Effect of Residual Water in Sediments on the CO2-CH4 Replacement Process
Abstract
1. Introduction
2. Materials and Methods
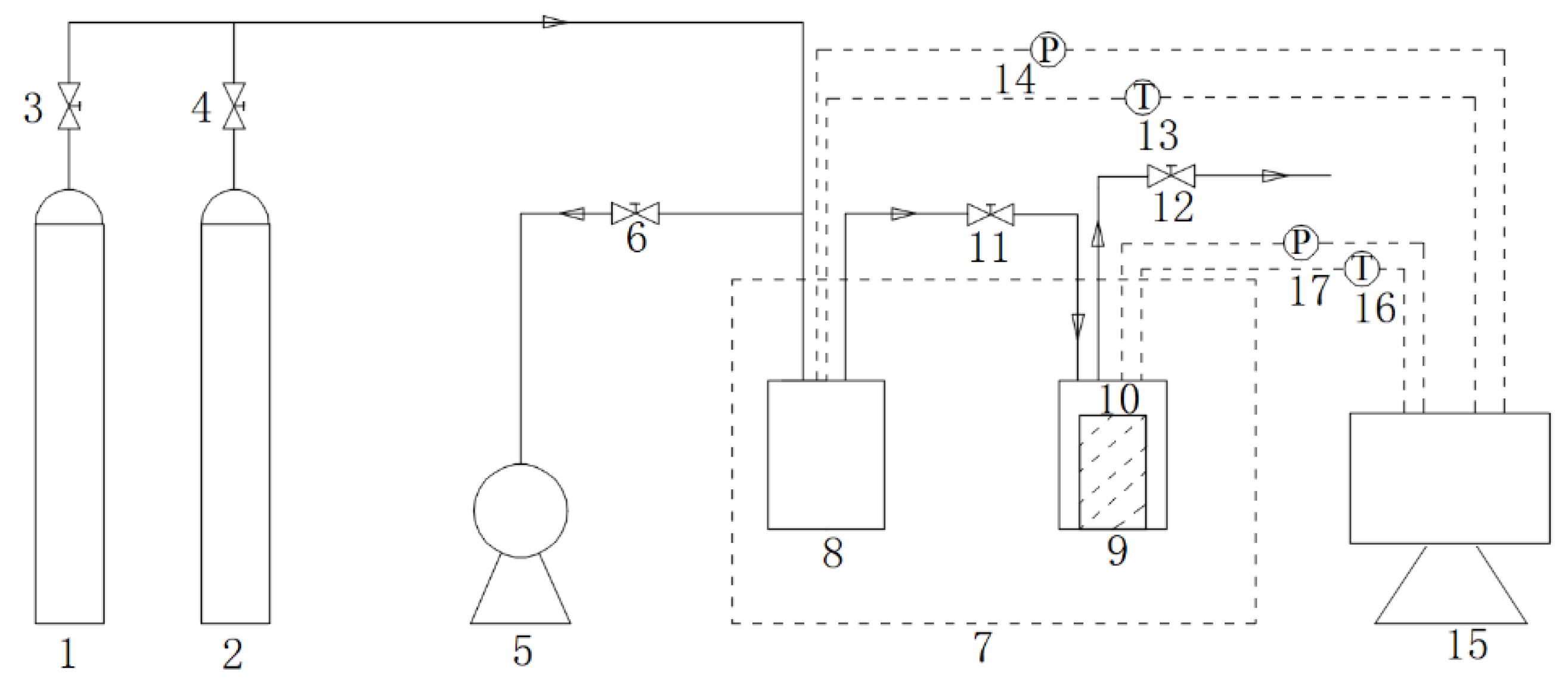
2.1. Experimental Apparatus
2.2. Experimental Materials
2.3. Experimental Procedures
2.3.1. Preparation of Methane Hydrate in Porous Media
2.3.2. Replacement Reaction Process
- Gaseous CO2 injection. The remaining CH4 in the reactor was quickly discharged, valve 11 was opened, and the reactor was purged with CO2 for 15 s to ensure that the CH4 gas in the reactor was completely discharged to the emptying pressure, and then valve 12 was closed. Subsequently, gaseous CO2 was injected for one minute. When the pressure was stable at 3 MPa, valve 11 was closed. This moment was recorded as time zero of the replacement reaction.
- Gas sample analysis. Gas samples were collected at time zero, and a gas chromatograph (GC9790, USA) was used to measure the gas sample components and content, record it as the content before the reaction, and then collect gas samples and measurements at time intervals. The volume of the single gas sample did not exceed 20 mL. The effects on temperature and pressure were negligible.
- Replacement reaction process. The replacement temperature was 277.15 K, the replacement pressure was 3 MPa, the replacement time was set to 4 days for each set of experiments, the temperature fluctuation during the reaction process did not exceed 0.1 K, and the pressure drop at the end of the reaction did not exceed 0.1 MPa.
