balloon Digester
5. The Physiochemical and Biological Variables Affecting AD Performance
5.1. Feedstock Particle Size
5.2. Total Solid Content
5.3. Carbon/Nitrogen (C/N) Ratio
5.4. Nutrients and Metals (Light and Heavy)
5.5. Presence of Inhibitory Compounds
5.5.1. Volatile Fatty Acids
5.5.2. Ammonia
5.6. pH
5.7. Temperature
5.8. Regular Agitation/Stirring
5.9. Hydraulic Retention Time
5.10. Organic Loading Rate
5.11. Pressure
6. Kinetics Modelling of AD
7. Knowledge Gap and Future Research
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozhiarasi, V.; Natarajan, T.S. Slaughterhouse and poultry wastes: Management practices, feedstocks for renewable energy production, and recovery of value added products. Biomass Convers. Biorefinery 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Meng, Y.; Li, S.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, A.; Jaffar, M.; Li, X. Evaluating biomethane production from anaerobic mono- and co-digestion of food waste and floatable oil (FO) skimmed from food waste. Bioresour. Technol. 2015, 185, 7–13. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). AR5 Climate Change 2014: Mitigation of Climate Change; Intergovernmental Panel on Climate Change (IPCC): New York, NY, USA, 2014; Available online: https://www.ipcc.ch/report/ar5/wg3/ (accessed on 10 March 2023).
- Agbede, O.O.; Aworanti, O.A.; Osuolale, F.N.; Adebayo, A.O.; Ogunleye, O.O.; Agarry, S.E.; Babatunde, K.A. Anaerobic Conversion of Biodegradable Municipal Solid Waste to Biogas: A Review. LAUTECH J. Civ. Environ. Stud. 2019, 3, 27–43. [Google Scholar] [CrossRef]
- Biodun, M.B.; Fayomi, O.S.I.; Okeniyi, J.O. The Possibility of Biogas production in Nigeria from Organic Waste Material: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012166. [Google Scholar] [CrossRef]
- Szilágyi, Á.; Bodor, A.; Tolvai, N.; Kovács, K.L.; Bodai, L.; Wirth, R.; Bagi, Z.; Szepesi, Á.; Markó, V.; Kakuk, B.; et al. A comparative analysis of biogas production from tomato bio-waste in mesophilic batch and continuous anaerobic digestion systems. PLoS ONE 2021, 16, e0248654. [Google Scholar] [CrossRef]
- Razzaq, L.; Mujtaba, M.A.; Soudagar, M.E.M.; Ahmed, W.; Fayaz, H.; Bashir, S.; Fattah, I.M.R.; Ong, H.C.; Shahapurkar, K.; Afzal, A.; et al. Engine performance and emission characteristics of palm biodiesel blends with graphene oxide nanoplatelets and dimethyl carbonate additives. J. Environ. Manag. 2021, 282, 111917. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Aworanti, O.A.; Agarry, S.E.; Aremu, M.O. Enhancement of animal waste biomethanation using fruit waste as co-substrate and chicken rumen as inoculums. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1653–1660. [Google Scholar] [CrossRef]
- Agbede, O.O.; Aworanti, O.A.; Ogunleye, O.O.; Agarry, S.E.; Babatunde, K.A.; Alagbe, S.O. Design and Fabrication of Electric Jacketed Anaerobic Digester. J. Pet. Environ. Biotechnol. 2020, 11, 403. [Google Scholar]
- Farooq, M.; Chaudhry, I.A.; Hussain, S.; Ramzan, N.; Ahmed, M. Biogas upgradation for power generation applications in Pakistan. J. Qual. Technol. Manag. 2012, 8, 107–118. [Google Scholar]
- Vögeli, Y.; Lohri, C.R.; Gallardo, A.; Diener, S.; Zurbrügg, C. Anaerobic Digestion of Biowaste in Developing Countries: Practical Information and Case Studies; Binkert Buag AG: Laufenburg, Switzerland, 2014; Available online: https://www.eawag.ch/fileadmin/Domain1/Abteilungen/sandec/publikationen/SWM/Anaerobic_Digestion/biowaste.pdf (accessed on 10 March 2023).
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and co-digestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Agarry, S.E.; Ogunleye, O.O. Biomethanization of Cattle Manure, Pig Manure and Poultry Manure Mixture in Co-digestion with Waste of Pineapple Fruit and Content of Chicken-Gizzard–Part I: Kinetic and Thermodynamic Modelling Studies. Open Biotechnol. J. 2017, 11, 36–53. [Google Scholar] [CrossRef]
- Patel, V.; Pandit, S.; Chandrasekhar, K. Basics of Methanogenesis in Anaerobic Digester. In Microbial Applications Vol.2: Biomedicine, Agriculture and Industry; Kalia, V.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 291–314. [Google Scholar]
- Lindkvist, E.; Johansson, M.T.; Rosenqvist, J. Methodology for Analysing Energy Demand in Biogas Production Plants—A Comparative Study of Two Biogas Plants. Energies 2017, 10, 1822. [Google Scholar] [CrossRef]
- Nnabuchi, M.N.; Akubuko, F.O.; Augustine, C.; Ugwu, G.Z. Assessment of the effect of co-digestion of chicken dropping and cow dung on biogas generation. Int. Res. J. Eng. Sci. Technol. Innov. (IRJESTI) 2012, 1, 238–243. [Google Scholar]
- Aragaw, T.; Andargie, M.; Gessesse, A. Co-digestion of cattle manure with organic kitchen waste to increase biogas production using rumen fluid as inoculums. Int. J. Phys. Sci. 2013, 8, 443–450. [Google Scholar]
- Ziauddin, Z.; Rajesh, P. Production and analysis of biogas from kitchen waste. Int. Res. J. Eng. Technol. 2015, 2, 622–632. [Google Scholar]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Enhancement of biogas production by pretreatment: A review. In Proceedings of the 4th International Conference on Advances in Energy Research Indian Institute of Technology Bombay, Mumbai, India, 10–12 December 2013; pp. 309–319. [Google Scholar]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Ho, W.S.; Klemes, J.J. The Kinetics for Mathematical Modelling on the Anaerobic Digestion of Organic Waste—A Review. Chem. Eng. Trans. 2017, 61, 1669–1674. [Google Scholar] [CrossRef]
- Morales-Polo, C.; Cledera-Castro, M.D.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]
- Uddin, M.N.; Siddiki, S.Y.A.; Mofijur, M.; Djavanroodi, F.; Hazrat, M.A.; Show, P.L.; Ahmed, S.F.; Chu, Y.-M. Prospects of Bioenergy Production From Organic Waste Using Anaerobic Digestion Technology: A Mini Review. Front. Energy Res. 2021, 9, 627093. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Rasul, M.G.; Akbar, D.; Narayanan, R.; Mofijur, M. A comprehensive review of the recent development and challenges of a solar-assisted biodigester system. Sci. Total Environ. 2021, 753, 141920. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Mailier, J.; Martin, C.; Rodríguez, J.; Aceves-Lara, C.A.; Wouwer, A.V. Model selection, identification and validation in anaerobic digestion: A review. Water Res. 2011, 45, 5347–5364. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, J.; Appels, L.; Thompson, I.P.; Degrève, J.; Van Impe, J.F.; Dewil, R. Mathematical modelling of anaerobic digestion of biomass and waste: Power and limitations. Prog. Energy Combust. Sci. 2013, 39, 383–402. [Google Scholar] [CrossRef]
