Catalytic Oxidation of Volatile Organic Compounds Using the Core–Shell Fe2O3-Cenospheric Catalyst in a Fluidised Bed Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Methodology for Testing the Catalytic Oxidation Process
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSC | Temperature inside the scrubber, °C |
| Volumetric flow rate at standard conditions, L·min−1 | |
| Mass flow rate (as function of time) of selected compound, kg·s−1 | |
| Temperature of the fluidised bed at time t, °C | |
| ΔTad | Increase in adiabatic temperature inside the reactor, °C |
| TExh1 | Flue gas temperature at fluidised reactor outlet (heat exchanger inlet), °C |
| TExh2 | Flue gas temperature at heat exchanger outlet, °C |
| TAir1 | Air temperature at the heat exchanger inlet, °C |
| TAir2 | Air temperature at the heat exchanger outlet (reactor inlet), °C |
| ΔTM | Mean temperature difference in heat exchanger, °C |
| T25% | Temperature of 25 %wt. VOCs decomposition, °C |
| T50% | Temperature of 50 %wt. VOCs decomposition, °C |
| T75% | Temperature of 75 %wt. VOCs decomposition, °C |
| Enthalpy of selected compound formation in the gaseous state, J·kg−1 | |
| Heat exchange area, cm2 | |
| Total heat transfer coefficient, W·m−2·K−1 | |
| Heat capacity of air at constant pressure as temperature function J·kg−1·°C−1 | |
| Air density as temperature function, kg·m−3 | |
| β | Efficiency coefficient, cm2·min−1·L−1 |
| γ | Ratio between the theoretical energy generated by the reaction in relation to the energy demand for heating the air |
| Heat generated at time t during catalytic oxidation of selected compound, W | |
| Heat demand to warm air from TAir1 to TAir2, W | |
| Heat taken from the exhaust during the cooling process from TExh1 to TExh2, W |
References
- Tokumura, M.; Nakajima, R.; Znad, H.T.; Kawase, Y. Chemical absorption process for degradation of VOC gas using heterogeneous gas-liquid photocatalytic oxidation: Toluene degradation by photo-Fenton reaction. Chemosphere 2008, 73, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Kolade, M.A.; Kogelbauer, A.; Alpay, E. Adsorptive reactor technology for VOC abatement. Chem. Eng. Sci. 2009, 64, 1167–1177. [Google Scholar] [CrossRef]
- Biard, P.F.; Couvert, A.; Renner, C.; Levasseur, J.P. Assessment and optimisation of VOC mass transfer enhancement by advanced oxidation process in a compact wet scrubber. Chemosphere 2009, 77, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, R.; Grévillot, G.; Marsteau, S.; Vallières, C. Values of the mass transfer coefficient of the linear driving force model for VOC adsorption on activated carbons. Chem. Eng. Res. Des. 2013, 91, 955–962. [Google Scholar] [CrossRef]
- Lhuissier, M.; Couvert, A.; Dabert, P.; Amrane, A.; Kane, A.; Audic, J.L.; Dumont, E. Removal of a Mixture of Seven Volatile Organic Compounds (VOCs) Using an Industrial Pilot-Scale Process Combining Absorption in Silicone Oil and Biological Regeneration in a Two-Phase Partitioning Bioreactor (TPPB). Energies 2022, 15, 4576. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Niu, R.; Xu, L.; Lu, Y.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Matsumoto, D.; Diniz, L.A.; Castro, L.S.; Teixeira, A.C.S.C.; Guardani, R.; de Paiva, J.L. Kinetic modeling and experimental validation of a photocatalytic fluidized bed reactor for n-hexane degradation. Braz. J. Chem. Eng. 2019, 36, 1561–1570. [Google Scholar] [CrossRef]