2.4. Calculations
2.4.1. Calculation of Methane Hydrate Saturation
2.4.2. Recovery Efficiency of CH4
2.4.3. Weight of Residual Water in the Sediment
2.4.4. CO2 Storage Efficiency
3. Results and Discussion
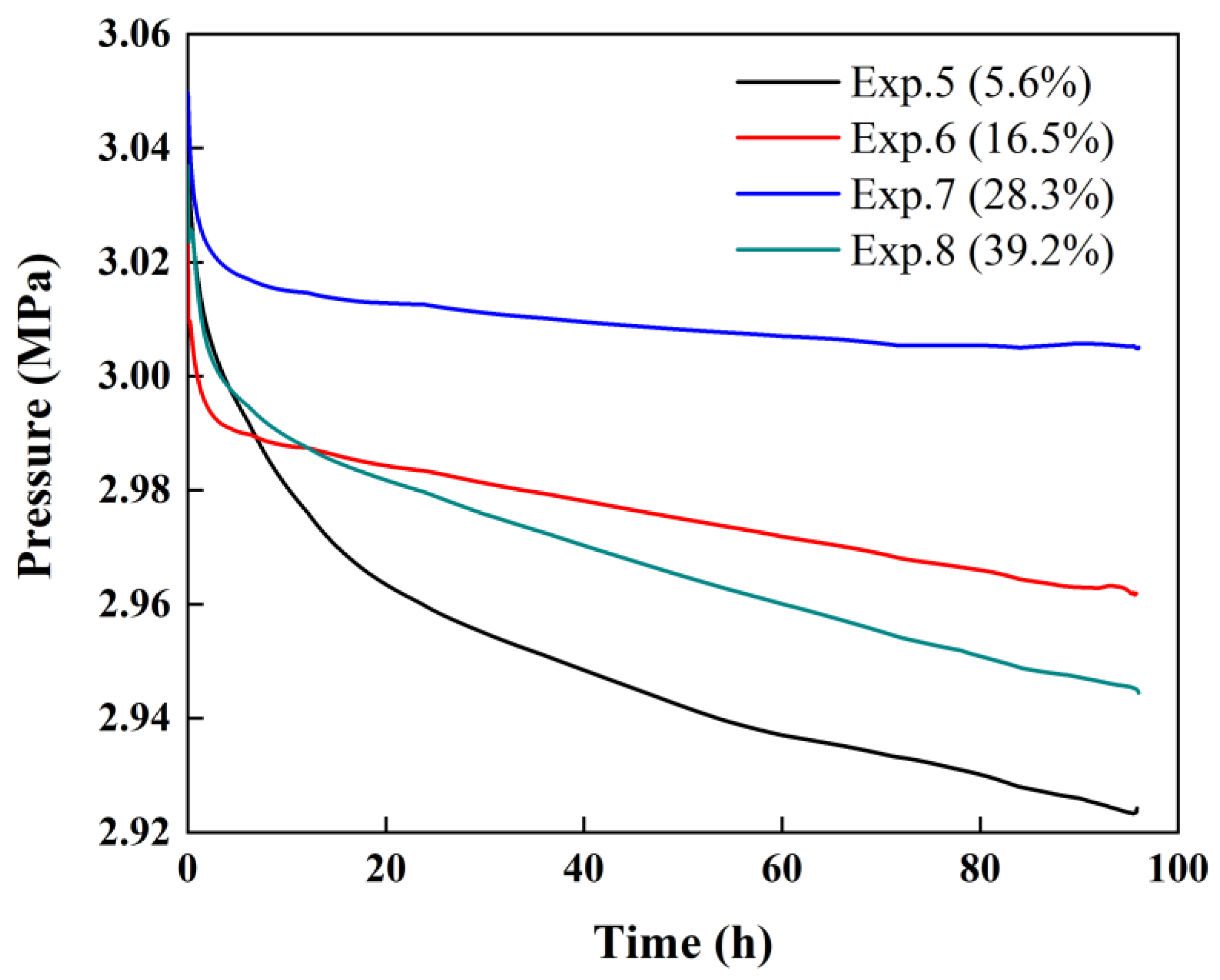
3.1. Variation in Pressure during the Replacement Process
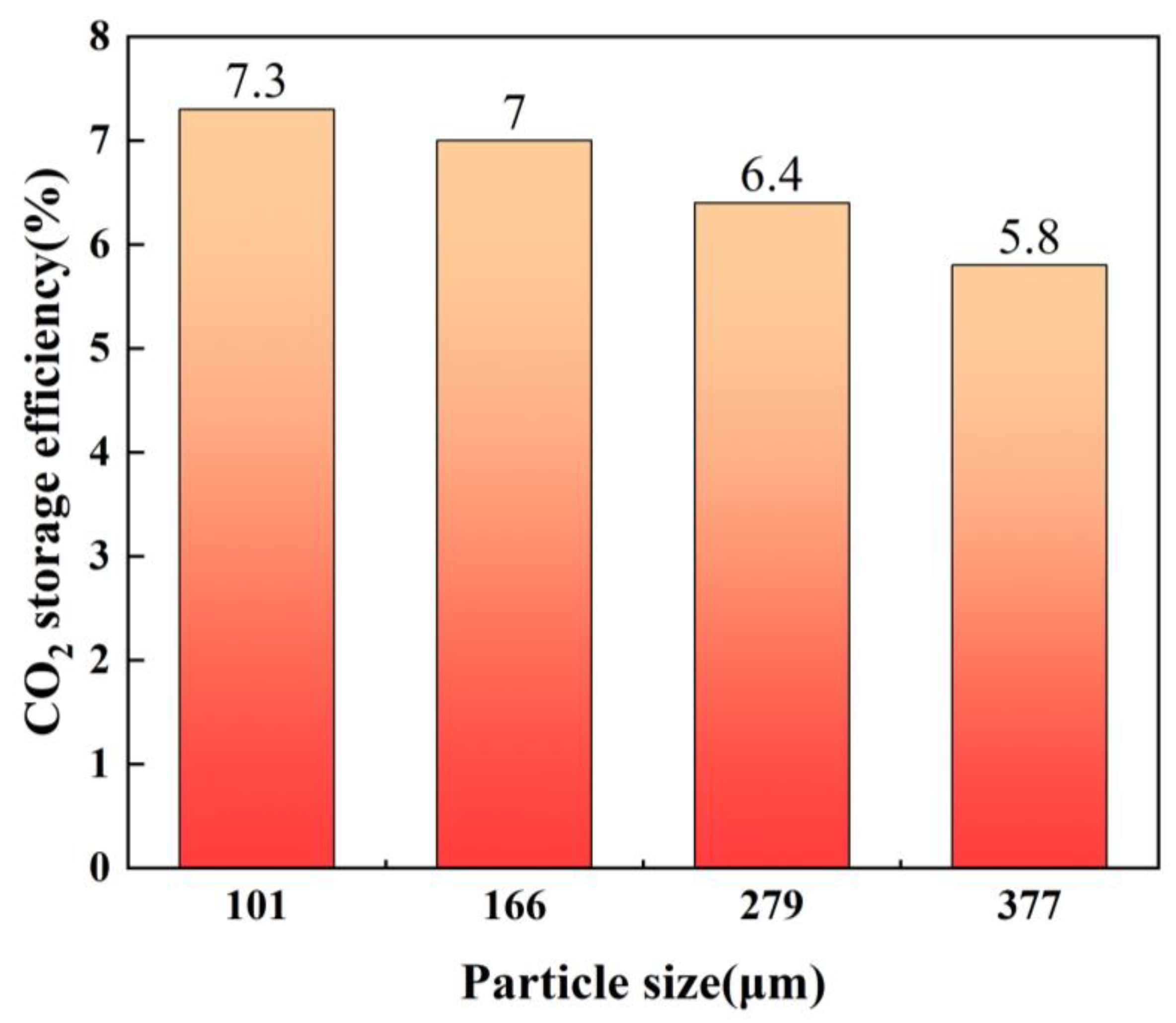
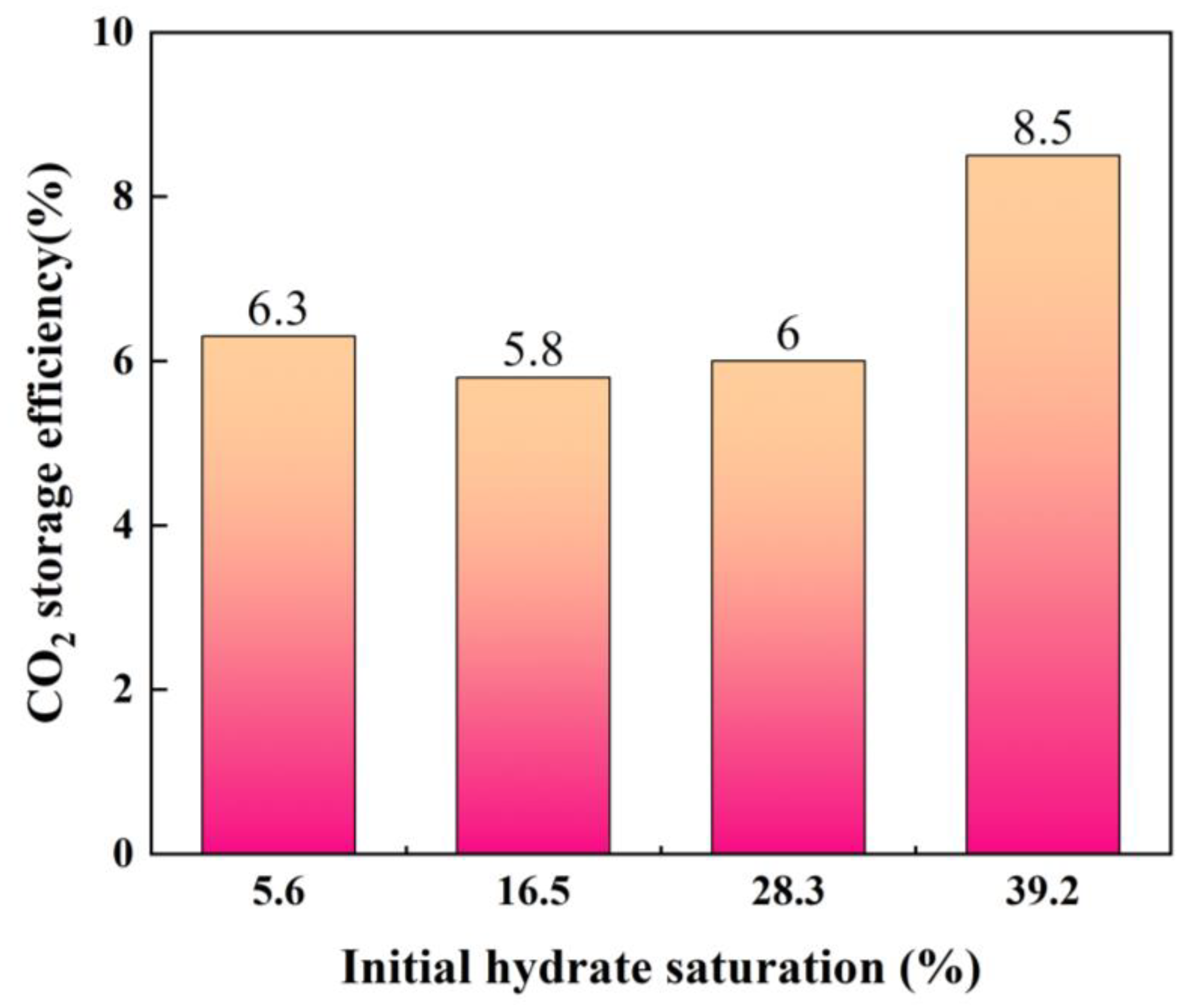
3.2. CO2 Storage Efficiency
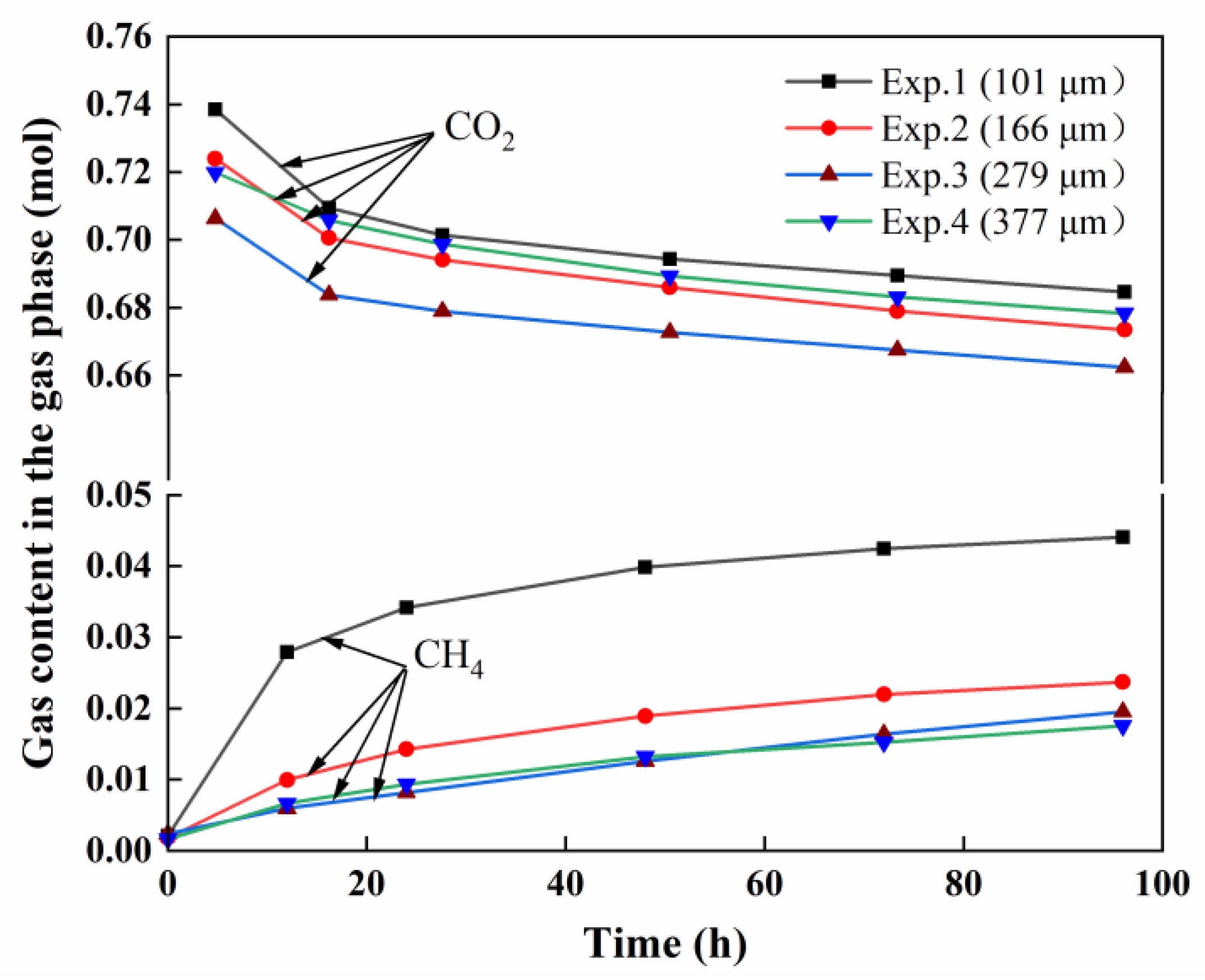
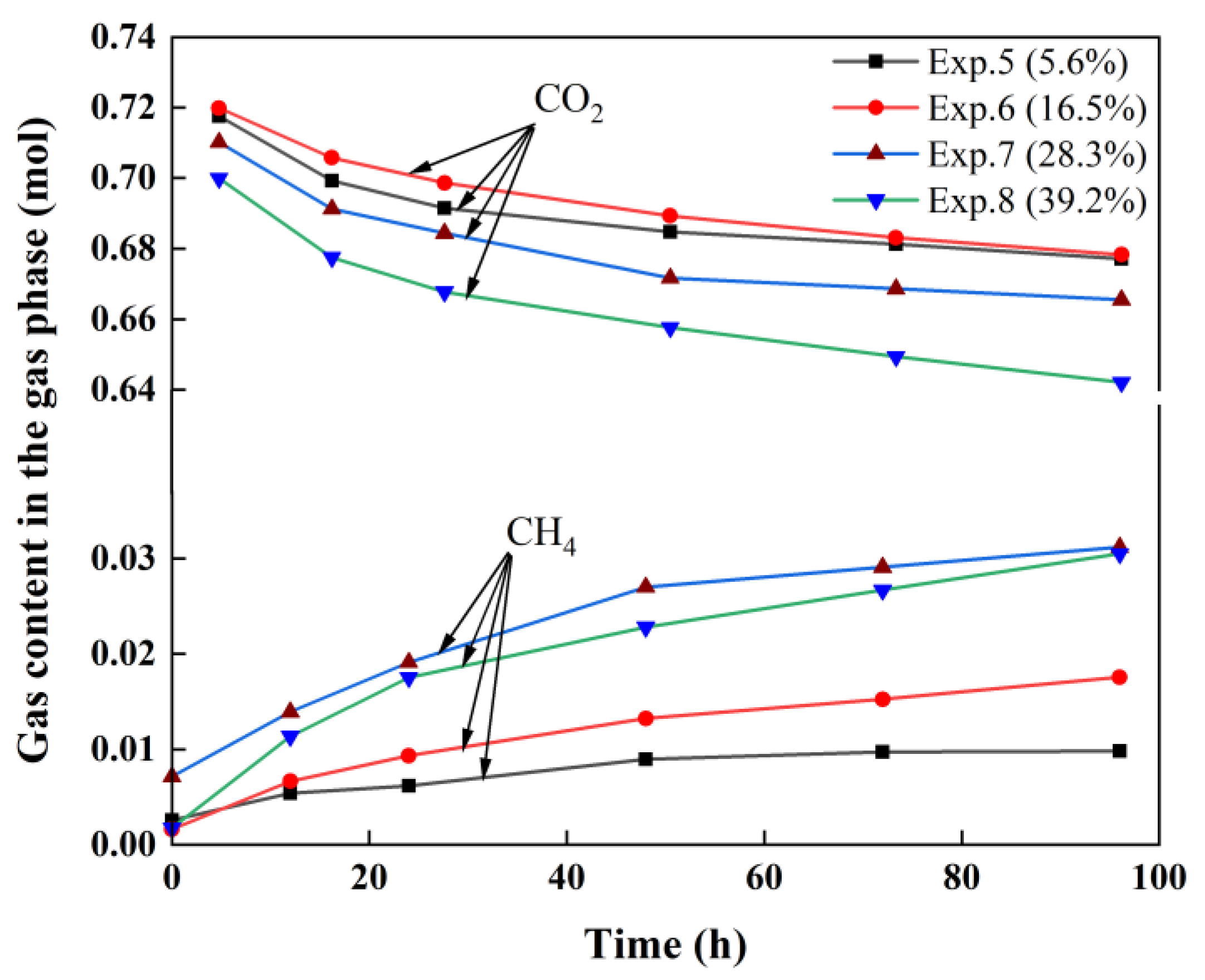
3.3. Variation in CO2 and CH4 in the Gas Phase
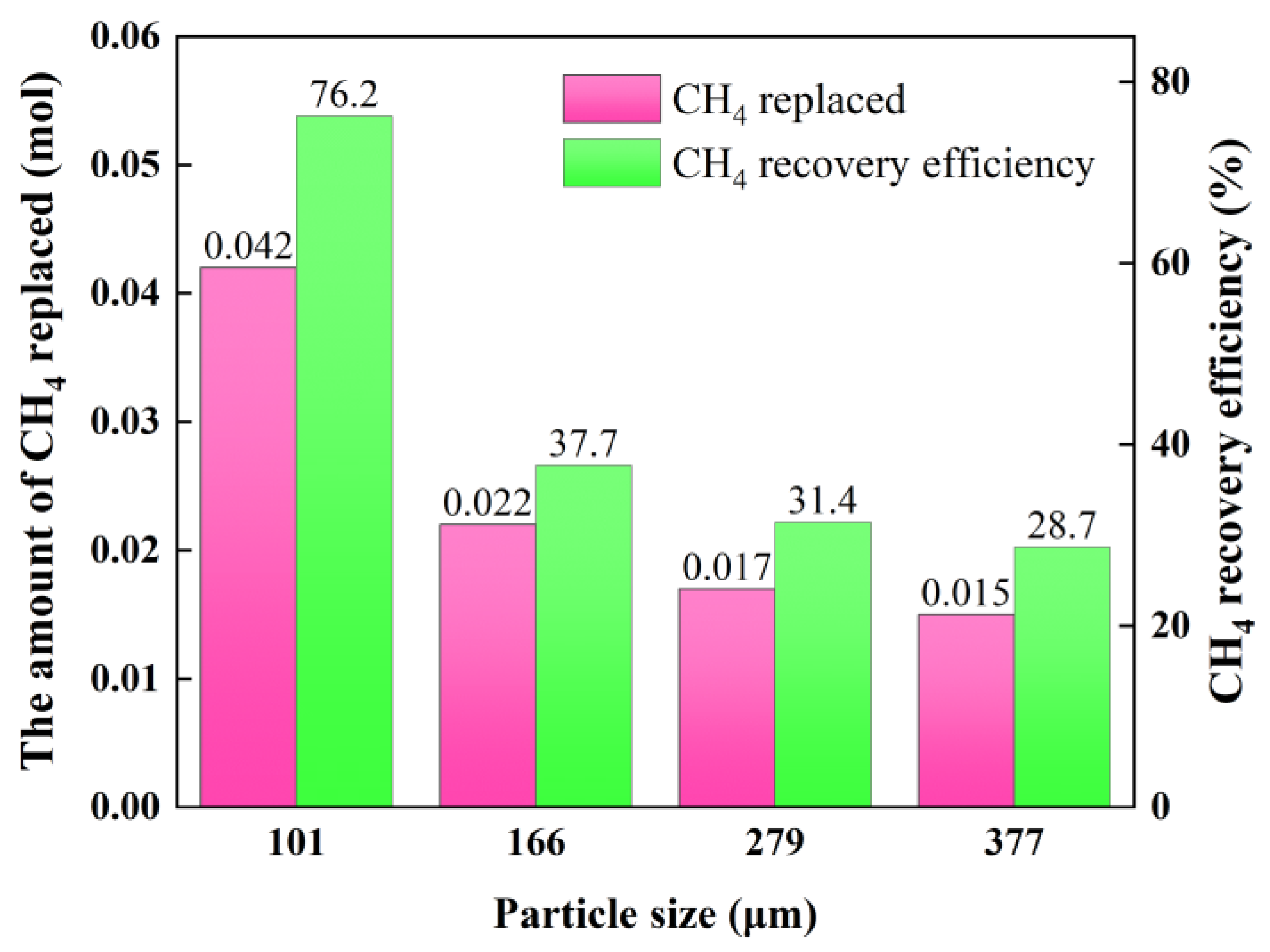
3.4. CH4 Recovery Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrates Hydrates of the Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and applications of gas hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Wang, Y.; Lang, X.; Fan, S.; Wang, S.; Yu, C.; Li, G. Review on Enhanced Technology of Natural Gas Hydrate Recovery by Carbon Dioxide Replacement. Energy Fuels 2021, 35, 3659–3674. [Google Scholar] [CrossRef]
- Collett, T.; Bahk, J.J.; Baker, R.; Boswell, R.; Divins, D.; Frye, M.; Goldberg, D.; Husebø, J.; Koh, C.; Malone, M.; et al. Methane Hydrates in Nature-Current Knowledge and Challenges. J. Chem. Eng. Data ACS J. Data 2015, 60, 329. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Yongxiu, B.; Shuangyuan, L. The Background, Challenges, Opportunities and Realization Paths of Double Carbon Goals. China Econ. Rev. 2021, 4, 10–13. [Google Scholar]