- Kythreotou, N.; Florides, G.; Tassou, S.A. A review of simple to scientific models for anaerobic digestion. Renew. Energy 2014, 71, 701–714. [Google Scholar] [CrossRef]
- Enitan, A.M.; Adeyemo, J.; Swalaha, F.M.; Kumari, S.; Bux, F. Optimization of biogas generation using anaerobic digestion models and computational intelligence approaches. Rev. Chem. Eng. 2017, 33, 309–335. [Google Scholar] [CrossRef]
- Ali, M.M.; Dia, N.; Bilal, B.; Ndongo, M. Theoretical models for prediction of methane production from anaerobic digestion: A critical review. Int. J. Phys. Sci. 2018, 13, 206–216. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Oranusi, U.S. Co-digestion of Food Waste and Human Excreta for Biogas Production. Br. Biotechnol. J. 2013, 3, 485–499. [Google Scholar] [CrossRef]
- Kader, F.; Baky, A.H.; Khan, M.N.H.; Chowdhury, H.A. Production of Biogas by Anaerobic Digestion of Food Waste and Process Simulation. Am. J. Mech. Eng. 2015, 3, 79–83. [Google Scholar]
- Usman, M.N.; Suleiman, M.A.; Binni, M.I. Anaerobic Digestion of Agricultural Wastes: A Potential Remedy for Energy Shortfalls in Nigeria. J. Waste Manag. Dispos. 2021, 4, 1–13. [Google Scholar]
- Zamri, M.F.M.A.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.H.; Mofijur, M.; Fattah, I.M.R.; Mahlia, T.M.I. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2021, 137, 110637. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Ahlberg-Eliasson, K.; Westerholm, M.; Isaksson, S.; Schnürer, A. Anaerobic Digestion of Animal Manure and Influence of Organic Loading Rate and Temperature on Process Performance, Microbiology, and Methane Emission From Digestates. Front. Energy Res. 2021, 9, 740314. [Google Scholar] [CrossRef]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J. Clean. Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Venkateswara Rao, P. Temperature-Phased Anaerobic Co-Digestion of Food Waste and Rice Husk Using Response Surface Methodology. In Water Resources and Environmental Engineering II; Springer: Singapore, 2019; pp. 137–146. [Google Scholar]
- Aldin, S.; Nakhla, G.; Ray, M.B. Modeling the Influence of Particulate Protein Size on Hydrolysis in Anaerobic Digestion. Ind. Eng. Chem. Res. 2011, 50, 10843–10849. [Google Scholar] [CrossRef]
- Moestedt, J.; Nilsson Påledal, S.; Schnürer, A. The effect of substrate and operational parameters on the abundance of sulphate-reducing bacteria in industrial anaerobic biogas digesters. Bioresour. Technol. 2013, 132, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Enhanced anaerobic digestion of piggery wastewater by ammonia stripping: Effects of alkali types. J. Hazard. Mater. 2010, 182, 536–543. [Google Scholar] [CrossRef]
- Karlsson, A.; Ejlertsson, J. Addition of HCl as a means to improve biogas production from protein-rich food industry waste. Biochem. Eng. J. 2012, 61, 43–48. [Google Scholar] [CrossRef]
- Tan, C.; Saritpongteeraka, K.; Kungsanant, S.; Charnnok, B.; Chaiprapat, S. Low temperature hydrothermal treatment of palm fiber fuel for simultaneous potassium removal, enhanced oil recovery and biogas production. Fuel 2018, 234, 1055–1063. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Tian, Y.; Wu, J.; Zhou, C.; Liu, B.; Zhao, L.; Fan, J.; Ren, N. Improvement of biogas production in plug flow reactor using biogas slurry pretreated cornstalk. Bioresour. Technol. Rep. 2020, 9, 100378. [Google Scholar] [CrossRef]
- Teghammar, A. Biogas Production from Lignocelluloses: Pretreatment, Substrate Characterization, Co-Digestion, and Economic Evaluation; Chalmers University of Technology: Göteborg, Sweden, 2013. [Google Scholar]
- Manikam, N. Biogas Production from Municipal Waste; Universiti Tunku Abdul Rahman: Petaling Jaya, Malaysia, 2012. [Google Scholar]
- The Biogas. Biogas Composition. Available online: https://www.biogas-renewable-energy.info/biogas_composition.html (accessed on 14 March 2023).
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Achinas, S.; Krooneman, J.; Euverink, G.J.W. Enhanced Biogas Production from the Anaerobic Batch Treatment of Banana Peels. Engineering 2019, 5, 970–978. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Investigation of the methane potential of horse manure. Agric. Eng. Int. CIGR J. 2013, 15, 161–172. [Google Scholar]
- Zhang, W.; Lang, Q.; Pan, Z.; Jiang, Y.; Liebetrau, J.; Nelles, M.; Dong, H.; Dong, R. Performance evaluation of a novel anaerobic digestion operation process for treating high-solids content chicken manure: Effect of reduction of the hydraulic retention time at a constant organic loading rate. Waste Manag. 2017, 64, 340–347. [Google Scholar] [CrossRef]
- Mukumba, P.; Makaka, G.; Mamphweli, S. Anaerobic digestion of donkey dung for biogas production. S. Afr. J. Sci. 2016, 112, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Silwadi, M.; Mousa, H.; Al-Hajji, B.Y.; Al-Wahaibi, S.S.; Al-Harrasi, Z.Z. Enhancing biogas production by anaerobic digestion of animal manure. Int. J. Green Energy 2023, 20, 257–264. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Ma, R.; Kumar, V.; Wah Tong, Y.; He, Y.; Mao, F. Mesophilic and thermophilic anaerobic digestion of animal manure: Integrated insights from biogas productivity, microbial viability and enzymatic activity. Fuel 2022, 320, 123990. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Velmurugan, B.; Ramanujam, R.A. Anaerobic Digestion of Vegetable Wastes for Biogas Production in a Fed-Batch Reactor. Int. J. Emerg. Sci. 2011, 1, 478–486. [Google Scholar]
- Goswami, R.; Chattopadhyay, P.; Shome, A.; Banerjee, S.N.; Chakraborty, A.K.; Mathew, A.K.; Chaudhury, S. An overview of physico-chemical mechanisms of biogas production by microbial communities: A step towards sustainable waste management. 3Biotech 2016, 6, 72. [Google Scholar] [CrossRef]
- Phetyim, N.; Wanthong, T.; Kannika, P.; Supngam, A. Biogas Production from Vegetable Waste by Using Dog and Cattle Manure. Energy Procedia 2015, 79, 436–441. [Google Scholar] [CrossRef]
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Ahlberg-Eliasson, K. Swedish Farm-Scale Biogas Production—Substrates and Operating Parameters; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018. [Google Scholar]