- Debono, O.; Thevenet, F.; Gravejat, P.; Hequet, V.; Raillard, C.; Lecoq, L.; Locoge, N. Toluene photocatalytic oxidation at ppbv levels: Kinetic investigation and carbon balance determination. Appl. Catal. B Environ. 2011, 106, 600–608. [Google Scholar] [CrossRef]
- Li, C.-y.; Jia, Y.-r.; Zhang, X.-c.; Zhang, S.-y.; Tang, A.-d. Photocatalytic degradation of formaldehyde using mesoporous TiO2 prepared by evaporation-induced self-assembly. J. Cent. South Univ. 2014, 21, 4066–4070. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS-PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S.; Crisafulli, C.; Galvagno, S. Catalytic combustion of volatile organic compounds over group IB metal catalysts on Fe2O3. Catal. Commun. 2001, 2, 229–232. [Google Scholar] [CrossRef]
- Kang, J.; Wang, Z.; Yang, Z.; Yan, Y.; Ran, J.; Guo, M. Catalytic Combustion of Low-Concentration Methane over Mx-Cu/γ-Al2O3 (M = Mn/Ce) Catalysts. Ind. Eng. Chem. Res. 2020, 59, 4291–4301. [Google Scholar] [CrossRef]
- Sophiana, I.C.; Topandi, A.; Iskandar, F.; Devianto, H.; Nishiyama, N.; Budhi, Y.W. Catalytic oxidation of benzene at low temperature over novel combination of metal oxide based catalysts: CuO, MnO2, NiO with Ce0.75Zr0.25O2 as support. Mater. Today Chem. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, F.; Tong, J.; Qi, C. Complete oxidation of benzene with cobalt oxide and ceria using the mesoporous support SBA-16. Appl. Catal. A Gen. 2013, 467, 1–6. [Google Scholar] [CrossRef]
- Leclercq, J.; Giraud, F.; Bianchi, D.; Fiaty, K.; Gaillard, F. Novel inductively-heated catalytic system for fast VOCs abatement, application to IPA in air. Appl. Catal. B Environ. 2014, 146, 131–137. [Google Scholar] [CrossRef]
- Kwong, K.Y.; Marek, E.J. Combustion of Biomass in Fluidized Beds: A Review of Key Phenomena and Future Perspectives. Energy Fuels 2021, 35, 16303–16334. [Google Scholar] [CrossRef]
- Żukowski, W.; Berkowicz, G. The combustion of liquids and low-density solids in a cenospheric fluidised bed. Combust. Flame 2019, 206, 476–489. [Google Scholar] [CrossRef]
- Okasha, F.M.; Zeidan, E.S.B. Experimental study on propane combustion in a novel fluidized bed configuration. Fuel Process. Technol. 2013, 116, 79–84. [Google Scholar] [CrossRef]
- Wang, L.; Wu, P.; Ni, X. Combustion of liquid petroleum gas in a fluidized bed furnace with a jetting-mixing distributor. Powder Technol. 2006, 170, 86–93. [Google Scholar] [CrossRef]
- Okasha, F.; Zaater, G.; El-Emam, S.; Awad, M.; Zeidan, E. Co-combustion of biomass and gaseous fuel in a novel configuration of fluidized bed: Combustion characteristics. Fuel 2014, 133, 143–152. [Google Scholar] [CrossRef]
- Miccio, F.; Raganati, F.; Ammendola, P.; Okasha, F.; Miccio, M. Fluidized bed combustion and gasification of fossil and renewable slurry fuels. Energies 2021, 14, 7766. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Sathasivam, S.; Williamson, B.A.D.; Althabaiti, S.A.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. Chemical Vapor Deposition Synthesis and Optical Properties of Nb2O5 Thin Films with Hybrid Functional Theoretical Insight into the Band Structure and Band Gaps. ACS Appl. Mater. Interfaces 2017, 9, 18031–18038. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Tomatis, M.; Xu, H.H.; He, J.; Zhang, X.D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 2016, 15. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 14 March 2023).
- Engineering ToolBox. Available online: https://www.engineeringtoolbox.com/ (accessed on 14 March 2023).