- Ota, M.; Abe, Y.; Watanabe, M.; Smith, R.L.; Inomata, H. Methane recovery from methane hydrate using pressurized CO2. Fluid Phase Equilibria 2005, 228–229, 553–559. [Google Scholar] [CrossRef]
- Li, Z.Z.; Guo, X.Q.; Wang, J.B.; Yang, L.Y. Experiment of developing different systems of CH4 hydrate by CO2 displacement method. Nat. Gas Ind. 2008, 28, 129–132. [Google Scholar]
- Zhou, W.; Fan, S.S.; Liang, D.Q.; Li, D.L.; Tang, C.P.; Tian, G.L. Effect of carbon dioxide pressure on methane hydrate replacement rate. J. Wuhan Univ. Technol. (Transp. Sci. Eng. Ed.) 2008, 32, 547–550. [Google Scholar]
- Zhou, X.; Fan, S.; Liang, D.; Du, J. Replacement of Methane from Quartz Sand-Bearing Hydrate with Carbon Dioxide-in-Water Emulsion. Energy Fuels 2008, 22, 1759–1764. [Google Scholar] [CrossRef]
- Lee, B.R.; Koh, C.A.; Sum, A.K. Quantitative measurement and mechanisms for CH4 production from hydrates with the injection of liquid CO2. Phys. Chem. Chem. Phys. 2014, 16, 14922–14927. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Zhang, X.; Chen, G.; Wang, W. Replacement mechanism of methane hydrate with carbon dioxide from microsecond molecular dynamics simulations. Energy Environ. Sci. 2012, 5, 7033–7041. [Google Scholar] [CrossRef]
- Xitang, Z.; Fan, S.; Liang, D. Kinetic Research on Replacement of Methane in Gas Hydrate with Carbon Dioxide Emulsion. Nat. Gas Geosci. 2013, 24, 259–264. [Google Scholar]