- Orlando, M.-Q.; Borja, V.-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Li, Y.; Achinas, S.; Zhao, J.; Geurkink, B.; Krooneman, J.; Willem Euverink, G.J. Co-digestion of cow and sheep manure: Performance evaluation and relative microbial activity. Renew. Energy 2020, 153, 553–563. [Google Scholar] [CrossRef]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. The Influence of Total Solid Contents on Biogas Yield from Cattle Manure Using Rumen Fluid Inoculum. Energy Res. J. 2010, 1, 6–11. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S. Co-Digestion of MSW, With Cow Manure & Poultry Waste: An innovative approach for Biogas Production. Int. J. Recent Dev. Eng. Technol. 2015, 4, 45–48. [Google Scholar]
- Abubakar, B.S.U.I.; Ismail, N. Anaerobic digestion of cow dung for biogas production. ARPN J. Eng. Appl. Sci. 2012, 7, 169–172. [Google Scholar]
- Mamhobu-Amadi, W.C.; Kinigoma, B.S.; Momoh, Y.L.O.; Oji, A.A. Abattoir operations and waste Management Options: A review. Int. J. Adv. Eng. Res. Sci. (IJAERS) 2019, 6, 226–231. [Google Scholar] [CrossRef]
- Selormey, G.K.; Barnes, B.; Kemausuor, F.; Darkwah, L. A review of anaerobic digestion of slaughterhouse waste: Effect of selected operational and environmental parameters on anaerobic biodegradability. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1073–1086. [Google Scholar] [CrossRef]
- Yoon, Y.-M.; Kim, S.-H.; Oh, S.-Y.; Kim, C.-H. Potential of anaerobic digestion for material recovery and energy production in waste biomass from a poultry slaughterhouse. Waste Manag. 2014, 34, 204–209. [Google Scholar] [CrossRef]
- Böjti, T.; Kovács, K.L.; Kakuk, B.; Wirth, R.; Rákhely, G.; Bagi, Z. Pretreatment of poultry manure for efficient biogas production as monosubstrate or co-fermentation with maize silage and corn stover. Anaerobe 2017, 46, 138–145. [Google Scholar] [CrossRef]
- Borowski, S.; Boniecki, P.; Kubacki, P.; Czyżowska, A. Food waste co-digestion with slaughterhouse waste and sewage sludge: Digestate conditioning and supernatant quality. Waste Manag. 2018, 74, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.Y.; Harvey, J.T.; Kim, J.; Lee, C. Improving Biomethanation of Chicken Manure by Co-Digestion with Ethanol Plant Effluent. Int. J. Environ. Res. Public Health 2019, 16, 5023. [Google Scholar] [CrossRef] [PubMed]
- Reyes, I.P.; Díaz, J.P.; Horváth, I.S. Anaerobic Biodegradation of Solid Substrates from Agroindustrial Activities—Slaughterhouse Wastes and Agrowastes. In Biodegradation and Bioremediation of Polluted Systems; Rolando, C., Francisca, R., Lorena, S., Eds.; IntechOpen: Rijeka, Croatia, 2015; pp. 31–64. [Google Scholar]
- Wang, F.; Pei, M.; Qiu, L.; Yao, Y.; Zhang, C.; Qiang, H. Performance of Anaerobic Digestion of Chicken Manure Under Gradually Elevated Organic Loading Rates. Int. J. Environ. Res. Public Health 2019, 16, 2239. [Google Scholar] [CrossRef] [PubMed]
- Bayr, S.; Rantanen, M.; Kaparaju, P.; Rintala, J. Mesophilic and thermophilic anaerobic co-digestion of rendering plant and slaughterhouse wastes. Bioresour. Technol. 2012, 104, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Taherzadeh, M.J.; Sárvári-Horváth, I.; Lundin, M. Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: Synergistic and antagonistic interactions determined in batch digestion assays. Chem. Eng. J. 2014, 245, 89–98. [Google Scholar] [CrossRef]
- Olaoye, J.O. An analysis of the environmental impacts of energy crops in Nigeria towards environmental sustainability. In Proceedings of the Nigerian Branch of International Soil Tillage Research Organization, Ilorin, Nigeria, 21–24 February 2011; pp. 204–212. [Google Scholar]
- Lim, J.S.; Abdul Manan, Z.; Wan Alwi, S.R.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Simonyan, K.J.; Fasina, O. Biomass resources and bioenergy potentials in Nigeria. Afr. J. Agric. Res. 2013, 8, 4975–4989. [Google Scholar]
- Frigon, J.-C.; Guiot, S.R. Biomethane production from starch and lignocellulosic crops: A comparative review. Biofuels Bioprod. Biorefin. 2010, 4, 447–458. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V. A review on current status of municipal solid waste management in India. J. Environ. Sci. 2015, 37, 206–217. [Google Scholar] [CrossRef]
- Brown, D.; Shi, J.; Li, Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. 2018. Available online: https://openknowledge.worldbank.org/entities/publication/d3f9d45e-115f-559b-b14f-28552410e90a (accessed on 13 March 2023).
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of Increasing Total Solids Contents on Anaerobic Digestion of Food Waste under Mesophilic Conditions: Performance and Microbial Characteristics Analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef] [PubMed]
- Nielfa, A.; Cano, R.; Vinot, M.; Fernández, E.; Fdz-Polanco, M. Anaerobic digestion modeling of the main components of organic fraction of municipal solid waste. Process Saf. Environ. Prot. 2015, 94, 180–187. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Nguyen, T.T.; Manassa, P.; Fitzgerald, S.K.; Dawson, M.; Vierboom, S. Co-digestion of sewage sludge and crude glycerol for on-demand biogas production. Int. Biodeterior. Biodegrad. 2014, 95, 160–166. [Google Scholar] [CrossRef]
- Pitk, P.; Kaparaju, P.; Palatsi, J.; Affes, R.; Vilu, R. Co-digestion of sewage sludge and sterilized solid slaughterhouse waste: Methane production efficiency and process limitations. Bioresour. Technol. 2013, 134, 227–232. [Google Scholar] [CrossRef]
- Ratanatamskul, C.; Wattanayommanaporn, O.; Yamamoto, K. An on-site prototype two-stage anaerobic digester for co-digestion of food waste and sewage sludge for biogas production from high-rise building. Int. Biodeterior. Biodegrad. 2015, 102, 143–148. [Google Scholar] [CrossRef]
- Meyer, T.; Edwards, E.A. Anaerobic digestion of pulp and paper mill wastewater and sludge. Water Res. 2014, 65, 321–349. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Grala, A.; Dudek, M. Algae biomass as an alternative substrate in biogas production technologies—Review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Tedesco, S.; Benyounis, K.Y.; Olabi, A.G. Mechanical pretreatment effects on macroalgae-derived biogas production in co-digestion with sludge in Ireland. Energy 2013, 61, 27–33. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Biogas Generation through Anaerobic Digetsion Process—An Overview. Res. J. Chem. Environ. 2014, 18, 80–93. [Google Scholar]
- Ma, J.; Zhao, Q.-B.; Laurens, L.L.M.; Jarvis, E.E.; Nagle, N.J.; Chen, S.; Frear, C.S. Mechanism, kinetics and microbiology of inhibition caused by long-chain fatty acids in anaerobic digestion of algal biomass. Biotechnol. Biofuels 2015, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Valijanian, E.; Tabatabaei, M.; Aghbashlo, M.; Sulaiman, A.; Chisti, Y. Biogas Production Systems. In Biogas: Fundamentals, Process, and Operation; Tabatabaei, M., Ghanavati, H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 95–116. [Google Scholar]