- Żukowski, W.; Migas, P.; Gwadera, M.; Larwa, B.; Kandafer, S. A numerical analysis of heat transfer in a cross- current heat exchanger with controlled and newly designed air flows. Open Chem. 2018, 16, 627–636. [Google Scholar] [CrossRef]

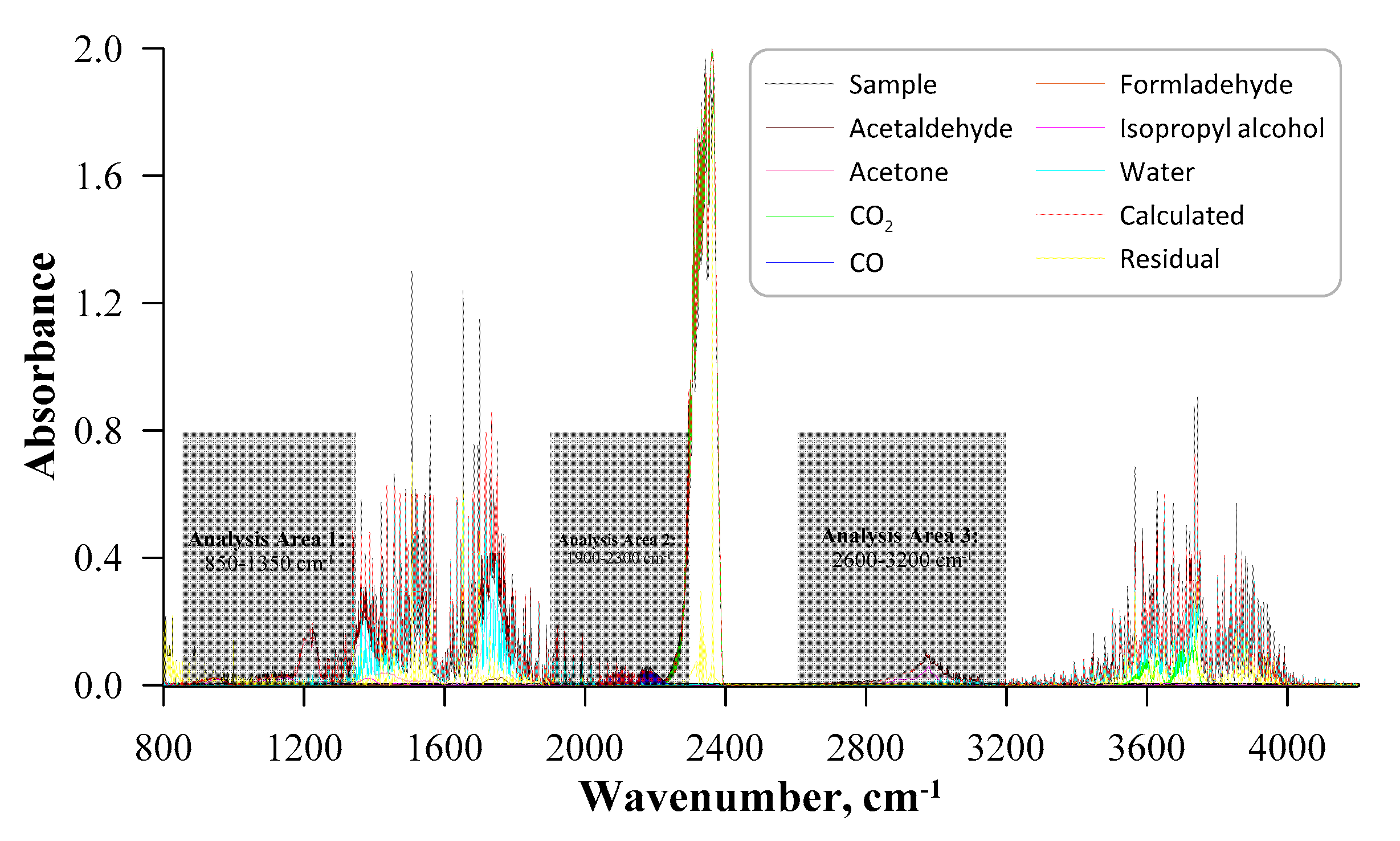
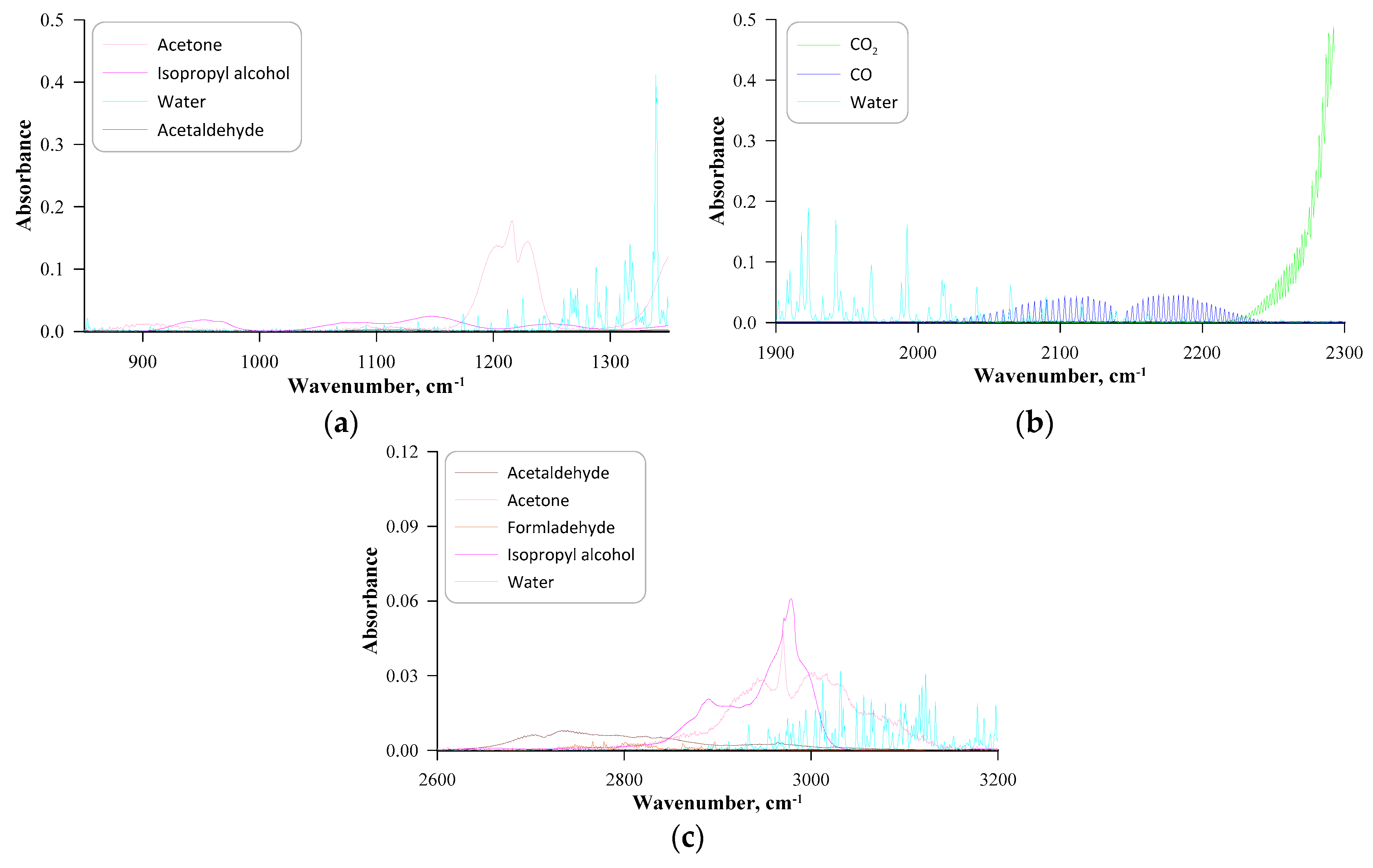
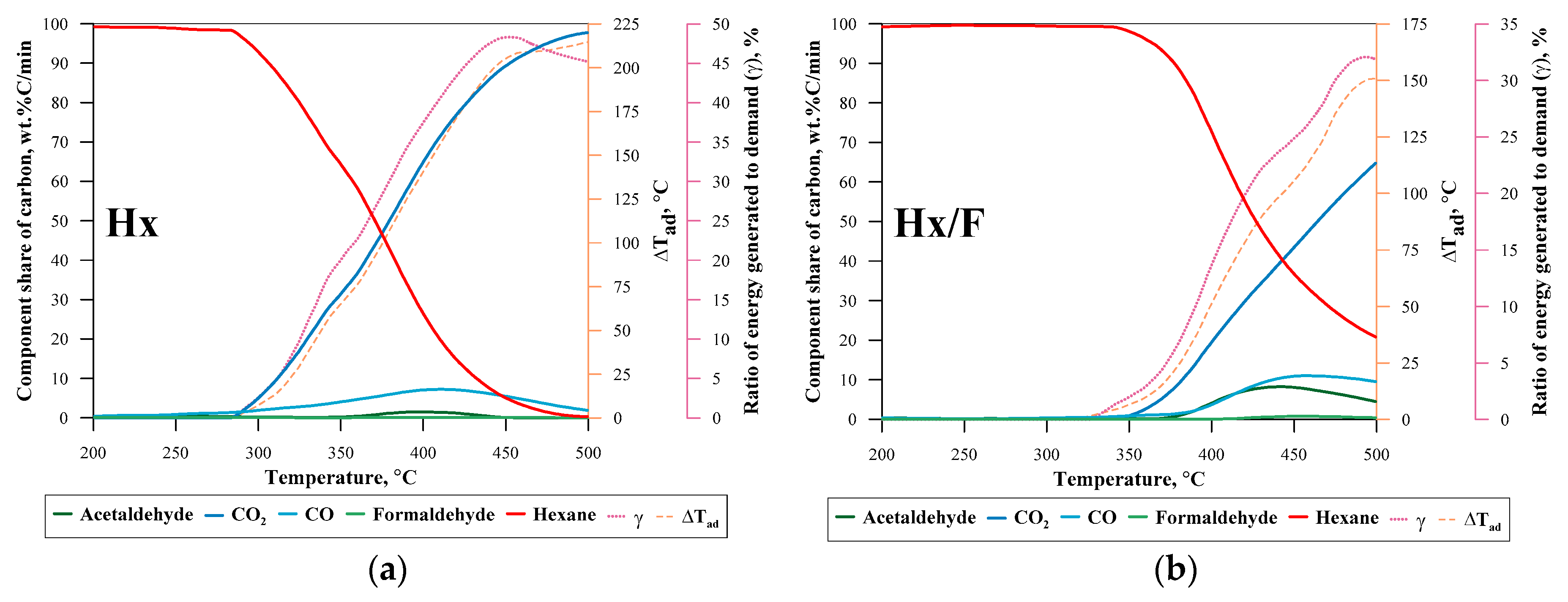
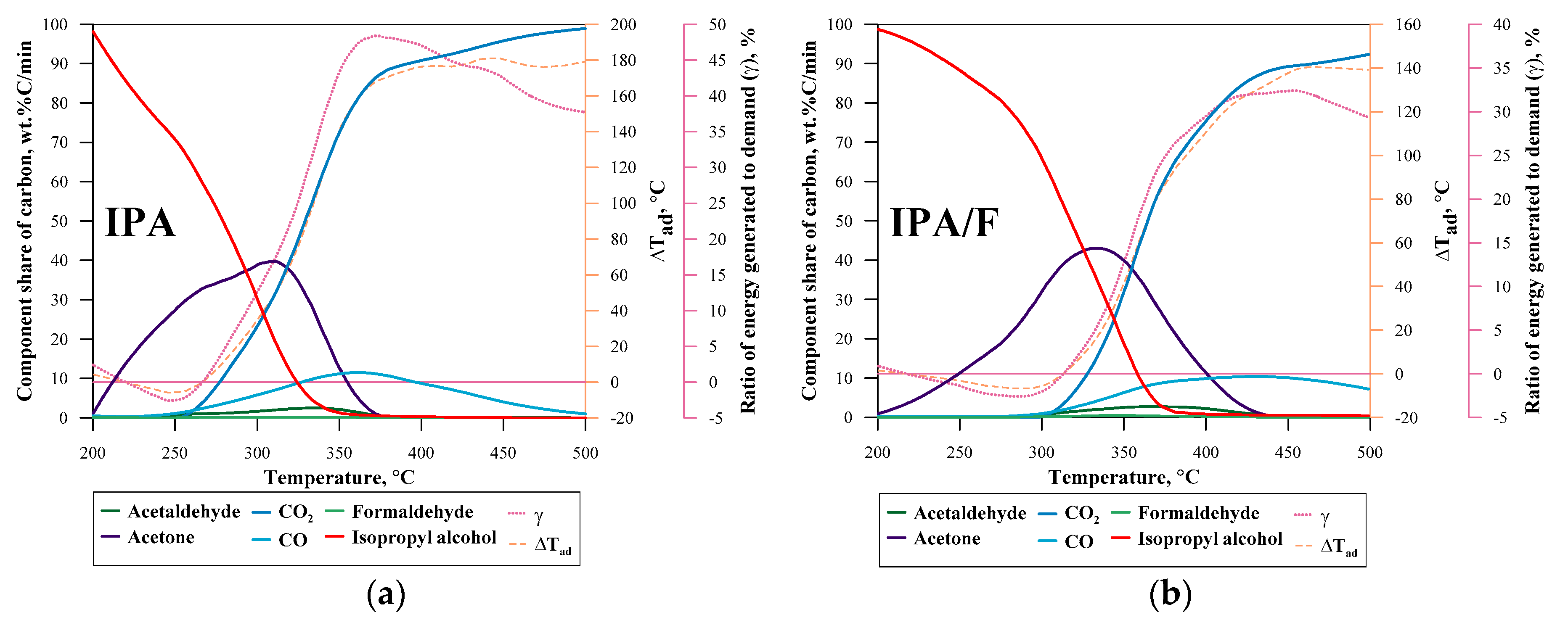
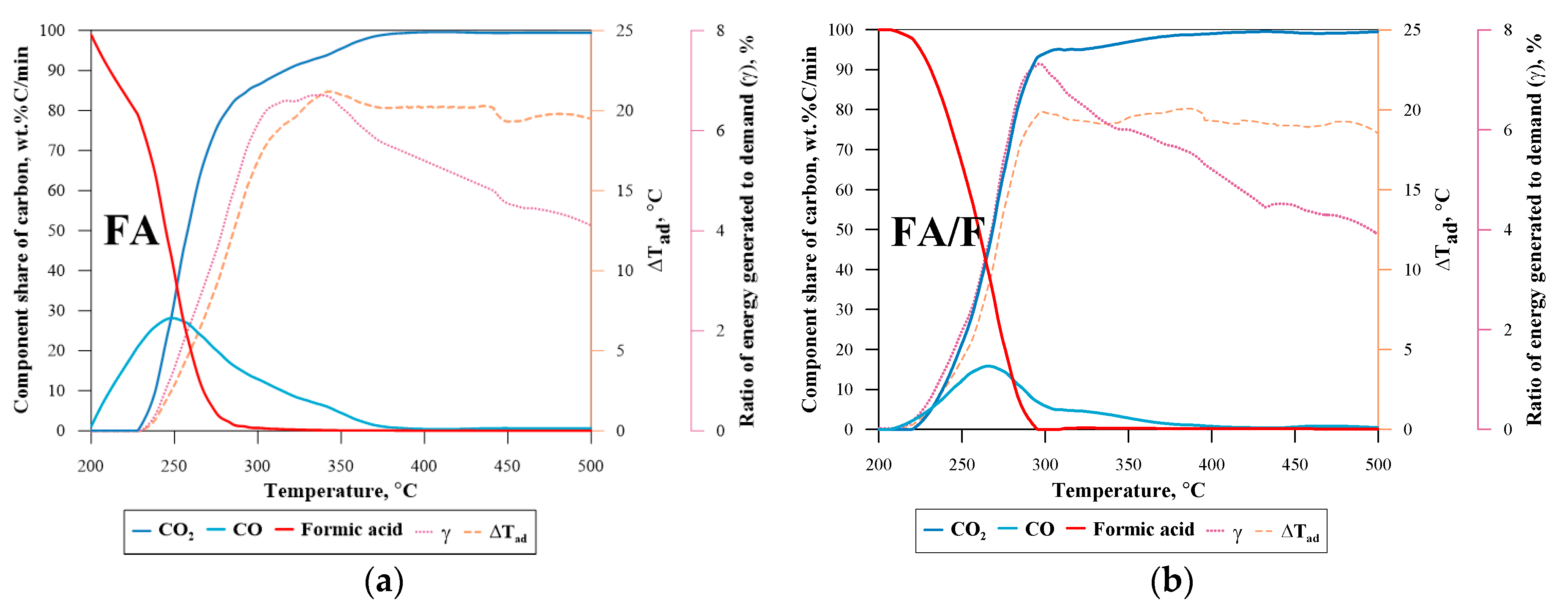
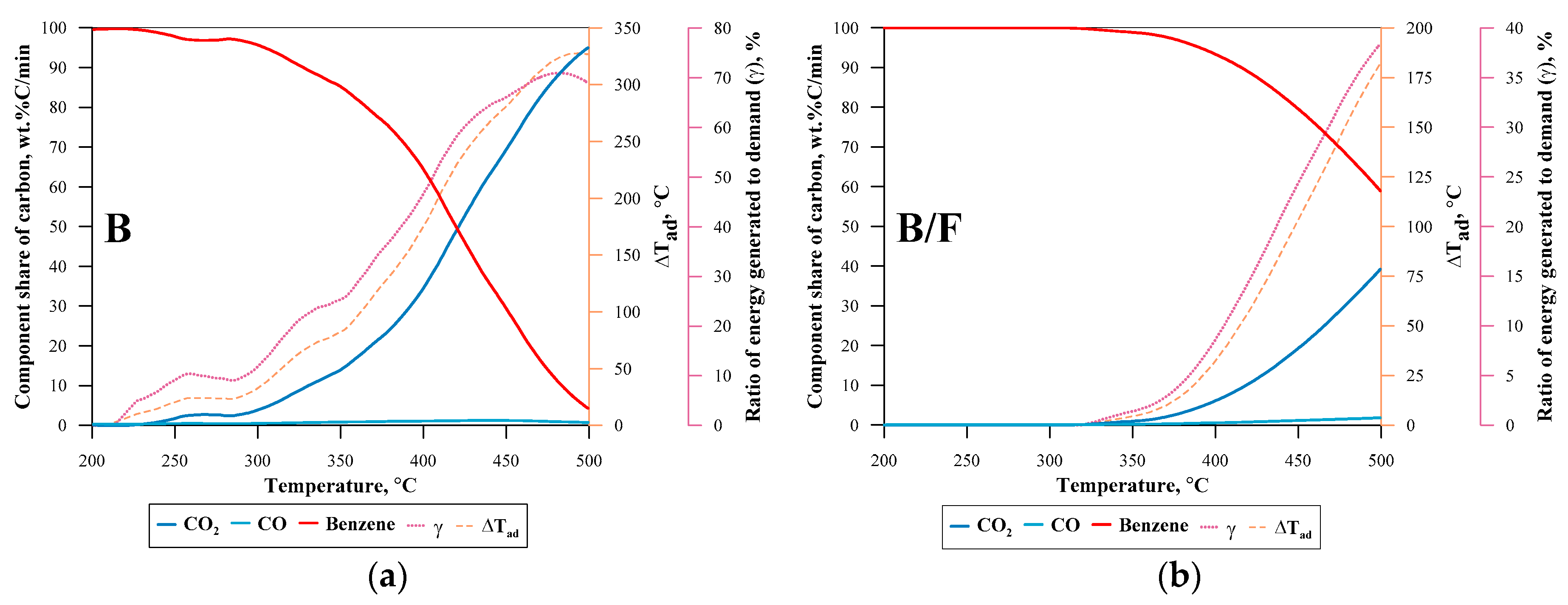
| No. | Label | VOC | TSC | Concentration of VOC, ppmv | 1a mL·min−1 | 1b L·min−1 | 1c L·min−1 |