- Xiaowen, W. Experimental Study on the Influence Factors of Exploiting Methane Hydrate by Replacement with CO2; China University of Petroleum (East China): Qingdao, China, 2014. [Google Scholar]
- Yuan, Q.; Sun, C.-Y.; Yang, X.; Ma, P.-C.; Ma, Z.-W.; Liu, B.; Ma, Q.-L.; Yang, L.-Y.; Chen, G.-J. Recovery of methane from hydrate reservoir with gaseous carbon dioxide using a three-dimensional middle-size reactor. Energy 2012, 40, 47–58. [Google Scholar] [CrossRef]
- Ren, L.L.; Jiang, M.; Wang, L.B.; Zhu, Y.J.; Li, Z.; Sun, C.Y.; Chen, G.J. Gas hydrate exploitation and carbon dioxide sequestration under maintaining the stiffness of hydrate-bearing sediments. Energy 2020, 194, 116869.1–116869.11. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, J.; Wu, Q.; Yang, L. Recovering CH4 from natural gas hydrate with CO2 in porous media below the freezing point. Pet. Sci. Technol. 2019, 37, 770–779. [Google Scholar] [CrossRef]
- Fan, S.; Wang, X.; Wang, Y.; Lang, X. Recovering methane from quartz sand-bearing hydrate with gaseous CO2. J. Energy Chem. 2017, 26, 655–659. [Google Scholar] [CrossRef]
- ConocoPhillips. ConocoPhillips Gas Hydrate Production Test; Oil & Natural Gas Technology, Progress Report First Half 2012; Office of Fossil Energy: Anchorage, AK, USA, 2012.
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Water Salinity as Potential Aid for Improving the Carbon Dioxide Replacement Process’ Effectiveness in Natural Gas Hydrate Reservoirs. Processes 2020, 8, 1298. [Google Scholar] [CrossRef]