- Ostrem, K. Greening Waste: Anaerobic Digestion for Treating the Organic Fraction of Municipal Solid Wastes; Columbia University: New York, NY, USA, 2004. [Google Scholar]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J. Biomethanation and its potential. Methods Enzym. 2011, 494, 327–351. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Suárez, J.L.; Arroyo, N.C.; González-Fernández, C. The Role of Anaerobic Digestion in Algal Biorefineries: Clean Energy Production, Organic Waste Treatment, and Nutrient Loop Closure. In Algae and Environmental Sustainability; Singh, B., Bauddh, K., Bux, F., Eds.; Springer: New Delhi, India, 2015; pp. 53–76. [Google Scholar]
- Stronach, S.M.; Rudd, T.; Lester, J.N. Anaerobic Digestion Processes in Industrial Wastewater Treatment; Aiba, S., Fan, L.T., Fiechter, A., de Klein, J., Schügerl, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Felchner-Zwirello, M. Propionic Acid Degradation by Syntrophic Bacteria during Anaerobic Biowaste Digestion; Karlsruher Institut für Technologie (KIT): Karlsruhe, Germany, 2014. [Google Scholar]
- André, L.; Ndiaye, M.; Pernier, M.; Lespinard, O.; Pauss, A.; Lamy, E.; Ribeiro, T. Methane production improvement by modulation of solid phase immersion in dry batch anaerobic digestion process: Dynamic of methanogen populations. Bioresour. Technol. 2016, 207, 353–360. [Google Scholar] [CrossRef]
- Ge, X.; Matsumoto, T.; Keith, L.; Li, Y. Biogas energy production from tropical biomass wastes by anaerobic digestion. Bioresour. Technol. 2014, 169, 38–44. [Google Scholar] [CrossRef]
- Paramaguru, G.; Kannan, M.; Lawrence, P. Effect of pH on Biogas Production through Anaerobic Digestion of Food Waste. J. Adv. Eng. Res. 2017, 4, 59–62. [Google Scholar]
- Oyejide, J.O.; Orhorhoro, E.K.; Atadious, D. Mathematical modeling of biogas yield from anaerobic co-digestion of organic waste and pig dung. Int. J. Eng. Sci. Invent. 2018, 7, 30–38. [Google Scholar]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Krishna, R.H. Role of factors influencing on anaerobic process for production of bio hydrogen: Future fuel. Int. J. Adv. Chem. 2013, 1, 31–38. [Google Scholar]
- Chen, X.; Yan, W.; Sheng, K.; Sanati, M. Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste. Bioresour. Technol. 2014, 154, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Jha, A.K.; Li, J.; Nies, L.; Zhang, L. Research advances in dry anaerobic digestion process of solid organic wastes. Afr. J. Biotechnol. 2011, 10, 14242–14253. [Google Scholar]
- Jiang, D.; Ge, X.; Zhang, Q.; Li, Y. Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour. Technol. 2016, 216, 60–68. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Le Hyaric, R.; Benbelkacem, H.; Bollon, J.; Bayard, R.; Escudié, R.; Buffière, P. Influence of moisture content on the specific methanogenic activity of dry mesophilic municipal solid waste digestate. J. Chem. Technol. Biotechnol. 2012, 87, 1032–1035. [Google Scholar] [CrossRef]
- Abbassi-Guendouz, A.; Brockmann, D.; Trably, E.; Dumas, C.; Delgenès, J.-P.; Steyer, J.-P.; Escudié, R. Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour. Technol. 2012, 111, 55–61. [Google Scholar] [CrossRef]
- Abu-Reesh, I.M. Kinetics of anaerobic digestion of labaneh whey in a batch reactor. Afr. J. Biotechnol. 2014, 13, 1745–1755. [Google Scholar]
- Anyaoku, C.C.; Baroutian, S. Decentralized anaerobic digestion systems for increased utilization of biogas from municipal solid waste. Renew. Sustain. Energy Rev. 2018, 90, 982–991. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D.; Jenicek, P. Thermophilic versus Mesophilic Anaerobic Digestion of Sewage Sludge: A Comparative Review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Dong, B.; Wu, B.; Dai, X. High-solid anaerobic digestion of sewage sludge under mesophilic conditions: Feasibility study. Bioresour. Technol. 2012, 104, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Slimane, K.; Fathya, S.; Assia, K.; Hamza, M. Influence of Inoculums/Substrate Ratios (ISRs) on the Mesophilic Anaerobic Digestion of Slaughterhouse Waste in Batch Mode: Process Stability and Biogas Production. Energy Procedia 2014, 50, 57–63. [Google Scholar] [CrossRef]
- Suhartini, S.; Heaven, S.; Banks, C.J. Comparison of mesophilic and thermophilic anaerobic digestion of sugar beet pulp: Performance, dewaterability and foam control. Bioresour. Technol. 2014, 152, 202–211. [Google Scholar] [CrossRef]
- Moset, V.; Poulsen, M.; Wahid, R.; Højberg, O.; Møller, H.B. Mesophilic versus thermophilic anaerobic digestion of cattle manure: Methane productivity and microbial ecology. Microb. Biotechnol. 2015, 8, 787–800. [Google Scholar] [CrossRef]
- Veluchamy, C.; Gilroyed, B.H.; Kalamdhad, A.S. Process performance and biogas production optimizing of mesophilic plug flow anaerobic digestion of corn silage. Fuel 2019, 253, 1097–1103. [Google Scholar] [CrossRef]
- Miramontes-Martínez, L.R.; Rivas-García, P.; Albalate-Ramírez, A.; Botello-Álvarez, J.E.; Escamilla-Alvarado, C.; Gomez-Gonzalez, R.; Alcalá-Rodríguez, M.M.; Valencia-Vázquez, R.; Santos-López, I.A. Anaerobic co-digestion of fruit and vegetable waste: Synergy and process stability analysis. J. Air Waste Manag. Assoc. 2021, 71, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hidaka, T.; Uchida, T.; Tsumori, J. Thermophilic anaerobic digestion of sewage sludge with high solids content. Water Sci. Technol. 2014, 69, 1949–1955. [Google Scholar] [CrossRef]
- Suksong, W.; Jehlee, A.; Singkhala, A.; Kongjan, P.; Prasertsan, P.; Imai, T.; O-Thong, S. Thermophilic solid-state anaerobic digestion of solid waste residues from palm oil mill industry for biogas production. Ind. Crops Prod. 2017, 95, 502–511. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Armato, C.; Pozo, C.; González-Martínez, A.; González-López, J. New concepts in anaerobic digestion processes: Recent advances and biological aspects. Appl. Microbiol. Biotechnol. 2018, 102, 5065–5076. [Google Scholar] [CrossRef] [PubMed]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Energy recovery from one- and two-stage anaerobic digestion of food waste. Waste Manag. 2017, 68, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Torretta, V. What Advanced Treatments Can Be Used to Minimize the Production of Sewage Sludge in WWTPs? Appl. Sci. 2019, 9, 2650. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, Y.; Yang, Y.; Sheng, H.; Li, X.; Li, T.; Song, Y.; Zhang, H.; Chen, S.; He, W.; et al. Water free anaerobic co-digestion of vegetable processing waste with cattle slurry for methane production at high total solid content. Energy 2014, 74, 309–313. [Google Scholar] [CrossRef]