|---|---|---|---|---|---|---|---|
| 1 | Hx | n-Hexane | 3.5–4.0 °C | 1700 | 100 | 1.5 | 0.4 |
| 2 | Hx/F | n-Hexane | 3.5–4.0 °C | 1500 | 100 | 1.5 | 0.8 |
| 3 | IPA | Isopropanol | 30.0–30.5 °C | 2800 | 180 | 0.9 | 0.4 |
| 4 | IPA/F | Isopropanol | 30.0–30.5 °C | 2600 | 180 | 0.9 | 0.8 |
| 5 | FA | Formic acid | 30.0–30.5 °C | 1200 | 100 | 1.5 | 0.4 |
| 6 | FA/F | Formic acid | 30.0–30.5 °C | 1100 | 100 | 1.5 | 0.8 |
| 7 | B | Benzene | 40.0–40.5 °C | 3500 | 180 | 0.9 | 0.4 |
| 8 | B/F | Benzene | 40.0–40.5 °C | 3700 | 180 | 0.9 | 0.8 |
| No. | Label | T25%, °C | T50%, °C | T75%, °C | WHSV, L·g−1·h−1 | HSV, h−1 |
|---|---|---|---|---|---|---|
| 1 | Hx | 332.6 | 370.7 | 402.1 | 23.7 | 755.9 |
| 2 | Hx/F | 397.4 | 427.0 | 482.3 | 23.7 | 755.9 |
| 3 | IPA | 240.8 | 279.1 | 305.3 | 16.0 | 510.2 |
| 4 | IPA/F | 286.6 | 317.5 | 344.0 | 16.0 | 510.2 |
| 5 | FA | 231.2 | 244.9 | 256.8 | 23.7 | 755.9 |
| 6 | FA/F | 243.9 | 260.1 | 273.0 | 23.7 | 755.9 |
| 7 | B | 379.5 | 419.6 | 456.7 | 16.0 | 510.2 |
| 8 | B/F | 462.2 | - | - | 16.0 | 510.2 |