- Mu, L.; Cui, Q. Experimental Study on the Dissociation Equilibrium of (CH4 + CO2) Hydrates in the (Quartz Sands + NaCl Solution) System. J. Chem. Eng. Data 2019, 64, 5806–5813. [Google Scholar] [CrossRef]
- Pan, D.; Zhong, X.; Zhu, Y.; Zhai, L.; Chen, C. CH4 recovery and CO2 sequestration from hydrate-bearing clayey sediments via CO2/N2 injection. J. Nat. Gas Sci. Eng. 2020, 83, 103503. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Wang, J.; Zhao, J.; Dong, H.; Yang, M.; Liu, Y.; Song, Y. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO 2 replacement of methane hydrate. Chem. Eng. J. 2017, 308, 40–49. [Google Scholar] [CrossRef]
- Pandey, J.S.; Karantonidis, C.; Karcz, A.P.; von Solms, N. Enhanced CH4-CO2 Hydrate Swapping in the Presence of Low Dosage Methanol. Energies 2020, 13, 5238. [Google Scholar] [CrossRef]
- Almenningen, S.; Graue, A.; Ersland, G. Experimental Investigation of Critical Parameters Controlling CH4–CO2 Exchange in Sedimentary CH4 Hydrates. Energy Fuels 2021, 35, 2468–2477. [Google Scholar] [CrossRef]
- Yuanxin, Y.; Xuebing, Z.; Dongliang, L.; Deqing, L. Experiments of CO2 gas sequestration on the seabed by hydrate method. Chem. Ind. Eng. Prog. 2021, 40, 3489–3498. [Google Scholar]