- Latinwo, G.K.; Agarry, S.E. Modelling the Kinetics of Biogas Production from Mesophilic Anaerobic Co-Digestion of Cow Dung with Plantain Peels. Int. J. Renew. Energy Dev. 2015, 4, 55–63. [Google Scholar] [CrossRef]
- Estevez, M.M.; Sapci, Z.; Linjordet, R.; Schnürer, A.; Morken, J. Semi-continuous anaerobic co-digestion of cow manure and steam-exploded Salix with recirculation of liquid digestate. J. Environ. Manag. 2014, 136, 9–15. [Google Scholar] [CrossRef]
- Gómez Camacho, C.E.; Ruggeri, B.; Mangialardi, L.; Persico, M.; Luongo Malavé, A.C. Continuous two-step anaerobic digestion (TSAD) of organic market waste: Rationalising process parameters. Int. J. Energy Environ. Eng. 2019, 10, 413–427. [Google Scholar] [CrossRef]
- Bouallagui, H.; Touhami, Y.; Ben Cheikh, R.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Lee, J.; Wang, C.-H.; Dai, Y.; Wah Tong, Y. Three-stage anaerobic co-digestion of food waste and horse manure. Sci. Rep. 2017, 7, 1269. [Google Scholar] [CrossRef] [PubMed]
- Voelklein, M.A.; O’ Shea, R.; Jacob, A.; Murphy, J.D. Role of trace elements in single and two-stage digestion of food waste at high organic loading rates. Energy 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Schievano, A.; Tenca, A.; Scaglia, B.; Merlino, G.; Rizzi, A.; Daffonchio, D.; Oberti, R.; Adani, F. Two-Stage vs Single-Stage Thermophilic Anaerobic Digestion: Comparison of Energy Production and Biodegradation Efficiencies. Environ. Sci. Technol. 2012, 46, 8502–8510. [Google Scholar] [CrossRef] [PubMed]
- Bertin, L.; Grilli, S.; Spagni, A.; Fava, F. Innovative two-stage anaerobic process for effective co-digestion of cheese whey and cattle manure. Bioresour. Technol. 2013, 128, 779–783. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Martín-Marroquín, J.M.; Sastre, E. Single-Phase and Two-Phase Anaerobic Co-Digestion of Residues from the Treatment Process of Waste Vegetable Oil and Pig Manure. BioEnergy Res. 2014, 7, 670–680. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Li, W.; Lim, J.W.; Dai, Y.; Tong, Y.W. Three-stage anaerobic digester for food waste. Appl. Energy 2017, 194, 287–295. [Google Scholar] [CrossRef]
- Rajendran, K.; Aslanzadeh, S.; Taherzadeh, M.J. Household Biogas Digesters—A Review. Energies 2012, 5, 2911–2942. [Google Scholar] [CrossRef]
- Ghosh, R.; Bhattacherjee, S. A review study on anaerobic digesters with an Insight to biogas production. Int. J. Eng. Sci. Invent. 2013, 2, 8–17. [Google Scholar]
- Akula, V.R. Wetland Biomass—Suitable for Biogas Production? Halmstad University: Halmstad, Sweden, 2013. [Google Scholar]
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Mungwe, J.N.; Colombo, E.; Adani, F.; Schievano, A. The fixed dome digester: An appropriate design for the context of Sub-Sahara Africa? Biomass Bioenergy 2016, 95, 35–44. [Google Scholar] [CrossRef]
- Walekhwa, P.N.; Lars, D.; Mugisha, J. Economic viability of biogas energy production from family-sized digesters in Uganda. Biomass Bioenergy 2014, 70, 26–39. [Google Scholar] [CrossRef]
- Saady, N.M.C.; Massé, D.I. High rate psychrophilic anaerobic digestion of high solids (35%) dairy manure in sequence batch reactor. Bioresour. Technol. 2015, 186, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.J.; Sooch, S.S. Comparative study of economics of different models of family size biogas plants for state of Punjab, India. Energy Convers. Manag. 2004, 45, 1329–1341. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Comparetti, A.; Febo, P.; Greco, C.; Orlando, S. Current state and future of biogas and digestate production. Bulg. J. Agric. Sci. 2013, 19, 1–14. [Google Scholar]
- Izumi, K.; Okishio, Y.-k.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Agyeman, F.O.; Tao, W. Anaerobic co-digestion of food waste and dairy manure: Effects of food waste particle size and organic loading rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Wu, G.; Healy, M.G.; Zhan, X. Effect of the solid content on anaerobic digestion of meat and bone meal. Bioresour. Technol. 2009, 100, 4326–4331. [Google Scholar] [CrossRef]
- Junaidi; Wijaya, A.; Rachmawan, A.; Andriyanto, M. Total Solid Content and Compound Properties from Different Collection Time of Latex. Acta Technol. Agric. 2019, 22, 104–108. [Google Scholar] [CrossRef]
- Budiyono; Syaichurrozi, I.; Sumardiono, S. Effect of Total Solid Content to Biogas Production Rate from Vinasse (RESEARCH NOTE). Int. J. Eng. 2014, 27, 177–184. [Google Scholar]
- Orhorhoro, E.K.; Ebunilo, P.O.; Sadjere, G.E. Experimental determination of effect of total solid (TS) and volatile solid (VS) on biogas yield. Am. J. Mod. Energy 2017, 3, 131–135. [Google Scholar] [CrossRef]
- Yavini, T.D.; Chia, A.I.; John, A. Evaluation of the effect of total solids concentration on biogas yields of agricultural wastes. Int. Res. J. Environ. Sci. 2014, 3, 70–75. [Google Scholar]
- Igoni, A.H.; Abowei, M.; Ayotamuno, M.; Eze, C. Effect of total solids concentration of municipal solid waste on the biogas produced in an anaerobic continuous digester. Agric. Eng. Int. CIGR J. 2008, 1–11. [Google Scholar]
- Sathish, S.; Chandrasekaran, M.; Solomon, G.R. Effect of total solids and agitation time on biogas yield, using rice husk. Int. J. Ambient Energy 2019, 40, 101–104. [Google Scholar] [CrossRef]
- Maamri, S.; Amrani, M. Biogas Production from Waste Activated Sludge Using Cattle Dung Inoculums: Effect of Total Solid Contents and Kinetics Study. Energy Procedia 2014, 50, 352–359. [Google Scholar] [CrossRef]