| VOC | Label | TAir1, °C | TAir2, °C | TExh1, °C | TExh2, °C | QExh, W | AH.E., cm2 | β, cm2·min−1·L−1 |
|---|---|---|---|---|---|---|---|---|
| n-Hexane | Hx | 25 | 280 | 500 | 245 | 2.2 | 33.1 | 82.7 |
| * n-Hexane | Hx/F | 25 | 380 | 600 | 245 | 6.2 | 94.6 | 118.2 |
| Isopropanol | IPA | 25 | 320 | 500 | 205 | 2.5 | 47.0 | 117.5 |
| * Isopropanol | IPA/F | 25 | 370 | 550 | 205 | 6.0 | 111.3 | 139.1 |
| Formic acid | FA | 25 | 380 | 400 | 45 | 3.0 | 504.1 | 1260.2 |
| Formic acid | FA/F | 25 | 380 | 400 | 45 | 6.0 | 1008.2 | 1260.2 |
| * Benzene | B | 25 | 190 | 520 | 355 | 1.4 | 14.2 | 35.5 |
| * Benzene | B/F | 25 | 340 | 670 | 355 | 5.6 | 56.5 | 70.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migas, P.; Żukowski, W.; Bradło, D. Catalytic Oxidation of Volatile Organic Compounds Using the Core–Shell Fe2O3-Cenospheric Catalyst in a Fluidised Bed Reactor. Energies 2023, 16, 2801. https://doi.org/10.3390/en16062801
Migas P, Żukowski W, Bradło D. Catalytic Oxidation of Volatile Organic Compounds Using the Core–Shell Fe2O3-Cenospheric Catalyst in a Fluidised Bed Reactor. Energies. 2023; 16(6):2801. https://doi.org/10.3390/en16062801
Chicago/Turabian StyleMigas, Przemysław, Witold Żukowski, and Dariusz Bradło. 2023. "Catalytic Oxidation of Volatile Organic Compounds Using the Core–Shell Fe2O3-Cenospheric Catalyst in a Fluidised Bed Reactor" Energies 16, no. 6: 2801. https://doi.org/10.3390/en16062801
APA StyleMigas, P., Żukowski, W., & Bradło, D. (2023). Catalytic Oxidation of Volatile Organic Compounds Using the Core–Shell Fe2O3-Cenospheric Catalyst in a Fluidised Bed Reactor. Energies, 16(6), 2801. https://doi.org/10.3390/en16062801