- Jianye, S.; Lele, L.; Xiaowen, W.; Feifei, W.; Changling, L. CO2 Replacement Experiment of Methane Hydrate in Sediment. Nat. Gas Ind. 2015, 35, 56–62. [Google Scholar]
- Yuan, Q.; Sun, C.-Y.; Liu, B.; Wang, X.; Ma, Z.-W.; Maa, Q.-L.; Yang, L.-Y.; Chen, G.-J.; Li, Q.-P.; Li, S.; et al. Methane recovery from natural gas hydrate in porous sediment using pressurized liquid CO2. Energy Convers. Manag. 2013, 67, 257–264. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ukita, T.; Fujii, T.; Noguchi, S.; Ripmeester, J.A. Particle size effect on the saturation of methane hydrate in sediments—Constrained from experimental results. Mar. Pet. Geol. 2011, 28, 1801–1805. [Google Scholar]
- Birkedal, K.A.; Hauge, L.P.; Graue, A.; Ersland, G. Transport Mechanisms for CO2-CH4 Exchange and Safe CO2 Storage in Hydrate-Bearing Sandstone. Energies 2015, 8, 4073–4095. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, Y.; Liu, L.; Qingtao, B.U.; Wang, Z.; Mao, P.; Gaowei, H.U. Research advances in gas-water relative permeability of hydrate-bearing sediments. Mar. Geol. Front. 2022, 38, 14–29. [Google Scholar]
- Kuang, Y.; Zhang, L.; Song, Y.; Yang, L.; Zhao, J. Quantitative determination of pore-structure change and permeability estimation under hydrate phase transition by NMR. AIChE J. 2020, 66, e16859. [Google Scholar] [CrossRef]
- Kumar, A.; Maini, B.; Bishnoi, P.R.; Clarke, M.; Zatsepina, O.; Srinivasan, S. Experimental determination of permeability in the presence of hydrates and its effect on the dissociation characteristics of gas hydrates in porous media. J. Pet. Sci. Eng. 2010, 70, 114–122. [Google Scholar] [CrossRef]