- Budiyono, B.; Syaichurrozi, I.; Suhirman, S.; Hidayat, T.; Jayanudin, J. Experiment and modeling to evaluate the effect of total solid on biogas production from the anaerobic co-digestion of Tofu liquid waste and rice straw. Pol. J. Environ. Stud. 2021, 30, 3489–3496. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and pH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Resch, C.; Wörl, A.; Waltenberger, R.; Braun, R.; Kirchmayr, R. Enhancement options for the utilisation of nitrogen rich animal by-products in anaerobic digestion. Bioresour. Technol. 2011, 102, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Facchin, V.; Cavinato, C.; Fatone, F.; Pavan, P.; Cecchi, F.; Bolzonella, D. Effect of trace element supplementation on the mesophilic anaerobic digestion of foodwaste in batch trials: The influence of inoculum origin. Biochem. Eng. J. 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Kangle, K.M.; Kore, S.V.; Kore, V.S.; Kulkarni, G.S. Recent Trends in Anaerobic Co-digestion: A Review. Univers. J. Environ. Res. Technol. 2012, 2, 210–219. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Dry thermophilic anaerobic digestion of the organic fraction of municipal solid wastes: Solid retention time optimization. Chem. Eng. J. 2014, 251, 435–440. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Dodd, I.C.; Herbert, B.M.J.; Li, H.; Ricketts, L.; Semple, K.T. High solid anaerobic digestion: Operational challenges and possibilities. Environ. Technol. Innov. 2015, 4, 268–284. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef]
- Massaccesi, L.; Sordi, A.; Micale, C.; Cucina, M.; Zadra, C.; Di Maria, F.; Gigliotti, G. Chemical characterisation of percolate and digestate during the hybrid solid anaerobic digestion batch process. Process Biochem. 2013, 48, 1361–1367. [Google Scholar] [CrossRef]
- Labatut, R.A.; Gooch, C.A. Monitoring of anaerobic digestion process to optimize performance and prevent system failure. In Proceedings of the Got Manure? Enhancing Environmental and Economic Sustainability Conference, Liverpool, NY, USA; 2014. [Google Scholar]
- Guendouz, J.; Buffière, P.; Cacho, J.; Carrère, M.; Delgenes, J.P. Dry anaerobic digestion in batch mode: Design and operation of a laboratory-scale, completely mixed reactor. Waste Manag. 2010, 30, 1768–1771. [Google Scholar] [CrossRef]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Sahu, N.; Deshmukh, S.; Chandrashekhar, B.; Sharma, G.; Kapley, A.; Pandey, R.A. Optimization of hydrolysis conditions for minimizing ammonia accumulation in two-stage biogas production process using kitchen waste for sustainable process development. J. Environ. Chem. Eng. 2017, 5, 2378–2387. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Long-term anaerobic digestion of food waste stabilized by trace elements. Waste Manag. 2012, 32, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A. Mini review: Update on bioaugmentation in anaerobic processes for biogas production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef]
- Ali, R.S. Biogas Production from Poultry Manure Using a Novel Solar Assisted System; University of Birzeit: Ramallah, Palestine, 2015. [Google Scholar]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Cerón-Vivas, A.; Cáceres, K.T.; Rincón, A.; Cajigas, Á.A. Influence of pH and the C/N ratio on the biogas production of wastewater. Rev. Fac. Ing. Univ. Antioq. 2019, 92, 70–79. [Google Scholar] [CrossRef]
- Khanal, S.K.; Li, Y. Biogas Production and Applications. In Bioenergy: Principles and Applications; Li, Y., Khanal, S.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 338–358. [Google Scholar]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Okoh, A.I.; Makaka, G.; Simon, M. Microbial anaerobic digestion (bio-digesters) as an approach to the decontamination of animal wastes in pollution control and the generation of renewable energy. Int. J. Environ. Res. Public Health 2013, 10, 4390–4417. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Gómez, X.; Morán, A.; García-González, M.C. Anaerobic co-digestion of livestock and vegetable processing wastes: Fibre degradation and digestate stability. Waste Manag. 2013, 33, 1332–1338. [Google Scholar] [CrossRef]
- Murphy, J.D.; Thanasit, T. 5—Fundamental science and engineering of the anaerobic digestion process for biogas production. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 104–130. [Google Scholar]
- Kennedy, Z.R.; Munisamy, P.; Murali, S.R. Enrichment of biogas production from mixture of rubber seed cake and cow dung using TiO2 catalyst and temperature. Int. J. Renew. Energy Technol. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Nelson, R. Methane Generation from Anaerobic Digesters: Considering Different Substrates; Iowa State University: Ames, IA, USA, 2010; pp. 1–11. [Google Scholar]
- Li, L.; He, Q.; Zhao, X.; Wu, D.; Wang, X.; Peng, X. Anaerobic digestion of food waste: Correlation of kinetic parameters with operational conditions and process performance. Biochem. Eng. J. 2018, 130, 1–9. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. Applications of the anaerobic digestion process. Adv. Biochem. Eng. Biotechnol. 2003, 82, 1–33. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Nandi, R.; Saha, C.; Huda, M.; Alam, M. Effect of mixing on biogas production from cow dung. Eco-Friendly Agric. J. 2017, 10, 7–13. [Google Scholar]
- Keanoi, N.; KanokornHussaro; Teekasap, S. Effect of with/without agitation of agricultural waste on biogas production from anaerobic co-digestion-A small scale. Am. J. Environ. Sci. 2014, 10, 74–85. [Google Scholar] [CrossRef]
- El-Bakhshwan, M.; El-Ghafar, A.; Zayed, M.; El-Shazly, A. Effect of mechanical stirring on biogas production efficiency in large scale digesters. J. Soil Sci. Agric. Eng. 2015, 6, 47–63. [Google Scholar] [CrossRef]
- Wang, B.; Wu, D.; Ekama, G.A.; Huang, H.; Lu, H.; Chen, G.-H. Optimizing mixing mode and intensity to prevent sludge flotation in sulfidogenic anaerobic sludge bed reactors. Water Res. 2017, 122, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Ghanimeh, S.; El Fadel, M.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012, 117, 63–71. [Google Scholar] [CrossRef]
- Rico, C.; Rico, J.L.; Muñoz, N.; Gómez, B.; Tejero, I. Effect of mixing on biogas production during mesophilic anaerobic digestion of screened dairy manure in a pilot plant. Eng. Life Sci. 2011, 11, 476–481. [Google Scholar] [CrossRef]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef]
- Lindmark, J.; Eriksson, P.; Thorin, E. The effects of different mixing intensities during anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2014, 34, 1391–1397. [Google Scholar] [CrossRef]
- Singh, B.; Kovács, K.L.; Bagi, Z.; Nyári, J.; Szepesi, G.L.; Petrik, M.; Siménfalvi, Z.; Szamosi, Z. Enhancing Efficiency of Anaerobic Digestion by Optimization of Mixing Regimes Using Helical Ribbon Impeller. Fermentation 2021, 7, 251. [Google Scholar] [CrossRef]
- Jaman, K.; Amir, N.; Musa, M.A.; Zainal, A.; Yahya, L.; Abdul Wahab, A.M.; Suhartini, S.; Tuan Mohd Marzuki, T.N.; Harun, R.; Idrus, S. Anaerobic Digestion, Co-digestion of Food Waste, and Chicken Dung: Correlation of Kinetic Parameters with Digester Performance and On-Farm Electrical Energy Generation Potential. Fermentation 2022, 8, 28. [Google Scholar] [CrossRef]