- Dangayach, S.; Singh, D.N.; Kumar, P.; Dewri, S.K.; Singh, J. Thermal instability of gas hydrate bearing sediments: Some issues. Mar. Pet. Geol. 2015, 67, 653–662. [Google Scholar] [CrossRef]
- Xu, J.; Bu, Z.; Li, H.; Li, S.; Sun, B. Pore-scale flow simulation on the permeability in hydrate-bearing sediments. Fuel J. Fuel Sci. 2022, 312, 122681. [Google Scholar] [CrossRef]
- Winters, W.J.; Pecher, I.A.; Waite, W.F.; Mason, D.H. Physical properties and rock physics models of sediment containing natural and laboratory-formed methane gas hydrate. Am. Mineral. 2004, 89, 00001221–00001227. [Google Scholar] [CrossRef]
- Jin, G.; Shuanshi, F.; Deqing, L.; Lihua, W.; Dongliang, L. Overview of Natural Gas Hydrate Exploration and Development. Adv. New Energy Renew. Energy 2019, 7, 10. [Google Scholar]
- Li, C.; Liu, C.; Hu, G.; Sun, J.; Hao, X.; Liu, L.; Meng, Q. Investigation on the multi-parameter of hydrate-bearing sands using nanofocus X-ray computed tomography. J. Geophys. Res. Solid Earth 2019, 124, 2296. [Google Scholar] [CrossRef]




| Number | Median Particle Size (μm) | Porosity (%) |
|---|---|---|
| 1 | 101 | 44.68 |
| 2 | 166 | 43.08 |
| 3 | 279 | 42.01 |
| 4 | 377 | 41.38 |
| NO. | Median Particle Size (μm) | Water Saturation (%) | Initial Hydrate Saturation (%) | Residual Water Weight (g) | CH4 Consumption (mol) |
|---|---|---|---|---|---|
| Exp.1 | 101 | 30 | 16.2 | 6.25 | 0.0552 |
| Exp.2 | 166 | 30 | 17.1 | 6.15 | 0.0581 |
| Exp.3 | 279 | 30 | 16.0 | 6.23 | 0.0550 |
| Exp.4 | 377 | 30 | 16.5 | 6.2 | 0.0556 |
| NO. | Median Particle Size (μm) | Water Saturation (%) | Initial Hydrate Saturation (%) | Residual Water Weight (g) | CH4 Consumption (mol) |
|---|---|---|---|---|---|
| Exp.5 | 377 | 20 | 5.6 | 6.23 | 0.0192 |
| Exp.6 | 377 | 30 | 16.5 | 6.20 | 0.0556 |
| Exp.7 | 377 | 40 | 28.3 | 6.18 | 0.0976 |
| Exp.8 | 377 | 50 | 39.2 | 6.24 | 0.1340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Zhou, X.; Huang, C.; Li, D.; Liang, D. Effect of Residual Water in Sediments on the CO2-CH4 Replacement Process. Energies 2023, 16, 3154. https://doi.org/10.3390/en16073154
Lu F, Zhou X, Huang C, Li D, Liang D. Effect of Residual Water in Sediments on the CO2-CH4 Replacement Process. Energies. 2023; 16(7):3154. https://doi.org/10.3390/en16073154
Chicago/Turabian StyleLu, Fuqin, Xuebing Zhou, Caili Huang, Dongliang Li, and Deqing Liang. 2023. "Effect of Residual Water in Sediments on the CO2-CH4 Replacement Process" Energies 16, no. 7: 3154. https://doi.org/10.3390/en16073154
APA StyleLu, F., Zhou, X., Huang, C., Li, D., & Liang, D. (2023). Effect of Residual Water in Sediments on the CO2-CH4 Replacement Process. Energies, 16(7), 3154. https://doi.org/10.3390/en16073154