- Tian, Z.; Chauliac, D.; Pullammanappallil, P. Comparison of non-agitated and agitated batch, thermophilic anaerobic digestion of sugarbeet tailings. Bioresour. Technol. 2013, 129, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.K.; Greenfield, P.F.; Pullammanappallil, P.C. Effect of mixing on biomethanation of cattle-manure slurry. Environ. Technol. 2002, 23, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. State of the art on mixing in an anaerobic digester: A review. Renew. Energy 2019, 141, 922–936. [Google Scholar] [CrossRef]
- Sulaiman, A.; Hassan, M.A.; Shirai, Y.; Abd-Aziz, S.; Tabatabaei, M.; Busu, Z.; Yacob, S. The effect of mixing on methane production in a semi-commercial closed digester tank treating palm oil mill effluent. Aust. J. Basic Appl. Sci. 2009, 3, 1577–1583. [Google Scholar]
- Rojas, C.; Fang, S.; Uhlenhut, F.; Borchert, A.; Stein, I.; Schlaak, M. Stirring and biomass starter influences the anaerobic digestion of different substrates for biogas production. Eng. Life Sci. 2010, 10, 339–347. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Harnisch, E.; Schwede, S.; Gerber, M.; Span, R. Different mixing modes for biogas plants using energy crops. Appl. Energy 2013, 112, 465–472. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Jensen, P.D.; Rabaey, K.; Tyson, G.W. Temperature and solids retention time control microbial population dynamics and volatile fatty acid production in replicated anaerobic digesters. Sci. Rep. 2015, 5, 8496. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Bachmann, N. Design and engineering of biogas plants. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 191–211. [Google Scholar]
- Bouallagui, H.; Ben Cheikh, R.; Marouani, L.; Hamdi, M. Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour. Technol. 2003, 86, 85–89. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-S.; Dong, J.-J.; Yu, J.-H.; Yin, H.; Hu, S.-M.; Huang, S.-X.; Yuan, X.-Z. Effect of Hydraulic Retention Time on Anaerobic Digestion of Wheat Straw in the Semicontinuous Continuous Stirred-Tank Reactors. BioMed Res. Int. 2017, 2017, 2457805. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Zhang, J. Enhanced biogas production from anaerobic digestion of solid organic wastes: Current status and prospects. Bioresour. Technol. Rep. 2019, 5, 280–296. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of sustainable approaches for converting the organic waste to bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, W.; Liu, X.; Lian, S.; Zheng, L. Effects of thermal pre-treatment on anaerobic co-digestion of municipal biowastes at high organic loading rate. Chemosphere 2014, 101, 66–70. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Anwar, N.; Ma, Z.; Liu, G.; Zhang, R. Effect of Organic Loading Rate on Anaerobic Digestion of Food Waste under Mesophilic and Thermophilic Conditions. Energy Fuels 2017, 31, 2976–2984. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Xie, D. Insights into the production potential and trends of China’s rural biogas. Int. J. Energy Res. 2015, 39, 1068–1082. [Google Scholar] [CrossRef]
- Ferguson, R.M.W.; Coulon, F.; Villa, R. Organic loading rate: A promising microbial management tool in anaerobic digestion. Water Res. 2016, 100, 348–356. [Google Scholar] [CrossRef]
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies—Developments and Innovations; IEA Bioenergy: Paris, France, 2009. [Google Scholar]
- Singh, G.; Jain, V.K.; Singh, A. Effect of Temperature and other factors on Anaerobic Digestion Process, responsible for Bio Gas Production. Int. J. Theor. Appl. Mech. 2017, 12, 637–657. [Google Scholar]
- Lindeboom, R.E.; Fermoso, F.G.; Weijma, J.; Zagt, K.; van Lier, J.B. Autogenerative high pressure digestion: Anaerobic digestion and biogas upgrading in a single step reactor system. Water Sci. Technol. 2011, 64, 647–653. [Google Scholar] [CrossRef]
- Sumantri, I.; Budiyono, B.; Purwanto, P. Kinetic Study of Anaerobic Digestion of Ketchup Industry Wastewater in a Three-stages Anaerobic Baffled Reactor (ABR). Bull. Chem. React. Eng. Catal. 2019, 14, 326–335. [Google Scholar] [CrossRef]
- Fdez.-Güelfo, L.A.; Álvarez-Gallego, C.; Sales Márquez, D.; Romero García, L.I. Dry-thermophilic anaerobic digestion of simulated organic fraction of Municipal Solid Waste: Process modeling. Bioresour. Technol. 2011, 102, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Obileke, K.; Mamphweli, S.; Meyer, E.L.; Makaka, G.; Nwokolo, N. Development of a Mathematical Model and Validation for Methane Production Using Cow Dung as Substrate in the Underground Biogas Digester. Processes 2021, 9, 643. [Google Scholar] [CrossRef]
- Almomani, F. Prediction of biogas production from chemically treated co-digested agricultural waste using artificial neural network. Fuel 2020, 280, 118573. [Google Scholar] [CrossRef]
- Mougari, N.E.; Largeau, J.F.; Himrane, N.; Hachemi, M.; Tazerout, M. Application of artificial neural network and kinetic modeling for the prediction of biogas and methane production in anaerobic digestion of several organic wastes. Int. J. Green Energy 2021, 18, 1584–1596. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, H.; Zhou, L.; Arhin, S.G.; Papadakis, V.G.; Goula, M.A.; Liu, G.; Zhang, Y.; Wang, W. Bioaugmentation with well-constructed consortia can effectively alleviate ammonia inhibition of practical manure anaerobic digestion. Water Res. 2022, 215, 118244. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Baldé, Y.M.; Diop, S.; Tebbani, S.; Kanté, C. Modeling of a Continuous Anaerobic Digestion of Wastes. In Proceedings of the 2020 24th International Conference on System Theory, Control and Computing (ICSTCC), Sinaia, Romania, 8–10 October 2020; pp. 596–601. [Google Scholar]
- Momodu, A.S.; Adepoju, T.D. System dynamics kinetic model for predicting biogas production in anaerobic condition: Preliminary assessment. Sci. Prog. 2021, 104, 368504211042479. [Google Scholar] [CrossRef]
- Gerber, M.; Span, R. An analysis of available mathematical models for anaerobic digestion of organic substances for production of biogas. Proc. IGRC Paris 2008, 1–30. Available online: https://www.researchgate.net/publication/283518957_An_analysis_of_available_mathematical_models_for_anaerobic_digestion_of_organic_substances_for_production_of_biogas (accessed on 10 March 2023).
- Dinh, P.V.; Fujiwara, T.; Phu, S.T.P.; Hoang, M.G. Kinetic of Biogas Production in Co-Digestion of Vegetable Waste, Horse Dung, and Sludge by Batch Reactors. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012041. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef]
- Orangun, A.; Kaur, H.; Kommalapati, R.R. Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Ghatak, M.D.; Mahanta, P. Comparison of kinetic models for biogas production rate from Saw Dust. Int. J. Res. Eng. Technol. 2014, 3, 248–254. [Google Scholar]
- Bakraouia, M.; Karouacha, F.; Ouhammoua, B.; Lahboubib, N.; Gnaouia, Y.E.; Aggoura, M.; Baria, H.E. Kinetics study of methane production from anaerobic digestion of sludge and wastewater recycled pulp and paper by different models simulation. Int. J. Smart Grid Clean Energy 2020, 9, 170–179. [Google Scholar] [CrossRef]
- Zhang, H.; An, D.; Cao, Y.; Tian, Y.; He, J. Modeling the Methane Production Kinetics of Anaerobic Co-Digestion of Agricultural Wastes Using Sigmoidal Functions. Energies 2021, 14, 258. [Google Scholar] [CrossRef]
- Prajapati, K.B.; Singh, R. Kinetic modelling of methane production during bio-electrolysis from anaerobic co-digestion of sewage sludge and food waste. Bioresour. Technol. 2018, 263, 491–498. [Google Scholar] [CrossRef]
- Lopes, J.O.; Rosa, A.P.; Sousa, I.d.P.; Oliveira, N.S.; Borges, A.C. Mathematical models for estimating methane production in covered lagoon biodigesters treating pig manure. Eng. Agrícola 2021, 41, 438–448. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; Dentice d’Accadia, M.; Infante, A.; Vicidomini, M. Modeling of the Anaerobic Digestion of Organic Wastes: Integration of Heat Transfer and Biochemical Aspects. Energies 2020, 13, 2720. [Google Scholar] [CrossRef]
- De Crescenzo, C.; Marzocchella, A.; Karatza, D.; Molino, A.; Ceron-Chafla, P.; Lindeboom, R.E.F.; van Lier, J.B.; Chianese, S.; Musmarra, D. Modelling of autogenerative high-pressure anaerobic digestion in a batch reactor for the production of pressurised biogas. Biotechnol. Biofuels Bioprod. 2022, 15, 20. [Google Scholar] [CrossRef]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Existing Empirical Kinetic Models in Biochemical Methane Potential (BMP) Testing, Their Selection and Numerical Solution. Water 2020, 12, 1831. [Google Scholar] [CrossRef]
- Ihoeghian, N.A.; Amenaghawon, A.N.; Ajieh, M.U.; Oshoma, C.E.; Ogofure, A.; Erhunmwunse, N.O.; Edosa, V.I.O.; Tongo, I.; Obuekwe, I.S.; Isagba, E.S.; et al. Anaerobic co-digestion of cattle rumen content and food waste for biogas production: Establishment of co-digestion ratios and kinetic studies. Bioresour. Technol. Rep. 2022, 18, 101033. [Google Scholar] [CrossRef]
- Jijai, S.; Siripatana, C. Kinetic Model of Biogas Production from Co-digestion of Thai Rice Noodle Wastewater (Khanomjeen) with Chicken Manure. Energy Procedia 2017, 138, 386–392. [Google Scholar] [CrossRef]
- Blasius, J.P.; Contrera, R.C.; Maintinguer, S.I.; Alves de Castro, M.C.A. Effects of temperature, proportion and organic loading rate on the performance of anaerobic digestion of food waste. Biotechnol. Rep. 2020, 27, e00503. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Pathak, V.V.; Bansal, M. Co-digestion of wheat straw and animal manure pretreated with calcium hydroxide for biomethane production: Kinetic study. Curr. Res. Green Sustain. Chem. 2021, 4, 100145. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of substrate pretreatment on biogas production through anaerobic digestion of food waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Abbà, A.; Domini, M.; Baldi, M.; Pedrazzani, R.; Bertanza, G. Ammonia Recovery from Livestock Manure Digestate through an Air-Bubble Stripping Reactor: Evaluation of Performance and Energy Balance. Energies 2023, 16, 643. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renew. Sustain. Energy Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]

| Agriculture | Communities | Industry |
|---|---|---|
| Manure | OFMSW | Food/beverage processing |
| Energy crops | Municipal solid waste | Dairy |
| Algal biomass | Sewage sludge | Starch industry |
| Harvest remains | Grass clippings/garden waste | Sugar industry |
| Food remains | Pharmaceutical industry | |
| Cosmetic industry | ||
| Biochemical industry | ||
| Pulp and paper | ||
| Slaughterhouse/rendering plant |
| Component (%vol) | Household Waste | Wastewater Sludge | Agricultural Wastes | Food Industry Waste |
|---|---|---|---|---|
| CH4 | 50–60 | 60–75 | 60–75 | 68 |
| CO2 | 38–34 | 33–19 | 33–19 | 26 |
| N2 | 0–5 | 0–1 | 0–1 | - |
| O2 | 0–1 | 0–0.5 | 0–0.5 | - |
| H2O | 6 (40 °C) | 6 (40 °C) | 6 (40 °C) | 6 (40 °C) |
| Total | 100 | 100 | 100 | 100 |
| Animal Manure | pH | Total Solids (%) | Volatile Solids (%) | Carbon/Nitrogen Ratio |
|---|---|---|---|---|
| Cattle manure | 7.1–8.6 | 14.5–22.7 | 11.9–72.0 | 14.6–18.9 |
| Sheep manure | 7.2–8.1 | 22.3–40.0 | 18.7–72.7 | 11.3–14.7 |
| Goat manure | 7.9 | 33.7–55.5 | 27.7–89.4 | 18.0 |
| Pig manure | 6.4–7.5 | 8.2–36.7 | 6.2–82.8 | 5.7–13.5 |
| Chicken manure | 6.9–7.4 | 20.0–92.6 | 18.3–84.1 | 7.5–9.8 |
| Model | Equation | Ref. |
|---|---|---|
| First-order kinetic model | [20] | |
| Gompertz model | [21] | |
| Modified Gompertz model | [22] | |
| Logistic model | [23] | |
| Modified logistic model | [24] | |
| Chen–Hashimoto model | [25] | |
| Richards model | [26] | |
| Modified Richards model | [27] | |
| Cone model | [28] | |
| Monod model | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aworanti, O.A.; Agbede, O.O.; Agarry, S.E.; Ajani, A.O.; Ogunkunle, O.; Laseinde, O.T.; Rahman, S.M.A.; Fattah, I.M.R. Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables. Energies 2023, 16, 3378. https://doi.org/10.3390/en16083378
Aworanti OA, Agbede OO, Agarry SE, Ajani AO, Ogunkunle O, Laseinde OT, Rahman SMA, Fattah IMR. Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables. Energies. 2023; 16(8):3378. https://doi.org/10.3390/en16083378
Chicago/Turabian StyleAworanti, Oluwafunmilayo Abiola, Oluseye Omotoso Agbede, Samuel Enahoro Agarry, Ayobami Olu Ajani, Oyetola Ogunkunle, Opeyeolu Timothy Laseinde, S. M. Ashrafur Rahman, and Islam Md Rizwanul Fattah. 2023. "Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables" Energies 16, no. 8: 3378. https://doi.org/10.3390/en16083378
APA StyleAworanti, O. A., Agbede, O. O., Agarry, S. E., Ajani, A. O., Ogunkunle, O., Laseinde, O. T., Rahman, S. M. A., & Fattah, I. M. R. (2023). Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables. Energies, 16(8), 3378. https://doi.org/10.3390/en16083378









